Abstract
Mesenchymal stem cells (MSCs) are being recognized as a viable cell source for cartilage repair, and there is growing evidence that mechanical signals play a critical role in the regulation of stem cell chondrogenesis and in cartilage development. In this study we investigated the effect of dynamic compressive loading on chondrogenesis, the production and distribution of cartilage specific matrix, and the hypertrophic differentiation of human MSCs encapsulated in hyaluronic acid (HA) hydrogels during long term culture. After 70 days of culture, dynamic compressive loading increased the mechanical properties, as well as the glycosaminoglycan (GAG) and collagen contents of HA hydrogel constructs in a seeding density dependent manner. The impact of loading on HA hydrogel construct properties was delayed when applied to lower density (20 million MSCs/ml) compared to higher seeding density (60 million MSCs/ml) constructs. Furthermore, loading promoted a more uniform spatial distribution of cartilage matrix in HA hydrogels with both seeding densities, leading to significantly improved mechanical properties as compared to free swelling constructs. Using a previously developed in vitro hypertrophy model, dynamic compressive loading was also shown to significantly reduce the expression of hypertrophic markers by human MSCs and to suppress the degree of calcification in MSC-seeded HA hydrogels. Findings from this study highlight the importance of mechanical loading in stem cell based therapy for cartilage repair in improving neocartilage properties and in potentially maintaining the cartilage phenotype.
Introduction
Mesenchymal stem cells (MSCs) have emerged as a clinically relevant cell source for regenerative medicine, particularly for cartilage repair. MSCs undergo chondrogenesis and deposit a cartilage specific matrix in pellet cultures and in a variety of natural and synthetic scaffold materials in the presence of the appropriate growth factors.1–3Mechanical signals have been identified as one of the key factors regulating embryonic limb development and adult musculoskeletal repair. More specifically, the rate of cartilage formation, mechanical properties, and cartilage matrix content of the formed cartilage is significantly reduced in immobilized embryos.4,5 As cartilage matures with load bearing use, matrix content and crosslinking increases substantially;6,7 however, these properties fail to increase when the tissue is removed from the load bearing environment.8 Previous studies have established that mechanical loading not only regulates the normal maintenance of articular cartilage in vivo9,10 and in vitro11–15 but also promotes cartilage matrix biosynthesis.16,17 Furthermore, recent studies have demonstrated that dynamic compression can enhance the expression of chondrogenic markers and cartilage matrix synthesis by MSCs encapsulated in various scaffold materials, including agarose,18 alginate,19 and fibrin gels.20
Previously, we demonstrated that the natural polysaccharide hyaluronic acid (HA) provides a stable 3D environment that is conducive to MSC chondrogenesis when processed into hydrogel form.21,22 We also showed that hydrogel crosslink density controls the distribution of cartilage matrix, with a lower density leading to more uniform matrix23 and that the controlled degradation of HA hydrogels could be used to modulate tissue distribution, as degradation opens up molecular porosity to allow for produced matrix to distribute throughout the hydrogel.22 Beyond tailoring the hydrogel component, recent studies also showed that mechanical loading promotes improved matrix distribution in engineered cartilage with an MSC-seeded agarose scaffold, leading to improved mechanical properties.24 Likewise, the MSC seeding density influences MSC chondrogenesis,25,26 with higher seeding densities and transient transforming growth factor (TGF) exposure improving properties in agarose and HA gels.27 Therefore, it is of great interest to evaluate the effect of dynamic compressive loading on MSC chondrogenesis and cartilage matrix production and distribution in HA scaffolds, in an attempt to improve overall neocartilage properties towards clinical utility.
While MSCs form cartilage-like tissue in vitro under chondrogenic induction with TGF-βs, and this transformation may be improved by dynamic loading and increased seeding density, transplantation to the uncontrolled in vivo environment commonly results in extensive calcification of the formed ECM.28–30 For example, we recently showed, using alginate microsphere delivery of TGF-β3, and direct subcutaneous implantation of human MSC seeded HA gels, considerable ectopic mineralization within 4 weeks.30 Another recent study reported that the delivery of TGF-β1 with MSCs results in increased subchondral trabecular bone formation in a rabbit osteochondral repair site.31 Mechanical signals may impact MSC transition to an osteogenic phenotype; loading regulates growth plate and articular chondrocyte hypertrophy via the IHH-PTHrP (India hedgehog, parathyroid hormone-related protein) pathway.32–35 However, it is difficult to accurately assess the effect of mechanical loading on hypertrophic differentiation of MSCs in vivo. A recent study demonstrated that MSC hypertrophy can be induced in vitro by withdrawing transforming growth factor beta 3 (TGF-β3), reducing the concentration of dexamethasone, and adding thyroid hormone (T3), providing a simplified in vitro model to study MSC hypertrophy.36 Therefore, the second objective of this study was to investigate the effect of dynamic compressive loading on the hypertrophic conversion of chondrogenic human MSCs in HA. Findings from this study provide important insight into stem cell based therapy for cartilage repair and regeneration.
Material and Methods
Macromer synthesis
Methacrylated HA (MeHA) was synthesized as previously reported.37 Briefly, methacrylic anhydride (methacrylic anhydride, 94%, FW: 154.17, Sigma-Aldrich, Haverhill, MA) was added to a solution of 1 wt% HA (sodium hyaluronate powder, research grade, MW ∼74 kDa, Lifecore, Chaska, MN) in deionized water, adjusted to a pH of 8 with 5 N NaOH, and reacted on ice for 24 h. The macromer solution was purified via dialysis (MW cutoff 6–8k) against deionized water for a minimum of 48 h with repeated changes of water. The final product was obtained by lyophilization, and stored at −20°C in powder form prior to use. The final macromer products were confirmed by 1H NMR to have a methacrylation of ∼27%. Lyophilized MeHA (1.5 wt%) was dissolved in phosphate buffered saline (PBS) containing 0.05 wt% 2-methyl-1-[4-(hydroxyethoxy) phenyl]-2-methyl-1-propanone (I2959, Ciba) for polymerization.
Sample preparation and in vitro culture
Human MSCs (Lonza, Basel, Switzerland) were expanded to passage 3 in growth media consisting of α-MEM with 16.7% FBS and 1% pen/strep. MSCs (20 or 60 million cells/ml) were photoencapsulated in 1.5 wt% MeHA hydrogel disks (Ø5 mm, 2.6 mm thickness) and cultured in chondrogenic medium (DMEM, 1% ITS+Premix, 50 μg/ml L-proline, 0.1 μM dexamethasone, 0.9 mM sodium pyruvate, penicillin/streptomycin, 50μg/ml ascorbate) supplemented with 10ng/ml TGF-β3 (R&D Systems, Minneapolis, MN), which was changed three times per week.38 To induce hypertrophy, constructs were first cultured in chondrogenic media for 2 weeks. Media was then switched to hypertrophic induction media (+T3: 1nM dexamethasone, 1nM triiodothyronine [T3] and 10mM β-glycerophosphate [β-gly]) or control media (−T3: without T3 or β-gly) from day 15 until day 30 of culture.36 Hypertrophic cultures were maintained as free swelling (FS) or exposed to dynamic loading conditions (DL), as described below. Cell viability was assessed using the LIVE/DEAD Assay Kit (Invitrogen, Carlsbad, CA), where live cells are stained green with calcein-AM and dead cells stained red with ethidium homodimer.
Mechanical loading
Compressive loading was applied as described previously.39,40 Briefly, the loading protocol consisted of a 10% peak compressive sinusoidal strain at 1 Hz frequency, superimposed on a 5% compressive tare strain. Loading was carried out in unconfined compression via impermeable loading platens, for 4 hours/day and 5 days/week. Dynamic compressive loading (DL) was carried out at 37°C and 5% CO2 in a humidified incubator. FS control cultures were positioned adjacent to the loading device during this period. DL was applied starting from day 3 and continued until the end of the study.
Mechanical testing
At set time points, samples were removed from the culture and the bulk mechanical properties of constructs were evaluated using a custom table top testing device as described previousl.y41 Briefly, samples were first equilibrated in creep to a tare load of 2 g by an impermeable loading platen in a loading chamber filled with PBS. From this offset, stress relaxation tests were performed with a single compression ramp at a speed of 10%/min until reaching 10% strain. The equilibrium Young's modulus (EY) was determined by the equilibrium load obtained after 1000 sec of relaxation under unconfined compression at 10% strain.
Gene expression analysis
For gene expression analysis, samples were homogenized in Trizol Reagent (Invitrogen) with a tissue grinder, RNA was extracted according to the manufacturer's instructions, and the RNA concentration was determined using an ND-1000 spectrophotometer (Nanodrop Technologies, Wilmington, DE). One microgram of RNA from each sample was reverse transcribed into cDNA using reverse transcriptase (Superscript II, Invitrogen) and oligoDT (Invitrogen). Polymerase chain reaction (PCR) was performed on an Applied Biosystems 7300 real-time PCR system using Taqman primers and probes specific for GAPDH (glyceraldehyde-3-phosphate dehydrogenase, housekeeping gene) and other genes of interest. Sequences of the primers and probes used are listed in Table 1. The relative gene expression was calculated using the ΔΔCT method, where fold difference was calculated using the expression 2ΔΔCt. Each sample was internally normalized to GAPDH, and each group was normalized to the expression levels of MSCs at the time of encapsulation (i.e., after expansion and before differentiation). Relative expression levels greater than 1 represent up-regulation with culture, while relative expression levels less than 1 represent down-regulation of that gene compared to that of initially encapsulated MSCs.
Table 1.
Sequences of Primers and Probes Used for Real-Time PCR; Sequences Related to Gene Type X Collagen are Proprietary to Applied Biosystems Inc. and Were Not Disclosed
| Gene | Forward primer | Reverse primer | Probe |
|---|---|---|---|
| GAPDH | AGGGCTGCTTTTAACTCTGGTAAA | GAATTTGCCATGGGTGGAAT | CCTCAACTACATGGTTTAC |
| COL I | AGGACAAGAGGCATGTCTGGTT | GGACATCAGGCGCAGGAA | TTCCAGTTCGAGTATGGC |
| COL II | GGCAATAGCAGGTTCACGTACA | CGATAACAGTCTTGCCCCACTT | CTGCACGAAACATAC |
| Aggrecan | TCGAGGACAGCGAGGCC | TCGAGGGTGTAGCGTGTAGAGA | ATGGAACACGATGCCTTTCACCACGA |
PCR, polymerase chain reaction, GAPDH, glyceraldehyde-3-phosphate dehydrogenase; COL, collagen.
Biochemical analysis
One-half of each construct was weighed wet, lyophilized, reweighed dry, and digested in 0.5 mg/ml Proteinase-K (Fisher Scientific, Hampton, NH) at 56°C for 16 h. The PicoGreen assay (Invitrogen) was used to quantify the DNA content of the constructs with lambda phage DNA (0–1 mg/ml) as a standard.42 For each sample, both the mass of the entire gel and the half gel used for the DNA assay were measured. The total amount of deoxyribonucleic acid (DNA) per sample was calculated by scaling the amount of DNA detected in the half gel by a weight ratio (total weight/half weight). The glycosaminoglycan (GAG) content was measured using the dimethylmethylene blue (DMMB, Sigma-Aldrich) dye-binding assay with shark chondroitin sulfate (0–50 mg/ml) as a standard.43 The overall collagen content was assessed by measuring the orthohydroxyproline (OHP) content via dimethylaminobenzaldehyde and chloramine T assay. Collagen content was calculated by assuming a 1:7.5 OHP-to-collagen mass ratio.44 The collagen and GAG contents were normalized to the disk wet weight. Calcium content was quantified using a commercial kit (BioVision, San Francisco, CA).
Histological analysis
The remaining halves of the constructs were fixed in 4% formalin for 24 h, embedded in paraffin, and processed using standard histological procedures. The histological sections (8 μm thick) were stained with alcian blue (pH=1.0) (proteoglycans) or picrosirius red (collagen), or were immunostained for targets of interest using the Vectastain ABC and DAB Substrate kits (Vector Labs, Burlingame, CA). Briefly, sections were predigested in 0.5 mg/ml hyaluronidase for 30 min at 37°C and incubated in 0.5 N acetic acid for 4 h at 4°C to swell the samples prior to overnight incubation with primary antibodies at dilutions of 1:100, 1:200, and 1:3 for chondroitin sulfate (mouse monoclonal anti-chondroitin sulfate, Sigma-Aldrich), and type I (mouse monoclonal anti-collagen type 1, Sigma-Aldrich) and type II collagen antibodies (mouse monoclonal anti-collagen type II, Developmental Studies Hybridoma Bank, Iowa City, IA), respectively. Non-immune controls underwent the same procedure without primary antibody incubation.
Statistical analysis
All data are presented as mean±standard deviation. Statistica (Statsoft, Tulsa, OK) was used to perform statistical analyses using two-way analysis of variance (ANOVA) and Tukey's honestly significant difference (HSD) post hoc test of the means (n=4 samples per group) with culture duration and experimental groups as independent factors.
Results and Discussion
Effect of dynamic compression on cartilage matrix production by MSCs in HA hydrogels
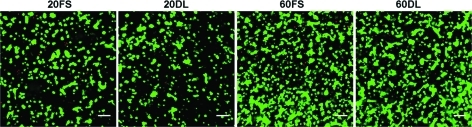
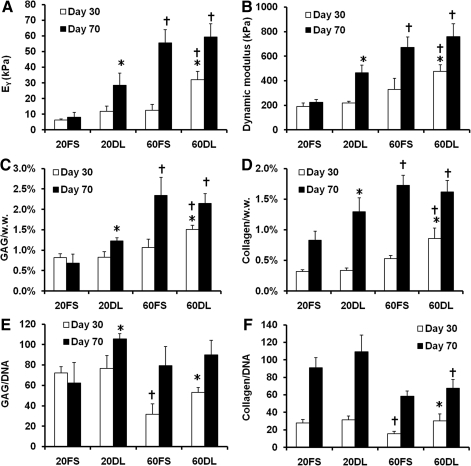
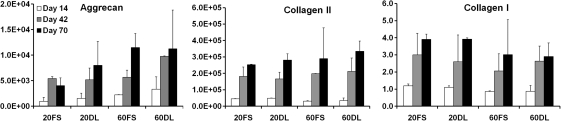
After 70 days of culture under either FS or DL conditions, MSCs encapsulated at both low (20 million cells/ml) and high (60 million cells/ml) seeding densities remained highly viable (>95%) (Fig. 1). While there were no differences between groups at day 30, by day 70 DL constructs seeded at a low density (20DL) exhibited significant increases in Young's modulus (28.3±7.8 kPa vs. 8.2±3.0 kPa), dynamic modulus (467±62k Pa vs. 225±22 kPa), GAG (1.2±0.1% vs. 0.7±0.2%) and collagen content (1.3±0.2% vs. 0.8±0.2%) compared to the free swelling (20FS) group (FIG. 2 A, B, C, D; Table 2). In contrast, in higher seeding density conditions (60DL), loading significantly increased the Young's modulus (32.0±5.3 kPa vs. 12.4±3.8 kPa), dynamic modulus (476±56 kPa vs. 329±93 kPa), GAG (1.5±0.1% vs. 1.1±0.2%) and collagen content (0.9±0.2% vs. 0.5±0.1%) at day 30 compared to free swelling construct (60FS). By day 70, constructs continued to increase in each of these parameters, but DL showed no impact at this later time point (Fig. 2 A, B, C, D; Table 2). On day 30, constructs at both MSC densities in free swelling condition (20FS, 60FS) had similar mechanical properties and biochemical content, whereas these parameters were statistically higher for the high density free swelling group (60FS) on day 70 (EY: 55.5±8.3 kPa vs. 8.2±3.0 kPa; dynamic modulus: 671±87 kPa vs. 225±22 kPa; GAG: 2.3±0.4% vs. 0.7±0.2%; collagen: 1.7±0.2% vs. 0.8±0.2%) (Fig. 2 A, B, C, D; Table 2). Under FS condition the high seeding density group (60FS) had lower GAG (31.7±10.1 vs. 72.2±6.2) and collagen (15.7±2.7 vs. 28.0±3.8) contents per cell than the low seeding density group (20FS) on day 30, but not on day 70 (Fig. 2 E, F; Table 2). DL significantly increased the GAG (day 30: 60FS @ 31.7±10.1 vs. 60DL @ 53.0±5.0; day 70: 20FS @ 62.3±19.9 vs. 20DL @ 105.7±4.9) and collagen (day 30: 60FS @ 15.6±2.7 vs. 60DL @ 30.1±7.9; day 70: 20FS @ 90.8±11.8 vs. 20DL @ 109.5±18.8) contents per cell in high and low seeding density constructs on day 30 and 70, respectively (Fig. 2 E, F; Table 2). High seeding density constructs exhibited less volumetric swelling compared to the low seeding density groups under free swelling conditions on day 30 (20FS @ 130.5±2.3% vs. 60FS @ 115.0±7.1%) (Table 2). The swelling of low seeding density constructs decreased over time under both free swelling and dynamic loading conditions (day 30: 20FS @ 130.5±2.3%, 20DL @ 124.9±7.5% vs. day 70 20FS @ 118.9±4.2%, 20DL @ 108.6±1.1%) (Table 2). Loading reduced swelling of low seeding density constructs on day 70 (20FS @ 118.9±4.2% vs. 20DL @ 108.6±1.1%) (Table 2). Although the expression of type II collagen and aggrecan was significantly upregulated in all groups compared to unencapsulated cells, there were no significant differences between FS and DL groups in the gene expression of aggrecan, type II and type I collagen on days 14, 42, and 70 of culture (Fig. 3).
FIG. 1.
Viability staining of mesenchymal stem cells (MSCs) seeded at 20 or 60 million cells/ml in hyaluronic acid (HA) hydrogels after 70 days of in vitro culture under free swelling (FS) or dynamic loading (DL) culture conditions. Green: live cells. Red: dead cells. Scale bar=100 μm. Color images available online at www.liebertonline.com/tea
FIG. 2.
Young's modulus (A), dynamic modulus (1Hz) (B), glycosaminoglycan (GAG) (C) and collagen (D) contents normalized to wet weight (w.w.), and GAG (E) and collagen (F) contents normalized to DNA (ng/ng). 20/60FS: constructs seeded with 20 or 60 million cells/ml and cultured under free swelling conditions; 20/60DL: constructs seeded with 20 or 60 million cells/ml and cultured under dynamic loading conditions, *p<0.05 vs. FS group at the same culture time (n=4), †p<0.05 vs. 20FS/DL group at the same culture time (n=4).
Table 2.
Young's Modulus (EY), Dynamic Modulus (1Hz), Glycosaminoglycan (GAG) and Collagen Contents Normalized to Wet Weight (w.w.); and GAG and Collagen Contents Normalized to DNA (ng/ng); and Construct Volume Normalized to Initial Volume on Day 0; 20/60FS: Constructs Seeded with 20 or 60 Million Cells/ml and Cultured Under Free Swelling (FS) Conditions; 20/60DL: Constructs Seeded with 20 or 60 Million Cells/ml and Cultured Under Dynamic Loading (DL) Conditions
| 20FS | 20DL | 60FS | 60DL | ||
|---|---|---|---|---|---|
| EY (kPa) | Day 30 | 6.3±0.7 | 11.8±3.3 | 12.4±3.8 | 32.0±5.3*† |
| Day 70 | 8.2±3.0 | 28.3±7.8* | 55.5±8.3† | 59.2±8.5† | |
| Dynamic modulus (kPa) | Day 30 | 190±28 | 218±14 | 329±93 | 476±56*† |
| Day 70 | 225±22 | 467±62* | 671±87† | 758±106† | |
| GAG/w.w. | Day 30 | 0.8±0.1% | 0.8±0.1% | 1.1±0.2% | 1.5±0.1%*† |
| Day 70 | 0.7±0.2% | 1.2±0.1%* | 2.3±0.4%† | 2.1±0.2%† | |
| Collagen/w.w. | Day 30 | 0.3±0.0% | 0.3±0.0% | 0.5±0.1% | 0.9±0.2%*† |
| Day 70 | 0.8±0.2% | 1.3±0.2%* | 1.7±0.2%† | 1.6±0.2%† | |
| GAG/DNA | Day 30 | 72.2±6.2 | 76.5±12.8 | 31.7±10.1† | 53.0±5.0* |
| Day 70 | 62.3±19.9 | 105.7±4.9* | 79.5±18.6 | 89.9±14.3 | |
| Collagen/DNA | Day 30 | 28.0±3.8 | 31.3±4.6 | 15.7±2.7† | 30.1±7.9* |
| Day 70 | 90.8±11.8 | 109.5±18.8 | 58.3±5.9 | 67.7±10.0† | |
| Normalized volume | Day 30 | 130.5±2.3% | 124.9±7.5% | 115.0±7.1%† | 119.6±5.7% |
| Day 70 | 118.9±4.2%‡ | 108.6±1.1%*‡ | 109.4±8.7% | 114.8±11.5% |
p<0.05 vs. FS group at the same culture time (n=4), †p<0.05 vs. 20FS/DL group at the same culture time (n=4), ‡p<0.05 vs. 20FS/DL group on Day 30 (n=4). Data presented as mean±standard deviation.
FIG. 3.
Expression of aggrecan, type II collagen and type I collagen in fold change (relative to day 0) after 14, 42, and 70 days of the culture (n=4) for either 20 or 60 million cells/ml encapsulation density and under FS or DL culture conditions.
Recent in vitro studies demonstrated successful chondrogenesis of MSCs photoencapsulated in HA hydrogels, formed through the crosslinking of HA modified with methacrylate groups.21–23 However, despite the advantages of HA as a scaffold material, the properties of the formed cartilage were inferior to that of native tissue. In this study, the results indicate that dynamic compressive loading enhances the synthesis of cartilage-specific extracellular matrix (ECM) by human MSCs seeded at both low and high density in HA hydrogels, leading to superior mechanical function of these constructs compared to free swelling controls.
An optimal cell seeding density is critical to the tissue engineering of cartilage, especially when MSCs are used. During development, the initial step of cartilage formation requires recruitment of and condensation of progenitor cells. The density of this condensation is directly related to the extent of the subsequent chondrogenic differentiation.45 On the other hand, cartilage cell density generally varies with distance from articular surface and with age. Adult human femoral condylar cartilage of has an overall density of ∼15 million cells/ml,46 whereas levels in fetal and juvenile cartilage can be many fold higher. In this study, two different seeding densities were investigated, one similar to adult cartilage and another threefold higher. Although there was no difference in mechanical stiffness and cartilage matrix content between the high and low seeding density constructs under free swelling conditions on day 30, the high seeding density constructs (60FS) eventually developed significantly higher mechanical properties and cartilage matrix content compared to the low seeding density constructs (20FS) on day 70.
Several previous studies showed lower cartilage matrix synthesis per cell at high seeding densities and a lack of difference between high and low MSC seeding density in bulk mechanical properties and cartilage matrix content under FS condition; however, these studies only included a shorter culture period of 3–5 weeks.27,47 In addition, the HA hydrogel scaffold used in this study may be more favorable to matrix remodeling and distribution compared to the scaffold materials used in these other studies. The GAG synthesis per cell in the high density group also increased to similar levels compared to the low density group on day 70. These results indicate that our HA scaffold supports chondrogenesis of MSCs at high seeding density, resulting in enhanced functional properties of the engineered cartilage compared to those of low seeding density constructs. A potential practical advantage of a high MSC seeding density in clinical translation is that tissue engineered constructs can attain mechanical functionality in a shorter period, which would expedite any in vitro culture prior to in vivo implantation.
The reduced volumetric swelling of high seeding density constructs compared to the low seeding density ones on day 30 is likely due to higher collagen content and more cellular contraction. As the low seeding density constructs matured over time with increasing collagen content, the swelling decreased. Dynamic loading further reduced swelling of low seeding density constructs on day 70 by increasing collagen deposition in the hydrogels. While a difference in total collagen content was detected between free swelling and dynamically loaded groups at certain time points, there was no significant difference in the gene expression of type II collagen evaluated at the selected time points. The biosynthetic pathway of collagen synthesis is very complex including steps such as mRNA processing, translation on the rough endoplasmic reticulum and post-translational modifications. The discrepancy between gene expression and actual protein production may be due to changes in one or several of these steps.
Effect of dynamic compression on cartilage matrix distribution by MSCs in HA hydrogels
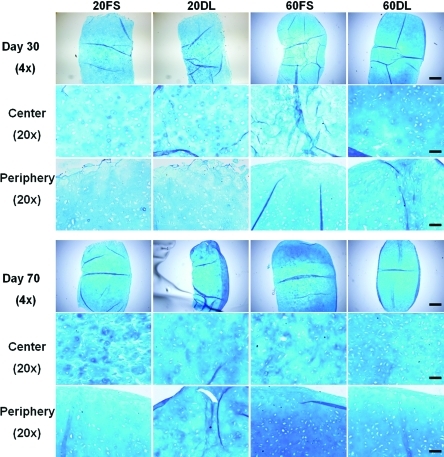
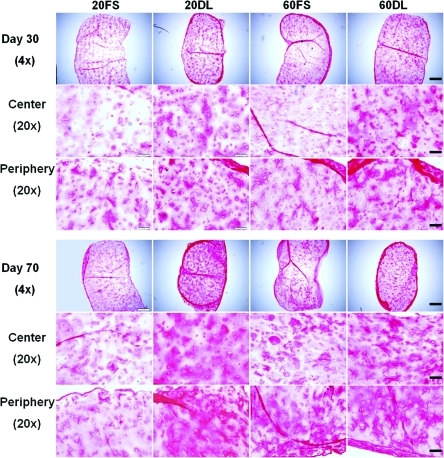
DL also significantly enhanced the spatial distribution of proteoglycans and collagen in HA hydrogels as evidenced by alcian blue and picrosirius red staining, respectively (Fig. 4, 5). 60DL and 20DL constructs exhibited significantly more diffuse staining compared to their free swelling counterparts, especially in the central area of the construct, on day 30 and 70, respectively (Fig. 4, 5). This is especially evident in the collagen production. Immunohistological staining against chondroitin sulfate and type II collagen showed similar trends (data not shown).
FIG. 4.
Alcian blue staining (pH=1.0) (proteoglycans) of all groups after 30 and 70 days of culture; 4× images show the entire construct cross-section, bar=500μm; 20× images show peripheral or central regions of construct, bar=100μm. Color images available online at www.liebertonline.com/tea
FIG. 5.
Picrosirius red staining (collagen) of all the groups after 30 and 70 days of culture; 4× images show entire construct cross-section, bar=500μm; 20× images show peripheral or central regions of the construct, bar=100μm. Color images available online at www.liebertonline.com/tea
In addition to elevated levels of GAG and collagen, the improved mechanical properties in the loaded constructs may also result from a more uniform matrix distribution between encapsulated cells. In contrast to previous studies performed with agarose scaffolds, dynamic loading initiated soon after MSC encapsulation (3 days after) did not hinder chondrogenesis or slow maturation in these MSC-seeded HA hydrogels,24,48 However, histology staining also revealed more cartilage matrix deposition in the periphery of the constructs compared to the interior under FS conditions, especially in high seeding density constructs. This uneven spatial distribution of cartilage matrix is likely due to diffusion limitations into the HA hydrogels, as reported previously.49,50 Previous studies showed that DL not only enhances chondrogenesis but may also increase nutrient transport into the hydrogel constructs, leading to significantly enhanced production and distribution of cartilage matrix.24,51 Histological staining showed that DL mitigated these large-scale spatial heterogeneities in matrix distribution within HA hydrogels, possibly by enhancing nutrient transport. The earlier response to loading by high density constructs compared to low density constructs could indicate a more pronounced beneficial effect of loading-induced transport of nutrients or TGF-β3 in high density constructs, which would likely suffer from more severe nutrient limitations at earlier time points compared to lower seeding densities. Findings from this study showed that these beneficial effects of mechanical loading are preserved in photocrosslinked HA hydrogels with varying MSC seeding densities. However, loading seemed to have diminishing effects on high density constructs over time. This indicates that a high seeding density of MSCs in HA hydrogels may enhance matrix distribution and therefore result in superior mechanical properties compared to low seeding density, independent of loading after long term culture.
Effect of dynamic compression on hypertrophy of chondrogenically induced MSCs
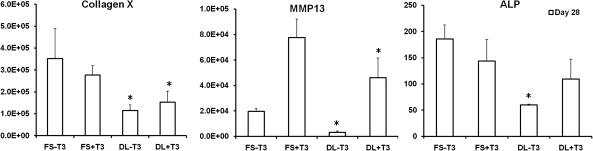
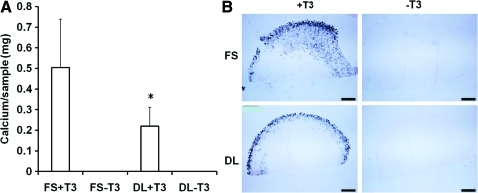
When compared to FS group, the expression of the primary hypertrophic markers, including type X collagen, matrix metalloproteinase 13, and alkaline phosphatase (ALP), were significantly decreased by dynamic loading in both the presence (+T3) or absence (−T3) of hypertrophic factors (T3, β-gly) (Fig. 6). Quantification of calcium content and Von Kossa staining showed that while no calcification was observed in the absence of T3 and β-gly, DL significantly reduced the degree of calcification in MSC-laden HA hydrogels in the presence of these factors (Fig. 7).
FIG. 6.
Expression of selected hypertrophic markers in fold change on day 28 for cells at the 20 million cells/ml seeding density, *p<0.05 vs. FS+T3/−T3 group (n=4).
FIG. 7.
Calcium content (A) and Von Kossa staining (B) of the HA hydrogels on day 30 for cells at the 20 million cells/ml seeding density, *p<0.05 vs. FS+T3 group (n=4); bar=500μm. Color images available online at www.liebertonline.com/tea
In addition to improving overall construct properties, DL suppressed hypertrophic conversion and calcium deposition within chondrogenically induced MSC-seeded HA constructs. To our knowledge, this is the first study to demonstrate the inhibitory effect of mechanical loading on MSC hypertrophy in tissue engineered cartilage under bioreactor culture. Interestingly, in the absence of T3 and β-glycerophosphate there is no calcification observed despite significant upregulation of hypertrophic markers such as alkaline phosphatase and type X collagen (FS-T3, DL-T3). Mineralization was only observed when T3 and β-glycerophosphate was supplemented in the media (FS+T3, DL+T3). Furthermore, the mineralization was mostly located in the periphery of the engineered cartilage constructs. Thus, while DL could act to dislodge forming calcification at these edges, this is unlikely to be the mechanism given the sharp decline in hypertrophic gene expression we observed with loading. In contrast to these in vitro findings, constructs previously implanted subcutaneously in nude mice for a similar duration showed extensive calcification throughout the entire construct.30 Thus, there is a dependence on culture environment on the distribution of calcification.
Mechanical signals have been shown to regulate the expression of IHH, PTHrP, TGF-β, and its receptor.32–35,52 The IHH-PTHrP negative feedback pathway regulates hypertrophic differentiation of both articular and growth plate chondrocytes to maintain the zonal structure of cartilage.53–56 Our unpublished data and previous work also showed that TGF-β is an inhibitor of MSC hypertrophy.57 These mechanically responsive molecular pathways could have contributed to the observed suppression in the gene expression of hypertrophic markers and mineralization by MSCs. However, mechanical loading failed to completely arrest the hypertrophic differentiation of MSCs. In this study, a loading regimen of 4 h per day was adopted due to its proved efficacy in promoting cartilage matrix production and distribution by MSCs. To further avert hypertrophy, longer loading durations could be pursued. Future studies to establish optimal loading regimens are warranted. A previous study by our group also showed that co-culture of MSCs and a small fraction of articular chondrocytes in HA hydrogels not only enhanced chondrogenesis of MSCs, but also reduced the expression of hypertrophic markers by MSCs.58 Thus, there may be a synergistic effect of coculture and mechanical loading on stabilizing the chondrogenic phenotype of MSCs.
The clinical implication of these findings is that joint loading, or in vitro loading in bioreactors in cases where constructs are to be pre-cultured before implantation, may enhance the maturation of engineered cartilage and inhibit hypertrophic differentiation of MSCs, resulting in superior outcomes compared to immobilization of joint. However, it should be noted that the highest mechanical stiffness of engineered cartilage achieved in this study is still well below that of the native cartilage tissue. On the other hand, for animal trials it is still to be determined what level of mechanical maturity will be required for the engineered cartilage implants to survive in load bearing joints. Additional factors, including the size and type of defects and the mobility of the operated joints after surgery, may also influence the survival of the implants. In regional defects of limited dimensions the implants will be subjected to partially confined loading due to lateral constraint from the surrounding native tissue and will likely exhibit higher resistance to compression than that measured in unconfined testing as reported in this study.
Conclusions
Our findings demonstrate that dynamic compressive loading significantly enhances cartilage matrix production and distribution by human MSCs in HA hydrogels, leading to improved properties. These outcomes were dependent on both the overall culture time and the density of cells initially encapsulated within the hydrogels. Furthermore, the expression of major hypertrophic genes and matrix mineralization were significantly reduced with daily compressive loading. These findings not only indicate the importance of mechanical preconditioning in achieving mature tissue engineered cartilage using MSCs prior to implantation, but also highlight the potential benefit of joint loading in the stabilization and development of implanted engineered cartilage in vivo.
Acknowledgment
This work was supported by National Institutes of Health grant R01 EB008722.
Disclosure Statement
No competing financial interests exist.
References
- 1.Erickson I.E. Huang A.H. Chung C. Li R.T. Burdick J.A. Mauck R.L. Differential maturation and structure-function relationships in mesenchymal stem cell- and chondrocyte-seeded hydrogels. Tissue Eng Part A. 2009;15:1041. doi: 10.1089/ten.tea.2008.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung C. Burdick J.A. Engineering cartilage tissue. Adv Drug Deliv Rev. 2008;60:243. doi: 10.1016/j.addr.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams C.G. Kim T.K. Taboas A. Malik A. Manson P. Elisseeff J. In vitro chondrogenesis of bone marrow-derived mesenchymal stem cells in a photopolymerizing hydrogel. Tissue Eng. 2003;9:679. doi: 10.1089/107632703768247377. [DOI] [PubMed] [Google Scholar]
- 4.Hosseini A. Hogg D.A. The effects of paralysis on skeletal development in the chick embryo. II. Effects on histogenesis of the tibia. J Anat. 1991;177:169. [PMC free article] [PubMed] [Google Scholar]
- 5.Mikic B. Isenstein A.L. Chhabra A. Mechanical modulation of cartilage structure and function during embryogenesis in the chick. Ann Biomed Eng. 2004;32:18. doi: 10.1023/b:abme.0000007787.39262.a7. [DOI] [PubMed] [Google Scholar]
- 6.Williamson A.K. Chen A.C. Masuda K. Thonar E.J. Sah R.L. Tensile mechanical properties of bovine articular cartilage: variations with growth and relationships to collagen network components. J Orthop Res. 2003;21:872. doi: 10.1016/S0736-0266(03)00030-5. [DOI] [PubMed] [Google Scholar]
- 7.Erickson I.E. van Veen S.C. Sengupta S. Kestle S.R. Mauck R.L. Cartilage Matrix Formation by Bovine Mesenchymal Stem Cells in Three-dimensional Culture Is Age-dependent. Clin Orthop Relat Res. 2011;469:2744. doi: 10.1007/s11999-011-1869-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williamson A.K. Masuda K. Thonar E.J. Sah R.L. Growth of immature articular cartilage in vitro: correlated variation in tensile biomechanical and collagen network properties. Tissue Eng. 2003;9:625. doi: 10.1089/107632703768247322. [DOI] [PubMed] [Google Scholar]
- 9.Palmoski M. Perricone E. Brandt K.D. Development and reversal of a proteoglycan aggregation defect in normal canine knee cartilage after immobilization. Arthritis Rheum. 1979;22:508. doi: 10.1002/art.1780220511. [DOI] [PubMed] [Google Scholar]
- 10.Setton L.A. Mow V.C. Howell D.S. Mechanical behavior of articular cartilage in shear is altered by transection of the anterior cruciate ligament. J Orthop Res. 1995;13:473. doi: 10.1002/jor.1100130402. [DOI] [PubMed] [Google Scholar]
- 11.Gray M.L. Pizzanelli A.M. Grodzinsky A.J. Lee R.C. Mechanical and physiocochemical determinants of the chondrocyte biosynthetic response. J Orthop Res. 1988;6:777. doi: 10.1002/jor.1100060602. [DOI] [PubMed] [Google Scholar]
- 12.Guilak F. Meyer B.C. Ratcliffe A. Mow V.C. The effects of matrix compression on proteoglycan metabolism in articular cartilage explants. Osteoarthritis Cartilage. 1994;2:91. doi: 10.1016/s1063-4584(05)80059-7. [DOI] [PubMed] [Google Scholar]
- 13.Sah R.L. Kim Y.J. Doong J.Y. Grodzinsky A.J. Plaas A.H. Sandy J.D. Biosynthetic response of cartilage explants to dynamic compression. J Orthop Res. 1989;7:619. doi: 10.1002/jor.1100070502. [DOI] [PubMed] [Google Scholar]
- 14.Valhmu W.B. Stazzone E.J. Bachrach N.M. Saed-Nejad F. Fischer S.G. Mow V.C., et al. Load-controlled compression of articular cartilage induces a transient stimulation of aggrecan gene expression. Arch Biochem Biophys. 1998;353:29. doi: 10.1006/abbi.1998.0633. [DOI] [PubMed] [Google Scholar]
- 15.Buschmann M.D. Kim Y-J. Wong M. Frank E. Hunziker E.B. Grodzinsky A.J. Stimulation of aggrecan synthesis in cartilage explants by cyclic loading is localized to regions of high interstitial fluid flow. Arch Biochem Biophys. 1999;366:1. doi: 10.1006/abbi.1999.1197. [DOI] [PubMed] [Google Scholar]
- 16.Kim Y.J. Sah R.L. Grodzinsky A.J. Plaas A.H. Sandy J.D. Mechanical regulation of cartilage biosynthetic behavior: physical stimuli. Arch Biochem Biophys. 1994;311:1. doi: 10.1006/abbi.1994.1201. [DOI] [PubMed] [Google Scholar]
- 17.Grodzinsky A.J. Levenston M.E. Jin M. Frank E.H. Cartilage tissue remodeling in response to mechanical forces. Annu Rev Biomed Eng. 2000;2:691. doi: 10.1146/annurev.bioeng.2.1.691. [DOI] [PubMed] [Google Scholar]
- 18.Mauck R.L. Byers B.A. Yuan X. Tuan R.S. Regulation of cartilaginous ECM gene transcription by chondrocytes and MSCs in 3D culture in response to dynamic loading. Biomech Model Mechanobiol. 2007;6:113. doi: 10.1007/s10237-006-0042-1. [DOI] [PubMed] [Google Scholar]
- 19.Campbell J.J. Lee D.A. Bader D.L. Dynamic compressive strain influences chondrogenic gene expression in human mesenchymal stem cells. Biorheology. 2006;43:455. [PubMed] [Google Scholar]
- 20.Pelaez D. Huang C.Y. Cheung H.S. Cyclic compression maintains viability and induces chondrogenesis of human mesenchymal stem cells in fibrin gel scaffolds. Stem Cells Dev. 2009;18:93. doi: 10.1089/scd.2008.0030. [DOI] [PubMed] [Google Scholar]
- 21.Chung C. Burdick J.A. Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis. Tissue Eng Part A. 2009;15:243. doi: 10.1089/ten.tea.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung C. Beecham M. Mauck R.L. Burdick J.A. The influence of degradation characteristics of hyaluronic acid hydrogels on in vitro neocartilage formation by mesenchymal stem cells. Biomaterials. 2009;30:4287. doi: 10.1016/j.biomaterials.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erickson I.E. Huang A.H. Sengupta S. Kestle S. Burdick J.A. Mauck R.L. Macromer density influences mesenchymal stem cell chondrogenesis and maturation in photocrosslinked hyaluronic acid hydrogels. Osteoarthritis Cartilage. 2009;17:1639. doi: 10.1016/j.joca.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang A.H. Farrell M.J. Kim M. Mauck R.L. Long-term dynamic loading improves the mechanical properties of chondrogenic mesenchymal stem cell-laden hydrogel. Eur Cell Mater. 2010;19:72. doi: 10.22203/ecm.v019a08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kavalkovich K.W. Boynton R.E. Murphy J.M. Barry F. Chondrogenic differentiation of human mesenchymal stem cells within an alginate layer culture system. In Vitro Cell Dev Biol Anim. 2002;38:457. doi: 10.1290/1071-2690(2002)038<0457:cdohms>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 26.Hui T.Y. Cheung K.M. Cheung W.L. Chan D. Chan B.P. In vitro chondrogenic differentiation of human mesenchymal stem cells in collagen microspheres: influence of cell seeding density and collagen concentration. Biomaterials. 2008;29:3201. doi: 10.1016/j.biomaterials.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Huang A.H. Stein A. Tuan R.S. Mauck R.L. Transient exposure to transforming growth factor beta 3 improves the mechanical properties of mesenchymal stem cell-laden cartilage constructs in a density-dependent manner. Tissue Eng Part A. 2009;15:3461. doi: 10.1089/ten.tea.2009.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pelttari K. Winter A. Steck E. Goetzke K. Hennig T. Ochs B.G., et al. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54:3254. doi: 10.1002/art.22136. [DOI] [PubMed] [Google Scholar]
- 29.Scotti C. Tonnarelli B. Papadimitropoulos A. Scherberich A. Schaeren S. Schauerte A., et al. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc Natl Acad Sci USA. 2010;107:7251. doi: 10.1073/pnas.1000302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bian L. Zhai D.Y. Tous E. Rai R. Mauck R.L. Burdick J.A. Enhanced MSC chondrogenesis following delivery of TGF-beta3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials. 2011;32:6425. doi: 10.1016/j.biomaterials.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo X. Park H. Young S. Kretlow J.D. van den Beucken J.J. Baggett L.S., et al. Repair of osteochondral defects with biodegradable hydrogel composites encapsulating marrow mesenchymal stem cells in a rabbit model. Acta Biomater. 2010;6:39. doi: 10.1016/j.actbio.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu Q-q. Zhang Y. Chen Q. Indian hedgehog is an essential component of mechanotransduction complex to stimulate chondrocyte proliferation. J Biol Chem. 2001;276:35290. doi: 10.1074/jbc.M101055200. [DOI] [PubMed] [Google Scholar]
- 33.Tang G.H. Rabie A.B. Hagg U. Indian hedgehog: a mechanotransduction mediator in condylar cartilage. J Dent Res. 2004;83:434. doi: 10.1177/154405910408300516. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka N. Ohno S. Honda K. Tanimoto K. Doi T. Ohno-Nakahara M., et al. Cyclic mechanical strain regulates the PTHrP expression in cultured chondrocytes via activation of the Ca2+ channel. J Dent Res. 2005;84:64. doi: 10.1177/154405910508400111. [DOI] [PubMed] [Google Scholar]
- 35.Chen X. Macica C.M. Nasiri A. Broadus A.E. Regulation of articular chondrocyte proliferation and differentiation by indian hedgehog and parathyroid hormone-related protein in mice. Arthritis Rheum. 2008;58:3788. doi: 10.1002/art.23985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mueller M.B. Tuan R.S. Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum. 2008;58:1377. doi: 10.1002/art.23370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smeds K.A. Pfister-Serres A. Miki D. Dastgheib K. Inoue M. Hatchell D.L., et al. Photocrosslinkable polysaccharides for in situ hydrogel formation. J Biomed Mater Res. 2001;54:115. doi: 10.1002/1097-4636(200101)54:1<115::aid-jbm14>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 38.Byers B.A. Mauck R.L. Chiang I.E. Tuan R.S. Transient exposure to transforming growth factor beta 3 under serum-free conditions enhances the biomechanical and biochemical maturation of tissue-engineered cartilage. Tissue Eng Part A. 2008;14:1821. doi: 10.1089/ten.tea.2007.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lima E.G. Bian L. Ng K.W. Mauck R.L. Byers B.A. Tuan R.S., et al. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-beta3. Osteoarthritis Cartilage. 2007;15:1025. doi: 10.1016/j.joca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mauck R.L. Soltz M.A. Wang C.C. Wong D.D. Chao P.H. Valhmu W.B., et al. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122:252. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 41.Mauck R.L. Soltz M.A. Wang CC-B. Wong D.D. Chao P-HG. Valhmu W.B., et al. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122:252. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 42.McGowan K.B. Kurtis M.S. Lottman L.M. Watson D. Sah R.L. Biochemical quantification of DNA in human articular and septal cartilage using PicoGreen and Hoechst 33258. Osteoarthritis Cartilage. 2002;10:580. doi: 10.1053/joca.2002.0794. [DOI] [PubMed] [Google Scholar]
- 43.Farndale R.W. Buttle D.J. Barrett A.J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochimica et biophysica acta. 1986;883:173. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 44.Hollander A.P. Heathfield T.F. Webber C. Iwata Y. Bourne R. Rorabeck C., et al. Increased damage to type II collagen in osteoarthritic articular cartilage detected by a new immunoassay. J Clin Invest. 1994;93:1722. doi: 10.1172/JCI117156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahrens P.B. Solursh M. Reiter R.S. Stage-related capacity for limb chondrogenesis in cell culture. Dev Biol. 1977;60:69. doi: 10.1016/0012-1606(77)90110-5. [DOI] [PubMed] [Google Scholar]
- 46.Stockwell R.A. The interrelationship of cell density and cartilage thickness in mammalian articular cartilage. J Anat. 1971;109:411. [PMC free article] [PubMed] [Google Scholar]
- 47.Ponticiello M.S. Schinagl R.M. Kadiyala S. Barry F.P. Gelatin-based resorbable sponge as a carrier matrix for human mesenchymal stem cells in cartilage regeneration therapy. J Biomed Mater Res. 2000;52:246. doi: 10.1002/1097-4636(200011)52:2<246::aid-jbm2>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 48.Thorpe S.D. Buckley C.T. Vinardell T. O'Brien F.J. Campbell V.A. Kelly D.J. Dynamic compression can inhibit chondrogenesis of mesenchymal stem cells. Biochemical and Biophysical Research Communications. 2008;377:458. doi: 10.1016/j.bbrc.2008.09.154. [DOI] [PubMed] [Google Scholar]
- 49.Kelly T.N. Ng K.W. Wang C-CB. Ateshian G.A. Hung C.T. Spatial and temporal development of chondroctye-seeded agarose constructs in free-swelling and dynamically loaded cultures. J Biomech. 2006;39:1489. doi: 10.1016/j.jbiomech.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 50.Bian L. Angione S.L. Ng K.W. Lima E.G. Williams D.Y. Mao D.Q., et al. Influence of decreasing nutrient path length on the development of engineered cartilage. Osteoarthritis Cartilage. 2009;17:677. doi: 10.1016/j.joca.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Albro M.B. Chahine N.O. Li R. Yeager K. Hung C.T. Ateshian G.A. Dynamic loading of deformable porous media can induce active solute transport. J Biomech. 2008;41:3152. doi: 10.1016/j.jbiomech.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang C.Y. Reuben P.M. Cheung H.S. Temporal expression patterns and corresponding protein inductions of early responsive genes in rabbit bone marrow-derived mesenchymal stem cells under cyclic compressive loading. Stem Cells. 2005;23:1113. doi: 10.1634/stemcells.2004-0202. [DOI] [PubMed] [Google Scholar]
- 53.Zhang M. Xie R. Hou W. Wang B. Shen R. Wang X., et al. PTHrP prevents chondrocyte premature hypertrophy by inducing cyclin-D1-dependent Runx2 and Runx3 phosphorylation, ubiquitylation and proteasomal degradation. J Cell Sci. 2009;122:1382. doi: 10.1242/jcs.040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo J. Chung U.I. Yang D. Karsenty G. Bringhurst F.R. Kronenberg H.M. PTH/PTHrP receptor delays chondrocyte hypertrophy via both Runx2-dependent and -independent pathways. Dev Biol. 2006;292:116. doi: 10.1016/j.ydbio.2005.12.044. [DOI] [PubMed] [Google Scholar]
- 55.St-Jacques B. Hammerschmidt M. McMahon A.P. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;13:2072. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vortkamp A. Lee K. Lanske B. Segre G.V. Kronenberg H.M. Tabin C.J. Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science. 1996;273:613. doi: 10.1126/science.273.5275.613. [DOI] [PubMed] [Google Scholar]
- 57.Mello M.A. anf Tuan R.S. Effects of TGF-beta1 and triiodothyronine on cartilage maturation: in vitro analysis using long-term high-density micromass cultures of chick embryonic limb mesenchymal cells. J Orthop Res. 2006;24:2095. doi: 10.1002/jor.20233. [DOI] [PubMed] [Google Scholar]
- 58.Bian L. Zhai D.Y. Mauck R.L. Burdick J.A. Coculture of human mesenchymal stem cells and articular chondrocytes reduces hypertrophy and enhances functional properties of engineered cartilage. Tissue Eng Part A. 2011;17:1137. doi: 10.1089/ten.tea.2010.0531. [DOI] [PMC free article] [PubMed] [Google Scholar]