Abstract
Bone marrow–derived mesenchymal stem cells (MSC) are a potential attractive source of cells for stem cell–based tissue regeneration, but the small number and reduced capabilities of MSC proliferation and differentiation due to in vitro replicative senescence and donor-associated pathophysiological factors, including age and estrogen depletion, severely restrict their potential usefulness in clinical applications. Glucocorticoids (GC) are well-known steroid hormones that regulate MSC proliferation and differentiation, but the defined effects and underlying mechanisms of endogenous glucocorticoids on MSC characteristics are not understood. This study investigated the effects of the blockage of endogenous GC using glucocorticoid receptor (GR) small interfering RNA (siRNA) delivered using biodegradable poly(lactic-co-glycolic acid) (PLGA) microparticles on proliferation and differentiation capabilities of human MSC in vitro. The results show that we can prepare PLGA microparticles as a delivery system for GR siRNA and maintain release of siRNA up to 40 days in vitro. Transfection of GR siRNA significantly downregulates GR and upregulates the expression of fibroblast growth factor-2 and Sox-11 of human MSC. MSC that have proliferated with endogenous GC blocked in vitro have greater proliferation rates and exhibit upregulated expression of osteogenic markers (alkaline phosphatase and core binding factor alpha 1) under differentiation stimulation after 1 week. Under adipogenic differentiation, MSC proliferated in vitro with siRNA transfection, resulting in significantly lower adipogenic markers (peroxisome proliferator-activated receptor and lipoprotein lipase) than controls. In conclusion, PLGA particles can serve as a tool for delivery of GR siRNA to effectively block the effects of endogenous GC on MSC, which has the potential to improve the capabilities of human MSC for clinical application by preventing replicative senescence.
Introduction
Adult stem cell–based tissue regeneration has emerged as a promising approach to substitute current clinical treatment of tissue defects and malfunction caused by trauma, tumor dissection, and congenital deficiency. Autologous bone marrow mesenchymal stem or stromal cells (MSC) are considered a potential cell source for this approach because of their capacity for high proliferation, multiple differentiations, and immune tolerance,1,2 although the natural population of MSC for feasible isolation is low.3 Pathophysiological factors including age, estrogen depletion, and arthritis further reduce the numbers and capacities of the MSCs.4–8 Although typical characteristics of MSC include self-renewal and multiple differentiations, MSC exhibit replicative senescence and lose proliferation and osteogenic differentiation capacity after cell expansion in current in vitro culture systems.9 Therefore, the development of therapeutic interventions to improve stem cell characteristics of MSC and prevent MSC senescence is necessary for clinical application of MSC.
Although mechanisms regulating stem cell characteristics of MSC are unclear, it is recognized that MSC lose their capability of self-renewal and multiple differentiations once they differentiate into a specific cell lineage or become senescent. Dexamethasone, a synthetic glucocorticoid (GC), has been demonstrated to effectively stimulate multiple pathways of MSC differentiation but inhibit the proliferation of MSC.10,11 The activity of glucocorticoid receptors (GR) determines the shift of hematopoietic stem cells from self-renewal into the differentiation state.12 Glucocorticoids have been demonstrated to downregulate telomerase activity and accelerate senescence.13,14 Elevated levels of endogenous GC were observed in age and estrogen depletion states associated with low populations and capabilities of MSC and accelerated senescence of MSC.15,16 Collectively, this previous evidence strongly indicated that natural physiological endogenous GC may cause MSC to lose stem cell characteristics in terms of self-renewal and multiple differentiations and hasten their senescence. Therefore, the blockage of endogenous GC may potentially be used to protect the stem cell characteristics of MSC and improve their capabilities.
Although GC appear to mediate some rapid, nongenomic cellular responses by interacting with membrane receptors, classical, intracellular GR that function as ligand-activated transcription factors mediate many GC responses. In this study, we used GR small interfering RNA (siRNA) to block the nuclear receptor pathway of GC effects. Systemic delivery of naked siRNA is widely reported to be inefficient because of rapid enzymatic degradation in serum.17 Furthermore, a high dose of naked siRNA is necessary, which can potentially stimulate undesirable inflammatory responses and immune responses that would ultimately decrease functional activity of siRNA.18 Thus, an appropriate delivery system is required for delivery of siRNA within the target cells. Several reviews have discussed viral and nonviral delivery systems for efficient delivery of siRNA to tissue cells.17,19–21 Here, we report on the use of a poly(lactic-co-glycolic) acid (PLGA) microparticle-based delivery system for efficient and sustained delivery of siRNA to MSC. PLGA microparticles are biocompatible and biodegradable and have been approved by the Food and Drug Administration. They have been developed to deliver multiple growth factors and steroids and have shown significant potential as scaffolds for tissue engineering applications.22–24 We discovered that the blockage of endogenous GC present in culture serum effectively upregulated fibroblast growth factor (FGF)-2, a stemness gene of MSC, and Sox-11 of MSC, a biomarker of MSC age.25,26 Human MSC that are proliferated with the blockage of endogenous GC in vitro have significantly higher proliferation and osteogenic differentiation but lower adipogenic differentiation capabilities. These results strongly suggested that the approach of blocking endogenous GC using GR siRNA may effectively prevent stem cell characteristics of MSC and improve the characteristics of MSC for clinical applications.
Materials and Methods
PLGA (75:25, inherent viscosity, 0.41 dL/g) was purchased from Lactel Absorbable Polymers (Cupertino, CA). Poly (vinyl alcohol) (PVA, 87–89 % hydrolyzed, molecular weight 30,000-67,000 Da) and rhodamine B (dye content ∼95 %) were purchased from Sigma-Aldrich (St. Louis, MO). Human GR siRNA was purchased commercially (Santa Cruz Biotechnology, Santa Cruz, CA). All other chemicals and solvents used, including dichloromethane (DCM) and acetonitrile, were of high-performance liquid chromatography analytical grade. Fresh human bone marrow was purchased from Allcells (Emeryville, CA). Invitrogen (Carlsbad, CA) provided all culture media and supplements.
Fabrication of PLGA microparticles
GR siRNA-loaded PLGA microparticles were fabricated using a (water-in-oil)-in-water double-emulsion solvent-evaporation method. Sonication was used to form the primary and secondary emulsions. Briefly, GR siRNA (50 μg) was added to 300 μL of a 1% aqueous solution of PVA ( water1 phase). The oil phase was prepared by dissolving 6.67% w/v of PLGA polymer in 2 mL of DCM. The water1 phase was emulsified into the oil phase using a microtip probe sonicator set at level 3 (Sonic Dismembrator Model 100, Fisher Scientific, Pittsburgh, PA). This primary water-in-oil emulsion was then immediately sonicated into 4 mL of aqueous phase containing 1% PVA for 30 seconds at the same setting as above to generate the secondary emulsion. The secondary emulsion was then dropped slowly into a beaker containing 25 mL of 1% aqueous solution of PVA and stirred using a magnetic stirrer until complete evaporation of DCM. The particles were collected by centrifugation at 5000 g for 5 minutes, washed three times with distilled water, and lyophilized overnight (Labconco FreeZone 4.5, Kansas City, MO).
Rhodamine-loaded microparticles were prepared and characterized using a similar single-emulsion solvent-evaporation methodology. For the primary emulsion, 300 μL of a 1% PVA aqueous solution was sonicated into an oil phase containing 200 mg of PLGA and 1 mg of rhodamine B dissolved in 2 mL of DCM. The primary emulsion was sonicated into a 1% aqueous solution of PVA, and particles were obtained as described above.
Characterizations of GR siRNA-loaded PLGA microparticles
Microparticles were suspended in distilled water at a concentration of 0.1 to 0.2 mg/mL, and the average particle size was determined using a particle size analyzer (Zetasizer Nano ZS, Malvern, Southborough, MA). Microparticle morphology was assessed using scanning electron microscopy (SEM, Hitachi S-4000). To determine the entrapment efficiency and loading efficiency, 10 mg of lyophilized GR siRNA-loaded microparticles was dissolved in 500 μL of DCM, and the precipitated siRNA was extracted by adding 2 mL of 10mM Tris-Cl, pH 7.5 and 1mM ethylenediaminetetraacetic acid. The extraction procedure was repeated two to three times, until no further active component could be detected in the aqueous extract. The siRNA content was analyzed using fluorescent spectroscopy as described below.
Loading efficiency was determined using the following equation:
 |
(1) |
where “Conc.” is the concentration of GR siRNA obtained in the aqueous extracts, and “Volume” is the total volume of the aqueous extract.
Entrapment efficiency was calculated using the following equation:
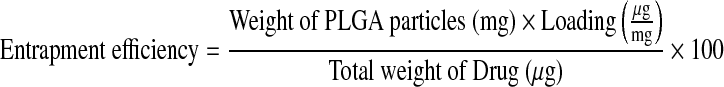 |
(2) |
where “Loading” is the loading efficiency as calculated from equation (1).
In vitro release profile of siRNA-incorporated PLGA particles
In vitro release profiles of siRNA were determined using PLGA microparticles loaded with a Cy3-labelled siRNA (Cy3, Integrated DNA Technologies, Coralville, IA). After preparation of Cy3 siRNA–loaded PLGA particles as described above, 30 to 40 mg of PLGA microparticles entrapping GR siRNA was suspended in 1.2 mL of phosphate buffered saline (PBS) (pH 7.4). The samples were shaken in a water bath at 37°C at 100 rpm. One mL of sample was withdrawn at predetermined time intervals. The sample was centrifuged using a microcentrifuge at 5,000 rpm for 5 minutes, and the supernatant was used for release analysis. The microparticle sediment was redispersed in 1 mL of the same release medium and put back into the release sample container. Released siRNA was assayed using fluorescence spectroscopy as described below. All measurements were performed in triplicate.
Quantification of siRNA using fluorescence spectroscopy
SiRNA concentration was measured using the Picogreen reagent (Invitrogen Corp., Carlsbad, CA), which is a fluorescent probe that allows for the quantitative analysis of oligonucleotides. The excitation and emission wavelengths were set at 480 and 520 nm, respectively, and relative fluorescence intensity was used to determine siRNA concentration. A standard solution of siRNA in a concentration range of 0.25 to 2 μg/mL was prepared by serial dilutions of a siRNA stock solution in PBS. The Picogreen detection reagent was prepared using a 200-time dilution in PBS. The samples (100 μL) were pipetted into microplate wells (Microwell-plates, Nunc, Germany) and mixed with 100 μL of detection reagent. The plates were incubated for 5 minutes at room temperature in the dark. The fluorescence intensity was then measured using a Microplate Reader (Spectramax, CA). Following the fluorescence assay of standard solutions of siRNA, a standard curve was generated from the linear relationship between relative fluorescence intensity and the concentration of siRNA. All measurements were done in triplicate.
Preparation of human bone marrow MSCs
Mononuclear cells of fresh human bone marrow were isolated using density gradient centrifugation (Histopaque-1.077, Sigma, St. Louis, MO). The cells were cultured with Dulbecco's modified Eagle medium (DMEM), 10% fetal bovine serum (FBS) (Atlanta Biologicals, Lawrenceville, GA) and 1% antibiotic-antimycotic (Sigma). On day 5, nonadherent cells were removed after medium exchange. The cells were collected when they reached 70% to 80% confluence to test the regulatory functions of intracellular release of GR siRNA-loaded PLGA particles.
Uptake of PLGA microparticles by human MSCs
Rhodamine-labeled PLGA microparticles were used to track the uptake of PLGA particles by human MSCs. Particles selected for these studies were similar in size, zeta potential, surface morphology, and shape to PLGA particles loaded with GR siRNA. MSC (104) were seeded on 8-well glass chamber slides overnight. After MSC were treated with 200 μg of particles per well for 1 or 24 hours, the cells were washed twice with PBS to remove suspended particles and fixed with 4% paraformaldehyde solution. After the cells were mounted with Vectashield containing 4',6-diamidino-2-phenylindole to bind DNA and locate the nucleuses (Vector Laboratories Inc., CA), a confocal microscope (Zeiss-701) was used to assess the uptake of PLGA particles by the human MSC using overlaying stacked images.
Quantification of PLGA microparticle uptake by human MSCs
To quantify uptake of particles, multiple images at the same magnification of randomly chosen areas of glass slides cultured with cells incubated with PLGA microparticles were acquired. Orthogonal sections from stacked images confirmed that particles were predominantly located inside the cell and not just on the surface. For quantitative analysis of the percentage of cells showing particle uptake, we used the following equation:
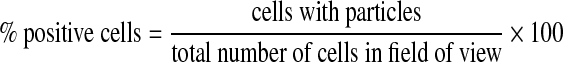 |
To quantify the extent of uptake, the total area of rhodamine particles was calculated using ImageJ software (National Institutes of Health, Bethesda, MD). The area was normalized using the total number of particle-positive cells to give the fraction area of each cell covered with particles. Thirty to 50 cells were studied at each time point and the quantification repeated in triplicate.
Effects of transfection using GR siRNA on the expression of GR stemness gene and senescent biomarker of human MSCs
One and 5 mg of GR siRNA-loaded microparticles, loaded with 0.12 and 0.6 μg of GR siRNA, respectively, were suspended in DMEM and added to human MSC cultured on a 100-mm petri dish for 24 hours. The MSC were then washed twice with PBS to remove suspended microparticles. MSC treated with PLGA particles without GR siRNA and prepared using identical double-emulsion solvent-evaporation methodologies served as controls. The gene expression of NR3C1, FGF-2, a stemness gene of MSC, and Sox-11, a gene that participates in MSC self-renewal25 and functions as a senescence marker of MSC,26 were measured after 2, 4, and 6 days using real-time polymerase chain reaction (PCR). Each measurement was performed in triplicate.
Proliferation capability of human MSC cultured with GR siRNA transfection in vitro
One and 5 mg of GR siRNA-loaded microparticles loaded with 0.12 and 0.6 μg of GR siRNA, respectively, were transfected to human MSC on a 100-mm petri dish for 24 hours. The MSC were then washed twice with PBS to remove suspended microparticles and cultured in DMEM with 10% FBS. MSC treated with PLGA particles without GR siRNA served as controls. After 1 week, the human MSC were collected and measured for proliferation rates. The MSC cells were plated in 96-well (1,000 cells/well) plates, and the amount of MSC DNA was measured using a FluoReporter Blue Fluorometric dsDNA Quantitation Kit (Invitrogen) according to the manufacturer's protocols every 2 days. This kit works on the principle that the bisbenzimidazole derivative Hoechst 33258 exhibits fluorescence enhancement upon binding to A-T-rich regions of double stranded DNA. DNA binding specificity is enhanced under high ionic strength conditions. The procedure is rapid and requires no extraction reagents for exposure of DNA to the fluorescent probe, and the assay is commonly used for proliferation studies including bone marrow MSC.27–29 The difference in DNA amounts between 2 days was used to identify the proliferation rate.
Differentiation capacity of human MSCs proliferated in vitro after transfection using GR siRNA
Because transfection using GR siRNA with 1 mg of GR siRNA–loaded PLGA microparticles significantly increases the proliferation rate of MSC, we further investigated the differentiation capabilities of MSC after proliferation in vitro after transfection using GR siRNA. One mg of GR siRNA–loaded microparticles was added to human MSC cultured on a 100-mm petri dish for 24 hours and subsequently cultured in DMEM supplemented with 10% FBS for 1 week. Human MSC treated with empty PLGA microparticles served as controls. The MSC were then collected, placed in 6-well plates at a density of 5×104 cells per well, exposed to osteogenic or adipogenic differentiation medium for up to 2 weeks. The differentiation medium for osteogenesis consisted of the basic medium supplemented with 100nM dexamethasone, 10mM β-glycerophosphate, and 0.05mM ascorbic acid-2-phosphate.19 For adipogenesis, the medium is supplemented with 500nM dexamethasone, 10μM insulin, and 0.5mM isobutyl-methylxanthine.30 Real-time PCR was used to quantify the gene expression of specific markers of MSC differentiations because it is a well-established technique to quantify the gene messenger RNA (mRNA) expressions of differentiation markers to reflect differentiation levels of MSC. The levels of mRNA are generally correlated to protein expression and are more sensitive than protein measurements for short-term observations. Alkaline phosphatase (ALP), core binding factor alpha (Cbfa)-1, and osteocalcin (OCN) were used to indicate osteogenic differentiation of MSC. Peroxisome proliferator-activated receptor gamma 2 (PPARγ2) and lipoprotein lipase (LPL) were used for adipogenic differentiation. Each measurement was performed in triplicate.
Real-time PCR analysis
RNA of cells was isolated using the Aqua Pure RNA Isolation Kit (Bio-Rad Laboratories, Inc., Hercules, CA) as recommended by the manufacturer. Real-time PCR amplification was performed on the iCycler iQ detection system, and the data were collected and analyzed using iCycler iQ version 3.0 software (Bio-Rad Laboratories, Inc.). Table 1 lists real-time PCR primers and probes for multiple differentiation markers of human MSC. The Syber Green real-time PCR primers and probe for human glyceraldehyde 3-phosphate dehydrogenase, FGF-2, and Sox-11 were purchased from ABI. Each measurement was performed in triplicate.
Table 1.
Real-Time PCR Primers and Probes of Human Bone Marrow–Derived Mesenchymal Stem Cells (MSC)
| Markers | 5′ to 3′ | 3′ to 5′ | probe |
|---|---|---|---|
| CBFA-1 | CAA CAA GACCCT GCC CGT | TCC CAT CTG GTA CCT CTC CG | CTT CAA GGT GGT AGC CC |
| OCN | TAG TGA AGA GAC CCA GGC GC | CAC AGT CCG GAT TGA GCT CA | TGT ATC AAT GGC TGG GAG CCC CAG |
| ALP | GGG AAC GAG GTC ACC TCC AT | TCG TGG TGG TCA CAA TGC C | TGG GCC AAG GAC GCT GGG AAA T |
| LPL | ACA CTT GCC ACC TCA TTC C | ACC CAA CTC TCA TAC ATT CCT G | AGT CCG TGG CTA CCT GTC ATT TCA ATC |
| PPARλ2 | CTC CTA TTG ACC CAG AAA GCG | GAG TGG TCT TCC ATT ACG GAG | ATC CAC GGA GCT GAT CCC AAA GTT |
| NR3C1 | CAT GCC GCT ATC GAA AAT G | CAG AGG TTT CTT GTG AGA CTC | CTG GAA TGA ACC TGG AAG CTC GAA AA |
PCR, polymerase chain reaction.
Statistical analysis
All quantitative data were expressed as means±standard deviations. The proliferation rate, FGF-2, and Sox-11 of each group with different doses of GR siRNA treatment were analyzed using one-way analysis of variance, and the differentiation markers of human MSC proliferated with transfection of GR siRNA were analyzed using paired Student t-tests with the use of commercially available statistical software (SPSS, Inc., Chicago, IL). P values less than 0.05 were considered significant.
Results
Characterization of GR siRNA–loaded PLGA microparticles and in vitro release
GR siRNA was encapsulated in PLGA microparticles using a (water-in-oil)-in-water double-emulsion solvent-evaporation technique. Figure 1A shows the photomicrograph of GR siRNA–loaded PLGA particles; particles were smooth and spherical (Fig. 1A). The average diameter of the particles was 2.1±0.36 μm with a polydispersity index (PDI) of 0.42±0.06 as determined by measurements on the Zetasizer Nano ZS. The loading efficiency of GR siRNA in PLGA particles was 53.6%, and the loading quantity was 0.12 μg of siRNA per mg of PLGA particles. Figure 1B shows the release profile of siRNA delivered using PLGA microparticles. The PLGA microparticles exhibit a small initial burst release phase, followed by a slower lag release phase. Approximately 80% of the total amount of siRNA was gradually released by 40 days (Fig. 1B).
FIG. 1.
Scanning electron microscopy photomicrographs (A) and in vitro release profile (B) of small interfering RNA (siRNA)-loaded poly(lactic-co-glycolic acid) (PLGA) particles.
Uptake of PLGA microspheres by human MSC
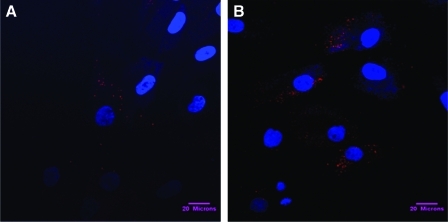
Figure 2 shows representative confocal microscopy images of MSC after 1 hour (Fig. 2A) and 24 hours (Fig. 2B) of incubation with rhodamine-loaded PLGA particles (1.8 μm±0.33, PDI 0.21±0.04). It was found that 33.7±16.8% of cells showed particle internalization after 1 hour of treatment, increasing to 64.6±24.1% of cells by 24 hours (Table 2). Rhodamine-loaded PLGA particle uptake per cell was also higher after 24 hours than at 1 hour (Table 2), confirming that cellular uptake of the particles is time dependent. To confirm that rhodamine-loaded PLGA particles were inside the cell, and not simply surface bound, we acquired confocal sections throughout the cell. Our previous study demonstrated that uptake of PLGA particles primarily occurs by endocytosis.19 No significant toxicity was observed by incubating the PLGA particles with human MSC.
FIG. 2.
Confocal microscopy images of human MSC after 1 (A) and 24 hours (B) of incubation with rhodamine-loaded PLGA particles visualized by overlaying images obtained using a Zeiss-701 confocal microscope. Blue: 4',6-diamidino-2-phenylindole staining of nucleus; Red: rhodamine staining for PLGA particles. Bar=20μm. Color images available online at www.liebertonline.com/tea
Table 2.
Quantification of the Uptake of Rhodamine-Loaded PLGA Particles by Human MSC
| |
Positive cells, % |
Area covered with particle/cell, μm2 |
|---|---|---|
| Mean±Standard Deviation | ||
| 1 Hour | 33.7±16.8 | 22.4±7.8 |
| 24 Hour | 64.6±24.1 | 233.4±100.5 |
Effects of transfection of GR siRNA on the expression of GR, FGF-2. and Sox-11
After 0.12 and 0.6 μg of GR siRNA carried by 1 and 5 mg of particles was used to transfect human MSC, GR expression of human MSC was downregulated after 2 and 4 days. GR expression was back to the parallel level of controls after 6 days (Fig. 3A). Figure 3B shows that transfection of GR siRNA also significantly upregulated FGF-2 expression after 2 days. After 6 days, FGF-2 expression of MSC from five donors with transfection of GR siRNA were notably higher than that of the controls. Because of individual variations, these increases were not statistically significant (Fig. 3B). Figure 3C shows that GR siRNA–loaded particles upregulated the expression of Sox-11 from day 2 after treatment and that the upregulation was statistically significantly higher than that of the controls after 4 days of transfection (Fig. 3C). These data indicated that blocking endogenous GC potentially upregulate the stemness gene and prevent MSC senescence. No significant difference was observed between different doses of GR siRNA on the expression of GR, FGF-2, and Sox-11.
FIG. 3.
Effects of blocking endogenous glucocorticoids (GC) on regulation of human MSC. Fold changes of the expressions of NR3C1 (A), fibroblast growth factor (FGF)-2 (B) and Sox-11 (C) of human MSC after transfection with GR siRNA for different time periods. n=6, Means±standard deviations (SDs); *p<0.05 versus control. The lines represent gene expression levels of controls.
Effects of transfection using GR siRNA on proliferation capability of human MSC
Figure 4 shows the 2-day proliferation rate of human MSC after proliferation in vitro for 1 week after transfection using 0.12 or 0.6 μg of GR siRNA. The MSC that had been transfected with GR siRNA had significantly higher proliferation capability than controls (Fig. 4), although no difference in proliferation rates was observed in the MSC treated with different doses of GR siRNA.
FIG. 4.
The increased percentage of DNA amount of human MSCs 2 days after proliferation in vitro with transfection of different concentrations of GR siRNA. n=6, Means±SDs; *p<0.05 versus controls.
The effects of transfection using GR siRNA on differentiation capacities of human MSC
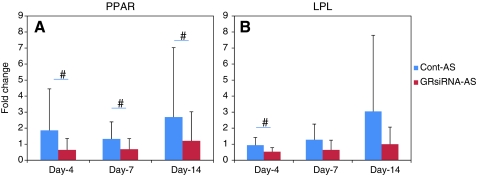
After cells were transfected with 1 mg of PLGA particles containing 0.12 μg of GR siRNA and cultured in DMEM for 1 week, human MSC were exposed to osteogenic or adipogenic differentiation medium for up to 2 weeks. After exposure to the differentiation medium, osteogenic differentiation markers of untreated MSC or MSC that had proliferated after transfection using GR siRNA, including ALP and OCN, were significantly upregulated (Fig. 5A,B). However, Cbfa-1 expression of human MSC that had proliferated after GR siRNA transfection were upregulated after 1 week of differentiation, whereas untreated control cells were not. Furthermore, the fold change of Cbfa-1 expression of human MSC proliferated after transfection with GR siRNA was significantly greater than that of controls (Fig. 5C), indicating that MSC that have proliferated after transfection with GR siRNA have higher osteogenic differentiation than controls. No significant difference in ALP and OCN expression after GR siRNA treatment was observed between MSC and controls. Adipogenic differentiation markers (PPAR and LPL) of MSC with or without transfection using GR siRNA were also upregulated after exposure to adipogenic differentiation medium (Fig. 6). However, human MSC that had proliferated after transfection using GR siRNA exhibited significantly lower expression of PPARγ-2 (Fig.6A) and LPL (Fig.6B) after differentiation than controls, indicating that MSC proliferated with blocking endogenous GC may inhibit adipogenic differentiation capability of human MSC.
FIG. 5.
Osteogenic differentiation capability of human MSC after proliferation in vitro after transfection using GR siRNA. Fold changes of alkaline phosphatase (ALP) (A), osteocalcin (OCN) (B), and core binding factor alpha (Cbfa)-1 (C) of human MSC after exposure to differentiation medium for different time periods. Cont-DMEM, control cell exposure to Dulbecco's modified Eagle medium; Cont-OS: control cell exposure to osteogenic stimulation medium; GR siRNA-DMEM, MSC proliferated with GR siRNA transfection exposure to DMEM; GR siRNA-OS, MSC proliferated with GR siRNA transfection exposure to osteogenic stimulation medium. n=6, Means±SDs; * p<0.05; #p<0.05. Color images available online at www.liebertonline.com/tea
FIG. 6.
Adipogenic differentiation capability of human MSC after proliferated in vitro with transfection of GR siRNA. Fold changes of peroxisome proliferator-activated receptor gamma (PPARγ) (A) and lipoprotein lipase (LPL) (B) of human MSC after exposure to differentiation medium for different time periods. Cont-AS, control cell exposure to adipogenic stimulation medium; GR siRNA-AS, MSCs proliferated with GR siRNA transfection exposure to adipogenic stimulation medium. n=6, Means±SDs; #, p<0.05. Color images available online at www.liebertonline.com/tea
Discussion
This study, for the first-time, demonstrated that blocking endogenous GC using GR siRNA effectively upregulates FGF-2 of human MSC during in vitro expansion. Human MSC exhibit enhanced proliferation and osteogenic capability even after short in vitro proliferation with blocking endogenous GC. Transfecting cells with GR siRNA significantly decreased adipogenic differentiation capability of MSC. Because MSC senescence initiates with the reduction of MSC proliferation and osteogenic differentiation capability and the increase of adipogenic differentiation,9,31 we presume that the blockage of endogenous GC resulted in an improvement with in vitro MSC expansion through prevention of MSC senescence. Upregulation of Sox-11 of human MSC during in vitro proliferation as a result of transfection using GR siRNA in the present study further supported the hypothesis that the blockage of endogenous GC may prevent MSC senescence. Collectively, these preliminary results strongly indicate that the approach of blocking endogenous GC may improve human MSC in vitro expansion capabilities, which are critical for their future clinical applications.
Although self-renewal of MSC is an important characteristic to maintain the high proliferation capacity of MSC, the regulation of GC on the self-renewal of MSC is unclear and its effects on proliferation of MSCs are controversial,32,33 although the function of GC to stimulate the differentiation of MSC indicates that GC may affect the stem cell characteristics of MSC in terms of self-renewal and multiple differentiations because MSC lose their stem cell characteristics once they commit to differentiating into specific lineages.34 Thus, endogenous GC may act to stimulate and force MSC into specific differentiations. In addition, the effects of GC, including onset of apoptosis and downregulation of telomerase activity, also suggest that endogenous GC may reduce the stem cell characteristics of MSC during in vitro expansion by accelerating senescence of MSC. Therefore, the blockage of endogenous GC during in vitro expansion theoretically protects the stem cell characteristics of MSC from the effect of endogenous GC and maintains or improves the stem cell characteristics of MSC. The present study revealed that the proliferation rate of MSC was greater after transfection using GR siRNA, demonstrating that blocking endogenous GC can enhance the proliferation capability of MSC during in vitro expansion. In addition, osteogenic and adipogenic differentiation markers of proliferated MSC after transfection using GR siRNA are upregulated, similar to control cells, after exposure to differentiation medium. This indicated that the MSC maintain their nature with multiple differentiation capacities after proliferation in vitro after transfection using GR siRNA. Furthermore, because Cbfa-1 is an essential transcription factor associated with osteoblast differentiation and bone formation, its faster upregulation with a higher amount in human MSC indicates that MSC that have proliferated after transfection using GR siRNA have higher osteogenic differentiation capabilities than controls. In contrast, the expression of PPAR and LPL of MSC that have proliferated with the blockage of endogenous GC is lower than that of the control, which suggests that blocking endogenous GC may inhibit adipogenic differentiation of MSC after in vitro expansion. Although the mechanisms underlying the effects of the blockage of endogenous GC on the regulation of MSC proliferation and differentiation need to be investigated further, one explanation is that FGF-2 is upregulated. FGF-2 is a well-known mitogenic growth factor and has been demonstrated to stimulate MSC proliferation. It has been demonstrated that disruption of the FGF-2 gene activates the adipogenic genes and suppresses the osteogenic program in bone marrow–derived MSC.35 Thus, the upregulation of FGF-2 in the present study may partially explain the mechanism underlying the effects of blocking endogenous glucocorticoid-mediated responses on human MSC proliferation and differentiation.
In addition, upregulation of FGF-2 and Sox-11 of MSC after transfection using GR siRNA also indicates that blocking endogenous GC may function to maintain the stemness of MSC and prevent their senescence. It has been demonstrated that, with more passages and longer time periods of MSC proliferation in vitro, MSC initiate the reduction of proliferation and osteogenic differentiation capability and increase adipogenic differentiation due to replicative senescence.7,31 FGF-2 is a well-known stemness gene that participates in self-renewal of MSC. FGF-2 treatment has been demonstrated to increase proliferation and inhibit the cellular senescence of MSCs.36–38 Thus, the upregulation of FGF-2 in the present study suggests that one of the potential mechanisms of blocking endogenous GC to enhance the capabilities of MSC proliferation and osteogenic differentiation and reduce the capability of adipogenic differentiation may be achieved through the upregulation of FGF-2 synthesis. Upregulated FGF-2 may further regulate and maintain the stem cell characteristics of MSC by autocrine or paracrine manners to postpone MSC senescence. It has also been demonstrated that Sox-11 participates in the self-renewal of MSC. Recent studies have demonstrated that Sox-11 expresses in the early stage of MSC and that its expression decreases and is eliminated after the increased passage of MSC expansion in vitro. It can function as a marker of replicative senescence for MSC during in vitro expansion.26 Upregulation of Sox-11 in MSC by transfection of GR siRNA in this study further supports the hypothesis that blocking endogenous GC potentially prevent replicative senescence of MSC in vitro.
Sustained delivery of several growth factors and steroids using biodegradable polymeric materials has shown significant potential for tissue engineering applications. Delivery of siRNA using PLGA particles has recently been demonstrated to sustain the release of siRNA and increase efficiency of transfection.39–46 In the current study, we demonstrated for the first time that PLGA microparticles can deliver GR siRNA and release it in human MSC. The release profile of siRNA from PLGA particles in this study was performed under physiological conditions (pH 7.4, 37°C), and the release of siRNA was sustained up to 40 days. Serum, cytoplasmic elements, and enzymes can potentially alter the release profile in vivo, but because the primary mechanism of degradation of PLGA is hydrolytic, we expect that the release profiles generated in vitro will broadly correlate with the release profiles observed in vivo and in the cytoplasm. We observed that transfection using GR siRNA delivered by PLGA microparticles significantly downregulated GR expression, indicating that this approach can be used to block the nuclear pathway of GC effect. Taken together, this study preliminarily demonstrated the function of blocking endogenous GC using GR siRNA delivered by PLGA microparticles to promote human MSC proliferation and differentiation and prevent MSC replicative senescence, although long-term observation and in vivo studies are necessary to further investigate the effects of GR siRNA on MSC. Although real-time PCR is more sensitive than protein measurements and suitable for short-term studies, it is possible that gene transcripts may not always exactly represent protein expression. Thus, protein measurement of MSC differentiation markers and histomorphometry of MSC-driven engineered tissue will be needed in future studies. Future and current studies will focus on optimizing the PLGA particle delivery systems, siRNA loading in PLGA particles, identifying optimal doses for in vivo studies, and clarifying the underlying mechanisms necessary to develop this approach to enhance capabilities of human MSCs for clinical applications.
Acknowledgments
We gratefully acknowledge support from Start-up funding from the Dows Institute for Dental Research, College of Dentistry, Institute for Clinical and Translational Science pilot grant, University of Iowa, the American Cancer Society (RSG-09-015-01-CDD), and the National Cancer Institute, National Institutes of Health (1R21CA13345-01/ 1R21CA128414-01A2/UI Mayo Clinic Lymphoma SPORE).
Disclosure Statement
No competing financial interests exist.
References
- 1.Pittenger M.F. Mackay A.M. Beck S.C. Jaiswal R.K. Douglas R. Mosca J.D. Moorman M.A. Simonetti D.W. Craig S. Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Barry F.P. Murphy J.M. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Mauney J.R. Volloch V. Kaplan D.L. Role of adult mesenchymal stem cells in bone tissue engineering applications: current status and future prospects. Tissue Eng. 2005;11:787. doi: 10.1089/ten.2005.11.787. [DOI] [PubMed] [Google Scholar]
- 4.Bellantuono I. Aldahmash A. Kassem M. Aging of marrow stromal (skeletal) stem cells and their contribution to age-related bone loss. Biochim Biophys Acta. 2009;1792:364. doi: 10.1016/j.bbadis.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Mueller S.M. Glowacki J. Age-related decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional collagen sponges. J Cell Biochem. 2001;82:583. doi: 10.1002/jcb.1174. [DOI] [PubMed] [Google Scholar]
- 6.Roobrouck V.D. Ulloa-Montoya F. Verfaillie C.M. Self-renewal and differentiation capacity of young and aged stem cells. Exp Cell Res. 2008;314:1937. doi: 10.1016/j.yexcr.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Rodríguez J.P. Montecinos L. Ríos S. Reyes P. Martínez J. Mesenchymal stem cells from osteoporotic patients produce a type I collagen-deficient extracellular matrix favoring adipogenic differentiation. J Cell Biochem. 2000;79:557. doi: 10.1002/1097-4644(20001215)79:4<557::aid-jcb40>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 8.Rodríguez J.P. Ríos S. Fernández M. Santibañez J.F. Differential activation of ERK1,2 MAP kinase signaling pathway in mesenchymal stem cell from control and osteoporotic postmenopausal women. J Cell Biochem. 2004;92:745. doi: 10.1002/jcb.20119. [DOI] [PubMed] [Google Scholar]
- 9.Vacanti V. Kong E. Suzuki G. Sato K. Canty J.M. Lee T. Phenotypic changes of adult porcine mesenchymal stem cells induced by prolonged passaging in culture. J Cell Physiol. 2005;205:194. doi: 10.1002/jcp.20376. [DOI] [PubMed] [Google Scholar]
- 10.Baksh D. Song L. Tuan R.S. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004;8:301. doi: 10.1111/j.1582-4934.2004.tb00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolf C.M. Cho E. Tuan R.S. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9:204. doi: 10.1186/ar2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wessely O. Deiner E.M. Beug H. von Lindern M. The glucocorticoid receptor is a key regulator of the decision between self-renewal and differentiation in erythroid progenitors. EMBO J. 1997;15:267. doi: 10.1093/emboj/16.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ichiyoshi H. Kiyozuka Y. Kishimoto Y. Fukuhara S. Tsubura A. Massive telomere loss and telomerase RNA expression in dexamethasone-induced apoptosis in mouse thymocytes. Exp Mol Pathol. 2003;75:178. doi: 10.1016/s0014-4800(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 14.Wolkowitz O.M. Epel E.S. Reus V.I. Mellon S.H. Depression gets old fast: do stress and depression accelerate cell aging? Depress Anxiety. 2010;27:327. doi: 10.1002/da.20686. [DOI] [PubMed] [Google Scholar]
- 15.Lupien S.J. Schwartz G. Ng Y.K. Fiocco A. Wan N. Pruessner J.C. Meaney M.J. Nair N.P. The Douglas Hospital Longitudinal Study of Normal and Pathological Aging: summary of findings. J Psychiatry Neurosci. 2005;30:328. [PMC free article] [PubMed] [Google Scholar]
- 16.Matthews S.G. Owen D. Banjanin S. Andrews M.H. Glucocorticoids, hypothalamo-pituitary-adrenal (HPA) development, and life after birth. Endocr Res. 2002;28:709. doi: 10.1081/erc-120016991. [DOI] [PubMed] [Google Scholar]
- 17.Sah D.W.Y. Bumcrot D. Manoharan M. Koteliansky V. RNAi therapeutics: a potential new class of pharmaceutical drugs. Nat Chem Biol. 2006;2:711. doi: 10.1038/nchembio839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Fougerolles A. Vornlocher H.P. Maraganore J. Lieberman J. Interfering with disease: a progress report on siRNA-based therapeutics. Nat Rev Drug Discov. 2007;6:443. doi: 10.1038/nrd2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang S.B. Zhao B. Jiang H.M. Wang B. Ma B.C. Cationic lipids and polymers mediated vectors for delivery of siRNA. J Control Release. 2007;123:1. doi: 10.1016/j.jconrel.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 20.Dowdy S.F. Meade B.R. Enhancing the cellular uptake of siRNA duplexes following noncovalent packaging with protein transduction domain peptides. Adv Drug Deliv Rev. 2008;60:530. doi: 10.1016/j.addr.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tseng Y.C. Mozumdar S. Huang L. Lipid-based systemic delivery of siRNA. Adv Drug Deliv Rev. 2009;61:721. doi: 10.1016/j.addr.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bala I. Hariharan S. Kumar M.N. PLGA nanoparticles in drug delivery: the state of the art. Crit Rev Ther Drug Carrier Syst. 2004;21:387. doi: 10.1615/critrevtherdrugcarriersyst.v21.i5.20. [DOI] [PubMed] [Google Scholar]
- 23.Birnbaum D.T. Kosmala J.D. Henthorn D.B. Brannon-Peppas L. Controlled release of beta-estradiol from PLAGA microparticles: the effect of organic phase solvent on encapsulation and release. J Control Release. 2000;3:375. doi: 10.1016/s0168-3659(99)00219-9. [DOI] [PubMed] [Google Scholar]
- 24.Hong L. Krishnamachari Y. Seabold D. Joshi V. Schneider G. Salem A.K. Intracellular release of 17-β estradiol from cationic polyamidoamine dendrimer surface-modified poly (lactic-co-glycolic acid) microparticles improves osteogenic differentiation of human mesenchymal stromal cells. Tissue Eng Part C Methods. 2011;17:319. doi: 10.1089/ten.tec.2010.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kubo H. Shimizu M. Taya Y. Kawamoto T. Michida M. Kaneko E. Igarashi A. Nishimura M. Segoshi K. Shimazu Y. Tsuji K. Aoba T. Kato Y. Identification of mesenchymal stem cell (MSC)-transcription factors by microarray and knockdown analyses, and signature molecule-marked MSC in bone marrow by immunohistochemistry. Genes Cells. 2009;14:407. doi: 10.1111/j.1365-2443.2009.01281.x. [DOI] [PubMed] [Google Scholar]
- 26.Larson B.L. Ylostalo J. Lee R.H. Gregory C. Prockop D.J. Sox11 is expressed in early progenitor human multipotent stromal cells and decreases with extensive expansion of the cells. Tissue Eng Part A. 2010;16:3385. doi: 10.1089/ten.tea.2010.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsiridis E. Ali Z. Bhalla A. Gamie Z. Heliotis M. Gurav N. Deb S. DiSilvio L. In vitro proliferation and differentiation of human mesenchymal stem cells on hydroxyapatite versus human demineralised bone matrix with and without osteogenic protein-1. Expert Opin Biol Ther. 2009;9:9. doi: 10.1517/14712590802622473. [DOI] [PubMed] [Google Scholar]
- 28.Tsiridis E. Ali Z. Bhalla A. Heliotis M. Gurav N. Deb S. DiSilvio L. In vitro and in vivo optimization of impaction allografting by demineralization and addition of rh-OP-1. J Orthop Res. 2007;25:1425. doi: 10.1002/jor.20387. [DOI] [PubMed] [Google Scholar]
- 29.Ivirico J.L. Salmerón-Sánchez M. Ribelles J.L. Pradas M.M. Soria J.M. Gomes M.E. Reis R.L. Mano J.F. Proliferation and differentiation of goat bone marrow stromal cells in 3D scaffolds with tunable hydrophilicity. J Biomed Mater Res B Appl Biomater. 2009;91:277. doi: 10.1002/jbm.b.31400. [DOI] [PubMed] [Google Scholar]
- 30.Hong L. Zhang G. Sultana H. Yu Y. Wei Z. The effects of 17-β estradiol on enhancing proliferation of human bone marrow mesenchymal stromal cells in vitro. Stem Cells Dev. 2011;20:925. doi: 10.1089/scd.2010.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moerman E.J. Teng K. Lipschitz D.A. Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell. 2004;3:379. doi: 10.1111/j.1474-9728.2004.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim C.H. Cheng S.L. Kim G.S. Effects of dexamethasone on proliferation, activity, and cytokine secretion of normal human bone marrow stromal cells: possible mechanisms of glucocorticoid-induced bone loss. J Endocrinol. 1999;162:371. doi: 10.1677/joe.0.1620371. [DOI] [PubMed] [Google Scholar]
- 33.Walsh S. Jordan G.R. Jefferiss C. Stewart K. Beresford J.N. High concentrations of dexamethasone suppress the proliferation but not the differentiation or further maturation of human osteoblast precursors in vitro: relevance to glucocorticoid-induced osteoporosis. Rheumatology (Oxford) 2001;40:74. doi: 10.1093/rheumatology/40.1.74. [DOI] [PubMed] [Google Scholar]
- 34.Dennis J.E. Merriam A. Awadallah A. Yoo J.U. Johnstone B. Caplan A.I. A quadripotential mesenchymal progenitor cell isolated from the marrow of an adult mouse. J Bone Miner Res. 1999;14:700. doi: 10.1359/jbmr.1999.14.5.700. [DOI] [PubMed] [Google Scholar]
- 35.Hanada K. Dennis J.E. Caplan A.I. Stimulatory effects of basic fibroblast growth factor and bone morphogenetic protein-2 on osteogenic differentiation of rat bone marrow-derived mesenchymal stem cells. J Bone Miner Res. 1997;12:1606. doi: 10.1359/jbmr.1997.12.10.1606. [DOI] [PubMed] [Google Scholar]
- 36.Ito T. Sawada R. Fujiwara Y. Seyama Y. Tsuchiya T. FGF-2 suppresses cellular senescence of human mesenchymal stem cells by down-regulation of TGF-beta2. Biochem Biophys Res Commun. 2007;20:108. doi: 10.1016/j.bbrc.2007.05.067. [DOI] [PubMed] [Google Scholar]
- 37.Dvorak P. Dvorakova D. Hampl A. Fibroblast growth factor signaling in embryonic and cancer stem cells. FEBS Lett. 2006;22:2869. doi: 10.1016/j.febslet.2006.01.095. [DOI] [PubMed] [Google Scholar]
- 38.Coutu D.L. François M. Galipeau J. Inhibition of cellular senescence by developmentally regulated FGF-receptors in mesenchymal stem cells. Blood. 2011;117:6801. doi: 10.1182/blood-2010-12-321539. [DOI] [PubMed] [Google Scholar]
- 39.Woodrow K.A. Cu Y. Booth C.J. Saucier-Sawyer J.K. Wood M.J. Saltzman W.M. Intravaginal gene silencing using biodegradable polymer nanoparticles densely loaded with small-interfering RNA. Nat Mater. 2009;8:526. doi: 10.1038/nmat2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cun D. Jensen D.K. Maltesen M.J. Bunker M. Whiteside P. Scurr D. Foged C. Nielsen H.M. High loading efficiency and sustained release of siRNA encapsulated in PLGA nanoparticles: quality by design optimization and characterization. Eur J Pharm Biopharm. 2011;77:26. doi: 10.1016/j.ejpb.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 41.Mountziaris P.M. Sing D.C. Chew S.A. Tzouanas S.N. Lehman E.D. Kasper F.K. Mikos A.G. Controlled release of anti-inflammatory siRNA from biodegradable polymeric microparticles intended for intra-articular delivery to the temporomandibular joint. Pharm Res. 2011;28:1370. doi: 10.1007/s11095-010-0354-9. [DOI] [PubMed] [Google Scholar]
- 42.Brunner T. Cohen S. Monsonego A. Silencing of proinflammatory genes targeted to peritoneal-residing macrophages using siRNA encapsulated in biodegradable microspheres. Biomaterials. 2010;31:2627. doi: 10.1016/j.biomaterials.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 43.Cun D. Foged C. Yang M. Frøkjaer S. Nielsen H.M. Preparation and characterization of poly(DL-lactide-co-glycolide) nanoparticles for siRNA delivery. Int J Pharm. 2010;390:70. doi: 10.1016/j.ijpharm.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 44.Braden A.R. Kafka M.T. Cunningham L. Jones H. Vishwanatha J.K. Polymeric nanoparticles for sustained down-regulation of annexin A2 inhibit prostate tumor growth. J Nanosci Nanotechnol. 2009;9:2856. doi: 10.1166/jnn.2009.028. [DOI] [PubMed] [Google Scholar]
- 45.Murata N. Takashima Y. Toyoshima K. Yamamoto M. Okada H. Anti-tumor effects of anti-VEGF siRNA encapsulated with PLGA microspheres in mice. J Control Release. 2008;20:246. doi: 10.1016/j.jconrel.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 46.Nguyen J. Steele T.W. Merkel O. Reul R. Kissel T. Fast degrading polyesters as siRNA nano-carriers for pulmonary gene therapy. J Control Release. 2008;18:243. doi: 10.1016/j.jconrel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]