Abstract
Tissue engineering approaches are promising to meet the increasing need for bone regeneration. Calcium phosphate cement (CPC) can be injected and self-set to form a scaffold with excellent osteoconductivity. The objectives of this study were to develop a macroporous CPC–chitosan–fiber construct containing alginate–fibrin microbeads encapsulating human umbilical cord mesenchymal stem cells (hUCMSCs) and to investigate hUCMSC release from the degrading microbeads and proliferation inside the porous CPC construct. The hUCMSC-encapsulated microbeads were completely wrapped inside the CPC paste, with the gas-foaming porogen creating macropores in CPC to provide for access to culture media. Increasing the porogen content in CPC significantly increased the cell viability, from 49% of live cells in CPC with 0% porogen to 86% of live cells in CPC with 15% porogen. The alginate–fibrin microbeads started to degrade and release the cells inside CPC at 7 days. The released cells started to proliferate inside the macroporous CPC construct. The live cell number inside CPC increased from 270 cells/mm2 at 1 day to 350 cells/mm2 at 21 days. The pore volume fraction of CPC increased from 46.8% to 78.4% using the gas-foaming method, with macropore sizes of approximately 100 to 400 μm. The strength of the CPC–chitosan–fiber scaffold at 15% porogen was 3.8 MPa, which approximated the reported 3.5 MPa for cancellous bone. In conclusion, a novel gas-foaming macroporous CPC construct containing degradable alginate–fibrin microbeads was developed that encapsulated hUCMSCs. The cells had good viability while wrapped inside the porous CPC construct. The degradable microbeads in CPC quickly released the cells, which proliferated over time inside the porous CPC. Self-setting, strong CPC with alginate–fibrin microbeads for stem cell delivery is promising for bone tissue engineering applications.
Introduction
The need for bone repair has increased as the world's population ages.1–5 Skeletal diseases, congenital malformations, trauma, and tumor resections often require bone reconstruction.6–10 In the United States, health care costs plus lost wages for people with musculoskeletal diseases reached approximately $849 billion in 2004, or 7.7% of the national gross domestic product.11 Tissue engineering approaches are being developed as alternatives to autogenous bone transplantation.12–16 Studies have shown promise in stem cell delivery using scaffolds for regenerative medicine applications.17–20 Calcium phosphate scaffolds mimic bone minerals and can facilitate cell attachment and function.14,21–23 They are bioactive and can bond to bone to form a functional interface.8,18,24,25 Calcium phosphate cements (CPCs) have good biocompatibility and osteoconductivity.26–32 CPCs have an advantage over prefabricated bioceramics, which require the surgeon to carve the surgical site and machine the graft to fit the bone cavity. CPCs are injectable and can set in situ in the bone site. The surgeon can handle the CPC in paste form and sculpt or shape it before it sets, which is useful for maxillofacial repairs to achieve esthetically pleasing results. The first CPC was developed in 1986 and approved in 1996 by the Food and Drug Administration for repairing craniofacial defects.26,33 Other CPC compositions have also become available.28–30,32 The mechanical properties and porosity of CPC were improved using degradable fibers, chitosan, and porogen.34–36
In addition to scaffolds, cells are another important component of tissue engineering. Human umbilical cord mesenchymal stem cells (hUCMSC) were derived for tissue engineering.37–42 Umbilical cords are an inexpensive and inexhaustible stem cell source, without the invasive procedure of harvesting human bone marrow mesenchymal stem cells (hBMSCs). hUCMSCs appeared to be primitive mesenchymal stem cells (MSCs), had high plasticity and developmental flexibility,43 and caused no immunorejection in preliminary animal studies.44 Only a few studies have used hUCMSCs for bone tissue engineering by examining the hUCMSCs on tissue culture polystyrene39 and polymer scaffolds,42,45 as well as in vivo.46
Osteoblasts and hUCMSCs were encapsulated in alginate hydrogel microbeads, and the microbeads were incorporated into CPC, which served as an injectable vehicle to deliver the cells.47–50 In these experiments, the cell-encapsulating microbeads were placed on the bottom of the culture well, and then the CPC paste was placed on top to cover the microbeads. The encapsulation in alginate enabled the cells to survive the CPC setting reaction. In this experimental setup, the upper side of the microbeads was in contact with the CPC paste, but the lower side of the microbeads was in contact with the tissue culture polystyrene.47,48 In addition, the CPC paste did not always cover the microbeads completely because some microbeads in the peripheral areas supported the paste and left small openings, which allowed culture media flow. This does not simulate the clinical application, in which the microbeads would be mixed in and surrounded by the CPC paste and in which the circulation of fluids would solely depend on the pores in the CPC matrix. Furthermore, the initial CPC hardening process takes several minutes, and the setting reaction is completed in approximately a day. Set CPC supports cell proliferation, so it would be desirable for the microbeads inside the CPC to degrade in several days to release the cells while concomitantly creating macropores for cell migration and biological function. However, a pilot study showed that the alginate microbeads of the previous studies showed no degradation after 21 days.
In this study, the hUCMSC-encapsulating microbeads were completely wrapped inside the CPC paste to simulate clinical applications in which the microbeads are mixed with CPC paste and injected or placed into a bone cavity. Novel degradable microbeads were developed that could release the cells inside CPC starting at 7 days. A gas-foaming method was used to create macropores in CPC to facilitate the cells' access to fluids and survival inside CPC. The objectives of this study were to develop macroporous CPC containing alginate–fibrin microbeads with hUCMSC delivery and to investigate cell release from the degradable microbeads inside CPC. It was hypothesized that: (1) The fast degradable alginate–fibrin microbeads will be able to release the hUCMSCs inside the CPC scaffold; (2) While the hUCMSCs will die inside the CPC without macropores, cells inside the macroporous CPC will have good viability; (3) Increasing the foaming agent amount will increase the CPC porosity, which will decrease its mechanical properties, while adding fibers will increase its mechanical properties.
Materials and Methods
Fabrication of gas-foaming CPC scaffold
The CPC powder consisted of an equimolar mixture of tetracalcium phosphate (TTCP) and dicalcium phosphate anhydrous (DCPA).33 Chitosan was used because it helps the CPC paste set quickly and increases the strength of CPC.35 Hence, the CPC liquid consisted of chitosan malate (Halosource, Redmond, WA) mixed with water at a chitosan/(chitosan+water) of 15% (by mass, unless noted otherwise).35 A degradable suture fiber (Vicryl, Ethicon, Somerville, NJ), a copolymer of glycolic and lactic acids, was cut to 3-mm filaments and used at a fiber volume fraction of 20% to reinforce the CPC.50 Previous studies have showed that this degradable suture fiber can increase the strength of CPC,35,50 although it has not been used to reinforce gas-foaming CPC.
A gas-foaming method was used to create macropores in CPC. The purpose of the macropores was to increase the fluid circulation and cell viability in the CPC scaffold. Following a previous study,51 sodium hydrogen carbonate (NaHCO3) and citric acid (citric acid monohydrate, C6H8O7·H2O) were added to CPC as the porogen. The acid–base reaction of citric acid with NaHCO3 produced carbon dioxide bubbles in CPC, resulting in macropores.52 NaHCO3 was added to the CPC powder at NaHCO3/(NaHCO3+CPC powder) mass fractions of 0%, 5%, 10%, 15%, 20%, 25%, and 30%. NaHCO3/(NaHCO3+CPC powder) is referred to as the mass fraction of foam porogen in CPC. The corresponding amount of C6H8O7·H2O was added to the CPC liquid to maintain a fixed NaHCO3/(NaHCO3+C6H8O7·H2O) mass fraction of 54.52%.52 CPC powder, chitosan, fiber, and porogen were sterilized in an ethylene oxide sterilizer (Andersen, Haw River, NC) for 12 hours and then degassed for 7 days, according to the manufacturer's specifications.
hUCMSC culture
The University of Maryland approved the use of hUCMSCs (ScienCell, Carlsbad, CA), which were harvested from Wharton's jelly in umbilical cords of healthy babies using an established method.37,41 Cells were cultured in low-glucose Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA) (control medium). At 80% to 90% confluence, hUCMSCs were detached and passaged, and passage 4 cells were used. The osteogenic medium was supplemented with 100nM dexamethasone, 10mM β-glycerophosphate, 0.05mM ascorbic acid, and 10nM 1α,25-dihydroxyvitamin (Sigma, St. Louis, MO).37,41,50
hUCMSC encapsulation in degradable alginate-fibrin hydrogel microbeads
Alginate can form an ionically crosslinked network under mild conditions, so alginate microbeads had been used to encapsulate cells previously.47–50 The purpose of the microbeads is to protect the cells from the mixing and injection stresses of the CPC paste. After the CPC is set, it is desirable for the microbeads to degrade and release the cells quickly so that the cells can attach to the CPC and proliferate inside the scaffold. However, a preliminary study showed that the alginate microbeads did not degrade significantly after 4 weeks in culture media and that few cells were released from the microbeads even when the alginate was partially oxidized. The preliminary study also found that adding fibrinogen to alginate yielded alginate–fibrin microbeads that could release the cells. Fibrin concentrations 0.2% and greater resulted in fibrin clots in the alginate because fibrin was sticky, a fibrin concentration of 0.05% yielded microbeads that were slow to degrade, but an intermediate fibrin concentration of 0.1% resulted in microbeads that could degrade successfully and release the cells in several days. Therefore, in the present study, fibrinogen (Sigma) at a mass fraction of 0.1% was added to the alginate solution.
Partially oxidizing the alginate was shown to enhance its degradability.53 The degree of oxidation (%) was the number of oxidized uronate residues per 100 uronate units in the alginate chain. In the present study, the reaction was performed at the correct stoichiometric ratio of sodium periodate to alginate to yield 7.5% of alginate oxidation. Following a previous study,53 1% of sodium alginate (molecular weight=75,000 to 220,000 g/mol, ProNova, Norway) was dissolved in water. For oxidization, 1.51 mL of 0.25 mol/L sodium periodate (Sigma) was added to 100 mL of alginate solution that was stirred to react in the dark at room temperature. At 24 hours, the oxidization reaction was stopped by adding 1 g of ethylene glycol and then 2.5 g of sodium chloride. Two hundred mL of ethanol was added to precipitate the product, which was then collected using centrifugation. The precipitates were redissolved in 100 mL of water and precipitated with 200 mL of ethanol. The second precipitates were collected and dissolved in 30 mL of water. The final product was freeze dried for 24 hours. The oxidized alginate thus obtained was dissolved in saline at a concentration of 1.2%. Fibrinogen from bovine plasma (Sigma) was added at a concentration of 0.1% and incubated at 37°C for 2 hours to yield a mixed alginate–fibrinogen solution. hUCMSCs were added at 1 million cells/mL in the alginate–fibrin solution. The solution was loaded into a syringe that was connected to a bead-generating device (Var J1, Nisco, Zurich, Switzerland), as described previously.50 Alginate droplets were sprayed into a beaker with a solution containing 125 mL of 100 mmol/L calcium chloride plus 125 NIH units of thrombin (Sigma). Calcium chloride caused the alginate to crosslink, and the reaction between fibrinogen and thrombin generated the fibrin, producing the oxidized alginate-fibrin microbeads. The microbeads were examined under a microscope (Eclipse TE-2000S, Nikon, Melville, NY).
Hydrogel microbead degradation
Degradation was characterized by examining the changes in the dry mass of microbeads without cells during incubation in phosphate buffered saline (PBS), following a previous study.54 The dry mass of the as-fabricated microbeads was used as the mass at day 0. The dry mass of the microbeads was measured at 0, 4, 7, 14, and 21 days of incubation in PBS at 37°C. At each time point, the samples were incubated in deionized water to remove buffer salts and freeze-dried using a Labconco freeze dry system. A decrease in microbead mass was indicative of degradation and loss of polymer chains from the network. Three materials were measured: alginate microbeads, oxidized alginate microbeads, and oxidized alginate-fibrin microbeads.
Viability of hUCMSCs encapsulated inside gas-foaming CPC
Two groups were tested. The purpose of group 1 was to investigate the effect of the gas-foaming porogen mass fraction on cell viability inside CPC. The following NaHCO3/(NaHCO3+CPC powder) mass fractions were used: 0%, 5%, 10%, 15%, 20%, 25%, and 30%. For each mass fraction, first a layer of CPC paste was placed on the bottom of a well of a 6-well plate. The CPC paste mass had a mass of 0.3 g, and it was shaped like a flat-bottomed cooking pan. Then, 0.3 g of the hUCMSC-encapsulating microbeads was placed in the “pan,” and the top was completely covered with a second layer of CPC paste (0.3 g). Efforts were made to ensure that the two layers of CPC bonded with each other so that the microbeads were completely wrapped inside CPC. An even more-ideal situation would be to mix the microbeads randomly with the CPC paste, as would be the case in potential clinical applications. However, when the interior of CPC was broken open for examination, it yielded a tortuous and rough surface. Hence, it was difficult to perform live/dead staining and take images to monitor cell viability. Therefore, the first layer of CPC was used in the shape of a flat-bottomed pan to provide a flat surface to perform live/dead staining and to take images for analysis.
After CPC construct hardening at 37°C for 30 minutes, 5 mL of osteogenic medium was added to each well to submerge the construct (n=5). After 1 day, the CPC construct was opened for live/dead staining (Molecular Probes, Eugene, OR). Live cells were stained green, and dead cells were stained red. The cells were observed using epifluorescence microscopy (Eclipse TE-2000S, Nikon, Melville, NY). Three random images per construct yielded 15 images for each porogen mass fraction. The percentage of live cells was P=NLIVE/(NLIVE+NDEAD), where NLIVE is the number of live cells, and NDEAD is the number of dead cells. The live cell density was D=NLIVE/A, where A was the area of the image where NLIVE was measured.55
The purpose of group 2 was to examine the effect of culture time from 1 day to 21 days on cell viability inside CPC. The results from group 1 showed that CPC with 15% foaming porogen yielded the best cell viability, so group 2 used 15% foaming porogen. The culture times were 1, 7, 14, and 21 days. At each time period, the CPC construct was opened and live/dead stained in the same manner as for group 1.
hUCMSC DNA quantification
Cell proliferation inside CPC was also measured by determining the DNA content of enzymatically digested scaffolds using a Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen). After being cultured for 1, 7, 14, and 21 days, the CPC scaffolds were broken carefully, and the cells were digested using M-PER reagent (Thermo Fisher Scientific). After digestion, 100 μL of sample was added to 100 μL of PicoGreen reagent solution (diluted 200-fold with TE buffer) in a 96-well microplate incubated for 5 minures in the dark at room temperature. Fluorescence at 535 nm was read on a fluorescence microplate reader to determine the DNA content per construct. Samples were compared with calf thymus DNA standards.
Measurement of gas-foaming CPC porosity and mechanical properties
Group 1 showed that the cell viability was high at foam porogen mass fractions of 10%, 15%, and 20% in CPC. Cell viability was lower at 0%, 5%, 25%, and 30% porogen. Hence, the porosity and mechanical properties were measured for scaffolds at foam porogen of 10%, 15%, and 20%, with 0% as control. To measure porosity, the CPC specimens were dried in a vacuum oven at 60°C for 24 hours. The density of CPC was measured using the specimen weight divided by volume.56 The volume was calculated according to the specimen dimensions measured using a micrometer, with each dimension being the average of three locations along the specimen. Six specimens were measured for each porogen content. Following a previous study,56 the pore volume fraction of CPC–chitosan specimens was obtained according to p=(dHA+CN – d)/dHA+CN, where p is porosity, and d is the measured density. The density of fully dense hydroxyapatite dHA is 3.14 g/cm3.56 The CPC contained 15% chitosan, and the fully dense hydroxyapatite–chitosan composite density dHA+CN was 2.82 g/cm3, as obtained in a previous study.56 The measured density, d, leads to the calculation of the porosity, p. A scanning electron microscope (SEM, FEI Quanta 200, Hillsboro, OR) was used to examine the pore morphology in specimens sputter coated with gold.
A three-point flexural test was used to fracture the bar specimens on a Universal Testing Machine (MTS, Eden Prairie, MN) using a span of 20 mm at a crosshead speed of 1 mm/min (ASTM, 2004). Flexural strength was calculated as 3FmaxL/(2bh2), where Fmax is the maximum load on the load-displacement (F-a) curve, L is span, b is specimen width, and h is thickness. Elastic modulus E equals (F/a) (L3/[4bh3]). Work-of-fracture (toughness) was the area under the F-d curve divided by the specimen's cross-sectional area.34
One-way and two-way analyses of variacne were performed to detect significant effects of the variables. Tukey's multiple comparison tests were used at p=0.05.
Results
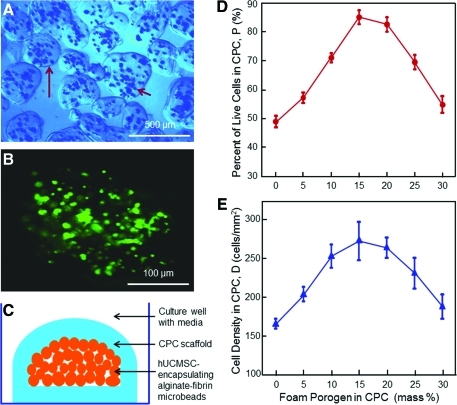
Figure 1 shows the experimental setup and the effect of foam on hUCMSC viability. A typical photo of hUCMSC-encapsulating microbeads is shown in Fig. 1A. Handling the microbeads and placing them into CPC paste indicated that they had sufficient integrity and were not damaged by handling. The microbeads were slightly elongated. The longest dimension of each microbead was measured and referred to as the length. The dimension perpendicular to the length was referred to as the width. Measurement of 100 random microbeads yielded a length of 105 to 566 μm (mean 314 μm). Microbead width ranged from 93 to 411 μm (mean 261 μm). An example of the live/dead staining image of a microbead is shown in Fig. 1B, indicating that the encapsulated cells were primarily alive.
FIG. 1.
(A) Optical photo of human umbilical cord mesenchymal stem cell (hUCMSC)-encapsulating alginate–fibrin microbeads. The long arrow indicates the boundary of a microbead. The short arrow indicates the cells inside the microbeads. (B) Live/dead image of an as-fabricated microbead. Live cells were stained green, which indicates that the encapsulated cells were mostly alive. (C) Schematic of hUCMSC-encapsulating microbeads inside calcium phosphate cement (CPC) scaffold immersed in culture media in a culture well. and Effects of gas-foam porogen mass fraction in CPC on the percentage of live cells (D) and live cell density (E). Each value is the mean of five measurements, with the error showing one standard deviation (mean±standard deviation (sd); n=5). Color images available online at www.liebertonline.com/tea
The schematic of microbeads inside the CPC is shown in Fig. 1C. After 1 day, the CPC construct was gently broken for live/dead staining on the bottom surface of CPC. In Fig. 1D, for CPC without porogen, the percentage of live cells was 49%, with many dead cells. The percentage of live cells was increased to 85% using 15% porogen. In Fig. 1E, the number of live cells was 166 cells/mm2 in CPC without porogen. It was increased to 272 cells/mm2 at 15% porogen and then started to decrease when the porogen was further increased.
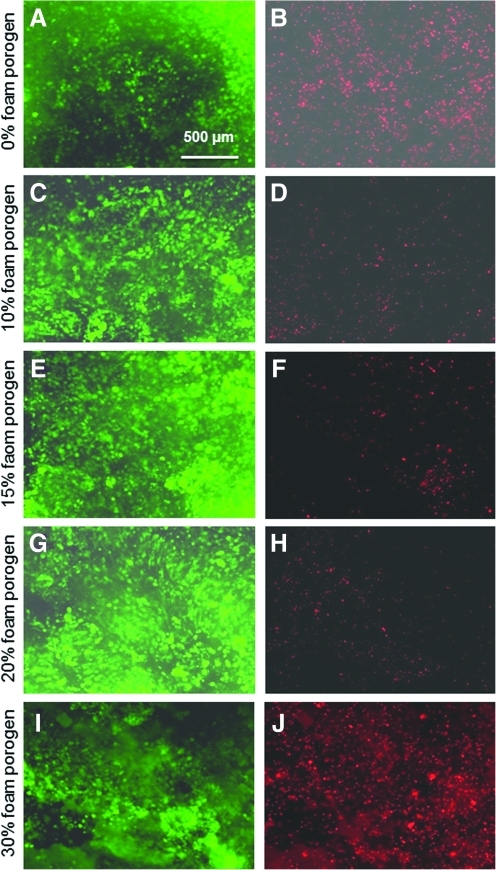
Typical live/dead stained photos at 1 day are shown in Fig. 2. At 1 day, the microbeads were still intact and not degraded. The cells were encapsulated in the microbeads and were not released yet, so a live cell showed as a green dot. Preliminary study showed that, after microbead degradation, the released cells would attach to the CPC surface and exhibit a spreading morphology. On the other hand, before microbead degradation, a cell encapsulated in the gel appeared as a green dot without a spreading morphology. In Fig. 2A and B, inside CPC without porogen, there was a large number of dead cells. The number of dead cells dramatically decreased when the porogen was increased to 10%, 15%, and 20%, as shown in Fig. 2B to H. Meanwhile, the live cell density increased, although when the foam porogen was further increased to 30%, the number of live cells decreased (Fig. 2I) and dead cells increased (Fig. 2J).
FIG. 2.
Live/dead stained photos of cells inside CPC. Live cells were stained green and dead cells were stained red. Labels on the left side indicate the porogen mass fraction in CPC. The number of live cells increased, and dead cells decreased, when the porogen was increased from 0% to 10%, 15%, and 20%. Color images available online at www.liebertonline.com/tea
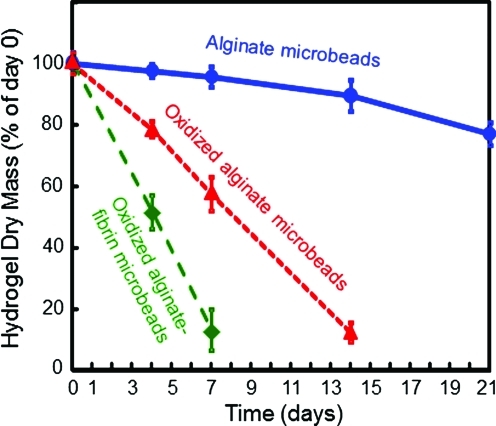
The three types of hydrogel microbead degradation results are plotted in Fig. 3. Each value is mean±standard deviation (n=5). Alginate microbeads had slow degradation and maintained approximately 80% of their mass by 21 days. Oxidized alginate microbeads had a faster degradation rate and lost most of the mass by 14 days. Oxidized alginate–fibrin microbeads had the fastest degradation and lost almost all their mass by 7 days.
FIG. 3.
Microbead degradation rates. Each value is mean±sd; n=5. Three types of microbeads were measured: alginate microbeads, oxidized alginate microbeads, and oxidized alginate-fibrin microbeads. The dry mass at 1, 4, 7, 14 and 21 days was divided by the original mass at 0 days to obtain the percentage of mass remaining at each time period. Color images available online at www.liebertonline.com/tea
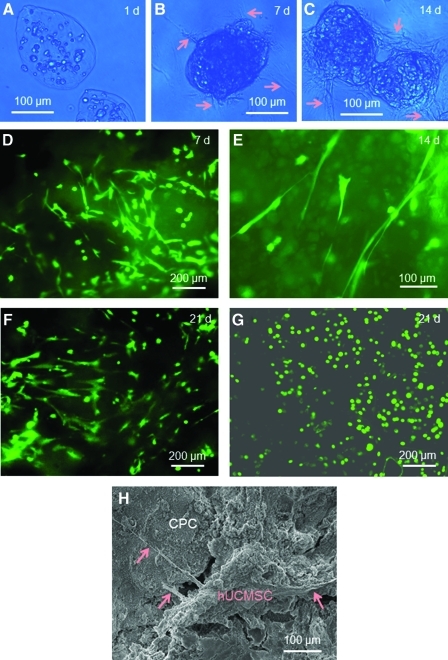
Figure 4 shows that, with time, the alginate–fibrin microbeads degraded and released the hUCMSCs. Optical photographs indicate no microbead degradation or cell release at 1 day (Fig. 4A), with degradation and some cell release (arrows) at 7 days (Fig. 4B) and numerous cells that were released and attached to CPC at 14 days (Fig. 4C). The live/dead staining images of the CPC bottom surface confirmed the cell release from microbeads, showing that, at 7 days (Fig. 4D), some cells appeared as green dots, indicating that they were still encapsulated in the fragments of the degraded microbeads. However, there were other areas where the cells showed a spreading morphology with an elongated spindle shape, typical of free (not encapsulated) MSCs attaching to a substrate. The spreading and elongated morphology was shown more clearly at higher magnification at 14 days (Fig. 4E). At 21 days, the number of released and attached cells had increased (Fig. 4F). In contrast, cells in alginate microbeads without fibrin appeared as green dots at 21 days (Fig. 4G). Furthermore, the alginate microbeads without fibrin could be collected with mechanical integrity after culturing for 21 days. However, the alginate–fibrin microbeads were degraded into fragments at 7 days and became invisible in the microscope at 14 days, and no integral microbeads could be found or collected. An example of the released cells attaching to the CPC bottom surface is shown in Fig. 4H at 7 days, where the arrows indicate the cytoplasmic extensions of the cell. Hence, the alginate–fibrin microbeads could degrade, the hUCMSCs could be released inside CPC at 7 days, and the released cells showed a healthy spreading morphology with good viability from 7 to 21 days.
FIG. 4.
Alginate–fibrin microbeads degradation and cell release. (A-C) Optical photos at 1, 7, and 14 days. A blue filter was used to enhance the contrast of the microbeads. Arrows in B and C indicate cell release from the microbeads. (D-G) Live/dead staining images of the bottom CPC surface. (D) At 7 days, some cells showed as green dots, indicating that they were still encapsulated in the fragments of microbeads. Other cells had a spread, spindled morphology, indicating that they were released and attached to CPC. (E) The spread morphology at 14 days. (F) At 21 days, the number of attached cells increased. (G) For comparison, alginate microbeads without fibrin did not degrade, with the cells remaining as green dots, and did not spread at 21 days. (H) Scanning electron microscopy (SEM) micrograph of released cells attaching to the CPC bottom surface. Arrows indicate the cytoplasmic extensions of the cells. Color images available online at www.liebertonline.com/tea
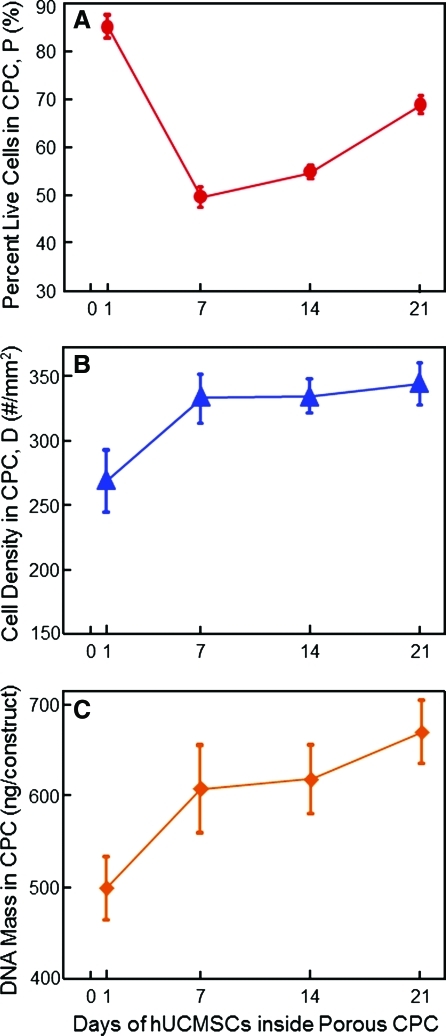
Figure 5 shows the effect of culture time from 1 to 21 days on hUCMSC viability inside CPC with 15% foam porogen. In Fig. 5A, the percentage of live cells decreased to 49% at 7 days and then started to increase, reaching 69% at 21 days. In Fig. 5B, the live cell density increased over time, from 270 cells/mm2 at 1 day to 350 cells/mm2 at 21 days (p<0.05). As shown in Fig. 5C, a similar significant increase was found in the DNA mass of the hUCMSCs inside CPC over time (p<0.05).
FIG. 5.
Effect of time on hUCMSCs inside CPC. Each CPC contained the same 15% porogen. (A) Percentage of live cells, (B) live cell density, and (C) DNA mass of the hUCMSCs. There was a decrease in the percentage of live cells in the first week, but cell proliferation increased the percentage of live cells as well as the live cell density and the DNA mass of the hUCMSCs over time. Each value is mean±sd; n=5. Color images available online at www.liebertonline.com/tea
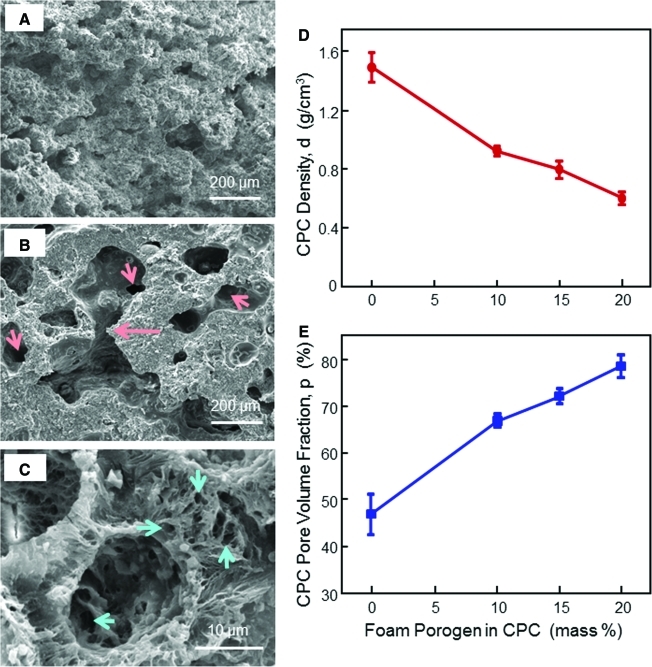
Figure 6 shows SEM micrographs of typical porosity in CPC with 0% (Fig. 6A), 10% Fig. 6B), and 20% (Fig. 6C) foam porogens. The macropores had sizes of approximately 100 to 400 μm. The long arrow in Fig. 6B indicates the interconnection between pores. The short arrows indicate opening fenestrations inside pores leading to the next pores. A higher magnification (Fig. 6C) showed numerous micropores on the macropore wall contributing to the interconnectivity. CPC matrix was full of micropores from submicron to several microns in sizes. Fig. 6D and E plots the density and porosity of CPC. The density decreased from 1.5 g/cm3 to 0.6 g/cm3 when the porogen in CPC was increased from 0% to 20%. Pore volume fraction was increased from 46.8% to 78.4%.
FIG. 6.
Gas-foaming CPC porosity. SEM micrograph at (A) 0%, (B) 10%, and (C) 20% foam porogen. An example of macropore interconnection is indicated by the long arrow in (B). The short arrows indicate openings inside macropores. Numerous micropores in CPC are shown in (C). (D, E) Effects of gas-foaming porogen on CPC density and porosity. The purpose was to measure the porosity in CPC matrix, so the specimens contained no fibers. When the foam agent was increased from 0% to 20%, the pore volume fraction in CPC increased from 46.8% (intrinsic microporosity in CPC) to 78.4% (microporosity+macroporosity). Each value is mean±sd; n=5. Color images available online at www.liebertonline.com/tea
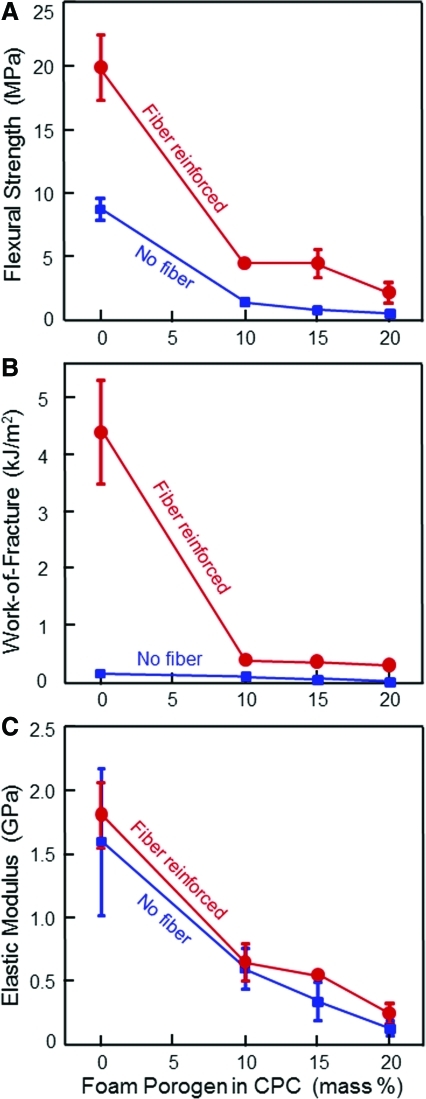
The mechanical properties of CPC and foam porogen content are plotted in Fig. 7. The strength was 8.6 MPa at 0% porogen, decreasing to 0.4 MPa when 20% foam porogen was used. Fiber reinforcement greatly improved the strength. The strength was increased to 19.9 MPa at 0% porogen, 3.8 MPa at 15% porogen, and 2.2 MPa at 20% porogen. Work-of-fracture of porous CPC was also substantially greater with fiber reinforcement. The elastic modulus also showed a decreasing trend with increasing foam porogen in CPC.
FIG. 7.
Effect of foam porogen content on CPC mechanical properties: (A) Flexural strength, (B) work-of-fracture, and (C) elastic modulus. For the group with fibers, suture fibers at a length of 3 mm and a volume fraction of 20% were used in CPC. Each value is mean±sd; n=5. Fiber reinforcement greatly increased the CPC mechanical properties. Color images available online at www.liebertonline.com/tea
Discussion
The present study investigated the encapsulation of hUCMSCs inside a novel injectable, gas-foaming CPC–chitosan–fiber construct for bone tissue engineering. The following aspects were performed: (1) Gas-foaming macroporous CPC–chitosan–fiber scaffolds were developed; (2) the cell microbeads were completely wrapped inside the CPC paste; (3) degradable alginate–fibrin microbeads encapsulating hUCMSCs released the cells inside CPC, and the cells remained viable with a healthy spreading morphology at 21 days. In previous studies,48,50 the cell-encapsulating microbeads were placed on the bottom of the culture well, and a layer of CPC paste was placed on top. The paste may not cover the microbeads completely at the periphery, and the bottom side of the microbeads was not in contact with CPC. Therefore, in previous studies, the effect of completely wrapping the microbeads in CPC paste with setting reaction on all sides was not investigated.47–50 In addition, the microbeads in previous studies were not degradable, and the cells were not released inside CPC.47–50
The microbeads were completely wrapped inside CPC, ensuring that the cells inside the CPC construct would rely solely on the porosity of CPC to access culture medium. This simulates clinical applications using a CPC paste mixed with the microbeads in which the cells would rely on the pores in the CPC scaffold for access to physiological fluids with nutrients and oxygen. This also allowed examination of how well the released cells could attach to the CPC bottom surface inside an enclosed CPC construct. In contrast, simply placing the microbeads on the bottom of a culture well, as in previous studies,50 would allow only the released cells to attach to the tissue culture polystyrene. Opening the CPC scaffold for examination of the cells would usually yield a tortuous fractured surface that would be difficult to perform live/dead staining and imaging analysis on. In this study, the complete wrapping method provided a flat bottom surface of CPC that the cells released from the degradable microbeads could attach to, enabling live/dead imaging and investigation of cell viability and proliferation.
The greater porosity in CPC using the gas-foaming method greatly improved hUCMSC viability inside CPC, resulting in a percentage of live cells of greater than 80%. This is likely because of the higher porosity and interconnectivity that provided access of culture medium with nutrition and oxygen to the cells inside CPC. The approximately 80% of live cells is consistent with the reported 70% to 80% of live cells in previous studies on cell encapsulation in hydrogels.57,58 However, when the foaming agent was further increased to 25% and 30%, the cell viability decreased. This was probably because the greater amounts of NaHCO3 and citric acid in CPC caused a stronger reaction, which harmed the cells. Considering the higher strength and work-of-fracture with 15% NaHCO3 than 20% NaHCO3, the CPC scaffold with 15% NaHCO3 foaming agent appeared to be optimal for encapsulating and delivering stem cells.
Fibrin is a fibrous protein that is an important element in the clotting of blood. It is polymerized from fibrinogen and thrombin into a "mesh" that, in conjunction with platelets, can form a hemostatic clot in a wound.59 Fibrin scaffolds have been engineered as tissue substitutes that successfully support cell survival, migration, proliferation, and differentiation.60 In the present study, alginate–fibrin microbeads were synthesized and incorporated into CPC for the first time. The reason that pure fibrin microbeads were not used was that they were sticky and formed agglomerates in preliminary studies. Alginate was used as the base hydrogel to make microbeads because of its fast polymerization and nonsticky characteristics. In preliminary studies, increasing the fibrin concentration in alginate increased the degradation rate of the microbeads, but the stickiness of the microbeads also increased. Decreasing the fibrin concentration in alginate resulted in microbeads with good mechanical integrity and ease of handling, but the degradation and release of cells were slowed when the fibrin concentration was decreased. Hence, a fibrin concentration of 0.1% was selected based on preliminary studies. The novel alginate–fibrin microbeads in CPC successfully degraded and released the cells starting at 7 days.
In previous studies, nonoxidized alginate microbeads were fabricated to encapsulate the cells.48,50 These studies established the encapsulation method and provided useful information on protecting the cells from the CPC setting reaction while rendering the CPC–microbead–stem cell paste injectable.50 However, the microbeads were not degradable and did not release the cells inside CPC, so the cells did not attach to CPC and did not proliferate. Partially oxidizing the alginate caused the hydrogel to degrade in weeks, which was suitable for tissue regeneration.53 For the present study, the purpose was for the microbeads to degrade even faster, in several days, to release the cells soon after the CPC construct was set. The unmodified alginate microbeads did not degrade or release the cells (Fig. 4D at 21 days), but the oxidized alginate–fibrin microbeads dramatically increased degradation and released the cells inside CPC starting at 7 days. While encapsulated in the hydrogel, a live cell was seen as a round green dot (Fig. 2), consistent with previous studies.47–50 When the cells were released from the degraded microbeads and attached to CPC, they showed a spreading, spindled morphology (Fig. 4A-C), consistent with previous studies on cell attachment on CPC.54
The released cells inside CPC not only started to attach to CPC with a spreading morphology, but also proliferated. Whereas the live cell percentage at 1 day was 86%, it decreased to 50% at 7 days (Fig. 3A). The initial hardening of CPC took only a few minutes, but the CPC setting reaction with ion activities could take several days to complete, which probably harmed the cells in the first week. However, the live cells proliferated, increasing the live cell percentage to 55% at 14 days and 69% at 21 days. This proliferation is corroborated by the live cell number per CPC area in Fig. 3B, with nearly 350 cells/mm2 at 21 days, significantly higher than 270 cells/mm2 at 1 day. The cell proliferation inside CPC observed here represents an improvement. This is because cells encapsulated in hydrogels generally do not proliferate. MSCs are anchorage-dependent and need a bioactive surface to attach and spread.61,62 Therefore, researchers used peptides to modify the hydrogels and achieved cell attachment and spreading in the bioactive gels.58,63 Nonetheless, the MSCs did not proliferate, whether inside bioinert or bioactive gels. The cell encapsulation inside a gel caused contact inhibition, and hence cell proliferation became arrested.62 In the present study, the encapsulated cells were released from the degradable microbeads and showed evidence of proliferation inside CPC for the first time. The ultimate function of the hUCMSC is to differentiate into the osteogenic lineage for bone regeneration. The present study focused on the development of a macroporous CPC encapsulating fast-degradable alginate–fibrin microbeads and the investigation of cell release, viability, and proliferation. Further study is needed to examine the osteogenic differentiation and bone matrix synthesis using the novel hUCMSC-encapsulating, macroporous CPC–microbead construct.
The mechanical properties of the CPC scaffold decreased precipitously with increasing porosity because the pores served as defects and crack initiation sites during fracture testing. The flexural strength of the CPC–chitosan–fiber scaffold at 15% gas-foaming porogen was 3.8 MPa, which approximated the tensile strength of 3.5 MPa for cancellous bone measured in a previous study.64 Another study measured a compressive strength of 3 to 17 MPa for cancellous bone.65 Finite element analysis yielded a compressive strength of 3 to 10 MPa for cancellous bone.66 In comparison, previous studies reported strengths of 0.1 to 0.7 MPa for injectable polymeric and hydrogel carriers for cell delivery, which were useful for non-load-bearing tissue engineering applications.67,68 The fibers and CPC paste were mixed manually using a spatula, and the paste was flowable and readily wetted and surrounded each fiber. A previous study performed the CPC–fiber mixing with three operators: one operator with extensive mixing experience, one operator with slight mixing experience, and one operator with no mixing experience who was given a 1-minute verbal instruction.69 All three operators produced CPC–fiber specimens with nearly the same mechanical properties.69 In the present study, although the cell response and mechanical properties were promising, it is important for CPC setting time to be acceptable. The setting time was measured for CPC with 0% porogen and 15% porogen, following a previous method.35 This yielded mean cement setting time±standard deviation (n=6) of 7.7±0.6 minutes for CPC with 0% porogen and 12.6±0.5 minutes for CPC with 15% porogen (p<0.05). Although the macroporous CPC had a slightly longer setting time, both cements are faster setting than traditional CPC with a setting time of approximately half an hour.26,33 Hence, the fast-setting CPC–chitosan–fiber with stem cells of the present study may be useful for moderate load–bearing tissue engineering applications. Animal studies are needed to investigate the bone regeneration of CPC constructs containing degradable alginate–fibrin microbeads with stem cell delivery.
Conclusions
This study developed a novel gas-foaming macroporous CPC–chitosan–fiber scaffold and fabricated degradable alginate–fibrin microbeads to deliver hUCMSCs inside the scaffold. The microbeads were completely wrapped inside the porous CPC scaffold. The hUCMSCs were rapidly released from the degradable microbeads, had good viability, and proliferated inside the scaffold. The gas-foaming method increased the porosity of CPC, improving hUCMSC viability inside the CPC construct. The percentage of live cells inside CPC was approximately 70% at 21 days. This matched the previously reported percentages of live cells in hydrogels. However, CPC is much stronger mechanically, with its strength matching that of cancellous bone. Adding fibrin to the oxidized alginate greatly facilitated cell release from the microbeads, enabling the cells to spread, attach, and proliferate inside the porous CPC. The potential benefits of this novel system include: (1) Stem cells could be delivered using a load-bearing and bioactive CPC that could be injected or placed as a paste to set in situ, (2) the paste could be sculpted to achieve good esthetic results for craniofacial repairs, and (3) the stem cells could be quickly released from the fast-degrading microbeads to remain viable and be able to proliferate inside the macroporous CPC. The gas-foaming CPC with degradable alginate–fibrin microbeads for rapid stem cell release may be useful in a wide range of orthopedic and craniofacial applications.
Acknowledgments
We thank Dr. L. C. Chow and Dr. S. Takagi at the Paffenbarger Research Center and Dr. C. G. Simon at the National Institute of Standards and Technology for discussions and help. We also thank Dr. X. Zhou and Dr. Q. Chen at the West China College of Stomatology for help. This study was supported by National Institutes of Health R01 grants DE17974 and DE14190 (HX), Maryland Stem Cell Fund (HX), University of Maryland Dental School, and the West China College of Stomatology.
Disclosure Statement
No competing financial interests exist.
References
- 1.Mao J.J. Vunjak-Novakovic G. Mikos A.G. Atala A. Regenerative Medicine: Translational Approaches and Tissue Engineering. Boston, MA: Artech House; 2007. [Google Scholar]
- 2.Russias J. Saiz E. Deville S. Gryn K. Liu G. Tomsia A.P. Fabrication and in vitro characterization of three-dimensional organic/inorganic scaffolds by robocasting. J Biomed Mater Res A. 2007;83:434. doi: 10.1002/jbm.a.31237. [DOI] [PubMed] [Google Scholar]
- 3.Bodde E.W. Boerman O.C. Russel F.G. Mikos A.G. Spauwen P.H. Jansen J.A. The kinetic and biological activity of different loaded rhBMP-2 calcium phosphate cement implants in rats. J Biomed Mater Res A. 2008;87:780. doi: 10.1002/jbm.a.31830. [DOI] [PubMed] [Google Scholar]
- 4.Atala A. Engineering organs. Curr Opin Biotechnol. 2009;20:575. doi: 10.1016/j.copbio.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Bohner M. Design of ceramic-based cements and putties for bone graft substitution. Eur Cell Mater. 2010;20:1. doi: 10.22203/ecm.v020a01. [DOI] [PubMed] [Google Scholar]
- 6.Gomes M.E. Mikos A.G. Reis R.L. Injectable polymeric scaffolds for bone tissue engineering. In: Reis R.L., editor; San Roman J., editor. Biodegradable Systems in Tissue Engineering and Regenerative Medicine. Boca Raton, FL: CRC Press; 2004. pp. 29–38. [Google Scholar]
- 7.Mikos A.G. Herring S.W. Ochareon P. Elisseeff J. Lu H.H. Kandel R. Schoen F.J. Toner M. Mooney D. Atala A. Van Dyke M.E. Kaplan D. Vunjak-Novakovic G. Engineering complex tissues. Tissue Eng. 2006;12:3307. doi: 10.1089/ten.2006.12.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deville S. Saiz E. Nalla R.K. Tomsia A.P. Freezing as a path to build complex composites. Science. 2006;311:515. doi: 10.1126/science.1120937. [DOI] [PubMed] [Google Scholar]
- 9.Ginebra M.P. Traykova T. Planell J.A. Calcium phosphate cements as bone drug-delivery systems: a review. J Controlled Release. 2006;113:102. doi: 10.1016/j.jconrel.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Johnson P.C. Mikos A.G. Fisher J.P. Jansen J.A. Strategic directions in tissue engineering. Tissue Eng. 2007;13:2827. doi: 10.1089/ten.2007.0335. [DOI] [PubMed] [Google Scholar]
- 11.United States Bone and Joint Decade (USBJD) 2002–2011. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008. The Burden of Musculoskeletal Diseases in the United States. [Google Scholar]
- 12.Hill E.E. Boontheekul T. Mooney D.J. Regulating activation of transplanted cells controls tissue regeneration. Proc Natl Acad Sci U S A. 2006;103:2494. doi: 10.1073/pnas.0506004103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mao J.J. Giannobile W.V. Helms J.A. Hollister S.J. Krebsbach P.H. Longaker M.T. Shi S. Craniofacial tissue engineering by stem cells. J Dent Res. 2006;85:966. doi: 10.1177/154405910608501101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruhe P.Q. Hedberg-Dirk E.L. Padron N.T. Spauwen P.H. Jansen J.A. Mikos A.G. Porous poly(DL-lactic-co-glycolic acid)/calcium phosphate cement composite for reconstruction of bone defects. Tissue Eng. 2006;12:789. doi: 10.1089/ten.2006.12.789. [DOI] [PubMed] [Google Scholar]
- 15.Salinas C.N. Anseth K.S. The influence of the RGD peptide motif and its contextual presentation in PEG gels on human mesenchymal stem cell viability. J Tissue Eng Regen Med. 2008;2:296. doi: 10.1002/term.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aoki T. Ohnishi H. Oda Y. Tadokoro M. Sasao M. Kato H. Hattori K. Ohgushi H. Generation of induced pluripotent stem cells from human adipose-derived stem cells without c-MYC. Tissue Eng A. 2010;16:2197. doi: 10.1089/ten.TEA.2009.0747. [DOI] [PubMed] [Google Scholar]
- 17.Benoit D.S.W. Nuttelman C.R. Collins S.D. Anseth K.S. Synthesis and characterization of a fluvastatin-releasing hydrogel delivery system to modulate hMSC differentiation and function for bone regeneration. Biomaterials. 2006;27:6102. doi: 10.1016/j.biomaterials.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 18.Reilly G.C. Radin S. Chen A.T. Ducheyne P. Differential alkaline phosphatase responses of rat and human bone marrow derived mesenchymal stem cells to 45S5 bioactive glass. Biomaterials. 2007;28:4091. doi: 10.1016/j.biomaterials.2007.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sundelacruz S. Kaplan D.L. Stem cell- and scaffold-based tissue engineering approaches to osteochondral regenerative medicine. Semin Cell Dev Biol. 2009;20:646. doi: 10.1016/j.semcdb.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varghese S. Hwang N.S. Ferran A. Hillel A. Theprungsirikul P. Canver A.C. Zhang Z. Gearhart J. Elisseeff J. Engineering musculoskeletal tissues with human embryonic germ cell derivatives. Stem Cells. 2010;28:765. doi: 10.1002/stem.325. [DOI] [PubMed] [Google Scholar]
- 21.Foppiano S. Marshall S.J. Marshall G.W. Saiz E. Tomsia A.P. The influence of novel bioactive glasses on in vitro osteoblast behavior. J Biomed Mater Res A. 2004;71:242. doi: 10.1002/jbm.a.30159. [DOI] [PubMed] [Google Scholar]
- 22.Ginebra M.P. Driessens F.C.M. Planell J.A. Effect of the particle size on the micro and nanostructural features of a calcium phosphate cement: a kinetic analysis. Biomaterials. 2004;25:3453. doi: 10.1016/j.biomaterials.2003.10.049. [DOI] [PubMed] [Google Scholar]
- 23.Radin S. Reilly G. Bhargave G. Leboy P.S. Ducheyne P. Osteogenic effects of bioactive glass on bone marrow stromal cells. J Biomed Mater Res A. 2005;73:21. doi: 10.1002/jbm.a.30241. [DOI] [PubMed] [Google Scholar]
- 24.Habib M. Baroud G. Gitzhofer F. Bohner M. Mechanisms underlying the limited injectability of hydraulic calcium phosphate paste. Acta Biomater. 2008;4:1465. doi: 10.1016/j.actbio.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Zuo Y. Yang F. Wolke J.G. Li Y. Jansen J.A. Incorporation of biodegradable electrospun fibers into calcium phosphate cement for bone regeneration. Acta Biomater. 2010;6:1238. doi: 10.1016/j.actbio.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 26.Friedman C.D. Costantino P.D. Takagi S. Chow L.C. BoneSource hydroxyapatite cement: a novel biomaterial for craniofacial skeletal tissue engineering and reconstruction. J Biomed Mater Res B. 1998;43:428. doi: 10.1002/(sici)1097-4636(199824)43:4<428::aid-jbm10>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 27.Ginebra M.P. Rilliard A. Fernández E. Elvira C. Román J.S. Planell J.A. Mechanical and rheological improvement of a calcium phosphate cement by the addition of a polymeric drug. J Biomed Mater Res. 2001;57:113. doi: 10.1002/1097-4636(200110)57:1<113::aid-jbm1149>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 28.Barralet J.E. Gaunt T. Wright A.J. Gibson I.R. Knowles J.C. Effect of porosity reduction by compaction on compressive strength and microstructure of calcium phosphate cement. J Biomed Mater Res B. 2002;63:1. doi: 10.1002/jbm.1074. [DOI] [PubMed] [Google Scholar]
- 29.Bohner M. Baroud G. Injectability of calcium phosphate pastes. Biomaterials. 2005;26:1553. doi: 10.1016/j.biomaterials.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 30.Jansen J.A. Vehof J.W.M. Ruhe P.Q. Kroeze-Deutman H. Kuboki Y. Takita H. Hedberg E.L. Mikos A.G. Growth factor-loaded scaffolds for bone engineering. J Control Release. 2005;101:127. doi: 10.1016/j.jconrel.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Kasten P. Beyen I. Niemeyer P. Luginbuhl R. Bohner M. Richter W. Porosity and pore size of beta-tricalcium phosphate scaffold can influence protein production and osteogenic differentiation of human mesenchymal stem cells: an in vitro and in vivo study. Acta Biomater. 2008;4:1904. doi: 10.1016/j.actbio.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 32.Bodde E.W. Habraken W.J. Mikos A.G. Spauwen P.H. Jansen J.A. Effect of polymer molecular weight on the bone biological activity of biodegradable polymer/calcium phosphate cement composites. Tissue Eng A. 2009;15:3183. doi: 10.1089/ten.TEA.2008.0694. [DOI] [PubMed] [Google Scholar]
- 33.Brown W.E. Chow L.C. A new calcium phosphate water setting cement. In: Brown P.W., editor. Cements Research Progress. Westerville, OH: American Ceramic Society; 1986. pp. 352–379. [Google Scholar]
- 34.Xu H.H.K. Quinn J.B. Calcium phosphate cement containing resorbable fibers for short-term reinforcement and macroporosity. Biomaterials. 2002;23:193. doi: 10.1016/s0142-9612(01)00095-3. [DOI] [PubMed] [Google Scholar]
- 35.Xu H.H.K. Takagi S. Quinn J.B. Chow L.C. Fast-setting and anti-washout calcium phosphate scaffolds with high strength and controlled macropore formation rates. J Biomed Mater Res A. 2004;68:725. doi: 10.1002/jbm.a.20093. [DOI] [PubMed] [Google Scholar]
- 36.Burguera E.F. Xu H.H.K. Weir M.D. Injectable and rapid-setting calcium phosphate bone cement with dicalcium phosphate dihydrate. J Biomed Mater Res B. 2006;77:126. doi: 10.1002/jbm.b.30403. [DOI] [PubMed] [Google Scholar]
- 37.Wang H.S. Hung S.C. Peng S.T. Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cells. 2004;22:1330. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- 38.Sarugaser R. Lickorish D. Baksh D. Hosseini M.M. Davies J.E. Human umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitors. Stem Cells. 2005;23:220. doi: 10.1634/stemcells.2004-0166. [DOI] [PubMed] [Google Scholar]
- 39.Baksh D. Yao R. Tuan R.S. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25:1384. doi: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- 40.Jäger M. Degistirici O. Knipper A. Fischer J. Sager M. Krauspe R. Bone healing and migration of cord blood-derived stem cells into a critical size femoral defect after xenotransplantation. J Bone Miner Res. 2007;22:1224. doi: 10.1359/jbmr.070414. [DOI] [PubMed] [Google Scholar]
- 41.Bailey M.M. Wang L. Bode C.J. Mitchell K.E. Detamore M.S. A comparison of human umbilical cord matrix stem cells and temporomandibular joint condylar chondrocytes for tissue engineering temporomandibular joint condylar cartilage. Tissue Eng. 2007;13:2003. doi: 10.1089/ten.2006.0150. [DOI] [PubMed] [Google Scholar]
- 42.Wang L. Singh M. Bonewald L.F. Detamore M.S. Signaling strategies for osteogenic differentiation of human umbilical cord mesenchymal stromal cells for 3D bone tissue engineering. J Tissue Eng Regen Med. 2009;3:398. doi: 10.1002/term.176. [DOI] [PubMed] [Google Scholar]
- 43.Can A. Karahuseyinoglu S. Concise review: human umbilical cord stroma with regard to the source of fetus-derived stem cells. Stem Cells. 2007;25:2886. doi: 10.1634/stemcells.2007-0417. [DOI] [PubMed] [Google Scholar]
- 44.Weiss M.L. Medicetty S. Bledsoe A.R. Rachakatla R.S. Choi M. Merchav S. Luo Y. Rao M.S. Velagaleti G. Troyer D. Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson's disease. Stem Cells. 2006;24:781. doi: 10.1634/stemcells.2005-0330. [DOI] [PubMed] [Google Scholar]
- 45.Wang L. Dormer N.H. Bonewald L.F. Detamore M.S. Osteogenic differentiation of human umbilical cord mesenchymal stromal cells in polyglycolic acid scaffolds. Tissue Eng A. 2010;16:1937. doi: 10.1089/ten.TEA.2009.0706. [DOI] [PubMed] [Google Scholar]
- 46.Diao Y. Ma Q. Cui F. Zhong Y. Human umbilical cord mesenchymal stem cells: osteogenesis in vivo as seed cells for bone tissue engineering. J Biomed Mater Res A. 2009;91:123. doi: 10.1002/jbm.a.32186. [DOI] [PubMed] [Google Scholar]
- 47.Simon C.G. Guthrie W.F. Wang F.W. Cell seeding into calcium phosphate cement. J Biomed Mater Res A. 2004;68:628. doi: 10.1002/jbm.a.20008. [DOI] [PubMed] [Google Scholar]
- 48.Weir M.D. Xu H.H.K. Simon C.G., Jr. Strong calcium phosphate cement-chitosan-mesh construct containing cell-encapsulating hydrogel beads for bone tissue engineering. J Biomed Mater Res A. 2006;77:487. doi: 10.1002/jbm.a.30626. [DOI] [PubMed] [Google Scholar]
- 49.Zhao L. Weir M.D. Xu H.H.K. Human umbilical cord stem cell encapsulation in calcium phosphate scaffolds for bone engineering. Biomaterials. 2010;31:3848. doi: 10.1016/j.biomaterials.2010.01.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao L. Weir M.D. Xu H.H.K. An injectable calcium phosphate-alginate hydrogel-umbilical cord mesenchymal stem cell paste for bone tissue engineering. Biomaterials. 2010;31:6502. doi: 10.1016/j.biomaterials.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hesaraki S. Moztarzadeh F. Sharifi D. Formation of interconnected macropores in apatitic calcium phosphate bone cement with the use of an effervescent additive. J Biomed Mater Res A. 2007;83:80. doi: 10.1002/jbm.a.31196. [DOI] [PubMed] [Google Scholar]
- 52.Hesaraki S. Zamanian A. Moztarzadeh F. The influence of the acidic component of the gas-foaming porogen used in preparing an injectable porous calcium phosphate cement on its properties: acetic acid versus citric acid. J Biomed Mater Res B. 2008;86:208. doi: 10.1002/jbm.b.31008. [DOI] [PubMed] [Google Scholar]
- 53.Bouhadir K.H. Lee K.Y. Alsberg E. Damm K.L. Anderson K.W. Mooney D.J. Degradation of partially oxidized alginate and its potential application for tissue engineering. Biotech Progress. 2001;17:945. doi: 10.1021/bp010070p. [DOI] [PubMed] [Google Scholar]
- 54.Hudalla G.A. Eng T.S. Murphy W.L. An approach to modulate degradation and mesenchymal stem cell behavior in poly(ethylene glycol) networks. Biomacromolecules. 2008;9:842. doi: 10.1021/bm701179s. [DOI] [PubMed] [Google Scholar]
- 55.Xu H.H.K. Zhao L. Detamore M.S. Takagi S. Chow L.C. Umbilical cord stem cell seeding on fast-resorbable calcium phosphate bone cement. Tissue Eng A. 2010;16:2743. doi: 10.1089/ten.tea.2009.0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Y. Xu H.H.K. Takagi S. Chow L.C. In-situ hardening hydroxyapatite-based scaffold for bone repair. J Mater Sci Mater Med. 2006;17:437. doi: 10.1007/s10856-006-8471-z. [DOI] [PubMed] [Google Scholar]
- 57.Kong H.J. Smith M.K. Mooney D.J. Designing alginate hydrogels to maintain viability of immobilized cells. Biomaterials. 2003;24:4023. doi: 10.1016/s0142-9612(03)00295-3. [DOI] [PubMed] [Google Scholar]
- 58.Salinas C.N. Cole B.B. Kasko A.M. Anseth K.S. Chondrogenic differentiation potential of human mesenchymal stem cells photoencapsulated within poly(ethylene glycol)-arginine-glycine-aspartic acid-serine thiol-methacrylate mixed-mode networks. Tissue Eng. 2007;13:1025. doi: 10.1089/ten.2006.0126. [DOI] [PubMed] [Google Scholar]
- 59.Atrah H.I. Fibrin glue. Br Med J. 1994;308:933. doi: 10.1136/bmj.308.6934.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ahmed T.A. Dare E.V. Hincke M. Fibrin: a versatile scaffold for tissue engineering applications. Tissue Eng Part B Rev. 2008;14:199. doi: 10.1089/ten.teb.2007.0435. [DOI] [PubMed] [Google Scholar]
- 61.Nuttelman C.R. Tripodi M.C. Anseth K.S. In vitro osteogenic differentiation of human mesenchymal stem cells photoencapsulated in PEG hydrogels. J Biomed Mater Res A. 2004;68:773. doi: 10.1002/jbm.a.20112. [DOI] [PubMed] [Google Scholar]
- 62.Markusen J.F. Mason C. Hull D.A. Town M.A. Tabor A.B. Clements M. Boshoff C.H. Dunnill P. Behavior of adult human mesenchymal stem cells entrapped in alginate-GRGDY beads. Tissue Eng. 2006;12:821. doi: 10.1089/ten.2006.12.821. [DOI] [PubMed] [Google Scholar]
- 63.Drury J.L. Mooney D.J. Review. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 64.Damien C.J. Parsons J.R. Bone graft and bone graft substitutes: a review of current technology and applications. J Appl Biomater. 1991;2:187. doi: 10.1002/jab.770020307. [DOI] [PubMed] [Google Scholar]
- 65.McCalden R.W. McGeough J.A. Court-Brown C.M. Age-related changes in the compressive strength of cancellous bone. The relative importance of changes in density and trabecular architecture. J Bone Joint Surg Am. 1997;79:421. doi: 10.2106/00004623-199703000-00016. [DOI] [PubMed] [Google Scholar]
- 66.Christiansen B.A. Kopperdahl D.L. Kiel D.P. Keaveny T.M. Bouxsein M.L. Mechanical contributions of the cortical and trabecular compartments contribute to differences in age-related changes in vertebral body strength in men and women assessed by QCT-based finite element analysis. J Bone Miner Res. 2011;26:974. doi: 10.1002/jbmr.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Drury J.L. Dennis R.G. Mooney D.J. The tensile properties of alginate hydrogels. Biomaterials. 2004;25:3187. doi: 10.1016/j.biomaterials.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 68.Shi X. Sitharaman B. Pham Q.P. Liang F. Wu K. Billups W.E. Wilson L.J. Mikos A.G. Fabrication of porous ultra-short single-walled carbon nanotube nanocomposite scaffolds for bone tissue engineering. Biomaterials. 2007;28:4078. doi: 10.1016/j.biomaterials.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu H.H.K. Simon C.G. Takagi S. Chow L.C. Eichmiller F.C. Strong, macroporous and in-situ hardening hydroxyapatite for bone tissue engineering. Biomater Forum. 2005;27:14. [Google Scholar]