Abstract
The blood–brain barrier (BBB) is the most significant obstacle to effective CNS drug delivery. It possesses structural and biochemical features (i.e., tight-junction protein complexes and, influx and efflux transporters) that restrict xenobiotic permeation. Pathophysiological stressors (i.e., peripheral inflammatory pain) can alter BBB tight junctions and transporters, which leads to drug-permeation changes. This is especially critical for opioids, which require precise CNS concentrations to be safe and effective analgesics. Recent studies have identified molecular targets (i.e., endogenous transporters and intracellular signaling systems) that can be exploited for optimization of CNS drug delivery. This article summarizes current knowledge in this area and emphasizes those targets that present the greatest opportunity for controlling drug permeation and/or drug transport across the BBB in an effort to achieve optimal CNS opioid delivery.
The blood–brain barrier (BBB) is the principal physical and biochemical barrier that separates the CNS from the systemic circulation. To this end, the BBB highly restricts flux of circulating substances in an effort to tightly control the CNS microenvironment and maintain cerebral homeostasis. In addition, the BBB is the most significant obstacle to drug delivery to the brain. In fact, many existing drugs have limited or no efficacy in the treatment of neurological diseases primarily due to a limited ability to traverse the BBB and accumulate within the CNS [1]. The BBB has evolved specific features that tightly control blood-to-brain xenobiotic permeability including highly dynamic tight-junction protein complexes between adjacent endothelial cells as well as expression of uptake carriers and efflux transporters at the endothelial cell surface. Current research in BBB biology has focused on understanding molecular mechanisms that determine CNS drug delivery. In particular, these studies have demonstrated how the BBB dynamically responds to pathophysiological stressors (i.e., pain, inflammation, hypoxia and stroke) and have identified and characterized molecular machinery involved in such changes [2–8]. In terms of pharmacotherapy, particularly treatment of peripheral inflammatory pain using opioid analgesics, these cutting-edge studies have identified novel targets that can be exploited for optimization of therapeutic drug delivery to the brain. Here, we provide a review of BBB properties that are directly involved in determining CNS drug delivery. In addition, we have summarized current knowledge on molecular regulation of these properties and provide insights on how the BBB can be targeted in an effort to optimize CNS opioid delivery for treatment of peripheral inflammatory pain.
Pain & opioid analgesics
Pain is a dominant symptom associated with inflammation resulting from acute tissue injury. In addition, inflammation localized to the site of damaged and/or affected nerves is a common underlying mechanism of neuropathic (i.e., chronic) pain [9]. According to the National Center for Health Statistics, pain affects 76.2 million people in the USA [301]. In fact, over 26% of Americans aged at least 20 years have reported that they have had a problem with pain that has lasted more than 24 h in duration [301]. Pain has a complex pathophysiology that involves production/secretion of immunological mediators, neurological inputs from the CNS and endocrine signaling via the hypothalamic–pituitary–adrenal (HPA) axis [10]. The immunological response to inflammatory pain is characterized by rapid production and release of cytokines, chemokines, cellular adhesion molecules, matrix metalloproteinases, kinins and prostaglandins at the site of tissue injury. The inflammatory component of pain is also characterized by increased vascular permeability, localized edema formation and redness, and increased leukocyte migration. The CNS utilizes neuronal pathways to signal the immune system. Neuronal responses to noxious stimulation involve both excitatory and inhibitory neurotransmission within sensory areas of the spinal cord, and the balance of these CNS responses determines the level of transmission of nociceptive signals to the brain [11]. Furthermore, proinflammatory mediators produced and released in the CNS following injury or inflammation in the periphery are involved in the centrally mediated pain response. Stress responses due to physical, emotional and environmental stimuli result in activation of the HPA axis and initiation of a ‘stress cascade’ [12]. Activation of the HPA axis leads to release of corticotrophin-releasing hormone (CRH) from the hypothalamus. Circulating CRH and vasopressin stimulate expression and release of adrenocorticotrophin (ACTH) from the anterior pituitary gland. ACTH circulates to the adrenal glands where it induces production and secretion of glucocorticoids, which are responsible for downregulation of the immune response [12]. Additionally, adrenal glucocorticoids can ‘prime’ glial cells in the spinal cord, thereby potentiating pain responses to subsequent noxious stimuli [13].
There are several pharmacological agents available that have analgesic properties. Of these drugs, opioids are the most effective analgesics used in pharmacological pain management regimens [14,15]. Opioids have been used for pain relief for thousands of years and continue to be one of the most commonly prescribed medications for treatment of acute and chronic pain [16]. In recent years, use of opioid analgesics for pain management has dramatically increased as evidenced by a 149% increase in overall opioid prescriptions in the USA from 1997 to 2007 [16]. Opioids exert their analgesic effect by binding to specific receptors (i.e., μ-, κ- and δ-opioid receptors) that are localized to neural tissue both within the CNS and in the periphery. Although opioids can provide some analgesia by binding to peripheral opioid receptors [17], optimal pharmacotherapy with these drugs requires the capability to access central opioid receptors [18,19]. Opioid receptors are G-protein coupled receptors and, therefore, have profound effects on ion gating, intracellular Ca2+ disposition, and protein phosphorylation. Since there are multiple subtypes of opioid receptors, a possibility for opioids to exert different pharmacological effects at different receptors exists. For example, morphine is a full agonist at the μ-opioid receptor but a weak agonist at both the κ- and δ-receptors. In contrast, codeine is a weak agonist at both the μ- and δ-receptors. Opioid analgesics have multiple actions in the CNS, not all of which are beneficial. They are known to cause euphoria that, in part, accounts for their abuse potential. Pharmacotherapy with opioids is associated with several adverse effects (i.e., respiratory depression, constipation, nausea, vomiting and rapid development of tolerance) [20]. This property may limit the opioid dose that can be administered as well as the level of analgesia that can be attained. In addition, these adverse events are enhanced by opioid-induced glial activation, which occurs via non-stereoselective activation of Toll-like receptor (TLR)4 at the glial cell surface [21]. Activation of glial TLR4 receptors is involved in pathogenesis of neuropathic pain and directly counteracts opioid analgesic efficacy [21]. Although opioid-mediated activation of glial TLR4 signaling can be blocked by co-administration of (+)-naloxone or (+)-naltrexone [22], it remains critical that opioid concentrations in the brain be maintained precisely to ensure efficacious management of pain and to limit adverse drug reactions. This therapeutic objective emphasizes the importance of understanding biological mechanisms involved in determining CNS delivery of opioid analgesics and how these mechanisms can be targeted in order to optimally deliver drugs to the brain for treatment of pain associated with peripheral inflammatory tissue injury.
The blood–brain barrier
The CNS is the most critical and sensitive organ system in the human body. Proper neuronal and glial function necessitates precise regulation of the brain extracellular milieu. Therefore, the interface between the CNS and the systemic circulation must be highly selective and possess effective mechanisms that can facilitate nutrient transport, exactly regulate ion balance, and provide a barrier to potentially toxic xenobiotics that may be present in the systemic circulation. The requirement for a physical and metabolic barrier is further emphasized by the extreme sensitivity of CNS tissues to exogenous solutes. Therefore, brain homeostasis must be tightly controlled by enabling some substances to permeate brain parenchyma with the exclusion of others. This homeostatic function of the cerebral microvasculature primarily occurs at the BBB at the level of the brain microvascular endothelium.
The first scientist to describe the possibility of a CNS diffusion barrier was Paul Ehrlich, who noted that water-soluble dyes injected intravenously stained all tissues except for the brain and spinal cord [23]. Ehrlich later attributed this observation to a low affinity of nervous tissue to the injected dyes [24]. Subsequent experiments by Ehrlich’s student Edwin Goldmann demonstrated that this conclusion was fallacy because injection of trypan blue directly into the CNS stained both the brain and spinal cord without permeating into the systemic circulation [25]. Taken together, these two studies indicated a barrier to circulating solutes that exists between the CNS and peripheral circulation. Lewandowsky was the first to use the term ‘bluthirnschranke’ (i.e., BBB) while studying limited permeation of potassium ferrocyanate into brain tissue [26].
Although Goldmann’s work strongly suggested a physical barrier between the CNS and the periphery, both the nature of the barrier and its actual existence were debated for many years. Several researchers noted that, because the composition of blood and cerebrospinal fluid (CSF) were considerably different, such properties could dramatically influence affinity of dyes for nervous tissue as well as their diffusion between blood and CSF [27]. These scientists argued that comparison of diffusion properties between a substance injected into the systemic circulation and a substance injected directly into CSF could not be made. Furthermore, experiments designed to assess CNS uptake of aniline dyes noted that basic dyes were able to stain brain tissue while acidic dyes could not. This led researchers to conclude that the apparent selective permeability of the brain–capillary barrier was due to individual physicochemical properties of injected substances [28]. The exact anatomical structures that determined selective permeability were also a source of considerable controversy until the advent of electron microscopy (EM). EM enabled more detailed anatomical studies of the brain and its associated vasculature. An early microscopy study by Maynard and colleagues indicated that brain interstitial space was virtually nonexistent and, therefore, the BBB was essentially an artifact [29]. Maynard argued that selective permeability to circulating solutes was merely a function of restricted uptake into glial cells and not due to presence of a specialized barrier. As the technique of EM was refined, Reese and Karnovsky made a critical discovery: at a resolution of 100,000×, they were able to clearly identify the existence of a CNS interstitial space between astrocytic end-feet and brain microvascular capillaries [30]. Furthermore, these researchers were the first to show existence of tight junctions that were localized to the interendothelial cleft and formed a continuous, impermeable membrane between the brain and the systemic circulation [30]. The impermeability of the brain microvascular endothelium was based upon studies with horseradish peroxidase, which was incapable of traversing the BBB when injected directly into the blood. In addition, the concept of selective permeability being due to limited glial permeation was refuted by additional experimentation with horseradish peroxidase, which demonstrated that this molecule, when injected directly into the CNS, can easily diffuse through the brain interstitial space [31].
Although the concept of the BBB has continued to be refined over the past 40 years, the current understanding of its basic structure is built primarily upon the work of Reese, Karnovsky, and Brightman in the late 1960s [30,31]. Anatomically, BBB endothelial cells are distinguished from those in the periphery by a lack of fenestrations, minimal pinocytotic activity, and presence of tight junctions [32]. Cerebral endothelial cells are demarcated by increased mitochondrial content as compared with other endothelium in the body [33]. This increased content of mitochondria may be required for transport of solutes into and out of the brain thereby contributing to maintenance of CNS homeostasis. Additionally, several receptors, ion channels, and influx/efflux transport proteins are prominently expressed in brain microvascular endothelial cells. Functionally, these transport systems are similar to well-characterized systems in other tissues (i.e., d-glucose transporter, l-amino acid carrier systems, Na+/K+ ATPase), although the capacity and rate of transport can vary. Transporters involved in transcellular flux of drugs have also been identified and characterized at the BBB endothelium. Examples of such transporters known to be expressed in brain endothelial cells include efflux transporters such as P-glycoprotein (P-gp) [34–37], multidrugresistance proteins 1–6 (MRP1–6 in humans; Mrp1–6 in rodents) [35,38–41], and breast-cancer resistance protein (BCRP in humans; Bcrp in rodents) [37,42,43]. Additionally, transporters that have been shown to facilitate drug permeation across the BBB include organic anion transporting polypeptides (OATPs in humans; Oatps in rodents) [8,44–46], organic anion transporters [39,47–49], monocarboxylate transporters [47,50], nucleoside transporters [51] and peptide transporters [52].
The highly specialized BBB phenotype is dependent upon surrounding cellular and structural components of neural tissue that directly interact with brain microvascular endothelium. Such a phenomenon has given rise to the concept of the neurovascular unit, which reflects involvement of glia (i.e., astrocytes and microglia), pericytes, neurons, and extracellular matrix in both development and physiology of the BBB [27,53,54]. Previous studies have shown that astrocytes, localized between neuronal cell bodies and endothelial cells and ensheathing over 99% of cerebral capillaries with their endfeet [27], are critical in the development and/or maintenance of BBB characteristics [55–59]. For example, studies using human umbilical vein endothelial cells showed that these cells could develop BBB properties when co-cultured with astrocytes, which implies that astrocytes secrete trophic factors critical to maintenance of the BBB phenotype [58]. Furthermore, injection of astrocyte-selective toxin 3-chloropropanediol into rodents caused focal loss of perivascular astrocytes that led to reduced BBB integrity that was characterized by decreased expression of the critical tight-junction protein occludin [59]. Astrocytes may be involved in transient regulation of cerebral microvascular permeability [60], in particular via dynamic Ca2+ signaling between astrocytes and the endothelium via gap junctions and purinergic transmission [61,62]. Recent evidence also suggests that astrocytes may play a critical role in regulating water and ion exchange across the brain microvascular endothelium [63,64]. In addition, astrocytes are also known to express drug-transport proteins including P-gp [65–67], MRP/Mrp isoforms [68–72] and Bcrp [42]. Studies in human glioma tissue have detected mRNA expression of various MRP and OATP isoforms [73]. The expression of multiple drug transporters in astrocytes suggests that these glial cells may act as a secondary barrier to CNS drug permeation. That is, the balance of transporters in astrocytes may either sequester drugs within the astrocyte cytoplasm, thereby preventing these compounds from reaching their site of action in the brain, or concentrate drugs in brain extracellular fluid. Pharmacological agents within brain extracellular space can be effluxed by active transport mechanisms at brain-barrier sites or via ‘sink’ effects of the CSF [74]. For more detailed information on drug-transport mechanisms in astrocytes, readers are referred to a recent review [74].
Microglia, which were first described by del Rio-Hortega in 1932 [75], are the primary cell type involved in innate and adaptive immunity in the CNS. In non-pathological conditions, microglia exist in a ramified state characterized by a small (5–10 µm) cell body and radial cell processes extending from the cell body. Ramified microglia contribute to BBB homeostasis by participating in extracellular fluid cleansing and neurotransmitter deactivation [74]. During disease or trauma, microglia may become activated and the degree of this activation is directly correlated to the type and severity of brain injury [76]. Microglia activation and proliferation is associated with dysfunction of the BBB characterized by changes in tight-junction protein expression and enhanced paracellular permeability [54]. Studies in a continuous rat microglia cell line (i.e., MLS-9) demonstrated functional expression of P-gp [77,78], Mrp1 [79] and Mrp4/Mrp5 [80]; however, the ability of microglia to contribute to drug permeation and/or distribution in the CNS requires further study. In addition to glia, pericytes play a crucial role in maintenance of BBB homeostasis [81]. Pericytes are flat, undifferentiated, contractile cells that attach at irregular intervals along capillary walls and communicate with other cell types of the neurovascular unit [81]. These cells, via secretion of pericyte-derived angiopoetin, induce expression of occludin at the BBB, which suggests that pericytes are directly involved in induction and/or maintenance of barrier properties [82]. Further evidence for the role of pericytes in maintenance of BBB phenotype comes from the observation that pericytes migrate away from the endothelium during hypoxia [83] or brain trauma [84], conditions that are associated with increased brain microvascular permeability. More recently, studies using adult-viable pericyte-deficient mouse mutants demonstrated that pericytes are critical in maintaining expression of BBB-specific genes in endothelial cells (i.e., transferrin receptor) and by inducing polarization of astrocyte end-feet adjacent to the cerebral microvasculature [85]. Furthermore, MRP isoforms (MRP1, MRP4 and MRP5) have been identified in pericytes in vitro, which implies that pericytes may contribute to regulation of BBB xenobiotic permeability [86].
In addition to glial cells and pericytes, there is considerable evidence for direct innervation of both brain microvascular endothelium and associated astrocyte processes. Noradrenergic [87,88], serotonergic [89], cholinergic [90,91], and GABAergic [92] neurons have been shown to make distinct connections with other cell types of the neurovascular unit. The need for direct innervation of brain microvasculature comes from the dynamic nature of neural activity and the metabolic requirements of nervous tissue, implying that the cerebral microcirculation must be highly responsive to the needs of CNS tissue. Indeed, ‘metabolic coupling’ of regional brain activity to blood flow is the basis of functional neuroimaging [93], although the cellular mechanisms of this process are not well understood [94]. Interestingly, disruption of BBB integrity induced by pathophysiological factors (i.e., inflammation, hypertension) often accompanies changes in cerebral blood flow and perfusion pressure [95,96] and there is evidence that such BBB opening may be a selective, compensatory event rather than a simple anatomical disruption. This implies that communication between neurons and the brain microvasculature may not simply regulate blood flow, but BBB permeability as well.
In addition to cellular components of the neurovascular unit, the extracellular matrix of the basal lamina interacts with the BBB endothelium. Disruption of extracellular matrix is strongly associated with increased BBB permeability in pathological states [97,98]. The extracellular matrix seems to serve as an anchor for the endothelium via interaction of laminin and other matrix proteins with endothelial integrin receptors [99]. Such cell–matrix interactions can stimulate a multitude of intracellular signaling pathways [100]. Matrix proteins can influence expression of tight-junction proteins [101,102], indicating that although the tight-junction protein complexes constitute the primary impediment to paracellular diffusion, the proteins of the basal lamina are likely involved in their maintenance.
Molecular characteristics of the BBB
Tight-junction protein complexes
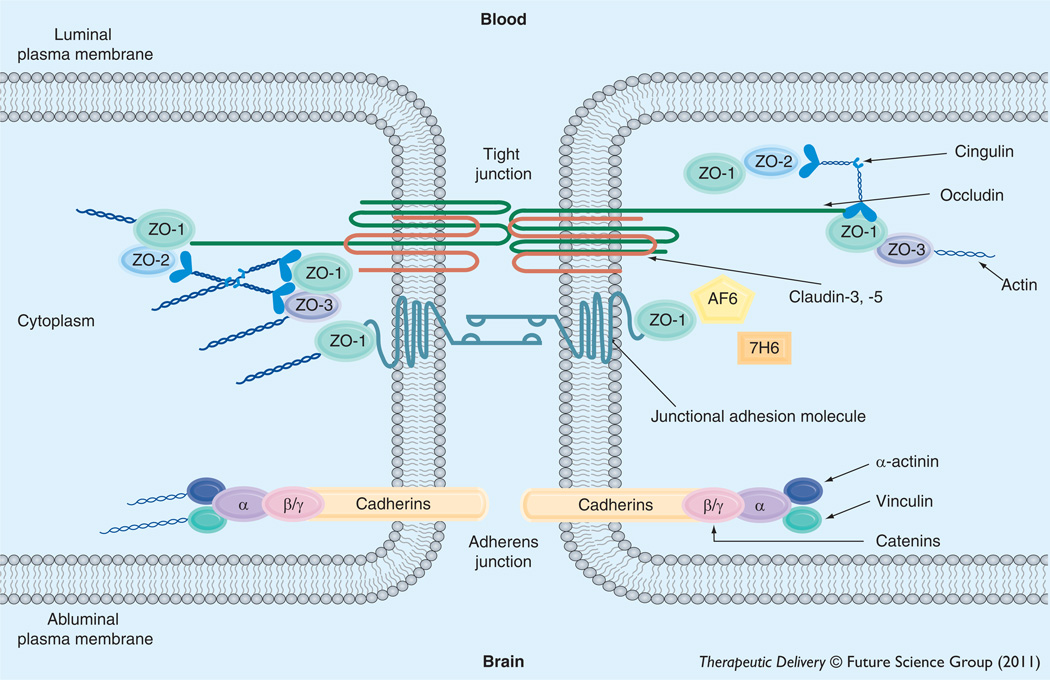
BBB endothelial cells are interconnected by tight junctions (Figure 1), large multiprotein complexes that are maintained by astrocytic trophic factors. Tight junctions form a continuous, almost impermeable barrier that limits paracellular flux of xenobiotics with the exception of small, lipid-soluble molecules [32]. The high BBB transendothelial resistance (1500– 2000 Ω/cm2) further restricts the free flow of water and solutes [103]. BBB tight junctions are formed by junctional adhesion molecules (JAMs), occludin and claudins (i.e., claudin-1, -3 and -5) and transmembrane proteins linked to the cytoskeleton through interactions with accessory proteins (i.e., zonula occluden [ZO]-1, -2 and -3) [27]. ZO proteins act as a scaffold for multiple intracellular signaling pathways and are involved in regulation of tight-junction function [104]. Additionally, other protein constituents (i.e., cingulin, AF6, 7H6 and epithelial membrane protein [EMP]-1) have been localized to the tight junction but their exact role has yet to be elucidated. A brief description of the primary proteins that constitute tight-junction protein complexes at the BBB is described below. Although endothelial cells of the BBB do also possess adherens junctions, which are ubiquitous in the vasculature and mediate inter-endothelial cell adhesion, these will not be discussed in this review.
Figure 1. Basic molecular organization of tight-junction protein complexes at the blood–brain barrier.
Adapted from Hawkins and Davis [27].
Junctional adhesion molecules
Several JAM isoforms have been identified at the mammalian BBB including JAM-1, JAM-2, and JAM-3 [27,105]. JAM-1 is a 40 kDa member of the IgG superfamily and is believed to mediate the early attachment of adjacent endothelial cells during development of the BBB [106]. In addition to their developmental roles, JAMs may regulate the transendothelial migration of leukocytes [105]. Although their function in a mature BBB is largely unknown, loss of JAM protein expression is directly correlated with BBB breakdown [107,108]. Studies in an immortalized human brain endothelial cell line (hCMEC/d3) showed that pro-inflammatory stimulation led to increased paracellular permeability to dextran 3000 that correlated with movement of JAM away from the tight junction, further suggesting that JAMs play a critical role in maintaining BBB functional integrity [109].
Occludin
Monomeric occludin is a 60–65 kDa protein that has four transmembrane domains with the carboxyl and amino terminals oriented to the cytoplasm and two extracellular loops that span the intercellular cleft [110]. It is highly expressed and consistently stains in a distinct, continuous pattern along endothelial cell margins in the cerebral vasculature [4,7,111]. In contrast, occludin distribution is considerably more diffuse in non-neural endothelia [112]. Occludin increases electrical resistance in tight junction-containing tissues [113]. This occurs via its two extracellular loops that interact with homologous segments on occludin molecules localized to adjacent endothelial cells, thus creating a tight seal that highly restricts paracellular diffusion of solutes [114,115]. The cytoplasmic C-terminal domain is likely involved in association of occludin with the cytoskeleton via ZO proteins [116,117]. Expression of C-terminal truncated versions of occludin leads to increased paracellular permeability to small-molecule xenobiotics [118]. Additionally, the C-terminal region of occludin is capable of forming disulfide bonds, a characteristic that enables occludin to assemble into dimers and higher order oligomeric assemblies [4,5,119]. Such oligomeric assemblies are essential for occludin function at the tight junction, both as a restrictor of paracellular permeability and as a mediator of intracellular signaling [4]. Previous knockout and knockdown experiments suggest that occludin is not essential for formation of tight junctions [120], despite the fact that altered expression of occludin is associated with disrupted BBB function in various diseases [6,121–125].
Claudins
Claudins have similar membrane topography to occludin but no sequence homology [126]. Claudins are 20–24 kDa proteins, of which at least 24 have been identified in mammals [27]. All claudins have similar sequence homology among themselves in the first and fourth transmembrane domains and extracellular loops [127]. The extracellular loops of claudins interact via homophilic and heterophilic interactions between cells [128]. Overexpression of claudin isoforms in fibroblasts can induce cell aggregation and formation of tight junction-like strands. Conversely, expression of occludin does not lead to formation of tight junctions; rather, occludin only localizes to tight junctions in cells that have already been transfected with claudins [129]. Thus, it is hypothesized that claudins form the primary ‘seal’ of the tight junction. In the cerebral microvascular endothelium, various isoforms of claudins have been detected including claudin-1, -3 and -5 [111,123,130–133].
Membrane-associated guanylate kinase-like proteins
Proper physiological functioning of the BBB, particularly restriction of paracellular solute transport, requires association of transmembrane constituents of tight-junction protein complexes with accessory proteins localized within the endothelial-cell cytoplasm. These include members of the membrane-associated guanylate kinase-like (MAGUK) family. In brain microvascular endothelial cells, MAGUK proteins are involved in coordination and clustering of tight-junction protein complexes to the cell membrane and in establishment of specialized domains within the membrane [134]. Three MAGUK proteins have been identified at the tight junction: ZO-1, -2 and -3.
ZO-1 was the first protein that was shown to be directly associated with tight-junction complexes [135]. It is a 220 kDa protein that links transmembrane proteins of the tight junction (i.e., occludin, claudins) to the actin cytoskeleton [117]. Previous studies have demonstrated that dissociation of ZO-1 from the junctional complex is associated with increased permeability [136–138], which implies that the ZO-1-transmembrane protein interaction is critical to tight-junction stability and function. ZO-1 may also act as a signaling molecule that communicates the state of the tight junction to the cellular interior, or vice versa. ZO-1 has been shown to localize to the nucleus under conditions of proliferation and injury [139], following Ca2+ depletion [140], and in response to nicotine [111]. It has also been colocalized with transcription factors [141] and various G-proteins [142].
ZO-2, a 160 kDa protein, has high sequence homology to ZO-1 [143] and is known to bind structural tight-junction constituents, signaling molecules, and transcription factors [144]. ZO-2 localizes to the nucleus during stress and proliferation, a property similar to ZO-1 [145,146]. In cultured brain microvessel endothelial cells, ZO-2 is localized along the plasma membrane at points of cell–cell contact [138], although it may be distributed more diffusely in whole cerebral microvessels [111]. Of particular note, ZO-2 may function somewhat redundantly with ZO-1 as it has been shown to facilitate formation of tight junctions that are morphologically intact in cultured epithelial cells lacking ZO-1 [147]. More recently, ZO-3, a 130 kDa protein, has been identified at the BBB [148] but its exact role in tight-junction formation and/or function has not been elucidated.
Other accessory proteins
In addition to MAGUK family members, other accessory proteins have been identified at the tight junction. These include cingulin, AF-6, 7H6 and EMP-1 [27,149]. Cingulin is a 140–160 kDa protein that associates with ZOs, JAM-1, and myosin [150] and is hypothesized to mediate interactions between the cytoskeleton and the tight junction. AF-6, a 180 kDa protein, interacts directly with ZO-1. This interaction is inhibited by inactivation of Ras, suggesting that disruption of ZO-1–AF-6 complex may be critical in modulaton of the tight junction by Ras-dependent pathways [151]. The function of 7H6 at the BBB is unknown [27] but it is known to reversibly dissociate from the tight junction under conditions of ATP depletion [152]. More recently, studies have identified a novel tightjunction protein known as EMP-1 [149]. Bangsow and colleagues showed that EMP-1 is enriched in porcine and murine brain microvessel endothelial cells as well as rat brain microvessels and colocalizes with occludin [149]. Of particular note, EMP-1 is upregulated under ischemic conditions while occludin is downregulated, which suggests that this protein may play a role in maintenance of barrier function during pathological conditions [149].
Transporters
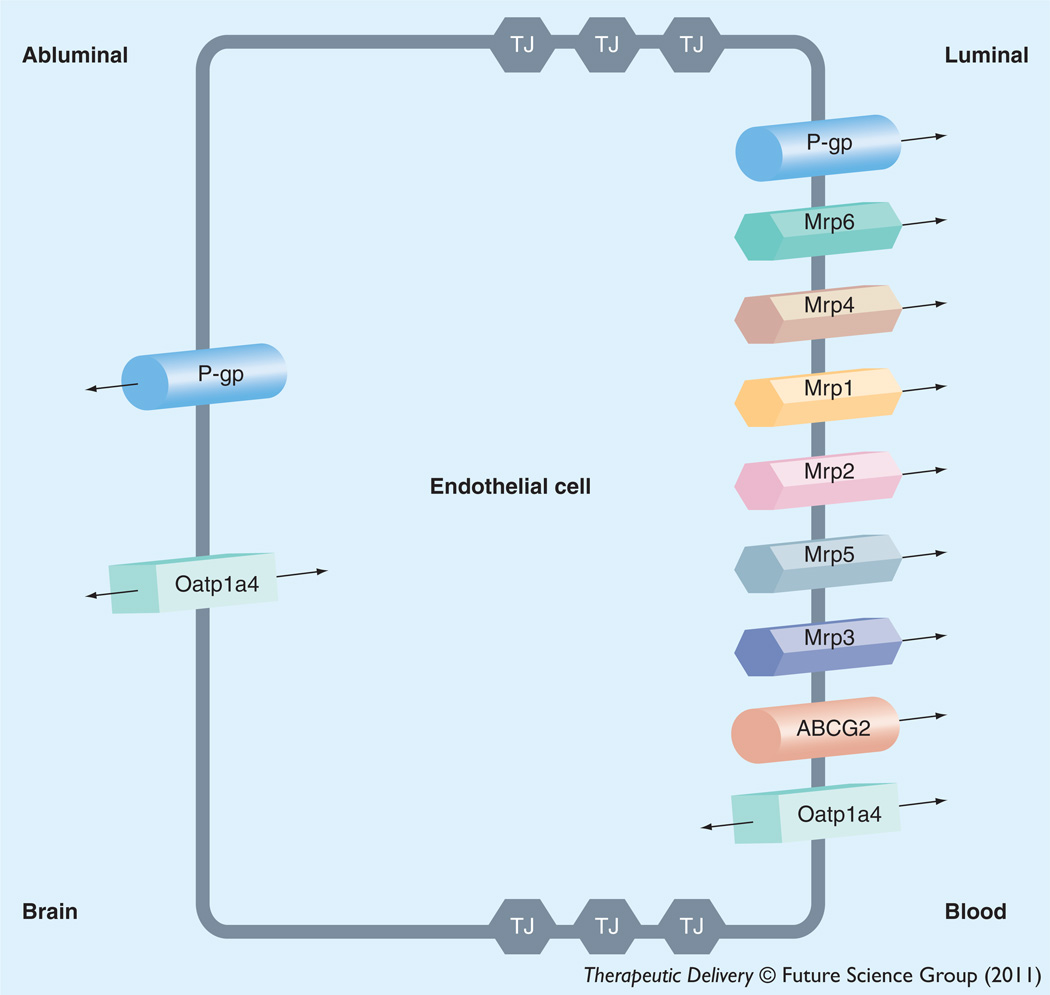
Pharmacological treatment of peripheral inflammatory pain requires that therapeutics attain efficacious concentrations in the brain. This objective requires that analgesic drugs (i.e., opioids) are able to successfully cross the BBB. Although opioids can bind to opioid receptors in the periphery, effective analgesia with opioids requires effective delivery to the CNS [18,19]. In general, small, nonionic, lipid-soluble compounds can easily enter the brain by passive diffusion while CNS permeation of larger, water-soluble, and/or ionic substances is less likely to occur by this mechanism. For many compounds, uptake into the brain and extrusion from the brain is governed by endogenously expressed transport proteins. Many transport proteins that have been shown to be involved in influx and/or efflux of opioid analgesics and/or opioid peptides (i.e., ATP-binding cassette [ABC] transporters, solute carrier [SLC] transporters) have been shown to be selectively expressed at the BBB endothelium [153,154]. In order to target transporters for optimization of CNS drug delivery, it is critical to understand localization (i.e., luminal versus abluminal) and functional expression of drug-transport proteins at the BBB endothelium. Below, we summarize current knowledge on such membrane transporters that are known to be involved in determining CNS delivery of therapeutic agents including opioid analgesics. Localization of specific transport proteins known to be involved in CNS delivery of opioids is depicted in Figure 2. Opioid analgesics and opioid peptides that are known to be substrates and/or inhibitors of endogenous BBB transporters are presented in Table 1.
Figure 2. Endothelial localization of drug transporters known to be involved in transport of opioids at the blood–brain barrier.
Table 1.
Opioid analgesics and opioid peptides that are known substrates and/or inhibitors for endogenous transport proteins at the blood–brain barrier.
| P-gp | MRP/Mrp | BCRP/Bcrp | OATP/Oatp | Peptide transport system-1 | |
|---|---|---|---|---|---|
| Opioid analgesic | |||||
| Alfentanil | S, I | ||||
| Buprenorphine | – | I | |||
| Codeine | – | ||||
| Fentanyl | S, I | S | |||
| Methadone | S, I | ||||
| Morphine | S, I | Morphine-3-glucuronide is a S for MRP2/MRP3 | |||
| Norbuprenorphine | S, I | I | |||
| Oxycodone | – | ||||
| Sufentanil | I | ||||
| Opioid peptide | |||||
| Deltorphin II | S | ||||
| DPDPE | S, I | S | |||
| Leu-enkephalin | I | S | |||
| Met-enkephalin | S | ||||
–: Known to be neither a substrate or inhibitor for a particular transporter; BCRP/Bcrp: Human/rodent breast cancer resistance protein I; Transport inhibitor; MRP/Mrp: Human/rodent multidrug resistance protein; OATP/Oatp: Human/rodent organic anion transporting polypeptides; S: Transport substrate.
ABC transporters
The ABC superfamily is among the largest and most ubiquitously expressed protein families known to date. ABC transporters are involved in translocation of opioids and their metabolites against their concentration gradient. The energy to transport xenobiotics is provided by binding and subsequent hydrolysis of ATP. In humans, 48 ABC genes have been identified and are classified according to seven subfamilies [155]. ABC drug transporters, specifically P-gp, MRPs in humans, Mrps in rodents and BCRP (also known as ABCG2) are known to be involved in cellular extrusion of therapeutic agents and thus constitute a considerable barrier to effective delivery of opioid analgesics to the brain. In general, P-gp transports cationic or basic and neutral compounds, whereas MRPs/Mrps are involved in cellular efflux of anionic drugs as well as their glucuronidated, sulfated, and glutathione-conjugated metabolites [74]. BCRP/Bcrp has significant overlap in substrate specificity profile with P-gp and has been shown to recognize a vast array of sulfoconjugated organic anions, hydrophobic, and amphiphilic compounds [156].
P-gp is a 170 kDa ATP-dependent integral membrane protein that was originally identified in colchicine-resistant Chinese Hamster Ovary cells [157]. It was designated as P-gp because of its inherent ability to affect permeability of biological membranes to xenobiotics that may be potentially toxic [157]. Physiologically, P-gp is believed to function as a biological defense mechanism against entry of toxic substances from the gut into the blood and for protection of vital organ systems such as the brain [158]. P-gp orthologues from different species have greater than 70% sequence identity [158] and are encoded by closely related genes (i.e., MDR genes), which have two isoforms in humans (MDR1 and MDR2) and three isoforms in both mice (i.e., mdr1, mdr2 and mdr3) and rats (i.e., mdr1a, mdr1b and mdr2). The human MDR2 gene and the murine/rodent mdr2 gene products are exclusively involved in hepatic transport of phosphatidylcholine and will not be further discussed. In contrast, human MDR1, murine mdr1/mdr3, and rodent mdr1a/mdr1b are involved in transport of therapeutic agents in several tissues including BBB endothelium. Specifically, P-gp expression has been identified at both the luminal [159,160] and abluminal membrane [66,161,162] of brain microvascular endothelial cells. Abluminal localization of P-gp has been identified on perivascular astrocyte foot processes [161,162] and on the abluminal plasma membrane of the endothelial cell itself [66].
The majority of P-gp transport substrates are weakly amphipathic and relatively hydrophobic. Additionally, many (but not all) substrates contain an aromatic ring and a positively charged tertiary nitrogen atom in their chemical structure [163]. Since its discovery, many opioids have been shown to be P-gp substrates including morphine [3,47,164–167], methadone [168–172], norbuprenorphine [172], fentanyl [173], alfentanil [174], and (d-penicillamine [2,5])-enkephalin (DPDPE) [167,175]. Additionally, these same opioids as well as sufentanil [176] are effective as P-gp transport inhibitors. In contrast, oxycodone [177] and buprenorphine [178] are opioids that do not interact with P-gp.
The mammalian MRP family belongs to the ABC subfamily C (ABCC) group of proteins, which contains 13 members including one ion channel (i.e., cystic fibrosis transmembrane conductance regulator [CFTR]), two surface receptors (i.e., SUR1 and 2) and a truncated protein that does not mediate transport (i.e., ABCC13) [38]. These proteins are not involved in drug transport and will not be further discussed. Many of the functionally characterized MRP isoforms that are known to be involved in drug transport have been localized to the mammalian BBB. These include MRP1/Mrp1, MRP2/Mrp2, MRP4/Mrp4, Mrp5 and Mrp6 [40,43,179–182]. The ability of various MRP isoforms to transport glucuronidated drug metabolites may have considerable consequences for opioid-mediated analgesia. Previous studies in isolated rat hepatocytes [183] and in a rodent model of cholestasis [184] have reported involvement of MRP2 and MRP3 in the efflux of morphine-3-glucuronide. Although morphine- 3-glucuronide is known to have minimal agonist activity at opioid receptors [185], it may act as an antagonist of analgesic effects induced by morphine and its active metabolite morphine- 6-glucuronide [186]. Therefore, MRP-mediated efflux of morphine-3-glucuronide may be a limiting factor in pharmacological activity of this metabolite. Unlike MRP1–3 and MRP6, MRP4 and MRP5 display a unique capacity to transport a variety of monophosphorylated nucleotides and nucleotide analogs [80,187,188]. More recently nonsteroidal anti-inflammatory drugs (NSAIDs), which are commonly included in treatment regimens for inflammatory pain conditions, have been shown to inhibit MRP4-mediated transport in peripheral blood lymphocytes [189]. The presence of multiple MRP homologues at the BBB may be a vital determinant in controlling the delivery of therapeutic agents, including opioids, to the brain.
A third ABC superfamily member that may be involved in xenobiotic efflux is BCRP. Several recent studies have demonstrated localization of BCRP at the brain microvasculature, particularly along the luminal side of the BBB [190–192]. In terms of transport activity, data from recent in vitro and in vivo studies have proved controversial. Although some studies have suggested that BCRP is not functional at the BBB [42,192,193] or plays a minimal role in xenobiotic efflux from the brain [194], more detailed analyses have confirmed that BCRP is a critical determinant of drug permeation across the BBB [37,195–197]. Opioids such as buprenorphine and norbuprenorphine have been recently shown to interact with BCRP at the BBB as inhibitors of xenobiotic transport [172]. Further studies are necessary to clarify the functional significance of this transporter at the BBB including the role of BCRP in CNS delivery of opioids.
Solute carrier transporters
The second major group of drug-transport proteins at the BBB endothelium is the SLC transporters. In contrast to ABC transporters, membrane transport of SLC family members is governed by either an electrochemical gradient utilizing an inorganic or organic solute as a driving force or the transmembrane concentration gradient of the substance actually being transported. To date, 319 SLC genes (i.e., SLC1 – SLC43 families) have been identified in humans [198]. Of the 43 known families of SLC transporters, members of SLC21A/SLCO and SLC22 are known to be expressed at the BBB and play a critical role in determining xenobiotic permeation across the brain microvascular endothelium [199].
Of the SLC transporters known to transport drugs at the BBB, perhaps the most viable candidates for transporter targeting are members of the SLC21A/SLCO family that includes OATPs/Oatps. OATPs/Oatps have distinct substrate preferences for amphipathic solutes [200]. For example, studies in Xenopus laevis oocytes have shown OATP1A2-mediated uptake of opioid peptides such as DPDPE and deltorphin II [201]. Data from this same study suggested that OATP1A2 may play a key role in drug–drug interactions associated with opioids because OATP1A2-mediated transport was inhibited by opiate antagonists (i.e., naloxone and naltrindole) as well as μ-opioid receptor agonist Tyr-d-Ala-Gly-N-methyl-Phe-glycinol and the endogenous peptide Leu-enkephalin [201]. Additionally, fentanyl brain:plasma ratios were reduced (four- to six-fold) in rats administered Oatp transport inhibitors (i.e., pravastatin and naloxone), which implies that delivery of this opioid analgesic to the brain occurs by an Oatp-mediated mechanism [202]. Although OATP isoforms are expressed in several tissues, not all exist at the BBB. Immunofluorescence staining of human brain frontal cortex demonstrated OATP1A2 (previously known as OATP-A) localization at the level of the brain microvascular endothelium [201]. In rodent brain, expression of Oatp1a4 and Oatp1c1 has been reported in capillary enriched fractions, capillary endothelial cells and/or isolated brain microvessels [8,44,45,153,203–205]. Oatp1c1 is selectively expressed at the BBB [205] and has relatively narrow substrate specificity and primarily transports thyroxine and conjugated sterols [44,45,205]. It has proposed that Oatp1a4, a rodent homologue of OATP1A2, is the primary drug-transporting Oatp isoform expressed at the rat BBB [200]. Using Oatp1a4−/− mice, Ose and colleagues demonstrated enhanced blood-to-brain transport of various Oatp substrates (i.e., pitavastatin, rosuvastatin, digoxin, taurocholate and ochratoxin A) as compared with wild-type controls [46]; however, the ability of Oatp1a4 to facilitate effective CNS drug delivery was previously indicated to be controversial [46].
Effect of peripheral inflammatory pain on paracellular drug uptake
It is established that the BBB may be compromised in response to various CNS pathologies [27,53,54]. Our laboratory has shown changes in BBB functional integrity during peripheral inflammatory pain [2,3,121,124,206–208]. For example, enhanced brain accumulation of 14C-sucrose, a vascular marker that does not typically cross the brain microvascular endothelium [209], was demonstrated in multiple in vivo models of peripheral inflammatory pain (i.e., subcutaneous injection of either formalin, λ-carrageenan or complete Freund’s adjuvant) suggesting that pain/inflammation in the periphery alters BBB permeability to circulating solutes [206]. In our studies, changes in brain solute uptake is not likely attributed to altered cerebral vascular blood volume because we have previously shown that blood volume changes are negligible in the λ-carrageenan model [206]. Changes in BBB permeability to 14C-sucrose were directly correlated with altered expression of constituent tight-junction proteins including occludin [6,121,124,206,207,210], claudin-3 [6,207], claudin-5 [6,124,207], JAM-1 [210] and ZO-1 [6,121,124,206]. Of paramount significance was the observation that peripheral inflammatory pain disrupted disulfide-bonded occludin oligomeric assemblies, thereby preventing monomeric occludin from forming an impermeable physical barrier to paracellular transport [4].
Our studies with 14C-sucrose implied that altered BBB paracellular permeability (i.e., leak) to vascular markers may have a significant impact on drug delivery to the CNS. Indeed, we demonstrated that opening of the paracellular route to circulating solutes does lead to increased drug uptake to the CNS for specific therapeutic drugs. One such drug is the moderate μ-opioid receptor agonist codeine. The therapeutic effects of codeine are centrallymediated thus indicating that this opioid analgesic must accumulate within the CNS in order to exert its analgesic and antitussive properties [2]. The physicochemical properties of codeine dictate that BBB transport primarily occurs via passive diffusion and is highly dependent on BBB transcellular permeability and blood flow [211]. Using microdialysis in rats injected with codeine intravenously, Xie and Hammarlund-Udenaes demonstrated that this opioid analgesic rapidly attained distributional equilibrium with equal unbound concentrations in brain and blood, which suggests that BBB codeine flux occurs via a passive process [211]. Furthermore, codeine transport did not show any evidence of dose-dependency or saturation kinetics, which suggests that a membrane transporter is unlikely to a determinant of codeine permeation at the BBB [211]. Although a recent study in an immortalized rat brain endothelial cell line (RBE4) demonstrated pH-dependent component of cellular codeine uptake, these data still were consistent with a codeine uptake mechanism involving passive diffusion of unionized codeine species [212]. Therefore, any pathophysiological stimulus that alters BBB tight-junction protein complexes and increases passive paracellular solute permeability will likely affect CNS codeine uptake. Using the well-established and highly reproducible λ-carrageenan model of peripheral inflammatory pain, we showed enhanced brain uptake of codeine at 3 h and 48 h post-injection of λ-carrageenan as compared with saline controls [2]. Capillary depletion analyses demonstrated that this enhanced uptake reflected increased accumulation of codeine within the brain extracellular milieu and was not due to vascular trapping [2]. Furthermore, antinociception studies (i.e., radiant-heat tail flick measurement) demonstrated that increased brain uptake of codeine led to an enhanced antinociceptive profile [2], suggesting that pain/inflammation in the periphery is an important consideration in therapeutic drug dosing and/or potential adverse drug reactions. It has been argued that much of the analgesic effect of codeine is attributed to hepatic metabolism to morphine, which is mediated by CYP2D6 [213]; however, this hypothesis has been seriously challenged in the literature. Indeed, codeine is metabolized to morphine by CYP2D6 in the liver but production of this metabolite accounts for only 10% of the total metabolism of codeine [214]. In fact, over 70% of codeine is metabolized to codeine-6-glucuronide, which is known to have significant analgesic properties [214]. This metabolite is produced via UGT2B7, which is extensively expressed in the brain in addition to the liver [215]. Therefore, codeine may be metabolized within the brain itself, which strongly suggests that increased brain delivery of codeine in response to pathological or pharmacological stimuli is a highly relevant clinical consideration.
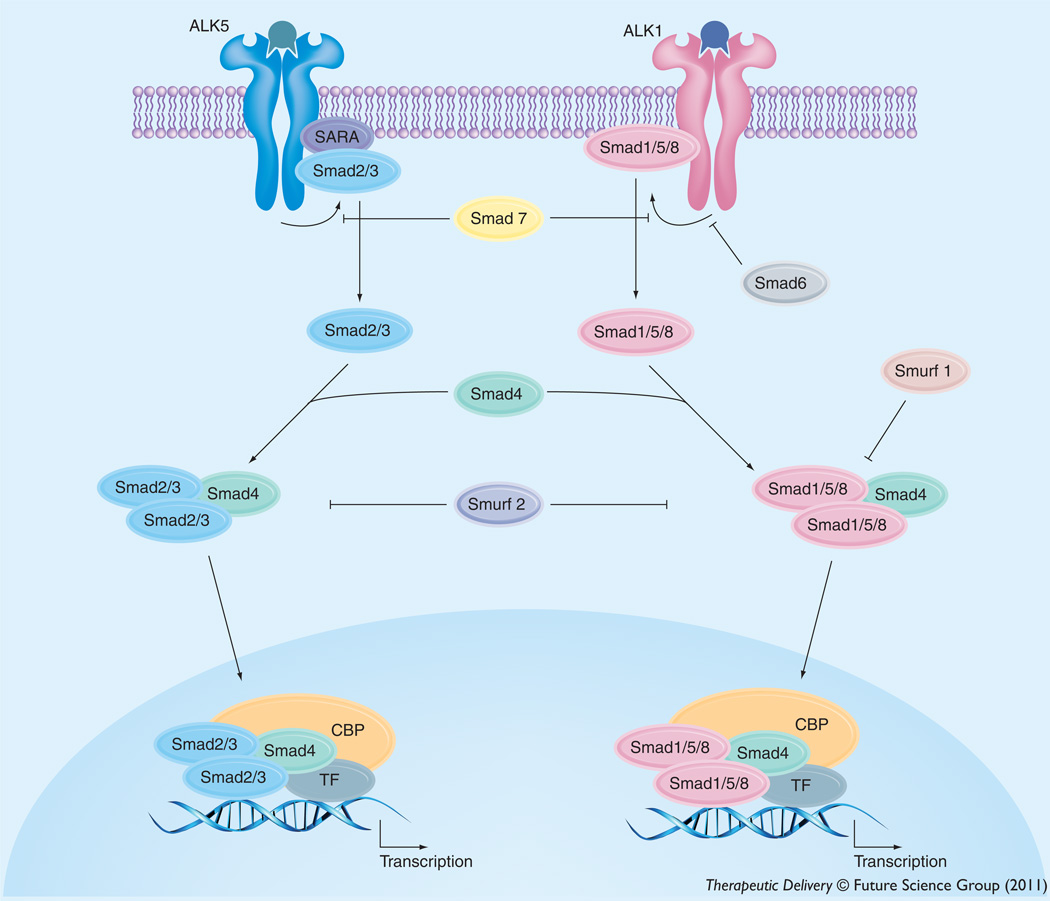
Despite the observation that peripheral inflammatory pain alters BBB paracellular permeability and tight-junction protein complex integrity, the intracellular signaling mechanisms involved had not been clearly elucidated until recently. We have shown involvement of TGF-β signaling in the regulation of BBB functional integrity and, by extension, paracellular drug uptake. TGF-β signaling regulates multiple cellular processes including vascular remodeling [216]. The TGF-β s are a family of pleiotropic cytokines that modulate cellular function by binding to a heterotetrameric complex of type I and type II serine/threonine kinase receptors [217]. The type I receptors, also known as activin receptor-like kinases (ALKs), propagate intracellular signals through phosphorylation of specific Smad proteins (i.e., receptor-regulated [R]-Smads) (Figures 3 & 4). Phosphorylated (R)-Smads form complexes with the common Smad (i.e., Smad4) enabling them to be translocated to the nucleus and regulate transcription of target genes [217].
Figure 3. Intracellular signaling molecules associated with TGF-β signaling at the blood–brain barrier.
Signals elicted by the TGF-β pathway involve two cell-surface receptors at the brain microvascular endothelium, which are designated ALK1 and ALK5. ALK1 transduces signals via phosphorylation of Smad proteins -1, -5, and -8 while ALK5 signals by phosphorylation of Smad2 and Smad3. Once phosphorylated, these Smad proteins bind to the common Smad (i.e., Smad4), thereby forming a protein complex that can translocate to the nucleus and affect transcription.
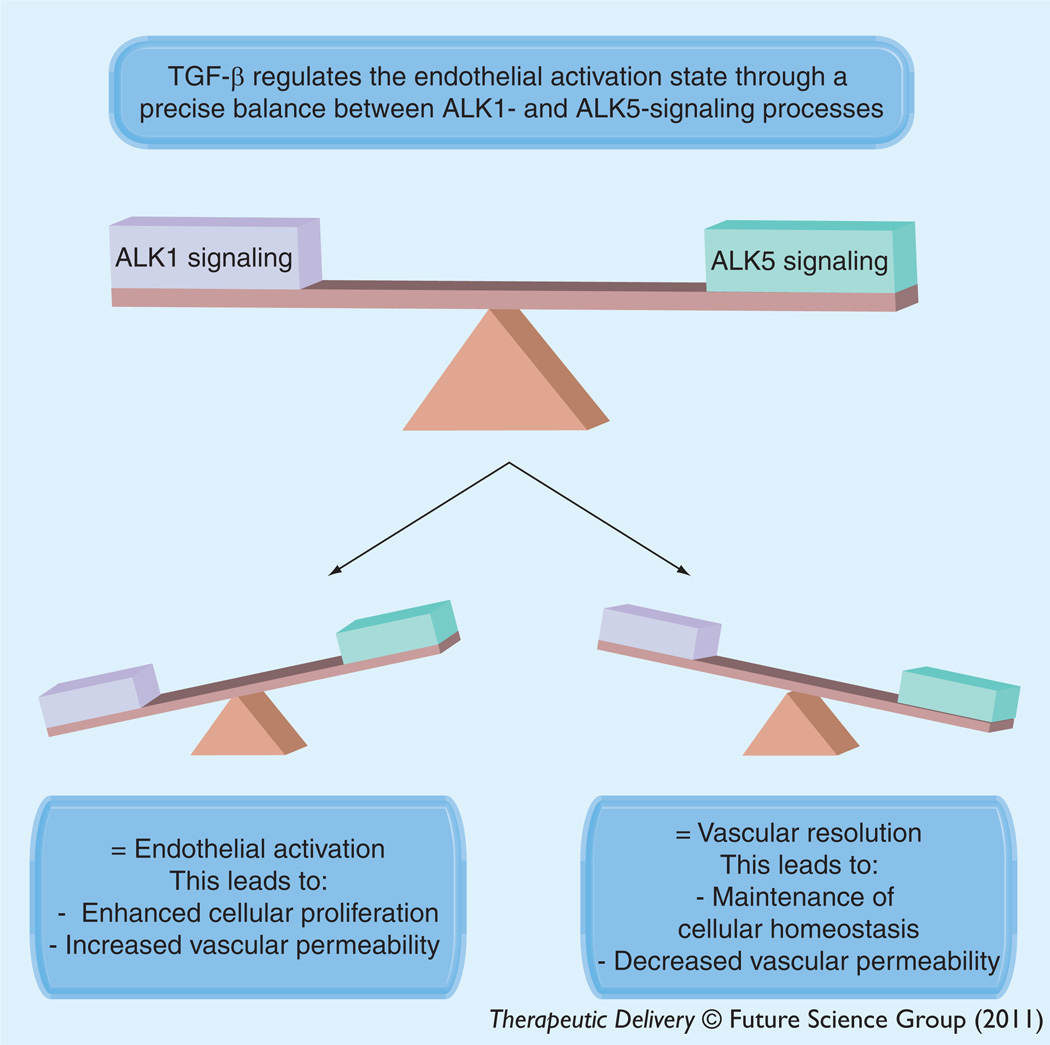
Figure 4. Physiological effect of divergent TGF-β signaling pathways at the brain microvascular endothelium.
ALK1 signaling mediates increased vascular permeability while ALK5 signaling mediates decreased vascular permeability. Endothelial homeostasis is maintained by a discrete balance between these two competing pathways.
In the majority of tissues, TGF-β signaling is mediated by ALK5 [218]; however, studies in cultured human endothelial cells [219] and in isolated arterial endothelium from ALK1-deficient mice [220] have indicated that ALK1 is also involved in vascular TGF-β signaling. TGF-β regulates the endothelial cell activation state through a precise balance between ALK1 and ALK5 signaling processes [219,221]. Whereas the ALK1 pathway leads to endothelial activation characterized by increased permeability, ALK5-mediated signaling promotes vascular resolution that is demarcated by decreased permeability [218,219]. Such effects on vascular permeability may be due to the ability of TGF-β signaling to alter expression of tight-junction constituent proteins. For example, claudin-5 expression was increased by pharmacological ALK5 inhibition in embryonic stem cells, suggesting involvement of TGF-β/ALK5 signaling in the regulation of tight-junction constituent proteins [222]. Additionally, studies using human glioblastoma cells cocultured with human brain endothelial cells showed that activation of TGF-β-mediated signaling decreased endothelial expression of occludin, claudin-1 and claudin-5 [223].
Based on these previous studies, we investigated the role of TGF-β signaling as a ‘triggering mechanism’ for BBB changes that occur during peripheral inflammatory pain [6]. Ultrasensitive ELISA analysis detected decreased levels of TGF-β 1 in serum collected from rats subjected to pain/inflammation as compared with control animals [6]. Expression of ALK1 was slightly reduced (1.3-fold) while ALK5 expression was profoundly decreased (4.0-fold) in brain microvessels isolated from rats injected with λ-carrageenan [6], which implies that TGF-β signaling may be reduced by peripheral inflammatory pain. Furthermore, the large magnitude of decrease in endothelial ALK5 expression indicates that TGF-β/ALK5 signaling may be reduced to a much greater degree than TGF-β/ALK1 signaling in animals subjected to peripheral inflammatory pain. Indeed, our data supports this hypothesis because Smad2/Smad3 nuclear accumulation was significantly reduced in rats administered λ-carrageenan while Smad1/5/8 nuclear expression was unchanged [6].
Since TGF-β/ALK5 signaling was reduced by peripheral inflammatory pain, we sought to determine if this decrease corresponded to an increase in paracellular solute permeability across the BBB. To meet this objective, we examined brain uptake of 14C-sucrose. CNS accumulation of 14C-sucrose was significantly enhanced in brain tissue collected from rats subjected to pain/inflammation as compared with saline controls [6]. Furthermore, 14C-sucrose uptake was further enhanced in the presence of SB431542 [6], a highly selective ALK5 inhibitor [224]. Our previous data showed that pretreatment with SB431542 prevented phosphorylation (i.e., activation) of Smad2 and Smad3 but did not affect Smad1/5/8 phosphorylation, further confirming specificity of this small-molecule ALK5 inhibitor [6]. These observations have profound implications for drug therapy, particularly opioid analgesia, because enhanced delivery across the BBB may improve analgesia but may also lead to an increased risk for adverse drug events. Therefore, we proposed that administration of a TGF-β/ALK5 receptor agonist may attenuate the increased paracellular permeability observed during peripheral inflammatory pain and protect the CNS from unwanted uptake of solutes from the systemic circulation. For these studies, we hypothesized that administration of exogenous TGF-β1 at a concentration that restored serum concentrations to control levels may protect BBB functional integrity in animals subjected to peripheral inflammatory pain. Indeed, pre-treatment with TGF-β1 (12.5 ng/kg) increased nuclear accumulation of phosphorylated Smad2/Smad3 and reduced brain uptake of 14C-sucrose during pain/inflammation [6]. These data emphasize the critical importance of TGF-β/ALK5-mediated signaling in maintaining BBB functional integrity and suggest that the ALK5 receptor may be a novel target that can be utilized to restore barrier function and control drug permeation during situations where brain endothelial integrity may be disrupted (i.e., inflammatory pain).
We have also investigated whether TGF-β/ALK5 signaling regulates BBB solute permeability during peripheral inflammatory pain by altering expression of constituent tight-junction proteins. Using the TGF-β/ALK5 inhibitor SB431542, we demonstrated that pharmacological blockade of ALK5 signaling led to increased expression of various tight-junction proteins (i.e., occludin, claudin-3, claudin-5 and ZO-1) in rat brain microvessels [6]. The complexity of TGF-β/ALK5 signaling at the BBB is further emphasized by the observation that occludin, claudin-3 and ZO-1 protein expression was increased in animals administered SB431542 and subjected to λ-carrageenan-induced peripheral inflammatory pain as compared with animals administered SB431542 alone [6]. These data support the hypothesis that impaired TGF-β/ALK5 signaling increases BBB permeability through alterations in protein constituents of tight-junction protein complexes; however, the different profile of tight-junction protein expression in animals subjected to pain/inflammation and in animals administered SB431542 underscores the complexity of intracellular signaling processes that modulate paracellular solute permeability. That is, different expression levels of ALK5 may alter tight-junction proteins to a different degree. It is critical to consider that time points beyond 3 h (i.e., chronic inflammatory pain) may reveal differential modulation of tight-junction proteins by varying levels of TGF-β/ALK5-mediated signaling, a possibility that requires further study.
It has been previously indicated that changes in paracellular permeability induced by peripheral inflammatory pain are not sufficient to enable CNS penetration of small-molecule therapeutics [225]. Specifically, Lu and colleagues utilized Evans blue dye as a model of a small molecule and, therefore, as an indicator of BBB integrity [225]. In the interpretation of these studies, it is important to consider that use of Evans blue dye is inappropriate in this context. Evans blue dye, when unconjugated to plasma proteins, has a molecular weight of 960.8 Da; however, it is well established that Evans blue dye irreversibly binds to serum albumin in vivo. This leads to the formation of a very large solute–protein complex (i.e., in excess of 60,000 Da) that can only traverse the BBB under considerable pathological stress such as that observed during ischemic stroke [226,227]. In context of peripheral inflammatory pain, changes in BBB permeability are not sufficient to allow leakage of large molecules such as Evans blue–albumin from the systemic circulation. In fact, we have shown that altered BBB functional integrity in response to peripheral inflammatory pain affects brain microvascular permeability only to small xenobiotics such as sucrose, codeine, and morphine [2,3,6,121,124,206,207,210]. In all of these studies, we measured CNS uptake of Evans blue–albumin and did not observe or detect any measureable uptake of this extremely large complex, which further clarifies that BBB opening during peripheral inflammatory pain will only affect paracellular uptake of small-molecule solutes such as opioid analgesics.
In addition to altered circulating levels of cytokines, it is important to consider that other pathophysiological processes can occur simultaneously. Such processes may also present therapeutic targets that can be utilized to regulate paracellular transport of drugs across the BBB during peripheral inflammatory pain. For example, production of reactive oxygen species (ROS) such as superoxide anion is a well-established component of both pain and inflammation [228]. Using the λ-carrageenan model of peripheral inflammatory pain, Wang and colleagues demonstrated that M40403, a superoxide dismutase mimetic, significantly attenuated all parameters of inflammation and hyperalgesia, which implies that superoxide anion is a critical component of the pathophysiological response to a peripheral inflammatory stimulus [229]. Similar observations were obtained in the λ-carrageenan model using the free radical scavenger and superoxide dismutase mimetic tempol [230]. More recently, our laboratory has shown that administration of tempol prevents changes in occludin localization and structure in an in vivo rodent component model of global hypoxia [7]. Taken together, these studies suggest that oxidative stress may play a prominent role in modulating BBB tight-junction complex structure and paracellular solute permeability during peripheral inflammatory pain. Therefore, further studies are critical to determine the role of ROS in BBB changes that occur during pain/inflammation and whether pharmacological scavenging of ROS may be a viable therapeutic approach for maintaining BBB functional integrity during acute and/or chronic inflammatory pain.
Effect of peripheral inflammatory pain on BBB transport proteins
The ability of a pharmacological agent (i.e., opioid analgesic and/or opioid peptide) to traverse the BBB endothelium and achieve efficacious concentrations within the CNS is dependent upon multiple mechanisms of transport. Such mechanisms include uptake into the brain via an influx transporter and/or extrusion from the CNS mediated by an efflux transporter. For many drugs, it is this discrete balance between influx and efflux that determines if a pharmacological agent will accumulate within the brain extracellular milieu and be able to elicit a therapeutic effect. The complexity of transport biology at the BBB is further underscored by the observation that functional expression of such transport proteins may be dramatically altered by pathophysiological stressors [3,8,231–234]. A thorough understanding of regulation and functional expression of endogenous BBB transporters in both healthly and diseased states is critical for optimization of opioid pharmacotherapy. Furthermore, such information will enable efficient targeting of transporters and/or transporter regulatory mechanisms, thus allowing endogenous BBB transport systems to be exploited for purposes of improving CNS drug delivery.
Perhaps the most critical transporter determinant of drug permeation to the CNS is P-gp. There are several reports in the literature that have shown altered functional expression of P-gp in various tissues by pathological stimuli such as viral proteins associated with HIV-1 infection [67,232], inflammation induced by bacterial endotoxin [235–238], and seizure activity associated with intractable epilepsy [239]. Using the λ-carrageenan model, we have shown increased functional expression of P-gp at the BBB in response to acute peripheral inflammatory pain [3]. This increase in brain microvascular P-gp expression in λ-carrageenan treated rats corresponded with a decrease in brain uptake of morphine [3], an established P-gp substrate [164,167]. Kinetically, this decrease in morphine uptake resulted in a 36% decrease in the apparent brain volume of distribution and a 50% increase in the kout coefficient, an approximation of the overall loss/efflux of morphine from the brain [3]. Involvement of P-gp was further established by the observation that the P-gp inhibitor cyclosporine A increased blood-to-brain transport of morphine in a concentration-dependent manner [3]. This observation is particularly critical as it emphasizes the paramount importance of P-gp as a determinant of CNS drug delivery. Furthermore, our functional studies with morphine in the λ-carrageenan model show that pathophysiological stressors such as peripheral inflammatory pain can upregulate P-gp at the BBB, which will similarly affect brain penetration of opioids that are known P-gp substrates. Altered P-gp functional activity in the context of peripheral inflammatory pain can have profound effects on opioid analgesic efficacy. Previously, Hamabe and colleagues studied inter-individual variability within the same strain of mice and reported that the level of morphine analgesia was inversely proportional to brain microvascular P-gp expression [18]. This paper emphasizes the fact that high P-gp expression levels at the BBB will have dramatic repercussions on morphine therapy. Therefore, it can be postulated that any stimulus that directly upregulates P-gp transport activity at the brain microvascular endothelium will likely exert a similar effect on efficacy of a P-gp substrate opioid. Using the radiant-tail flick test, a well-accepted method of measuring analgesic effect, our group demonstrated a significant decrease in morphine analgesia in animals subjected to λ-carrageenan induced inflammatory pain as compared with saline controls [3]. Decreased morphine efficacy following pain/inflammation was further highlighted by reduced AUC in λ-carrageenan treated animals as compared with saline controls [3]. Our studies on morphine antinociception differ from previous work by Ossipov and colleagues that showed increased morphine analgesia following peripheral inflammatory pain [240]. This discrepancy may be explained by gender differences because we used female Sprague-Dawley rats in our studies and the work of Ossipov and colleagues utilized male rats. Androgens have been shown to repress transporters including P-gp [241], which suggests that the level of morphine analgesia measured by Ossipov and colleagues may have been subject to a considerably reduced P-gp effect. Nonetheless, the results of our P-gp studies with morphine clearly demonstrate that pathophysiological mechanisms are critical in determining opioid brain uptake and analgesic efficacy.
To date, most studies on involvement of drug transporters in CNS drug delivery have focused on mechanisms that prevent drugs from accumulating in the brain. An alternative approach for delivering drugs (i.e., opioids) to the CNS is to target endogenous BBB transporters known to be involved in blood-to-brain xenobiotic transport. An intriguing candidate is Oatp1a4, which is well-known to transport opioids [46,167,201]. For example, studies in human embryonic kidney cells transfected with rat Oatp1a4 demonstrated saturable DPDPE uptake [46]. Recently, we reported for the first time increased functional expression of Oatp1a4 at the BBB in rats subjected to peripheral inflammatory pain [8]. Evidence for increased Oatp1a4 transport at the BBB included:
-
▪
Increased brain accumulation of taurocholate, a selective Oatp substrate [242];
-
▪
Attenuation of taurocholate uptake by Oatp transport inhibitors (i.e., digoxin, estrone-3-sulfate and fexofenadine);
-
▪
Increase in KIN for taurocholate during peripheral pain, which implies increased blood-to-brain transport;
-
▪
An increase in taurocholate accumulation within brain interstitial fluid but no change in taurocholate sequestration within the BBB endothelium itself [8].
In order to determine if Oatp1a4 could effectively facilitate CNS drug delivery, we studied BBB transport of the opioid peptide DPDPE. Brain uptake of DPDPE is governed by multiple mechanisms in addition to Oatp1a4-mediated transport including transcytosis [243] and P-gp-mediated efflux [167]. Although we showed increased Oatp1a4 functional expression at the BBB in animals subjected to peripheral inflammatory pain, we did not see any change in blood-to-brain DPDPE transport [8]. In light of our previous work with P-gp [3], we proposed that Oatp1a4 influx transport was negated by P-gp efflux. This implies that the relative contribution of Oatp1a4 to overall brain uptake of DPDPE could only be determined in the absence of P-gp-mediated transport activity. When we inhibited P-gp efflux transport using reversin 205, a selective P-gp inhibitory peptide [244], we observed that the relative contribution of Oatp1a4 to brain uptake of DPDPE increased from 56% in saline controls to 71% in animals subjected to peripheral inflammatory pain [245]. These data are particularly critical because they showed, for the first time, that Oatp1a4 can be targeted for delivering opioids and/or opioid peptides to the brain.
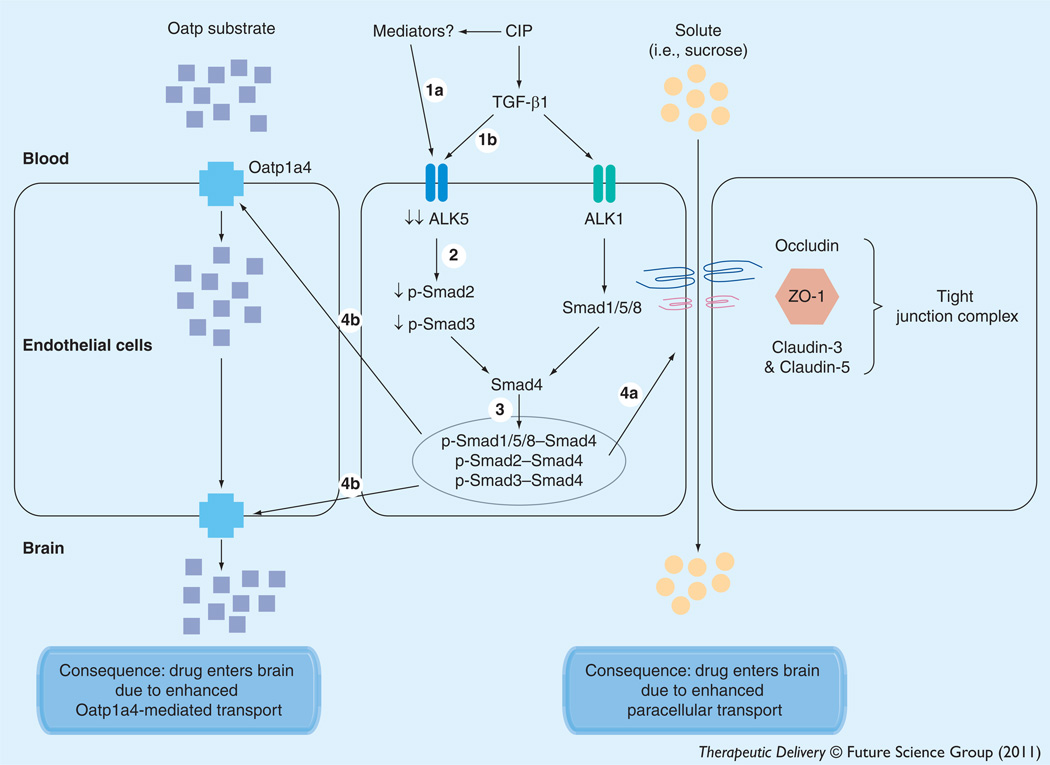
In order to successfully target a transporter system for optimization of CNS drug delivery, it is crucial to determine how a transport of interest is regulated at the molecular level. In the context of peripheral inflammatory pain, this includes identification and characterization of biological mechanisms that enable peripheral pain to ‘transmit’ signals upstream and alter BBB drug transporters. Based on our previous work [6], we proposed that this signal may involve alterations in serum concentrations of cytokines such as TGF-β1, a critical regulator of brain microvascular homeostasis [218]. Of paramount significance, we showed that administration of diclofenac, a commonly prescribed NSAID, prevented decreases in serum TGF-β as well as reduced microvascular expression of ALK1/ALK5, suggesting that inflammation in the periphery is directly involved in overall regulation of the TGF-β signaling pathway [8]. Furthermore, pharmacological inhibition of TGF-β/ALK5 signaling using SB431542 increased Oatp1a4 functional expression both in animals subjected to peripheral inflammatory pain and in corresponding saline controls [8]. Although studies in immortalized mouse brain endothelial cells (MBE4) have shown involvement of ALK5-mediated signaling in P-gp regulation [246], we are the first to report TGF-β/ALK5 signaling regulation of an endogenous BBB drug-uptake transporter. Our work on TGF-β/ALK5 signaling demonstrated that this pathway can regulate permeability at the BBB both by altering the structure of tightjunction protein complexes and by increasing functional expression of an influx transporter (Figure 5). Furthermore, these studies highlight the potential of the TGF-β/ALK5 pathway as a pharmacological target that can be utilized for optimization of drug delivery to the CNS.
Figure 5. Proposed effect of blood–brain barrier changes in TGF-β signaling on CNS drug delivery during peripheral inflammatory pain.
Acute inflammatory pain is characterized by an increase in production of inflammatory mediators and decrease in circulating levels of TGF-β1, which leads to decreased expression of ALK5 (1a,1b). In turn, this leads to a decrease in signaling through the ALK5 receptor and decreased phosphorylation of Smad2 and Smad3 (2). The decreased expression of p-Smad2 and p-Smad3 results in a lower level of translocation of these Smad isoforms to the nucleus and a corresponding downregulation of TGF-β/ALK5- mediated signaling (3). Our data also show decreased expression of the ALK1 receptor but this may be a compensatory response of the endothelium to maintain balance between ALK1- and ALK5-mediated signaling events. Interestingly, expression of p-Smad1/5/8 does not change, suggesting that the TGF-β/ALK1 pathway may predominate at the BBB during acute inflammatory pain. Overall, this causes dysregulation of the tight junction complex that is demarcated by altered expression of claudin-3, claudin-5, occludin and ZO-1 (4a). Additionally, functional expression of Oatp1a4 is increased (4b), leading to enhanced brain uptake of Oatp substrate drugs. The result of this signaling mechanism is an increase in blood–brain permeability and influx transport that is characterized by enhanced CNS drug delivery via the paracellular route or by Oatp1a4-mediated transport.
Similar to the tight junction, complexity of transporter regulation at the BBB is underscored by the fact that multiple signaling pathways are activated in response to peripheral inflammatory pain. We have shown that acute peripheral inflammatory pain significantly increases circulating levels of IL-6 [Ronaldson PT, Davis TP. Unpublished Observation]. Inflammatory responses involving IL-6 secretion may activate JAK, a family of non-receptor tyrosine kinases that transduce cytokine-mediated signals. Activation of these intracellular enzymes results in phosphorylation of STAT proteins such as STAT3. In vivo, STAT3 has been shown to be the principal signal transducer of the IL-6 receptor [247]. With respect to drug transporters, there are few studies that have shown a correlation between altered mRNA expression and STAT3 signaling. Using human epidermal keratinocytes and dermal fibroblasts, Dreuw and colleagues reported an association between activation of STAT3 and an increase in MRP1, MRP3, MRP4, and MRP5 mRNA levels [248]. Recently, in vivo studies have demonstrated increased STAT3 DNA binding and reduced hepatic expression of Mrp2 and Oatp isoforms in IL-6+/+ mice in comparison to IL-6−/− mice [249]. Studies must be done to fully characterize the relationship between JAK/STAT3 signaling and regulation of drug-transport proteins at the BBB. A thorough understanding of this pathway in the context of peripheral inflammatory pain may point to novel strategies to optimize CNS delivery of opioid analgesic drugs.
In addition to pathophysiological factors, endogenous BBB transporters may also be regulated by pharmacological factors. Patients aff licted by peripheral inflammatory pain are often prescribed multiple drugs as part of their therapeutic regimen. This may lead to altered analgesic efficacy of opioids, the primary therapeutic drug class used to treat pain/inflammation, and/or unexpected drug–drug interactions. Such effects may be mediated by nuclear receptors, ligand-activated transcription factors that are known to be activated by a broad spectrum of xenobiotics [250]. Some of these receptors, specifically pregnane-X-receptor (PXR) and constitutive androstane receptor (CAR), are known to be expressed by brain microvascular endothelial cells and are implicated in regulation of ABC transporters (i.e., P-gp, MRPs/Mrps and BCRP) and OATPs/Oatps [170,251–256]. The pharmacological impact of PXR-mediated up-regulation of P-gp was demonstrated in a human PXR transgenic mouse model where PXR activation with two specific ligands (i.e., ifampin and hyperforin) reduced CNS accumulation of methadone, an established P-gp substrate [170]. Studies in primary cultures of rat brain microvessel endothelial cells demonstrated that dexamethasone treatment increased functional expression of P-gp, Mrp2 and Bcrp via a PXR-dependent mechanism [254]. Additionally, studies in an immortalized rat brain endothelial cell line demonstrated that activation of CAR with specific ligands (i.e., carbamazepine and phenobarbital) significantly increased functional activity of P-gp, Mrp1, and Mrp2 [253]. Furthermore, studies in isolated mouse and rat capillaries showed increased expression and activity of P-gp, Mrp2, and Bcrp when tissues were exposed to 1,4-bis-[2-(3,5-dichloropyridyloxy] benzene (TCPOBOP, a mouse-specific CAR agonist) and phenobarbital (i.e., a rat-specific CAR agonist) respectively [256]. These data suggest that PXR/CAR activation may tighten the BBB to opioid analgesics by altering functional expression of ABC transporters. Studies in the liver have shown that nuclear receptors can also regulate functional expression of Oatps [257,258]; however, the role of PXR and/or CAR on regulation of Oatp1a4 expression at the BBB remains poorly characterized.
It has been estimated that unwanted activation of nuclear receptors may account for up to 60% of drug–drug interactions [252]. Although such interactions involving PXR and/or CAR have not been directly studied in the CNS, much of this information may prove to be clinically relevant with respect to treatment of peripheral inflammatory pain. Many drugs included in pain management regimens, particularly anti-inflammatory drugs, have been reported to interact with PXR and/or CAR in both in vitro and in vivo model systems. For example, dexamethasone, an anti-inflammatory glucocorticoid and established PXR activator [259], is often used in the management of pain conditions [260,261]. Dexamethasone has also been shown to reduce antinociceptive effects of opioids such as morphine and β-endorphin [262,263]; however, these reports did not determine if reduced analgesic effects in animals administered dexamethasone involved changes in CNS opioid concentrations. Acetaminophen is also commonly prescribed for management of acute or chronic peripheral inflammatory pain. Toxicity associated with acetaminophen is, in part, related to depletion of cellular concentrations of glutathione [264], a well-established substrate for MRP/Mrp isoforms [68,70,265]. Since acetaminophen is a known CAR activator [266], it may mediate its own toxicity via increased MRP/Mrp functional activity and glutathione efflux. Morphine metabolites (i.e., morphine- 3-glucuronide) are also transport substrates for MRP/Mrp isoforms [183]. Combined, these data suggest that inclusion of anti-inflammatory drugs (i.e., dexamethasone, acetaminophen) in a pain-treatment regimen may activate nuclear receptors and alter drug-transport proteins known to be involved in CNS delivery of opioids. Additionally, activation of nuclear receptors may facilitate clinically significant drug–drug interactions leading to modified opioid analgesia and adverse drug events associated with opioid toxicity.
Future perspective
The field of BBB biology, particularly the study of tight-junction protein complexes and endogenous transport systems, has rapidly advanced over the past two decades. For example, it is now well established that tight-junction protein complexes are dynamic in nature and can organize and reorganize in response to various pathological stimuli (i.e., peripheral inflammatory pain). These changes in tight-junction protein complexes can lead to increased BBB permeability to small-molecule drugs via the paracellular route. Additionally, many previous studies reported on the controversial ability of transporters (i.e., Oatp1a4) to act as facilitators of brain drug uptake. Now, it is beginning to be appreciated that endogenous BBB transporters can facilitate uptake of xenobiotics from the blood to the brain, thereby rendering these transport proteins potential targets for optimizing CNS drug delivery. Furthermore, molecular machinery involved in regulating endogenous BBB transport systems (i.e., TGF-β/ALK5 signaling, nuclear receptor systems) are just now being identified and characterized. These critical discoveries have identified multiple molecular targets that can be exploited for optimization of CNS delivery of therapeutic agents, including opioids. Such studies are particularly critical for newly developed peptides that can act as opioid-receptor agonists. In fact, many novel opioid peptides have been recently produced and have shown considerable analgesic efficacy [267,268]; however, molecular mechanisms involved in their CNS delivery have yet to be identified. Discovery of mechanisms that facilitate brain permeation of these peptides will undoubtedly enable more efficient analgesia and an improved utility as potential therapeutics. Perhaps targeting of novel opioid peptides to influx transporters such as Oatp1a4, which is already known to be involved in opioid-peptide transport at the BBB [8], will lead to significant advancements in the field of opioid pharmacology and pain management. Additionally, identification and characterization of intracellular signaling pathways that can regulate functional expression of uptake transporters provides yet another approach for pharmacological control of drug-transporter systems in an effort to deliver therapeutics to the CNS. Future work will continue to provide more insight on the interplay of tight-junction protein complexes, transporters, and intracellular signaling pathways at the BBB and how these systems can be effectively targeted. Ultimately, data derived from these studies will enable achievement of more precise drug (i.e., opioid) concentrations within the CNS and improved treatment for pathological conditions such as peripheral inflammatory pain.
Executive summary.
-
▪
The blood–brain barrier (BBB) is the most significant obstacle to effective CNS drug delivery. In fact, many currently available drugs have limited or no efficacy in the treatment of neurological diseases primarily because of limited ability to traverse the BBB and attain therapeutically efficacious concentrations in the CNS.
-
▪
Pain, a dominant symptom associated with acute and chronic inflammation that affects 76.2 million Americans, is most effectively treated with opioid analgesics. Opioid concentrations in the CNS must be precisely controlled in order to achieve effective analgesia and limit potentially dangerous central adverse effects. This therapeutic objective emphasizes the need to further characterize biological mechanisms that facilitate opioid delivery across the BBB in both healthly and diseased states and to understand how these systems can be exploited in an effort to optimize CNS opioid delivery.
-
▪
The highly specialized BBB phenotype requires interplay of the brain microvascular endothelium with surrounding astrocytes, microglia, pericytes, neurons, and extracellular matrix. This is often referred to as the neurovascular unit, which reflects involvement of multiple cellular and structural components in the physiology of the BBB including regulation of xenobiotic permeation.
-
▪
Critical structural features of the BBB that restrict blood-to-brain transport of pharmacological agents such as opioids include tight-junction protein complexes as well as endogenous transporters involved in drug influx and/or efflux.
-
▪
Functional integrity of the BBB is altered in response to pathophysiological stressors. Specifically, peripheral inflammatory pain increases paracellular permeability to vascular markers (i.e., sucrose) and therapeutic drugs (i.e., codeine) by changing expression of protein constituents of BBB tight junctions (i.e. claudin-3, claudin-5, occludin and ZO-1).
-
▪
During peripheral inflammatory pain, changes in paracellular permeability and tight-junction protein expression are mediated by decreased TGF-β/ALK5 signaling. Pharmacological inhibition of the ALK5 receptor during pain/inflammation leads to changes in BBB functional integrity that are greater than observed under conditions of peripheral inflammatory pain only. Exogenous administration of TGF-β1 restored TGF-β/ALK5 signaling and paracellular sucrose permeability to levels identical to control. These data suggest that the ALK5 receptor can be targeted in an effort to pharmacologically control BBB functional integrity.
-
▪
P-glycoprotein (P-gp), an efflux transporter, expression and activity at the BBB is markedly increased during peripheral inflammatory pain. Such changes lead to decreased brain accumulation of drugs such as morphine, an opioid analgesic. Reduced brain uptake of morphine is manifested by decreased analgesia.
-
▪
Organic anion transporting polypeptide 1a4 (Oatp1a4), a sodium-independent transporter governed by the transmembrane concentration gradient of its substrates, expression and activity at the BBB is significantly increased during peripheral inflammatory pain. Such changes lead to an enhanced contribution of Oatp1a4 in the brain uptake of d-penicillamine [2,5]-enkephalin, an opioid peptide analgesic. These data suggest that Oatp1a4 is a potential target that can be exploited for optimization of CNS drug delivery.
-
▪
Oatp1a4 functional expression at the BBB is regulated by TGF-β/ALK5 signaling. These data point to a regulatory mechanism that can be utilized to control Oatp1a4 expression and/or activity for purposes of optimizing CNS delivery of therapeutics.
-
▪
Future studies will likely aim to further characterize the interplay between transporter regulation and intracellular signaling systems. Additionally, identification of novel transporters and/or signaling systems involved in transporter regulation will provide new opportunities in the field of CNS drug delivery. Such studies will benefit opioid pharmacotherapy by enabling more precise control of CNS concentrations and improved treatment for conditions such as peripheral inflammatory pain.
Acknowledgments
This work was supported by NIH Grants R01-NS42652 and R01-DA11271 to TP Davis.
Key Terms
- Blood–brain barrier
A selective barrier between the CNS and the systemic circulation that maintains brain homeostasis by controlling fluid and electrolyte balance, regulating nutrient transport, and determining blood-borne solute permeability.
- Tight junction
In the context of the blood–brain barrier, tight junctions are a complex network of proteins that span the apical region of the intercellular cleft between two apposing endothelial cells. Tight junctions enable asymmetric distribution of membrane constituents (i.e., apical vs basolateral) and limit paracellular solute permeability.
- Opioid analgesic
A class of therapeutic agents that are currently the most effective drugs for pain relief. Opioids produce analgesia by binding to specific opioid receptors in the brain as well as in the periphery. Effective therapy with opioids requires delivery to the brain, where concentrations must be precisely controlled to produce effective analgesia while limiting unwanted side effects such as respiratory depression, tolerance, constipation, nausea and vomiting.
- P-glycoprotein
An ATP-dependent, membrane-bound drug efflux transporter that is endogenously expressed at the brain microvascular endothelium and is one of the most critical determinants of drug permeation across the BBB.
- Oatp1a4
A member of the larger family of solute-carrier transporters, Oatp1a4 is endogenously expressed at the BBB and is involved in bidirectional transport of various drugs including opioid peptides. The human homologue of Oatp1a4 is OATP1A2.
- TGF-β signaling pathway
An intracellular signaling pathway that is prominently expressed by brain microvascular endothelial cells. At the blood–brain barrier, TGF-β signaling is regulated by two distinct surface receptors designated activin receptor-like kinase-1 (ALK1) and ALK5. Stimulation of the ALK1 receptor increases vascular permeability to circulating solutes while ALK5 receptor activation decreases permeability. Integrity of the BBB is maintained, in part, by a precise balance between signaling mediated by ALK1 and signaling mediated by ALK5.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
- 1.Hartz AM, Bauer B. Regulation of ABC transporters at the blood–brain barrier: new targets for CNS therapy. Mol. Interv. 2010;10(5):293–304. doi: 10.1124/mi.10.5.6. [DOI] [PubMed] [Google Scholar]
- 2.Hau VS, Huber JD, Campos CR, Davis RT, Davis TP. Effect of lambda-carrageenan-induced inflammatory pain on brain uptake of codeine and antinociception. Brain Res. 2004;1018(2):257–264. doi: 10.1016/j.brainres.2004.05.081. [DOI] [PubMed] [Google Scholar]
- 3.Seelbach MJ, Brooks TA, Egleton RD, Davis TP. Peripheral inflammatory hyperalgesia modulates morphine delivery to the brain: a role for P-glycoprotein. J. Neurochem. 2007;102(5):1677–1690. doi: 10.1111/j.1471-4159.2007.04644.x. [DOI] [PubMed] [Google Scholar]
- 4.McCaffrey G, Seelbach MJ, Staatz WD, et al. Occludin oligomeric assembly at tight junctions of the blood–brain barrier is disrupted by peripheral inflammatory hyperalgesia. J. Neurochem. 2008;106(6):2395–2409. doi: 10.1111/j.1471-4159.2008.05582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCaffrey G, Willis CL, Staatz WD, et al. Occludin oligomeric assemblies at tight junctions of the blood–brain barrier are altered by hypoxia and reoxygenation stress. J. Neurochem. 2009;110(1):58–71. doi: 10.1111/j.1471-4159.2009.06113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ronaldson PT, DeMarco KM, Sanchez-Covarrubias L, Solinsky CM, Davis TP. Transforming growth factor-β signaling alters substrate permeability and tight junction protein expression at the blood–brain barrier during inflammatory pain. J. Cereb. Blood Flow Metab. 2009;29(6):1084–1098. doi: 10.1038/jcbfm.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lochhead JJ, McCaffrey G, Quigley CE, et al. Oxidative stress increases blood–brain barrier permeability and induces alterations in occludin during hypoxia-reoxygenation. J. Cereb.Blood Flow Metab. 2010;30(9):1625–1636. doi: 10.1038/jcbfm.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ronaldson PT, Finch JD, DeMarco KM, Quigley CE, Davis TP. Inflammatory pain signals an increase in functional expression of organic anion transporting polypeptide 1a4 at the blood–brain barrier. J. Pharmacol. Exp. Ther. 2011;336(3):827–839. doi: 10.1124/jpet.110.174151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vallejo R, Tilley DM, Vogel L, Benyamin R. The role of glia and the immune system in the development and maintenance of neuropathic pain. Pain Pract. 2010;10(3):167–184. doi: 10.1111/j.1533-2500.2010.00367.x. [DOI] [PubMed] [Google Scholar]
- 10.Wolka AM, Huber JD, Davis TP. Pain and the blood–brain barrier: obstacles to drug delivery. Adv. Drug Deliv. Rev. 2003;55(8):987–1006. doi: 10.1016/s0169-409x(03)00100-5. [DOI] [PubMed] [Google Scholar]
- 11.Dickinson T, Mitchell R, Robberecht P, Fleetwood-Walker SM. The role of VIP/PACAP receptor subtypes in spinal somatosensory processing in rats with an experimental peripheral mononeuropathy. Neuropharmacology. 1999;38(1):167–180. doi: 10.1016/s0028-3908(98)00171-3. [DOI] [PubMed] [Google Scholar]
- 12.Miller DB, O’Callaghan JP. Neuroendocrine aspects of the response to stress. Metabolism. 2002;51(6 Suppl. 1):5–10. doi: 10.1053/meta.2002.33184. [DOI] [PubMed] [Google Scholar]
- 13.Loram LC, Taylor FR, Strand KA, et al. Prior exposure to glucocorticoids potentiates lipopolysaccharide induced mechanical allodynia and spinal neuroinflammation. Brain Behav. Immun. 2011 doi: 10.1016/j.bbi.2011.04.013. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chou R. 2009 Clinical Guidelines from the American Pain Society and the American Academy of Pain Medicine on the use of chronic opioid therapy in chronic noncancer pain: what are the key messages for clinical practice? Pol. Arch. Med. Wewn. 2009;119(7–8):469–477. [PubMed] [Google Scholar]
- 15.Gloth FM., III Pharmacological management of persistent pain in older persons: focus on opioids and nonopioids. J. Pain. 2011;12(3 Suppl. 1):S14–S20. doi: 10.1016/j.jpain.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Manchikanti L, Fellows B, Ailinani H, Pampati V. Therapeutic, use, abuse, and nonmedical use of opioids: a ten-year perspective. Pain Physician. 2010;13(5):401–435. [PubMed] [Google Scholar]
- 17.Vadivelu N, Mitra S, Hines RL. Peripheral opioid receptor agonists for analgesia: a comprehensive review. J. Opioid. Manag. 2011;7(1):55–68. doi: 10.5055/jom.2011.0049. [DOI] [PubMed] [Google Scholar]
- 18.Hamabe W, Maeda T, Kiguchi N, Yamamoto C, Tokuyama S, Kishioka S. Negative relationship between morphine analgesia and P-glycoprotein expression levels in the brain. J. Pharmacol. Sci. 2007;105(4):353–360. doi: 10.1254/jphs.fp0071287. [DOI] [PubMed] [Google Scholar]
- 19.Labuz D, Mousa SA, Schafer M, Stein C, Machelska H. Relative contribution of peripheral versus central opioid receptors to antinociception. Brain Res. 2007;1160:30–38. doi: 10.1016/j.brainres.2007.05.049. [DOI] [PubMed] [Google Scholar]
- 20.Haas DA. An update on analgesics for the management of acute postoperative dental pain. J. Can. Dent. Assoc. 2002;68(8):476–482. [PubMed] [Google Scholar]
- 21.Watkins LR, Hutchinson MR, Rice KC, Maier SF. The ‘toll’ of opioid-induced glial activation: improving the clinical efficacy of opioids by targeting glia. Trends Pharmacol. Sci. 2009;30(11):581–591. doi: 10.1016/j.tips.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hutchinson MR, Zhang Y, Brown K, et al. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of Toll-like receptor 4 (TLR4) Eur. J Neurosci. 2008;28(1):20–29. doi: 10.1111/j.1460-9568.2008.06321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ehrlich P. Eine Farbenanalytische Studie. Hirschwald: 1885. Das sauerstufbudurfnis des organismus. [Google Scholar]
- 24.Ehrlich P. Gesammelte Arbeiten zur Immunitaetsforschung. Vol. 574. Hischwald: 1904. Ueber die beziehungen von chemischer constitution, verteilung und pharmakologischer wirkung. [Google Scholar]
- 25.Goldmann EE. Vitalfarbung am zentralnervensystem. Abhandl Konigl preuss Akad Wiss. 1913;1:1–60. [Google Scholar]
- 26.Lewandowsky M. Zur lehre von der cerebrospinalflussigkeit. Z. Klin. Med. 1900;40:480–494. [Google Scholar]
- 27.Hawkins BT, Davis TP. The blood–brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2005;57(2):173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 28.Tschirgi RD. Blood–brain barrier: fact or fancy? Fed. Proc. 1962;21:665–671. [PubMed] [Google Scholar]
- 29.Maynard EA, Schultz RL, Pease DC. Electron microscopy of the vascular bed of rat cerebral cortex. Am. J Anat. 1957;100(3):409–433. doi: 10.1002/aja.1001000306. [DOI] [PubMed] [Google Scholar]
- 30.Reese TS, Karnovsky MJ. Fine structural localization of a blood–brain barrier to exogenous peroxidase. J. Cell Biol. 1967;34(1):207–217. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brightman MW, Reese TS. Junctions between intimately apposed cell membranes in the vertebrate brain. J. Cell Biol. 1969;40(3):648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abbott NJ. Dynamics of CNS barriers: evolution, differentiation, and modulation. Cell Mol. Neurobiol. 2005;25(1):5–23. doi: 10.1007/s10571-004-1374-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oldendorf WH, Cornford ME, Brown WJ. The large apparent work capability of the blood–brain barrier: a study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat. Ann. Neurol. 1977;1(5):409–417. doi: 10.1002/ana.410010502. [DOI] [PubMed] [Google Scholar]
- 34.Ose A, Kusuhara H, Yamatsugu K, et al. P-glycoprotein restricts the penetration of oseltamivir across the blood–brain barrier. Drug Metab. Dispos. 2008;36(2):427–434. doi: 10.1124/dmd.107.018556. [DOI] [PubMed] [Google Scholar]
- 35.Roberts LM, Black DS, Raman C, et al. Subcellular localization of transporters along the rat blood–brain barrier and blood–cerebralspinal fluid barrier by in vivo biotinylation. Neuroscience. 2008;155(2):423–438. doi: 10.1016/j.neuroscience.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 36.Yousif S, Saubamea B, Cisternino S, et al. Effect of chronic exposure to morphine on the rat blood–brain barrier: focus on the P-glycoprotein. J. Neurochem. 2008;107(3):647–657. doi: 10.1111/j.1471-4159.2008.05647.x. [DOI] [PubMed] [Google Scholar]
- 37.Kodaira H, Kusuhara H, Ushiki J, Fuse E, Sugiyama Y. Kinetic analysis of the cooperation of P-glycoprotein (P-gp/Abcb1) and breast cancer resistance protein (Bcrp/Abcg2) in limiting the brain and testis penetration of erlotinib, flavopiridol, and mitoxantrone. J. Pharmacol. Exp. Ther. 2010;333(3):788–796. doi: 10.1124/jpet.109.162321. [DOI] [PubMed] [Google Scholar]
- 38.Dallas S, Miller DS, Bendayan R. Multidrug resistance-associated proteins: expression and function in the central nervous system. Pharmacol. Rev. 2006;58(2):140–161. doi: 10.1124/pr.58.2.3. [DOI] [PubMed] [Google Scholar]
- 39.Hawkins BT, Ocheltree SM, Norwood KM, Egleton RD. Decreased blood–brain barrier permeability to fluorescein in streptozotocin-treated rats. Neurosci. Lett. 2007;411(1):1–5. doi: 10.1016/j.neulet.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bauer B, Hartz AM, Lucking JR, Yang X, Pollack GM, Miller DS. Coordinated nuclear receptor regulation of the efflux transporter, Mrp2, and the phase-II metabolizing enzyme, GSTpi, at the blood–brain barrier. J. Cereb. Blood Flow Metab. 2008;28(6):1222–1234. doi: 10.1038/jcbfm.2008.16. [DOI] [PubMed] [Google Scholar]
- 41.Dauchy S, Miller F, Couraud PO, et al. Expression and transcriptional regulation of ABC transporters and cytochromes P450 in hCMEC/D3 human cerebral microvascular endothelial cells. Biochem. Pharmacol. 2009;77(5):897–909. doi: 10.1016/j.bcp.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 42.Lee G, Babakhanian K, Ramaswamy M, Prat A, Wosik K, Bendayan R. Expression of the ATP-binding cassette membrane transporter, ABCG2, in human and rodent brain microvessel endothelial and glial cell culture systems. Pharm. Res. 2007;24(7):1262–1274. doi: 10.1007/s11095-007-9244-1. [DOI] [PubMed] [Google Scholar]
- 43.Uchida Y, Ohtsuki S, Katsukura Y, et al. Quantitative targeted absolute proteomincs of human blood–brain barrier transporters and receptors. J. Neurochem. 2011;117(2):333–345. doi: 10.1111/j.1471-4159.2011.07208.x. [DOI] [PubMed] [Google Scholar]
- 44.Westholm DE, Stenehjem DD, Rumbley JN, Drewes LR, Anderson GW. Competitive inhibition of organic anion transporting polypeptide 1c1-mediated thyroxine transport by the fenamate class of nonsteroidal antiinflammatory drugs. Endocrinology. 2009;150(2):1025–1032. doi: 10.1210/en.2008-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Westholm DE, Salo DR, Viken KJ, Rumbley JN, Anderson GW. The blood–brain barrier thyroxine transporter organic anion-transporting polypeptide 1c1 displays atypical transport kinetics. Endocrinology. 2009;150(11):5153–5162. doi: 10.1210/en.2009-0769. [DOI] [PubMed] [Google Scholar]
- 46.Ose A, Kusuhara H, Endo C, et al. Functional characterization of mouse organic anion transporting peptide 1a4 in the uptake and efflux of drugs across the blood–brain barrier. Drug Metab. Dispos. 2010;38(1):168–176. doi: 10.1124/dmd.109.029454. [DOI] [PubMed] [Google Scholar]
- 47.Kusch-Poddar M, Drewe J, Fux I, Gutmann H. Evaluation of the immortalized human brain capillary endothelial cell line BB19 as a human cell culture model for the blood–brain barrier. Brain Res. 2005;1064(1–2):21–31. doi: 10.1016/j.brainres.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 48.Ose A, Ito M, Kusuhara H, et al. Limited brain distribution of [3R,4R,5S]-4-acetamido-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxyl ate phosphate (Ro 64–0802), a pharmacologically active form of oseltamivir, by active efflux across the blood–brain barrier mediated by organic anion transporter 3 (Oat3/Slc22a8) and multidrug resistance-associated protein 4 (Mrp4/Abcc4) Drug Metab. Dispos. 2009;37(2):315–321. doi: 10.1124/dmd.108.024018. [DOI] [PubMed] [Google Scholar]
- 49.Miyajima M, Kusuhara H, Fujishima M, Adachi Y, Sugiyama Y. Organic anion transporter 3 mediates the efflux transport of an amphipathic organic anion, dehydroepiandrosterone sulfate, across the blood–brain barrier in mice. Drug Metab. Dispos. 2011;39(5):814–819. doi: 10.1124/dmd.110.036863. [DOI] [PubMed] [Google Scholar]
- 50.Batrakova EV, Zhang Y, Li Y, et al. Effects of pluronic P85 on GLUT1 and MCT1 transporters in the blood–brain barrier. Pharm. Res. 2004;21(11):1993–2000. doi: 10.1023/b:pham.0000048189.79606.6e. [DOI] [PubMed] [Google Scholar]
- 51.Chishty M, Begley DJ, Abbott NJ, Reichel A. Interaction of nucleoside analogues with nucleoside transporters in rat brain endothelial cells. J. Drug Target. 2004;12(5):265–272. doi: 10.1080/10611860410001731398. [DOI] [PubMed] [Google Scholar]
- 52.Dogrukol-Ak D, Kumar VB, Ryerse JS, et al. Isolation of peptide transport system-6 from brain endothelial cells: therapeutic effects with antisense inhibition in Alzheimer and stroke models. J. Cereb. Blood Flow Metab. 2009;29(2):411–422. doi: 10.1038/jcbfm.2008.131. [DOI] [PubMed] [Google Scholar]
- 53.Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD. Blood–brain barrier: structural components and function under physiologic and pathologic conditions. J. Neuroimmune.Pharmacol. 2006;1(3):223–236. doi: 10.1007/s11481-006-9025-3. [DOI] [PubMed] [Google Scholar]
- 54.Zlokovic BV. The blood–brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 55.Janzer RC, Raff MC. Astrocytes induce blood–brain barrier properties in endothelial cells. Nature. 1987;325(6101):253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- 56.Tao-Cheng JH, Nagy Z, Brightman MW. Tight junctions of brain endothelium in vitro are enhanced by astroglia. J. Neurosci. 1987;7(10):3293–3299. doi: 10.1523/JNEUROSCI.07-10-03293.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Neuhaus J, Risau W, Wolburg H. Induction of blood–brain barrier characteristics in bovine brain endothelial cells by rat astroglial cells in transfilter coculture. Ann. NY Acad. Sci. 1991;633:578–580. doi: 10.1111/j.1749-6632.1991.tb15667.x. [DOI] [PubMed] [Google Scholar]
- 58.Hayashi Y, Nomura M, Yamagishi S, Harada S, Yamashita J, Yamamoto H. Induction of various blood–brain barrier properties in non-neural endothelial cells by close apposition to co-cultured astrocytes. Glia. 1997;19(1):13–26. [PubMed] [Google Scholar]
- 59.Willis CL, Nolan CC, Reith SN, et al. Focal astrocyte loss is followed by microvascular damage, with subsequent repair of the blood–brain barrier in the apparent absence of direct astrocytic contact. Glia. 2004;45(4):325–337. doi: 10.1002/glia.10333. [DOI] [PubMed] [Google Scholar]
- 60.Ballabh P, Braun A, Nedergaard M. The blood–brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol. Dis. 2004;16(1):1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 61.Goldberg M, De Pitta M, Volman V, Berry H, Ben Jacob E. Nonlinear gap junctions enable long-distance propagation of pulsating calcium waves in astrocyte networks. PLoS Comput. Biol. 2010;6(8):ii. doi: 10.1371/journal.pcbi.1000909. e1000909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pelligrino DA, Vetri F, Xu HL. Purinergic mechanisms in gliovascular coupling. Semin. Cell Dev. Biol. 2011;22(2):229–236. doi: 10.1016/j.semcdb.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 2006;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 64.Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia. 2010;58(9):1094–1103. doi: 10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- 65.Ronaldson PT, Bendayan M, Gingras D, Piquette-Miller M, Bendayan R. Cellular localization and functional expression of P-glycoprotein in rat astrocyte cultures. J. Neurochem. 2004;89(3):788–800. doi: 10.1111/j.1471-4159.2004.02417.x. [DOI] [PubMed] [Google Scholar]
- 66.Bendayan R, Ronaldson PT, Gingras D, Bendayan M. In situ localization of P-glycoprotein (ABCB1) in human and rat brain. J. Histochem. Cytochem. 2006;54(10):1159–1167. doi: 10.1369/jhc.5A6870.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ronaldson PT, Bendayan R. HIV-1 viral envelope glycoprotein gp120 triggers an inflammatory response in cultured rat astrocytes and regulates the functional expression of P-glycoprotein. Mol. Pharmacol. 2006;70(3):1087–1098. doi: 10.1124/mol.106.025973. [DOI] [PubMed] [Google Scholar]
- 68.Hirrlinger J, Dringen R. Multidrug resistance protein 1-mediated export of glutathione and glutathione disulfide from brain astrocytes. Methods Enzymol. 2005;400:395–409. doi: 10.1016/S0076-6879(05)00023-6. [DOI] [PubMed] [Google Scholar]
- 69.Minich T, Riemer J, Schulz JB, Wielinga P, Wijnholds J, Dringen R. The multidrug resistance protein 1 (Mrp1), but not Mrp5, mediates export of glutathione and glutathione disulfide from brain astrocytes. J. Neurochem. 2006;97(2):373–384. doi: 10.1111/j.1471-4159.2006.03737.x. [DOI] [PubMed] [Google Scholar]
- 70.Ronaldson PT, Bendayan R. HIV-1 viral envelope glycoprotein gp120 produces oxidative stress and regulates the functional expression of multidrug resistance protein-1 (Mrp1) in glial cells. J. Neurochem. 2008;106(3):1298–1313. doi: 10.1111/j.1471-4159.2008.05479.x. [DOI] [PubMed] [Google Scholar]
- 71.Ronaldson PT, Ashraf T, Bendayan R. Regulation of multidrug resistance protein 1 by tumor necrosis factor α in cultured glial cells: involvement of nuclear factor-κB and c-Jun N-terminal kinase signaling pathways. Mol. Pharmacol. 2010;77(4):644–659. doi: 10.1124/mol.109.059410. [DOI] [PubMed] [Google Scholar]
- 72.Nies AT, Jedlitschky G, Konig J, et al. Expression and immunolocalization of the multidrug resistance proteins, MRP1–MRP6 (ABCC1–ABCC6), in human brain. Neuroscience. 2004;129(2):349–360. doi: 10.1016/j.neuroscience.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 73.Bronger H, Konig J, Kopplow K, et al. ABCC drug efflux pumps and organic anion uptake transporters in human gliomas and the blood–tumor barrier. Cancer Res. 2005;65(24):11419–11428. doi: 10.1158/0008-5472.CAN-05-1271. [DOI] [PubMed] [Google Scholar]
- 74.Ronaldson PT, Persidsky Y, Bendayan R. Regulation of ABC membrane transporters in glial cells: relevance to the pharmacotherapy of brain HIV-1 infection. Glia. 2008;56(16):1711–1735. doi: 10.1002/glia.20725. [DOI] [PubMed] [Google Scholar]
- 75.del Rio-Hortega P. Microglia. In: Penfield W, editor. Cytology and Cellular Pathology of the Nervous System. Hoecher: 1932. pp. 481–584. [Google Scholar]
- 76.Speth C, Dierich MP, Sopper S. HIV-infection of the central nervous system: the tightrope walk of innate immunity. Mol. Immunol. 2005;42(2):213–228. doi: 10.1016/j.molimm.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 77.Lee G, Schlichter L, Bendayan M, Bendayan R. Functional expression of P-glycoprotein in rat brain microglia. J. Pharmacol. Exp. Ther. 2001;299(1):204–212. [PubMed] [Google Scholar]
- 78.Ronaldson PT, Lee G, Dallas S, Bendayan R. Involvement of P-glycoprotein in the transport of saquinavir and indinavir in rat brain microvessel endothelial and microglia cell lines. Pharm. Res. 2004;21(5):811–818. doi: 10.1023/b:pham.0000026433.27773.47. [DOI] [PubMed] [Google Scholar]
- 79.Dallas S, Zhu X, Baruchel S, Schlichter L, Bendayan R. Functional expression of the multidrug resistance protein 1 in microglia. J. Pharmacol. Exp. Ther. 2003;307(1):282–290. doi: 10.1124/jpet.103.054304. [DOI] [PubMed] [Google Scholar]
- 80.Dallas S, Schlichter L, Bendayan R. Multidrug resistance protein (MRP) 4- and MRP 5-mediated efflux of 9-(2-phosphonylmethoxyethyl)adenine by microglia. J. Pharmacol. Exp. Ther. 2004;309(3):1221–1229. doi: 10.1124/jpet.103.063966. [DOI] [PubMed] [Google Scholar]
- 81.Dore-Duffy P, Cleary K. Morphology and properties of pericytes. Methods Mol. Biol. 2011;686:49–68. doi: 10.1007/978-1-60761-938-3_2. [DOI] [PubMed] [Google Scholar]
- 82.Hori S, Ohtsuki S, Hosoya K, Nakashima E, Terasaki T. A pericyte-derived angiopoietin-1 multimeric complex induces occludin gene expression in brain capillary endothelial cells through Tie-2 activation in vitro. J. Neurochem. 2004;89(2):503–513. doi: 10.1111/j.1471-4159.2004.02343.x. [DOI] [PubMed] [Google Scholar]
- 83.Gonul E, Duz B, Kahraman S, Kayali H, Kubar A, Timurkaynak E. Early pericyte response to brain hypoxia in cats: an ultrastructural study. Microvasc. Res. 2002;64(1):116–119. doi: 10.1006/mvre.2002.2413. [DOI] [PubMed] [Google Scholar]
- 84.Dore-Duffy P, Owen C, Balabanov R, Murphy S, Beaumont T, Rafols JA. Pericyte migration from the vascular wall in response to traumatic brain injury. Microvasc. Res. 2000;60(1):55–69. doi: 10.1006/mvre.2000.2244. [DOI] [PubMed] [Google Scholar]
- 85.Armulik A, Genove G, Mae M, et al. Pericytes regulate the blood–brain barrier. Nature. 2010;468(7323):557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 86.Berezowski V, Landry C, Dehouck MP, Cecchelli R, Fenart L. Contribution of glial cells and pericytes to the mRNA profiles of P-glycoprotein and multidrug resistance-associated proteins in an in vitro model of the blood–brain barrier. Brain Res. 2004;1018(1):1–9. doi: 10.1016/j.brainres.2004.05.092. [DOI] [PubMed] [Google Scholar]
- 87.Ben Menachem E, Johansson BB, Svensson TH. Increased vulnerability of the blood–brain barrier to acute hypertension following depletion of brain noradrenaline. J. Neural Transm. 1982;53(2–3):159–167. doi: 10.1007/BF01243407. [DOI] [PubMed] [Google Scholar]
- 88.Cohen Z, Molinatti G, Hamel E. Astroglial and vascular interactions of noradrenaline terminals in the rat cerebral cortex. J. Cereb. Blood Flow Metab. 1997;17(8):894–904. doi: 10.1097/00004647-199708000-00008. [DOI] [PubMed] [Google Scholar]
- 89.Cohen Z, Bonvento G, Lacombe P, Hamel E. Serotonin in the regulation of brain microcirculation. Prog. Neurobiol. 1996;50(4):335–362. doi: 10.1016/s0301-0082(96)00033-0. [DOI] [PubMed] [Google Scholar]
- 90.Vaucher E, Hamel E. Cholinergic basal forebrain neurons project to cortical microvessels in the rat: electron microscopic study with anterogradely transported Phaseolus vulgaris leucoagglutinin and choline acetyltransferase immunocytochemistry. J. Neurosci. 1995;15(11):7427–7441. doi: 10.1523/JNEUROSCI.15-11-07427.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tong XK, Hamel E. Regional cholinergic denervation of cortical microvessels and nitric oxide synthase-containing neurons in Alzheimer’s disease. Neuroscience. 1999;92(1):163–175. doi: 10.1016/s0306-4522(98)00750-7. [DOI] [PubMed] [Google Scholar]
- 92.Vaucher E, Tong XK, Cholet N, Lantin S, Hamel E. GABA neurons provide a rich input to microvessels but not nitric oxide neurons in the rat cerebral cortex: a means for direct regulation of local cerebral blood flow. J. Comp Neurol. 2000;421(2):161–171. [PubMed] [Google Scholar]
- 93.Buxton RB, Frank LR. A model for the coupling between cerebral blood flow and oxygen metabolism during neural stimulation. J. Cereb. Blood Flow Metab. 1997;17(1):64–72. doi: 10.1097/00004647-199701000-00009. [DOI] [PubMed] [Google Scholar]
- 94.Paemeleire K. The cellular basis of neurovascular metabolic coupling. Acta Neurol. Belg. 2002;102(4):153–157. [PubMed] [Google Scholar]
- 95.Vital SA, Terao S, Nagai M, Granger DN. Mechanisms underlying the cerebral microvascular responses to angiotensin II-induced hypertension. Microcirculation. 2010;17(8):641–649. doi: 10.1111/j.1549-8719.2010.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cucullo L, Couraud PO, Weksler B, et al. Immortalized human brain endothelial cells and flow-based vascular modeling: a marriage of convenience for rational neurovascular studies. J. Cereb. Blood Flow Metab. 2008;28(2):312–328. doi: 10.1038/sj.jcbfm.9600525. [DOI] [PubMed] [Google Scholar]
- 97.Sood RR, Taheri S, Candelario-Jalil E, Estrada EY, Rosenberg GA. Early beneficial effect of matrix metalloproteinase inhibition on blood–brain barrier permeability as measured by magnetic resonance imaging countered by impaired long-term recovery after stroke in rat brain. J. Cereb. Blood Flow Metab. 2008;28(2):431–438. doi: 10.1038/sj.jcbfm.9600534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.del Zoppo GJ. The neurovascular unit, matrix proteases, and innate inflammation. Ann. NY Acad. Sci. 2010;1207:46–49. doi: 10.1111/j.1749-6632.2010.05760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 100.Tilling T, Engelbertz C, Decker S, Korte D, Huwel S, Galla HJ. Expression and adhesive properties of basement membrane proteins in cerebral capillary endothelial cell cultures. Cell Tissue Res. 2002;310(1):19–29. doi: 10.1007/s00441-002-0604-1. [DOI] [PubMed] [Google Scholar]
- 101.Tilling T, Korte D, Hoheisel D, Galla HJ. Basement membrane proteins influence brain capillary endothelial barrier function in vitro. J. Neurochem. 1998;71(3):1151–1157. doi: 10.1046/j.1471-4159.1998.71031151.x. [DOI] [PubMed] [Google Scholar]
- 102.Savettieri G, Di LI, Catania C, et al. Neurons and ECM regulate occludin localization in brain endothelial cells. Neuroreport. 2000;11(5):1081–1084. doi: 10.1097/00001756-200004070-00035. [DOI] [PubMed] [Google Scholar]
- 103.Butt AM, Jones HC, Abbott NJ. Electrical resistance across the blood–brain barrier in anaesthetized rats: a developmental study. J. Physiol. 1990;429:47–62. doi: 10.1113/jphysiol.1990.sp018243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Haskins J, Gu L, Wittchen ES, Hibbard J, Stevenson BR. ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J. Cell Biol. 1998;141(1):199–208. doi: 10.1083/jcb.141.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Del Maschio A, De Luigi A, Martin-Padura I, et al. Leukocyte recruitment in the cerebrospinal fluid of mice with experimental meningitis is inhibited by an antibody to junctional adhesion molecule (JAM) J. Exp. Med. 1999;190(9):1351–1356. doi: 10.1084/jem.190.9.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dejana E, Lampugnani MG, Martinez-Estrada O, Bazzoni G. The molecular organization of endothelial junctions and their functional role in vascular morphogenesis and permeability. Int. J Dev. Biol. 2000;44(6):743–748. [PubMed] [Google Scholar]
- 107.Yeung D, Manias JL, Stewart DJ, Nag S. Decreased junctional adhesion molecule-A expression during blood–brain barrier breakdown. Acta Neuropathol. 2008;115(6):635–642. doi: 10.1007/s00401-008-0364-4. [DOI] [PubMed] [Google Scholar]
- 108.Hoffman WH, Stamatovic SM, Andjelkovic AV. Inflammatory mediators and blood–brain barrier disruption in fatal brain edema of diabetic ketoacidosis. Brain Res. 2009;1254:138–148. doi: 10.1016/j.brainres.2008.11.100. [DOI] [PubMed] [Google Scholar]
- 109.Haarmann A, Deiss A, Prochaska J, et al. Evaluation of soluble junctional adhesion molecule-A as a biomarker of human brain endothelial barrier breakdown. PLoS One. 2010;5(10):e13568. doi: 10.1371/journal.pone.0013568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Furuse M, Hirase T, Itoh M, et al. Occludin: a novel integral membrane protein localizing at tight junctions. J. Cell Biol. 1993;123(6 Pt 2):1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hawkins BT, Abbruscato TJ, Egleton RD, et al. Nicotine increases in vivo blood–brain barrier permeability and alters cerebral microvascular tight junction protein distribution. Brain Res. 2004;1027(1–2):48–58. doi: 10.1016/j.brainres.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 112.Hirase T, Staddon JM, Saitou M, et al. Occludin as a possible determinant of tight junction permeability in endothelial cells. J. Cell Sci. 1997;110(Pt 14):1603–1613. doi: 10.1242/jcs.110.14.1603. [DOI] [PubMed] [Google Scholar]
- 113.McCarthy KM, Skare IB, Stankewich MC, et al. Occludin is a functional component of the tight junction. J. Cell Sci. 1996;109(Pt 9):2287–2298. doi: 10.1242/jcs.109.9.2287. [DOI] [PubMed] [Google Scholar]
- 114.Lacaz-Vieira F, Jaeger MM, Farshori P, Kachar B. Small synthetic peptides homologous to segments of the first external loop of occludin impair tight junction resealing. J. Membr. Biol. 1999;168(3):289–297. doi: 10.1007/s002329900518. [DOI] [PubMed] [Google Scholar]
- 115.Feldman GJ, Mullin JM, Ryan MP. Occludin: structure, function and regulation. Adv. Drug Deliv. Rev. 2005;57(6):883–917. doi: 10.1016/j.addr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 116.Furuse M, Itoh M, Hirase T, et al. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J. Cell Biol. 1994;127(6 Pt 1):1617–1626. doi: 10.1083/jcb.127.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J. Biol. Chem. 1998;273(45):29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- 118.Balda MS, Whitney JA, Flores C, Gonzalez S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical–basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J. Cell Biol. 1996;134(4):1031–1049. doi: 10.1083/jcb.134.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.McCaffrey G, Staatz WD, Quigley CA, et al. Tight junctions contain oligomeric protein assembly critical for maintaining blood–brain barrier integrity in vivo. J. Neurochem. 2007;103(6):2540–2555. doi: 10.1111/j.1471-4159.2007.04943.x. [DOI] [PubMed] [Google Scholar]
- 120.Saitou M, Furuse M, Sasaki H, et al. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol. Biol. Cell. 2000;11(12):4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Huber JD, Hau VS, Borg L, Campos CR, Egleton RD, Davis TP. Blood–brain barrier tight junctions are altered during a 72-h exposure to λ-carrageenan-induced inflammatory pain. Am. J Physiol. Heart Circ. Physiol. 2002;283(4):H1531–H1537. doi: 10.1152/ajpheart.00027.2002. [DOI] [PubMed] [Google Scholar]
- 122.Brown RC, Davis TP. Hypoxia/aglycemia alters expression of occludin and actin in brain endothelial cells. Biochem. Biophys. Res. Commun. 2005;327(4):1114–1123. doi: 10.1016/j.bbrc.2004.12.123. [DOI] [PubMed] [Google Scholar]
- 123.Nag S, Venugopalan R, Stewart DJ. Increased caveolin-1 expression precedes decreased expression of occludin and claudin-5 during blood–brain barrier breakdown. Acta Neuropathol. 2007;114(5):459–469. doi: 10.1007/s00401-007-0274-x. [DOI] [PubMed] [Google Scholar]
- 124.Campos CR, Ocheltree SM, Hom S, Egleton RD, Davis TP. Nociceptive inhibition prevents inflammatory pain induced changes in the blood–brain barrier. Brain Res. 2008;1221:6–13. doi: 10.1016/j.brainres.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tai LM, Holloway KA, Male DK, Loughlin AJ, Romero IA. Amyloid-β-induced occludin down-regulation and increased permeability in human brain endothelial cells is mediated by MAPK activation. J. Cell Mol. Med. 2010;14(5):1101–1112. doi: 10.1111/j.1582-4934.2009.00717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J. Cell Biol. 1998;141(7):1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Heiskala M, Peterson PA, Yang Y. The roles of claudin superfamily proteins in paracellular transport. Traffic. 2001;2(2):93–98. doi: 10.1034/j.1600-0854.2001.020203.x. [DOI] [PubMed] [Google Scholar]
- 128.Furuse M, Sasaki H, Tsukita S. Manner of interaction of heterogeneous claudin species within and between tight junction strands. J. Cell Biol. 1999;147(4):891–903. doi: 10.1083/jcb.147.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kubota K, Furuse M, Sasaki H, et al. Ca2+-independent cell-adhesion activity of claudins, a family of integral membrane proteins localized at tight junctions. Curr. Biol. 1999;9(18):1035–1038. doi: 10.1016/s0960-9822(99)80452-7. [DOI] [PubMed] [Google Scholar]
- 130.Witt KA, Mark KS, Hom S, Davis TP. Effects of hypoxia-reoxygenation on rat blood–brain barrier permeability and tight junctional protein expression. Am. J Physiol. Heart Circ. Physiol. 2003;285(6):H2820–H2831. doi: 10.1152/ajpheart.00589.2003. [DOI] [PubMed] [Google Scholar]
- 131.Wolburg H, Wolburg-Buchholz K, Kraus J, et al. Localization of claudin-3 in tight junctions of the blood–brain barrier is selectively lost during experimental autoimmune encephalomyelitis and human glioblastoma multiforme. Acta Neuropathol. 2003;105(6):586–592. doi: 10.1007/s00401-003-0688-z. [DOI] [PubMed] [Google Scholar]
- 132.Forster C, Burek M, Romero IA, Weksler B, Couraud PO, Drenckhahn D. Differential effects of hydrocortisone and TNFα on tight junction proteins in an in vitro model of the human blood–brain barrier. J. Physiol. 2008;586(7):1937–1949. doi: 10.1113/jphysiol.2007.146852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang P, Liu Y, Shang X, Xue Y. CRM197-induced blood–brain barrier permeability increase is mediated by upregulation of caveolin-1 protein. J. Mol. Neurosci. 2011;43(3):485–492. doi: 10.1007/s12031-010-9471-5. [DOI] [PubMed] [Google Scholar]
- 134.Gonzalez-Mariscal L, Bnzos A, Avila-Flores A. MAGUK proteins: structure and role in the tight junction. Semin.Cell Dev. Biol. 2000;11(4):315–324. doi: 10.1006/scdb.2000.0178. [DOI] [PubMed] [Google Scholar]
- 135.Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J. Cell Biol. 1986;103(3):755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Abbruscato TJ, Lopez SP, Mark KS, Hawkins BT, Davis TP. Nicotine and cotinine modulate cerebral microvascular permeability and protein expression of ZO-1 through nicotinic acetylcholine receptors expressed on brain endothelial cells. J. Pharm. Sci. 2002;91(12):2525–2538. doi: 10.1002/jps.10256. [DOI] [PubMed] [Google Scholar]
- 137.Fischer S, Wobben M, Marti HH, Renz D, Schaper W. Hypoxia-induced hyperpermeability in brain microvessel endothelial cells involves VEGF-mediated changes in the expression of zonula occludens-1. Microvasc. Res. 2002;63(1):70–80. doi: 10.1006/mvre.2001.2367. [DOI] [PubMed] [Google Scholar]
- 138.Mark KS, Davis TP. Cerebral microvascular changes in permeability and tight junctions induced by hypoxia-reoxygenation. Am. J Physiol. Heart Circ. Physiol. 2002;282(4):H1485–H1494. doi: 10.1152/ajpheart.00645.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gottardi CJ, Arpin M, Fanning AS, Louvard D. The junction-associated protein, zonula occludens-1, localizes to the nucleus before the maturation and during the remodeling of cell–cell contacts. Proc. Natl Acad. Sci. USA. 1996;93(20):10779–10784. doi: 10.1073/pnas.93.20.10779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Riesen FK, Rothen-Rutishauser B, Wunderli-Allenspach H. A ZO1-GFP fusion protein to study the dynamics of tight junctions in living cells. Histochem.Cell Biol. 2002;117(4):307–315. doi: 10.1007/s00418-002-0398-y. [DOI] [PubMed] [Google Scholar]
- 141.Balda MS, Matter K. The tight junction protein ZO-1 and an interacting transcription factor regulate ErbB-2 expression. EMBO J. 2000;19(9):2024–2033. doi: 10.1093/emboj/19.9.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Meyer TN, Schwesinger C, Denker BM. Zonula occludens-1 is a scaffolding protein for signaling molecules. Gα(12) directly binds to the Src homology 3 domain and regulates paracellular permeability in epithelial cells. J. Biol. Chem. 2002;277(28):24855–24858. doi: 10.1074/jbc.C200240200. [DOI] [PubMed] [Google Scholar]
- 143.Gumbiner B, Lowenkopf T, Apatira D. Identification of a 160 kDa polypeptide that binds to the tight junction protein ZO-1. Proc. Natl Acad. Sci. USA. 1991;88(8):3460–3464. doi: 10.1073/pnas.88.8.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bnzos A, Huerta M, Lopez-Bayghen E, Azuara E, Amerena J, Gonzalez-Mariscal L. The tight junction protein ZO-2 associates with Jun, Fos and C/EBP transcription factors in epithelial cells. Exp. Cell Res. 2004;292(1):51–66. doi: 10.1016/j.yexcr.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 145.Islas S, Vega J, Ponce L, Gonzalez-Mariscal L. Nuclear localization of the tight junction protein ZO-2 in epithelial cells. Exp. Cell Res. 2002;274(1):138–148. doi: 10.1006/excr.2001.5457. [DOI] [PubMed] [Google Scholar]
- 146.Traweger A, Fuchs R, Krizbai IA, Weiger TM, Bauer HC, Bauer H. The tight junction protein ZO-2 localizes to the nucleus and interacts with the heterogeneous nuclear ribonucleoprotein scaffold attachment factor-B. J. Biol. Chem. 2003;278(4):2692–2700. doi: 10.1074/jbc.M206821200. [DOI] [PubMed] [Google Scholar]
- 147.Umeda K, Matsui T, Nakayama M, et al. Establishment and characterization of cultured epithelial cells lacking expression of ZO-1. J. Biol. Chem. 2004;279(43):44785–44794. doi: 10.1074/jbc.M406563200. [DOI] [PubMed] [Google Scholar]
- 148.Takenaga Y, Takagi N, Murotomi K, Tanonaka K, Takeo S. Inhibition of Src activity decreases tyrosine phosphorylation of occludin in brain capillaries and attenuates increase in permeability of the blood–brain barrier after transient focal cerebral ischemia. J. Cereb.Blood Flow Metab. 2009;29(6):1099–1108. doi: 10.1038/jcbfm.2009.30. [DOI] [PubMed] [Google Scholar]
- 149.Bangsow T, Baumann E, Bangsow C, et al. The epithelial membrane protein 1 is a novel tight junction protein of the blood–brain barrier. J. Cereb.Blood Flow Metab. 2008;28(6):1249–1260. doi: 10.1038/jcbfm.2008.19. [DOI] [PubMed] [Google Scholar]
- 150.Citi S, Sabanay H, Kendrick-Jones J, Geiger B. Cingulin: characterization and localization. J. Cell Sci. 1989;93(Pt 1):107–122. doi: 10.1242/jcs.93.1.107. [DOI] [PubMed] [Google Scholar]
- 151.Yamamoto T, Harada N, Kawano Y, Taya S, Kaibuchi K. In vivo interaction of AF-6 with activated Ras and ZO-1. Biochem. Biophys. Res. Commun. 1999;259(1):103–107. doi: 10.1006/bbrc.1999.0731. [DOI] [PubMed] [Google Scholar]
- 152.Zhong Y, Enomoto K, Isomura H, et al. Localization of the 7H6 antigen at tight junctions correlates with the paracellular barrier function of MDCK cells. Exp. Cell Res. 1994;214(2):614–620. doi: 10.1006/excr.1994.1299. [DOI] [PubMed] [Google Scholar]
- 153.Li JY, Boado RJ, Pardridge WM. Blood–brain barrier genomics. J. Cereb. Blood Flow Metab. 2001;21(1):61–68. doi: 10.1097/00004647-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 154.Li JY, Boado RJ, Pardridge WM. Rat blood–brain barrier genomics. II. J. Cereb. Blood Flow Metab. 2002;22(11):1319–1326. doi: 10.1097/01.WCB.0000040944.89393.0f. [DOI] [PubMed] [Google Scholar]
- 155.Leslie EM, Deeley RG, Cole SP. Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol. Appl. Pharmacol. 2005;204(3):216–237. doi: 10.1016/j.taap.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 156.Robey RW, To KK, Polgar O, et al. ABCG2: a perspective. Adv. Drug Deliv. Rev. 2009;61(1):3–13. doi: 10.1016/j.addr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim. Biophys. Acta. 1976;455(1):152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- 158.Gottesman MM, Hrycyna CA, Schoenlein PV, Germann UA, Pastan I. Genetic analysis of the multidrug transporter. Annu. Rev. Genet. 1995;29:607–649. doi: 10.1146/annurev.ge.29.120195.003135. [DOI] [PubMed] [Google Scholar]
- 159.Beaulieu E, Demeule M, Ghitescu L, Beliveau R. P-glycoprotein is strongly expressed in the luminal membranes of the endothelium of blood vessels in the brain. Biochem.J. 1997;326(Pt 2):539–544. doi: 10.1042/bj3260539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Virgintino D, Robertson D, Errede M, et al. Expression of P-glycoprotein in human cerebral cortex microvessels. J. Histochem. Cytochem. 2002;50(12):1671–1676. doi: 10.1177/002215540205001212. [DOI] [PubMed] [Google Scholar]
- 161.Golden PL, Pardridge WM. P-glycoprotein on astrocyte foot processes of unfixed isolated human brain capillaries. Brain Res. 1999;819(1–2):143–146. doi: 10.1016/s0006-8993(98)01305-5. [DOI] [PubMed] [Google Scholar]
- 162.Schlachetzki F, Pardridge WM. P-glycoprotein and caveolin-1α in endothelium and astrocytes of primate brain. Neuroreport. 2003;14(16):2041–2046. doi: 10.1097/00001756-200311140-00007. [DOI] [PubMed] [Google Scholar]
- 163.Sharom FJ. Shedding light on drug transport: structure and function of the P-glycoprotein multidrug transporter (ABCB1) Biochem. Cell Biol. 2006;84(6):979–992. doi: 10.1139/o06-199. [DOI] [PubMed] [Google Scholar]
- 164.Letrent SP, Polli JW, Humphreys JE, Pollack GM, Brouwer KR, Brouwer KL. P-glycoprotein-mediated transport of morphine in brain capillary endothelial cells. Biochem. Pharmacol. 1999;58(6):951–957. doi: 10.1016/s0006-2952(99)00180-x. [DOI] [PubMed] [Google Scholar]
- 165.Cisternino S, Rousselle C, Dagenais C, Scherrmann JM. Screening of multidrug-resistance sensitive drugs by in situ brain perfusion in P-glycoprotein-deficient mice. Pharm. Res. 2001;18(2):183–190. doi: 10.1023/a:1011080418027. [DOI] [PubMed] [Google Scholar]
- 166.Dagenais C, Zong J, Ducharme J, Pollack GM. Effect of mdr1a P-glycoprotein gene disruption, gender, and substrate concentration on brain uptake of selected compounds. Pharm. Res. 2001;18(7):957–963. doi: 10.1023/a:1010984110732. [DOI] [PubMed] [Google Scholar]
- 167.Dagenais C, Graff CL, Pollack GM. Variable modulation of opioid brain uptake by P-glycoprotein in mice. Biochem. Pharmacol. 2004;67(2):269–276. doi: 10.1016/j.bcp.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 168.Stormer E, Perloff MD, von Moltke LL, Greenblatt DJ. Methadone inhibits rhodamine123 transport in Caco-2 cells. Drug Metab. Dispos. 2001;29(7):954–956. [PubMed] [Google Scholar]
- 169.Wang JS, Ruan Y, Taylor RM, Donovan JL, Markowitz JS, DeVane CL. Brain penetration of methadone (R)- and (S)-enantiomers is greatly increased by P-glycoprotein deficiency in the blood–brain barrier of Abcb1a gene knockout mice. Psychopharmacology (Berl.) 2004;173(1–2):132–138. doi: 10.1007/s00213-003-1718-1. [DOI] [PubMed] [Google Scholar]
- 170.Bauer B, Yang X, Hartz AM, et al. In vivo activation of human pregnane X receptor tightens the blood–brain barrier to methadone through P-glycoprotein up-regulation. Mol. Pharmacol. 2006;70(4):1212–1219. doi: 10.1124/mol.106.023796. [DOI] [PubMed] [Google Scholar]
- 171.Ortega I, Rodriguez M, Suarez E, Perez-Ruixo JJ, Calvo R. Modeling methadone pharmacokinetics in rats in presence of P-glycoprotein inhibitor valspodar. Pharm. Res. 2007;24(7):1299–1308. doi: 10.1007/s11095-007-9251-2. [DOI] [PubMed] [Google Scholar]
- 172.Tournier N, Chevillard L, Megarbane B, Pirnay S, Scherrmann JM, Decleves X. Interaction of drugs of abuse and maintenance treatments with human P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) Int. J Neuropsychopharmacol. 2010;13(7):905–915. doi: 10.1017/S1461145709990848. [DOI] [PubMed] [Google Scholar]
- 173.Hamabe W, Maeda T, Fukazawa Y, et al. P-glycoprotein ATPase activating effect of opioid analgesics and their P-glycoprotein-dependent antinociception in mice. Pharmacol. Biochem. Behav. 2006;85(3):629–636. doi: 10.1016/j.pbb.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 174.Kalvass JC, Olson ER, Pollack GM. Pharmacokinetics and pharmacodynamics of alfentanil in P-glycoprotein-competent and P-glycoprotein-deficient mice: P-glycoprotein efflux alters alfentanil brain disposition and antinociception. Drug Metab. Dispos. 2007;35(3):455–459. doi: 10.1124/dmd.106.011445. [DOI] [PubMed] [Google Scholar]
- 175.Dagenais C, Ducharme J, Pollack GM. Uptake and efflux of the peptidic μ-opioid receptor agonist. Neurosci. Lett. 2001;301(3):155–158. doi: 10.1016/s0304-3940(01)01640-8. [DOI] [PubMed] [Google Scholar]
- 176.Wandel C, Kim R, Wood M, Wood A. Interaction of morphine, fentanyl, sufentanil, alfentanil, and loperamide with the efflux drug transporter P-glycoprotein. Anesthesiology. 2002;96(4):913–920. doi: 10.1097/00000542-200204000-00019. [DOI] [PubMed] [Google Scholar]
- 177.Bostrom E, Simonsson US, Hammarlund-Udenaes M. Oxycodone pharmacokinetics and pharmacodynamics in the rat in the presence of the P-glycoprotein inhibitor PSC833. J. Pharm. Sci. 2005;94(5):1060–1066. doi: 10.1002/jps.20327. [DOI] [PubMed] [Google Scholar]
- 178.Hassan HE, Myers AL, Coop A, Eddington ND. Differential involvement of P-glycoprotein (ABCB1) in permeability, tissue distribution, and antinociceptive activity of methadone, buprenorphine, and diprenorphine: in vitro and in vivo evaluation. J. Pharm. Sci. 2009;98(12):4928–4940. doi: 10.1002/jps.21770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Miller DS, Nobmann SN, Gutmann H, Toeroek M, Drewe J, Fricker G. Xenobiotic transport across isolated brain microvessels studied by confocal microscopy. Mol. Pharmacol. 2000;58(6):1357–1367. doi: 10.1124/mol.58.6.1357. [DOI] [PubMed] [Google Scholar]
- 180.Leggas M, Adachi M, Scheffer GL, et al. Mrp4 confers resistance to topotecan and protects the brain from chemotherapy. Mol. Cell Biol. 2004;24(17):7612–7621. doi: 10.1128/MCB.24.17.7612-7621.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhang Y, Schuetz JD, Elmquist WF, Miller DW. Plasma membrane localization of multidrug resistance-associated protein homologs in brain capillary endothelial cells. J. Pharmacol. Exp. Ther. 2004;311(2):449–455. doi: 10.1124/jpet.104.068528. [DOI] [PubMed] [Google Scholar]
- 182.Bandler PE, Westlake CJ, Grant CE, Cole SP, Deeley RG. Identification of regions required for apical membrane localization of human multidrug resistance protein 2. Mol. Pharmacol. 2008;74(1):9–19. doi: 10.1124/mol.108.045674. [DOI] [PubMed] [Google Scholar]
- 183.van de WK, Zelcer N, Kuil A, et al. Multidrug resistance proteins 2 and 3 provide alternative routes for hepatic excretion of morphine-glucuronides. Mol. Pharmacol. 2007;72(2):387–394. doi: 10.1124/mol.107.035592. [DOI] [PubMed] [Google Scholar]
- 184.Hasegawa Y, Kishimoto S, Takahashi H, Inotsume N, Takeuchi Y, Fukushima S. Altered expression of MRP2, MRP3 and UGT2B1 in the liver affects the disposition of morphine and its glucuronide conjugate in a rat model of cholestasis. J. Pharm. Pharmacol. 2009;61(9):1205–1210. doi: 10.1211/jpp/61.09.0010. [DOI] [PubMed] [Google Scholar]
- 185.Penson RT, Joel SP, Gloyne A, Clark S, Slevin ML. Morphine analgesia in cancer pain: role of the glucuronides. J. Opioid. Manag. 2005;1(2):83–90. doi: 10.5055/jom.2005.0021. [DOI] [PubMed] [Google Scholar]
- 186.Penson RT, Joel SP, Bakhshi K, Clark SJ, Langford RM, Slevin ML. Randomized placebo-controlled trial of the activity of the morphine glucuronides. Clin. Pharmacol. Ther. 2000;68(6):667–676. doi: 10.1067/mcp.2000.111934. [DOI] [PubMed] [Google Scholar]
- 187.Schuetz JD, Connelly MC, Sun D, et al. MRP4: a previously unidentified factor in resistance to nucleoside-based antiviral drugs. Nat. Med. 1999;5(9):1048–1051. doi: 10.1038/12487. [DOI] [PubMed] [Google Scholar]
- 188.Jedlitschky G, Burchell B, Keppler D. The multidrug resistance protein 5 functions as an ATP-dependent export pump for cyclic nucleotides. J. Biol. Chem. 2000;275(39):30069–30074. doi: 10.1074/jbc.M005463200. [DOI] [PubMed] [Google Scholar]
- 189.Clemente MI, Alvarez S, Serramia MJ, et al. Non-steroidal anti-inflammatory drugs increase the antiretroviral activity of nucleoside reverse transcriptase inhibitors in HIV type-1-infected T-lymphocytes: role of multidrug resistance protein 4. Antivir. Ther. 2009;14(8):1101–1111. doi: 10.3851/IMP1468. [DOI] [PubMed] [Google Scholar]
- 190.Cooray HC, Blackmore CG, Maskell L, Barrand MA. Localisation of breast cancer resistance protein in microvessel endothelium of human brain. Neuroreport. 2002;13(16):2059–2063. doi: 10.1097/00001756-200211150-00014. [DOI] [PubMed] [Google Scholar]
- 191.Hori S, Ohtsuki S, Tachikawa M, et al. Functional expression of rat ABCG2 on the luminal side of brain capillaries and its enhancement by astrocyte-derived soluble factor(s) J. Neurochem. 2004;90(3):526–536. doi: 10.1111/j.1471-4159.2004.02537.x. [DOI] [PubMed] [Google Scholar]
- 192.Lee YJ, Kusuhara H, Jonker JW, Schinkel AH, Sugiyama Y. Investigation of efflux transport of dehydroepiandrosterone sulfate and mitoxantrone at the mouse blood–brain barrier: a minor role of breast cancer resistance protein. J. Pharmacol. Exp. Ther. 2005;312(1):44–52. doi: 10.1124/jpet.104.073320. [DOI] [PubMed] [Google Scholar]
- 193.van Herwaarden AE, Jonker JW, Wagenaar E, et al. The breast cancer resistance protein (Bcrp1/Abcg2) restricts exposure to the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Cancer Res. 2003;63(19):6447–6452. [PubMed] [Google Scholar]
- 194.Zhao R, Raub TJ, Sawada GA, et al. Breast cancer resistance protein interacts with various compounds in vitro, but plays a minor role in substrate efflux at the blood–brain barrier. Drug Metab. Dispos. 2009;37(6):1251–1258. doi: 10.1124/dmd.108.025064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.de Vries NA, Zhao J, Kroon E, Buckle T, Beijnen JH, van Tellingen O. P-glycoprotein and breast cancer resistance protein: two dominant transporters working together in limiting the brain penetration of topotecan. Clin. Cancer Res. 2007;13(21):6440–6449. doi: 10.1158/1078-0432.CCR-07-1335. [DOI] [PubMed] [Google Scholar]
- 196.Zhou L, Schmidt K, Nelson FR, Zelesky V, Troutman MD, Feng B. The effect of breast cancer resistance protein and P-glycoprotein on the brain penetration of flavopiridol, imatinib mesylate (Gleevec®), prazosin, and 2-methoxy-3-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)phenyl)propanoic acid (PF-407288) in mice. Drug Metab. Dispos. 2009;37(5):946–955. doi: 10.1124/dmd.108.024489. [DOI] [PubMed] [Google Scholar]
- 197.Agarwal S, Sane R, Ohlfest JR, Elmquist WF. The role of the breast cancer resistance protein (ABCG2) in the distribution of sorafenib to the brain. J. Pharmacol. Exp. Ther. 2011;336(1):223–233. doi: 10.1124/jpet.110.175034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sugiura T, Kato Y, Tsuji A. Role of SLC xenobiotic transporters and their regulatory mechanisms PDZ proteins in drug delivery and disposition. J. Control Release. 2006;116(2):238–246. doi: 10.1016/j.jconrel.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 199.Kusuhara H, Sugiyama Y. Active efflux across the blood–brain barrier: role of the solute carrier family. NeuroRx. 2005;2(1):73–85. doi: 10.1602/neurorx.2.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hagenbuch B, Meier PJ. Organic anion transporting polypeptides of the OATP/SLC21 family: phylogenetic classification as OATP/ SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch. 2004;447(5):653–665. doi: 10.1007/s00424-003-1168-y. [DOI] [PubMed] [Google Scholar]
- 201.Gao B, Hagenbuch B, Kullak-Ublick GA, Benke D, Aguzzi A, Meier PJ. Organic anion-transporting polypeptides mediate transport of opioid peptides across blood–brain barrier. J. Pharmacol. Exp. Ther. 2000;294(1):73–79. [PubMed] [Google Scholar]
- 202.Elkiweri IA, Zhang YL, Christians U, Ng KY, Tissot van Patot MC, Henthorn TK. Competitive substrates for P-glycoprotein and organic anion protein transporters differentially reduce blood organ transport of fentanyl and loperamide: pharmacokinetics and pharmacodynamics in Sprague-Dawley rats. Anesth. Analg. 2009;108(1):149–159. doi: 10.1213/ane.0b013e31818e0bd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sugiyama D, Kusuhara H, Taniguchi H, et al. Functional characterization of rat brain-specific organic anion transporter (Oatp14) at the blood–brain barrier: high affinity transporter for thyroxine. J. Biol. Chem. 2003;278(44):43489–43495. doi: 10.1074/jbc.M306933200. [DOI] [PubMed] [Google Scholar]
- 204.Taogoshi T, Nomura A, Murakami T, Nagai J, Takano M. Transport of prostaglandin E1 across the blood–brain barrier in rats. J. Pharm.Pharmacol. 2005;57(1):61–66. doi: 10.1211/0022357055173. [DOI] [PubMed] [Google Scholar]
- 205.Chu C, Li JY, Boado RJ, Pardridge WM. Blood–brain barrier genomics and cloning of a novel organic anion transporter. J. Cereb. Blood Flow Metab. 2008;28(2):291–301. doi: 10.1038/sj.jcbfm.9600538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Huber JD, Witt KA, Hom S, Egleton RD, Mark KS, Davis TP. Inflammatory pain alters blood–brain barrier permeability and tight junctional protein expression. Am. J Physiol. Heart Circ. Physiol. 2001;280(3):H1241–H1248. doi: 10.1152/ajpheart.2001.280.3.H1241. [DOI] [PubMed] [Google Scholar]
- 207.Brooks TA, Hawkins BT, Huber JD, Egleton RD, Davis TP. Chronic inflammatory pain leads to increased blood–brain barrier permeability and tight junction protein alterations. Am. J Physiol. Heart Circ. Physiol. 2005;289(2):H738–H743. doi: 10.1152/ajpheart.01288.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Huber JD, Campos CR, Mark KS, Davis TP. Alterations in blood–brain barrier ICAM-1 expression and brain microglial activation after lambda-carrageenan-induced inflammatory pain. Am. J Physiol. Heart Circ. Physiol. 2006;290(2):H732–H740. doi: 10.1152/ajpheart.00747.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Bhattacharjee AK, Nagashima T, Kondoh T, Tamaki N. Quantification of early blood–brain barrier disruption by in situ brain perfusion technique. Brain Res.Brain Res. Protoc. 2001;8(2):126–131. doi: 10.1016/s1385-299x(01)00094-0. [DOI] [PubMed] [Google Scholar]
- 210.Brooks TA, Ocheltree SM, Seelbach MJ, et al. Biphasic cytoarchitecture and functional changes in the BBB induced by chronic inflammatory pain. Brain Res. 2006;1120(1):172–182. doi: 10.1016/j.brainres.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Xie R, Hammarlund-Udenaes M. Blood–brain barrier equilibration of codeine in rats studied with microdialysis. Pharm. Res. 1998;15(4):570–575. doi: 10.1023/a:1011929910782. [DOI] [PubMed] [Google Scholar]
- 212.Fischer W, Bernhagen J, Neubert RH, Brandsch M. Uptake of codeine into intestinal epithelial (Caco-2) and brain endothelial (RBE4) cells. Eur. J Pharm. Sci. 2010;41(1):31–42. doi: 10.1016/j.ejps.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 213.Kirchheiner J, Schmidt H, Tzvetkov M, et al. Pharmacokinetics of codeine and its metabolite morphine in ultra-rapid metabolizers due to CYP2D6 duplication. Pharmacogenomics J. 2007;7(4):257–265. doi: 10.1038/sj.tpj.6500406. [DOI] [PubMed] [Google Scholar]
- 214.Lotsch J, Skarke C, Schmidt H, et al. Evidence for morphine-independent central nervous opioid effects after administration of codeine: contribution of other codeine metabolites. Clin. Pharmacol. Ther. 2006;79(1):35–48. doi: 10.1016/j.clpt.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 215.King CD, Rios GR, Assouline JA, Tephly TR. Expression of UDP-glucuronosyltransferases (UGTs) 2B7 and 1A6 in the human brain and identification of 5-hydroxytryptamine as a substrate. Arch. Biochem. Biophys. 1999;365(1):156–162. doi: 10.1006/abbi.1999.1155. [DOI] [PubMed] [Google Scholar]
- 216.Pepper MS. Transforming growth factor-β: vasculogenesis, angiogenesis, and vessel wall integrity. Cytokine Growth Factor Rev. 1997;8(1):21–43. doi: 10.1016/s1359-6101(96)00048-2. [DOI] [PubMed] [Google Scholar]
- 217.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature. 2003;425(6958):577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 218.Lebrin F, Deckers M, Bertolino P, ten Dijke P. TGF-β receptor function in the endothelium. Cardiovasc. Res. 2005;65(3):599–608. doi: 10.1016/j.cardiores.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 219.Wu X, Ma J, Han JD, Wang N, Chen YG. Distinct regulation of gene expression in human endothelial cells by TGF-β and its receptors. Microvasc. Res. 2006;71(1):12–19. doi: 10.1016/j.mvr.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 220.Seki T, Yun J, Oh SP. Arterial endothelium-specific activin receptor-like kinase 1 expression suggests its role in arterialization and vascular remodeling. Circ. Res. 2003;93(7):682–689. doi: 10.1161/01.RES.0000095246.40391.3B. [DOI] [PubMed] [Google Scholar]
- 221.Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P. Balancing the activation state of the endothelium via two distinct TGF-β type I receptors. EMBO J. 2002;21(7):1743–1753. doi: 10.1093/emboj/21.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Watabe T, Nishihara A, Mishima K, et al. TGF-β receptor kinase inhibitor enhances growth and integrity of embryonic stem cell-derived endothelial cells. J. Cell Biol. 2003;163(6):1303–1311. doi: 10.1083/jcb.200305147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ishihara H, Kubota H, Lindberg RL, et al. Endothelial cell barrier impairment induced by glioblastomas and transforming growth factor β2 involves matrix metalloproteinases and tight junction proteins. J. Neuropathol. Exp. Neurol. 2008;67(5):435–448. doi: 10.1097/NEN.0b013e31816fd622. [DOI] [PubMed] [Google Scholar]
- 224.Inman GJ, Nicolas FJ, Callahan JF, et al. SB-431542 is a potent and specific inhibitor of transforming growth factor-β superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol. Pharmacol. 2002;62(1):65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- 225.Lu P, Gonzales C, Chen Y, et al. CNS penetration of small molecules following local inflammation, widespread systemic inflammation or direct injury to the nervous system. Life Sci. 2009;85(11–12):450–456. doi: 10.1016/j.lfs.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 226.Nagaraja TN, Keenan KA, Fenstermacher JD, Knight RA. Acute leakage patterns of fluorescent plasma flow markers after transient focal cerebral ischemia suggest large openings in blood–brain barrier. Microcirculation. 2008;15(1):1–14. doi: 10.1080/10739680701409811. [DOI] [PubMed] [Google Scholar]
- 227.Strbian D, Durukan A, Pitkonen M, et al. The blood–brain barrier is continuously open for several weeks following transient focal cerebral ischemia. Neuroscience. 2008;153(1):175–181. doi: 10.1016/j.neuroscience.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 228.Salvemini D, Doyle TM, Cuzzocrea S. Superoxide, peroxynitrite and oxidative/nitrative stress in inflammation. Biochem. Soc. Trans. 2006;34(Pt 5):965–970. doi: 10.1042/BST0340965. [DOI] [PubMed] [Google Scholar]
- 229.Wang ZQ, Porreca F, Cuzzocrea S, et al. A newly identified role for superoxide in inflammatory pain. J. Pharmacol. Exp. Ther. 2004;309(3):869–878. doi: 10.1124/jpet.103.064154. [DOI] [PubMed] [Google Scholar]
- 230.Khattab MM. TEMPOL, a membrane-permeable radical scavenger, attenuates peroxynitrite- and superoxide anion-enhanced carrageenan-induced paw edema and hyperalgesia: a key role for superoxide anion. Eur. J Pharmacol. 2006;548(1–3):167–173. doi: 10.1016/j.ejphar.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 231.van Vliet EA, Redeker S, Aronica E, Edelbroek PM, Gorter JA. Expression of multidrug transporters MRP1, MRP2, and BCRP shortly after status epilepticus, during the latent period, and in chronic epileptic rats. Epilepsia. 2005;46(10):1569–1580. doi: 10.1111/j.1528-1167.2005.00250.x. [DOI] [PubMed] [Google Scholar]
- 232.Hayashi K, Pu H, Tian J, et al. HIV-Tat protein induces P-glycoprotein expression in brain microvascular endothelial cells. J. Neurochem. 2005;93(5):1231–1241. doi: 10.1111/j.1471-4159.2005.03114.x. [DOI] [PubMed] [Google Scholar]
- 233.Hayashi K, Pu H, Andras IE, et al. HIV-TAT protein upregulates expression of multidrug resistance protein 1 in the blood–brain barrier. J. Cereb. Blood Flow Metab. 2006;26(8):1052–1065. doi: 10.1038/sj.jcbfm.9600254. [DOI] [PubMed] [Google Scholar]
- 234.Pekcec A, Unkruer B, Stein V, et al. Overexpression of P-glycoprotein in the canine brain following spontaneous status epilepticus. Epilepsy Res. 2009;83(2–3):144–151. doi: 10.1016/j.eplepsyres.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 235.Hartmann G, Cheung AK, Piquette-Miller M. Inflammatory cytokines, but not bile acids, regulate expression of murine hepatic anion transporters in endotoxemia. J. Pharmacol. Exp. Ther. 2002;303(1):273–281. doi: 10.1124/jpet.102.039404. [DOI] [PubMed] [Google Scholar]
- 236.Goralski KB, Hartmann G, Piquette-Miller M, Renton KW. Downregulation of mdr1a expression in the brain and liver during CNS inflammation alters the in vivo disposition of digoxin. Br. J Pharmacol. 2003;139(1):35–48. doi: 10.1038/sj.bjp.0705227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Hartz AM, Bauer B, Fricker G, Miller DS. Rapid modulation of P-glycoprotein-mediated transport at the blood–brain barrier by tumor necrosis factor-α and lipopolysaccharide. Mol. Pharmacol. 2006;69(2):462–470. doi: 10.1124/mol.105.017954. [DOI] [PubMed] [Google Scholar]
- 238.Salkeni MA, Lynch JL, Otamis-Price T, Banks WA. Lipopolysaccharide impairs blood–brain barrier P-glycoprotein function in mice through prostaglandin- and nitric oxide-independent pathways. J. Neuroimmune. Pharmacol. 2009;4(2):276–282. doi: 10.1007/s11481-008-9138-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Volk H, Potschka H, Loscher W. Immunohistochemical localization of P-glycoprotein in rat brain and detection of its increased expression by seizures are sensitive to fixation and staining variables. J. Histochem. Cytochem. 2005;53(4):517–531. doi: 10.1369/jhc.4A6451.2005. [DOI] [PubMed] [Google Scholar]
- 240.Ossipov MH, Kovelowski CJ, Porreca F. The increase in morphine antinociceptive potency produced by carrageenan-induced hindpaw inflammation is blocked by naltrindole, a selective δ-opioid antagonist. Neurosci. Lett. 1995;184(3):173–176. doi: 10.1016/0304-3940(94)11199-s. [DOI] [PubMed] [Google Scholar]
- 241.Cui YJ, Cheng X, Weaver YM, Klaassen CD. Tissue distribution, gender-divergent expression, ontogeny, and chemical induction of multidrug resistance transporter genes (Mdr1a, Mdr1b, Mdr2) in mice. Drug Metab. Dispos. 2009;37(1):203–210. doi: 10.1124/dmd.108.023721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Noe B, Hagenbuch B, Stieger B, Meier PJ. Isolation of a multispecific organic anion and cardiac glycoside transporter from rat brain. Proc. Natl Acad. Sci. USA. 1997;94(19):10346–10350. doi: 10.1073/pnas.94.19.10346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Egleton RD, Davis TP. Transport of the δ-opioid receptor agonist [d-penicillamine2,5] enkephalin across the blood–brain barrier involves transcytosis. J. Pharm. Sci. 1999;88(4):392–397. doi: 10.1021/js980410+. [DOI] [PubMed] [Google Scholar]
- 244.Sharom FJ, Yu X, Lu P, et al. Interaction of the P-glycoprotein multidrug transporter (MDR1) with high affinity peptide chemosensitizers in isolated membranes, reconstituted systems, and intact cells. Biochem. Pharmacol. 1999;58(4):571–586. doi: 10.1016/s0006-2952(99)00139-2. [DOI] [PubMed] [Google Scholar]
- 245.Ronaldson PT, Tran CS, Rasaiah VPA, Bendayan R. Increased functional expression of multidrug resistance protein 1 (Mrp1) in cultured glial cells treated with the HIV-1 viral envelope protein gp120 and cytokines. Can. J Infect. Dis. 2007;18:13B. [Google Scholar]
- 246.Dohgu S, Yamauchi A, Takata F, et al. Transforming growth factor-β1 upregulates the tight junction and P-glycoprotein of brain microvascular endothelial cells. Cell Mol. Neurobiol. 2004;24(3):491–497. doi: 10.1023/B:CEMN.0000022776.47302.ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Levy DE, Lee CK. What does STAT3 do? J. Clin. Invest. 2002;109(9):1143–1148. doi: 10.1172/JCI15650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Dreuw A, Hermanns HM, Heise R, et al. Interleukin-6-type cytokines upregulate expression of multidrug resistance-associated proteins in NHEK and dermal fibroblasts. J. Invest Dermatol. 2005;124(1):28–37. doi: 10.1111/j.0022-202X.2004.23499.x. [DOI] [PubMed] [Google Scholar]
- 249.Andrejko KM, Raj NR, Kim PK, Cereda M, Deutschman CS. IL-6 modulates sepsis-induced decreases in transcription of hepatic organic anion and bile acid transporters. Shock. 2008;29(4):490–496. doi: 10.1097/shk.0b013e318150762b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kullak-Ublick GA, Becker MB. Regulation of drug and bile salt transporters in liver and intestine. Drug Metab Rev. 2003;35(4):305–317. doi: 10.1081/dmr-120026398. [DOI] [PubMed] [Google Scholar]
- 251.Bauer B, Hartz AM, Fricker G, Miller DS. Pregnane X receptor up-regulation of P-glycoprotein expression and transport function at the blood–brain barrier. Mol. Pharmacol. 2004;66(3):413–419. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- 252.Urquhart BL, Tirona RG, Kim RB. Nuclear receptors and the regulation of drug-metabolizing enzymes and drug transporters: implications for interindividual variability in response to drugs. J. Clin. Pharmacol. 2007;47(5):566–578. doi: 10.1177/0091270007299930. [DOI] [PubMed] [Google Scholar]
- 253.Lombardo L, Pellitteri R, Balazy M, Cardile V. Induction of nuclear receptors and drug resistance in the brain microvascular endothelial cells treated with antiepileptic drugs. Curr. Neurovasc. Res. 2008;5(2):82–92. doi: 10.2174/156720208784310196. [DOI] [PubMed] [Google Scholar]
- 254.Narang VS, Fraga C, Kumar N, et al. Dexamethasone increases expression and activity of multidrug resistance transporters at the rat blood–brain barrier. Am. J Physiol. Cell Physiol. 2008;295(2):C440–C450. doi: 10.1152/ajpcell.00491.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Zastre JA, Chan GN, Ronaldson PT, et al. Up-regulation of P-glycoprotein by HIV protease inhibitors in a human brain microvessel endothelial cell line. J. Neurosci. Res. 2009;87(4):1023–1036. doi: 10.1002/jnr.21898. [DOI] [PubMed] [Google Scholar]
- 256.Wang X, Sykes DB, Miller DS. Constitutive androstane receptor-mediated up-regulation of ATP-driven xenobiotic efflux transporters at the blood–brain barrier. Mol. Pharmacol. 2010;78(3):376–383. doi: 10.1124/mol.110.063685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Cheng X, Maher J, Dieter MZ, Klaassen CD. Regulation of mouse organic anion-transporting polypeptides (Oatps) in liver by prototypical microsomal enzyme inducers that activate distinct transcription factor pathways. Drug Metab. Dispos. 2005;33(9):1276–1282. doi: 10.1124/dmd.105.003988. [DOI] [PubMed] [Google Scholar]
- 258.Stedman CA, Liddle C, Coulter SA, et al. Nuclear receptors constitutive androstane receptor and pregnane X receptor ameliorate cholestatic liver injury. Proc. Natl Acad. Sci. USA. 2005;102(6):2063–2068. doi: 10.1073/pnas.0409794102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Matheny CJ, Ali RY, Yang X, Pollack GM. Effect of prototypical inducing agents on P-glycoprotein and CYP3A expression in mouse tissues. Drug Metab. Dispos. 2004;32(9):1008–1014. [PubMed] [Google Scholar]
- 260.Stein A, Yassouridis A, Szopko C, Helmke K, Stein C. Intraarticular morphine versus dexamethasone in chronic arthritis. Pain. 1999;83(3):525–532. doi: 10.1016/S0304-3959(99)00156-6. [DOI] [PubMed] [Google Scholar]
- 261.Kardash KJ, Sarrazin F, Tessler MJ, Velly AM. Single-dose dexamethasone reduces dynamic pain after total hip arthroplasty. Anesth. Analg. 2008;106(4):1253–1257. doi: 10.1213/ANE.0b013e318164f319. [DOI] [PubMed] [Google Scholar]
- 262.Pieretti S, Di Giannuario A, Domenici MR, et al. Dexamethasone-induced selective inhibition of the central μ-opioid receptor: functional in vivo and in vitro evidence in rodents. Br. J Pharmacol. 1994;113(4):1416–1422. doi: 10.1111/j.1476-5381.1994.tb17155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Capasso A, Loizzo A. Functional interference of dexamethasone on some morphine effects: hypothesis for the steroid–opioid interaction. Recent Pat. CNS Drug Discov. 2008;3(2):138–150. doi: 10.2174/157488908784534612. [DOI] [PubMed] [Google Scholar]
- 264.Oak S, Choi BH. The effects of glutathione glycoside in acetaminophen-induced liver cell necrosis. Exp. Mol. Pathol. 1998;65(1):15–24. doi: 10.1006/exmp.1998.2221. [DOI] [PubMed] [Google Scholar]
- 265.Rius M, Hummel-Eisenbeiss J, Hofmann AF, Keppler D. Substrate specificity of human ABCC4 (MRP4)-mediated cotransport of bile acids and reduced glutathione. Am. J Physiol. Gastrointest. Liver Physiol. 2006;290(4):G640–G649. doi: 10.1152/ajpgi.00354.2005. [DOI] [PubMed] [Google Scholar]
- 266.Zhang J, Huang W, Chua SS, Wei P, Moore DD. Modulation of acetaminophen-induced hepatotoxicity by the xenobiotic receptor CAR. Science. 2002;298(5592):422–424. doi: 10.1126/science.1073502. [DOI] [PubMed] [Google Scholar]
- 267.Yamamoto T, Nair P, Ma SW, et al. The biological activity and metabolic stability of peptidic bifunctional compounds that are opioid receptor agonists and neurokinin-1 receptor antagonists with a cystine moiety. Bioorg. Med. Chem. 2009;17(20):7337–7343. doi: 10.1016/j.bmc.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Largent-Milnes TM, Yamamoto T, Nair P, et al. Spinal or systemic TY005, a peptidic opioid agonist/neurokinin 1 antagonist, attenuates pain with reduced tolerance. Br. J Pharmacol. 2010;161(5):986–1001. doi: 10.1111/j.1476-5381.2010.00824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Website
- 301.American Pain Foundation. Pain facts and figures. 2011 doi: 10.3109/15360288.2011.599920. www.painfoundation.org/media/resource/pain-facts-figures.html. [DOI] [PubMed]