Abstract
Circadian dysfunction has long been implicated in the etiology of mood disorders. The gene Clock and related molecules (e.g. Per1, Per2) represent key regulators of circadian rhythmicity, and their targeted disruption in mutant mice produces potentiated reward drive, novelty-seeking, impulsivity, disrupted sleep, reduced depression and anxiety– a behavioral profile highly reminiscent of our selectively-bred High Responder (bHR) rats compared to bred Low Responders (bLRs). The current study evaluated potential diurnal bHR-bLR differences in behavior, gene expression, and neuroendocrinology. Relative to bHRs, bLRs showed diminished homecage locomotion during the dark (but not light) phase and a delayed corticosterone peak. In situ hybridizations in hypothalamus, amygdala, and hippocampus at Zeitgeber Time (ZT)2 and ZT14 revealed distinct bHR-bLR day-night gene expression fluctuations. bHRs exhibited altered day-night patterns of corticotrophin releasing hormone (CRH) and arginine vasopression (AVP) mRNA in the hypothalamus, and perturbed hippocampal MR:GR ratios relative to bLR rats. bHR-bLR rats showed disparate day-night Clock expression in the suprachiasmatic nucleus, a master circadian oscillator, with bHRs showing higher levels at ZT14 versus ZT2 and bLRs showing the opposite pattern. Clock, Per1 and Per2 were assessed in the substantia nigra pars compacta (SNc) and ventral tegmental area (VTA) since disruption of these genes induces “bHR-like” behavior in mutant mice. Clock and Per1 did not differ between strains, but there were robust Per2 differences, with bHRs having reduced Per2 in VTA and SNc. These findings resonate with earlier work demonstrating that perturbation of Clock and related molecules contributes to disturbances of emotional and addictive behaviors.
Keywords: bred High Responder, bred Low Responder, circadian, Clock, Per1, Per2, AVP, CRH, glucocorticoid receptor, mineralocorticoid receptor, corticosterone
Introduction
Personality differences often manifest as variations in temperament or emotional reactivity along a continuum from behavioral over-control (e.g. shyness) to behavioral under-control (e.g. thrill-seeking). These differences profoundly influence how individuals respond to stress, and under certain circumstances may put them at risk for developing specific types of stress-induced psychopathology. Accordingly, individuals that exhibit behavioral over-control are at greater risk for depression and anxiety, while those expressing behavioral under-control are more likely to exhibit emotional lability, impulsivity, and aggression (Kendler et al., 1997; Krueger et al., 2007).
While the underlying neurobiological mechanisms of these phenomena are likely multi-faceted, emerging evidence in both humans and rodents suggests that disturbances of the circadian system may contribute to such individual differences in emotionality (Germain and Kupfer, 2008; McClung, 2007). For example, recent data demonstrate that altered expression of Clock, one of the core circadian genes, potentiates reward drive, novelty-seeking, and impulsivity, disrupts sleep patterns, and reduces depression- and anxiety-like behaviors in mice (Le-Niculescu et al., 2008; Roybal et al., 2007), suggesting an important link between the circadian system and different aspects of emotionality. Furthermore, clinical literature indicates that polymorphisms in Clock and related genes are associated with mood disorders (Benedetti et al., 2003; Mansour et al., 2006; Serretti et al., 2003), and that manipulation of circadian cues can ameliorate symptoms of depression and mania. For instance, short-term sleep deprivation, circadian phase advance, and bright light therapy have been demonstrated to improve depressive symptoms (Gillin et al., 1980; Wu et al., 2008). Likewise, stabilizing the daily presentation of environmental cues (i.e. zeitgebers) that entrain the circadian system is the basis of interpersonal social rhythm therapy, an effective mood stabilizing behavioral intervention in bipolar disorder patients (Frank et al., 2005; Frank et al., 2007). Finally, mood symptoms are often accompanied by significant differences in the diurnal rhythmicity of the limbic-hypothalamic-pituitary-adrenal (LHPA) axis. For instance, many depressed individuals exhibit dampened fluctuations in 24 h cortisol levels (Burke et al., 2005; Gold and Chrousos, 2002). Likewise, human postmortem studies have documented gene expression alterations within brain regions that regulate the LHPA axis, including increased corticotrophin-releasing hormone (CRH) and arginine vasopressin (AVP) mRNA in the paraventricular nucleus of the hypothalamus (PVN) (Bao et al., 2008), and altered ratios of glucocorticoid (GR) and mineralocorticoid (MR) receptor mRNAs in the hippocampus of depressed patients (Lopez et al., 1998).
In recent years, our laboratory developed a rodent model of inborn differences in emotionality and environmental reactivity by selectively breeding Sprague-Dawley rats for differences in locomotor response to novelty. Rats exhibiting exaggerated novelty-induced locomotion, termed bred High Responders (bHRs), were selected over many successive generations, while rats showing diminished locomotion in a novel environment, termed bred Low Responders (bLRs), were likewise bred for several generations. The bHR/bLR behavioral differences stabilized by the fourth breeding generation, with over 95% of offspring from each line exhibiting novelty-induced locomotor responses similar to their parents (Stead et al., 2006). Subsequent investigations revealed pronounced differences in several aspects of emotional behavior, including increased reward drive, impulsivity (Flagel et al., 2010), and aggression (Abraham et al., 2006) in bHR rats. In contrast, bLR rats display increased depression- and anxiety-like behaviors compared to bHRs (Orr et al., 2008; Stead et al., 2006). These findings suggest that the bHR/bLR rat model recapitulates certain aspects of temperament differences in humans, specifically as they relate to behavioral over-control (bLRs) versus under-control (bHRs). Furthermore, the behavioral profile of bHR rats (compared to their bLR counterparts) is strikingly similar to that of the Clock-mutant mice, pointing to potential differences in circadian function in these animals.
Based on these observations, we hypothesized bHR/bLR differences in the expression of Clock and related genes Per1 and Per2, as well as differences in diurnal locomotor behavior and LHPA axis function. To test this notion, we first evaluated diurnal patterns of homecage locomotor activity in bHR versus bLR animals. Next, we assessed Clock, Per1 and Per2 mRNA expression in select brain areas of bHR/bLR animals. Lastly, we examined circulating levels of corticosterone, as well as day-night gene expression patterns for several LHPA axis-related markers (AVP, CRH, MR, and GR) in the hippocampus, hypothalamus, and amygdala – all key regions that participate in the regulation of the LHPA axis, circadian system, and emotionality.
Methods
All experiments were approved by the University Committee on the Use and Care of Animals at the University of Michigan and were conducted in accordance with the National Institute of Health (NIH) Guide for the Care and Use of Laboratory Animals, dictated by the National Research Council in 1996.
Animals
Male bHR and bLR animals were acquired from our in-house breeding colony where the bred lines have been maintained for several generations. We previously published a description of our breeding strategy and initial behavioral characterization of the bHR/bLR lines (Stead et al., 2006), and have continued to examine the many facets of the bHR-bLR behavioral phenotypes (Flagel et al., 2010). Our original founding population was composed of 60 male and 60 female Sprague-Dawley rats purchased from three separate Charles River Laboratory breeding colonies. Animals were screened for locomotor response to novelty, and males and females with the highest and lowest scores from locomotion testing were bred together to generate the first generation bHR and bLR lines, respectively. At the first generation, animals with the top and bottom 20% of locomotion scores from our initial colony were selected for breeding. For each selected line, twelve litters were maintained at each generation. Adult males and females from each generation were screened for locomotor response to novelty, and the most extreme bHR and bLR animals from each family were selected to perpetuate the colony. For more details, please see (Stead et al., 2006).
For the current behavior experiment, an additional comparison group of male Sprague-Dawley rats was purchased from Charles River (Wilmington, MA) and allowed to acclimate to our housing facilities for at least 1 week prior to any behavioral testing. All rats were housed in a 12:12 light–dark cycle (lights on at 6 a.m./Zeitgeber Time (ZT) 0, and lights off at 6 p.m./ZT12). Animals were pair-housed and food and water were available ad libitum. As noted below, these purchased rats were screened for locomotor response to novelty in order to classify them as “Purchased High Responder (HR)” or Purchased Low Responder (LR)” rats.
Screening for Locomotor Response to Novelty
Prior to all other experiments, animals from the bHR/bLR colony as well as commercially-purchased Sprague-Dawley rats were screened to assess novelty-induced locomotor activity as previously described (Stead et al., 2006). Rats were individually placed in standard clear acrylic cages (43 × 21.5 × 25.5 cm high) equipped with infrared photocell emitters mounted 2.3 and 6.5 cm above the floor to record horizontal and rearing movement. The test chambers were located in a behavioral testing room separate from the housing quarters, and the rats were exposed to the test chamber for the first time on the day of testing. Horizontal and rearing movements were monitored in 5-min intervals over a period of 60-min via computer. All testing was performed between ZT2 (8 a.m.) and ZT5 (11 a.m.). Novelty-induced locomotor scores for each rat were calculated by adding the total number of horizontal and rearing movements over the 60-min test period. bHR rats whose scores fell one standard deviation below the bHR group average and bLR rats whose scores fell one standard deviation above the bLR group average were not used for the present studies. For the purchased Sprague-Dawley rats, an animal was classified as a High-Responder (HR) if its locomotor score fell above the median of the purchased rat population locomotor score; conversely, the animal was considered a Low-Responder (LR) if its locomotor score fell below the median score of the purchased rat population.
Monitoring Home-Cage Diurnal Locomotor Activity
Homecage locomotor activity was assessed over a 24-h period using the same locomotor monitoring device described above. The recording device was brought into the housing room and animals’ cages were placed on the test rack to acclimate for 5 days. On the day of testing, bLR and bHR males from the 7th generation of our colony (N=25 per group) and the purchased Sprague-Dawley males (N=36) were singly housed to monitor individual activity levels. Locomotor activity was monitored in 15 minute intervals for 24 h (from ZT6 on one day until ZT6 the following day) via a computer. Final locomotion scores were determined by summing horizontal and rearing movements for each 1-h interval over the 24-h period.
In Situ Hybridization
A set of bHR/bLR animals from the 12th generation of our colony was sacrificed by rapid decapitation at ZT2, ZT6, ZT10, ZT14, ZT16, ZT18, ZT20, and ZT22 (n = 8-10 per phenotype per timepoint). Brains were removed, snap frozen, and stored at −80° C until processing for in situ hybridization. Trunk blood samples were collected in EDTA-coated tubes to assess basal diurnal corticosterone levels (see next section). Harvested brains from bLR and bHR rats sacrificed at ZT2 and ZT14 were cryostat sectioned at 15 μm, and sections were immediately thaw-mounted onto Fisherbrand Superfrost/Plus microscope slides (Fisher Scientific, www.fishersi.com). These two timepoints were selected since bHR/bLR behavioral and neuroendocrine differences are prominent at these times. Sections taken at 240 μm intervals from the hypothalamus through the midbrain were prepared for in situ hybridization as previously described (Kabbaj et al., 2000b). Briefly, sections were fixed in 4% paraformaldehyde at room temperature for 1 h. The slides were then washed three times in room temperature 2x SSC (300mM NaCl/30mM sodium citrate, pH 7.2), 5 min each wash. Next, the slides were placed in a solution containing acetic anhydride (0.25%) in triethanolamine (0.1 M), pH 8.0, for 10 min at room temperature, rinsed in distilled water, and dehydrated through graded ethanol washes (50%, 75%, 85%, 95%, and 100%). After air-drying, separate sets of sections were hybridized with a 35S-labeled cRNA probe for each of the following genes: Clock (length 796 nt., accession # AB019258, pos. 1250-2046); Per1 (length 950 nt., accession # AB002107), Per2 (length 1000 nt., accession # AB016532, pos. 3575-4583), CRH (length 762 nt., accession # M54987, pos. 1,283 - 2,045), AVP (length 235 nt., accession # M64785, pos. 692-923), MR (length 410 nt., accession # M36074, pos. 570-980), or GR (length 451 nt., accession # M14053, pos. 2364-2815). Sections through the midbrain were also processed with a riboprobe for tyrosine hydroxylase (TH; length 274, accession # M10244, pos. 89-363) to help delineate substantia nigra pars compacta (SNc) and the ventral tegmental area (VTA) for the Clock/Per1/Per2 studies.
The probes were labeled in a reaction mixture consisting of 1 μg of linearized plasmid, 1x transcription buffer (Epicenter Technologies, Madison, WI), 125 μCi of 35S-labeled-UTP, 125 μCi of 35S-CTP, 150 μM ATP and GTP, 12.5uM dithiothreitol, 0.5 μl of RNase inhibitor, and 1.5 μl of T3 RNA polymerase. The reactions were incubated for 120 min at 37° C, and then 1 μl of DNAse (RNAse free) was added to the reaction to incubate for another 15 min at room temperature. The labeled probes were purified using Micro Bio-Spin P-30 Tris Spin Columns (Bio-Rad Laboratories), then diluted in hybridization buffer (containing 50% formamide, 10% dextran sulfate, 3x SSC, 50mM sodium phosphate buffer, pH 7.4, 1x Denhardt’s solution, 0.1 mg/ml yeast tRNA, and 10mM dithiothreitol) to yield 106 dpm/70 μl. A cover slip with 70 μl of diluted riboprobe was placed on each slide. Slides were placed in a humidified box with filter paper saturated with 50% formamide buffer, and incubated overnight at 55° C. Following 20 h of hybridization, coverslips were removed and the slides were washed in room temperature 2xSSC for 5 min, and then incubated for 1 h in RNaseA (200 mg/ml in 10mM Tris buffer containing 0.5M NaCl, pH 8) at 37° C. The slides were then washed in increasingly stringent SSC solutions: 2x, 1x, and 0.5x for 5 min each at room temperature, followed by incubation for 1 h in 0.1x SSC at 65 1C. Finally, slides were rinsed in distilled water and dehydrated through graded ethanol washes, air-dried, and apposed to Kodak XAR film (Eastman Kodak, Rochester, NY) for 7 days for the Clock probe, 14 days for Per1 and Per2 probes, 20 h for the AVP probe, 7 days for the CRH probe, and 80 h for the MR and GR probes.
Autoradiograms were digitized using a ScanMaker 1000XL Pro (Microtek, Carson, CA) with LaserSoft Imaging software (AG, Kiel, Germany). Digitized images were analyzed using ImageJ Analysis Software for PC (http://rsbweb.nih.gov/ij/). Specific signal, defined as 3.5 × the standard deviation of individual pixel signal values above mean background signal, was converted to optical density and multiplied by the area of signal to produce integrated optical density (IOD).
We first assessed Clock, Per1 and Per2 mRNA levels in the suprachiasmatic nucleus (SCN) of the hypothalamus, and later measured these genes in the VTA and SNc, focusing on these two dopamine-rich areas, in part, because manipulation of Clock in mutant mice alleviated their “HR-like” behavioral phenotype. Because Clock, Per1 and Per 2 are widely expressed in the brain (albeit at low levels in the VTA and SNc), we used tyrosine hydroxylase (TH) mRNA expression as a marker of the SNc and VTA, and thus processed one set of sections for TH and adjacent sections for Clock, Per1, and Per2. Signal and IOD measurements for all 4 probes were taken across the entire rostro-caudal extent of the SNc and VTA.
A second set of in situ hybridization studies aimed to assess the expression of several LHPA axis markers including CRH within PVN and central nucleus of amygdala (CeA) and AVP in PVN and supraoptic nucleus (SON). IODs from 5-6 sections spaced 240 μm across the entire extent of PVN or SON were summed together to yield sum IOD measure for the expression of AVP in SON and PVN, and CRH in PVN and CeA. Because PVN is functionally heterogeneous, it was further subdivided into its magnocellular (mPVN) and parvocellular (pPVN) compartments. These subregions differ in their projections to the pituitary, with mPVN projecting to the posterior pituitary, and pPVN projecting to the anterior pituitary (Sawchenko et al., 1996). Finally, we also examined the expression of GR and MR in the hippocampus (CA1-3 and dentate gyrus). These measurements were made in 8-10 sections spaced 240 μm apart across the rostro-caudal extent of the hippocampus.
Hormone Assays for Diurnal Corticosterone Levels
As described above, a set of bHR/bLR animals from the 12th generation of our colony was sacrificed by rapid decapitation at ZT2, ZT6, ZT10, ZT14, ZT16, ZT18, ZT20, and ZT22 (n = 8-10 per phenotype per timepoint). Trunk blood samples were collected in EDTA-coated tubes to assess basal diurnal corticosterone levels. Blood samples were separated by centrifugation (1,000g for 10 min. at 4° C), and plasma was removed, frozen and stored at −80° C. Plasma corticosterone was measured using commercially available radioimmunoassay kits (MP Biomedicals, Solon, OH) according to manufacturer instructions. The sensitivity of the assay was 12.5 ng/ml and intra- and inter- assay coefficients of variation were less than 5%.
Statistical Analyses
In situ hybridization data analysis was carried out separately for each region and probe, using two-way ANOVA (phenotype × timepoint). ANOVAs were followed by Fisher’s post-hoc comparisons when necessary. Repeated measures ANOVA was used to measure diurnal locomotion (phenotype × 1-h time interval) in bHR, bLR, and purchased animals. The corticosterone assay data was analyzed by two-way ANOVA (phenotype × timepoint). All data were analyzed using Statview 5.0.1 for Windows, and for all tests, α=0.05.
Results
Homecage 24-h Locomotor Activity
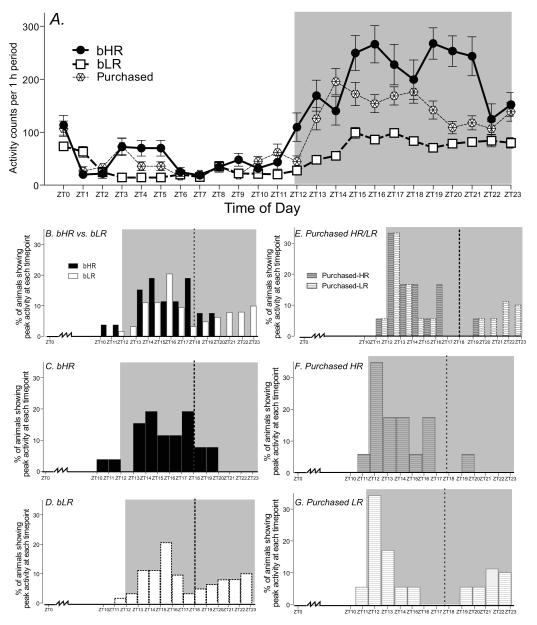
Homecage activity was measured over a 24-hr period in bHR, bLR and purchased Sprague-Dawley rats (Fig. 1). All animals were much more active during the dark phase compared to the light phase, and repeated measures ANOVA revealed significant main effects of phenotype (F1, 2 = 64.14, p<0.0001) and time interval (F1, 22 = 56.47, p<0.0001) as well as a significant phenotype × time interval interaction (F1, 44 = 8.78, p<0.0001). Post-hoc analysis showed that all three groups differed from one another, with bHR rats being more active than both purchased and bLR animals (p<0.0001), and with bLR rats being significantly less active than purchased rats (p<0.0001; Fig. 1A).
Figure 1.
Diurnal homecage locomotor activity in bHR, bLR (N=25 per group) and purchased Sprague-Dawley rats (N=36). (A) Top graph shows locomotor activity counts per 1 h period over 24-h for bHRs, bLRs and purchased animals. While all three groups displayed relatively low levels of activity during the light phase (Zeitgeber Time (ZT)0-ZT12), the groups diverged during the dark phase (ZT12-24), with bHRs showing the highest activity levels, bLRs showing very low activity levels, and purchased animals exhibiting an intermediate level of activity. The lower panels (B-G) display patterns of peak locomotor activity in bHR, bLR and purchased Sprague-Dawley rats. For each animal we determined the 1-h period of peak locomotor activity across the 24-h cycle. (B) Combined distribution of bHR and bLR peak locomotor scores over 24 h. (C) 80% of bHR animals showed peak locomotor activity relatively early in the dark phase (prior to ZT18.), while the remaining 20% of bHRs peaked later. (D) bLRs exhibited a different pattern, with only 66% peaking earlier in the dark phase, and 34% peaking much later (as late as ZT23). (E) Purchased animals were classified as HR and LR based on locomotor response to novelty, and we then compared the distribution of 24-h peak activity in the two purchased groups. (F) Purchased HRs resembled bHRs in that the majority (94%) showed peak activity earlier in the dark cycle, while only 6% peaked later. (G) Similarly, the purchased LRs showed a similar peak activity distribution as bLRs, with 66% peaking earlier and 34% peaking later in the cycle.
Heterogeneity in Peak Locomotor Activity
We observed individual bHR/bLR differences in patterns of peak locomotor activity over the 24-hr cycle. For each animal we determined the 1-h period of peak locomotor activity across the 24-h cycle. The histograms in Figure 1B-1G show the percentage of animals from each experimental group (bHR, bLR, purchased HR and LR) that exhibited peak activity at each 1-h time interval. Data are presented in two ways. Combined data are shown for bHR/bLR groups in Fig. 1B and data for purchased HR/LR are shown in Fig. 1E; subsequent panels display data for individual groups (bHR Fig. 1C; bLR Fig. 1D; purchased HR Fig. 1F and purchased LR Fig. 1G). The majority (80%) of bHR animals peaked relatively early in the dark phase, showing the highest activity within the first half of the dark phase between ZT2 and ZT18 (Fig. 1B and 1C). In contrast, bLR rats exhibited a bimodal pattern of peak activity, with 66% peaking between ZT2 and ZT18, while the other 34% of the animals peaked between ZT18-ZT24 (Fig. 1B and 1D). Chi-square analysis confirmed the distinct distribution of “early” versus “late” peakers in the bHR and bLR groups (χ=4.53, d.f. 1, p<0.05).
We found a similar distribution of peak activity in the purchased HR and LR animals. Like the bHRs, a vast majority (94%) of the purchased (non-selectively bred) HR rats showed peak activity between ZT12 and ZT18 (Fig. 1E and 1F). Similarly, within the purchased LR group, we observed a bimodal distribution of activity peaks that closely resembled the bimodal distribution of peak activity levels observed in bLR rats. Accordingly, 66% of purchase LR animals peaked within the first half of the dark phase, while 34% peaked after ZT18 (Fig. 1E and 1G). Chi-square analysis confirmed the distinct distribution of “early” versus “late” peakers in the purchased HR and LR groups (χ=23.59, d.f. 1, p<0.0001).
Clock, Per1 and Per2 gene expression
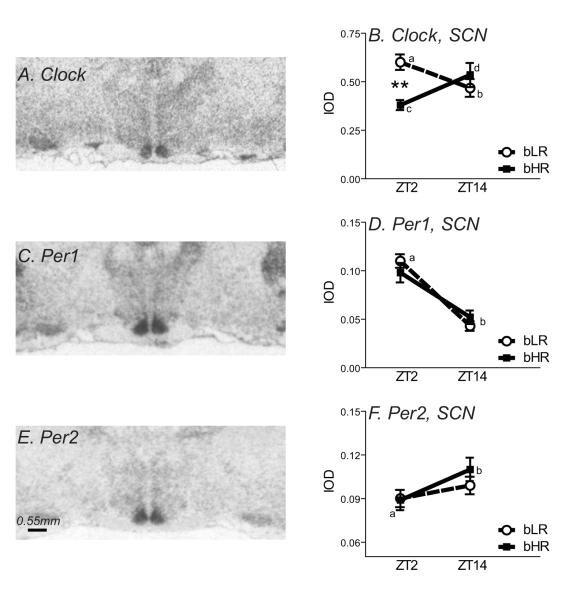
Expression of Clock, Per1 and Per2 was first assessed in the SCN. We focused on the SCN due to its central role in setting the brain’s circadian system. Consistent with previous reports (Shieh et al., 2005), Clock, Per1 and Per2 mRNA were all widely expressed throughout the brain, with particular enrichment in the SCN (Fig. 2A, 2C, and 2E).
Figure 2.
Expression of Clock, Per1 and Per2 mRNA in the suprachiasmatic nucleus of bHR/bLR animals (N=8 per group). Left panels show autoradiograms from representative X-ray films exposed for 7 d after in situ hybridization with an antisense riboprobe against rat Clock (A), Per1 (C), or Per2 (E) mRNA. Clock, Per1 and Per2 expression was quantified in the suprachiasmatic nucleus (SCN). There was a significant interaction between bHR/bLR phenotype and time of day for Clock in the SCN (B). Clock expression was higher in the morning (Zeitgeber Time (ZT) 2) versus evening (ZT14) in bLR rats (differences indicated by a versus b), but bHRs showed the opposite pattern, with lower levels at ZT2 and higher levels at ZT14 (indicated by c versus d). At ZT2, bLRs showed higher levels of Clock expression compared to bHRs (B). There was a time of day effect on the expression of Per1, with higher levels at ZT2 versus ZT14, although there were no bHR/bLR differences (D). Likewise, there was no bHR/bLR difference in Per2 expression in the SCN, although there was an effect of time of day, with lower levels at ZT2 compared to ZT14 (F). **indicates p<0.01.
Within SCN, there was a significant phenotype x timepoint interaction in Clock expression (F1, 29= 8.87, p < 0.01), but no main effect of bHR/bLR phenotype or time of day. Post-hoc testing revealed opposite day-night differences in Clock expression in the two rat lines. In bLR rats, Clock expression was higher at ZT2 versus ZT14 (F1, 29= 4.39, p < 0.05), while this difference was reversed in bHR animals (F1, 29= 4.89, p < 0.05) (Fig. 2B). Also, bLRs showed higher Clock levels compared to bHR at ZT2 (F1, 29= ; 5.85, p < 0.05), but similar levels at ZT14.
For Per1, there was a main effect of time of day (F1, 29= 59.80, p < 0.0001), with Per1 levels higher in the morning (ZT2) versus evening (ZT14) (Fig. 2D). There was no effect of bHR/bLR phenotype, and no phenotype x time of day interaction. For Per2, there was a main effect of time of day (F1, 29= 4.78, p < 0.05), with Per2 levels lower at ZT2 versus ZT14 (Fig. 2F). Like Per1, there was no effect of bHR/bLR phenotype, and no phenotype x time of day interaction.
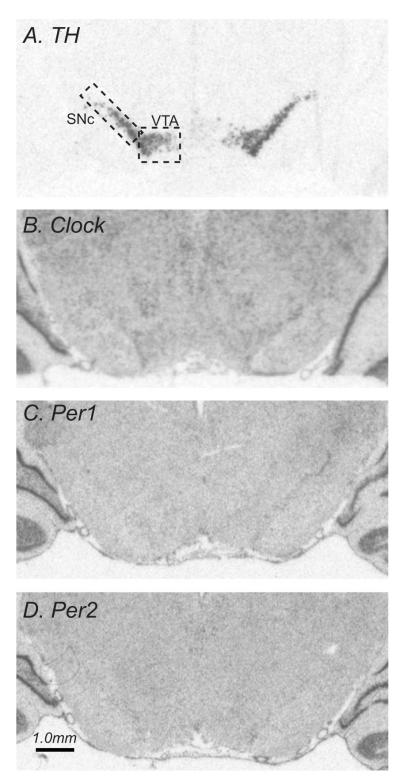
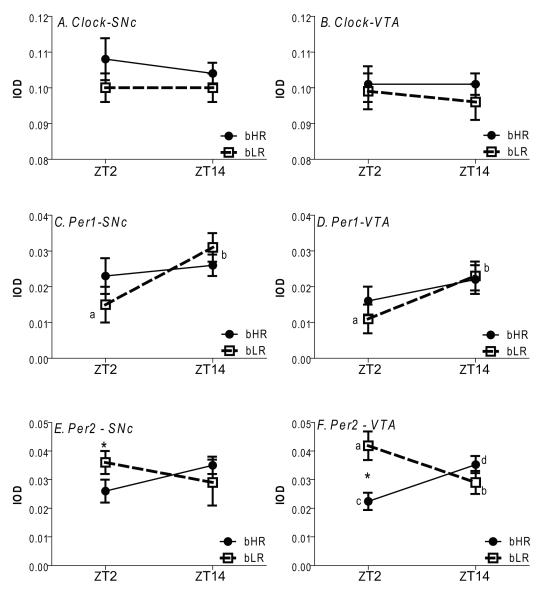
We also assessed the expression of Clock, Per1 and Per2 in the dopamine-rich SNc and VTA, since prior work in mutant mice demonstrated that decreased expression and/or function of Clock and related molecules in these regions produces an “HR-like” behavioral phenotype. To facilitate the identification of the SNc and VTA in the Clock, Per1 and Per2 studies, adjacent sections were processed for TH mRNA. Figure 3 shows the expression patterns for TH, Clock, Per1 and Per2 mRNAs in the SNc, VTA, and surrounding areas at one representative rostro-caudal level (dashed boxes outline the sampled area within VTA and SNc). TH mRNA was restricted to the dopamine cell-containing regions of the SN and VTA. Clock, Per1 and Per2 mRNAs were all widely expressed across several brain structures, with relatively low levels present in the SNc and VTA (Fig. 3). There was a main effect of bHR/bLR phenotype on TH mRNA levels in the SNc, with bHRs having higher TH expression compared to bLRs (F1, 17 = 6.83, p<0.05), but there was no effect of time of day, and no phenotype x time of day interaction. There were no bHR/bLR differences in TH mRNA expression in the VTA, no effect of time of day, and no phenotype x time of day interaction (data not shown). Clock levels were similar in bHR/bLR rats in the SNc and VTA at ZT2 and ZT14 and there was no day-night fluctuation in either region, with no main effects of phenotype or time of day, and no phenotype x time interaction (Fig. 4A-B). Per1 levels were lower at ZT2 versus ZT14 (main effect of time of day, F1, 17 = 5.52, p<0.05), but did not differ between strains (Fig. 4C-D). Per2 mRNA levels were higher in bLR versus bHR at ZT2 in both SNc (main effect of phenotype, F1, 17 = 3.83, p<0.05), and the VTA (main effect of phenotype, F1, 17 = 11.06, p<0.05). There was also a significant phenotype x time of day interaction (F1, 17 = 11.90, p<0.01) since the two strains showed opposite day-night Per2 expression patterns. While this effect was apparent in both SNc and the VTA, the effect was only statistically significant in the VTA, with bLRs having higher Per2 at ZT2 versus ZT14 (Fig. 4F a versus b, p<0.05) and bHRs having the opposite pattern (Fig. 4F c versus d).
Figure 3.
Expression of tyrosine hydoxylase (TH), Clock, Per1 and Per2 in the midbrain. Panels A-D show autoradiograms from representative tissue sections processed by in situ hybridization with antisense riboprobes against rat TH (A), Clock (B), Per1 (C), or Per2 mRNA. (D) TH, Clock, Per1 and Per2 expression was quantified in the ventral tegmental area (VTA) and substantia nigra pars compacta (SNc) (indicated with dashed lines). Since Clock, Per1 and Per2 expression levels were relatively low in the VTA and SNc, the TH signal from adjacent sections was used to ensure precise identification of and measurement within these regions.
Figure 4.
Clock, Per1 and Per2 mRNA expression in bHR versus bLR (N=8 per group). Clock, Per1 and Per2 mRNA was measured in the substantia nigra pars compacta (SNc; graphs in left column) and ventral tegmental area (VTA, graphs in right column) during the morning (Zeitgeber Time (ZT)2) versus evening (ZT14). There were no day-night differences in Clockexpression in either brain region, and no bHR/bLR phenotype differences (A-B). While there were no bHR/bLR differences in Per1 in the SNc and VTA, there was a time of day effect, with Per1 levels being lower at ZT2 versus ZT14 in both brain regions (C-D). bLR animals had higher Per2 levels in the SNc compared to bHRs, although this effect only occurred at ZT2 (E). Within the VTA, bLRs again had higher Per2 levels at ZT2 compared to bHRs. Interestingly, bHRs and bLRs also showed opposite day-night patterns of Per2 expression, with bLRs showing higher levels in the morning versus night (indicated by a versus b), while bHRs showed low levels at ZT2 and higher levels at AT14 (indicated by c versus d) (F).
LHPA axis: day-night gene expression and 24-h corticosterone secretion
Based on our observations of divergent 24-h locomotor behavior and altered circadian genes in bHR versus bLR, we hypothesized that the phenotypes may also exhibit diurnal differences in the LHPA axis. Specifically, we sought to quantify expression of genes that either stimulate (i.e. CRH, AVP) or inhibit (i.e. GR, MR) LHPA axis function (Brown et al., 1999). Expression of these genes was quantified in brain regions that play key roles in regulating the LHPA axis (i.e. PVN, hippocampus). Expression of AVP and CRH was also quantified in the SON and CeA, respectively, since these two brain regions also participate in the integration of stress-elicited endocrine and behavioral responses (Engelmann and Ludwig, 2004; Roozendaal et al., 1997). We also assessed LHPA axis output by measuring corticosterone levels at several timepoints across a 24-h period. Our gene expression studies focused on two brain/blood collection timepoints - ZT2 and ZT14, as these represented times when animals exhibited either a trough or peak in corticosterone and locomotor activity (ZT2 and ZT14, respectively).
AVP
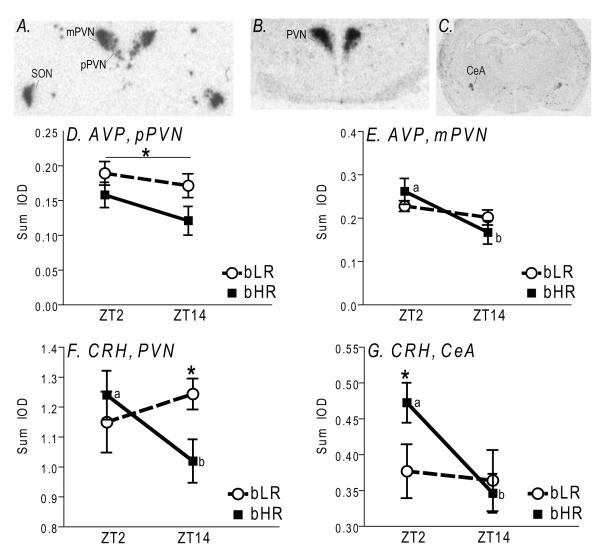
Consistent with previous reports (Wotjak et al., 1996), AVP expression was largely confined to PVN and SON (Fig. 5A). Within PVN, clear regional differences in AVP expression were apparent, with dorsolateral areas of heavy, dense signal corresponding to the magnocellular subdivision (mPVN), and loosely-appearing, less intense signal located medially and ventrally corresponding to the parvocellular subdivision (pPVN; Fig. 5A). Distinct AVP expression patterns were observed within the two PVN subdivisions. bLR rats exhibited significantly greater AVP mRNA levels specifically in the pPVN compared to bHR animals (F1, 29 = 4.99, p<0.05; Fig. 5D), although there was no effect of timepoint or phenotype × timepoint interaction. In contrast, there were no bHR/bLR AVP differences in the mPVN, but there was a significant interaction between phenotype and timepoint (F1, 29 = 7.26, p<0.02), with bHR animals expressing significantly higher levels of AVP mRNA at ZT2 compared to ZT14 (p<0.01; Fig. 5E). In the SON, there was a main effect of phenotype (F1, 29 = 26.16, p<0.0001), as bLR rats expressed overall higher AVP mRNA levels compared to bHRs (data not shown).
Figure 5.
Arginine vasopression (AVP) and corticotropin releasing hormone (CRH) mRNA expression bHR/bLR rats (N=8 per group). Top panels show autoradiograms from representative X-ray films after in situ hybridization with antisense riboprobes against rat AVP (A) or CRH (B-C) mRNA. AVP levels were measured within the supraoptic nucleus of the hypothalamus (SON) and two subregions of the paraventricular nucleus (PVN) of hypothalamus – the parvocellular (pPVN) and magnocellular (mPVN) regions (A). CRH mRNA expression was assessed in the PVN (B) and central nucleus of the amygdala (CeA; C). In the pPVN, bLRs expressed higher AVP levels compared to bHRs (D). In the mPVN, AVP mRNA levels were higher in the morning (Zeitgeber Time (ZT)2) versus evening (ZT14), but this effect was only observed in bHR animals (indicated by point a versus b; E). CRH mRNA levels in the PVN were also higher in the morning (ZT2) compared to evening (ZT14), but this effect was again only observed in bHR animals (indicated by point a versus b). Also, at the ZT14 timepoint, bHRs had lower levels of CRH mRNA in the PVN relative to bLRs (F). In the CeA, CRH mRNA levels were higher in the morning (ZT2) versus evening (ZT14), but again this effect was only observed in bHR animals (indicated by point a versus b). During the ZT2 timepoint, bHRs expressed higher levels of CRH in the CeA compared to bLRs (G). *indicates p<0.05.
CRH
Radioactive in situ hybridization revealed robust CRH expression in PVN (Fig. 5B), and the signal appeared to be confined to its parvocellular subdivision, which is consistent with previous reports (Swanson and Simmons, 1989). Statistical analysis revealed a significant phenotype × timepoint interaction (F1, 64 = 8.79, p<0.01), but no main effects of either factor alone (Fig. 5F). Post-hoc analysis showed a significant time of day effect in bHR, but not bLR rats, with greater CRH mRNA at ZT2 vs. ZT14 (p<0.01). This day-night difference led to significantly greater CRH mRNA in bLR versus bHR animals at ZT14 (p<0.01), but not ZT12 (Fig. 5F). In the amygdala, CRH expression was largely confined to CeA (Fig. 5C). As in PVN, CRH expression was significantly impacted by a phenotype × timepoint interaction (F1, 64 = 4.23, p<0.05), in addition to a main effect of timepoint (F1, 64 = 6.38, p<0.02). Post-hoc tests revealed a time of day effect in bHRs only, however, unlike in PVN, this day-night difference led to lower CRH levels in bLR versus bHR rats at ZT2 (p<0.01).
MR and GR
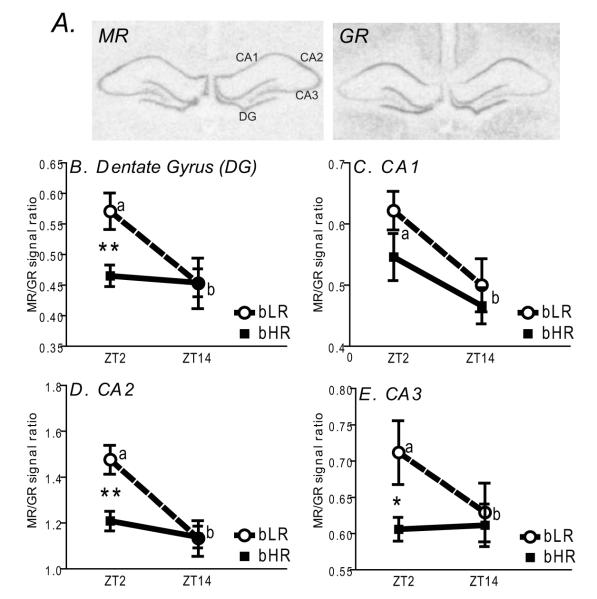
MR and GR mRNAs were assessed in the dorsal hippocampus where we observed robust expression levels in CA1, CA2, CA3 and dentate gyrus (DG) subfields (Fig. 6A). Consistent with previous observations (Lopez et al., 1998), levels of these mRNAs differed across the hippocampal subfields, with CA2-3 showing relatively low expression of GR and relatively high expression of MR (Fig. 6A).
Figure 6.
Expression of mineralicorticoid receptor (MR) and glucocorticoid receptor (GR) mRNAs in hippocampus of bHR/bLR animals (N=8 per group). Top panels show autoradiograms from representative X-ray films exposed for 7 d after in situ hybridization with antisense riboprobes against rat MR and GR mRNAs. MR and GR expression was quantified in 4 subregions of the hippocampus: Cornu Ammonis fields CA1-CA3, and the dentate gyrus (DG) (A). For each animal at either the morning (Zeitgeber Time (ZT)2 or evening (ZT14)) timepoint, we calculated a ratio of the MR:GR in situ hybridization signals. Graphs in panels B-E display average MR:GR ratios for bHR and bLR animals in each subfield at ZT2 and ZT14. There was a time of day effect within all hippocampal subfields, with a higher MR:GR ratio in the morning versus evening, although this was only observed in bLR animals (differences indicated by a versus b, panels B-E). During the morning, bLR animals exhibited a higher MR:GR ratio compared to bHRs in the DG (B), CA2 (D), and CA3 (E). **indicates p<0.01; *indicates p<0.05.
We focused our quantitative analyses on potential alterations in the MR/GR ratio since the relative levels of these two receptors, rather their individual levels, appear to be most closely linked to alterations in mood and affect in humans and in rodent models (De Kloet et al., 1998; Lopez et al., 1998). Significant phenotype × timepoint interactions were detected in DG (F1, 30 = 3.86, p<0.05), CA2 (F1, 30 = 6.32, p<0.01), and CA3 (F1, 30 = 3.87, p<0.05), with significant day-night differences in MR/GR ratios in bLR rats only. Post-hoc analyses revealed significant elevations in the MR/GR ratio in these subfields in bLR rats at ZT2 compared to bHR rats (see Fig. 6 B, D, and E). Within CA1, no bHR/bLR differences in expression were observed, however there was a significant decrease in the MR/GR ratio at ZT14 versus ZT2 within both rat lines (F1,1 = 9.54, p<0.01; Fig. 6C).
24-h corticosterone secretion
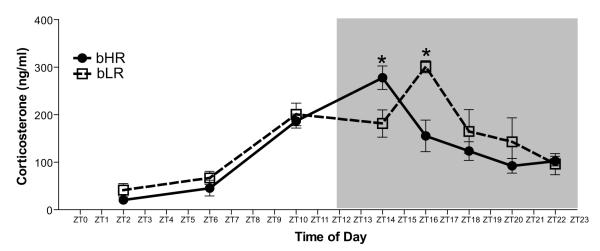
Plasma corticosterone levels in bHR/bLR rats were determined at eight different timepoints throughout the 24-h cycle: ZT2, ZT6, ZT10, ZT14, ZT16, ZT18, ZT20, and ZT22. We detected a significant main effect of timepoint (F1, 7 = 22.43, p<0.0001), and a significant phenotype × timepoint interaction (F1, 7 = 3.41, p<0.01), within the corticosterone data. Post-hoc analysis showed that corticosterone levels were significantly greater (p<0.05) in bHR than bLR at ZT14, but this difference was reversed at ZT16 (Fig. 7), indicating a delay in peak corticosterone secretion in bLR versus bHR rats.
Figure 7.
Corticosterone secretion in bHR/bLR rats over the 24-h cycle (N=8-10 per group per timepoint). Trunk blood samples were taken from bHR and bLR rats at eight different timepoints (Zeitgeber Time (ZT)2, ZT6, ZT10, ZT14, ZT16, ZT18, ZT20, and ZT22) across the 24-h cycle. bHR and bLR animals displayed relatively similar patterns of corticosterone secretion over the 24-h period, although bHRs showed higher levels than bLRs at ZT14 and lower levels at ZT16, suggesting a shift in peak corticosterone secretion for bLR versus bHR animals. * indicates p<0.05.
Discussion
The present study utilized selectively-bred bLR/bHR rats to demonstrate that inborn differences in novelty-induced locomotion and emotionality predict divergent diurnal patterns of homecage locomotor behavior, circadian gene transcript expression, and markers of the LHPA axis. Specifically, we observed marked bHR/bLR differences in nocturnal homecage activity, with bLR rats showing diminished nighttime activity compared to bHRs along with increased heterogeneity in the timing of their peak activity levels. Our in situ hybridization studies for Clock and related genes Per1 and Per2 revealed opposite day-night patterns of Clock mRNA, but similar patterns of Per1 and Per2 expression in the SCN, the brain’s master biological clock. We also observed opposite patterns of Per2 expression in the VTA, and overall reduced Per2 levels in the SNc and VTA of bHRs compared to bLRs. These data are consistent with earlier work in mutant mice where a downregulation of Clock and related molecules produces an “HR-like” behavior phenotype (Abarca et al., 2002; Roybal et al., 2007; Spanagel et al., 2005). Our LHPA axis studies revealed strikingly different diurnal gene expression patterns, with bHRs showing day-night differences in genes that potentiate ACTH secretion (i.e. AVP and CRH), while bLR rats exhibited diurnal fluctuations in genes regulating negative feedback of the LHPA axis (i.e. MR and GR). Interestingly, LHPA output does not dramatically differ between strains, although bLRs show a delayed corticosterone peak compared to bHRs. Taken together, our current findings suggest that bHR/bLR behavioral and neuroendocrine differences may be associated with fundamental differences in their circadian systems.
Diurnal homecage locomotor activity
We observed significant differences in homecage locomotion among bHR, bLR, and purchased Sprague-Dawley rats in the dark phase (ZT12-24). However, no such differences were detected in the light phase (ZT0-12). We previously documented increased impulsivity and aggression together with decreased depressive- and anxiety-like behaviors in bHR vs. bLR rats(Abraham et al., 2008; Flagel et al., 2010; Orr et al., 2009; Stead et al., 2006), but assays that evaluate these behaviors, such as Porsolt’s forced swim test and the Elevated Plus Maze test, may be confounded by baseline differences in locomotor behavior. Importantly, the present study demonstrates that bHR/bLR animals do not exhibit homecage locomotion differences during the light phase, which is when these animals demonstrate their behavioral differences on tests of depression, anxiety, impulsivity, and aggression. Therefore, our present observations suggest that bHR/bLR behavioral differences represent divergent emotional profiles rather than alterations in the baseline locomotor drive. Differences in homecage activity during the active (dark) phase may be related to different styles of interaction with the environment, and may indicate that bHR rats are somehow more engaged by environmental stimuli in home as well as in novel environments. This then raises the important question as to whether bHR/bLR behavioral differences (e.g. anxiety, depression) are apparent during both the light and dark phases. Since the extensive behavioral characterization of these lines has only been conducted during the light phase, future efforts should determine whether these behavioral profiles remain consistent across both the light-phase and dark-phase.
In addition to bHR/bLR group differences, we observed increased individual heterogeneity in diurnal patterns of locomotion in bLR rats. When compared to bHR rats, a greater fraction of bLR animals exhibited peak locomotion during the latter half of the dark phase (ZT18-ZT24). Interestingly, this bimodal distribution of peak locomotion was also present in purchased (non-selectively-bred) LR animals. An important caveat of these data is the fact that the reported homecage activity patterns represent only a single day for each animal. It would have been ideal to monitor animals across multiple days to determine the consistency of daily peak activity, so future experiments will pursue this type of analysis. That said, although we cannot comment on the consistency of a given animal’s peak activity over multiple days, we can comment on the consistency of the population differences as two subsequent experiments revealed the exact same distribution of early/late peakers (80% early versus 20% late peakers in bHR but 66% early versus 34% late peakers in bLRs; data not shown). Similar population differences were also observed in the purchased LR and HR rats, further indicating differences in the timing of activity peaks in animals with innate differences in environmental reactivity.
“Clock Gene” Expression
Our findings discussed above indicate distinct bHR/bLR diurnal patterns of behavior, suggesting underlying differences in circadian functioning in each of these types of rat. In support of this notion, we observed a decrease from ZT2 to ZT14 in the expression of Clock in the SCN in bLR rats. This day-night difference was reversed in bHR animals, leading to higher expression of Clock at ZT2 in bLR rats as compared to their bHR counterparts. We found no bHR/bLR differences in Per1 and Per2 expression in the SCN. Both groups showed higher Per1 levels during the light versus dark phase, and the opposite day-night pattern for Per2 mRNA levels, which is consistent with previous work (Shieh et al., 2005).
SCN is the master circadian oscillator in the brain, and as such, it generates endogenous circadian rhythm (Welsh et al., 2010). Its function is regulated by complex molecular machinery, within which Clock plays a key role in transcriptional regulation of other core circadian genes, including Cry1/2, Per1/2, and Rev-ErbA (Takahashi et al., 2008). A recent study reported that Clock-mutant mice manifest alterations in affective behaviors, including increased reward drive, increased propensity for drug self-administration, and decreased anxiety-like behavior (Roybal et al., 2007). This behavioral profile is reminiscent of the bHR rats, suggesting that bHR/bLR behavioral differences may, in part, be related to their Clock expression differences. Though we observed opposite day-night fluctuations in Clock expression in bHR/bLR rats, it is unlikely that their circadian clocks are in opposite phase because: 1) no Per1 or Per2 expression differences in the SCN were detected in these animals, and 2) we did not observe differences in their overall behavior patterns. One functional consequence of the Clock expression differences maybe an alteration in the length of the circadian cycle, since extensive evidence indicates that Clock lengthens the circadian period (Takahashi et al., 2008). Clock is also a histone acetyltransferase, which determines chromatin remodeling and likely regulates transcription of many other genes, beyond just the “core” circadian genes (Doi et al., 2006). Such genes may include those that regulate behavior and stress, and may contribute to behavioral differences in bHR/bLR animals.
In contrast to Clock, no bHR/bLR differences in Per1/2 expression in the SCN were observed. However, these genes appeared to be out of phase with each other. While this observation may be surprising as both of these transcripts are under the influence of Clock:Bmal1 dimers (Takahashi et al., 2008), it has previously been reported that the cycle of expression for these genes is 6-8 h out of phase. Shieh, et al. have demonstrated that Per1 reaches its peak expression near ZT4 and nadir near ZT16, while Per2 does so at ZT12 and ZT20, respectively (Shieh et al., 2005), a pattern that is closely matched by our observations. The most likely explanation for these differences in cycling is that expression of Period genes is regulated by a complex interaction of multiple factors, including: stress, inflammation, dopamine, feeding, and steroid hormones (Hood et al., 2010; Perrin et al., 2006; Takahashi et al., 2001; Wakamatsu et al., 2001).
Earlier mutant mouse work showed that manipulation of Clock (Roybal et al., 2007) or relatedmolecules Per1 and Per2 (Abarca et al., 2002) specifically within the mesolimbic dopamine system produced animals with an “HR-like” behavioral phenotype. We therefore sought to examine the expression of Clock, Per1 and Per2 in the VTA and SNc of bHR versus bLR animals. bHR/bLR animals showed similar Clock and Per1 expression in the VTA and SNc, but exhibited marked Per2 differences, particularly in the VTA, with bLRs having greater Per2 compared to bHRs. The earlier study in mutant mice showed that reduced Per2 function produces an enhanced behavioral sensitivity to cocaine (Abarca et al., 2002). There is abundant literature documenting bHR/bLR (and purchased HR/LR) differences in addictive behaviors, including differences in cocaine sensitization and self-administration, with bHRs/HRs demonstrating a markedly enhanced proclivity for addiction (Davis et al., 2008; Flagel et al., 2010; Hooks et al., 1991; Piazza et al., 1989). Our present Per2 findings suggest a possible neurobiological mechanism that may contribute to their distinctive phenotypes. Future studies will explore these possibilities to determine if (a) increasing Per2 levels in bHRs ameliorate their addictive tendencies, and (b) decreasing Per2 in bLRs makes them more sensitive to cocaine. Alternatively, the observed Per2 differences may be related to bHR/bLR differences in dopaminergic tone, since recent work by Hood et. al. elegantly demonstrated how striatal Per2 gene and protein expression is regulated via activation of D2 dopamine receptors (Hood et al., 2010).
LHPA axis markers – gene expression and diurnal corticosterone secretion
We observed significant bHR/bLR differences in the day-night expression of genes that regulate the LHPA axis, depressive- and anxiety-like behaviors. Interestingly, day-night expression differences were detected across different brain regions. For example, expression of CRH decreased from the morning until night in bHR animals in the PVN and CeA, while bLRs had no such fluctuation. In the hippocampus, bLRs showed a marked morning to night decrease in MR/GR across all hippocampal subfields, while bHRs did not. These observations also suggest that diurnal regulation of the LHPA axis is very different in bLR/bHR animals, so that in bHRs, diurnal fluctuations in corticosterone levels are likely due to fluctuations in the hypothalamic drive to anterior pituitary (i.e. CRH in PVN), while in bLRs they are likely due to day-night differences in the negative feedback loop (MR/GR ratio in hippocampus).
These differences in day-night patterns of gene expression resulted in bHR/bLR expression differences that were time of day-dependent. For instance, CRH expression in the PVN was greater in bLRs at ZT14, but not at ZT2, while its upregulation in bHRs in CeA was only apparent at ZT2. Similarly, MR/GR ratio was higher in bLRs in the dentate gyrus, CA2, and CA3 at ZT2, but not ZT14. In addition, AVP expression in pPVN was significantly greater in bLR rats, but this effect was not dependent on the time of day. Interestingly, in the CeA, bHR exhibited greater CRH mRNA levels, but only during the light phase. This finding conflicts with a previous study in commercially-purchased (non-selectively bred) HR/LR animals, which do not differ in their baseline CRH expression in CeA the light phase (Kabbaj et al., 2000a). Thus, while bHR and bLR animals exhibit similar behavioral profiles to those of purchased HR and LR rats, the underlying neurobiological mechanisms differences for their distinct behavioral phenotypes may be different.
In addition to their role in the regulation of ACTH and corticosterone secretion, MR and GR have also been implicated in emotional regulation. For instance, mice that over express GR in the forebrain exhibit behaviors suggestive of increased emotional lability (Wei et al., 2004), while those that over express MR exhibit alterations in their anxiety-like behavior (Rozeboom et al., 2007). Previous studies have also linked altered MR/GR balance to depression and suicide (De Kloet et al., 1998; Lopez et al., 1998), and to stress-elicited depressive-like behavior in rodents (Lopez et al., 1998). Within CA1-3 and DG, we observed significant day-night differences in the MR/GR ratio in bLR rats, with greater levels at ZT2 compared to ZT14. In contrast, we found no time of day MR/GR ratio differences in bHRs within CA2, CA3, and DG. This difference in diurnal fluctuation resulted in a significant elevation of the MR/GR ratio in bLRs versus bHRs at ZT2 within these hippocampal subfields. These day-night patterns differed from those observed for CRH expression in PVN and CeA, which varied with time of day in bHR but not bLR animals. Taken together, these observations indicate distinct patterns of diurnal regulation of the LHPA axis, with bHR animals exhibiting day-night differences in its stimulatory (i.e. CRH), but not inhibitory (i.e. MR/GR), component and vice versa in bLR rats. Unfortunately, no other studies to date have reported day-night MR/GR ratio differences in “normal” (i.e. non selectively-bred) rats. Therefore, it is unclear whether it is the bHR or bLR animals that exhibit typical/normal patterns of expression. Future experiments could examine these measures in commercially available/non-selected Sprague-Dawley rats to investigate this issue.
While no differences in 24-h corticosterone secretion were detected, we observed a significant delay in the timing of its peak secretion in bLR rats (at ZT16 vs. ZT14 in bHRs). It is feasible that this difference is due to a greater proportion of “late-peaking” bLR rats compared to “late-peaking” bHRs. Future studies aimed at monitoring 24-h corticosterone levels within individual animals together with homecage locomotion will be required to test this hypothesis. Apart from this, though, it is interesting that in spite of the marked LHPA differences apparent in the brain (e.g. differences in CRH, AVP and MR/GR ratios) of bHR/bLR animals, the final output of the LHPA axis is fairly similar between strains, with only a slight shift in timing of corticosterone release. This is further evidence of the robustness of the LHPA axis, highlighting the fact that (a) evaluating plasma levels of stress hormones are not enough to ensure that the same neural pattern is in place, and (b) the LHPA system can achieve what looks like a normal circadian rhythm via different mechanisms.
Conclusions
In summary, our findings in the bHR/bLR model indicate that individual differences in emotionality predict distinct diurnal patterns of behavior, hormone secretion, and brain gene expression. These observations expand our existing knowledge of bHR/bLR differences in baseline LHPA axis function and importantly suggest that circadian functioning in rodents with behavioral under-control (i.e. bHR) may be fundamentally different than in animals exhibiting behavioral over-control (i.e. bLR). An important limitation of our study is that these observations are correlational, thus, future studies in which different aspects of circadian functioning are manipulated are required to evaluate this notion.
Acknowledgements
This study was supported by NIMH 5K99MH081927 (IAK), NARSAD Young Investigator award (IAK), NIMH 1K99MH085859 (SMC), Office of Naval Research N00014-09-1-0598 (HA and SJW), and NIDA PPG 5P01DA021633-02 (HA and SJW). The authors would like to thank Sue Miller for excellent technical assistance.
References
- Abarca C, Albrecht U, Spanagel R. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proc Natl Acad Sci U S A. 2002;99:9026–30. doi: 10.1073/pnas.142039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham A, Clinton SM, Watson SJ, Akil H. Individual Differences in Novelty-Seeking Correlate with Differences in Aggressive Behavior; Society for Neuroscience 36th Annual Meeting; Atlanta, GA. 2006. [Google Scholar]
- Abraham AD, Clinton SM, Kerman IA, Bedrosian TA, Watson SJ, Akil H. Selectively-bred low novelty-seeking rats exhibit exaggerated anxiety- and depression-like behavior: a novel animal model of depression?. Society for Neuroscience 38th Annual Meeting; Washington, DC.. 2008. [Google Scholar]
- Bao AM, Meynen G, Swaab DF. The stress system in depression and neurodegeneration: focus on the human hypothalamus. Brain Res Rev. 2008;57:531–53. doi: 10.1016/j.brainresrev.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Serretti A, Colombo C, Barbini B, Lorenzi C, Campori E, Smeraldi E. Influence of CLOCK gene polymorphism on circadian mood fluctuation and illness recurrence in bipolar depression. Am J Med Genet B Neuropsychiatr Genet. 2003;123B:23–6. doi: 10.1002/ajmg.b.20038. [DOI] [PubMed] [Google Scholar]
- Brown ES, Rush AJ, McEwen BS. Hippocampal remodeling and damage by corticosteroids: implications for mood disorders. Neuropsychopharmacology. 1999;21:474–84. doi: 10.1016/S0893-133X(99)00054-8. [DOI] [PubMed] [Google Scholar]
- Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology. 2005;30:846–56. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Davis BA, Clinton SM, Akil H, Becker JB. The effects of novelty-seeking phenotypes and sex differences on acquisition of cocaine self-administration in selectively bred High-Responder and Low-Responder rats. Pharmacol Biochem Behav. 2008;90:331–8. doi: 10.1016/j.pbb.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- Doi M, Hirayama J, Sassone-Corsi P. Circadian regulator CLOCK is a histone acetyltransferase. Cell. 2006;125:497–508. doi: 10.1016/j.cell.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Ludwig M. The activity of the hypothalamo-neurohypophysial system in response to acute stressor exposure: neuroendocrine and electrophysiological observations. Stress. 2004;7:91–6. doi: 10.1080/10253890410001677240. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P, Phillips PE, Akil H. An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: implications for addiction. Neuropsychopharmacology. 2010;35:388–400. doi: 10.1038/npp.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank E, Kupfer DJ, Thase ME, Mallinger AG, Swartz HA, Fagiolini AM, Grochocinski V, Houck P, Scott J, Thompson W, Monk T. Two-year outcomes for interpersonal and social rhythm therapy in individuals with bipolar I disorder. Arch Gen Psychiatry. 2005;62:996–1004. doi: 10.1001/archpsyc.62.9.996. [DOI] [PubMed] [Google Scholar]
- Frank E, Swartz HA, Boland E. Interpersonal and social rhythm therapy: an intervention addressing rhythm dysregulation in bipolar disorder. Dialogues Clin Neurosci. 2007;9:325–32. doi: 10.31887/DCNS.2007.9.3/efrank. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain A, Kupfer DJ. Circadian rhythm disturbances in depression. Hum Psychopharmacol. 2008;23:571–85. doi: 10.1002/hup.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillin JC, Sitaram N, Duncan WC, Gershon ES, Nurnberger J, Post RM, Murphy DL, Wehr T, Goodwin FK, Bunney WE., Jr. Sleep disturbance in depression: diagnostic potential and pathophysiology [proceedings] Psychopharmacol Bull. 1980;16:40–2. [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry. 2002;7:254–75. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- Hood S, Cassidy P, Cossette MP, Weigl Y, Verwey M, Robinson B, Stewart J, Amir S. Endogenous dopamine regulates the rhythm of expression of the clock protein PER2 in the rat dorsal striatum via daily activation of D2 dopamine receptors. J Neurosci. 2010;30:14046–58. doi: 10.1523/JNEUROSCI.2128-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB., Jr. Response to novelty predicts the locomotor and nucleus accumbens dopamine response to cocaine. Synapse. 1991;9:121–8. doi: 10.1002/syn.890090206. [DOI] [PubMed] [Google Scholar]
- Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. J Neurosci. 2000a;20:6983–8. doi: 10.1523/JNEUROSCI.20-18-06983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. Journal of Neuroscience. 2000b;20:6983–8. doi: 10.1523/JNEUROSCI.20-18-06983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Davis CG, Kessler RC. The familial aggregation of common psychiatric and substance use disorders in the National Comorbidity Survey: a family history study. Br J Psychiatry. 1997;170:541–8. doi: 10.1192/bjp.170.6.541. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Benning SD, Kramer MD. Linking antisocial behavior, substance use, and personality: an integrative quantitative model of the adult externalizing spectrum. J Abnorm Psychol. 2007;116:645–66. doi: 10.1037/0021-843X.116.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le-Niculescu H, McFarland MJ, Ogden CA, Balaraman Y, Patel S, Tan J, Rodd ZA, Paulus M, Geyer MA, Edenberg HJ, Glatt SJ, Faraone SV, Nurnberger JI, Kuczenski R, Tsuang MT, Niculescu AB. Phenomic, convergent functional genomic, and biomarker studies in a stress-reactive genetic animal model of bipolar disorder and co-morbid alcoholism. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:134–66. doi: 10.1002/ajmg.b.30707. [DOI] [PubMed] [Google Scholar]
- Lopez JF, Chalmers DT, Little KY, Watson SJ. A.E. Bennett Research Award. Regulation of serotonin1A, glucocorticoid, and mineralocorticoid receptor in rat and human hippocampus: implications for the neurobiology of depression. Biol Psychiatry. 1998;43:547–73. doi: 10.1016/s0006-3223(97)00484-8. [DOI] [PubMed] [Google Scholar]
- Mansour HA, Wood J, Logue T, Chowdari KV, Dayal M, Kupfer DJ, Monk TH, Devlin B, Nimgaonkar VL. Association study of eight circadian genes with bipolar I disorder, schizoaffective disorder and schizophrenia. Genes Brain Behav. 2006;5:150–7. doi: 10.1111/j.1601-183X.2005.00147.x. [DOI] [PubMed] [Google Scholar]
- McClung CA. Circadian genes, rhythms and the biology of mood disorders. Pharmacol Ther. 2007;114:222–32. doi: 10.1016/j.pharmthera.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr H, Clinton SM, Abraham AD, Bedrosian TA, Watson SJ, Akil H. Low Novelty-Seeking Rats Exhibit Exaggerated Anxiety- and Depression-Like Behavior Compared to High Novelty-Seekers: Impact of Chronic Paxil Treatment. Society for Neuroscience 38th Annual Meeting; Washington, D.C. 2008. [Google Scholar]
- Orr HR, Bedrosian TA, Clinton SM, Kerman IA, Abraham AD, Watson SJ, Akil H. High Novelty-seeking rats show exaggerated aggression and distinct activation of forebrain limbic circuits following an aggressive encounter. Society for Neuroscience Meeting 39th Annual Meeting; Chicago, IL. 2009. [Google Scholar]
- Perrin JS, Segall LA, Harbour VL, Woodside B, Amir S. The expression of the clock protein PER2 in the limbic forebrain is modulated by the estrous cycle. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:5591–5596. doi: 10.1073/pnas.0601310103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–3. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Koolhaas JM, Bohus B. The role of the central amygdala in stress and adaption. Acta Physiol Scand Suppl. 1997;640:51–4. [PubMed] [Google Scholar]
- Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, Chakravarty S, Peevey J, Oehrlein N, Birnbaum S, Vitaterna MH, Orsulak P, Takahashi JS, Nestler EJ, Carlezon WA, Jr., McClung CA. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci U S A. 2007;104:6406–11. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozeboom AM, Akil H, Seasholtz AF. Mineralocorticoid receptor overexpression in forebrain decreases anxiety-like behavior and alters the stress response in mice. Proc Natl Acad Sci U S A. 2007;104:4688–93. doi: 10.1073/pnas.0606067104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawchenko PE, Brown ER, Chan RK, Ericsson A, Li HY, Roland BL, Kovacs KJ. The paraventricular nucleus of the hypothalamus and the functional neuroanatomy of visceromotor responses to stress. Prog Brain Res. 1996;107:201–22. doi: 10.1016/s0079-6123(08)61866-x. [DOI] [PubMed] [Google Scholar]
- Serretti A, Benedetti F, Mandelli L, Lorenzi C, Pirovano A, Colombo C, Smeraldi E. Genetic dissection of psychopathological symptoms: insomnia in mood disorders and CLOCK gene polymorphism. Am J Med Genet B Neuropsychiatr Genet. 2003;121B:35–8. doi: 10.1002/ajmg.b.20053. [DOI] [PubMed] [Google Scholar]
- Shieh KR, Yang SC, Lu XY, Akil H, Watson SJ. Diurnal rhythmic expression of the rhythm-related genes, rPeriod1, rPeriod2, and rClock, in the rat brain. J Biomed Sci. 2005;12:209–17. doi: 10.1007/s11373-004-8176-6. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, Lascorz J, Depner M, Holzberg D, Soyka M, Schreiber S, Matsuda F, Lathrop M, Schumann G, Albrecht U. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat Med. 2005;11:35–42. doi: 10.1038/nm1163. [DOI] [PubMed] [Google Scholar]
- Stead JD, Clinton SM, Neal C, Schneider J, Jama A, Miller S, Vazquez DM, Watson SJ, Akil H. Selective Breeding for Divergence in Novelty-seeking Traits: Heritability and Enrichment in Spontaneous Anxiety-related Behaviors. Behavioral Genetics. 2006;36:697–712. doi: 10.1007/s10519-006-9058-7. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Simmons DM. Differential steroid hormone and neural influences on peptide mRNA levels in CRH cells of the paraventricular nucleus: a hybridization histochemical study in the rat. J Comp Neurol. 1989;285:413–35. doi: 10.1002/cne.902850402. [DOI] [PubMed] [Google Scholar]
- Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9:764–75. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Yokota S, Hara R, Kobayashi T, Akiyama M, Moriya T, Shibata S. Physical and inflammatory stressors elevate circadian clock gene mPer1 mRNA levels in the paraventricular nucleus of the mouse. Endocrinology. 2001;142:4910–7. doi: 10.1210/endo.142.11.8487. [DOI] [PubMed] [Google Scholar]
- Wakamatsu H, Yoshinobu Y, Aida R, Moriya T, Akiyama M, Shibata S. Restricted-feeding-induced anticipatory activity rhythm is associated with a phase-shift of the expression of mPer1 and mPer2 mRNA in the cerebral cortex and hippocampus but not in the suprachiasmatic nucleus of mice. Eur J Neurosci. 2001;13:1190–6. doi: 10.1046/j.0953-816x.2001.01483.x. [DOI] [PubMed] [Google Scholar]
- Wei Q, Lu XY, Liu L, Schafer G, Shieh KR, Burke S, Robinson TE, Watson SJ, Seasholtz AF, Akil H. Glucocorticoid receptor overexpression in forebrain: a mouse model of increased emotional lability. Proc Natl Acad Sci U S A. 2004;101:11851–6. doi: 10.1073/pnas.0402208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol. 2010;72:551–77. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wotjak CT, Kubota M, Kohl G, Landgraf R. Release of vasopressin from supraoptic neurons within the median eminence in vivo. A combined microdialysis and push-pull perfusion study in the rat. Brain Res. 1996;726:237–41. [PubMed] [Google Scholar]
- Wu JC, Gillin JC, Buchsbaum MS, Schachat C, Darnall LA, Keator DB, Fallon JH, Bunney WE. Sleep deprivation PET correlations of Hamilton symptom improvement ratings with changes in relative glucose metabolism in patients with depression. J Affect Disord. 2008;107:181–6. doi: 10.1016/j.jad.2007.07.030. [DOI] [PubMed] [Google Scholar]