Abstract
This two PHASE evaluation documented the delivery and effectiveness of evidenced-based health education methods by regular staff to pregnant smokers. During PHASE 1, 436 Medicaid patients were screened and 416 (95%) gave consent: 334 non-smokers and 102 smokers. This historical Comparison (C) group was assessed to document the “normal” pre-Trial smoking prevalence, patient non-disclosure (deception), and cessation rates at the 1st prenatal visit and during care. After this study, a Formative Evaluation of SCRIPT methods was conducted among 139 Experimental group patients and 126 Control group patients. During the PHASE 2, 6514 patients were screened over a 36 month period: 1736 (27%) were smokers and 1340 (77%) gave consent. After randomization, 247 became ineligible. The remaining 1093 smokers received brief routine advice to quit. The Experimental group (N=544) also received: a “Commit To Quit” video, “A Pregnant Woman’s Guide to Quit Smoking”, and counseling. Self-reports and saliva were collected at baseline, ≥ 60 days, and ≤ 90 days postpartum for cotinine analyses to document cessation and significant reduction (SR) rates. The PHASE 1 Formative Evaluation documented a 24% non-disclosure rate at the onset of care. It also confirmed a significantly higher Experimental (17.3%) versus Control group (8.8%) cessation rate and Experimental versus Control group SR rates of 22% and 16%. During PHASE 2, unplanned policy changes, and delivery of E group counseling procedures to 15%–20% of C group patients, resulted in a final E group cessation rate of 12% and C group rate of 10%. The E group SR rate of 18%, however, was significantly higher than the C group SR rate of 13%. Effectiveness varied by the stability of clinic infrastructure, and degree of fidelity of staff performance of assessment and intervention procedures. The methods and results of this study will assist future health education programs for pregnant smokers to plan and conduct process and impact evaluations in prenatal care.
Keywords: effectiveness, health education methods, SCRIPT, Medicaid prenatal care, pregnant smokers
BACKGROUND
Exposure to tobacco smoke during pregnancy is the most salient cause of infant morbidity and mortality in the United States (US Department of Health and Health Services [DHHS], 1990, 2000; Hasselmeyer, Meyer, & Catz, 1979). A dose-response relationship exists between smoking and low birth weight (LBW): rates increase 53% for light and 130% for heavy smokers (DHHS, 1990; Hasselmeyer, Meyer, & Catz, 1979). Smoking during and after pregnancy also causes infant respiratory diseases (DHHS, 1990). Despite nationwide initiatives, the National 2000 Health Objectives for cessation and LBW during pregnancy were not achieved (DHHS, 2000). The National 2010 Health Objectives to increase cessation during pregnancy to 99% and reduce LBW incidence to ≤ 5% (DHHS, 2000) were also not achieved. Because of consistently large differences between smoking rates reported in the last decade by the CDC of 10.2% (DHHS, 2006) and SAMSHA rates of 17.3% (Office of Applied Studies, 2006), well documented high patient non-disclosure rates (Russell et al. 2005; Windsor, Boyd, & Orleans, 1998), and a lack of biochemical confirmation of self-reports in any national survey during pregnancy, limited progress has been made to reduce the smoking rate during pregnancy in the U.S. All national and state smoking rates based only on patient self-reports are very inaccurate.
The need to disseminate and evaluate the level of adoption by regular staff and the effectiveness and cost-effectiveness of evidence-based intervention methods is well recognized (Kendrick, Zahniser, Miller, et al., 1995; Li, Windsor, Lowe, et al., 1992; Windsor, Cutter, Morris, et al., 1985; Windsor, Lowe, Perkins, et al., 1993; Windsor, Li, Lowe, et al., 1993; Windsor, Boyd, & Orleans, 1998; Windsor, Woodby, Miller, 2000). Windsor, Orleans, and Boyd (1998), the Agency for Health Care Policy and Research (AHCPR, Fiore et al., 1996), the Agency for Healthcare Research and Quality (AHRQ, Fiore, Bailey, Cohen, et al.,2000, 2008), and Cochrane-Lumley, Oliver, & Waters, 2008 meta-analyses confirm that health education methods for pregnant smokers are modestly efficacious. While rates vary by population characteristics, typical cessation rates of 2–10% during care from brief advice (Ask-Advise-Assess) may be increased to 10–20% by delivery of best practices methods (Ask-Advise-Assess-Assist-Arrange) by trained providers (Fiore, Bailey, Cohen, et al., 1996, 2000, 2008; Li et al, 1992; Lumley, Oliver, & Waters, 2006; Windsor et al., 1985, 1993a, 1993b, 2000a; Windsor, Boyd, & Orleans, 1998; Windsor, 2010).
The Smoking Cessation and Reduction In Pregnancy Treatment (SCRIPT) Trial III was conducted as a sequel to two successful evaluations, Trial I (Windsor, et al., 1985) and Trial II (Windsor, et al.,1993a). Trials I and II had confirmed the successful delivery of the health education intervention by Health Education Specialists, and its efficacy and cost-effectiveness (Windsor, Warner, and Cutter, 1988, Windsor, et al, 1993a, Windsor, 2003). Biochemically confirmed effectiveness rates attributable to best practice methods delivered by regular prenatal care staff have not been documented for a large representative state-wide sample of pregnant Medicaid-supported smokers.
Trial III assessed the fidelity of the delivery of SCRIPT methods by regular staff at 10 prenatal clinics by public health nurses, social workers, and nutritionists, and evaluated the effectiveness of the methods among a large cohort of patients representative of a state Medicaid population. Our primary hypothesis was that the AHCPR, 1996 and AHRQ, 2000 recommended SCRIPT methods would significantly increase the cessation and significant reduction rates beyond the normal, pre-Trial III rates for Medicaid pregnant smokers. This report is a comprehensive presentation of the methods and results of the SCRIPT Trial III.
METHODS
This Trial was a five-year, two PHASE collaborative study between the University of Alabama at Birmingham (UAB) and Alabama Department of Public Health (ADPH). During PHASE 1, organizational development and site selection were completed. A pre-Trial, non-experimental smoking history study was conducted to document smoking prevalence and the patient non-disclosure (deception) rate at the onset of care (Russell et al, 2005). The historical comparison (C) group was designed to confirm in year 1 the “normal” effectiveness (cessation and significant reduction) rates, from the delivery of brief cessation counseling by staff before the new SCRIPT methods were introduced at the clinics by regular prenatal care staff. After the smoking history study was completed, Staff at each site received a standardized training program to prepare them to implement the new SCRIPT and assessment procedures. A Formative Evaluation was conducted among 265 smokers to determine the feasibility of the delivery of the new intervention methods and to document behavioral impact in years 1–2. PHASE 1 evaluation and intervention methods and results have been published (Windsor et al., 2000a; Crawford et al. 2004).
In years 2–5 the PHASE 2 Effectiveness Evaluation of the SCRIPT Program was implemented. Trial III methods were based on the AHCPR (Fiore et al., 1996) and AHRQ (Fiore et al., 2000) Tobacco Treatment Guidelines, the experiences and results of SCRIPT Trial I and Trial II in Alabama representing 1223 Medicaid patients (Windsor et al., 1985, 1993a; Windsor, Boyd, & Orleans, 1998), and five other successful, independent evaluations of SCRIPT methods (Sweden: Hjalmarson, Hahan, & Svanberg, 1991; Canada: O’Connor, Davies, Dulberg, et al., 1992; Norway: Valbo & Nylander, 1994; Valbo & Schioldborg, 1994; North Carolina: Hartmann, Thorpe, Pahel-Short, et al., 1996; and Ohio: Gebauer, Kwo, Haynes, et al., 1998).
Organizational Development
Involvement of public health program leadership and clinic staff in planning and policymaking processes in the introduction and evaluation of new health education methods for routine prenatal care practice is essential. A science-policy-practice partnership of UAB Investigators and Bureau of Family Health Services (BFHS) Program Directors, the SCRIPT Policy and Management Committee (PMC), was established to develop the proposal, and direct project implementation. A Practice Advisory Committee, one staff member per prenatal clinic, was created to ensure routine practice input to the PMC.
Site Selection
A site selection process was designed to yield a representative sample of the state’s pregnant Medicaid population who smoked. Eligible sites were identified in the 67 counties using self-reported smoking rates from annual vital statistics (Woolbright & Henderson, 1997). Because a county needed ≥ 50 smokers/year to participate (≥ 1 smoker/week), 50 counties were ineligible. Because one of the 17 eligible counties had a very large annual maternity census, two moderate-sized, eligible counties were combined to create one large matched cohort. After stratification of the 16 eligible counties by number of smokers and percent black and white, eight matched dyads were created. One county per dyad was randomly selected to participate in the SCRIPT Trial III. Clinic and staff participation was voluntary. There were 10 prenatal care clinics and 28 regular members of the staff in the eight counties selected.
The eight counties selected, one from each of the eight Public Health Areas, provided services annually to > 15% of pregnant Medicaid patients in Alabama (Woolbright & Henderson, 1997). A comparison of statistics for the year prior to Trial III confirmed the comparability of the eight SCRIPT counties selected, the nine SCRIPT eligible counties not selected, and the 50 non-eligible counties for the following rates: (1) infant mortality; (2) LBW; (3) percent receiving WIC; (4) percent in family planning; (5) percent of high school graduates in the county; and, (6) percent black and white. We were successful in identifying a sample of counties representative of the Alabama Medicaid supported prenatal care population.
After each county was recruited to participate, staff orientation sessions and patient flow assessments (PFA) were conducted at all clinics during the first six months of the Trial. A PFA documented the type of services and health education methods provided to pregnant women, smokers and non-smokers, and time per provider at the 1st visit (DHHS, 1993). New SCRIPT procedures were introduced during the training as a collaborative partnership, reflecting a continuous quality improvement (CQI) philosophy (Solberg, Kottke, Brekke, et al., 1996). Because smoking status assessment and brief counseling were part of routine care, our training objective was to collaborate with staff to refine the normal counseling structure, process and content. Total training time was three hours each during two on-site sessions. Associated costs were modest (Windsor, Warner, & Cutter, 1988; Windsor et al., 1993).
PROGRAM EVALUATION DESIGN
Historical Comparison (C) Group Design
During PHASE 1 a representative sample, a non-experimental historical comparison (C) group, of all new patients, smokers and non-smokers from all sites, was assessed over a one month period at their 1st visit and at ≥ 60 days. The notation (C) is used to indicate that the group was not randomized and was created before staff was trained to deliver the new SCRIPT methods. This study documented the “normal” pre-Trial self-reported and cotinine-confirmed prevalence and non-disclosure (deception) rates at the 1st visit. It also confirmed the “normal” pre-Trial cessation and significant reduction rates during care attributable to the brief informational methods provided by staff to smokers. The smoking history study was conducted before SCRIPT staff training and before the intervention was introduced to the Experimental group patients during the Formative and Effectiveness Evaluations.
The pre-Trial historical comparison (C) group study was conducted because we anticipated that the Trial III Control group cessation and significant reduction rates would be 5% higher than the “normal”, historical comparison (C) group cessation and significant reduction rates for these clinics and patients. In SCRIPT Trial II (Windsor et al., 1993a), we conducted the same pre-Trial comparison group study as Trial III. A representative sample of 406, 100 smokers and 306 non-smokers, new patients (94% of eligible patients) was recruited during one month from the four largest clinics in Birmingham, AL.
After completing the Formative Evaluation, an Effectiveness Evaluation was conducted. Smokers were randomly assigned at each clinic to an Experimental group or Control group after screening, consent, and baseline assessment. Participants continued to receive regular obstetrical care from Medicaid reimbursed providers in their home county.
SCRIPT Procedures
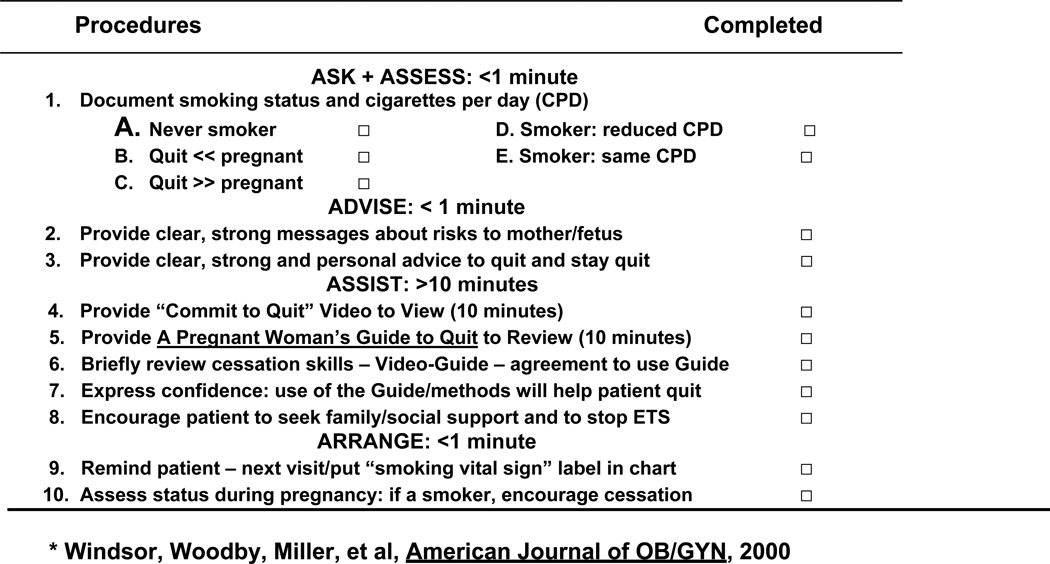
All Experimental and Control Group patients received (Ask-Advise-Assess-Arrange) Procedures #1, #2, #3, #9 and #10 (Fiore et al., 1996, 2000; Windsor et al., 1985, 1993a). As noted in Form 1, Experimental group patients also received (Assist) Procedures #4 through #8:
A “Commit to Quit Smoking During and After Pregnancy” Video (Windsor, Crawford, & Woodby, 1998)
A Pregnant Woman’s Guide to Quit Smoking (Windsor, 1985), and
A ≤ 10 minute counseling session (Laine & Davidoff, 1996; Leopold, Cooper, & Clancy,1996).
Form 1.
SCRIPT PROCEDURES *
After an introduction, the Guide was given to patients to review while watching a 14-minute Video (edited: 10 minutes post-Trial). It presents three motivational themes by ex-pregnant smokers: (1) promotion of self-efficacy; (2) strong visual messages about maternal, fetal, and infant risk; and (3) cessation skill demonstration. The Guide (5th edition, Windsor, 2005) has a 6th grade reading level and uses a 10 day plan with daily cessation skills. The final SCRIPT process and content was derived from meta-evaluations (AHCPR, 1996; Windsor, Boyd, & Orleans, 1998), patient flow analyses (CDC, 1993), and patient interviews (Crawford, et al, 2005).
Measurement Procedures
All patients completed standardized baseline and follow-up forms and provided a saliva sample for analysis during Trial III. A smoker was defined as a patient who reported ≥1 cigarette (even one puff) in the last 7 days, or had a cotinine ≥ 20ng/mL. Follow-up assessments were performed ≥ 60 days after the 1st visit, and ≤ 90 days postpartum. A patient lost-to-follow-up (LTF) was counted as a smoker. All forms were transmitted daily by staff at each clinic to the UAB Data Coordinator using the Teleform software (Hardin et al., 2005).
Significant Reduction (SR) Measurement
In the SCRIPT Trial II (Windsor et al., 1993a), a significant reducer was defined as a patient with a baseline saliva cotinine ≥ 30ng/mL and ≥ 50% reduction at follow-up. SR was documented among 16.8% among 400 Experimental group patients in Trial II. As predicted, while Trial II quitters had infants on average 200+ grams heavier than smokers, a strong, positive association between SR and an increased, adjusted birth weight of 96 grams was documented (Li, Windsor, Perkins, et al., 1993). Patients who significantly reduced had heavier babies than smokers. Trial II evidence suggested that significant reduction may be a secondary indicator of impact and harm reduction (Li et al., 1993; Windsor et al., 1993a; Windsor, Li, Boyd, et al., 1999).
In Trial III, a baseline saliva cotinine had to be ≥ 50ng/mL and follow-up had to be ≤ 50% lower than the baseline to classify a patient as a significant reducer. The revised definition increased the validity of the biochemical estimate of SR by eliminating patients with ultra low baseline levels of exposure. Quitters could not be counted as a significant reducer.
RESULTS
Smoking History Study
During the pre-Trial smoking history study, a representative sample of 436 new patients, 334 non-smokers and 102 smokers, were screened at all clinics for one month. Ninety-five percent (N = 416) gave consent, and provided a baseline: 90% (N = 375) had a follow-up assessment and gave a saliva sample. Among the 324 patients who said at their 1st visit “they were not smokers,” a 24% non-disclosure (deception) rate was confirmed (Russell et al., 2005). This study also confirmed a 4.2% cessation rate and 8.5% significant reduction rate among the pre-Trial III historical comparison (C) group of smokers in year 1. These data documented the “normal” pre-Trial impact of the brief informational methods before the 28 regular clinic staff were trained to deliver the new SCRIPT methods and before smokers were randomized during the Effectiveness Evaluation in years 2–5.
During the Effectiveness Evaluation, over a 36 month period 6514 patients were screened at their 1st visit: 1736 (27%) were smokers. Of the 1736, 1340 (77%) consented and were randomized. After randomization of the 1340 to the Control group or Experimental group, 247 patients (18%) from two counties, equally distributed in the Control and Experimental groups, became ineligible for Trial III participation because the Medicaid primary care contracts at those care sites were rebid by the Department of Health in years 3 and 4. A comparison of the 1093 Trial III eligible participants to the 247 ineligible patients confirmed a significant difference for only the mean baseline cotinine value.
Table 1 presents descriptive data about the 1093 patients who gave consent and were randomized. As noted, the 452 Experimental group and 449 Control group patients and the patients lost-to-follow-up (LTF), 95 Experimental group and 97 Control group, were not significantly different for multiple baseline smoking predictors (Dolan-Mullen, Ramirez, & Groff, 1994; Windsor, Boyd, & Orleans; 1998; Woodby, Windsor, Snyder, et al., 1999). These data documented high rates of eligible patient participation (77%), and low attrition rates (18%) for a Medicaid population. These rates and the lack of significant differences between participants who continued or dropped out of the evaluation provided strong support for a small degree of selection bias in Trial III. In combination with the random selection of counties, these data provided strong support for the representativeness of the study cohort and external validity of Trial III results.
Table 1.
Baseline Comparability of SCRIPT Trial III Patients
| Control Group (n = 449) |
Control LTF (n = 97) |
Experimental Group (n = 452) |
Experimental LTF (n = 95) |
Ineligible a | Significance | |
|---|---|---|---|---|---|---|
| Mean age (years) | 22.4 | 24.0 | 22.2 | 23.0 | 22.0 | ns |
| Black | 15.7% | 19.6% | 15.4% | 14.7% | 17.8% | ns |
| Cigarettes per day | 9.8 | 10.3 | 10.4 | 12 | 9.6 | ns |
| Lives with smoker | 69.8% | 75.3% | 73.7% | 66.0% | 72.0% | ns |
| Mean cotinine | 163 ng/mL | 181 ng/mL | 181 ng/mL | 178 ng/mL | 230 ng/mL b | 0.05 |
| Estimated gestational age (weeks) | 10.0 | 9.2 | 9.2 | 9.6 | 10.8 | ns |
LTF = lost to follow-up
Ineligible = No longer received care at clinic sites
Experimental + control group vs. ineligible
Staff Performance Measurement and Process Evaluation Results
A process evaluation confirmed that 6514 patients were screened over a 36 month period: 77% of eligible smokers (1340/1736) agreed to participate. Twenty-eight staff at the 10 clinics implemented Trial III patient assessment and intervention procedures for a high percentage of patients as part of their routine practice without additional compensation. They performed 100% of baseline and 82% of follow-up assessments, and collected 99% of baseline and 72% of the follow-up saliva samples. Based on patient follow-up reports, staff provided the Video to 95%, the Guide to 99%, and counseling methods to 97% of the E group (Windsor, Whiteside, Solomon, et al., 2000; Windsor, Clark, Boyd, Goodman, 2004).
Our process evaluation data and discussions about delivery of SCRIPT methods to Control group patients with our Practice Advisory Committee at our annual two-day Progress Review meeting in years 3 and 4 confirmed Control group exposure to SCRIPT methods. Some of clinic staff admitted to having provided some or all SCRIPT methods to a significant proportion of their Control group patients. For example: at the largest Trial III clinic with 194 patients (17.8% of Trial III participants), we confirmed a Control group rate of 17.5% and an Experimental group cessation rate of 22.7%. A large percentage of Control group patients at this site had to have received most/all of the SCRIPT methods to produce these impact rates. The Experimental and Control group cessation rates at this Trial site were among the highest rates for pregnant smokers cited in meta-analyses (Fiore et al., 1996, 2000; Lumley, Oliver, & Waters, 2009; Windsor, Boyd, & Orleans, 1998, Windsor, 2010).
Cessation Rates
Data in Table 2 from the effectiveness evaluation confirmed that the Experimental group group cessation rate of 12.0% was not significantly higher than the 10.0% Control group rate. Data in Table 2 also confirmed that the Control group cessation rate of 10.0% was also significantly higher (P > 0.03) than the normal 4.2% cessation rate of the pre-Trial historical comparison (C) group. The 5.8% cessation rate difference (10.0% minus 4.2%) in the Control group versus the historical comparison (C) group cessation rates confirmed that a significantly lower percentage of patients were quitting from the brief counseling methods provided by clinic staff before training and before randomization and delivery of the SCRIPT methods to Experimental group patients. The Experimental group (12%) versus Control group (10%) cessation rates in Table 2 were also in contrast to the published Formative Evaluation results. The Experimental group (n = 136) cessation rate of 17.3% was significantly higher than the Control group (n = 129) cessation rate of 8.8% (Windsor, Woodby, Miller, et al, 2000a) in year 1–2.
Table 2.
Trial III Comparison (C), Control, and Experimental Group Smoking Status at Follow-Up
| (C) Groupa (n = 96) |
Control Group (n = 549) |
Experimental Group (n = 544) |
z Scoreb | p Valueb | |
|---|---|---|---|---|---|
| Cessation | 10.0%c | 12.0c | 1.02c | 0.31c | |
| 4.2%d | 10.0%d | 1.85d | 0.03d | ||
| Significant reduction | 13.2%c | 18.2c | 1.96c | 0.02c | |
| 8.5%d | 13.2%d | 1.63d | 0.052d |
Historical comparison (C) group
One-tailed tests
Experimental group vs. control group
Comparison (C) group vs. control group
Because there were no significant differences in any of the predictors of smoking behavior change during pregnancy among Experimental and Control groups LTF patients, it is implausible that all patients LTF were smokers. Although we appropriately applied an “Intent to Treat” policy in the computation of our impact rates, among the 786 of 1093 patients (72%) with two cotinine tests, the Experimental group cessation rate was 16.2% and Control group rate was 13.3%. While there was probably a small degree of selection bias between the two cohorts, these data suggested that the true cessation rates of the Experimental and the Control groups were most likely higher than the impact rates presented in Table 2.
Significant Reduction (SR) Rates
Analysis of the significant reduction rates in Table 2 confirmed that the SCRIPT methods produced a statistically higher (P > 0.02) Experimental group (18.2%) versus Control group (13.2%) significant reduction rate. Data in Table 2 also confirmed that the 8.5% significant reduction rates of the comparison (C) group was 4.7% lower than the Trial III Control group significant reduction rate of 13.2% (P > 0.05). Data in Table 3 confirmed that a percentage of heavy, Experimental and Control groups smokers (≥ 266 ng/mL) dramatically reduced (≥ 70%) their tobacco exposure levels during their pregnancy.
Table 3.
Control and Experimental Group Cotinine Values (ng/ML) at Baseline and Follow-Up
| Control Group | Experimental Group | |||
|---|---|---|---|---|
| Baseline | Follow-Up | Baseline | Follow-Up | |
|
Cessation |
(n = 55) 60 ng/mL |
4 ng/mL |
(n = 65) 85 ng/mL |
2 ng/mL |
|
Significant reductiona |
(n = 65) 271 ng/mL |
60 ng/mL (<79%) |
(n = 87) 263 ng/mL |
86 ng/mL (<67%) |
|
Insignificant change |
(n = 429) 174 ng/mL |
192 ng/mL |
(n = 392) 180 ng/mL |
191 ng/mL |
Excludes patients who quit
Although we also applied an “Intent to Treat” policy to compute significant reduction rates, assuming LTF patients had not significantly reduced, this is also implausible. There were no significant differences in smoking predictors among the 452 Experimental group, 449 Control group, 95 Experimental LTF and 97 Control LTF patients. After excluding the 110 Experimental and Control group patients with two cotinine tests who quit, the significant reduction rates were: Experimental group SR = 24.0% and Control group SR = 18.0%. The true significant reduction rates were higher than the reported rates. These data confirmed that a large proportion of patients with the highest baseline cotinine levels were able to substantially reduce exposure to tobacco smoke. The follow-up cotinine levels also confirmed that SR patients were not compensating in their daily pattern of tobacco use.
Non-Disclosure (Deception) Rates
The Experimental and Control group follow-up non-disclosure (deception) rate among smokers in Table 2, defined as patients who said they quit but had a saliva cotinine of ≥ 20ng/ml, was 10%. This rate was much lower than the deception rate of 24% in patients in the Trial III smoking history study who said they were “non-smokers” at the 1st visit (Windsor, et al, 2000, Russell et al., 2005). Trial III data were consistent with the non-disclosure rate of 25% among “non-smokers” from Trial II (Windsor, et al, 1993a, Windsor, Boyd, & Orleans; 1998). Trial II and III data from 2000+ patients indicated that even after informed consent by a provider, patients were much more sensitive at their 1st visit about not reporting smoking status than during care and as a participant in an evaluation. Systematic reviews of completed evaluations have consistently reported the highest rates of non-disclosure at the 1st prenatal visit among patients in Medicaid-supported care (Windsor, Boyd, and Orleans, 1998).
Trial III and Trial II Control Group Exposure to SCRIPT and Estimated Impact
In an evaluation and interpretation of Trial III results, data from the pre-Trial historical comparison (C) group combined with the published (C) group results from Trial II (Windsor, Lowe, Perkins, et al, 1993), need to be examined. Trial II applied the same assessment, measurement, and intervention methods as Trial III for a representative sample (n = 914) of pregnant smokers recruited over a 36 month period in Birmingham, Alabama. Trial II process evaluation confirmed successful implementation. As noted in Table 4, among the historical comparison (C) groups of Medicaid patients who smoked before both Trials, the normal pre-Trial II cessation rate was 3.0% and pre-Trial III cessation rate was 4.2%. The normal pre-Trial II significant reduction rate was 9.3% and 8.5% in Trial III. As noted in Table 4, both comparison (C) group rates were significantly lower (P > 0.05) than the Trial II Control group cessation rate (3.0% vs 8.5%), and Trial III Control group cessation rate (10% vs 4.2%).
Table 4.
SCRIPT Trial II and Trial III Comparison (C), Control and Experimental Group Cessation and SR Rates
| Group | |||||
|---|---|---|---|---|---|
| Comparison (C) | Control | Experimental | Differencea | ||
| SCRIPT Trial II | |||||
| Cessation | 3.0%*1,2 | 8.5%*1 | 14.3%*2 | +11.3 | |
| SR | 9.3%*2 | 12.3% | 16.8%*2 | +7.5 | |
| Sample size (n) | 100 | 414 | 400 | ||
| SCRIPT Trial III | |||||
| Cessation | 4.2%*1,2 | 10.0%*1 | 12.0%*2 | +7.8 | |
| SR | 8.7%*2 | 13.1% | 18.2%*2 | +9.5 | |
| Sample size (n) | 96 | 549 | 544 | ||
Note: SR = significant reduction
Experimental group rates minus pretrial comparison (C) group rates
Comparison (C) group rate vs. control group: z scores *p > .05
Comparison (C) group rate vs. experimental group: z scores *p > .05
One plausible explanation for the large increase in both Trial II and Trial III Control group cessation rates was staff participation in health education training programs designed to improve tobacco treatment counseling skills. It also increased the quality, frequency, and intensity of the delivery of counseling messages to all Experimental and Control group patients after training. In addition, while patients in Trial III were randomized to the Control group, a large proportion were NOT true Control group patients. We confirmed that many Control group patients, especially at the clinic with the largest Trial III census (18%), received SCRIPT methods: Video + Guide + counseling. The Experimental group cessation rate of 22.7% and Control group cessation rate of 17.5% indicates that probably the majority of patients in the Control group at this prenatal care clinic received most or all SCRIPT methods.
We concluded that the significantly higher final Control group rates versus the Comparison (C) group rates in Trial III and Trial II in Table 4 were, at least in part, attributable to organizational development and communication activities in year 1–5, staff training and increased counseling, and attention to smoking behavior during the “Effectiveness Evaluations” of both Trials.
As noted in Table 4, an analysis of the pre-Trial comparison (C) group, the Control group, and Experimental group cessation and significant reduction rates in Trial III and Trial II confirmed significant differences for all six rates. When all Trial III and Trial II data, issues, and results were considered, we came to the following conclusion. The SCRIPT methods delivered to the Trial III Experimental group were perceived to be significantly more effective than the brief intervention methods delivered by the same staff at the same clinics to the two Comparison (C) groups before Trial III and Trial II training sessions and before patient randomization.
DISCUSSION
The following discussion further examines our results and conclusion. In contrast to the Formative Evaluation conducted in year 1–2, significant unplanned events during year 3-4-5 of the Effectiveness Evaluation produced structural and process changes at many sites. Medicaid contracts were re-bid twice: two counties and 247 patients became ineligible. These patients received care at new practice sites. Two other salient factors contributed to the lack of significant differences in the final Experimental and Control group effectiveness rates in Trial III. They were: a lower patient follow-up assessment rate of 82% and saliva collection follow-up rate of 72% in years 3-4-5 compared to the 90% baseline patient assessment rate and cotinine follow-up rates in year 1-2, and unplanned exposure to SCRIPT methods by 15–20% of Control group patients in years 3-4-5. Over a 3–5 year period, the quality, time, and fidelity of delivery of the SCRIPT methods (or any counseling methods) are likely to be attenuated. The routinization of new methods and a combination of other historical factors may diminish behavioral intervention effectiveness over time.
All of these internal and external historical events represent plausible, partial explanations for a lack of significant differences between the Experimental and Control groups in Table 2 in contrast to the Formative Evaluation results. The opportunity to assert control over inter-site variations in staffing, and implementation procedures represent common issues and differences that contrast “Efficacy Evaluations: Trial I and Trial II” with “Effectiveness Evaluations: Trial III”. After training and randomization in both five-year studies, the normal, pre-Trial comparison (C) group cessation rates significantly increased from 3.0% to 8.5% in the Trial II Control group and significantly increased from 4.2% to 10.0% in Trial III Control group. The normal pre-Trial comparison (C) group significant reduction rate increased from 9.3% to 12.3% in the Trial II Control group and 8.5% to 13.1% in the Trial III Control group. The magnitude of these increases reduced the opportunity and probability of documenting statistically significant differences in effectiveness rates.
The two cohorts of pre-Trial comparison (C) group patients with biochemical confirmation were representative samples from the same clinics and Medicaid populations. The two pre-Trial comparison (C) group cohorts were not significantly different for multiple predictors of smoking behavior during pregnancy, and were demographically comparable to the Control groups of both Trials. The same staff delivered the same methods over each five year Trial period. These data were derived from the combined Trial II and III cohort of 2007 smokers from 14 Medicaid supported prenatal care clinics in the same state. The individual and combined data of the two largest Trials in this area of research confirmed that significantly lower cessation and significant reduction rates were occurring among the historical Comparison (C) groups of patients prior to staff training to deliver SCRIPT methods and prior to randomization in Trial II and Trial III.
Our process and qualitative methods confirmed that a large number of Control group patients were exposed to SCRIPT methods. During SCRIPT Trials I and II there were no reports from Control group patients at the end of pregnancy assessments that they had been provided specific SCRIPT methods by specialty staff (Efficacy Evaluation). The Trial III process evaluation data indicated that when regular staff are trained and have the responsibility to routinely deliver new SCRIPT methods (Effectiveness Evaluation), over time, they may be less likely to follow policies about what counseling methods patients should receive or not receive.
The aggregate results of Trial III and Trial II indicated that a prenatal care program will probably assist an additional 6–8% of patients in quitting when staffing and resources are relatively stable and follow-up rates are high (≥ 90%) and the SCRIPT methods are delivered with fidelity by trained regular staff. They will also probably assist an additional 8–10% of patients who are very heavy smokers (≥ 200 ng/ml) who cannot quit to significantly reduce tobacco exposure by approximately 70%. These levels of behavioral impact fall well within the range of effectiveness identified by the AHRQ, 2008 and Cochrane Review, 2009 meta-analyses.
The decision to randomize patients within clinics, and not match and randomize clinics, was selected because of the successful implementation of procedures in Trials I and II representing 1223 pregnant smokers from clinics in Birmingham, AL. Randomization of clinics may eliminate the Trial III exposure problem. A salient, practical barrier to randomization of counties, however, was the limitation of resources. We did not have the funding to recruit, match, and randomize the 20+ prenatal clinics/counties (≥ 10 Experimental group and ≥ 10 Control group sites) with a minimum of > 50 pregnant smokers/site/year needed to have sufficient sample size and statistical power for a group-randomized clinical trial and analysis (Murray, 1998).
The data from Trials II and III produced very strong and consistent evidence and insight for future SCRIPT evaluations. All evaluation research studies need to biochemically confirm “normal” pre-Trial prevalence and non-disclosure rates at the 1st visit, and to document the “normal” pre-Trial behavior change rates during care attributable to the existing counseling methods by using a comparison (C) group representative of all prenatal care practice sites. These two types of evaluation studies must be conducted before training intervention staff and before randomization of patients or sites. Future SCRIPT evaluations should also consider addressing the Control group intervention exposure/contamination issue by staff by selecting and applying a matched group/clinic randomized design with an adequate number of sites/subjects and power (Murray, 1998).
Trial II and III results from a very large, combined sample of 2007 Medicaid patients representative of a mid-sized city and a state prenatal care population respectively, produced biochemically validated evidence of significant reduction (SR). The SR rates were higher than cessation rates in both Trials. These two studies unequivocally confirmed that at least 10–12% of Control group patients and significantly higher percentages, at least 16–18%, of Experimental group patients who smoked the heaviest, were able to dramatically reduce (270ng to 80ng/mL) their daily tobacco exposure. Trial II and Trial III cotinine data confirmed that compensation, such as more intense and complete smoking of each cigarette, was not occurring among these patients. Patient self-reports of cigarettes per day were not used to determine significant reduction because they are very inaccurate.
The literature has described ≤ 80 ng/ml as a “threshold of addiction” (Benowitz & Henningfield, 1994). Patients who reduce to < 100 ng/mL, from an addiction perspective, should be more able and receptive to targeted interventions. Almost all patients (90%) in either the Experimental or Control group of Trial II and Trial III who quit had a baseline cotinine of ≤ 100 ng/mL. Patients with very high biological and psychological addiction levels are very unlikely to quit. Because of the enhanced motivation levels produced by being pregnant, however, 18–24% in the Experimental group were able to substantially reduce their daily tobacco use.
The large sample size, representativeness, study quality, and the magnitude and consistency of Trial II and III results confirmed that significant reduction occurred among these patients. Given the well-established dose-response relationship and relative and attributable risks between smoking and multiple adverse clinical outcomes, SR rates may represent an additional measure of impact for pregnant smokers. It may be a potential harm reduction indicator of the effectiveness of health education methods. At present, the American College of Obstetricians and Gynecologists (www.acog.org) recommends that if a pregnant smoker cannot quit, cutting back to as few cigarettes as possible would be beneficial.
There continue to be a lack of consensus, however, about the validity of significant reduction as a valid measure of “harm reduction” (England, Kendrick, Wilson, et al., 2001; Li et al., 1993; Windsor et al., 1993a, 1999). Future evaluation research is needed to compare a significant reduction intervention + cessation intervention versus a cessation only intervention to determine if this behavioral approach can significantly increase cessation rates among pregnant smokers. Additional epidemiologic research is needed among a large, representative, prospective cohort of pregnant smokers and non-smokers (≥ 2000 patients). A study with saliva cotinine and CO confirmation of tobacco exposure levels at the 1st visit, end of pregnancy, and postpartum is needed to examine the harm reduction potential and relationship between SR and infant birth weight, and other salient perinatal outcomes.
The complexity of intervention procedures and time per patient are enduring barriers to routine provider use (Davis, 1997; National Committee for Quality Assurance, 2009; Ockene & Zapka, 1997; Muramoto, Connoly, Strayer, et al., 2000; Orleans, Barker, Kaufman, et al., 2000; Zapka, Pbert, Stoddard, et al., 2000; Latts, Prochazka, Sala, et al., 2002; Ershoff, 2004). SCRIPT training methods were successfully implemented especially during years 1 and 2, using a science-practice partnership philosophy. Staff was perceived as local experts and collaborators in training and decision making to reduce barriers to routine delivery. Implementation success was also achieved because of strong administrative leadership support by the Alabama Department of Public Health and at the local clinics. The successful application of a consensus building philosophy by leadership and colleagues in practice, in contrast to a top down/policy driven approach, should enhance future SCRIPT dissemination and evaluation initiatives.
Our process evaluation methods using Teleform software provided daily and weekly confirmation of the performance levels of each of the 28 members of the staff. They provided essential data to prepare monthly and quarterly feedback to individual providers and clinics. They documented the consistency and inconsistency of delivery (fidelity) of the SCRIPT methods (Hardin et al., 2005). If prenatal care service providers want to document delivery of SCRIPT methods, Health-Plan Employer Data and Information Set (HEDIS) performance standards and measures, process evaluation methods used in Trials II and III must be included as part of any evaluation of a new tobacco treatment for pregnant women (Windsor, Whiteside, Solomon, et al. 2000, Windsor et al., 1993a, 2000a, 2000b, NCQA, 2009).
Although the Formative and Effectiveness Evaluation results were mixed and other dissemination and evaluation challenges needed to be addressed (Ahluwalia, 2004), the Bureau of Family Health Services (BFHS) recommended dissemination of SCRIPT methods. This decision was made because of the Formative Evaluation results (Windsor et al., 2000b; Crawford et al., 2004), the AHCPR (Fiore et al., 1996) and AHRQ 2000 Guidelines (Fiore et al., 2000), and the success of SCRIPT evaluations in Alabama (Windsor et al., 1985, 1993a; Windsor, Warner, & Cutter; 1988) and in other domestic (Ohio-Gebauer, Kwo, Haynes, et al., 1998; North Carolina-Hartmann, Thorpe, Pahel-Short, et al., 1996) and foreign systems of care (Sweden: Hjalmarson, Hahn, & Svanberg, 1991; Canada: O’Connor et al., 1992; Norway: Valbo & Nylander, 1994; Valbo & Schioldborg, 1994). The BFHS revised the pregnant smoker counseling policies of the Alabama Medicaid Maternity Care Program–Operational Manual (Alabama Medicaid Maternity Care Program, 2002) requiring SCRIPT to be routinely provided by public health staff and other primary care providers.
IMPLICATIONS FOR HEALTH EDUCATION PRACTICE
The SCRIPT Trial III planning, organizational and staff development procedures and process and impact evaluation methods should provide useful information and insight to health education and maternal and child health program leadership and stakeholders. They should help colleagues, program planners and managers, and health education specialists, who are responsible for planning, managing, and evaluating counseling interventions in prenatal care settings. This evaluation reinforced the importance of blending evaluation-technical dimensions of health education practice and leadership skills essential to establish productive collaborative relationships with colleagues in policy, management, and practice settings for a statewide Medicaid program. The project provided examples of how to introduce and conduct rigorous process and impact evaluations of evidence-based health education methods with regular providers and prenatal clinics for a state-wide program.
Because cessation during pregnancy continues to be a major problem in the US for which limited progress has been made in the last decade, this study has implications for future program and evaluation initiatives. The high rates of participation and retention, and the routine delivery of the health education counseling methods by regular staff were encouraging. The time to deliver the methods, and materials and training costs were modest if carbon monoxide (CO) monitoring and self-reports were used to assess cessation, because these practical methods to evaluate new counseling interventions are available at a low cost.
The methods and results of this project, other evaluations of interventions for pregnant smokers, and the most recent Agency for Healthcare Research and Quality Tobacco Treatment Guidelines indicate a consensus (AHRQ, 2008). SCRIPT methods need to be disseminated to systems of prenatal care. The next generation of health education methods needs to focus on reducing the barriers to the dissemination, adoption, and evaluation of a wider variety of evidenced based methods by a greater variety of colleagues in practices and program settings who serve pregnant women (AHRQ, 2008, Windsor, 2010)
Acknowledgments
Success was achieved because of the contributions of the many colleagues. UAB: Dr Myra Crawford for project planning, and intervention development, and the UAB Trial III staff, Robert Azadian, MPH, Toya Russell, PhD, Stephanie Chisolm, PhD, Baoyi Zheng, PhD, Wendy Horn, PhD, and Karen Cole, BA; ADPH: Don Williamson, MD, State Health Officer; ADPH-BFHS--Sherry George, Denise Donald, Phyllis Gilchrist, Laurie Stout, Sharon Gerogiannis, Connie McMicheal, Wendy Blackmon, and Martha Kreauter; ADP Practice Advisory Committee: Vanessa Mills, Addie Hightower, Yvonne McKinnen, June Johnson, Lora Harris, Carrie Capps, Jackie Strickland, Leslie Turner, Lesa Cotton, Gayle Whatley, Ava Rozelle, Angel Cook, Candace Adkins, Marla Odom, Suzie Harrison, Jeanie Pritchard, Reba Brannon, and Blyth Keith; and Trial III Consultant: We especially thank our colleague, Dr. Carlo Di Clemente, for his input and during the Formative Evaluation.
Funding: This study was funded by the National Heart, Lung and Blood Institute, NIH. Grant #HL R01 560110b 02: Division of Epidemiology and Clinical Applications
Footnotes
Competing Interests: The authors do not have any conflicts of interest in this study.
References
- Alabama Medicaid Maternity Care Program. Operational Manual. PO Box 5624, 50 Dexter Avenue, Montgomery, AL: Bureau of Family Health Services; 2002. [Google Scholar]
- Ahluwalia J. Reaching the medically underserved with the AHCPR Guideline. Tobacco Control. 1997;6(1):S29–S32. [PubMed] [Google Scholar]
- Benowitz N, Henningfield J. Establishing a nicotine threshold for addiction: the implications for tobacco regulation. New England Journal of Medicine. 1994;331(2):123–125. doi: 10.1056/NEJM199407143310212. [DOI] [PubMed] [Google Scholar]
- Crawford M, Woodby L, Russell T, Windsor R. Using formative evaluation to improve a smoking cessation intervention for pregnant women. Health Communication. 2005;17(3):265–281. doi: 10.1207/s15327027hc1703_4. [DOI] [PubMed] [Google Scholar]
- Davis R. Healthcare report cards and tobacco measures. Tobacco Control. 1997;6(1):570–577. [PubMed] [Google Scholar]
- Dolan-Mullen P, Ramirez G, Groff J. A meta-analysis of randomized trials of prenatal smoking cessation interventions. J. of Obstetrics and Gynecology. 1994;171:1328–1334. doi: 10.1016/0002-9378(94)90156-2. [DOI] [PubMed] [Google Scholar]
- England L, Kendrick J, Wilson H, et al. Effects of smoking reduction during pregnancy on the birth weight of term infants. American Journal of Epidemiology. 2001;154(8):694–701. doi: 10.1093/aje/154.8.694. [DOI] [PubMed] [Google Scholar]
- Ershoff D. Helping pregnant women quit smoking: progress and future directions (supplemental issue) Nicotine and Tobacco Research. 2004;6(2) doi: 10.1080/14622200410001669204. Guest Editior. [DOI] [PubMed] [Google Scholar]
- Fiore M, Bailey W, Cohen S, et al. Smoking cessation: clinical practice guideline. Rockville, MD: AHCPR; 1996. [Google Scholar]
- Fiore M, Bailey W, Cohen S, et al. Treating tobacco use and dependence: a clinical practice guideline. Rockville, MD: AHRQ; 2000. [Google Scholar]
- Fiore M, Bailey W, Cohen S. Treating tobacco use and dependence: 2008 update--clinical practice guideline. Rockville, MD: AHRQ; 2008. [Google Scholar]
- Gebauer C, Kwo C, Haynes E, et al. A nurse managed smoking cessation intervention during pregnancy. J Obstet Gynecol Nursing. 1998;21:47–53. doi: 10.1111/j.1552-6909.1998.tb02590.x. [DOI] [PubMed] [Google Scholar]
- Hardin J, Woodby L, Crawford M, Windsor R. Data Collection and Management in A Multi-Site Effectiveness Project: The SCRIPT Study. Public Health Nursing. 2005;22(4):347–351. doi: 10.1111/j.0737-1209.2005.220410.x. [DOI] [PubMed] [Google Scholar]
- Hasselmeyer E, Meyer M, Catz C. Smoking and health: a report of the surgeon general. Washington, DC: USGPO; 1979. Chapter 8, Pregnancy and infant health. [Google Scholar]
- Hjalmarson A, Hahn L, Svanberg B. Stopping smoking in pregnancy: effect of a self help manual in a controlled trial. British Journal of OB/GYN. 1991;98:260–264. doi: 10.1111/j.1471-0528.1991.tb13390.x. [DOI] [PubMed] [Google Scholar]
- Hartmann K, Thorpe J, Pahel-Short L, Koch M. A randomized controlled trial of smoking cessation intervention in pregnancy in an academic clinic. Obstetrics and Gynaecology. 1996;87:621–626. doi: 10.1016/0029-7844(95)00492-0. [DOI] [PubMed] [Google Scholar]
- Kendrick J, Zahniser S, Miller, et al. Integrating smoking cessation into routine public prenatal care: the SCIP project. American Journal of Public Health. 1995;85(2):217–222. doi: 10.2105/ajph.85.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine C, Davidoff F. The patient-physician relationship: Patient-centered medicine, a professional evolution. Journal of the American Medical Association. 1996;275:152–156. [PubMed] [Google Scholar]
- Latts L, Prochazka A, Salas N, Young D. Smoking cessation in pregnancy: failure in an HMO pilot project to improve guideline implementation. Nicotine and Tobacco Research. 2004;4(1):525–530. doi: 10.1080/14622200210128054. [DOI] [PubMed] [Google Scholar]
- Leopold N, Cooper J, Clancy C. Characteristics of primary care: Sustained partnership in primary care. Journal of Family Practice. 1996;42f:129–137. [PubMed] [Google Scholar]
- Li C, Windsor R, Lowe J, Goldenberg R. Evaluation of the impact of Dissemination of smoking cessation methods on the low birth-weight rate and health care costs in the U. S. : Achieving the 2000 infant health objectives. American Journal of Preventive Medicine. 1992:171–177. [PubMed] [Google Scholar]
- Li C, Windsor R, Perkins L, Goldenberg R, Lowe J. The impact on infant birth weight and gestational age of cotinine-validated smoking reduction during pregnancy. Journal of the American Medical Association. 1993;269(12):1519–1524. [PubMed] [Google Scholar]
- Lumley J, Oliver S, Waters E. Interventions for promoting smoking cessation during pregnancy. Cochrane Database of Systematic Reviews. 2009 doi: 10.1002/14651858.CD001055.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramoto M, Connoly T, Strayer L, et al. Tobacco cessation skills certification in Arizona: application of a state wide, community based model for diffusion of evidence-based practice guidelines. Tobacco Control. 2000;9(4):408–414. doi: 10.1136/tc.9.4.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray D. Group randomized designs and analysis. New York, NY: Oxford U. Press; 1998. [Google Scholar]
- National Committee for Quality Assurance NCQA. 1100 13th St., NW, Suite 1000 Washington, DC 20005: ( www.ncqa.org or 1-888-275-7585) [Google Scholar]
- O’Connor A, Davies B, Dulberg C, Buhler P, Nadon C, et al. Effectiveness of a pregnancy smoking cessation program. J. of OB/GYN and Neonatal Nursing. 1992;21:385–392. doi: 10.1111/j.1552-6909.1992.tb01755.x. [DOI] [PubMed] [Google Scholar]
- Ockene J, Zapka J. Changing provider behaviour: provider education and training. Tobacco Control. 1997;6(1):S63–S67. [PubMed] [Google Scholar]
- Office of Applied Studies. Results from the 2006 national survey of drug use and health: national findings. Rockville, MD: SAMHSA; 2007. HHS Pub No. SMA 07-4293. [Google Scholar]
- Orleans T, Barker D, Kaufman N, Marx J. Helping pregnant smokers quit: meeting the challenge in the next decade. Tobacco Control. 2000;9(suppl. III):iii 6–iii 11. doi: 10.1136/tc.9.suppl_3.iii6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg L, Kottke T, Brekke M, Calomeni C, Conn S, Davidson G. Using continuous quality improvement to increase preventive services in clinical practice: going beyond guidelines. Preventive Medicine. 1996;25:259–267. doi: 10.1006/pmed.1996.0055. [DOI] [PubMed] [Google Scholar]
- Russell T, Crawford M, Woodby L. Measuring tobacco smoke exposure during pregnancy: Why simply asking isn’t enough. Nicotine & Tobacco Research. 2004;6(2):141–151. doi: 10.1080/14622200410001669141. [DOI] [PubMed] [Google Scholar]
- US DHHS. The health benefits of smoking cessation: a report of the surgeon general. Rockville, MD: US Public Health Service, CDC, Office on Smoking Health; 1990. [Google Scholar]
- US DHHS. Data collection manual. Rockville, Md: CDC, National Center for Chronic Disease Prevention and Health Promotion, Clinical Management Unit; 1993. Patient flow analysis. [Google Scholar]
- US DHHS. Chartbook on trends in the health of Americans. Washington, DC: 2006. Table 12: Smoking during pregnancy by race, Hispanic origin, age, and education of mother by selected years, 1989–2004. [Google Scholar]
- US DHHS. Healthy People 2010: national health promotion and disease prevention objectives. Washington, DC: US DHHS; 2000. [PMC free article] [PubMed] [Google Scholar]
- Valbo A, Nylander G. Smoking cessation in pregnancy: intervention among heavy smokers. Acta Obstetrica et Gynaecogica Scandanavia. 1994;73:215–219. doi: 10.3109/00016349409023442. [DOI] [PubMed] [Google Scholar]
- Valbo A, Schioldborg P. Smoking cessation in pregnancy: the effect of self help manuals. Journal of Maternal Fetal Investigation. 1994;4:167–170. [Google Scholar]
- Windsor R, Cutter G, Morris J, Reese Y, Manzella B, et al. The effectiveness of smoking cessation methods for smokers in public health maternity clinics: A randomized trial. American Journal of Public Health. 1985;75:1389–1392. doi: 10.2105/ajph.75.12.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windsor R. A Pregnant Woman’s Guide to Quit Smoking. ISBN 0-935105-01-08. Washington, DC: Society for Public Health Education; 1985 &2005. www.sophe.org. [Google Scholar]
- Windsor R, Warner K, Cutter G. A cost-effectiveness analysis of self-help smoking cessation methods for pregnant women. Public Health Reports. 1988;103(1):83–88. [PMC free article] [PubMed] [Google Scholar]
- Windsor R, Lowe J, Perkins L, et al. Health education for pregnant smokers: Its behavioral impact and cost benefit. American Journal of Public Health. 1993;83:201–206. doi: 10.2105/ajph.83.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windsor R, Li C, Lowe J, Perkins L, Ershoff D, Glynn T. Dissemination of smoking cessation methods for pregnant women: achieving the year 2000 objectives. American Journal of Public Health. 1993;83:173–178. doi: 10.2105/ajph.83.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windsor R, Boyd R, Orleans T. A meta-evaluation of smoking cessation intervention research among pregnant women: improving the science and art. Health Education Research Theory Practice. 1998;18:419–438. doi: 10.1093/her/13.3.419. [DOI] [PubMed] [Google Scholar]
- Windsor R, Crawford M, Woodby L. Commit to quit smoking during and after pregnancy. Washington, DC: 1998. [Video]. UAB Medical Television. Edited 2004, Society for Public Health Education, SOPHE.org. [Google Scholar]
- Windsor R, Li C, Boyd N, Hartmann K. The use of significant reduction rates to evaluate health education methods for pregnant smokers: A new harm reduction behavioral indicator? Health Education and Behavior. 1999;26(5):648–662. doi: 10.1177/109019819902600506. [DOI] [PubMed] [Google Scholar]
- Windsor R, Woodby L, Miller T, et al. Effectiveness of AHCPR clinical practice guideline and patient education methods for pregnant smokers in Medicaid maternity care. American Journal of Obstetrics and Gynecology. 2000;182:68–75. doi: 10.1016/s0002-9378(00)70492-3. [DOI] [PubMed] [Google Scholar]
- Windsor R, Whiteside H, Solomon L, et al. A process evaluation model for patient education methods for pregnant smokers. Tobacco Control. 2000;9(3):29–35. doi: 10.1136/tc.9.suppl_3.iii29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windsor R, Clark N, Boyd R, Goodman R. Evaluation of Health Promotion, Health Education and Disease Prevention Programs. 3rd ed. New York, NY: McGraw-Hill; 2004. [Google Scholar]
- Windsor R. SCRIPT Methods: A Meta-Evaluation of the Impact of Dissemination. American J. of Medical Sciences. 2003;326(4):216–222. doi: 10.1097/00000441-200310000-00013. [DOI] [PubMed] [Google Scholar]
- Windsor R. Women’s reproductive and perinatal health: interventions and evidence for reducing racial and ethnic disparities. Chicago, Illinois: Springer Publishing; 2010. Behavioral Treatment Methods for Pregnant Smokers: the evidence base for prenatal care programs and professional practice. Chapter. [Google Scholar]
- Woodby L, Windsor R, Snyder S, et al. Predictors of smoking cessation during pregnancy. Addiction. 1999;94(2):283–292. doi: 10.1046/j.1360-0443.1999.94228311.x. [DOI] [PubMed] [Google Scholar]
- Woolbright A, Henderson N. Maternal-Child Statistics Data Set: 1996. Montgomery, AL: Alabama Department of Public Health, Center for Health Statistics; 1997. [Google Scholar]
- Zapka J, Pbert L, Stoddard A, et al. Smoking cessation counseling with pregnant and Post-partum women: a survey of community health center providers. American Journal of Public Health. 2000;90(1):78–84. doi: 10.2105/ajph.90.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]