Abstract
Taste neophobia refers to a reduction in consumption of a novel taste relative to when it is familiar. To gain more understanding of the neural basis of this phenomenon, the current study examined whether a novel taste (0.5% saccharin) supports a different pattern of c-Fos expression than the same taste when it is familiar. Results revealed that the taste of the novel saccharin solution evoked more Fos immunoreactivity than the familiar taste of saccharin in the basolateral region of the amygdala, central nucleus of the amygdala, gustatory portion of the thalamus, and the gustatory insular cortex. No such differential expression was found in the other examined areas, including the bed nucleus of stria terminalis, medial amygdala, and medial parabrachial nucleus. The present results are discussed with respect to a forebrain taste neophobia system.
Keywords: c-Fos, Central gustatory system, Unknown taste, Neophobic reaction, Rat
1. Introduction
Food intake is modulated by its post-ingestive consequences. For example, when the consequence is gastrointestinal illness the food will be avoided on future encounters. This phenomenon, in which the taste (conditioned stimulus; CS) of a poisonous or toxic food is associated with the ensuing gastrointestinal illness (unconditioned stimulus; US) via Pavlovian conditioning, is termed conditioned taste aversion (CTA) learning (Garcia, Kimeldorf, & Koelling, 1955; Revusky & Garcia, 1970; for recent reviews see Reilly & Schachtman, 2009). Obviously, for a food with a novel taste, experiential knowledge about post-ingestive consequences is absent. Is this novel food safe or is it dangerous? Some tastes are innately liked (e.g., sucrose) and therefore, although novel, are avidly consumed as though they are familiar. The adaptive response to an unknown, novel taste is to consume relatively little on initial encounter and to wait in order that the post-ingestive consequences can be experienced thereby permitting a proper evaluation of the food. This phenomenon, the reluctance to consume these types of novel, potentially toxic, tastes, has been termed neophobia (e.g., Barnett, 1956, Barnett, 1958; Domjan, 1977) or, more specifically, taste neophobia. In the absence of ensuing aversive post-ingestive consequences, the avoidance response to the taste stimulus begins to habituate and, over trials, more and more of the food is consumed until an asymptote of intake is achieved for this now familiar and safe stimulus. It should be noted that the intake of some aqueous stimuli might never reach the level of water intake. Such stimuli are considered non-preferred.
Given the survival value of taste neophobia, research has examined the neural circuits mediating the phenomenon. In an early study, Nachman and Ashe (1974) found that electrolytic lesions of the basolateral region of the amygdala (BLA) attenuated the neophobic reaction to a novel 0.25% saccharin solution. The additional finding that these same BLA-lesioned (BLAX) rats also showed lower intake of the familiar saccharin at asymptote than neurologically intact animals compromised accurate determination of the nature of the deficit. Did these non-selective BLA lesions disrupt the perception of taste danger/novelty or did they change the perceived intensity/quality of the taste stimulus? More recently, Lin, Roman, St. Andre and Reilly (2009) found that excitotoxic lesions of the BLA lesions attenuated taste neophobia without influencing the level of intake at asymptote of the now familiar 0.5% saccharin solution. In addition, these investigators found a similar deficit in animals with excitotoxic lesions of either the medial amygdala (MeA) or the insular cortex (IC). Importantly, none of these lesions affected the neophobic reaction to an aqueous odor or an aqueous trigeminal stimulus. This pattern of results indicates that the three brain areas (BLA, MeA and IC) play a critical role in the perception of, or responsivity to, novel and potentially dangerous taste stimuli.
To obtain a more comprehensive understanding of the neural circuit underlying taste neophobia, we need to determine if other brain structures contribute to this particular innate avoidance behavior. To pursue this issue, the current experiment examined which brain areas are activated by a novel taste using Fos-like immunoreactivity. As an immediate-early gene, c-Fos is expressed during neural activation (Morgan & Curran, 1991). Therefore, c-Fos has been wildly used to investigate the neural substrates of various behavioral phenomena, such as spatial learning (Gill, Bernstein & Mizumori, 2007), conditioned place preference (Honsberger & Leri, 2008), olfactory learning (Solov'eva, Lagutina, Antonova & Anokhin, 2007). Unsurprisingly, c-Fos also has also been used to explore the brain areas that are involved in taste-related learning such as CTA. For example, c-Fos has been utilized to explore the brain areas activated by an intraorally infused taste before CTA conditioning (e.g., Houpt, Philopena, Wessel, Joh & Smith 1994; Yamamoto, Sako, Sakai & Iwafune 1997), by the lithium chloride US (see Gu, Gonzalez, Chin & Deutsch 1993; Sakai & Yamamoto 1997; St. Andre, Albanos & Reilly, 2007), and by the taste CS after CTA conditioning (Swank & Bernstein, 1994; Swank, Schafe & Bernstein 1995; Koh & Bernstein, 2005). Following the rationale of these studies, we expect that if a brain area is involved in the neophobic reaction to a novel and potentially dangerous taste stimulus then that area should show significantly more c-Fos expression as revealed by immunostaining conducted after ingestion of a novel taste relative to the same taste when, following repeated exposures, it has become familiar and safe.
In additions to the areas already implicated in taste neophobia (BLA, MeA and IC), we examined the medial parabrachial nucleus (mPBN) and three forebrain areas to which the mPBN relays ascending taste information: the gustatory region of the thalamus (GT), the central nucleus of the amygdala (CNA) and the bed nucleus of the stria terminalis (BNST). To afford comparability with our previous work (Lin et al., 2009), we used the same 15-min voluntary intake design. Earlier work using c-Fos to examine the neural substrates of neophobia (e.g., Houpt et al., 1994; Yamamoto et al., 1997) infused the taste stimuli directly into the mouth, an approach that may not be ideal for the assessment of a voluntary avoidance behavior. Furthermore, CTA acquisition following intraorally infused CSs is known to engage a somewhat different neural substrate than CSs which are voluntarily consumed (Fresquet, Angst & Sandner, 2004; Schafe, Thiele & Bernstein, 1998; St. Andre & Reilly, 2007; for a review see Reilly, 2009) and, as shown by Wilkins and Bernstein (2006), each CS delivery method causes a different pattern of c-Fos expression. Accordingly, the present experiment investigated c-Fos expression in the BLA, BNST, CNA, GT, IC, MeA and mPBN in rats voluntarily drinking a novel taste or, after repeated exposures, the same taste when familiar.
2. Results
2.1. Behavioral
Group Novel was given access to a novel 0.5% saccharin solution whereas the rats in Group Familiar were given five 15-min exposure trials to 0.5% saccharin prior to the final limited-intake test trial. Table 1 shows the average saccharin intake on each trial for the Novel and Familiar groups. As shown in the table, both groups of rats consumed about 5 ml on their first exposure to the novel and potentially dangerous saccharin solution. As the trials continued for group Familiar, saccharin intake gradually increased (i.e., habituation of neophobia occurred) and reached asymptote at about 17 ml. A one-way ANOVA, conducted on data from rats in group Familiar (Trials 1 - 5), found a significant main effect of trial, F(4,20) = 25.55, p < .001. Post hoc comparisons (Fisher LSD test) further revealed that intake on Trial 1 was significantly lower than on Trials 2 - 5 (p < .05) and that there was no significant intake differences on Trials 3 - 5 (p > .05), indicating asymptotic level of consumption for the now familiar and safe saccharin solution.
Table 1.
Mean (±SE) 0.5% saccharin intake (ml) of rats in the Novel and Familiar groups across all trials of the experiment.
| Trial | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Novel | ||||||
| Mean | 4.83 | - | - | - | - | - |
| SE | 1.70 | |||||
| Familiar | ||||||
| Mean | 5.00 | 13.75* | 15.50* | 17.33* | 17.00* | 5.00 |
| SE | 0.77 | 1.63 | 0.95 | 1.12 | 0.72 | 0.00 |
Notes: Intake was capped at 5.00 ml on Trail 6.
Significantly different from Trial 1 at p < .05.
2.2. Immunohistology
For the groups that consumed a novel or a familiar 0.5% saccharin solution, Fig. 1 shows the number of c-Fos positive nuclei in the seven brain regions that were examined. Separate independent t-tests were conducted on the data obtained from each brain area to determine which structures participate in the neophobic reaction to a novel taste stimulus. Significant effects of group were found for the BLA, t(10) = 2.94, p < .05, CNA, t(10) = 1.91, p < .05; GT, t(10) = 2.86, p < .05, and IC, t(10) = 2.00, p < .05. That is, the BLA, CNA, GT and IC each exhibited increased c-Fos expression following consumption of novel saccharin relative to familiar saccharin. No effect of group was obtained in the BNST, t(10) = 1.80, p = .051; MeA, t(10) = 0.71, p > .05; or mPBN, t(10) = 1.41, p > .05. Fig. 2 shows representative photomicrographs of the c-Fos expression in each of the target nuclei consequent to ingestion of a novel or familiar saccharin solution.
Fig 1.
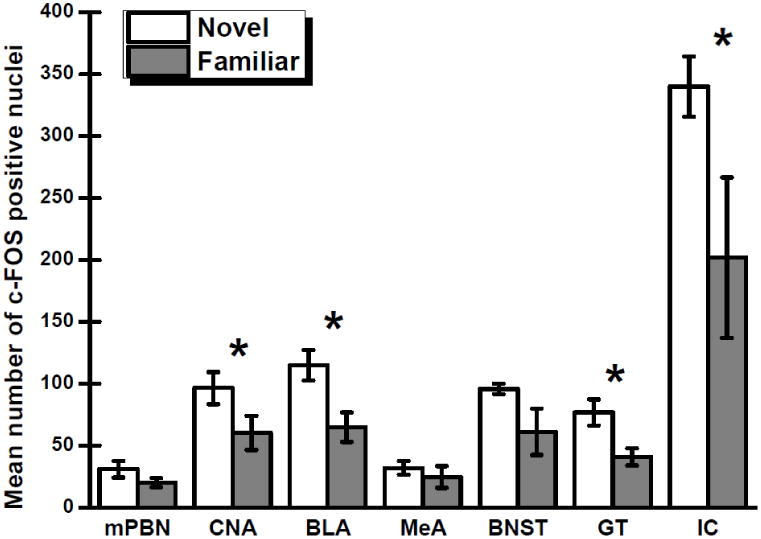
Mean numbers of nuclei positive for c-Fos (±SE) in the medial parabrachial nucleus (mPBN), central nucleus of the amygdala (CNA), basolateral region of the amygdala (BLA), medial amygdala (MeA), bed nucleus of the stria terminalis (BNST), gustatory thalamus (GT), and insular cortex (IC) following consumption of a novel or familiar 0.5% saccharin solution. * Significant at p < 0.05.
Fig 2.
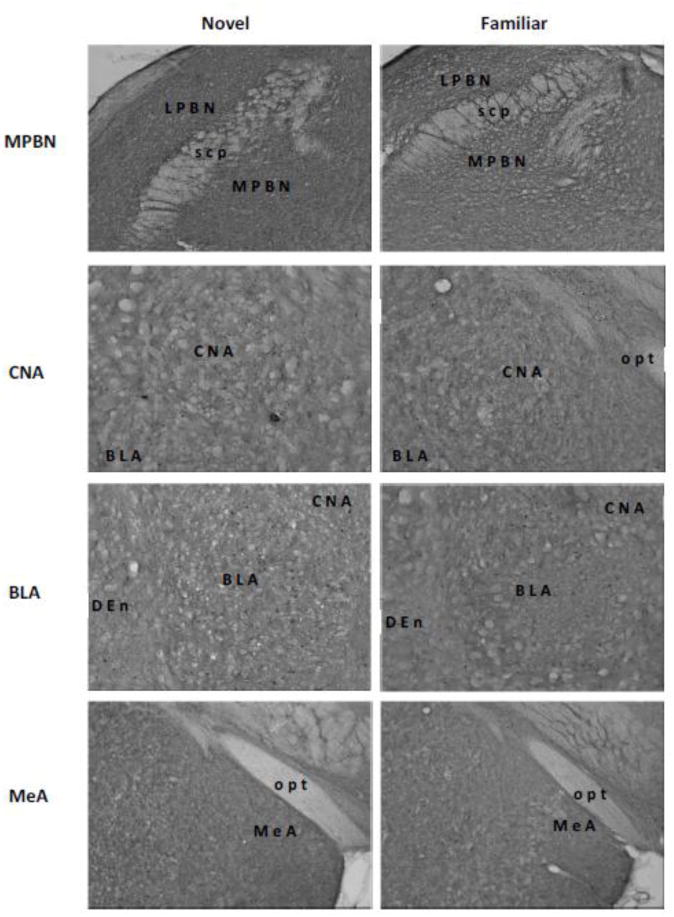
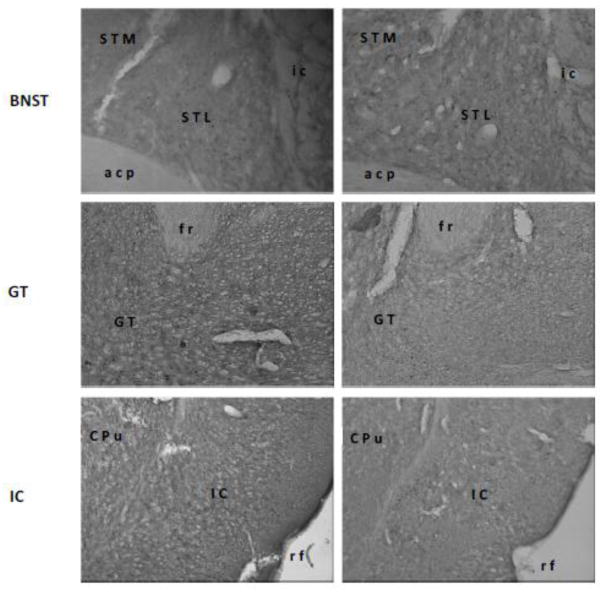
Representative digital photomicrographs of c-Fos activity in the medial parabrachial nucleus (MPBN), central nucleus of the amygdala (CNA), basolateral amygdala (BLA), medial amygdala (MeA), bed nucleus of stria terminalis (BNST), gustatory thalamus (GT), and insular cortex (IC). Relative to a familiar taste (right panels), a novel taste (left panels) induced higher level of c-Fos expressions only in the BLA and GT. Abbreviations: acp, posterior portion of the anterior commissure; CPu, caudate putamen; Den, dorsal endopiriform nucleus; fr, fasciculus retroflexus; ic, internal capsule; LPBN, lateral parabrachial nucleus; opt, optic tract; rf, rhinal fissure; scp, superior cerebella peduncle; STL, lateral division of the bed nucleus of the stria terminalis; STM, medial division of the bed nucleus of the stria terminalis.
3. Discussion
Significant taste novelty-dependent elevations in c-Fos expression emerged in four nuclei: the BLA, CNA, GT and IC. However, no such difference was found in the other three target structures (viz.: BNST, MeA and mPBN). Interpretation of this pattern of results will be discussed by brain region, beginning with those regions that showed positive results.
The increased activation in the BLA and IC following a novel taste stimulus provides further support for the view that these two structures contribute to the expression of taste neophobia, which has previously been indicated on the basis of lesion (Lin et al., 2009) and pharmacological (for the BLA, Figueroa-Guzmán & Reilly, 2008; Miranda, LaLumiere, Buen, Bermudez-Rattoni & McGaugh, 2003; for the IC, Figueroa-Guzmán, Kuo, & Reilly, 2006; Gutiérrez, Téllez, & Bermúdez-Rattoni, 2003) manipulations. Moreover, the finding that lesions of either structure also disrupt the acquisition of CTAs to a novel but not a familiar taste CS (e.g., Aggleton, Petrides, & Iversen, 1981; Roman, Lin, & Reilly, 2009; Shimai & Hoshishima, 1982; St. Andre & Reilly, 2007) provides converging lines of evidence that the BLA and IC each have roles in the perception of, or responsivity to, taste novelty. It is surprising, then, that Koh, Wilkins & Bernstein (2003; Experiment 1), using a design that was highly similar to that of the present experiment, did not find an elevation of c-Fos expression in the BLA consequent to voluntary consumption of a novel taste solution. Although we have no ready explanation for this null result, it is noted that the overall levels of c-Fos expression in the Koh et al. study were substantially lower than in the present study which encourages the speculation that floor effects might have tended to obscure detection of group differences.
The present c-Fos data indicate that the GT has a significant role in the taste neophobia system. This conclusion is consistent with the earlier finding that GT-lesioned (GTX) rats drank significantly more of the taste CS on the first preexposure trial of a latent inhibition experiment than non-lesioned control subjects (Reilly, Bornovalova, Dengler & Trifunovic, 2003). However, this GT lesion-induced taste neophobia deficit had no influence on the subsequent acquisition of a lithium chloride-induced CTA to either a novel or a familiar CS (Reilly et al., 2003). Indeed, there are numerous reports of normal CTA acquisition in GTX rats (Flynn, Grill, Schulkin, & Norgren, 1991; Grigson, Lyuboslavsky, & Tanase, 2000; Mungarndee, Lundy, & Norgren, 2006; Reilly & Pritchard, 1996; Scalera, Grigson, & Norgren, 1997). Thus, for the BLA, IC and GT, the c-Fos data are consistent with the lesion data that shows elevated intake of a novel taste on initial encounter. However, whereas lesions of the BLA or IC delay CTA acquisition, rats with GT lesions show normal CTA acquisition. This pattern of results suggests that the GT has a different function in the taste neophobia system than either the BLA or IC.
Elevated c-Fos expression in the CNA following consumption of novel saccharin was unexpected because previous work found that rats with lesions of the CNA showed normal taste neophobia, normal CTA acquisition to a novel taste CS and normal CTA acquisition to a familiar taste CS (St. Andre & Reilly, 2007). The finding of taste novelty induced c-Fos expression in the CNA might be considered a false positive except Koh et al. (2003) reported a similar result. Thus, it is not immediately obvious how to reconcile the inconsistent findings from c-Fos immunoreactivity and lesion experiments.
The finding that the BNST and mPBN did not yield significant changes in c-Fos expression consequent to the consumption of a novel taste stimulus requires little comment. The null result for the BNST is consistent with other findings that show that lesions of the structure cause neither over-consumption on initial encounter with a taste CS nor influence the acquisition of CTAs to a novel taste (Roman, Nebieridze, Sastre, & Reilly, 2006; YaOmamoto & Fujimoto, 1991). On the other hand, although lesions of the mPBN abolish CTA acquisition (DiLorenzo 1988; Grigson, Reilly, Shimura & Norgren, 1998; Scalera, Spector & Norgren, 1995; Spector, Norgren, & Grill, 1992; Reilly, Grigson, & Norgren, 1993; Trifunovic & Reilly, 2002), it is widely accepted that this deficit reflects a failure of associative learning such that the taste CS is not linked with the aversive US (for reviews see Reilly, 1999, 2009). Thus, it was not surprising that null results were obtained from the mPBN in the present experiment.
Relative to the null results for the BNST and mPBN, it was, however, surprising that there were no changes in c-Fos expression in the MeA following consumption of a novel taste. This result was unexpected because rats with excitotoxic lesions of the MeA have a known taste neophobia deficit (Lin et al., 2009). Moreover, the deficit induced by MeA lesions was specific to taste; the rats showed normal neophobic reactions to a novel aqueous odor stimulus as well as a novel aqueous trigeminal stimulus (Lin et al., 2009). At first glance, then, the c-Fos and lesions data provide conflicting views of the involvement of the MeA in taste neophobia. It may, however, be possible to reconcile these seemingly disparate sets of findings. One of the recognized constraints of the c-Fos technique is that it can only be used to detect excitatory activity; inhibitory neural activity cannot be distinguished from background or baseline activity (Kovacs, 2008; Stark, Davies, Williams, & Luckman, 2006). Perhaps, then, the absence of novelty-dependent c-Fos expression in the MeA simply reflects an inhibitory role for the subnucleus in taste neophobia. Admittedly speculative, this hypothesis can readily be tested by recording neuronal activity in the MeA during ingestion of a novel taste solution using in vivo techniques such as microdialysis or electrophysiology.
In summary, converging lines of evidence implicate four structures (BLA, GT, IC and MeA) and possibly a fifth (the CNA) in detection and/or responsivity to a novel and potentially dangerous taste stimulus when it is first encountered and consumed. However, it would appear that none of these structures function in an identical manner since there is no evidence of behavioral compensation when one of these areas is lesioned. That lesions of each area cause a behavioral deficit suggests that each area has a different, albeit inter-dependent, function in the taste neophobia system. Further research, using a variety of techniques and methods (including c-Fos, asymmetric permanent lesions, temporary inactivation, and other types of pharmacological manipulations), will seek to determine with more specificity the nature of the function(s) performed by each structure as well as how these functions are orchestrated into the seamless but multifaceted neophobic reactions that are triggered by an unknown taste stimulus.
4. Methods
4.1. Animals
Twelve naïve male Sprague-Dawley rats (Charles River Laboratories) weighing ∼300 g served as subjects. On arrival in the laboratory the animals were individually housed in stainless steel hanging cages in a temperature controlled room (21° C) on a 12:12 h light-dark cycle (lights on at 0700 hours). Rats were given ad libitum food and water until the experiment started when, as described below, water access was restricted. All rats were treated in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (1986) and the University of Illinois at Chicago Institutional Animal Care and Use Committee approved the experimental protocols.
4.2. Procedure
Behavioral testing occurred in the home cages. To afford comparability with our previous research on neophobia and taste aversion learning, all rats in the present experiment were acclimated to our standard deprivation schedule: 15-min access to water each day. When fluid consumption stabilized (∼7 days), subjects were matched for water intake and separated into two groups: Novel (n = 6) and Familiar (n = 6). All taste trials were 15-min in duration. On Trial 1, all rats received access to 0.5% saccharin from a calibrated bottle that was attached to the front of the home cage. The rats in the Novel group were perfused 90 min later. Animals in the Familiar group continued to receive saccharin every third day until asymptote was reached on Trial 5; 15-min water access was provided on the intervening days. On Trial 6, saccharin intake was capped at 5 ml (to match to amount of saccharin consumed by the Novel group on Trial 1) and these rats were perfused 90 min later.
4.3. Histology
Rats were anesthetized using sodium pentobarbital and perfused trans-cardially with phosphate-buffered saline followed by formalin. Brains were removed and stored in formalin followed by 30% sucrose at 4°C. Subsequently, the brains were sliced at 60 μm using a cryostat and immunoassayed for c-Fos expression. All the brain samples were processed in one immunoassay to ensure consistency of c-Fos staining across subjects
4.4. Immunohistology
The c-Fos protocol was identical to that employed by St. Andre et al. (2007). Briefly, slices were pre-treated for 20 min with 0.3% H2O2, rinsed, and then transferred to a solution of 3% normal goat serum and 0.5% Triton X-100 for 30 min. The tissue was then refrigerated for 48 h in a bath containing the c-Fos antibody (Santa Cruz Biotechnology, Santa Cruz, CA). The sections were rinsed and incubated in a secondary antibody (biotinylated goat anti-rabbit IgG; Vector Laboratories, Burlingame, CA) for 2 h. Both antibody solutions were mixed in a solution of 1% normal goat serum, 0.5% Triton X-100, and phosphate-buffered saline. The tissue was then rinsed, processed with ABC (Vector Laboratories, Burlingame, CA), rinsed again, and visualized using a 3,3′-diaminobenzidine (DAB; Vector Laboratories, Burlingame, CA). Following this last step, the sections were rinsed, mounted on gelatin-subbed slides, rehydrated with ethanol and xylenes, and cover-slipped. Photographs were taken of the BLA, BNST, CNA, GT, IC, MeA, and mPBN in each brain. Four individuals served as raters, who counted the number of c-Fos expressing cells in the areas of interest in each image. The scores by each person were significantly positively correlated with each other, rs > .80; ps < .001, and therefore were averaged for the subsequent analyses.
Research Highlights.
We examined c-Fos expression in the central gustatory system after either novel or familiar saccharin was consumed.
Relative to a familiar one, a novel taste induced higher levels of c-Fos expression in the basolateral amygdala, central nucleus of amygdala, gustatory thalamus, and gustatory insular cortex
The potentiated c-Fos expression induced by novel tastes was not found in the other examined areas, including the medial parabrachial nucleus, bed nucleus of stria terminalis, medial amygdala.
Acknowledgments
This research was supported by grant DC 04341 and DC06456 from the National Institute of Deafness and Other Communication Disorders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jian-You Lin, Email: jlin2@uic.edu.
Steve Reilly, Email: sreilly@uic.edu.
References
- Aggleton JP, Petrides M, Iversen SD. Differential effects of amygdaloid lesions on conditioned taste aversion learning by rats. Physiol Behav. 1981;27:397–400. doi: 10.1016/0031-9384(81)90322-x. [DOI] [PubMed] [Google Scholar]
- Barnett SA. Behaviour of wild rats in the laboratory. Med Biol Illustration. 1956;6:104–111. [PubMed] [Google Scholar]
- Barnett SA. Experiments on neophobia in wild and laboratory rats. Brit J Psychol. 1958;49:195–201. doi: 10.1111/j.2044-8295.1958.tb00657.x. [DOI] [PubMed] [Google Scholar]
- DiLorenzo PM. Long-delay learning in rats with parabrachial pontine lesions. Chem Senses. 1988;13:219–229. [Google Scholar]
- Domjan M. Attenuation and enhancement of neophobia for edible substances. In: Barker LM, Best MR, Domjan M, editors. Learning mechanisms in food selection. New York: Baylor: University Press; 1977. pp. 151–179. [Google Scholar]
- Figueroa-Guzmán Y, Kuo JS, Reilly S. NMDA receptor antagonist MK-801 infused into the insular cortex prevents the attenuation of gustatory neophobia in rats. Brain Res. 2006;1114:183–186. doi: 10.1016/j.brainres.2006.07.036. [DOI] [PubMed] [Google Scholar]
- Figueroa-Guzmán Y, Reilly S. NMDA receptors in the basolateral amygdala and gustatory neophobia. Brain Res. 2008;1210:200–203. doi: 10.1016/j.brainres.2008.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn FW, Grill HJ, Schulkin J, Norgren R. Central gustatory lesions: II. Effects on sodium appetite, taste aversion learning, and feeding behaviors. Behav Neurosci. 1991;105:944–954. doi: 10.1037//0735-7044.105.6.944. [DOI] [PubMed] [Google Scholar]
- Fresquet N, Angst MJ, Sandner G. Insular cortex lesions alter conditioned taste avoidance in rats differentially when using two methods of sucrose delivery. Behav Brain Res. 2004;153:357–365. doi: 10.1016/j.bbr.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Garcia J, Kimeldorf DJ, Koelling R. Conditioned aversion to saccharin resulting from exposure to gamma radiation. Science. 1955;122:157–158. [PubMed] [Google Scholar]
- Gill KM, Bernstein IL, Mizumori SJY. Immediate early gene activation in hippocampus and dorsal striatum: effects of explicit place and response training. Neurobiol Learn Mem. 2007;87:583–596. doi: 10.1016/j.nlm.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Lyuboslavsky P, Tanase D. Bilateral lesions of the gustatory thalamus disrupt morphine- but not LiCl-induced intake suppression in rats: evidence against the conditioned taste aversion hypothesis. Brain Res. 2000;858:327–337. doi: 10.1016/s0006-8993(00)01939-9. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Reilly S, Shimura T, Norgren R. Ibotenic acid lesions of the parabrachial nucleus and conditioned taste aversion: further evidence for an associative deficit in rats. Behav Neurosci. 1998;112:160–171. [PubMed] [Google Scholar]
- Gu Y, Gonzalez MF, Chin DY, Deutsch JA. Expression of c-fos in brain subcortical structures in response to nauseant lithium chloride and osmotic pressure in rats. Neurosci Lett. 1993;157:49–52. doi: 10.1016/0304-3940(93)90640-7. [DOI] [PubMed] [Google Scholar]
- Gutiérrez R, Téllez LA, Bermúdez-Rattoni F. Blockade of cortical muscarinic but not NMDA receptors prevents a novel taste from becoming familiar. Eur J Neurosci. 2003;17:1556–1562. doi: 10.1046/j.1460-9568.2003.02608.x. [DOI] [PubMed] [Google Scholar]
- Honsberger MJM, Leri F. Fos expression in mesocorticolimbic areas during heroin place conditioning. Neuroreport. 2008;19:63–67. doi: 10.1097/WNR.0b013e3282f31d82. [DOI] [PubMed] [Google Scholar]
- Houpt TA, Philopena JM, Wessel TC, Joh TH, Smith GP. Increased c-fos expression in nucleus of the solitary tract correlated with conditioned taste aversion to sucrose in rats. Neurosci Lett. 1994;172:1–5. doi: 10.1016/0304-3940(94)90648-3. [DOI] [PubMed] [Google Scholar]
- Koh MT, Bernstein IL. Mapping conditioned taste aversion associations using c-Fos reveals a dynamic role for insular cortex. Behav Neurosci. 2005;119:388–398. doi: 10.1037/0735-7044.119.2.388. [DOI] [PubMed] [Google Scholar]
- Koh MT, Wilkins EE, Bernstein IL. Novel tastes elevate c-fos expression in the central amygdala and insular cortex: implication for taste aversion learning. Behav Neurosci. 2003;117:1416–1422. doi: 10.1037/0735-7044.117.6.1416. [DOI] [PubMed] [Google Scholar]
- Kovács KJ. Measurement of immediate-early gene activation- c-fos and beyond. J Neuroendocrinol. 2008;20:665–672. doi: 10.1111/j.1365-2826.2008.01734.x. [DOI] [PubMed] [Google Scholar]
- Lin JY, Roman C, St Andre J, Reilly S. Taste, olfactory and trigeminal neophobia in rats with forebrain lesions. Brain Res. 2009;1251:195–203. doi: 10.1016/j.brainres.2008.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda MI, LaLumiere RT, Buen TV, Bermudez-Rattoni F, McGaugh JL. Blockade of noradrenergic receptors in the basolateral amygdala impairs taste memory. European J Neurosci. 2003;18:2605–2610. doi: 10.1046/j.1460-9568.2003.03008.x. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Ann Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- Mungarndee SS, Lundy RF, Jr, Norgren R. Central gustatory lesions and learned taste aversions: Unconditioned stimuli. Physiol Behav. 2006;87:542–551. doi: 10.1016/j.physbeh.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachman M, Ashe JH. Effects of basolateral amygdala lesions on neophobia, learned taste aversions, and sodium appetite in rats. J Comp Physiol Psychol. 1974;87:622–643. doi: 10.1037/h0036973. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Guide for the care and use of laboratory animals (DHEW Publication No 86-23) Government Printing Office; Washington, DC, US: 1986. [Google Scholar]
- Reilly S. The parabrachial nucleus and conditioned taste aversion. Brain Res Bull. 1999;48:239–254. doi: 10.1016/s0361-9230(98)00173-7. [DOI] [PubMed] [Google Scholar]
- Reilly S. Central gustatory system lesions and conditioned taste aversion. In: Reilly S, Schachtman TR, editors. Conditioned taste aversion: Behavioral and neural processes. New York: Oxford University Press; 2009. pp. 309–327. [Google Scholar]
- Reilly S, Bornovalova MA, Dengler C, Trifunovic R. Effects of excitotoxic lesions of the gustatory thalamus on latent inhibition and blocking of conditioned taste aversion in rats. Brain Res Bull. 2003;62:117–128. doi: 10.1016/j.brainresbull.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Reilly S, Grigson PS, Norgren R. Parabrachial nucleus lesions and conditioned taste aversion: Evidence supporting an associative deficit. Behav Neurosci. 1993;107:1005–1017. doi: 10.1037//0735-7044.107.6.1005. [DOI] [PubMed] [Google Scholar]
- Reilly S, Pritchard TC. Gustatory thalamus lesions in the rat: II. Aversive and appetitive taste conditioning. Behav Neurosci. 1996;110:746–759. [PubMed] [Google Scholar]
- Reilly S, Schachtman TR, editors. Conditioned taste aversion: Behavioral and neural processes. New York: Oxford University Press; 2009. [Google Scholar]
- Revusky S, Garcia J. Learned aversions over long delays. In: Bower GM, Spence JT, editors. Psychology of learning and motivation: Advances in research and theory. Vol. 4. New York: Academic Press; 1970. pp. 1–84. [Google Scholar]
- Roman C, Lin JY, Reilly S. Conditioned taste aversion and latent inhibition following extensive taste preexposure in rats with insular cortex lesions. Brain Res. 2009;1259:68–73. doi: 10.1016/j.brainres.2008.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman C, Nebieridze N, Sastre A, Reilly S. Effects of lesions of the bed nucleus of the stria terminalis, lateral hypothalamus, or insular cortex on conditioned taste aversion and conditioned odor aversion. Behav Neurosci. 2006;120:1257–1267. doi: 10.1037/0735-7044.120.6.1257. [DOI] [PubMed] [Google Scholar]
- Sakai N, Yamamoto T. Conditioned taste aversion and c-fos expression in the rat brainstem after administration of various USs. Neuroreport. 1997;8:2215–2220. doi: 10.1097/00001756-199707070-00025. [DOI] [PubMed] [Google Scholar]
- Scalera G, Spector AC, Norgren R. Excitotoxic lesions of the parabrachial nuclei prevent conditioned taste aversions and sodium appetite in rats. Behav Neurosci. 1995;109:997–1008. [PubMed] [Google Scholar]
- Scalera G, Grigson PS, Norgren R. Gustatory functions, sodium appetite, and conditioned taste aversion survive excitotoxic lesions of the thalamic taste area. Behav Neurosci. 1997;111:633–645. [PubMed] [Google Scholar]
- Schafe GE, Thiele TE, Bernstein IL. Conditioning method dramatically alters the role of amygdala in taste aversion learning. Learn Mem. 1998;5:481–492. [PMC free article] [PubMed] [Google Scholar]
- Shimai S, Hoshishima K. Effects of bilateral amygdala lesions on neophobia and conditioned taste aversion in mice. Percept Motor Skills. 1982;54:127–130. doi: 10.2466/pms.1982.54.1.127. [DOI] [PubMed] [Google Scholar]
- Solov'eva NA, Lagutina LV, Antonova LV, Anokhin KV. Regulation of c-Fos gene expression in the rat olfactory bulb during olfactory learning. Neurosci Behav Physiol. 2007;37:697–704. doi: 10.1007/s11055-007-0070-z. [DOI] [PubMed] [Google Scholar]
- Spector AC, Norgren R, Grill HJ. Parabrachial gustatory lesions impair taste aversion learning in rats. Behav Neurosci. 1992;106:147–161. doi: 10.1037//0735-7044.106.1.147. [DOI] [PubMed] [Google Scholar]
- St Andre J, Albanos K, Reilly S. C-fos expression in the rat brain following lithium chloride-induced illness. Brain Res. 2007;1135:122–128. doi: 10.1016/j.brainres.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Andre J, Reilly S. Effects of central and basolateral amygdala lesions on conditioned taste aversion and latent inhibition. Behav Neurosci. 2007;121:90–99. doi: 10.1037/0735-7044.121.1.90. [DOI] [PubMed] [Google Scholar]
- Stark JA, Davies KE, Williams SR, Luckman SM. Functional magnetic resonance imaging and c-Fos mapping in rats following an anorectic dose of m-chlorophenylpiperazine. NeuroImage. 2006;31:1228–1237. doi: 10.1016/j.neuroimage.2006.01.046. [DOI] [PubMed] [Google Scholar]
- Swank MW, Bernstein IL. c-Fos induction in response to a conditioned stimulus after single trial taste aversion learning. Brain Res. 1994;636:202–208. doi: 10.1016/0006-8993(94)91018-9. [DOI] [PubMed] [Google Scholar]
- Swank MW, Schafe GE, Bernstein IL. c-Fos induction in response to taste stimuli previously paired with amphetamine or LiCl during taste aversion learning. Brain Res. 1995;673:251–261. doi: 10.1016/0006-8993(94)01421-d. [DOI] [PubMed] [Google Scholar]
- Trifunovic R, Reilly S. Medial versus lateral parabrachial nucleus lesions in the rat: effects on mercaptoacetate-induced feeding and conditioned taste aversion. Brain Res Bull. 2002;58:107–113. doi: 10.1016/s0361-9230(02)00766-9. [DOI] [PubMed] [Google Scholar]
- Wilkins EE, Bernstein IL. Conditioning method determines patterns of c-fos expression following novel taste-illness pairing. Behav Brain Res. 2006;169:93–97. doi: 10.1016/j.bbr.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Sako N, Sakai N, Iwafune A. Gustatory and visceral inputs to the amygdala of the rat: conditioned taste aversion and induction of c-fos-like immunoreactivity. Neurosci Lett. 1997;226:127–130. doi: 10.1016/s0304-3940(97)00265-6. [DOI] [PubMed] [Google Scholar]


