Abstract
Hypoxic (low oxygen) and reperfusion (post-hypoxic reoxygenation) phases of stroke promote an increase in microvascular permeability at tight junctions (TJs) of the blood–brain barrier (BBB) that may lead to cerebral edema. To investigate the effect of hypoxia (Hx) and reoxygenation on oligomeric assemblies of the transmembrane TJ protein occludin, rats were subjected to either normoxia (Nx, 21% O2, 60 min), Hx (6% O2, 60 min), or hypoxia/reoxygenation (H/R, 6% O2, 60 min followed by 21% O2, 10 min). After treatment, cerebral microvessels were isolated, fractionated by detergent-free density gradient centrifugation, and occludin oligomeric assemblies associated with plasma membrane lipid rafts were solubilized by perfluoro-octanoic acid (PFO) exclusively as high molecular weight protein complexes. Analysis by non-reducing and reducing sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis/western blot of PFO-solubilized occludin revealed that occludin oligomeric assemblies co-localizing with ‘TJ-associated’ raft domains contained a high molecular weight ‘structural core’ that was resistant to disassembly by either SDS or a hydrophilic reducing agent ex vivo, and by Hx and H/R conditions in vivo. However, exposure of PFO-solubilized occludin oligomeric assemblies to SDS ex vivo revealed the non-covalent association of a significant amount of dimeric and monomeric occludin isoforms to the disulfide-bonded inner core, and dispersal of these non-covalently attached occludin subunits to lipid rafts of higher density in vivo was differentially promoted by Hx and H/R. Our data suggest a model of isoform interaction within occludin oligomeric assemblies at the BBB that enables occludin to simultaneously perform a structural role in inhibiting paracellular diffusion, and a signaling role involving interactions of dimeric and monomeric occludin isoforms with a variety of regulatory molecules within different plasma membrane lipid raft domains.
Keywords: blood–brain barrier, density gradient, lipid raft, occludin, tight junction
Stroke is the second leading cause of death worldwide (Donnan et al. 2008). On average, every 40 s someone suffers a stroke, and stroke is a leading cause of serious, long-term disability in the United States (http://www.strokeassociation.org). Stroke involves a cerebral blood vessel blockage, the consequence of which is that a particular region of the brain is deprived for a period of time of oxygen and nutrients. During the ischemic (hypoxic) and reperfusion (reoxygenation) phases of stroke there is a breach (i.e., leak) of the blood–brain barrier (BBB) (Sandoval and Witt 2008). The BBB is the critical interface between the CNS and the periphery. Anatomically comprised of approximately 20 m2 of cerebral microvascular endothelial cells (per 1.3 kg brain), the BBB forces water-soluble substances to pass from the systemic circulation to the brain by either a transcellular route (through microvascular endothelial cells) or a paracellular route (between microvascular endothelial cells) (Abbott et al. 2006). Paracellular diffusion of solutes, water, and ions between adjacent microvascular endothelial cells is severely restricted by tight junctions (TJs), and changes in TJ integrity during stroke directly promote the cerebral edema that is a leading cause of death subsequent to ischemic stroke (Bounds et al. 1981; Heo et al. 2005; Sandoval and Witt 2008). TJs are large, multiprotein complexes that extend into the interendothelial space to create a physical barrier to paracellular diffusion. Current understanding of the molecular composition of BBB TJs describes a framework of integral transmembrane proteins that interacts with cytoplasmic accessory, signaling, and regulatory proteins to generate a barrier to paracellular diffusion which is capable of rapid disassembly in response to extracellular stressors, such as pain, inflammation, and hypoxia (Hx) (Huber et al. 2001; Wolka et al. 2003; Hawkins and Davis 2005; Balda and Matter 2008; Forster 2008; Paris et al. 2008).
The transmembrane protein occludin is critical for BBB TJ function (Harhaj and Antonetti 2004; Hawkins and Davis 2005). Its M-shaped topology, characterized by a four transmembrane helix architecture with cytoplasmic N- and C- termini (Furuse et al. 1993; Sanchez-Pulido et al. 2002), facilitates both structural and signaling roles at the BBB. Through its two extracellular loops, it interacts with homologous segments of occludin molecules on adjacent microvascular endothelial cell membranes to enable the fusion of the apposing cell membranes that creates a tight, interendothelial (TJ) seal to restrict paracellular diffusion (Lacaz-Vieira et al. 1999; Feldman et al. 2005). Through its C-terminus, it interacts with accessory proteins, zonula occludens (ZO-1, ZO-2 and ZO-3), thereby establishing a link to the underlying actin cytoskeleton (Furuse et al. 1994; Fanning et al. 1998). Also through its C-terminus, occludin interacts not only with other occludin molecules to form dimers (Blasig et al. 2006) but also with serine–threonine protein kinase C-zeta, tyrosine kinase c-Yes, regulatory (p85) subunit of phosphatidylinositol 3-kinase, gap junction component connexin-26 (Nusrat et al. 2000a), tyrosine kinase c-Src (Elias et al. 2009), casein kinase I epsilon (McKenzie et al. 2006), and casein kinase II (Smales et al. 2003). Other signaling and regulatory proteins reported to interact with occludin include Rab13 (Morimoto et al. 2005), Rho kinase (Yamamoto et al. 2008), caveolin (Nusrat et al. 2000b), clathrin (Ivanov et al. 2004), 33 kDa Vamp-associated protein (Lapierre et al. 1999), protein phosphatases PP2A and PP1 (Seth et al. 2007), E3 ubiquitin-protein ligase itch (Traweger et al. 2002), and transforming growth factor beta receptors I and II (Barrios-Rodiles et al. 2005).
A relationship between microvascular endothelial paracellular permeability and occludin has been demonstrated in numerous in vivo and in vitro studies that employed a variety of Hx model systems and examined occludin localization and/or expression and post-translational modification (Mark and Davis 2002; Witt et al. 2003; Kago et al. 2006; Wang et al. 2007; Bangsow et al. 2008). Hypoxic stress-induced changes in occludin have been shown to be influenced by matrix metalloproteinases (Lohmann et al. 2004; Reijerkerk et al. 2006; Rosenberg and Yang 2007; Yang et al. 2007), hepatocyte growth factor (Date et al. 2006), phospholipase C gamma, phosphatidylinositol 3-kinase and protein kinase G (Fischer et al. 2004), antioxidants (Martin et al. 2006; Xu et al. 2007; Handa et al. 2008), clusterin (Kim et al. 2007), calcium (Park et al. 1999; Brown and Davis 2005), mitogen-activated protein kinase (Kevil et al. 2000; Fischer et al. 2005; Krizbai et al. 2005; Reijerkerk et al. 2008), chemokine (C-C motif) ligand 2 (Dimitrijevic et al. 2006), interleukin-1beta (Bolton et al. 1998; Yamagata et al. 2004), and reactive oxygen species (Kevil et al. 2000; Lee et al. 2004; Schreibelt et al. 2007).
How occludin performs on a molecular level, both structural and signaling roles at the BBB TJ, is incompletely understood. Previously, we demonstrated the use of a neutral pH, detergent-free, isosmotic OptiPrep density gradient method to fractionate intact cerebral microvessels to isolate oligomeric occludin within the context of its normal plasma membrane lipid raft environment (McCaffrey et al. 2007). In this study, we employ this subcellular fractionation technique to investigate the effect of Hx and H/R on occludin oligomeric assemblies at the TJ. Using perfluoro-octanoic acid (PFO) to solubilize occludin oligomeric assemblies so that hydrophobic interactions are maintained (Ramjeesingh et al. 1999; Mitic et al. 2003), we used non-reducing and reducing SDS–PAGE/western blot analysis to investigate if Hx and H/R promote changes in the structural integrity and isoform composition of occludin multiprotein complexes at TJs of the BBB. We incorporated the use of an in vivo rat model of global H/R in which anesthetized animals were subjected for 1 h to an acute, moderate-hypoxic stress (inhaled 6% O2), and then exposed for a brief period (10 min) to normal atmospheric conditions (21% O2). Previous work in our laboratory showed that anesthetized animals subjected to these conditions of global Hx and reoxygenation exhibited increased paracellular permeability (i.e., leak) to [14C]sucrose, vasogenic brain edema, and altered expression of occludin isoforms in cerebral micro-vessel homogenates (Witt et al. 2003, 2008). Our data reveal, for the first time, that treatment of animals with Hx and H/R promotes a dynamic reorganization of occludin oligomeric assemblies in which changes occur in the appearance/disappearance of occludin isoforms of different molecular weights, in the association with different occludin isoforms with lipid rafts of different density, and in the extent of non-covalent (hydrophobic) and covalent (disulfide bond) interactions between occludin subunits within higher order structures. In addition, our data support the hypothesis that occludin oligomeric assemblies at TJs of the BBB in vivo are comprised of a backbone of disulfide-bonded subunits (engaged in a critical structural role) that is adorned with non-covalently attached dimeric and monomeric subunits whose greater accessibility to signaling and regulatory molecules, and ease of disassociation from the parent oligomeric core and subsequent association with different raft domains, allows them to perform a signaling role that incorporates diversity and amplification in the case of hypoxic stress.
Materials and methods
Chemicals and reagents
OptiPrep was purchased from Accurate Chemical (Westbury, NY, USA). EDTA-free Complete Proteinase inhibitor was obtained from Roche (Indianapolis, IN, USA). Criterion XT (10% Bis–Tris) gels, 20× 3-morpholinopropanesulfonic acid running buffer, 4× XT sample loading buffer, 20× XT reducing agent [tris(2-carboxyethyl) phosphine hydrochloride, TCEP], and Precision Plus pre-stained molecular weight markers were obtained from Bio-Rad (Hercules, CA, USA). Polyvinylidene difluoride (PVDF, 0.45 µm) and Western Lightning Chemiluminescence Reagent Plus were purchased from Perkin Elmer (Waltham, MA, USA). Blue autoradiography film was bought from Genesee Scientific (San Diego, CA, USA). Bicinchoninic acid protein assay reagent and albumin standard were purchased from Pierce (Rockford, IL, USA). All other chemicals and reagents were obtained from either Fisher Scientific (Fairlawn, NJ, USA) or Sigma (St. Louis, MO, USA).
Antibodies
Normal goat serum was obtained from DAKO A/S (Glostrup, Denmark). Mouse monoclonal occludin antibody (33-1500), rabbit polyclonal occludin antibody (71-1500), and non-specific mouse IgG1 control antibody (08-6599) were purchased from Zymed (South San Francisco, CA, USA). Monoclonal antibody to platelet endothelial cell adhesion molecule-1 (PECAM-1) was obtained from Serotec (Raleigh, NC, USA), and mouse monoclonal antibody against caveolin-1 (610406) was obtained from BD Transduction Laboratories (San Jose, CA, USA). Alexa-Fluor-488 and 568, and ProLong Gold antifade were obtained from Invitrogen (Carlsbad, CA, USA). The goat polyclonal antibody against Rab13 (sc-30374) and the rabbit polyclonal antibody against nucleoporin (sc-25523) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Animals and treatments
Female Sprague–Dawley rats (250–300 g) were purchased from Harlan Sprague–Dawley (Indianapolis, IN, USA), housed under standard 12 h light/12 h dark conditions, and given food and water ad libitum. Animals were randomly assigned to each treatment group, and all treatment protocols were approved by the University of Arizona Institutional Animal Care and Use Committee and abide by National Institutes of Health guidelines. In vivo hypoxic conditions were created in an oxygen-controlled normobaric Hx chamber (COY Laboratory Products, Grass Lake, MI, USA) as previously described (Witt et al. 2003, 2005, 2008). Rats received 1.0 mL/kg i.p. of anesthetic cocktail (in mg/mL: 78.3 ketamine, 3.1 xylazine, and 0.6 acepromazine). Anesthetized animals were subjected to Nx (21% O2 for 60 min), Hx (6% O2 for 60 min), or H/R (Hx for 60 min followed by Nx for 10 min). Body temperature was maintained at 37°C with a heating pad (Harvard Apparatus, South Watwick, MA, USA). At the end of the treatment, blood was collected from the descending aorta via heparinized tubes (BD Vacutainer; Franklin Lakes, NJ, USA) and immediately analyzed for blood gas and electrolyte concentrations using an ABL77 blood gas analyzer (Radiometer, Copenhagen, Denmark) to validate the degree of hypoxic insult at 6% O2 compared with normoxic controls (n = 6 rats). Animals were then subjected to transcardiac perfusion with 0.9% saline, killed by decapitation, and cerebral microvessels were isolated as previously described (McCaffrey et al. 2007, 2008).
Confocal microscopy
Rat cerebral microvessels were placed on slides, air-dried, and fixed in 100% ethanol for 10 min. Microvessels were washed in phosphate-buffered saline (PBS), followed by 1% bovine serum albumin/0.2% Tween-20 in PBS (buffer 1) and incubated in normal goat serum (1 : 50) in buffer 1 for 30 min. Microvessels were incubated with the polyclonal antibody to occludin (2–5 µg/mL) and monoclonal antibody to PECAM-1 (0.25 µg/mL) for 90 min, washed in buffer 1, and then incubated with purified goat anti-mouse IgG and goat anti-rabbit IgG secondary antibodies conjugated to either Alexa-Fluor-488 or 568 (4 µg/mL) for 1 h in the dark. Finally, microvessels were washed in buffer 1 followed by PBS, and then mounted in ProLong Gold antifade. All incubations were carried out at 22°C. Microvessels were assessed for changes in occludin and PECAM-1 immunoreactivity using a Zeiss LSM 510 laser scanning confocal microscope (Zeiss, Thornwood, NY, USA). Scans were made sequentially through the microvessels and maximum projection images obtained were exported and viewed using Paint Shop Pro 7.0 (Jasc Software, Eden Prairie, MN, USA) and uniformly adjusted to optimize brightness and contrast.
Microvessel fractionation and PAGE/western blot analysis
Rat cerebral microvessels were fractionated by an adaptation of the detergent-free, OptiPrep method of Macdonald and Pike (2005), as previously described (McCaffrey et al. 2007, 2008). A BioComp Gradient Station (BioComp, Fredericton, NB, Canada) was used to collect 15 one mL fractions from the top of the gradient. Fractions were assayed for refractive index, protein content, or mixed with XT sample loading buffer (containing XT reducing agent TCEP) and subjected to SDS–PAGE on 10% Bis-Tris Criterion XT pre-cast gels using 3-morpholinopropanesulfonic acid buffer, electrophoretically transferred to PVDF membranes, probed for Rab13, caveolin, and nucleoporin, and analyzed as previously described (McCaffrey et al. 2007, 2008). Equal volume aliquots of gradient fractions were also incubated at 22°C for 30 min with equal volumes of 2× PFO extraction buffer (100 mM Tris, 4% PFO, pH 8). Following centrifugation (11 000 g for 10 min), supernatants containing PFO-solubilized material were mixed with equal volumes of PFO sample buffer (100 mM Tris, 20% glycerol, 4% PFO, and 0.005% bromophenol blue, pH 8), incubated at 22°C for 30 min, and then subjected to PFO–PAGE on 10% Bis-Tris Criterion XT pre-cast gels using 25 mM Tris, 192 mM glycine, and 0.1% PFO (pH 8.3) (Ramjeesingh et al. 1999; Coyne et al. 2003). Supernatants containing PFO-solubilized material were also mixed with equal volumes of XT sample loading buffer (with or without reducing TCEP), heated for 10 min at 70°C, and subjected to SDS–PAGE (as described above).
Results
In vivo model of hypoxia-reoxygenation
Our laboratory has previously characterized an in vivo rat model of global H/R stress that promotes increased BBB TJ permeability and vasogenic brain edema. In this model, anesthetized animals were subjected to acute Hx (6% O2 for 60 min) followed by a brief period of reoxygenation (21% O2 for 10 min) (Witt et al. 2003, 2005, 2008). In the present study, identical conditions of H/R were used, and for comparison, additional sets of anesthetized animals were subjected either to Nx (21% O2 for 60 min) or Hx alone (6% O2 for 60 min). Immediately following treatment, blood chemistries were determined to provide a measure of the hypoxic insult (Table 1).
Table 1.
Blood gas and electrolyte measurements during hypoxia-reoxygenation treatment
| Treatment | |||
|---|---|---|---|
| Nx | Hx | H/R | |
| pH | 7.42 ± 0.01 | 7.53 ± 0.02 | 7.42 ± 0.02 |
| PCO2 (mmHg) | 41.0 ± 2.5 | 15.8 ± 0.7 | 28.2 ± 2.4 |
| PO2 (mmHg) | 113.7 ± 8.8 | 31.2 ± 1.1 | 106.8 ± 5.0 |
| Na+ (mM) | 138.5 ± 0.7 | 135.3 ± 0.6 | 136.3 ± 0.8 |
| K+ (mM) | 4.27 ± 0.15 | 4.20 ± 0.20 | 3.33 ± 0.08 |
| Ca2+ (mM) | 1.34 ± 0.02 | 1.16 ± 0.01 | 1.22 ± 0.02 |
| Cl− (mM) | 106.7 ± 1.0 | 104.3 ± 1.0 | 105.5 ± 1.1 |
| O2 Saturation (%) | 98.2 ± 0.5 | 70.7 ± 2.4 | 98.6 ± 0.2 |
Anesthetized animals subjected to normoxia (Nx; 21% O2 for 60 min), hypoxia (Hx; 6% O2 for 60 min) or H/R (Hx for 60 min followed by Nx for 10 min), following which blood collected from the descending aorta was analyzed for blood gas and electrolyte concentrations. Data combined from two independent experiments. Values are means ± SE; n = 6 rats.
The partial pressure of O2 decreased 73% during Hx, returning to Nx levels during H/R. The percent saturation of hemoglobin for O2 decreased during Hx from 98% to 71%, returning to 98% with H/R. The partial pressure of CO2 decreased 61% during Hx, returning to 31% below Nx levels during reoxygenation. Blood pH increased from 7.42 ± 0.01 (Nx) to 7.53 ± 0.02 (Hx), then returned to 7.42 ± 0.02 (H/R). Ca2+ concentration decreased approximately 13% during Hx and remained 9% below Nx levels in H/R. The K+ concentration, on the other hand, remained at Nx levels during Hx but decreased 22% during H/R. Na+ and Cl− concentrations did not change significantly during the course of Hx or H/R.
Confocal microscopy of isolated cerebral microvessels
To provide a global assessment of Hx- and H/R-induced changes in localization of the TJ transmembrane protein occludin, cerebral microvessels were isolated from Nx-, Hx-, and H/R-treated animals and analyzed by confocal microscopy following immunostaining for both occludin (using the Zymed 71-1500 polyclonal antibody directed against the C-terminus of occludin; Fig. 1a–c) and PECAM-1 (Fig. 1a–c insets). Occludin staining in cerebral microvessels from Nx-treated animals showed a continuous, sharply defined pattern of a single line marking the paracellular clefts of adjacent PECAM-1 immunoreactive endothelial cells. Hx treatment greatly reduced occludin immunoreactivity within cerebral microvessels, promoting a loss of occludin staining from the paracellular clefts and the fragmenting of occludin staining along the length of the microvessel. H/R treatment increased occludin staining at the paracellular cleft compared with that observed following Hx treatment. Although H/R treatment re-established sharply defined paracellular occludin immunoreactivity along stretches of the microvessel, this was accompanied with the appearance of diffuse occludin immunoreactivity within (and also immediately beneath) the plasma membrane that was punctuated by sites of more intense staining (Fig. 1c). No change was observed in microvascular PECAM-1 staining during Hx or H/R.
Fig. 1.
Hypoxia and post-hypoxic reoxygenation differentially affect occludin localization in isolated cerebral microvessels. Cerebral microvessels isolated from brains of either Nx-, Hx-, or H/R-treated animals were imaged by confocal microscopy following immunostaining for occludin and PECAM-1. Occludin immunoreactivity in normoxic tissue (a) shows a continuous, sharply defined pattern along the length of a cerebral microvessel as visualized by PECAM-1 immunoreactivity (inset). Following 1 h of hypoxic stress, occludin immunoreactivity in the microvessels was greatly reduced (b). After 10 min reoxygenation, occludin immunoreactivity showed greater expression at the paracellular cleft when compared with microvessels from hypoxic-treated rats. In addition there was diffuse cytoplasmic occludin expression within PECAM-1 immunopositive microvessels (c). Data shown are representative of two independent experiments (n = 10 rats). Scale Bar = 10 µm.
Density gradient fractionation of cerebral microvessels
To investigate on a molecular level, the effects of Hx and H/R on occludin oligomeric assemblies at TJs of the BBB, intact cerebral microvessels from Nx-, Hx-, and H/R-treated animals were fractionated by detergent-free OptiPrep density gradient centrifugation. This procedure separated ‘TJ-associated’ plasma membrane lipid rafts co-localizing with occludin multiprotein complexes from non-raft membranous structures and soluble cytoplasmic components (Macdonald and Pike 2005; McCaffrey et al. 2007, 2008). Fractionation of Nx-, Hx-, and H/R microvessel samples produced gradients with almost identical profiles for fraction density and protein content (Fig. 2a). Average fraction density (ρ) increased linearly from 1.042 g/mL (top of gradient) to 1.161 g/mL (bottom of gradient).
Fig. 2.
Density gradient fractionation of cerebral microvessels. Rat cerebral microvessels prepared from brains of either Nx-, Hx-, or H/R-treated animals were subjected to detergent-free density gradient fractionation, following which 15 one mL fractions were collected from the top of the gradient. (a) Graphs showing average density (solid squares) and protein (open circles) for all three treatments. (b) SDS–PAGE/western blots from a representative gradient (for each animal treatment) probed for Rab13 and caveolin-1 (‘TJ-associated’ plasma membrane lipid raft markers) and nucleoporin (nuclear membrane marker). Fraction density and protein data in (a) and western blot data for Rab13 and caveolin-1 in (b) are representative of three independent experiments (n = 15 rats) for each treatment condition. Western blot data for nucleoporin are representative of two independent experiments (n = 10 rats) for each treatment condition.
Screening of density gradient fractions by SDS–PAGE/western blot analysis for Rab13 and caveolin-1 (previously shown to co-localize with ‘TJ-associated’ plasma membrane lipid rafts (McCaffrey et al. 2007, 2008), and nucleoporin (nuclear membrane marker) confirmed the separation of low density, plasma membrane lipid raft domains (fractions 5–10; ρ = 1.067–1.111 g/mL) from higher density, and non-raft membranous structures (fractions 12–15; ρ = 1.121–1.147 g/mL) (Fig. 2b). During steady-state (Nx), staining for Rab13 was restricted to fractions 7 and 8. Hx simultaneously promoted an increase of Rab13 staining in the lower density fractions 5 and 6, and a decrease in Rab13 staining in fractions 7 and 8. H/R reversed this trend, decreasing the amount of Rab13 staining in the lower density fractions and increasing the amount in fractions 7 and 8. Similar to Rab13, caveolin-1 staining was differentially modulated by Hx and H/R, with Hx inducing a decrease in association of this scaffolding protein with plasma membrane lipid rafts and H/R reversing this trend.
PFO–PAGE of solubilized occludin oligomeric assemblies
Previously, our laboratory used subcellular fractionation and SDS–PAGE/western blot analysis of isolated cerebral microvessels to identify the presence of oligomeric (>150 kDa), dimeric (~110–120 kDa), monomeric (53–65 kDa), and low molecular weight (LMW, 37–25 kDa) occludin isoforms within occludin oligomeric assemblies at TJs of the BBB. Treatment of density gradient fractions with the hydrophobic reducing agent 1,2-ethanedithiol (EDT) effectively reduced oligomeric and dimeric occludin isoforms to monomeric and LMW isoforms, confirming a role for hydrophobic disulfide bonds in formation of higher order occludin oligomeric structures (McCaffrey et al. 2007, 2008). In this study, to provide more detailed information about the structural integrity and molecular composition of raft-associated occludin oligomeric assemblies, density gradient fractions were first extracted with PFO, and then the PFO extracts were subjected to either non-reducing PFO–PAGE or non-reducing or reducing SDS–PAGE. Unlike the strongly ionic detergent SDS which breaks non-covalent interactions between proteins and promotes disassembly of multimeric proteins, PFO maintains hydrophobic interactions between subunits of multiprotein complexes and preserves the quaternary structure of multimeric proteins (Ramjeesingh et al. 1999). The optimal concentration of PFO necessary to solubilize occludin was determined by control experiments using equal protein aliquots of microvessel homogenates in which increasing amounts of PFO were used to solubilize occludin. PFO extracts were subjected to reducing SDS–PAGE in the presence of TCEP, and the corresponding western blots were probed with the Zymed 71-1500 antibody to the C-terminus of occludin. As the concentration of PFO increased from 0.5% to 4%, the amount of occludin in the supernatant and corresponding pellet increased and decreased, respectively, and 2% PFO was found sufficient to solubilize all occludin present within microvessel homogenates (data not shown).
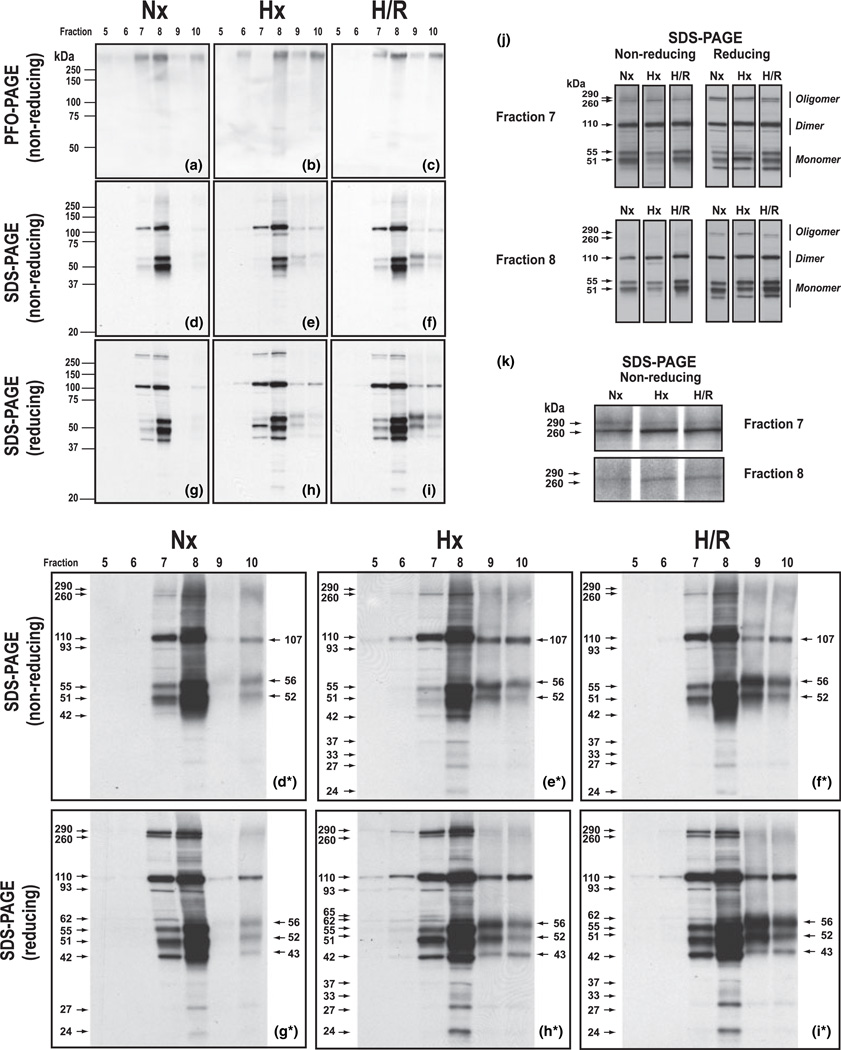
Aliquots of density gradient fractions 5–10 (plasma membrane lipid rafts, ρ = 1.067–1.111 g/mL) solubilized by 2% PFO were subjected to non-reducing PFO–PAGE (Fig. 3a–c) to provide confirmation of the solubilization of occludin as high molecular weight (>250 kDa) multiprotein occludin structures associated with plasma membrane lipid rafts.
Fig. 3.
Hypoxia and reoxygenation reveal plasticity of occludin oligomeric assemblies. Rat cerebral microvessels isolated from brains of either Nx-, Hx-, or H/R-treated animals were subjected to detergent-free, OptiPrep density gradient fractionation, and occludin oligomeric assemblies were extracted from gradient fractions 5–10 (associated with plasma membrane lipid rafts) using 2% PFO and then subjected to non-reducing PFO–PAGE (a–c) or SDS–PAGE in the absence (d–f) or presence (g–i) of the hydrophilic reducing agent tris(2-carboxyethyl)phosphine hydrochloride (TCEP). Western blots were probed with an antibody to the C-terminus of occludin. Prolonged film exposures (d*–i*), side-by-side alignment (j), and enlarged views (k) are included to enhance analysis. Data shown are representative of three independent experiments (n = 15 rats) for each treatment condition.
In Nx samples (Fig. 3a), occludin oligomeric assemblies were prominently detected in the low density fractions 7 and 8 (ρ = 1.081–1.100 g/mL) and to a lesser extent in the higher density fraction 10 (ρ = 1.111 g/mL). Density measurements of western blots revealed that Hx treatment promoted a dramatic reduction in the amount of PFO-solubilized occludin oligomeric assemblies that could be detected in fraction 7 (Fig. 3b), the previously characterized low density ‘TJ-associated’ lipid raft domain (McCaffrey et al. 2007, 2008). H/R treatment increased the detectability of PFO-solubilized oligomeric occludin in fraction 7 (Fig. 3c). Hx treatment also decreased the amount of PFO-solubilized occludin oligomer detectable in fraction 8, concomitantly increasing the amount of PFO-solubilized occludin oligomer detectable in fractions 6, 9, and 10 (Fig. 3b). H/R treatment almost completely restored the amount of PFO-solubilized occludin oligomer present in fraction 8 to its pre-Hx value (Fig. 3c).
Non-reducing SDS–PAGE of PFO-solubilized occludin oligomeric assemblies
To identify Hx- and H/R-induced changes in the type and relative amounts of monomeric occludin isoforms that were attached by non-covalent (hydrophobic) interactions to higher order occludin structures at the TJ and adjacent plasma membrane domains, aliquots of PFO extracts of density gradient fractions 5–10 (plasma membrane lipid rafts, ρ = 1.067–1.111 g/mL) that were visualized by non-reducing PFO–PAGE (Fig. 3a–c) were heated for 10 min at 70°C in the presence of 2% SDS, and then subjected to non-reducing SDS–PAGE. The resultant western blots were probed with the Zymed 71-1500 antibody to the C-terminus of occludin (Fig. 3d–f). Multiple film exposures were taken for each set of blots (containing Nx, Hx, and H/R samples) to enhance detection of less-abundant occludin isoforms, and to ensure that for a particular occludin isoform, qualitative assessment of relative densities could be made within the linear range of the film. Western blots shown in Fig. 3d*–f* represent prolonged film exposures of western blots shown in Fig. 3d–f.
Non-reducing SDS–PAGE/western blot analysis of PFO-solubilized occludin from Nx samples (Fig. 3d) showed that during steady-state, the bulk of detectable occludin was associated with the ‘TJ-associated’ fraction 7 (ρ = 1.081 g/mL) and the slightly higher density fraction 8 (ρ = 1.100 g/mL). Occludin oligomeric assemblies in fractions 7 and 8 were revealed to have appreciable amounts of dimeric (110 kDa) and monomeric (55 and 51 kDa) occludin isoforms attached by SDS-sensitive (hydrophobic) interactions to higher order occludin structures. This finding was more evident in the prolonged film exposure shown in Fig. 3d*, which in addition revealed the presence in fraction 10 of dimeric (107 kDa) and monomeric (56 and 52 kDa) occludin isoforms. To more readily discern relationships between oligomeric, dimeric, and monomeric occludin isoforms within either fraction 7 or 8, Fig. 3j was constructed to show western blots for each fraction separately aligned according to SDS–PAGE conditions (non-reducing and reducing) and treatment conditions (Nx, Hx, and H/R). In addition, Fig. 3k was prepared using enhanced film exposures of western blots of non-reducing SDS–PAGE for fractions 7 and 8 in which the oligomeric (~290 and ~260 kDa) occludin isoforms could more easily be detected. Taken together, Fig. 3d, 3d*, 3j and 3k show that the lower density, more tightly-packed ‘TJ-associated’ lipid rafts (fraction 7) not only had detectable amounts of both the ~290 and ~260 kDa occludin oligomeric isoforms, but also relatively higher oligomeric/dimeric and dimeric/monomeric occludin isoform ratios, than did the higher density, less tightly-packed lipid rafts (fraction 8).
The amount of non-covalently bound, monomeric (55 and 51 kDa) occludin isoforms present in the ‘TJ-associated’ fraction 7 was dramatically reduced by Hx treatment, and this was accompanied by a reduction in the ~290 kDa occludin isoform and an increase in the ~260 kDa occludin isoform (Fig. 3e, 3e*, 3j, 3k). Hx treatment also caused a discernible decrease in the amount of monomeric (55 and 51 kDa) occludin isoforms in fraction 8, and the additional appearance of occludin isoforms at 93 and 42 kDa (Fig. 3e), and at 37, 27, and 24 kDa (Fig. 3e*). Reduced staining of monomeric (55 and 51 kDa) occludin isoforms in the lower density fractions 7 and 8 occurred simultaneously with marked increases in the amount of dimeric (107 kDa) and monomeric (56 and 52 kDa) occludin isoforms detectable in the higher density fractions 9 and 10, and of dimeric (110 kDa) occludin isoforms visible in the lower density fraction 6 (Fig. 3e*). Western blots of non-reducing SDS–PAGE from H/R samples (Fig. 3f, 3f*) showed that H/R treatment dramatically restored the staining of monomeric (55 and 51 kDa) occludin isoforms in the ‘TJ-associated’ fraction 7. Simultaneously, H/R increased the amount of these monomeric isoforms detected in fraction 8, and the amount of the 56 and 52 kDa monomeric isoforms in fractions 9 and 10. The amount of dimeric (110 kDa) occludin isoforms in fraction 6 was reduced by H/R as was the visibility of the 37 kDa occludin isoform in fraction 8. The relative amounts of the presumed dimeric (107 kDa) and monomeric (56 and 52 kDa) occludin isoforms in fractions 9 and 10 were altered such that, especially in fraction 9, a distinct decrease in the dimeric (107 kDa) occludin isoform coincided with a marked increase in the monomeric (56 and 52 kDa) occludin isoforms.
Reducing SDS–PAGE of PFO-solubilized occludin oligomeric assemblies
To identify Hx- and H/R-induced changes in the type and relative amounts of occludin isoforms that were attached by covalent (disulfide bond) interactions to higher order occludin structures at the TJ, replicate aliquots of PFO extracts of density gradient fractions 5–10 were also heated for 10 min at 70°C in the presence of 2% SDS and the hydrophilic reducing agent TCEP, and then subjected to SDS–PAGE/western blot and probed with the Zymed 71-1500 antibody to the C-terminus of occludin (Fig. 3g–i). Western blots shown in Fig. 3g*–i* represent prolonged film exposures of western blots shown in Fig. 3g–i. Reducing SDS–PAGE/western blot analysis of PFO extracts from Nx samples (fraction 7, Fig. 3g) revealed that exposure of ‘TJ-associated’ occludin oligomeric assemblies to the hydrophilic reducing agent TCEP markedly increased the detectability of the oligomeric (~290 and ~260 kDa) occludin isoforms (Fig. 3g* and j). In addition, TCEP reduction slightly increased the staining of the 55 and 51 kDa occludin isoforms (compare Fig. 3g* and d*) and promoted the appearance of 93, 62, and 42 kDa occludin isoforms (Fig. 3g*). In contrast, TCEP reduction of PFO-solubilized occludin oligomeric assemblies associated with higher density lipid rafts (fraction 10) reduced the staining for oligomeric (>250 kDa) occludin isoforms, increased staining for dimeric (110 kDa) occludin isoforms, and induced the appearance of a 43 kDa occludin isoform (Fig. 3g*). Interestingly, following TCEP reduction, all dimeric occludin isoforms detected in fractions 7–10 appeared at the same molecular weight (110 kDa); monomeric (56, 52, and 43 kDa) occludin isoforms were detected only in fraction 10, and LMW (27 and 24 kDa) occludin isoforms were discernible only in fraction 8 (Fig. 3g*).
Reducing SDS–PAGE/western blot analysis of ‘TJ-associated’ PFO-solubilized occludin oligomeric assemblies from Hx samples emphatically increased the staining of monomeric (51 kDa) occludin isoforms (fraction 7, Fig. 3h). Moreover, comparison of non-reducing (fraction 7, Fig. 3e*) and reducing (fraction 7, Fig. 3h*) western blots for TJ-associated’ PFO-solubilized occludin oligomeric assemblies from Hx samples showed that exposure to TCEP enhanced staining for oligomeric (~290 and ~260 kDa) occludin isoforms as well as the staining for occludin isoforms of 93, 65, 62, and 42 kDa. In addition, staining of 55 and 51 kDa occludin isoforms was markedly increased. TCEP reduction of PFO-solubilized oligomeric assemblies from Hx samples also increased staining of oligomeric (~290 kDa) and dimeric (110 kDa) occludin isoforms in fraction 6 and allowed detection of dimeric (110 kDa) occludin isoforms in fraction 5 (Fig 3h*). In contrast, TCEP reduction of PFO-solubilized oligomeric assemblies from Hx samples associated with relatively higher density lipid rafts (fractions 9 and 10) caused simultaneously reduced staining for oligomeric (>250 kDa) occludin isoforms, increased staining for dimeric (110 kDa) and monomeric (56 and 52 kDa) occludin isoforms and the appearance of the 43 kDa occludin isoform (Fig. 3e* and h*). In fraction 8, comparison of non-reducing (Fig. 3e) and reducing (Fig. 3h) western blots for TJ-associated’ PFO-solubilized occludin oligomeric assemblies from Hx samples showed that TCEP reduction not only markedly increased staining for the higher molecular weight oligomeric (~290 kDa) occludin isoform but also caused the disappearance of the dimeric (93 kDa) occludin isoform and the enhanced detection of the monomeric (55, 51, and 42 kDa) occludin isoforms. Moreover, comparison of band densities for monomeric (55, 51, and 42 kDa) occludin isoforms detected in non-reducing (Fig. 3e and e*) and reducing (Fig. 3h and h*) blots for fractions 7 and 8 showed that TCEP reduction of PFO-solubilized occludin oligomeric assemblies from Hx samples produced distinctly different patterns for the ‘TJ-associated’ lipid rafts (fraction 7) and lipid rafts of a slightly higher density (fraction 8).
Reducing SDS–PAGE/western blot analysis of PFO-solubilized occludin oligomeric assemblies from H/R samples greatly increased staining for oligomeric (~290 and ~260 kDa) occludin isoforms in fractions 7 and 8 (compare Fig. 3f* and i*). However, in comparison to Hx samples, in both fractions 7 and 8, staining for the ~290 occludin isoform was decreased, and staining for the ~260 kDa occludin isoform was increased (Fig. 3j). In addition, there was decreased staining of the ~290 kDa occludin isoform in the lower density fraction 6, and increased staining of higher molecular weight (>250 kDa) occludin isoforms in the higher density fractions 9 and 10 (compare Fig. 3h* and i*). Detection of dimeric (110 kDa) occludin isoforms with lower density fractions 5 and 6 was also decreased by H/R, simultaneously with slight increases in detection of dimeric (110 kDa) occludin isoforms in the higher density fractions 9 and 10 (compare Fig. 3h* and i*). TCEP reduction increased staining in fractions 7 and 8 for monomeric (62, 55, 51, and 43 kDa) occludin isoforms, and staining in fractions 9 and 10 for monomeric (56, 52, and 43 kDa) occludin isoforms (compare Fig. 3f* and i*). In addition, TCEP reduction increased staining in fraction 8 for LMW (37, 33, 27, and 24 kDa) occludin isoforms. Comparison of Fig. 3i* and h* showed that although H/R caused the disappearance of the 65 kDa occludin isoform, the staining for LMW (37, 33, 27, and 24 kDa) occludin isoforms was not altered.
Discussion
In this study, we used a non-invasive in vivo rat model of global ischemia to investigate the sensitivity of occludin oligomeric assemblies at TJs of the BBB to acute changes in Hx. Intact cerebral microvessels obtained from animals subjected to either Nx (21% O2 for 60 min), Hx (6% O2 for 60 min), or H/R (6% O2 for 60 min followed by 21% O2 for 10) were fractionated by detergent-free, OptiPrep density gradient centrifugation in order to isolate plasma membrane lipid rafts from non-raft membranous structures and soluble cytoplasmic components. The structural integrity and isoform composition of raft-associated occludin oligomeric assemblies solubilized with PFO was examined by non-reducing and reducing SDS–PAGE. Our data showed for the first time that ‘TJ-associated’ occludin oligomeric assemblies responded to acute changes in oxygen availability with alterations in the type and extent of occludin isoforms attached by non-covalent (hydrophobic) and covalent (disulfide bond) interactions.
Stroke-related brain damage and mortality is a major health concern worldwide (Donnan et al. 2008). Changes in paracellular permeability during the ischemic (hypoxic) and reperfusion (reoxygenation) phases of stroke directly promotes the cerebral edema that leads to increased intracranial pressure and death (Petty and Lo 2002; Sandoval and Witt 2008). Molecular understanding of the mechanism of TJ dysfunction at the BBB during Hx and post-hypoxic reoxygenation is a research priority in the identification of targets suitable for therapeutic manipulation to prevent increases in paracellular permeability and the development of cerebral edema (Neuwelt et al. 2008). To investigate the effect of Hx and H/R on paracellular permeability at the BBB, our laboratory has established an in vivo rat model of global H/R in which anesthetized animals were first subjected for 1 h to an acute, moderate hypoxic stress (inhaled 6% O2), and then exposed for various periods of time to normal atmospheric conditions (21% O2). In situ brain perfusion studies demonstrated a biphasic response to H/R, with significant increases in the uptake of [14C]sucrose into brain parenchyma occurring at 10 min and 6–18 h post-H/R (Witt et al. 2003). Additional capillary depletion, cerebral blood flow, and brain water content (cerebral edema) analyses indicated that the increased BBB permeability was because of increased paracellular diffusion (i.e., leak) through the TJ (Witt et al. 2003, 2008), and western blot analyses of microvessel homogenates showed altered expression of the TJ transmembrane protein occludin (Witt et al. 2003). In the present study, we used our in vivo model of global Hx and reoxygenation model, combined with confocal microscopy and subcellular fractionation of isolated cerebral microvessels, to further investigate the response of occludin to acute changes in oxygen availability.
Blood gas and electrolyte analysis (Table 1) performed immediately following the treatment of anesthetized animals with either Nx (21% O2 for 60 min), Hx (6% O2 for 60 min), or H/R (6% O2 for 60 min followed by 21% O2 for 10 min) confirmed that animals in this study subjected to an acute, moderate-to-severe hypoxic insult showed a classic acute respiratory response to oxygen deprivation, responding in a similar manner to those in previous studies in which in situ brain perfusion studies and cerebral edema measurements revealed significant TJ dysfunction at the BBB (Witt et al. 2003, 2008). Global assessment of occludin localization within microvascular endothelial cells at the BBB in Nx-, Hx-, and H/R-treated animals using confocal microscopy of isolated cerebral microvessels probed with the Zymed 71-1500 polyclonal antibody to the C-terminus of occludin (Fig. 1) demonstrated that a unique pattern for occludin immunostaining was associated with each treatment. Whereas a clearly-defined, continuous pattern of occludin immunostaining was observed in cerebral microvessels isolated from Nx-treated animals, Hx treatment greatly reduced occludin immunoreactivity to the extent that it was only weakly present in an ill-defined, fragmented fashion along the length of the microvessel. H/R treatment promoted a dramatic increase in microvascular occludin immunoreactivity, causing the reappearance of extended regions of sharply defined paracellular occludin immunoreactivity and, in addition, the irregular appearance of isolated spots of relatively more intense occludin immunoreactivity within regions of the plasma membrane and directly adjacent cytoplasm. Taken together, blood chemistry and confocal microscopy data demonstrated that Nx, Hx, and H/R represent three distinct physiological states, each of which exhibited a unique pattern of occludin localization within endothelial microvessels. Our finding of decreased occludin staining at the endothelial cell plasma membrane during hypoxic stress was in agreement with previous reports of decreased microvascular endothelial occludin immunoreactivity following hypoxic stress (Mark and Davis 2002; Brown and Davis 2005; Date et al. 2006; Wang et al. 2007; Bangsow et al. 2008).
Recently, we have characterized a neutral pH, detergent-free, isosmotic OptiPrep density gradient centrifugation method to fractionate intact cerebral microvessels so as to separate low density plasma membrane lipid rafts from higher density non-raft plasma membrane domains and subcellular membranous components. Absence of detergent in this fractionation procedure enabled the isolation of TJ-associated occludin within the context of its normal plasma membrane lipid raft environment. Western blots of density gradient fractions (subjected to reducing SDS–PAGE with a hydrophilic reducing agent) when probed for occludin using an antibody directed to the C-terminus, revealed a complex distribution profile within microvascular endothelial plasma membrane lipid rafts of different density of oligomeric, dimeric, monomeric, and LMW occludin isoforms ranging in molecular weight from approximately 25 to 300 kDa. Use of the strong hydrophobic reducing agent EDT during SDS–PAGE was shown to significantly reduce oligomeric (>250 kDa) and dimeric (~100–120 kDa) occludin isoforms and simultaneously increase the amount of monomeric isoforms. By so doing, EDT reduction revealed the importance of disulfide bonds within hydrophobic regions of occludin in the maintenance of higher order occludin structures (McCaffrey et al. 2007). In a follow-up study, density gradient fractionation of isolated cerebral microvessels, combined with both reducing and non-reducing SDS– PAGE/western blot analysis, showed the stress of 3 h of peripheral inflammatory hyperalgesia provoked a disruption of disulfide-bonded occludin oligomeric assemblies and a redistribution of occludin isoforms within lipid raft domains of different density (McCaffrey et al. 2008). In the current study, following density gradient fractionation of isolated cerebral microvessels to isolate plasma membrane lipid rafts, we investigated if intact occludin oligomeric assemblies could be solubilized from lipid rafts with PFO. PFO is a mild ionic detergent that has been used to solubilize multimeric protein complexes of human ATP-binding cassette G2 (Xu et al. 2004), vanilloid receptor 1 (Kedei et al. 2001), and the TJ transmembrane protein claudin-4 (Mitic et al. 2003). Effective solubilization of occludin was achieved with 2% PFO, and PFO-solubilized occludin oligomeric assemblies were visualized exclusively as high molecular weight (>250 kDa) structures by non-reducing PFO–PAGE/western blot (using the Zymed 71-1500 C-terminal occludin antibody).
Non-reducing PFO–PAGE of PFO extracts showed that the distribution of occludin among plasma membrane lipid rafts (density gradient fractions 5–10; ρ = 1.067–1.111 g/mL) was differentially modulated by Nx, Hx, and H/R (Fig. 3a–c). During steady-state (Nx), the majority of oligomeric occludin was associated with plasma membrane lipid rafts of density ρ = 1.081–1.100 g/mL (fractions 7 and 8; Fig. 3a). This finding was in agreement with density gradient fraction screening (Fig. 2b) which showed a similar concentration within fractions 7 and 8 of the intact TJ markers, Rab13 and oligomeric caveolin-1. Similar to confocal microscopy data (Fig. 1), Hx caused a dramatic reduction of ‘TJ-associated’ PFO-solubilized occludin oligomeric assemblies (Fig. 3b), and H/R substantially (but not completely) reversed this (Fig. 3c). Evidence that staining for Rab13 and caveolin-1 within fractions 7 and 8 was decreased by Hx and increased by H/R (Fig. 2b) provided corroboration that during these two physiological states there was differential remodeling of low density plasma membrane lipid rafts associated with TJs.
The rapid reappearance of occludin immunoreactivity following H/R treatment (Figs. 1 and 3c), which differed from Hx treatment by the addition of only 10 min of ambient air, cannot be accounted for by de novo occludin synthesis. Consequently, decreased occludin staining in Hx samples is not believed the result of significant in situ proteolysis. Because the intensity of protein staining in western blot analysis is directly proportional to the extent of antigen binding by the probing antibody, decreased occludin immunoreactivity may reflect either a loss of protein or a change in protein conformation which effectively masks antigenic sites. To begin to address this issue, we used non-reducing and reducing SDS–PAGE/western blot analysis of PFO-solubilized occludin to examine the effect of Hx and H/R on the extent and type of occludin isoforms bound to higher order structures by non-covalent (hydrophobic) and covalent (disulfide bond) interactions. PFO extracts of density gradient fractions 5–10 from Nx samples, upon being analyzed by either non-reducing (Fig. 3d and d*) or reducing (Fig. 3g and g*) SDS–PAGE, revealed that PFO-solubilized occludin oligomeric assemblies associated with plasma membrane lipid rafts of different densities were differently sensitive to SDS and SDS + TCEP because unique profiles for occludin isoforms ranging in molecular weight from 24 to ~290 kDa were observed. Hx and H/R differentially altered the profiles of non-covalent and covalently bound occludin isoforms for different plasma membrane lipid raft domains (Fig. 3h, i, h* and i*). Comprehensive analysis of non-reducing and reducing SDS–PAGE/western blot data enabled us to hypothesize that occludin oligomeric assemblies at TJs of the BBB contained both an inner core of covalently attached occludin subunits (>250 kDa) and an outer shell of non-covalently attached occludin subunits (ranging in molecular weight from 24 to 110 kDa) (Fig. 4).
Fig. 4.
Form defines function: proposed model of isoform interaction within occludin oligomeric assemblies enables performance of both structural and signaling roles. Occludin oligomeric assemblies at TJ of the BBB are hypothesized to be comprised an inner ‘structural core’ of covalently bonded subunits that is adorned with a variety of non-covalently attached monomeric and dimeric subunits. Change in conformation of selected subunits within the inner core (perhaps because of limited disruption of disulfide bonds between occludin molecules on different sides of the paracellular cleft) may cause not only a breach in the transmembrane protein diffusion barrier but also conformational changes in selected non-covalently bound subunits that may render the latter more accessible to signaling and regulatory molecules and/or more readily disassociated from the parent oligomeric occludin structure. Trafficking of occludin isoforms away from ‘TJ-associated’ lipid rafts to other plasma membrane raft domains increases the exposure of occludin to different signaling and regulatory molecules.
Focusing on ‘TJ-associated’ occludin oligomeric assemblies (fraction 7, ρ = 1.081–1.100 g/mL), our data revealed the presence of a core oligomeric structure comprised two major components at ~260 and ~290 kDa that was remarkably resistant to disruption by not only SDS or SDS + TCEP, but also Hx- and H/R stress. Evidence of this structural resilience was seen in the comparatively slight changes in staining intensities for oligomeric occludin (in either non-reducing or reducing SDS–PAGE) in Nx, Hx, and H/R samples. Occludin oligomeric structural stability was also affirmed by the fact that exposure of PFO-solubilized oligomeric occludin assemblies to SDS + TCEP caused a marked increase (not decrease) in immunoreactivity at the same molecular weights (~290 and ~260 kDa), which revealed that although conformational changes were induced (involving reduction of component disulfide bonds) which unmasked antigenic sites within the C-terminus of occludin, the integrity of the high molecular weight structures was not impaired to the extent that the overall complex was forcibly collapsed into LMW subunits. In contrast to oligomeric occludin isoform staining, dramatic changes in staining of non-covalently bound monomeric isoforms was caused not only by SDS and SDS + TCEP, but also by Hx and H/R. Comparison of non-reducing (Fig. 3d and d*) and reducing (Fig. 3g and g*) SDS–PAGE/western blot analysis of ‘TJ-associated’ PFO-solubilized occludin oligomeric assemblies from Nx samples showed that whereas a subset of non-covalently bound dimeric (110 kDa) and monomeric (55 and 51 kDa) occludin isoforms could readily be disassociated from higher order structures by SDS, another subset of dimeric (110 and 93 kDa) and monomeric (62, 55, 51, and 42 kDa) occludin isoforms attached by covalent (hydrophobic) interactions could be removed by SDS + TCEP. Comparison of non-reducing SDS–PAGE of ‘TJ-associated’ PFO-solubilized occludin oligomeric assemblies (Fraction 7) from Hx (Fig. 3e and e*) and H/R (Fig. 3f and f*) samples dramatically revealed that Hx and H/R caused the apparent disappearance and reappearance, respectively, of a subset of monomeric (55 and 51 kDa) occludin isoforms attached by non-covalent (hydrophobic) interactions. Assuming that this subset of non-covalently bound occludin monomeric subunits had C-termini that was not involved in coiled coil and/or disulfide bridge interactions and therefore accessible to a C-terminal occludin antibody during western blot analysis, their absence (during Hx) may contribute to diminished occludin immunoreactivity at the TJ. Accessibility of the C-termini of this subset of non-covalently bound monomeric occludin subunits to interaction with specific regulatory and/or signaling molecules could also lead to changes in post-translational modification (such as phosphorylation) that result in conformational changes and reassociation with plasma membrane lipid rafts of higher density. Comparison of profiles for monomeric occludin isoforms in fractions 7–10 in non-reducing SDS–PAGE (Fig. 3e and e*) showed that a decrease in staining for 55 and 51 kDa occludin isoforms in the lower density fractions 7 and 8 occurred simultaneously with an increase in staining for 56 and 52 kDa occludin isoforms in the higher density fractions 9 and 10. Taken together, these data suggested that during Hx there occurred conformational changes and/or disulfide bond disruptions in the underlying structural core of oligomeric occludin subunits (evidenced by increased immunoreactivity of oligomeric occludin), and post-translational modifications of non-covalently attached 55 and 51 kDa monomeric occludin subunits (e.g., phosphorylation), the end result of which there was a reassociation of modified occludin subunits to higher density plasma membrane lipid raft domains. In addition, removal of an outer shell of closely attached monomeric occludin subunits appeared to increase the vulnerability of the occludin oligomeric assembly to SDS + TCEP, because the latter was able to disassociate a comparatively greater amount of monomeric (65, 62, 55, 51, and 42 kDa) occludin isoforms (compare Fig. 3g*/3d* with Fig. 3h* and e*). Similar to SDS + TCEP, H/R induced disulfide bond breakage within ‘TJ-associated’ occludin oligomeric assemblies, resulting in the generation of additional non-covalently attached monomeric (55 and 51 kDa) occludin subunits that could enable enhanced occludin immunoreactivity (during western blot analysis) and interact with additional regulatory/signaling molecules to intensify occludin signaling pathways.
In summary, we used density gradient fractionation of isolated cerebral microvessels, combined with PFO- and SDS–PAGE/western blot analysis of PFO-solubilized occludin oligomeric assemblies to investigate if acute changes in oxygen availability (i.e., Hx) affect occludin oligomeric assemblies at TJs of the BBB. Our data confirm that each physiological state or stressor (Nx, Hx, and H/R) differentially affects the organization of occludin isoforms within plasma membrane lipid rafts of different densities. ‘TJ-associated’ occludin oligomeric assemblies were revealed to contain a backbone core of high molecular weight, disulfide-bonded units (believed to be engaged in a structural role) that is adorned with a variety of non-covalently attached, LMW occludin isoforms which has C-termini that is readily accessible to interact with regulatory and/or signaling molecules. These exciting data which supported a model for ‘TJ-associated’ occludin oligomeric assemblies that incorporates both stable and mobile elements were in agreement with a recent assessment of TJ protein dynamics using live-cell imaging in epithelial cells which showed that approximately 70% of occludin diffused rapidly within the TJ during steady-state (Shen et al. 2008). Future studies involving detailed investigation of the effect of hypoxic and reoxygenation stress on protein–protein interactions of particular occludin isoforms will reveal how different signaling and regulatory molecules modulate occludin oligomeric assemblies to promote changes in TJ integrity at the BBB.
Acknowledgements
This work was supported by NIH/NINDS grants RO1 NS-39592 to TPD and CA 09820-0251 to GM. The authors thank Dr. Patrick Ronaldson and Dr. Robert Kuester for their helpful advice on this manuscript.
Abbreviations used
- BBB
blood–brain barrier
- EDT
1,2-ethanedithiol
- H/R
hypoxia reoxygenation
- Hx
hypoxia
- LMW
low molecular weight
- Nx
normoxia
- PECAM-1
platelet endothelial cell adhesion molecule-1
- PFO
perfluoro-octanoic acid
- SDS–PAGE
sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- TCEP
tris(2-carboxyethyl)phosphine hydrochloride
- TJ
tight junction
- ZO-1
zonula occludens-1
References
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Balda MS, Matter K. Tight junctions at a glance. J. Cell Sci. 2008;121:3677–3682. doi: 10.1242/jcs.023887. [DOI] [PubMed] [Google Scholar]
- Bangsow T, Baumann E, Bangsow C, Jaeger MH, Pelzer B, Gruhn P, Wolf S, von Melchner H, Stanimirovic DB. The epithelial membrane protein 1 is a novel tight junction protein of the blood-brain barrier. J. Cereb. Blood Flow Metab. 2008;28:1249–1260. doi: 10.1038/jcbfm.2008.19. [DOI] [PubMed] [Google Scholar]
- Barrios-Rodiles M, Brown KR, Ozdamar B, et al. High-throughput mapping of a dynamic signaling network in mammalian cells. Science (New York, NY) 2005;307:1621–1625. doi: 10.1126/science.1105776. [DOI] [PubMed] [Google Scholar]
- Blasig IE, Winkler L, Lassowski B, et al. On the self-association potential of transmembrane tight junction proteins. Cell. Mol. Life Sci. 2006;63:505–514. doi: 10.1007/s00018-005-5472-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton SJ, Anthony DC, Perry VH. Loss of the tight junction proteins occludin and zonula occludens-1 from cerebral vascular endothelium during neutrophil-induced blood-brain barrier breakdown in vivo. Neuroscience. 1998;86:1245–1257. doi: 10.1016/s0306-4522(98)00058-x. [DOI] [PubMed] [Google Scholar]
- Bounds JV, Wiebers DO, Whisnant JP, Okazaki H. Mechanisms and timing of deaths from cerebral infarction. Stroke. 1981;12:474–477. doi: 10.1161/01.str.12.4.474. [DOI] [PubMed] [Google Scholar]
- Brown RC, Davis TP. Hypoxia/aglycemia alters expression of occludin and actin in brain endothelial cells. Biochem. Biophys. Res. Commun. 2005;327:1114–1123. doi: 10.1016/j.bbrc.2004.12.123. [DOI] [PubMed] [Google Scholar]
- Coyne CB, Gambling TM, Boucher RC, Carson JL, Johnson LG. Role of claudin interactions in airway tight junctional permeability. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003;285:L1166–L1178. doi: 10.1152/ajplung.00182.2003. [DOI] [PubMed] [Google Scholar]
- Date I, Takagi N, Takagi K, Tanonaka K, Funakoshi H, Matsumoto K, Nakamura T, Takeo S. Hepatocyte growth factor attenuates cerebral ischemia-induced increase in permeability of the blood-brain barrier and decreases in expression of tight junctional proteins in cerebral vessels. Neurosci. Lett. 2006;407:141–145. doi: 10.1016/j.neulet.2006.08.050. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic OB, Stamatovic SM, Keep RF, Andjelkovic AV. Effects of the chemokine CCL2 on blood-brain barrier permeability during ischemia-reperfusion injury. J. Cereb. Blood Flow Metab. 2006;26:797–810. doi: 10.1038/sj.jcbfm.9600229. [DOI] [PubMed] [Google Scholar]
- Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- Elias BC, Suzuki T, Seth A, et al. Phosphorylation of Tyr-398 and Tyr-402 in occludin prevents its interaction with ZO-1 and destabilizes its assembly at the tight junctions. J. Biol. Chem. 2009;284:1559–1569. doi: 10.1074/jbc.M804783200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J. Biol. Chem. 1998;273:29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- Feldman GJ, Mullin JM, Ryan MP. Occludin: structure, function and regulation. Adv. Drug Deliv. Rev. 2005;57:883–917. doi: 10.1016/j.addr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Fischer S, Wiesnet M, Marti HH, Renz D, Schaper W. Simultaneous activation of several second messengers in hypoxia-induced hyperpermeability of brain derived endothelial cells. J. Cell. Physiol. 2004;198:359–369. doi: 10.1002/jcp.10417. [DOI] [PubMed] [Google Scholar]
- Fischer S, Wiesnet M, Renz D, Schaper W. H2O2 induces paracellular permeability of porcine brain-derived microvascular endothelial cells by activation of the p44/42 MAP kinase pathway. Eur. J. Cell Biol. 2005;84:687–697. doi: 10.1016/j.ejcb.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Forster C. Tight junctions and the modulation of barrier function in disease. Histochem. Cell Biol. 2008;130:55–70. doi: 10.1007/s00418-008-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J. Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Itoh M, Hirase T, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J. Cell Biol. 1994;127:1617–1626. doi: 10.1083/jcb.127.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa O, Stephen J, Cepinskas G. Role of endothelial nitric oxide synthase-derived nitric oxide in activation and dysfunction of cerebrovascular endothelial cells during early onsets of sepsis. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H1712–H1719. doi: 10.1152/ajpheart.00476.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harhaj NS, Antonetti DA. Regulation of tight junctions and loss of barrier function in pathophysiology. Int. J. Biochem. Cell Biol. 2004;36:1206–1237. doi: 10.1016/j.biocel.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Heo JH, Han SW, Lee SK. Free radicals as triggers of brain edema formation after stroke. Free Radic. Biol. Med. 2005;39:51–70. doi: 10.1016/j.freeradbiomed.2005.03.035. [DOI] [PubMed] [Google Scholar]
- Huber JD, Egleton RD, Davis TP. Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neurosci. 2001;24:719–725. doi: 10.1016/s0166-2236(00)02004-x. [DOI] [PubMed] [Google Scholar]
- Ivanov AI, Nusrat A, Parkos CA. Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment. Mol. Biol. Cell. 2004;15:176–188. doi: 10.1091/mbc.E03-05-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kago T, Takagi N, Date I, Takenaga Y, Takagi K, Takeo S. Cerebral ischemia enhances tyrosine phosphorylation of occludin in brain capillaries. Biochem. Biophys. Res. Commun. 2006;339:1197–1203. doi: 10.1016/j.bbrc.2005.11.133. [DOI] [PubMed] [Google Scholar]
- Kedei N, Szabo T, Lile JD, Treanor JJ, Olah Z, Iadarola MJ, Blumberg PM. Analysis of the native quaternary structure of vanilloid receptor 1. J. Biol. Chem. 2001;276:28613–28619. doi: 10.1074/jbc.M103272200. [DOI] [PubMed] [Google Scholar]
- Kevil CG, Oshima T, Alexander B, Coe LL, Alexander JS. H(2)O(2)-mediated permeability: role of MAPK and occludin. Am. J. Physiol. 2000;279:C21–C30. doi: 10.1152/ajpcell.2000.279.1.C21. [DOI] [PubMed] [Google Scholar]
- Kim JH, Yu YS, Kim JH, Kim KW, Min BH. The role of clusterin in in vitro ischemia of human retinal endothelial cells. Curr. Eye Res. 2007;32:693–698. doi: 10.1080/02713680701487871. [DOI] [PubMed] [Google Scholar]
- Krizbai IA, Bauer H, Bresgen N, Eckl PM, Farkas A, Szatmari E, Traweger A, Wejksza K, Bauer HC. Effect of oxidative stress on the junctional proteins of cultured cerebral endothelial cells. Cell. Mol. Neurobiol. 2005;25:129–139. doi: 10.1007/s10571-004-1378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacaz-Vieira F, Jaeger MM, Farshori P, Kachar B. Small synthetic peptides homologous to segments of the first external loop of occludin impair tight junction resealing. J. Membr. Biol. 1999;168:289–297. doi: 10.1007/s002329900518. [DOI] [PubMed] [Google Scholar]
- Lapierre LA, Tuma PL, Navarre J, Goldenring JR, Anderson JM. VAP-33 localizes to both an intracellular vesicle population and with occludin at the tight junction. J. Cell Sci. 1999;112(Pt 21):3723–3732. doi: 10.1242/jcs.112.21.3723. [DOI] [PubMed] [Google Scholar]
- Lee HS, Namkoong K, Kim DH, Kim KJ, Cheong YH, Kim SS, Lee WB, Kim KY. Hydrogen peroxide-induced alterations of tight junction proteins in bovine brain microvascular endothelial cells. Microvasc. Res. 2004;68:231–238. doi: 10.1016/j.mvr.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Lohmann C, Krischke M, Wegener J, Galla HJ. Tyrosine phosphatase inhibition induces loss of blood–brain barrier integrity by matrix metalloproteinase-dependent and -independent pathways. Brain Res. 2004;995:184–196. doi: 10.1016/j.brainres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Macdonald JL, Pike LJ. A simplified method for the preparation of detergent-free lipid rafts. J. Lipid Res. 2005;46:1061–1067. doi: 10.1194/jlr.D400041-JLR200. [DOI] [PubMed] [Google Scholar]
- Mark KS, Davis TP. Cerebral microvascular changes in~permeability and tight junctions induced by hypoxiareoxygenation. Am. J. Physiol. Heart Circ. Physiol. 2002;282:H1485–H1494. doi: 10.1152/ajpheart.00645.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TA, Das T, Mansel RE, Jiang WG. Synergistic regulation of endothelial tight junctions by antioxidant (Se) and polyunsaturated lipid (GLA) via Claudin-5 modulation. J. Cell. Biochem. 2006;98:1308–1319. doi: 10.1002/jcb.20860. [DOI] [PubMed] [Google Scholar]
- McCaffrey G, Staatz WD, Quigley CA, Nametz N, Seelbach MJ, Campos CR, Brooks TA, Egleton RD, Davis TP. Tight junctions contain oligomeric protein assembly critical for maintaining blood–brain barrier integrity in vivo. J. Neurochem. 2007;103:2540–2555. doi: 10.1111/j.1471-4159.2007.04943.x. [DOI] [PubMed] [Google Scholar]
- McCaffrey G, Seelbach MJ, Staatz WD, Nametz N, Quigley C, Campos CR, Brooks TA, Davis TP. Occludin oligomeric assembly at tight junctions of the blood–brain barrier is disrupted by peripheral inflammatory hyperalgesia. J. Neurochem. 2008;106:2395–2409. doi: 10.1111/j.1471-4159.2008.05582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie JA, Riento K, Ridley AJ. Casein kinase I epsilon associates with and phosphorylates the tight junction protein occludin. FEBS Lett. 2006;580:2388–2394. doi: 10.1016/j.febslet.2006.03.048. [DOI] [PubMed] [Google Scholar]
- Mitic LL, Unger VM, Anderson JM. Expression, solubilization, and biochemical characterization of the tight junction transmembrane protein claudin-4. Protein Sci. 2003;12:218–227. doi: 10.1110/ps.0233903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto S, Nishimura N, Terai T, et al. Rab13 mediates the continuous endocytic recycling of occludin to the cell surface. J. Biol. Chem. 2005;280:2220–2228. doi: 10.1074/jbc.M406906200. [DOI] [PubMed] [Google Scholar]
- Neuwelt E, Abbott NJ, Abrey L, et al. Strategies to advance~translational research into brain barriers. Lancet Neurol. 2008;7:84–96. doi: 10.1016/S1474-4422(07)70326-5. [DOI] [PubMed] [Google Scholar]
- Nusrat A, Chen JA, Foley CS, Liang TW, Tom J, Cromwell M, Quan C, Mrsny RJ. The coiled-coil domain of occludin can act to organize structural and functional elements of the epithelial tight junction. J. Biol. Chem. 2000a;275:29816–29822. doi: 10.1074/jbc.M002450200. [DOI] [PubMed] [Google Scholar]
- Nusrat A, Parkos CA, Verkade P, Foley CS, Liang TW, Innis-Whitehouse W, Eastburn KK, Madara JL. Tight junctions are membrane microdomains. J. Cell Sci. 2000b;11310(Pt):1771–1781. doi: 10.1242/jcs.113.10.1771. [DOI] [PubMed] [Google Scholar]
- Paris L, Tonutti L, Vannini C, Bazzoni G. Structural organization of the tight junctions. Biochim. Biophys. Acta. 2008;1778:646–659. doi: 10.1016/j.bbamem.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Park JH, Okayama N, Gute D, Krsmanovic A, Battarbee H, Alexander JS. Hypoxia/aglycemia increases endothelial permeability: role of second messengers and cytoskeleton. Am. J. Physiol. 1999;277:C1066–C1074. doi: 10.1152/ajpcell.1999.277.6.C1066. [DOI] [PubMed] [Google Scholar]
- Petty MA, Lo EH. Junctional complexes of the blood–brain barrier: permeability changes in neuroinflammation. Prog. Neurobiol. 2002;68:311–323. doi: 10.1016/s0301-0082(02)00128-4. [DOI] [PubMed] [Google Scholar]
- Ramjeesingh M, Huan LJ, Garami E, Bear CE. Novel method for evaluation of the oligomeric structure of membrane proteins. Biochem. J. 1999;342(Pt 1):119–123. [PMC free article] [PubMed] [Google Scholar]
- Reijerkerk A, Kooij G, van der Pol SM, Khazen S, Dijkstra CD, de Vries HE. Diapedesis of monocytes is associated with MMP-mediated occludin disappearance in brain endothelial cells. FASEB. J. 2006;20:2550–2552. doi: 10.1096/fj.06-6099fje. [DOI] [PubMed] [Google Scholar]
- Reijerkerk A, Kooij G, van der Pol SM, Leyen T, van Het Hof B, Couraud PO, Vivien D, Dijkstra CD, de Vries HE. Tissue-type plasminogen activator is a regulator of monocyte diapedesis through the brain endothelial barrier. J. Immunol. 2008;181:3567–3574. doi: 10.4049/jimmunol.181.5.3567. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Yang Y. Vasogenic edema due to tight junction disruption by matrix metalloproteinases in cerebral ischemia. Neurosurg. Focus. 2007;22:E4. doi: 10.3171/foc.2007.22.5.5. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pulido L, Martin-Belmonte F, Valencia A, Alonso MA. MARVEL: a conserved domain involved in membrane apposition events. Trends Biochem. Sci. 2002;27:599–601. doi: 10.1016/s0968-0004(02)02229-6. [DOI] [PubMed] [Google Scholar]
- Sandoval KE, Witt KA. Blood–brain barrier tight junction permeability and ischemic stroke. Neurobiol. Dis. 2008;32:200–219. doi: 10.1016/j.nbd.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Schreibelt G, Kooij G, Reijerkerk A, et al. Reactive oxygen species alter brain endothelial tight junction dynamics via RhoA, PI3 kinase, and PKB signaling. FASEB. J. 2007;21:3666–3676. doi: 10.1096/fj.07-8329com. [DOI] [PubMed] [Google Scholar]
- Seth A, Sheth P, Elias BC, Rao R. Protein Phosphatases 2A and 1 interact with occludin and negatively regulate the assembly of tight junctions in the CACO-2 cell monolayer. J. Biol. Chem. 2007;282:11487–11498. doi: 10.1074/jbc.M610597200. [DOI] [PubMed] [Google Scholar]
- Shen L, Weber CR, Turner JR. The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady-state. J. Cell Biol. 2008;181:683–695. doi: 10.1083/jcb.200711165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smales C, Ellis M, Baumber R, Hussain N, Desmond H, Staddon JM. Occludin phosphorylation: identification of an occludin kinase in brain and cell extracts as CK2. FEBS Lett. 2003;545:161–166. doi: 10.1016/s0014-5793(03)00525-8. [DOI] [PubMed] [Google Scholar]
- Traweger A, Fang D, Liu YC, Stelzhammer W, Krizbai IA, Fresser F, Bauer HC, Bauer H. The tight junction-specific protein occludin is a functional target of the E3 ubiquitin-protein ligase itch. J. Biol. Chem. 2002;277:10201–10208. doi: 10.1074/jbc.M111384200. [DOI] [PubMed] [Google Scholar]
- Wang YL, Hui YN, Guo Band, Ma JX. Strengthening tight junctions of retinal microvascular endothelial cells by pericytes under normoxia and hypoxia involving angiopoietin-1 signal way. Eye (London, England) 2007;21:1501–1510. doi: 10.1038/sj.eye.6702716. [DOI] [PubMed] [Google Scholar]
- Witt KA, Mark KS, Hom S, Davis TP. Effects of hypoxia-reoxygenation on rat blood–brain barrier permeability and tight junctional protein expression. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H2820–H2831. doi: 10.1152/ajpheart.00589.2003. [DOI] [PubMed] [Google Scholar]
- Witt KA, Mark KS, Huber J, Davis TP. Hypoxia-inducible factor and nuclear factor kappa-B activation in blood–brain barrier endothelium under hypoxic/reoxygenation stress. J. Neurochem. 2005;92:203–214. doi: 10.1111/j.1471-4159.2004.02871.x. [DOI] [PubMed] [Google Scholar]
- Witt KA, Mark KS, Sandoval KE, Davis TP. Reoxygenation stress on blood–brain barrier paracellular permeability and edema in the rat. Microvasc. Res. 2008;75:91–96. doi: 10.1016/j.mvr.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolka AM, Huber JD, Davis TP. Pain and the blood–brain barrier: obstacles to drug delivery. Adv. Drug Deliv. Rev. 2003;55:987–1006. doi: 10.1016/s0169-409x(03)00100-5. [DOI] [PubMed] [Google Scholar]
- Xu J, Liu Y, Yang Y, Bates S, Zhang JT. Characterization of oligomeric human half-ABC transporter ATP-binding cassette G2. J. Biol. Chem. 2004;279:19781–19789. doi: 10.1074/jbc.M310785200. [DOI] [PubMed] [Google Scholar]
- Xu Y, Gong B, Yang Y, Awasthi YC, Woods M, Boor PJ. Glutathione-S-Transferase protects against oxidative injury of endothelial cell tight junctions. Endothelium. 2007;14:333–343. doi: 10.1080/10623320701746263. [DOI] [PubMed] [Google Scholar]
- Yamagata K, Tagami M, Takenaga F, Yamori Y, Itoh S. Hypoxia-induced changes in tight junction permeability of brain capillary endothelial cells are associated with IL-1beta and nitric oxide. Neurobiol. Dis. 2004;17:491–499. doi: 10.1016/j.nbd.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Ramirez SH, Sato S, Kiyota T, Cerny RL, Kaibuchi K, Persidsky Y, Ikezu T. Phosphorylation of claudin-5 and occludin by rho kinase in brain endothelial cells. Am. J. Pathol. 2008;172:521–533. doi: 10.2353/ajpath.2008.070076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J. Cereb. Blood Flow Metab. 2007;27:697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]