Abstract
The use of tendon-derived stem cells (TDSCs) as a cell source for musculoskeletal tissue engineering has not been compared with that of bone marrow stromal cells (BMSC). This study compared the mesenchymal stem cell (MSC) and embryonic stem cells (ESC) markers, clonogenicity, proliferative capacity, and multilineage differentiation potential of rat TDSC and BMSC in vitro. The MSC and ESC marker profiles of paired TDSC and BMSC were compared using flow cytometry and quantitative real-time polymerase chain reaction (qRT-PCR), respectively. Their clonogenicity and proliferative capacity were compared using colony-forming and 5-bromo-2′-deoxyuridine assays, respectively. The expression of tenogenic, osteogenic, and chondrogenic markers at basal state were examined using qRT-PCR. Their osteogenic, chondrogenic, and adipogenic differentiation potentials were compared using standard assays. TDSC and BMSC showed similar expression of CD90 and CD73. TDSC expressed higher levels of Oct4 than BMSC. TDSC exhibited higher clonogenicity, proliferated faster, and expressed higher tenomodulin, scleraxis, collagen 1 α 1 (Col1A1), decorin, alkaline phosphatase, Col2A1, and biglycan messenger RNA levels than BMSC. There was higher calcium nodule formation and osteogenic marker expression in TDSC than BMSC upon osteogenic induction. More chondrocyte-like cells and higher glycosaminoglycan deposition and chondrogenic marker expression were observed in TDSC than BMSC upon chondrogenic induction. There were more oil droplets and expression of an adipogenic marker in TDSC than BMSC upon adipogenic induction. TDSC expressed higher Oct4 levels, which was reported to positively regulate mesendodermal lineage differentiation, showed higher clonogenicity and proliferative capacity, and had greater tenogenic, osteogenic, chondrogenic, and adipogenic markers and differentiation potential than BMSC. TDSC might be a better cell source than BMSC for musculoskeletal tissue regeneration.
Introduction
Tissue engineering has received close attention recently for the development of functional replacement tissue. Numerous studies have been conducted to evaluate the optimal cell source, biomaterial, and environment for musculoskeletal tissue engineering.1,2 Commonly used cell types for musculoskeletal tissue engineering include the terminated differentiated cells in the target tissue, adult mesenchymal stem cells (MSC), and embryonic stem cells (ESC).3 Of these, there are some advantages to the use of adult MSC for musculoskeletal repair because these cells maintain some self-renewal potential and can differentiate into different tissues originating from the mesoderm, including bone, cartilage, muscle, tendon, and fat. The synthetic and proliferative abilities of these cells are also robust, along with good modification potential by modern molecular biology techniques.4–6 They can be easily expanded in vitro while maintaining phenotypic stability.7
The most common MSC type that has been studied for musculoskeletal tissue repair is the bone marrow–derived MSC (BMSC) because they are easily accessible. Recently, MSC have also been isolated in other tissues, such as adipose,8 umbilical cord,9 periodontal ligament,10 articular cartilage,11 muscle,12 periosteum,13 synovium,14 and tendon.15,16 Although stem cells that originate from different tissues share some common stem cell characteristics, they might also exhibit some tissue-specific properties and hence functional differences.17 This has implications for the selection of an appropriate cell source and conditions for the engineering of specific tissues.18–27 For instance, MSC isolated from alveolar bone showed less chondrogenic and adipogenic potential than MSCs isolated from iliac bone.27 Higher telomerase activity and hence greater longevity of osteophyte-derived mesenchymal cells were observed than in patient-matched bone marrow stromal cells.18 Adipose tissue–derived stem cells (ADSCs) were reported to possess higher proliferative potential and deposit significantly more calcified extracellular matrix than BMSC upon osteogenic induction,19 although the use of ADSC, but not BMSC, resulted in the growth of fat tissue structures in a calvarial defect repair model.19 Umbilical cord–derived MSCs seeded on polyglycolic acid (PGA) scaffolds produced more glycosaminoglycans and collagen but less collagen type II than BMSC.22 In another study, amniotic fluid–derived stem cells produced less cartilage matrix than BMSC.23 Kern et al.24 has done a comparative analysis of MSC from bone marrow, umbilical cord blood, and adipose tissue. The study reported that MSCs derived from umbilical cord blood formed the fewest colonies after isolation but had the highest proliferative capacity; ADSC had the highest clonogenicity, and BMSC had the shortest culture period and the lowest proliferative capacity.24 Moreover, umbilical cord blood–derived MSC were unable to undergo adipogenic differentiation.24 In another study comparing rat MSC derived from bone marrow, synovium, periosteum, adipose tissue, and muscle, synovium-derived cells had the highest yield, colony-forming efficiency, proliferation, and chondrogenesis.26
We hypothesized that tendon being an organ that functions to connect bone and muscle, its resident stem cells should possess high tenogenic, osteogenic, and chondrogenic differentiation potential and responsiveness to the induction signals to regenerate tendon and junctional tissue. Therefore, it may be a better alternative cell source than BMSC for musculoskeletal tissue repair. The potentials of tendon-derived stem cells (TDSC) as a better cell source for musculoskeletal tissue engineering than BMSC have not been well characterized. This study therefore aimed to compare the MSC and ESC marker expression, clonogenicity, proliferative capacity, and multilineage differentiation potential of rat TDSC and BMSC in vitro.
Materials and Methods
Isolation and culture of rat TDSC and BMSC
The Animal Research Ethics Committee, the Chinese University of Hong Kong approved all experiments. Four- to 6-week-old male outbred green fluorescent protein Sprague-Dawley rats (SD-Tg (CAG-EGFP) Cz-004Osb) weighing 150 to 220 g were used in this study. TDSC and BMSC were isolated from the same animals. The procedures for the isolation of TDSC and BMSC has been previously established.16,28 For the isolation of TDSC, the midsubstance of patellar tendons was excised from healthy rats overdosed with 2.5% intraperitoneal sodium phenobarbital (1.0 mL/400 g). Care was taken that only the midsubstance of patellar tendon tissue, but not the tissue in the tendon–bone junction, was collected. Peritendinous connective tissue was carefully removed, and the tissue was stored in sterile phosphate buffered saline (PBS). The tissues were minced, digested with type I collagenase (3 mg/mL; Sigma-Aldrich, St. Louis, MO) and passed through a 70-μm cell strainer (Becton Dickinson, Franklin Lakes, NJ) to yield a single-cell suspension. The released cells were washed in PBS and resuspended in low-glucose Dulbecco's modified Eagle medium (LG-DMEM) (Gibco) supplemented with 10% fetal bovine serum (FBS), 10 U/mL of penicillin, 100 μg/mL of streptomycin, and 2mM L-glutamine (complete basal culture medium) (all from Invitrogen Corp., Carlsbad, CA). The isolated nucleated cells were plated at the optimal low plating cell density (500 cells/cm2) for the isolation of stem cells and cultured at 37°C, 5% carbon dioxide (CO2) to form colonies. On day 2 after initial plating, the cells were washed twice with PBS to remove the nonadherent cells. On days 7 to 10, they were trypsinized and mixed together as passage 0 (P0).
For the isolation of BMSCs, tibiae and femurs were removed from healthy rats and dissected free of muscle. The bones were rinsed in sterilized PBS before being cut in half; with the cut surface facing the bottom of the centrifuge tube, the tube was spin at 800 g for 15 minutes. The bones were removed, and the bone marrow tissues were washed with PBS. The mononuclear cells were then isolated using density gradient centrifugation (850 g, 30 minutes) using Lymphoprep and resuspended in culture medium containing alpha minimum essential medium (α-MEM; Gibco), 10% FBS, 100 U/mL of penicillin, 100 μg/mL of streptomycin, and 2mM L-glutamine (all from Invitrogen Corp.). These mononuclear cells were plated at an optimal low cell density (105 cells/cm2) for the isolation of stem cells and cultured in a humidified atmosphere at 37°C, 5% CO2 to form colonies. On day 2 after initial plating, the cells were washed twice with PBS to remove the nonadherent cells. On days 7 to 10, they were trypsinized and mixed together as P0.
The optimal initial seeding density for TDSC and BMSC isolation was determined using the colony-forming assay based on the same criteria: that colony-to-colony contact inhibition did not affect colony size and that the greatest number of colonies per nucleated cell was obtained, with colonies that were smaller than 2 mm in diameter and faintly stained being ignored. The optimal initial cell density thus determined was 500/cm2 for the isolation of TDSC from patellar tendon and 105/cm2 for the isolation of BMSC from bone marrow.
TDSC and BMSC were subcultured when they reached 80% to 90% confluence. Medium was changed every 3 days. Except for the chondrogenic differentiation assays, for which cells at P7 were used, all of the assays were performed with cells from P3 to P5. Our previous study showed that there was increase in cellular senescence and loss of multilineage differentiation potential of TDSC with passaging,29 but TDSC at P5 and P10 formed chondrocyte-like cells, although there was decrease in the immunohistochemical staining of collagen type II and Safranin O staining in TDSC from P5 to P10 upon chondrogenic induction for 21 days.29 There was no difference in the messenger RNA (mRNA) expression of collagen type II A 1 (Col2A1) and aggrecan (Acan) in TDSC at P5 and P10 after chondrogenic induction.29 Hence the use of cells at P7 for chondrogenesis was regarded as acceptable. The clonogenicity and multidifferentiation potential of the isolated TDSC and BMSC were confirmed before experiments using colony forming assays and osteogenic, adipogenic, and chondrogenic differentiation assays as described previously.16,28
For the purpose of tracking the fate of transplanted TDSC and BMSC in other on-going and future studies, cells isolated from green fluorescent protein (GFP) rats were used in this study. The GFP rats have the same genetic background as the wild-type Sprague-Dawley rats, except for the expression of GFP, and hence we did not expect any differences in the results obtained with TDSC and BMSC isolated from GFP animals for the purpose of this study.
Fluorescence-activated cell sorting analysis
For study of the expression of CD90 and CD45, 5×105 TDSC and BMSC at P3 to P5 were incubated with 1μg of phycoerythrin (PE)-conjugated mouse anti-rat CD90 monoclonal antibodies (BD Biosciences, Franklin Lakes, NJ) and PE-Cy5-conjugated mouse anti-rat CD45 monoclonal antibodies (BD Biosciences) for 1 hour at 4°C. TDSC and BMSC incubated with PE-Cy5-conjugated isotype-matched IgG1 (BD Biosciences) were used as negative controls. For study of the expression of CD73, 1×106 TDSC and BMSC at P3 to P5 were incubated with 1 μg of unconjugated mouse anti-CD73 monoclonal antibodies (BD Biosciences) for 1 hour at 4°C, followed by incubation with 1 μg of allophycocyanin (APC)-conjugated rat anti-mouse secondary antibodies (BD Biosciences) for 1 hour at 4°C. TDSC and BMSC incubated with APC-conjugated rat anti-mouse secondary antibodies only were used as negative controls. After washing with PBS and being centrifuged at 400 g for 5 minutes, the stained cells were resuspended in 500 μL of ice-cold PBS (with 10% FBS and 1% sodium azide) and analyzed using fluorescence-activated cell sorting (FACS) (BD Biosciences); 104 events were counted for each sample. The percentage of cells with a positive signal and the mean geometric fluorescence value of the positive population were calculated using the WinMDI Version 2.9 program (Scripps Research Institute, La Jolla, CA).
Colony-forming assay
TDSC and BMSC at P3 were plated at 100 cells per 10-cm2 dish and cultured for 10 days. The cells were stained with 0.5% crystal violet (Sigma, St. Louis, MO) to count the number of cell colonies. Colonies that were smaller than 2 mm in diameter and faintly stained were ignored. The number of colonies in each plate was reported.
Cell proliferation assay
TDSC and BMSC at P3 were plated at 2,000 cells/well (cell growth area 0.32cm2/well) in a 96-well plate and incubated at 37°C, 5% CO2. At day 2, cell proliferation was assessed using a 5-bromo-2′-deoxyuridine assay kit (Roche Applied Science, Indianapolis, IN) according to the manufacturer's instruction. The absorbance was measured at 370 to 490 nm and reported.
Expression of osteogenic, chondrogenic, and tenogenic markers at basal state using real-time quantitative polymerase chain reaction
The expression of osteogenic, chondrogenic, and tenogenic markers at basal state was measured using quantitative real-time polymerase chain reactin (qRT-PCR) as previously described.30 Briefly, TDSC and BMSC at P3 were harvested and homogenized for RNA extraction using the RNeasy mini kit (Qiagen, Germany) in triplicate. The mRNA was reverse transcribed to complementary DNA (cDNA) using the First Strand cDNA kit (Promega, Madison, WI). Five μL of cDNA from each sample were amplified in a 25-μL reaction mix containing the Platinum SYBR Green qPCR SuperMix-UDG and specific primers for tenogenic markers (Tnmd, scleraxis (Scx), Col1A), Col3A1, decorin (Dcn), tenascin C (Tnc)), chondrogenic markers (Acan, biglycan (Bgn), Col2A1), and osteogenic marker (alkaline phosphatase (Alpl)) or β-actin using the ABI StepOne Plus system (all from Applied Biosystems, CA) (Table 1). Cycling conditions were denaturation at 95°C for 10 minutes, 45 cycles at 95°C for 20 seconds, optimal annealing temperature for 25 seconds, 72°C for 30 seconds, and 60°C to 95°C with a heating rate of 0.1°C/s. The expression of target gene was normalized to that of β-actin. Relative gene expression was calculated using the 2–ΔCT formula.
Table 1.
Primer Sequences and Conditions for Qualitative Real-Time Polymerase Chain Reaction
| Gene | Gene name | Primer nucleotide sequence | Product size (bp) | Annealing temperature, °C | Accession number |
|---|---|---|---|---|---|
| β-actin | β-actin | 5′-ATCGTGGGCCGCCCTAGGCA-3′ (Forward) 5′-TGGCCTTAGGGTTCAGAGGGG-3′ (Reverse) |
243 | 52 | NM_031144.2 |
| Tenomodulin | Tnmd | 5′-GTGGTCCCACAAGTGAAGGT-3′ (Forward) 5′-GTCTTCCTCGCTTGCTTGTC-3′ (Reverse) |
60 | 52 | NM_022290.1 |
| Scleraxis | Scx | 5′-AACACGGCCTTCACTGCGCTG-3′ (Forward) 5′-CAGTAGCACGTTGCCCAGGTG-3′ (Reverse) |
102 | 58 | NM_001130508.1 |
| Collagen type 1 α 1 | Col1A1 | 5′-CATCGGTGGTACTAAC-3′ (Forward) 5′-CTGGATCATATTGCACA-3′ (Reverse) |
238 | 50 | NM_053356.1 |
| Collagen type 3 α 1 | Col3A1 | 5′-GATGGCTGCACTAAAC-3′ (Forward) 5′-CGAGATTAAAGCAAGAG-3′ (Reverse) |
225 | 50 | NM_032085.1 |
| Decorin | Dcn | 5′-ATGATTGTCATAGAACTGGGC-3′ (Forward) 5′-TTGTTGTTATGAAGGTAGAC-3′ (Reverse) |
382 | 55 | NM_024129.1 |
| Tenascin C | Tnc | 5′-CAGAAGCTGAACCGGAAGTTG-3′ (Forward) 5′-GGCTGTTGTTGCTATGGCACT-3′ (Reverse) |
278 | 55 | NM_053861.1 |
| Alkaline phosphatase | Alpl | 5′-TCCGTGGGTCGGATTCCT-3′ (Forward) 5′-GCCGGCCCAAGAGAGAA-3′ (Reverse) |
85 | 58 | NM_013059.1 |
| Collagen type 2 α 1 | Col2A1 | 5′-CCGGACTGTGAGGTTAGGAT-3′ (Forward) 5′-AACCCAAAGGACCCAAATAC-3′ (Reverse) |
101 | 58 | NM_012929.1 |
| Aggrecan | Acan | 5′-CTTGGGCAGAAGAAAGATCG-3′ (Forward) 5′-GTGCTTGTAGGTGTTGGGGT-3′ (Reverse) |
159 | 58 | NM_022190.1 |
| Biglycan | Bgn | 5′-TCTACATCTCCAAGAACCACCTGG-3′ (Forward) 5′-TTGGTGATGTTGTTGGAGTGCAGA-3′ (Reverse) |
513 | 55 | NM_017087.1 |
| Sox9 | Sox9 | 5′-AGAGCGTTGCTCGGAACTGT-3′ (Forward) 5′-TCCTGGACCGAAACTGGTAAA-3′ (Reverse) |
67 | 60 | XM_343981.2 |
| Runt-related transcription factor 2 | Runx2 | 5′-CCGATGGGACCGTGGTT-3′ (Forward) 5′-CAGCAGAGGCATTTCGTAGCT-3′ (Reverse) |
74 | 60 | NM_053470.1 |
| Bone morphogenetic protein-2 | Bmp2 | 5′-TAGTGACTTTTGGCCACGACG-3′ (Forward) 5′-GCTTCCGCTGTTTGTGTTTG-3′ (Reverse) |
81 | 58 | NM_017178.1 |
| Osteopontin | Spp1 | 5′-TCCAAGGAGTATAAGCAGCGGGCCA-3′ (Forward) 5′-CTCTTAGGGTCTAGGACTAGCTTCT-3′ (Reverse) |
199 | 55 | NM_012881.2 |
| Osteocalcin | Bglap | 5′-GAGCTGCCCTGCACTGGGTG-3′ (Forward) 5′-TGGCCCCAGACCTCTTCCCG-3′ (Reverse) |
263 | 60 | M23637.1 |
| CCAAT enhancer binding protein alpha | C/EBPα | 5′-AAGGCCAAGAAGTCGGTGGA-3′ (Forward) 5′-CAGTTCGCGGCTCAGCTGTT-3′ (Reverse) |
189 | 55 | NM_012524.2 |
| Peroxisome proliferator-activated receptor gamma 2 | PPARγ2 | 5′-CGGCGATCTTGACAGGAAAG-3′ (Forward) 5′-GCTTCCACGGATCGAAACTG-3′ (Reverse) |
174 | 59 | AB019561 |
| Oct4 | Oct4 | 5′-CATCTGCCGCTTCGAG-3′ (Forward) 5′-CTCAATGCTAGTCCGCTTTC-3′ (Reverse) |
166 | 55 | NM_001009178.2 |
| Nanog | Nanog | 5′-GCCCTGATTCTTCTAGCAAT-3′ (Forward) 5′-AGAACACAGTCCGCATCTT-3′ (Reverse) |
120 | 55 | NM_001100781.1 |
| Sox2 | Sox2 | 5′-CCCACCTACAGCATGTCCTA-3′ (Forward) 5′-TGGAGTGGGAGGAAGAGGTA-3′ (Reverse) |
124 | 60 | NM_001109181.1 |
| GAPDH | Gapdh | 5′-GGTCGGTGTGAACGGATTTGG-3′ (Forward) 5′-GCCGTGGGTAGAGTCATACTGGAAC-3′ (Reverse) |
148 | 55 | NM_017008.3 |
ESC marker analysis
The expression of ESC markers, including Oct4, Nanog, and Sox2, in TDSC and BMSC at P5 were compared using qRT-PCR as described above using primers listed in Table 1. The expression of target gene was normalized to that of glyceraldehyde 3-phosphate dehydrogenase.
Osteogenic differentiation assays
TDSC and BMSC at P3 were plated at 4×103 cells/cm2 in a 6-well plate and cultured in basal complete culture medium until the cells reached confluence. They were then incubated in basal complete medium or osteogenic medium, which was basal complete culture medium supplemented with 1nM dexamethasone, 50mM ascorbic acid, and 20mM β-glycerophosphate (all from Sigma-Aldrich). At day 21, the calcium nodule formation in TDSC and BMSC was assessed using an Alizarin red S staining assay, and the mRNA expression of osteogenic markers (Alpl, Runx2, bone morphogenetic protein 2 (Bmp2), osteopontin (Spp1), osteocalcin (Bglap)) were assessed using qRT-PCR as described above using primers listed in Table 1.
Alizarin red S staining assay
The cell–matrix layer was washed with PBS, fixed with 70% ethanol, and stained with 0.5% Alizarin red S (pH 4.1, Sigma). To quantitate the amount of Alizarin red S bound to the mineralized nodules, the cells were rinsed with water and extracted with 10% (w/v) cetylpyridinium chloride (CPC) in 10mM sodium phosphate, pH 7.0 for 15 minutes at room temperature. The dye concentration in the extracts was determined at optical density (OD) 562 nm.
Chondrogenic differentiation assays
A pellet culture system was used to compare the chondrogenic differentiation of TDSC and BMSC; 8×105 cells at P7 were pelleted into a micromass by centrifugation at 450 g for 10 minutes in a 15-mL conical polypropylene tube and cultured in basal complete culture medium or chondrogenic medium at 37° C, 5% CO2, which contained LG-DMEM (Gibco), supplemented with 10 ng/mL of transforming growth factor beta 3 (R&D Systems, Minneapolis, MN), 500 ng/mL of bone morphogenetic protein-2 (R&D Systems, Inc., Minneapolis, MN), 10−7M dexamethasone, 50 μg/mL of ascorbate-2-phosphate, 40 μg/mL of proline, 100 μg/mL of pyruvate (all from Sigma-Aldrich), and 1:100 diluted ITS+Premix (6.25 mg/mL insulin, 6.25 mg/mL transferrin, 6.25 mg/mL selenious acid, 1.25 mg/mL bovine serum albumin, 5.35 mg/mL linoleic acid) (Becton Dickinson). At days 14 and 21, the pellet was fixed for hematoxylin and eosin staining for the identification of chondrocyte phenotype and Alcian blue staining for the examination of glycosaminoglycan deposition. The mRNA expression of Col2A1, Acan, and Sox9 were studied at days 0, 7, 14, and 21 using qRT-PCR as described above using primers listed in Table 1 and presented as the ratio of gene expression in chondrogenic medium and basal medium.
Histologic assay
The cell pellet was fixed in 4% paraformaldehyde, dehydrated, and embedded in paraffin. Sections were cut at a thickness of 5 μm and stained with hematoxylin and eosin or Alcian blue after deparaffination and viewed using a LEICA Q500MC microscope (Leica Cambridge Ltd, Cambridge, UK).
Adipogenic differentiation assays
TDSC and BMSC at P5 were plated at 4x103 cells/cm2 in a 6-well culture plate and cultured until the cells reached confluence. The medium was then replaced with basal complete culture medium or adipogenic medium, which was basal complete culture medium supplemented with 500nM dexamethasone, 0.5mM isobutylmethylxanthine, 50μM indomethacin, and 10μg/mL of insulin (all from Sigma-Aldrich). The cells were cultured for another 21 days to assess the formation of oil droplets using the Oil Red O staining assay and the mRNA expression of CCAAT enhancer binding protein alpha (C/EBPα) and peroxisome proliferator-activated receptor gamma 2 (PPARγ2) using qRT-PCR as described above using primers listed in Table 1. To perform the Oil Red O staining assay, the cells were fixed with 70% ethanol for 10 minutes, stained with 0.3% fresh Oil Red O solution (Sigma-Aldrich) for 2 hours, and viewed using a LEICA Q500MC microscope (Leica Cambridge Ltd).
Data analysis
Quantitative data was shown in box plots. The clonogenicity and proliferative potential of TDSC and BMSC were compared using the unpaired t-test. Otherwise, the comparison of two groups was done using the Mann-Whitney U-test, and the comparison of more than two groups was done using the Kruskal-Wallis test, followed by the Mann-Whitney U-test in the post hoc comparison. All of the data analysis was done using SPSS analysis software (version 16.0, SPSS Inc., Chicago, IL). p≤0.05 was regarded as statistically significant.
Results
Immunophenotypic profile
TDSC and BMSC expressed CD90 and CD73 but were negative for CD45 (Fig. 1). Although they showed similar percentages of cells with a positive signal, the mean geometric fluorescence values of CD90 and CD73 seemed to be higher in TDSC than BMSC (Fig. 1).
FIG. 1.
Histograms showing the expression of CD90, CD73, and CD45 in paired bone marrow–derived stem cells (BMSC) and tendon-derived stem cells (TDSC). Filled area shows the expression of target marker; open open area shows the expression of the corresponding isotype control (phycoerythrin (PE) or allophycocyanin (APC)). Gm, geometric mean fluorescence value of the positive population; Cv, coefficient of variation. The percentage of cells showing positive expression is shown in brackets.
Clonogenicity and proliferative potential
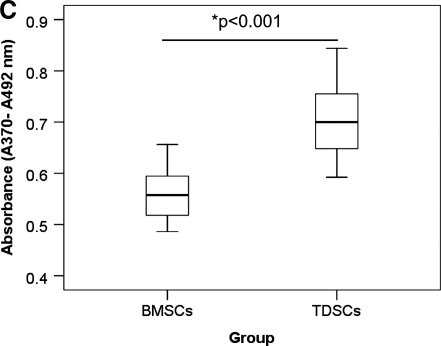
TDSC formed more colonies (p=0.01) (Fig. 2A, B) and proliferated faster (p<0.001) (Fig. 2C) than BMSC.
FIG. 2.
(A) Photographs and (B) box plot comparing the number of colony forming units (CFU) of paired TDSC and BMSC at day 10. (C) Box plot comparing the proliferative potential of paired TDSC and BMSC at day 2 with 5-bromo-2′-deoxyuridine assay. *p≤0.050.
Expression of osteogenic, chondrogenic, and tenogenic markers at basal state
TDSC expressed higher mRNA level of tenogenic (Tnmd, Scx, Col1A1, Dcn), osteogenic (Alp), and chondrogenic (Col2A1, Bgn) markers than paired BMSC in complete culture medium (all p=0.05) (Fig. 3). There was a trend toward lower mRNA expression of Col3A1 and higher expression of Tnc and Acan in TDSC than in BMSC, but the difference was not statistically significant (Fig. 3).
FIG. 3.
Box plots showing the messenger RNA (mRNA) expression of (A) Tnmd, (B) Scx, (C) Col1A1, (D) Col3A1, (E) Col1A1/Col3A1, (F) Dcn, (G) Tnc, (H) Alpl, (I) Col2A1, (J) Acan, and (K) Bgn in paired TDSC and BMSC in basal complete medium. *p≤0.05.
ESC marker analysis
TDSC expressed higher levels of Oct4 (p=0.02, Fig. 4A) than BMSC. There was no significant difference in the expression of Nanog (p=0.52, Fig. 4B) or Sox2 (p=0.42, Fig. 4C) in TDSC and BMSC.
FIG. 4.
Box plots showing the mRNA expression of (A) Oct4, (B) Nanog, and (C) Sox2 in paired TDSC and BMSC in basal complete medium. *p≤0.05.
Osteogenic differentiation potential
There was significant increase in matrix mineralization in BMSC and TDSC upon osteogenic induction for 21 days, as demonstrated using Alizarin red S staining (Fig. 5A-E; both p=0.004). More calcium nodules were formed in TDSC than BMSC upon osteogenic induction (Fig. 5C-E, p=0.004). There were significant increases in runt-related transcription factor) (Runx2) (p=0.00) (Fig. 45G), Spp1 (p=0.004) (Fig. 5I), and Bglap (p=0.004) (Fig. 5J) but a decrease in Alpl (p=0.025) (Fig. 5F) in TDSC at day 21 upon osteogenic induction. Under the same conditions, only the expression of Alpl and Bmp2 increased in BMSC (both p=0.006, Fig. 5F, H). There was significantly higher expression of Alpl, Runx2, Bmp2, and Bglap in TDSC than in BMSC at day 21 in basal medium (all p=0.004, Fig. 5F, G, H, J). There was significantly higher expression of Alpl (p=0.006), Runx2 (p=0.006), Bmp2 (p=0.01), Spp1 (p=0.006), and Bglap (p=0.006) in TDSC than in BMSC at day 21 upon osteogenic induction (Fig. 5 F-J).
FIG. 5.
Photomicrographs showing the Alizarin red S staining of calcium nodules in (A, C) BMSC and (B, D) TDSC in (A, B) basal or (C, D) osteogenic induction media after 21 days. Magnification: ×100. Scale bar=100μm (E) Box plot showing the quantitative analysis of the Alizarin red S stain bound to calcium nodules. Box plots showing the mRNA expression of (F) Alpl, (G) Runx2, (H) Bmp2, (I) Spp1, and (J) Bglap in paired TDSC and BMSC in basal medium or osteogenic medium for 21 days. *p≤0.05. Color images available online at www.liebertonline.com/tea
Chondrogenic differentiation potential
Cell pellets were formed at days 14 and 21 upon chondrogenic induction in both cell types (Fig. 6A-D), although more chondrocyte-like cells were observed in the cell pellets formed by TDSC at days 14 (Fig. 6A, C, insert, arrows) and 21 (Fig. 6B, D, insert, arrows). There was more glycosaminoglycan deposition in the cell pellets formed by TDSC than in the cell pellets formed by BMSC at days 14 (Fig. 6E, G, insert) and 21 (Fig. 6F, H, insert). There was significantly higher expression of Col2A1 (Fig. 6I) and Acan (Fig. 6J) but not Sox9 (Fig. 6K) expression ratios in TDSC than BMSC upon chondrogenic induction.
FIG. 6.
Photomicrographs showing the presence of chondrocyte phenotype (arrows) in paired (A, B) TDSC and (C, D) BMSC after chondrogenic induction for (A, C) 14 and (B, D) 21 days. Stain, hematoxylin and eosin; magnification, ×200x; insert, ×400. Photomicrographs showing the glycosaminoglycan deposition in paired (E, F) TDSC and (G, H) BMSC after chondrogenic induction for (E, G) 14 and (F, H) 21 days. Stain, Alcian blue; magnification, ×200; insert, ×400. Box plots showing the ratio of mRNA expression of (I) Col2A1, (J) Acan, (K) Sox9 in basal medium or chondrogenic medium in paired TDSC and BMSC at days 0, 7, 14, and 21. *p≤0.05. Color images available online at www.liebertonline.com/tea
Adipogenic differentiation potential
More oil droplets were formed in TDSC than BMSC upon adipogenic induction for 21 days (Fig. 7C, D). The expression of PPARγ2 and C/EBPα increased in TDSC and BMSC upon adipogenic induction (all p=0.004; Fig. 7E). There was significantly higher expression of PPARγ2 (p=0.006; Fig. 7E) but not C/EBPα (p=0.262; Fig. 7F) in TDSC than BMSC upon adipogenic induction.
FIG. 7.
Photomicrographs showing the Oil Red O staining of oil droplets in (A, C) BMSC and (B, D) TDSC in (A, B) basal or (C, D) adipogenic induction medium for 21 days. Magnification, ×100. Scale bar=100μm. Box plots showing the mRNA expression of (E) PPARγ2 and (F) C/EBPα in paired TDSC and BMSC in basal medium or adipogenic medium for 21 days. *p≤0.05.
Discussion
Adult MSC isolated from different tissues have been studied for musculoskeletal tissue regeneration. The selection of appropriate cell sources is an important issues in musculoskeletal tissue engineering, given that stem cells isolated from different sources, although sharing some common stem cell characteristics, also have some tissue-specific properties that may influence the outcome of tissue engineering. Tendon stem and progenitor cells (TSPC) have been isolated recently in tendon.15,16 As stem cells residing in tendons, these cells may be a good cell source for musculoskeletal repair because they might retain epigenetic memory similar to induced pluripotent stem cells (iPSC)31 and other tissue-specific MSCs,32 which might facilitate tissue repair. This study therefore aimed to compare the MSC and ESC markers, colony-forming ability, proliferative potential, and multilineage differentiation potential of TDSC and BMSC isolated from rats.
Our results showed that TDSC culture proliferated faster and recruited more-primitive cells than BMSC culture. Our results regarding the proliferative capacity of TDSC was consistent with the result of Bi et al.,15 who reported that human and mouse TSPC proliferated faster than BMSC isolated from the same person or animal. The number of population doublings of mouse TSPC was also higher than that of BMSC, but this was not observed for human TSPC. Our findings in this study thus have a significant bearing on the in vitro isolation and expansion of stem cells for musculoskeletal tissue engineering.
We report higher mRNA expression of tenogenic, chondrogenic, and osteogenic markers in TDSC than BMSC at basal state. We further showed that TDSC surpassed BMSC in osteogenesis, chondrogenesis, and adipogenesis upon induction. This corroborated with the findings of Bi et al.,15 who reported that mouse TPSC expressed higher mRNA levels of Scx, Comp, Sox9, and Runx2 than mouse BMSC, whereas human TPSC expressed higher levels of Tnmd than human BMSC. More mouse TSPC expressed Comp and Tnc proteins than mouse BMSC. All mouse TSPC expressed collagen type I, whereas only a certain population of BMSC expressed this protein. In addition, mouse and human TSPC were reported to have higher osteogenic and adipogenic differentiation potentials than BMSC upon induction, although the chondrogenic differentiation potential of TPSC and BMSC was not compared in their study. Thomson et al.33 showed that Oct4 and Sox2 were critical for germ layer fate choice in addition to their roles in maintaining the pluripotency and self-renewal capacity of ESC. Differentiation signals modulated the expression of Oct4 and Sox2 such that induction of Oct4 suppressed neural ectodermal differentiation and promoted mesendodermal differentiation, whereas induction of Sox2 inhibited mesendodermal differentiation and promoted neural ectodermal differentiation.33 The higher expression of Oct4 in TDSC than in BMSC, as observed in our study, might favor mesendodermal lineage choice of TDSC. Our results suggested that TDSC are a more-promising therapeutic cell source for musculoskeletal tissue repair than BMSC. Further studies comparing the effect of TDSC and BMSC on the regeneration of different musculoskeletal tissues in relevant animal models are necessary.
The origin of TDSC is not clear. Seeing as we observed higher colony-forming ability and proliferative and multilineage differentiation potential upon induction and higher osteogenic, chondrogenic, and tenogenic marker expression at basal state in TDSC,s than BMSC, it is likely that TDSC are a distinct cell type from BMSC. TDSC may be imprinted under the influence of local environmental milieu so that they are more likely to produce tendon and junctional tissues, although the possibility that TDSC and BMSC are different stages of a common MSC cannot be excluded. Previous studies suggested that the perivascular niche is a source of stem cells and that all MSC are pericytes that gradually assume tissue-specific phenotypes under the influence of the local niche.34,35 Whether TDSC are pericytes or are resident at the perivascular niche need further study. Rat TDSC expressed αSMA, a pericyte-associated marker, suggesting their possible relationship to perivascular cells.16 The availability of a panel of surface markers that can differentiate TDSC from BMSC will facilitate the study of the origin of TDSC.
Despite the fact that TDSC might show good potential for musculoskeletal tissue repair, the application of TDSC for tissue repair is feasible only with the use of an allogeneic cell source because it is difficult to obtain autologous TDSC without causing donor site morbidity. On the other hand, allogeneic TDSC can be easily isolated from the waste tendon tissue during tendon and ligament surgery, such as the residual tendon graft tissue in anterior cruciate ligament (ACL) reconstruction and total knee replacement, and an allogeneic TDSC bank can be established for future clinical application. Our laboratory is currently isolating TDSC from human patellar tendons and human hamstring tendons during ACL reconstruction for other experiments, indicating that it is practical. The use of allogeneic stem cells for tendon repair also allows earlier treatment for patients thanth the use of an autologous cell source. Unlike bone marrow aspiration for the isolation of BMSC, the harvest of residual tendon tissue does not require a separate surgery and does not impose additional pain on donors. Mouse patellar tendons and human hamstring tendons contain 3% to 4% TDSC.15 Rat flexor tendons contain approximately 1% to 2% TDSC.16 Approximately 5% to 6% of human fetal Achilles TDSC at passage 2 were able to form colonies.36 Approximately 0.001% to 0.01% of total nucleated cells of human bone marrow aspirates were BMSC.37,38 The percentage of stem cells in tendon therefore exceeds that in bone marrow aspirates by at least three orders of magnitude. Moreover, the amount of BMSC obtained depends on the volume of aspiration. The concentration of BMSC obtained per mL decreased with increased volume of aspirated marrow for each puncture because of dilution of the bone marrow sample with peripheral blood.39 These major drawbacks have inspired many investigators to explore alternative tissues for more-abundant and -accessible sources of MSC with less-invasive collection procedures. Our unpublished result showed that TDSC lacked major histocompatability complex class II and that TDSC from an allgoneic source promoted tendon regeneration in a patellar tendon window injury model in rats.40 Experiments are underway to confirm the hypo-immunogenicity of TDSC. Because of their higher proliferative rate and multilineage differentiation potential, we believe that TDSC are a cell source competitive with BMSC for musculoskeletal tissue engineering.
In conclusion, TDSCs showed higher Oct4; clonogenicity; proliferative capacity; and tenogenic, osteogenic, chondrogenic, and adipogenic differentiation markers and potential than BMSC. TDSC might be a better cell source than BMSC for musculoskeletal tissue regeneration.
Acknowledgments
This study was supported by equipment / resources donated by the Hong Kong Jockey Club Charities Trust and the CUHK Direct Grant (2009.1.043).
Disclosure Statement
No competing financial interests exist.
References
- 1.Deans T.L. Elisseeff J.H. Stem cells in musculoskeletal engineered tissue. Curr Opin Biotechnol. 2009;20:537. doi: 10.1016/j.copbio.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Leo A.J. Grande D.A. Mesenchymal stem cells in tissue engineering. Cells Tissues Organs. 2006;183:112. doi: 10.1159/000095985. [DOI] [PubMed] [Google Scholar]
- 3.Tuan R.S. Boland G. Tuli R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res Ther. 2003;5:32. doi: 10.1186/ar614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu H. Fan H. Toh S.L. Goh J.C.H. A comparison of rabbit mesenchymal stem cells and anterior cruciate ligament fibroblasts responses on combined silk scaffolds. Biomaterials. 2008;29:1443. doi: 10.1016/j.biomaterials.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 5.Van Eijk F. Saris D.B. Riesle J. Willems W.J. Van Blitterswijk C.A. Verbout A.J. Dhert W.J. Tissue engineering for ligaments: a comparison of bone marrow stromal cells, anterior cruciate ligament, and skin fibroblasts as cell source. Tissue Eng. 2004;10:893. doi: 10.1089/1076327041348428. [DOI] [PubMed] [Google Scholar]
- 6.Ge Z. Goh J.C. Lee E.H. Selection of cell source for ligament tissue engineering. Cell Transplant. 2005;14:573. doi: 10.3727/000000005783982819. [DOI] [PubMed] [Google Scholar]
- 7.Pittenger M.F. Mackay A.M. Beck S.C. Jaiswal R.K. Douglas R. Mosca J.D. Moorman M.A. Simonetti D.W. Craig S. Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 8.Lin T.M. Chang H.W. Wang K.H. Kao A.P. Chang C.C. Wen C.H. Lai C.S. Lin S.D. Isolation and identification of mesenchymal stem cells from human lipoma tissue. Biochem Biophys Res Commun. 2007;361:883. doi: 10.1016/j.bbrc.2007.07.116. [DOI] [PubMed] [Google Scholar]
- 9.Kim J.W. Kim S.Y. Park S.Y. Kim Y.M. Kim J.M. Lee M.H. Ryu H.M. Mesenchymal progenitor cells in the human umbilical cord. Ann Hematol. 2004;83:733. doi: 10.1007/s00277-004-0918-z. [DOI] [PubMed] [Google Scholar]
- 10.Seo B.M. Miura M. Gronthos S. Bartold P.M. Batouli S. Brahim J. Young M. Robey P.G. Wang C.Y. Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 11.Dowthwaite G.P. Bishop J.C. Redman S.N. Khan I.M. Rooney P. Evans D.J. Haughton L. Bayram Z. Boyer S. Thomson B. Wolfe M.S. Archer C.W. The surface of articular cartilage contains a progenitor cell population. J Cell Sci. 2004;117(Pt 6):889. doi: 10.1242/jcs.00912. [DOI] [PubMed] [Google Scholar]
- 12.Qu-Petersen Z. Deasy B. Jankowski R. Ikezawa M. Cummins J. Pruchnic R. Mytinger J. Cao B. Gates C. Wernig A. Huard J. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157:851. doi: 10.1083/jcb.200108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Bari C. Dell'Accio F. Vanlauwe J. Eyckmans J. Khan I.M. Archer C.W. Jones E.A. McGonagle D. Mitsiadis T.A. Pitzalis C. Luyten F.P. Mesenchymal multipotency of adult human periosteal cells demonstrated by single-cell lineage analysis. Arthritis Rheum. 2006;54:1209. doi: 10.1002/art.21753. [DOI] [PubMed] [Google Scholar]
- 14.De Bari C. Dell'Accio F. Tylzanowski P. Luyten F.P. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 15.Bi Y. Ehirchiou D. Kilts T.M. Inkson C.A. Embree M.C. Sonoyama W. Li L. Leet A.I. Seo B.M. Zhang L. Shi S. Young M.F. Identification of tendon stem / progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 16.Rui Y.F. Lui P.P.Y. Li G. Fu S.C. Lee Y.W. Chan K.M. Isolation and characterization of multi-potent rat tendon-derived stem cells. Tissue Eng Part A. 2010;16:1549. doi: 10.1089/ten.TEA.2009.0529. [DOI] [PubMed] [Google Scholar]
- 17.Sakaguchi Y. Sekiya I. Yagishita K. Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 18.Singh S. Dhaliwal N. Crawford R. Xiao Y. Cellular senescence and longevity of osteophyte-derived mesenchymal stem cells compared to patient-matched bone marrow stromal cells. J Cell Biochem. 2009;108:839. doi: 10.1002/jcb.22312. [DOI] [PubMed] [Google Scholar]
- 19.Lin L. Shen Q. Wei X. Hou Y. Xue T. Fu X. Duan X. Yu C. Comparison of osteogenic potentials of BMP4 transduced stem cells from autologous bone marrow and fat tissue in a rabbit model of calvarial defects. Calcif Tissue Int. 2009;85:55. doi: 10.1007/s00223-009-9250-x. [DOI] [PubMed] [Google Scholar]
- 20.Baksh D. Yao R. Tuan R.S. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25:1384. doi: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi O. Katsube Y. Hirose M. Ohgushi H. Ito H. Comparison of osteogenic ability of rat mesenchymal stem cells from bone marrow, periosteum, and adipose tissue. Calcif Tissue Int. 2008;82:238. doi: 10.1007/s00223-008-9112-y. [DOI] [PubMed] [Google Scholar]
- 22.Wang L. Tran I. Seshareddy K. Weiss M.L. Detamore M.S. A comparison of human bone marrow-derived mesenchymal stem cells and human umbilical cord-derived mesenchymal stromal cells for cartilage tissue engineering. Tissue Eng Part A. 2009;15:2259. doi: 10.1089/ten.tea.2008.0393. [DOI] [PubMed] [Google Scholar]
- 23.Kolambkar Y.M. Peister A. Soker S. Atala A. Guldberg R.E. Chondrogenic differentiation of amniotic fluid-derived stem cells. J Mol Histol. 2007;38:405. doi: 10.1007/s10735-007-9118-1. [DOI] [PubMed] [Google Scholar]
- 24.Kern S. Eichler H. Stoeve J. Kluter H. Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 25.Cheng M.T. Liu C.L. Chen T.H. Lee O.K. Comparison of potentials between stem cells isolated from human anterior cruciate ligament and bone marrow for ligament tissue engineering. Tissue Eng Part A. 2010;16:2237. doi: 10.1089/ten.TEA.2009.0664. [DOI] [PubMed] [Google Scholar]
- 26.Yoshimura H. Muneta T. Nimura A. Yokoyama A. Koga H. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007;327:449. doi: 10.1007/s00441-006-0308-z. [DOI] [PubMed] [Google Scholar]
- 27.Matsubara T. Tsutsumi S. Pan H. Hiraoka H. Oda R. Nishimura M. Kawaguchi H. Nakamura K. Kato Y. A new technique to expand human mesenchymal stem cells using basement membrane extracellular matrix. Biochem Biophys Res Commun. 2004;313:503. doi: 10.1016/j.bbrc.2003.11.143. [DOI] [PubMed] [Google Scholar]
- 28.Rui Y.F. Lui P.P.Y. Ni M. Chan L.S. Lee Y.W. Chan K.M. Mechanical loading increased BMP-2 expression which promoted osteogenic differentiation of tendon-derived stem cells. J Orthop Res. 2011;29:390. doi: 10.1002/jor.21218. [DOI] [PubMed] [Google Scholar]
- 29.Tan Q. Lui P.P. Rui Y.F. Effect of in-vitro passaging on the stem cell-related properties of tendon-derived stem cells—implication in tissue engineering. Stem Cells Dev. 2011 Aug 8; doi: 10.1089/scd.2011.0160. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lui P.P.Y. Chan L.S. Lee Y.W. Fu S.C. Chan K.M. Sustained expression of proteoglycans and collagen type III/Type I ratio in a calcified tendinopathy model. Rheumatology (Oxford) 2010;49:231. doi: 10.1093/rheumatology/kep384. [DOI] [PubMed] [Google Scholar]
- 31.Quattrocelli M. Palazzolo G. Floris G. Schoffski P. Anastasia L. Orlacchio A. Vandendriessche T. Chuah M.K. Cossu G. Verfaillie C. Sampaolesi M. Intrinsic cell memory reinforces myogenic commitment of pericyte-derived iPSCs. J Pathol. 2011;223:593. doi: 10.1002/path.2845. [DOI] [PubMed] [Google Scholar]
- 32.Liu A. Han Y.R. Li J., et al. The glial or neuronal fate choice of oligodendrocyte progenitors is modulated by their ability to acquire an epigenetic memory. J Neurosci. 2007;27:7339. doi: 10.1523/JNEUROSCI.1226-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomson M. Liu S.J. Zou L.N. Smith Z. Meissner A. Ramanathan S. Pluripotency factors in embryonic stem cells regulate differentiation into germ layers. Cell. 2011;145:875. doi: 10.1016/j.cell.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caplan A.I. All MSCs are pericytes? Cell Stem Cell. 2008;3:229. doi: 10.1016/j.stem.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 35.da Silva Meirelles L. Chagastelles P.C. Nardi N.B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119(Pt 11):2204. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 36.Yin Z. Chen X. Chen J.L. Shen W.L. Hieu Nguyen T.M. Ouyang H.W. The regulation of tendon stem cell differentiation by the alignment of nanofibers. Biomaterials. 2010;31:2163. doi: 10.1016/j.biomaterials.2009.11.083. [DOI] [PubMed] [Google Scholar]
- 37.Stenderup K. Justesen J. Eriksen E.F. Rattan S.I. Kassem M. Number and proliferative capacity of osteogenic stem cells are maintained during aging and in patients with osteoporosis. J Bone Miner Res. 2001;16:1120. doi: 10.1359/jbmr.2001.16.6.1120. [DOI] [PubMed] [Google Scholar]
- 38.Pittenger M.F. Mackay A.M. Beck S.C. Jaiswal R.K. Douglas R. Mosca J.D. Moorman M.A. Simonetti D.W. Craig S. Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 39.Muschler G.F. Boehm C. Easley K. Aspiration to obtain osteoblast progenitor cells from human bone marrow: the influence of aspiration volume. J Bone Joint Surg Am. 1997;79:1699. doi: 10.2106/00004623-199711000-00012. [DOI] [PubMed] [Google Scholar]
- 40.Ni M. Lui P.P.Y. Rui Y.F. Lee Y.W. Lee Y.W. Tan Q. Wong Y.M. Kong S.K. Lau P.M. Li G. Chan K.M. Tendon-derived stem cells (TDSCs) promote tendon repair in a rat patellar tendon window defect model. J Orthop Res. 2011 Sep 16; doi: 10.1002/jor.21559. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]