Abstract
Calcium-based minerals have consistently been shown to stimulate osteoblastic behavior in vitro and in vivo. Thus, use of such minerals in biomaterial applications has become an effective method to enhance bone tissue engineered constructs. In the present study, for the first time, human bone marrow stromal cells (hBMSC) were osteogenically differentiated on scaffolds consisting only of hydroxyapatite (HAp)-loaded poly(d,l-lactic acid-co-glycolic acid) (PLGA) microspheres of high monodispersity. Scaffold formulations included 0, 5, 10, and 20 wt% Hap, and the hBMSC were cultured for 6 weeks. Results demonstrated suppression of some osteogenic genes during differentiation in the HAp group, but higher end-point glycosaminoglycan and collagen content in 10% and 20% HAp samples, as evidenced by biochemical tests, histology, and immunohistochemistry. After 6 weeks of culture, constructs with 0% and 5% HAp had average compressive moduli of 0.7±0.2 and 1.5±0.9 kPa, respectively, whereas constructs with 10% and 20% HAp had higher average moduli of 17.6±4.6 and 18.9±8.1 kPa, respectively. The results of this study indicate that HAp inclusion in microsphere-based scaffolds could be implemented as a physical gradient in combination with bioactive signal gradients seen in previous iterations of these microsphere-based scaffolds to enhance osteoconduction and mechanical integrity of a healing site.
Introduction
Osteochondral tissue engineering, the field of tissue engineering focusing on regenerative solutions for bone and cartilage, has provided myriad potential designs and unique applications.1–8 Many osteochondral tissue engineering studies use three-dimensional (3-D) structures made not only of biocompatible materials, but also of materials that occur naturally in the tissues they aim to mimic and regenerate.
For bone tissue engineering, approaches have involved the incorporation of calcium-based minerals such as tricalcium phosphate (TCP)9–12 and hydroxyapatite (HAp).10,13–15 The osteoblastic response to HAp is well documented and has been shown to enhance implant integration in vivo.10,13,16,17 With regard to osteochondral designs, mineral additives are typically isolated to the bone-like side of 3-D scaffolds, yielding stratified or continuously graded designs. Stratified designs exhibit a sharp physical transition between bone-like and cartilage-like regions, whereas continuous gradients employ a smooth transition between scaffolding layers.18–24 Although microspheres have been used in tissue engineering for stratified designs,25–27 the use of continuously graded microsphere-based scaffolds is still limited.28,29 The combination of continuously graded microsphere-only scaffolds with mineral incorporation, which provides a continuous gradient in macroscopic material composition, has previously been demonstrated as a method,30 but the long-term response of cells in this environment has not been investigated. To address osteoblastic cell response, the most logical step is to evaluate the 3-D performance of homogenous microsphere-based scaffolds with mineral incorporation before using a continuously-graded design, as in the current study. The advantages of this approach relate to the ability to tightly control a gradient in physical or chemical properties across the length of the scaffold by using each single microsphere as a “building block.” The current study, for the first time, used a precision particle fabrication technology28,30–32 to make poly(d,l-lactic acid-co-glycolic acid) (PLGA) microspheres loaded with HAp nanoparticles. The microspheres were used to construct 3-D scaffolds with various HAp contents on which human bone marrow stromal cells (hBMSC) were osteogenically differentiated for 6 weeks. The objective was to determine how HAp incorporation might affect osteogenesis in terms of gene expression, biochemical activity, tissue synthesis, and mechanical integrity. The main hypothesis was that greater HAp content would enhance osteoblastic behavior, including bone-relevant biochemical synthesis and gene upregulation. The results have implications for how best to use HAp in combination with a previously reported continuously graded design using controlled release of growth factors.28
Materials and Methods
Preparation of HAp-loaded microspheres
HAp (Sigma Aldrich, St. Louis, MO) was mixed with PLGA (50:50 lactic acid:glycolic acid, acid end group, molecular weight ∼38,000 Da, Lakeshore Biomaterials, Birmingham, AL) dissolved in dichloromethane (DCM) so that the total solids content was 20 w/v%. The final mixture was then sonicated (50% amplitude, 30 seconds). Using continuously stirred PLGA-HAp emulsions, uniform HAp-loaded PLGA microspheres with a nominal diameter of approximately 240 μm were prepared using technology from our recent studies.28,31,32 Briefly, using acoustic excitation produced using an ultrasonic transducer, regular jet instabilities were created in the polymer stream that produced uniform polymer droplets. An annular carrier nonsolvent stream of 0.5% w/v poly (vinyl alcohol) (PVA, 88% hydrolyzed, 25,000 Da, Polysciences, Inc., Warrington, PA) in deionized water (DI H2O) surrounding the droplets was produced using a nozzle coaxial to the needle. The emanated polymer–HAp–carrier streams flowed into a beaker containing the nonsolvent at 0.5% w/v, with an additional 1.25% w/v Pluronic F-127 (Sigma Aldrich) in DI H2O to prevent aggregation of the particles. Incipient polymer droplets were stirred for 3 to 4 hours to allow solvent evaporation and then filtered and rinsed with DI H2O to remove residual PVA and stored at −20°C (Fig. 1). Blank control microspheres were prepared in a similar manner with only PLGA. After 48 hours of lyophilization, the size distribution of microsphere preparations was determined using a Coulter Multisizer 3 (Beckman Coulter, Inc., Fullerton, CA) equipped with a 560-μm aperture.
FIG. 1.
Microsphere and scaffold fabrication process. Top left: Precision particle fabrication makes microspheres with high monodispersity. Top right: Microspheres are loaded into a mold, wetted, and sintered for 60 minutes with an ethanol–acetone mixture. Bottom: Microsphere-based scaffolds with varying weight fractions of hydroxyapatite (HAp) are seeded with pooled human bone marrow stromal cells (hBMSC) from three different donors.
Scaffold fabrication
Scaffolds were prepared using our recently reported technology.28,32 Briefly, lyophilized HAp-loaded microspheres were loaded into a cylindrical mold (3.4-mm diameter, 3.0 cm high). Using a filter (particle retention>3 μm) at the bottom of the mold, DI H2O was pushed through the mold to wet the microsphere stacks, which were approximately 2.5 mm high (Fig. 1). The stacked microspheres were then sintered using 5% v/v acetone in ethanol treatment for 1 hour.32 The molds (containing the scaffolds) were freeze-dried for 48 hours and then retrieved and stored at −20°C. Blank scaffolds with no HAp were prepared in a similar manner. Four experimental scaffold groups were investigated, with varying weight fractions of HAp in PLGA (0, 5, 10, and 20 wt% HAp; Fig. 1).
Cell expansion and seeding
hBMSC from three different donors at passage 1 (P1) (plated once) were purchased from StemCell Technologies (Vancouver, Canada). Frozen hBMSC were thawed, plated at a density of 4,000 cells/cm2, and cultured. The culture medium for hBMSC consisted of low-glucose Dulbecco's modified Eagle medium (DMEM), 1% penicillin–streptomycin (P/S), and 10% fetal bovine serum (FBS, certified, cat # 16000-036) (all from Invitrogen Life Technologies, Carlsbad, CA). When the cells were 80% to 90% confluent, they were trypsinized and re-plated at 4,000 cells/cm2. Seeding was performed when cells reached P4. Scaffolds (diameter 3.2 mm, height 2.5 mm) were sterilized using ethylene oxide for 12 hours, allowed to ventilate overnight after sterilization, and placed in a 24-well plate. Cells (P4) were resuspended in medium at a concentration of approximately 22×106 cells/mL. Next, 10 μL (∼50% of the scaffold volume, approximately corresponding to the pore volume32) of cell solution was placed directly onto the top of the scaffold, which infiltrated the scaffold via capillary action. Cells were allowed to attach to the scaffolds for 1 hour. Then 1.5 mL of culture medium was added, and the scaffolds were cultured statically for 24 hours. After the initial 24 hours (Week 0), the culture medium was completely replaced with 1.5 mL of osteogenic medium consisting of low-glucose DMEM, 1% P/S, 50 μg/mL L-ascorbic acid (Sigma Aldrich), 10mM β-glycerophosphate (disodium salt, pentahydrate; Calbiochem, San Diego, CA), and 100 nM dexamethasone (Sigma Aldrich). Every 48 hours for 6 weeks, two-thirds of the culture medium was replaced with fresh osteogenic medium.
Quantitative polymerase chain reaction
In preparation for reverse transcriptase polymerase chain reaction (PCR), samples (n=4) at 0, 1, 2, 3, and 6 weeks were first homogenized in 1 mL of Trizol reagent (Invitrogen), and the RNA was isolated according to the manufacturer's guidelines. Isolated RNA was cleaned using an RNeasy spin column method (Qiagen, Valencia, CA) and converted to complementary DNA using a TaqMan High Capacity kit (Applied Biosystems, Foster City, CA) in a BioRad ThermoCycler. TaqMan Gene expression assays from Applied Biosystems for glyceraldehyde 3-phosphate dehydrogenase (GAPDH, Hs99999905_m1), bone gamma-carboxyglutamate protein (BGLAP, osteocalcin, Hs01587813_g1), collagen type I (COL1A1, Hs00164004_m1), integrin-binding sialoprotein (IBSP, Hs00173720_m1), runt-related transcription factor 2 (RUNX2, Hs00231692_m1), and secreted phosphoprotein 1 (SPP1, osteopontin, Hs00959010_m1) were run in an Applied Biosystems 7500 Fast Real-time PCR System. A 2−ΔΔCt method was used to evaluate the relative level of expression for each target gene.33 For quantification, the blank constructs were designated as a calibrator group and GAPDH expression as an endogenous control.
Biochemical analyses
Constructs (n=4) were analyzed for matrix production using biochemical assays at 0, 1, 2, 3, and 6 weeks. First, a digestion solution consisting of 125 μg/mL papain (from papaya latex), 5mM N-acetyl cysteine, 5mM ethylenediaminetetraacetic acid, and 100mM potassium phosphate buffer (20mM monobasic potassium phosphate, 79mM dibasic potassium phosphate) in DI H2O was mixed (all reagents from Sigma Aldrich). Constructs were removed from culture in a sterile manner, placed in microcentrifuge tubes, homogenized with the papain solution, and allowed to digest overnight in a 60°C water bath. The digested scaffolds were then centrifuged at 10,000 rpm for 5 minutes to pellet fragments of polymer and other impurities and stored at −20°C. Later, the supernatant was used to determine DNA, glycosaminoglycan (GAG), and hydroxyproline (HYP) content. DNA content was quantified using the Picogreen assay (Molecular Probes, Eugene, OR) according to the manufacturer's instructions. A conversion factor of 8.5 pg/cell, based on earlier preliminary studies, may be used to convert DNA content to cell number. GAG content was measured using a dimethylmethylene blue assay as recommended by the vendor (Biocolor, Newtownabbey, Northern Ireland). Alkaline phosphatase (ALP) activity was estimated by determining liberated p-nitrophenol (p-NITRO) rate (concentration/μg protein per minute), as described elsewhere.34 Net HYP content was assessed using a modified HYP assay,35 as described in our previous publications.36 A conversion factor of 11.5 can be used to convert HYP mass to collagen mass based on our preliminary studies. In the cases of GAG and HYP content, results for normalized (to DNA) content was analyzed.
Histological and immunohistochemical staining
At 6 weeks, samples (n=2) were fixed in 10% neutral-buffered formalin (NBF) before being equilibrated in optimal cutting temperature embedding medium (Tissue-Tek) overnight at 37°C. The samples were then frozen at −20°C, sectioned at 14 μm perpendicular to the longitudinal axis of the construct, and collected on SuperFrost Plus slides (all materials from Fisher Scientific, Pittsburgh, PA). Alizarin red staining for calcium deposition and von Kossa staining for calcium phosphate was done as described elsewhere.37 Masson's trichrome staining was performed according to the manufacturer's instructions (all stains from Sigma Aldrich). Collagen I immunohistochemistry (IHC) was performed using a Vectastain Elite ABC kit and a VIP visualization reagent (Vector Laboratories, Burlingame, CA), as recommended by the supplier. Primary antibody for collagen type I was purchased through Accurate Chemical (Westbury, NY). Slides were then dehydrated in graded alcohol and cleared in xylene for mounting (solutions from Sigma Aldrich).
Mechanical testing
Mechanical characterization of the constructs (n=3 - 4) was performed using a uniaxial testing apparatus (50 N load cell, Instron Model 5848, Canton, MA) under unconfined compression. Tare-loaded (0.05 N) constructs were compressed to 70% strain at a rate of 10% per minute. Testing was done on dry scaffolds without cells and in phosphate buffered saline (138mM sodium chloride, 2.7mM potassium chloride) at 37°C after 6 weeks of culture. Moduli of elasticity were obtained from the initial linear regions of the stress–strain curves, as previously described.28,30,32
Statistical analyses
Statistical analyses were performed using a single factor analysis of variance (ANOVA) in PASW 18.0 software (SPSS, Inc., Chicago, IL), followed by a Tukey's honestly significant difference post hoc test when significance was detected below the p=0.05 value. All quantitative results (numerical values and representative diagrams) are expressed as the average±standard deviation.
Results
BGLAP expression
Relative expression of BGLAP (Fig. 2) in HAp-loaded constructs did not change during the culture period. BGLAP expression of control (0% HAp) constructs was higher than 5% (p<0.05) and 20% HAp (p<0.05) constructs at week 0 and higher than 10% (p<0.005) and 20% (p<0.005) HAp constructs at week 6. BGLAP expression in control samples dropped between weeks 0 and 1 (p<5×10−5), rose between weeks 1 and 2 (p<0.001), and decreased between weeks 2 and 3 (p<5×10−4).
FIG. 2.
Bone gamma-carboxyglutamate protein (BGLAP) expression. Expression of BGLAP was cyclic for the control groups, with peaks at weeks 0, 2, and 6. BGLAP expression for the HAp groups were largely suppressed from the initial time point through week 6. At week 6, BGLAP expression of 10% and 20% HAp samples was not evident. All values are expressed as the average±standard deviation (n=4), p<0.05, @statistically significant difference from week 0 value, #statistically significant difference from the previous week, *statistically significant difference from the control at that time point.
COL1A1 expression
COL1A1 expression (Fig. 3) in control (0% HAp) constructs exhibited a decrease at week 1 (p<5×10−5) but a subsequent increase at weeks 2 (p<1×10−4) and 6 (p<5×10−4). COL1A1 expression in 5% HAp constructs remained unchanged throughout culture and was less than the control at week 6 (p<1×10−11). In 20% HAp constructs, COL1A1 expression was suppressed (on average, about 20% of the control) during the entire culture period (p<5×10−4). Although COL1A1 expression in 10% HAp constructs was suppressed at week 0 (p<5×10−5), expression increased at week 2 (p<1×10−5) to 3 times the week 0 value and then exhibited a 50% decrease the following week (p<5×10−5).
FIG. 3.
Collagen I (COL1A1) expression. Expression of COL1A1 was cyclic for the control groups, with peaks at weeks 0, 2, and 6. COL1A1 expression for the HAp groups was largely suppressed from the initial time point through week 6. At week 3, COL1A1 expression of 10% HAp samples peaked to a value similar to that of the control then exhibited a decrease the following week. All values are expressed as the average±standard deviation (n=4), p<0.05, @statistically significant difference from week 0 value, #statistically significant difference from the previous week, *statistically significant difference from the control at that time point.
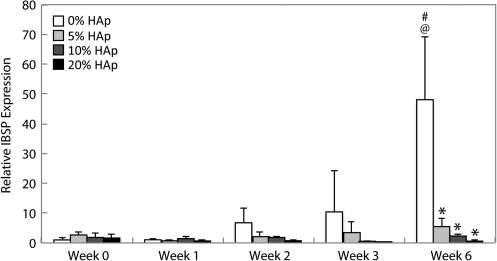
IBSP expression
There were no changes in IBSP expression (Fig. 4) until week 6, when the control (0% HAp) constructs exhibited an expression that was 50 times the value at week 0 (p<1 x 10−11). At week 6, the IBSP expression of all HAp-containing constructs was less than in the control (p<1×10−10).
FIG. 4.
Integrin-binding sialoprotein (IBSP) expression. Expression of IBSP was suppressed in all groups until week 6, when IBSP expression in the control group increased to an average of 50 times the week 0 value. IBSP expression in all HAp groups was suppressed throughout culture. The IBSP expression in HAp samples was less than that in the control at week 6. All values are expressed as the average±standard deviation (n=4), p<0.05, @statistically significant difference from week 0 value, #statistically significant difference from the previous week, *statistically significant difference from the control at that time point.
RUNX2 expression
Increases in RUNX2 expression (Fig. 5) did not occur in any group until week 2 (p<0.005). After week 2, RUNX2 expression in 5% HAp constructs continued to be higher than the week 0 value (p<0.005). At week 2, RUNX2 expression in 10% and 20% HAp constructs was also greater than at week 1 (p<0.001). After peaking at week 2, RUNX2 expression in 10% and 20% HAp constructs decreased by 50% at week 3 (p<0.05).
FIG. 5.
Runt-related transcription factor 2 (RUNX2) expression. RUNX2 expression peaked in all groups at week 2, with the 10% and 20% HAp groups also having expression values higher than the previous week. After the peak in RUNX2 expression, the 10% and 20% HAp groups exhibited a decrease in expression, whereas the control and 5% HAp groups did not. All values are expressed as the average±standard deviation (n=4), p<0.05, @statistically significant difference from week 0 value, #statistically significant difference from the previous week, *statistically significant difference from the control at that time point.
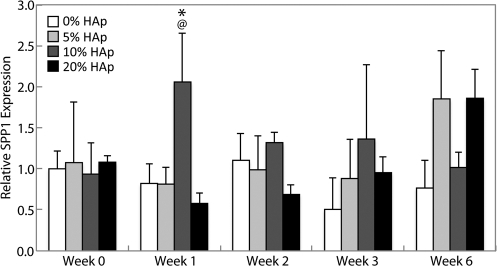
SPP1 expression
There were no statistically significant changes in SPP1 expression (Fig. 6) during the culture period in any group except in 10% HAp constructs, in which expression was 2.5 times the control at week 1 (p<0.05).
FIG. 6.
Secreted phosphoprotein 1 (SPP1) expression. Expression of SPP1 was largely unchanged for all groups throughout the entire culture period, with the exception of a peak in IBSP expression from the 10% HAp group at week 1, which was also higher than that of the control. All values are expressed as the average±standard deviation (n=4), p<0.05, @statistically significant difference from week 0 value, #statistically significant difference from the previous week, *statistically significant difference from the control at that time point.
DNA content
The initial cell seeding efficiency was approximately 59.3±4.5%, with an average cell density of 6.2±0.5 million cells/mL of scaffold in all groups at week 0 (Fig. 7). After week 0, all groups exhibited lower DNA content throughout culture (p<5×10−4). Weekly decreases in DNA content were uniform between groups and were statistically significant at weeks 1 (p<5×10−4) and 2 (p<0.05).
FIG. 7.
DNA content. Statistically significant decreases in DNA content were found uniformly in all groups through week 2. Although DNA content at weeks 3 and 6 was found to be overall lower than at week 0, there was no statistically significant indication that DNA levels continued to decrease past week 2. All values are expressed as the average±standard deviation (n=4), p<0.05 @statistically significant difference from week 0 value, #statistically significant difference from the previous week, *statistically significant difference from the control at that time point.
ALP activity
Constructs with 5% and 20% HAp peaked in ALP activity (Fig. 8) at week 1 (p<0.005) and continued to have activity greater than the week 0 value through week 3 (p<0.05). The ALP activity of 20% HAp constructs at week 1 was greater than in the control (p<0.05). Constructs with 0% and 10% HAp had greater ALP activity at week 3 than at week 0 (p<5×10−7) and the previous week (p<0.005), after which the ALP activity of 10% HAp constructs decreased by week 6 (p<5 ×10−7) snf was lower than the control (p<0.01). The ALP activity of 0% HAp constructs was did not differ significantly at week 6 from week 3.
FIG. 8.
Alkaline phosphatase (ALP) activity. ALP activity in 5% and 20% HAp groups peaked at week 1 and continued to be higher than the week 0 value. ALP activity in the control and 10% HAp groups peaked at week 3. ALP activity of the control group did not decrease by week 6, but that of the 10% HAp group did. All values are expressed as the average±standard deviation (n=4), p<0.05 @statistically significant difference from week 0 value, #statistically significant difference from the previous week, *statistically significant difference from the control at that time point.
GAG production
An increase in GAG/DNA production (Fig. 9) from the week 0 value was evident in 0% HAp constructs as early as week 2 (p<0.02). Constructs with HAp generally did not exhibit overall increases in GAG/DNA production until week 6 (p<5×10−4). At week 6, GAG/DNA production in HAp constructs was also greater than at week 3 (p<0.05). Constructs with 10% and 20% HAp had GAG/DNA levels 3 times greater than the control at week 6 (p<1×10−11). With regard to total GAG content (Fig. 9), there was an increase in the 20% HAp group at week 2 (p<0.05) over the week 0 and 1 values and then a decrease the following week (p<0.001), with no other statistically significant differences.
FIG. 9.
Glycosaminoglycan (GAG) production. Top: Normalized GAG production demonstrated that GAG synthesis per cell was highest in the 10% and 20% HAp samples by week 6, even though the control group had increases over the week 0 value as early as week 2. Bottom: Net GAG production shows a peak in GAG mass at 2 weeks for the 10% HAp group only and a subsequent decrease at week 3. All values are expressed as the average±standard deviation (n=4), p<0.05 @statistically significant difference from week 0 value, #statistically significant difference from the previous week, *statistically significant difference from the control at that time point.
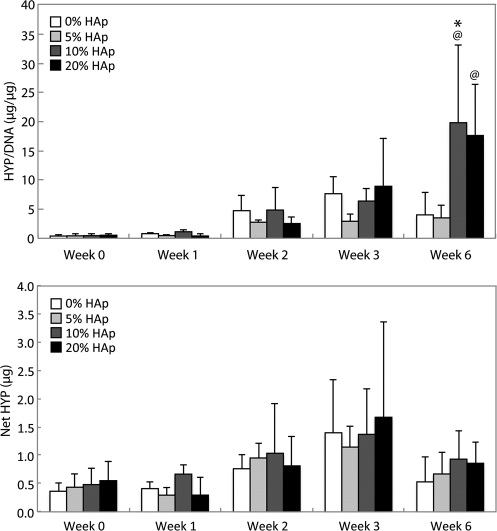
HYP content
There were no increases in HYP/DNA content (Fig. 10) until week 6. At week 6, only constructs with 10% and 20% HAp were found to have greater HYP/DNA content than the week 0 value (p<0.005). The HYP/DNA content for 10% HAp samples at week 6 was 4 times as great as the control (p<0.05). There were no statistically significant changes in net HYP content throughout culture (Fig. 10).
FIG. 10.
Hydroxyproline (HYP) content. Top: Normalized HYP content demonstrated that HYP synthesis per cell was highest in the 10% and 20% HAp samples by week 6. Bottom: There were no statistically significant changes in net HYP content during culture, which may be because of large standard deviations between samples. All values are expressed as the average±standard deviation (n=4), p<0.05 @statistically significant difference from week 0 value, #statistically significant difference from the previous week, *statistically significant difference from the control at that time point.
Histological and immunohistochemical staining
Visual inspection of histological samples showed that construct sizes varied considerably after the culture period (Figs. 11 and 12). All constructs exhibited a decrease in diameter, with the 10% HAp constructs being approximately 2.5 mm in diameter. The 5% and 20% HAp constructs had a diameter of approximately 2.0 mm, with 0% HAp constructs shrinking to 1.0 mm in diameter. The original cylindrical shape was preserved much more uniformly in HAp-containing samples.
FIG. 11.
Histological and immunohistochemical staining. Alizarin red (AR) staining showed large calcium ion nodules in all HAp groups, with 10% and 20% HAp groups having a more-dispersed mineral distribution (red). Von Kossa (VK) staining showed a calcium phosphate distribution similar to that of calcium (brown-black). Masson's trichrome (MT) showed collagen (blue) in all samples, with bright staining in the control group and the appearance of more GAG (orange-red) in the 10% and 20% HAp samples. Collagen type I (CI) showed more-intense staining in the 10% and 20% HAp samples (blue–purple) than in the negative control (CN). Scale bar=500 μm. Color images available online at www.liebertonline.com/tea
FIG. 12.
High-magnification histological and immunohistochemical staining. Alizarin red (AR) staining showed large calcium ion nodules in all HAp groups, with 10% and 20% HAp groups having a more-dispersed mineral distribution (red). Von Kossa (VK) staining showed a calcium phosphate distribution similar to that of calcium (brown–black). Masson's trichrome (MT) showed collagen (blue) in all samples, with bright staining in the control group and an appearance of more GAG (orange-red) in the 10% and 20% HAp samples. Collagen type I (CI) showed more-intense staining in the 10% and 20% HAp samples (blue-purple) than in the negative control (CN). Scale bar=200 μm. Color images available online at www.liebertonline.com/tea
Alizarin red staining for calcium ions in control samples revealed uniform deposition throughout the construct, although staining was much more intense in HAp-loaded constructs. Samples with 5% HAp had a high density of interconnected calcium nodules, whereas these nodules were sparse in 10% and 20% HAp-loaded constructs. The intensity of staining was darkest in the 20% HAp group. Von Kossa staining for calcium phosphate revealed trends commensurate with those of Alizarin red, with light staining in the control constructs and a nodular morphology in HAp-containing samples.
Masson's trichrome also revealed a faint collagen (blue) and GAG (orange-red) network, which outlined the mineral nodules. Cell distribution (dark blue–black) was higher around mineral nodules as well. Collagen staining was most intense in 0% HAp samples, although the area of staining was much larger in 10% and 20% HAp constructs. In the control and 5% HAp samples, the GAG network was more localized to the perimeter of the cylindrical constructs. IHC for collagen I revealed only slight differences in staining intensity from the negative control for 0% and 5% HAp constructs. The presence of collagen I in samples with 10% and 20% HAp was more apparent.
Mechanical testing
Dry scaffolds (Fig. 13) with 0% HAp exhibited an average elastic modulus of 12.0±4.3 MPa, whereas dry scaffolds with 5%, 10%, and 20% HAp had significantly lower average moduli of 0.8±0.8 (p<5×10−4), 3.6±2.1 (p<0.005), and 2.4±1.6 MPa (p<0.001), respectively. After 6 weeks of culture, constructs with 0% and 5% HAp had average moduli of 0.7±0.2 and 1.5±0.9 kPa, respectively, which were not found to be significantly different from one another. Constructs with 10% and 20% HAp had average moduli of 17.6±4.6 (p<0.005) and 18.9±8.1 kPa (p<0.005), respectively, at week 6.
FIG. 13.
Mechanical integrity. Top: Dry construct compression testing showed that HAp inclusion yielded a lower elastic modulus than poly(d,l-lactic acid-co-glycolic acid)-only microsphere-based scaffolds. Bottom: After 6 weeks of cell culture, the trend in elastic modulus was reversed, with 10% and 20% HAp constructs exhibiting the highest elastic moduli under hydrated conditions. All values are expressed as the average±standard deviation (n=3 - 4), p<0.05 *statistically significant difference from the control at that time point.
Discussion
The current study used precision particle fabrication technology to make PLGA microspheres loaded with nanoparticles of HAp. Although there are many studies using hBMSCs and scaffolds of calcium-based minerals, this was the first to evaluate the response of hBMSCs to HAp during osteogenic differentiation on microparticle-based scaffolds created using an ethanol sintering technique. The results have implications for how best to integrate HAp into microparticle-based scaffolds of continuous chemical signal gradients. Expression of BGLAP and COL1A1 in HAp samples was largely suppressed throughout culture. Alternatively, BGLAP and COL1A1 in control samples (PLGA without HAp) had fluctuating expression, with peaks at 0, 2, and 6 weeks. If the fluctuating expression of BGLAP and COL1A1 in control samples were representative of a cyclic process of osteoblast matrix and mineral production, it could be inferred that HAp presence inhibited expression of BGLAP and COL1A1 by creating a substrate environment that was already high in mineral content. In the case of IBSP, inclusion of HAp significantly inhibited gene expression in a dose-independent manner. The considerable difference in expression between the control and experimental samples at later times may suggest that HAp presence on the substrate surface was a strong indicator of IBSP activity during osteogenesis. The largely unchanging expression of SPP1 in most of the experimental groups lends little to the conclusions in this study, except that HAp inclusion might not have had any effect on the gene expression of this terminal-phase protein. Overall, there was an abundance of gene expression evidence, indicating that differentiation down an osteogenic lineage occurred more traditionally within the control group, although gene expression of cells in HAp-loaded constructs suggested that the gene response was altered in many ways because of the mineral presence. Gene expression is only a small facet of a much larger outcome, including biochemical synthesis, matrix production, and eventually, actual performance in vivo. Despite some indications that HAp presence might have adversely affected gene expression, biochemical synthesis demonstrated a protein-level cellular response that may support the inclusion of HAp. Endpoint matrix production per cell was significantly higher than in the control in constructs with HAp. Groups with 10% and 20% HAp delayed the GAG per cell (GAG/DNA) increases until 6 weeks, although this increase was almost 3 times as high as the control at 6 weeks. Similar behavior was seen with HYP production, with 10% and 20% HAp samples having, on average, approximately 4 times the amount of collagen per cell (HYP/DNA) as the control at 6 weeks. ALP activity (p-NITRO liberation) for all HAp groups also increased during culture from the week 0 value. With the prescribed medium conditions in this study, it was shown that ALP activity peaked during the pre-osteoblast stage of differentiation.38
All constructs possessed a mineral matrix of calcium and calcium phosphate (demonstrated by Alizarin red and von Kossa), with HAp groups exhibiting the most-intense staining. These constructs were expected to stain positive for mineral, because HAp is the primary mineral component in mature bone.39 Masson's trichrome and collagen I IHC further contextualized the mineral stains, gene expression, and biochemical synthesis. With regard to trichrome staining, all groups showed evidence of collagen production, with a greater density of GAGs with greater HAp content. IHC also demonstrated that collagen I density increased with HAp concentration, with the most intense staining in 20% HAp constructs. Using the factor of 11.5 to convert HYP mass to collagen mass, the biochemical data supported the notion that collagen mass was at least 2 times as great as GAG mass. Despite suppressed gene expression of COL1A1 in HAp groups during the sampled time points, 10% and 20% HAp constructs produced a modest collagenous matrix by the conclusion of the 6-week culture period.
Mechanical testing was performed on dry scaffolds after fabrication and on mature constructs after 6 weeks of culture. Unconfined compression revealed that the elastic modulus of the control group in dry scaffolds was at least 3 times as large as that of any HAp group. Intuitively, one might be inclined to project that HAp nanoparticle incorporation might increase the elastic modulus proportionally to mineral content, but the phenomenon of a lower elastic modulus in composite scaffolds has been observed in a previous investigation with the microparticle design, which used calcium carbonate and titanium dioxide.30 An explanation for the elastic modulus paradox may relate to poor adhesion and surface tension between the mineral phase and bulk (polymer) phase. A detailed analysis of composite mineral–polymer formulations, including scanning electron microscopy images, will be necessary when developing a method for increasing adhesion between material phases, but after 6 weeks of culture, the elastic modulus of 10% and 20% HAp scaffolds were superior to those of the control. A single reason for this result is difficult to discern without further targeted investigation, but biochemical analysis, histology, and immunohistochemistry indicated a denser mineral, collagen, and GAG network in HAp samples, which may provide a structural insight into observed mechanical integrity. Lastly, because the 6-week elastic moduli were not directly proportional to HAp content, there may be support for the idea that having a minimum of 10% HAp created a synergistic effect between moderating breakdown and creating a denser collagenous network. Overall, 10% and 20% HAp constructs exhibited the most-favorable mechanical integrity after 6 weeks.
With regard to cell number (DNA content), the initial cell seeding efficiency was at best approximately 65%, with an average cell population density of approximately 7 million cells/mL of scaffold at week 0. The initial cell density and seeding efficiency in the current study was similar to those in a previous in vitro investigation using PLGA microparticle–based scaffolds without HAp.28 In the previous in vitro study, cell populations increased over the course of 6 weeks, to an average of 3 times the initial value, but in the current study, statistically significant decreases in cell number were found uniformly in all groups through week 2. Because the decrease in cell numbers was uniform among groups, there is support for the idea that the decrease may have been due to microenvironmental factors independent of HAp presence, such as cell density. The main difference between the current study and the previous in vitro investigation was the height-to-width ratio of the scaffolds. For the current study, this value was approximately 0.8, whereas in the previous report, it was approximately 0.6. Thus, the penetrable distance along the longitudinal axis of the scaffold in the current study was greater, and cellular travel into the center may have been impeded, although histological sections taken perpendicular to the longitudinal axis did not indicate a heterogeneous cellular infiltration pattern.
Lastly, size changes in the scaffolds may have related to mineral presence. The hydroxyapatite used was a nanopowder with a particle size less than 200 μm, with a mostly spherical shape. The physical travel path of the nanoparticles was not explicitly monitored during the study, but it could be reasoned that, during PLGA degradation, the nanoparticles would be free to diffuse into the culture medium. Drastic size changes were most likely due to PLGA. During the earliest stages of PLGA breakdown, the polymeric microspheres take up water and swell. After water creates pores within each microsphere, the bulk begins to degrade more rapidly, which the acidic byproducts of PLGA (lactic acid and glycolic acid) auto-catalyze. The end result is breakdown and diffusion of LA and GA chains into the culture medium and a smaller scaffold. Biosynthesis of GAGs and collagen can also alter the overall size of the constructs.
Additionally, even though there have been many other tissue engineering studies evaluating and supporting the inclusion of HAp to enhance osteogenesis, some investigations have provided evidence that there may be HAp-to-cell concentrations40–42 or types of HAp (sintered vs non-sintered HAp)43 past which there are no dose-dependent advantages to cell growth and viability, gene expression, or biochemical synthesis, concepts that were not explicitly addressed in the current study. Moreover, surface functionalization with arginine-glycine-aspartic acid residues44–48 of the microspheres may lead to greater retention of initial cell number.
Conclusion
The present study evaluated the osteogenic differentiation of hBMSC on HAp-loaded microsphere-based PLGA scaffolds in vitro. Although gene expression revealed that HAp inclusion could have suppressive and supportive roles in osteogenesis of hBMSCs, protein production relevant to bone matrix synthesis, such as GAGs and collagen type I, was more favorable with higher concentrations of HAp. In addition, histological and immunohistochemical characterization revealed bone-relevant tissue synthesis and the maintenance of a mineral network consisting of calcium and calcium phosphate. After 6 weeks of culture, the mechanical integrity of constructs with larger percentages of HAp had higher elastic moduli than the control, with shape and size retention. The major finding was that cells sensed and responded to micro-encapsulated HAp nanoparticles, as evidenced by gene expression, histology, and biochemical content, and that scaffold morphology was affected, as evidenced by mechanical integrity and scaffold dimensions. Only further experimentation will reveal whether HAp inclusion in polymeric microsphere-based scaffolds provides significant advantages for in vivo applications. With the evidence presented, it is reasonable to conclude that HAp addition to microparticle-based scaffolds could easily be used in a gradient-based design,28,32 in which HAp or other calcium-based minerals might enhance the end-point biochemical and mechanical performance of the bone-like region of 3-D tissue engineered constructs, as well as providing “raw materials” for the regenerating tissue, ultimately reducing patient recovery time.
Acknowledgments
The authors would like to acknowledge funding from the National Institutes of Health (NIH R01 AR056347) and from the Pharmaceutical Aspects of Biotechnology Training Grant (NIH/NIGMS T32-GM008359) to support N. H. Dormer.
Disclosure Statement
No competing financial interests exist.
References
- 1.Mikos A. Herring S. Ochareon P. Elisseeff J. Lu H. Kandel R. Schoen F. Toner M. Mooney D. Atala A. Engineering complex tissues. Tissue Eng. 2006;12:3307. doi: 10.1089/ten.2006.12.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mano J. Reis R. Osteochondral defects: present situation and tissue engineering approaches. J Tissue Eng Regen Med. 2007;1:261. doi: 10.1002/term.37. [DOI] [PubMed] [Google Scholar]
- 3.Martin I. Miot S. Barbero A. Jakob M. Wendt D. Osteochondral tissue engineering. J Biomech. 2007;40:750. doi: 10.1016/j.jbiomech.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Chan G. Mooney D. New materials for tissue engineering: towards greater control over the biological response. Trends Biotechnol. 2008;26:382. doi: 10.1016/j.tibtech.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Grayson W. Martens T. Eng G. Radisic M. Vunjak-Novakovic G. Biomimetic approach to tissue engineering. Sem Cell Dev Biol. 2009;20:665. doi: 10.1016/j.semcdb.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hutmacher D. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21:2529. doi: 10.1016/s0142-9612(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 7.Hutmacher D. Schantz J. Lam C. Tan K. Lim T. State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. J Tissue Eng Regen Med. 2007;1:245. doi: 10.1002/term.24. [DOI] [PubMed] [Google Scholar]
- 8.Lee J. Cuddihy M.J. Kotov N.A. Three-dimensional cell culture matrices: state of the art. Tissue Eng. 2008;14:61. doi: 10.1089/teb.2007.0150. [DOI] [PubMed] [Google Scholar]
- 9.Erisken C. Kalyon D. Wang H. Functionally graded electrospun polycaprolactone and β-tricalcium phosphate nanocomposites for tissue engineering applications. Biomaterials. 2008;29:4065. doi: 10.1016/j.biomaterials.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 10.Ahn J.-H. Lee T.-H. Oh J.-S. Kim S.-Y. Kim H.-J. Park I.-K. Choi B.-S. Im G.-I. Novel hyaluronate-atelocollagen/beta-TCP-hydroxyapatite biphasic scaffold for the repair of osteochondral defects in rabbits. Tissue Eng Part A. 2009;15:2595. doi: 10.1089/ten.TEA.2008.0511. [DOI] [PubMed] [Google Scholar]
- 11.Cheng L. Ye F. Yang R. Lu X. Shi Y. Li L. Fan H. Bu H. Osteoinduction of hydroxyapatite/β-tricalcium phosphate bioceramics in mice with a fractured fibula. Acta Biomater. 2009;6:1569. doi: 10.1016/j.actbio.2009.10.050. [DOI] [PubMed] [Google Scholar]
- 12.Gotterbarm T. Richter W. Jung M. Berardi Vilei S. Mainil-Varlet P. Yamashita T. Breusch S. An in vivo study of a growth-factor enhanced, cell free, two-layered collagen–tricalcium phosphate in deep osteochondral defects. Biomaterials. 2006;27:3387. doi: 10.1016/j.biomaterials.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 13.Im G.-I. Ahn J.-H. So-Young K. Choi B.-S. Lee S.-W. A hyaluronate-atelocollagen/beta-TCP-hydroxyapatite biphasic scaffold for the repair of osteochondral defects: a porcine study. Tissue Engi Part A. 2010;16:1189. doi: 10.1089/ten.TEA.2009.0540. [DOI] [PubMed] [Google Scholar]
- 14.Liu C. Han Z. Czernuszka J. Gradient collagen/nanohydroxyapatite composite scaffold: development and characterization. Acta Biomater. 2009;5:661. doi: 10.1016/j.actbio.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 15.Shen H. Hu X. Yang F. Bei J. Wang S. An injectable scaffold: rhBMP-2-loaded poly (lactide-co-glycolide)/hydroxyapatite composite microspheres. Acta Biomater. 2009;6:455. doi: 10.1016/j.actbio.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 16.Daugaard H. Elmengaard B. Bechtold J.E. Jensen T. Soballe K. The effect on bone growth enhancement of implant coatings with hydroxyapatite and collagen deposited electrochemically and by plasma spray. J Biomed Mater Res A. 2009;92:913. doi: 10.1002/jbm.a.32303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kon E. Delcogliano M. Filardo G. Pressato D. Busacca M. Grigolo B. Desando G. Marcacci M. A novel nano-composite multi-layered biomaterial for treatment of osteochondral lesions: technique note and an early stability pilot clinical trial. Injury. 2010;41:693. doi: 10.1016/j.injury.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 18.Keeney M. Pandit A. The osteochondral junction and its repair via bi-phasic tissue engineering scaffolds. Tissue Eng Part B Rev. 2009;15:55. doi: 10.1089/ten.teb.2008.0388. [DOI] [PubMed] [Google Scholar]
- 19.Leong K. Chua C. Sudarmadji N. Yeong W. Engineering functionally graded tissue engineering scaffolds. J Mechan Behav Biomed Mater. 2008;1:140. doi: 10.1016/j.jmbbm.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Lu H. Jiang J. Interface tissue engineering and the formulation of multiple-tissue systems. Adv Biochem Eng Biotechnol. 2006;102:91. [PubMed] [Google Scholar]
- 21.O'Shea T. Miao X. Bilayered scaffolds for osteochondral tissue engineering. Tissue Eng Part B Rev Tissue Eng Part B Rev. 2008;14:447. doi: 10.1089/ten.teb.2008.0327. [DOI] [PubMed] [Google Scholar]
- 22.Sharma B. Elisseeff J. Engineering structurally organized cartilage and bone tissues. Ann Biomed Eng. 2004;32:148. doi: 10.1023/b:abme.0000007799.60142.78. [DOI] [PubMed] [Google Scholar]
- 23.Dormer N.H. Berkland C.J. Detamore M.S. Emerging techniques in stratified designs and continuous gradients for tissue engineering of interfaces. Ann Biomed Eng. 2010;38:2121. doi: 10.1007/s10439-010-0033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh M. Berkland C. Detamore M.S. Strategies and applications for incorporating physical and chemical signal gradients in tissue engineering. Tissue Eng Part B Rev. 2008;14:341. doi: 10.1089/ten.teb.2008.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo X. Park H. Young S. Kretlow J.D. van den Beucken J.J. Baggett L.S. Tabata Y. Kasper F.K. Mikos A. Jansen J.A. Repair of osteochondral defects with biodegradable hydrogel composites encapsulating marrow mesenchymal stem cells in a rabbit model. Acta Biomater. 2010;6:39. doi: 10.1016/j.actbio.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holland T. Bodde E. Baggett L. Tabata Y. Osteochondral repair in the rabbit model utilizing bilayered, degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds. J Biomed Mater Res. 2005;75:156. doi: 10.1002/jbm.a.30379. [DOI] [PubMed] [Google Scholar]
- 27.Spalazzi J. Doty S. Moffat K. Levine W. Lu H. Development of controlled matrix heterogeneity on a triphasic scaffold for orthopedic interface tissue engineering. Tissue Eng. 2006;12:3497. doi: 10.1089/ten.2006.12.3497. [DOI] [PubMed] [Google Scholar]
- 28.Dormer N.H. Singh M. Wang L. Berkland C.J. Detamore M.S. Osteochondral interface tissue engineering using macroscopic gradients of bioactive signals. Ann Biomed Eng. 2010;38:2167. doi: 10.1007/s10439-010-0028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X. Wenk E. Zhang X. Meinel L. Vunjak-Novakovic G. Kaplan D. Growth factor gradients via microsphere delivery in biopolymer scaffolds for osteochondral tissue engineering. J Control Rel. 2009;134:81. doi: 10.1016/j.jconrel.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh M. Dormer N. Salash J. Three-dimensional macroscopic scaffolds with a gradient in stiffness for functional regeneration of interfacial tissues. J Biomed Mater Res A. 2010;94:870. doi: 10.1002/jbm.a.32765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berkland C. Kim K. Pack D.W. Fabrication of PLG microspheres with precisely controlled and monodisperse size distributions. J Control Rel. 2001;73:59. doi: 10.1016/s0168-3659(01)00289-9. [DOI] [PubMed] [Google Scholar]
- 32.Singh M. Morris C.P. Ellis R.J. Detamore M.S. Berkland C. Microsphere-based seamless scaffolds containing macroscopic gradients of encapsulated factors for tissue engineering. Tissue Eng Part C Methods. 2008;14:299. doi: 10.1089/ten.tec.2008.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Livak K.J. Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 34.Boyan B.D. Schwartz Z. Bonewald L.F. Swain L.D. Localization of 1,25-(OH)2D3-responsive alkaline phosphatase in osteoblast-like cells (ROS 17/2.8, MG 63, and MC 3T3) and growth cartilage cells in culture. J Biol Chem. 1989;264:11879. [PubMed] [Google Scholar]
- 35.Edwards C. O'Brien W., Jr Modified assay for determination of hydroxyproline in a tissue hydrolyzate. Clin Chim Acta. 1980;104:161. doi: 10.1016/0009-8981(80)90192-8. [DOI] [PubMed] [Google Scholar]
- 36.Wang L. Tran I. Seshareddy K. Weiss M.L. Detamore M.S. A comparison of human bone marrow-derived mesenchymal stem cells and human umbilical cord-derived mesenchymal stromal cells for cartilage tissue engineering. Tissue Eng Part A. 2009;15:1009. doi: 10.1089/ten.tea.2008.0393. [DOI] [PubMed] [Google Scholar]
- 37.An Y. Martin K. Handbook of Histology Methods for Bone and Cartilage. Totowa, NJ: Humana Press; 2003. [Google Scholar]
- 38.Jaiswal N. Haynesworth S.E. Caplan A.I. Bruder S.P. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295. [PubMed] [Google Scholar]
- 39.Martin R.B. Burr D.B. Sharkey N.A. Skeletal Tissue Mechanics. New York: Springer; 1998. [Google Scholar]
- 40.Kim K. Dean D. Lu A. Mikos A. Early osteogenic signal expression of rat bone marrow stromal cells is influenced by both hydroxyapatite nanoparticle content and initial cell seeding density in in biodegradable nanocomposite scaffolds. Acta Biomater. 2010;7:1249. doi: 10.1016/j.actbio.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leong N.L. Lu H.H. Polymer-ceramic composite scaffold induces osteogenic differentiation of human mesenchymal stem cells. Conf Proc IEEE Eng Med Biol Soc. 2006;1:2651. doi: 10.1109/IEMBS.2006.259459. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y.K. Wang G.C. Cai Y.R. Ji H.J. Zhou G.S. Zhao X.L. Tang R.K. Zhang M. In vitro effects of nanophase hydroxyapatite particles on proliferation and osteogenic differentiation of bone marrow-derived mesenchymal stem cells. J Biomed Mater Res. 2009;90:1083. doi: 10.1002/jbm.a.32192. [DOI] [PubMed] [Google Scholar]
- 43.Malafaya P. Reis R. Bilayered chitosan-based scaffolds for osteochondral tissue engineering: influence of hydroxyapatite on in vitro cytotoxicity and dynamic bioactivity studies in a specific double-chamber bioreactor. Acta Biomater. 2009;5:644. doi: 10.1016/j.actbio.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 44.Hersel U. Dahmen C. Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003;24:4385. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 45.Benoit D. Anseth K. The effect on osteoblast function of colocalized RGD and PHSRN epitopes on PEG surfaces. Biomaterials. 2005;26:5209. doi: 10.1016/j.biomaterials.2005.01.045. [DOI] [PubMed] [Google Scholar]
- 46.Ho M.-H. Hou L.-T. Tu C.-Y. Hsieh H.-J. Lai J.-Y. Chen W.-J. Wang D.-M. Promotion of cell affinity of porous PLLA scaffolds by immobilization of RGD peptides via plasma treatment. Lab Invest. 2006;6:90. doi: 10.1002/mabi.200500130. [DOI] [PubMed] [Google Scholar]
- 47.Jin Yoon J. Ho Song S. Sung Lee D. Park T. Immobilization of cell adhesive RGD peptide onto the surface of highly porous biodegradable polymer scaffolds fabricated by a gas foaming/salt leaching method. Biomaterials. 2004;25:5613. doi: 10.1016/j.biomaterials.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 48.Kim T. Park T. Biomimicking extracellular matrix: cell adhesive RGD peptide modified electrospun poly (D, L-lactic-co-glycolic acid) nanofiber mesh. Tissue Eng. 2006;12:221. doi: 10.1089/ten.2006.12.221. [DOI] [PubMed] [Google Scholar]