Abstract
Scaffolds for tissue engineering must be designed to direct desired events such as cell attachment, growth, and differentiation. The incorporation of extracellular matrix-derived peptides into biomaterials has been proposed to mimic biochemical signals. In this study, three synthetic fragments of fibronectin, vitronectin, and stromal-derived factor-1 were investigated for the first time as potential adhesive sequences for cardiomyocytes (CMs) compared to smooth muscle cells. CMs are responsive to all peptides to differing degrees, demonstrating the existence of diverse adhesion mechanisms. The pretreatment of nontissue culture well surfaces with the (Arginine-Glycine-Aspartic Acid) RGD sequence anticipated the appearance of CMs' contractility compared to the control (fibronectin-coated well) and doubled the length of cell viability. Future prospects are the inclusion of these sequences into biomaterial formulation with the improvement in cell adhesion that could play an important role in cell retention during dynamic cell seeding.
Introduction
Cardiovascular disease is responsible for a preponderance of health problems in developed countries, where almost one quarter of the population lives with coronary heart disease, congenital cardiovascular defects, or congestive heart failure. Cardiovascular diseases result in substantial disability and contribute greatly to the escalation of healthcare costs.
Myocardium does not possess the ability to regenerate after injury. Cell-based therapies are aimed at the creation of novel curative treatments either by the use of cells alone or by scaffold-based tissue engineering cardiac constructs that can be surgically applied to the diseased cardiac muscle. While the integration of cells into scar tissue is designated for treating small-scale injuries, tissue engineering may provide substitutes to treat large defects. Additionally—in contrast to the cells-only approach—engineered tissues have the potential advantage of immediate functionality1 targeted at restoring tissue function with substrates able to direct desired cell fates such as attachment, growth, and differentiation.2 Mimicking the best natural scaffold, the extracellular matrix (ECM), represents an optimal strategy to reproduce biochemical signals inserted in a three-dimensional (3D) environment, many of which exist on a nanoscale.3–7 Cells integrate with the ECM in vivo as a physiologic mechanism involving bi-directional interactions between the intracellular environment, through cell-surface receptors, and the surrounding ECM itself. Integrin receptors, in particular, are responsible for modulating signaling events that are essential for cell adhesion, proliferation, spreading, and eventually differentiation.8 On one hand, these interactions induce specific behaviors and differentiation of individual cells; on the other hand, they provide the signals that sustain tissue formation and turnover of ECM proteins.9
Recent strategies in tissue-engineered biomaterial development have focused on incorporating ECM-derived peptides into biomaterials in order to mimic natural matrix.10 The use of peptides, instead of native proteins, gives some advantages in the design of a chemoselective ligation strategy and in the resultant ordered orientation of the sequences on surfaces or within scaffolds.11 Cell adhesion to fibronectin is mediated by the RGD motif (Arginine-Glycine-Aspartic Acid) bound by specific integrins.12,13 These latter bind to the RGD motif of fibronectin dissociating from focal adhesions and translocating along actin stress fibers toward the cell center, where they form a new adhesion structure called fibrillar adhesions.14
The covalent grafting of an adhesive sequence containing one RGD motif in a polymeric scaffold15 or in a photopolymerizable polyethylene glycol matrix16 enhanced adhesion and growth of smooth muscle cells (SMCs).
Additionally, it was demonstrated that an RGD peptide immobilized in an alginate scaffold was able to increase neonatal rat cardiomyocytes' (CMs) adhesion, preventing cell apoptosis and accelerating cardiac tissue regeneration.17 To identify novel peptides for the aforementioned applications, three synthetic peptides reproducing sequences of fibronectin, vitronectin, and, for the first time, the novel stromal cell-derived factor-1 (SDF-1)β were investigated regarding their potential to enhance the adhesion of neonatal rat CMs compared to rat neonatal SMCs from abdominal aorta. Before moving to the implementation of tridimensional scaffolds with these synthetic peptides, we aimed in this study at understanding their basic ability to promote cell adhesion on plastic surfaces. These kinds of cells, in addition to endothelial cells and fibroblasts, constitute the majority of differentiated adherent elements for the cardiovascular system. The fibronectin-derived peptide is a linear sequence that repeats the native fragment GRGDSP four times and contains, in its C-terminus, a Lysine residue. This peptide was demonstrated to increase cell adhesion fourfold compared to GRGDSPK or other RGD sequences.18 Hereafter, we will refer to the RGD peptide as the GRGDSP sequence derived for specifically for this study.
Vitronectin is a 75-kDa monomeric protein present in the blood, and in its oligomeric form in the ECM, which contains multiple domains capable of binding integrins, collagens, plasminogen, insulin-like growth factors, and the urokinase receptor.19 The vitronectin-derived sequence, named as human vitronectin protein (HVP), displays a heparin binding site (sequence 351–359) and was used with success in the preparation of biomimetic titania surfaces.20 The use of this sequence may also be suitable for CM and SMC because, in addition to binding of RGD peptide by integrins, the engagement of the heparin binding sites of adhesive glycoproteins with glycosaminoglycans (GAGs) on the cell surface is also involved in the adhesion of these kinds of cells.21
Finally, we propose, as an adhesion sequence, a fragment (sequence 51–72) of SDF-1β.19 SDF-1 (two types of SDF-1, named α and β, derived by alternative splicing) and its receptor, CXCR4, were found to be present in the heart, specifically in CMs and fibroblasts. SDF-1 is an important homing signal, and hypoxic induction of SDF-1 expression serves to protect the heart from ischemia/reperfusion injury by activating survival signaling pathways.22 In the adhesion assays, reported herein, these peptides were adsorbed on tissue culture (TC) plate wells.
CMs were cultured on either nitrocellulose pretreated or non-pretreated surfaces. For SMC adhesion assays, the experiments were carried out in the presence or absence of fetal bovine serum (FBS) to limit and evaluate the contribution of blood proteins to the biomimetic coverage of the well. The results allowed the authors to confirm the modulation of adhesion of both the selected cell populations induced by peptides and to predict their utility in conditioning strategies for cell cultures and for cardiac tissue engineering applications.
Materials and Methods
Peptide synthesis
All peptides were synthesized by solid phase methods using a fully automated peptide synthesizer (Applied Biosystems 431A) via Fmoc chemistry.18,20,23 The following solid supports were used: Fmoc-Lys(Boc)-Wang resin (0.77 mmol/g) for RGD and SDF peptides; Fmoc-Tyr (tBu)-Sasrin resin for the HVP peptide. The following side chain protections were used: Lys, Boc; Arg, Pmc; Asn, Gln and His, Trt; Asp and Glu, OtBu; Ser and Tyr, tBu; Trp, Boc. At the end of the synthesis and after the Fmoc-deprotection of the last residue, the resin was treated with the following solutions: 2,2,2-trifluoroacetic acid (TFA):triethylsilane (TES):water=95:0.25:0.25 for 90 min for HVP and RGD and TFA:ethandithiole:water:TES=94.5:2.5:2.5:1 for 90 min for SDF. After filtration the crude product was precipitated from the concentrated solution with cold diethyl ether. Crude peptides were purified using reversed-phase high performance liquid chromatography technique (RP-HPLC). Identity and homogeneity of all the synthetic products were evaluated using analytical reversed-phase chromatography, capillary electrophoresis, amino acid analysis after acid hydrolysis, and mass spectrometric analysis. The homogeneity of each peptide proved to be over 98%.18,24
Determination of adsorbed peptide
Each well of a nontissue culture (nTC) plate (96-wells) was filled with 60 μL of 0.01 mM peptide solution, corresponding to 2.0 nmoles/cm2 or with 60 μL of 0.001 mM peptide solution, corresponding to 0.2 nmoles/cm2. After 24 h incubation at 37°C, each peptide solution was drawn up for quantification. The residual peptide concentration was determined by the integration of the area of RP-HPLC peak corresponding to the peptide. The area of the peak (mean value of three injection) was correlated to peptide nmoles using three calibration curves, one for each peptide. The nmoles of the adsorbed peptide were obtained as the difference between the initial quantity used for incubation and the residual peptide quantity. This quantification was performed only for the 2.0 nmoles/cm2 concentration of the three peptides, as the HPLC analysis was not sensitive enough to provide data for the absorption of the 0.2 nmoles/cm2–coated wells.
Preparation of the biomimetic surface
The cell adhesion experiment was performed on nTC multiwell plate functionalized with the peptide solutions. RGD, HVP, and SDF 1β were dissolved in phosphate-buffered saline (PBS) and used at two different concentrations, 0.2 and 2.0 nmoles/cm2, for both SMCs and CMs.
Commercially available fibronectin, from human foreskin fibroblasts (Sigma), was diluted in PBS to a final concentration of 0.005 nM and used as a positive control. Peptides and fibronectin were incubated at 37°C for 24 h in order to favor their adsorption on the plastic surface. Later, extensive washings were performed to remove nonadsorbed peptides and protein, and a further treatment with 1 mg/mL bovine serum albumin (BSA) at 37°C for 2 h was carried out, followed by new washings, before cell seeding.
For CMs only, a preliminary incubation with a solution of nitrocellulose (RPN303D Amersham) was performed as indicated in Figure 1B. The solution was prepared by cutting a 0.1-cm2 piece of solid nitrocellulose and dissolving it in 1 mL of methanol.
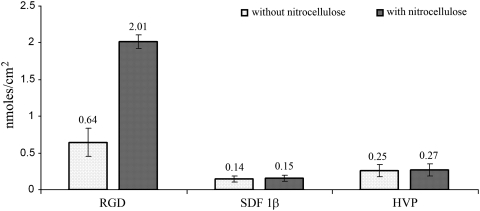
FIG. 1.
Absorption of the different peptides on the surface of nTC plates when using solution of 2.0 nmoles/cm2 both in the presence and in the absence of nitrocellulose. nTC, nontissue culture.
Cell cultivation and seeding on the multiwell plates
SMCs were isolated from the abdominal aorta of 18 syngeneic Fischer 344 rats, 2 days after birth using the Oakes' method.25 Cell characterization was performed by flow cytometry using the FITC-conjugated (GeneTex) primary antibodies anti-caldesmon (Abcam), anti-SM actin (Millipore), anti-MyHC (Abcam); positive cells were sorted and seeded onto 75-cm2 flasks to be amplified. SMCs, at passage P3–P5, were then seeded on the peptide-coated multiwell using Dulbecco's Modified Eagle Medium (DMEM) with nutrient mixture F12. The experiments were performed using 7500, 15,000, and 30,000 cells/cm2 over a culture time of 5 h in the presence or absence of FBS.26
The primary cultures of rat CMs used in our experiments were purchased from Lonza,27 which derived them from newborn Sprague Dawley rat ventricular myocardium at passage P1–P2.
According to data reported in the literature28 and empirical tests done in the lab, 200,000 cells/cm2 were seeded on each multiwell. At 4 h postseeding, 80% of the medium volume was replaced with the same amount of fresh medium (DMEM F12 HEPES modification, containing heat inactivated 7.5% FBS and 7.5% horse serum) with the addition of 61.5 mM bromodeoxyuridine (Sigma), and the cultures were carried on for a total time of 14 h.
SMC and CM adhesion assays
Cell adhesion was quantified using a colorimetric reaction known as the method of Landegren.29,30 This protocol measures the cell absorbance developed using a chromogenic substrate (p-nitrophenol-N-acetyl-beta-D-glucoseamminide) that binds the endogenous lysosomal enzyme N-acetyl-β-D-hexosaminidase. Once cells in culture were lysed with a citrate buffer, Landegren's solution was added. The intensity of the developed yellow color intensity was directly proportional to the amount of lysosomal enzyme, which, in turn, is directly proportional to the cell number. The optical density was measured with a spectrophotometer (DV-990B6; NT Laboratory—IT) at λ=405 nm.
SMCs growth curve
SMCs were counted after 5, 24, 50, and 72 h from culture on fibronectin-, RGD-, SDF 1β-, and HVP-coated wells (n=3 for each time points for each peptide). The number of viable cells (stained with Trypan Blue) was obtained using Automated Cell Counter (Countess™; Invitrogen).
Cell morphology and immunocytochemistry
The SMC morphology was evaluated by seeding cells, at a concentration of 7500 cells/cm2, in basic conditions (TC plate with PBS as culture medium) and after the coating of nTC plates with one of RGD, SDF-1β, or HVP solutions at 2.0 nmoles/cm2. Cells were seeded and cultured over different intervals (1, 5, and 72 h). CMs' morphology was also investigated by seeding 200,000 cells/cm2 on nTC plates with or without nitrocellulose coating and preconditioned with a solution of 0.2 nmoles/cm2 RGD or 2.0 nmoles/cm2 RGD or fibronectin used as control substrate. Seeding was also carried out to investigate the maintenance of cell contractility over a 24-h period both in the conditions suggested by the cell retailer and in the presence of the RGD peptide. SMC and CM morphology and CM beating were photographed and filmed by means of a camera coupled to an inverted phase-contrast microscope (Olympus Ulwcd 0.30; Wetzlar D).
Immunocytochemistry stainings were performed on CMs seeded on 96-well plates in the same conditions used for the adhesion assay. After 14-h culture, each well was washed twice with PBS and cells were fixed thereafter with a cold solution composed of 95% ethanol and 5% glacial acetic acid for 7 min. Further washings in PBS were performed before the addition of 2% BSA in a PBS solution for 30 min at room temperature. For immunolocalization experiments, double labeling for focal adhesion kinase (FAK) and for myosin heavy chain II (MHC II) was performed. Rabbit anti-rat FAK antibody (Upstate Millipore) was incubated 1:80 for 1 h at room temperature, while mouse anti-rat MHC II (Abcam) was incubated for 1 h at 37°C. Secondary rhodamine-conjugated anti-rabbit antibody for FAK was incubated 1:500 for 30 min at 37°C. Secondary FITC-conjugated anti-mouse IgG (H+L) antibody for MHC II was incubated 1:1000 for 30 min at 37°C. Cells were counterstained with 4,6-diamidino-2-phenylindole (DAPI; 1 μg/mL) for 10 min. Another single fluorescence set of staining was used for immunolocalization of Proline-rich tyrosine kinase 2 protein (Pyk-2) on CMs. Rabbit anti-rat PYK-2 antibody (Upstate Millipore) was incubated 1:100 for 1 h at 37°C. After washing the wells in PBS a secondary FITC anti-rabbit antibody (GeneTex) was incubated 1:1000 for 30 min at 37°C. Cells were counterstained with DAPI for 10 min.
Images were acquired using a Leica DM4000 fluorescence microscope.
Statistics
Differences were studied by one-way analysis of variance (ANOVA) and unpaired two-tails t-test. p-value below 0.05 was considered significant. p-value below 0.001 was taken as zero.
Results
The peptide synthesis and purification did not present particular difficulties. RGD, SDF-1β, and HVP peptides were adsorbed on the surface of nTC plates according to Figure 1.
When the three peptide solutions were poured on a nitrocellulose-pretreated surface RGD showed the greatest amount of adsorption (100% of the initial amount); this value could be explained by the lower net charge of the RGD peptide, in comparison with SDF-1β and HVP. In fact it is well known that the interaction between nitrocellulose and proteins occurs by hydrophobic interactions.
Through the development of Landegren's colorimetric reaction, qualitative and quantitative analyses were carried out providing information on the effect of the peptides on cell adhesion. The results are presented as the comparison of the average absorbance, achieved for each condition, to the mean absorbance at baseline of the same cells seeded on nonfunctionalized wells and culture in PBS medium. The latter value was arbitrarily defined as 100% cell adhesion (control). Therefore, in the graphs, the mean absorbance for each condition is expressed as percentages of the control. Moreover, in order to ensure greater adhesion to the culture medium, the optimal condition is defined as that of serum deprivation.31
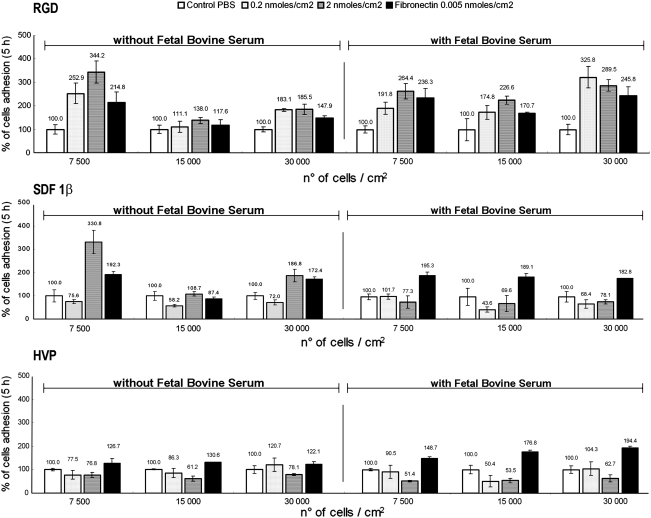
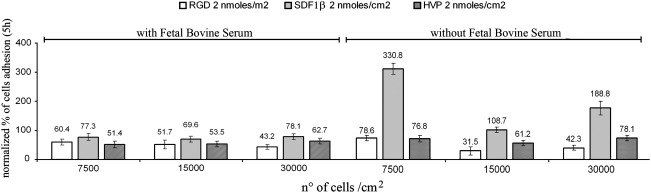
In accordance with data published in the literature,32–34 we used three different concentrations for SMC seeding: 7500, 15,000, and 30,000 cells/cm2. It was evident that 5 h after seeding, the RGD and SDF-1β peptides specifically enhanced adhesion of SMCs (Fig. 2). Cell culture on the HVP peptide, either in the presence or in the absence of FBS, did not show any bioactivity (Fig. 2). SDF-1β resulted in a strong enhancement of cell attachment compared to fibronectin-coated wells only when the medium was serum free and the peptide was at a concentration of 0.2 or 2.0 nmoles/cm2. Especially at 7500 cells/cm2, the increase in adhesion was very evident. Without FBS the optical density was the highest at this concentration due to the increase in cell adhesion. This cell quantity (the lowest used in the experimental set with SMCs), increased significantly (p<0.05) with 2.0 nmoles/cm2 of RGD and the cell adhesive properties were 3.5 times higher compared to control wells and 1.5 times higher than fibronectin.
FIG. 2.
Percentage adhesion of SMCs on different concentrations of peptide-pretreated wells after 5 h. The increase in cell adhesion was observed in both the presence and in the absence of fetal bovine serum. SMC, smooth muscle cell.
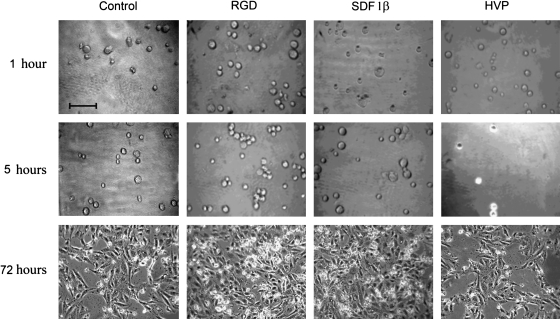
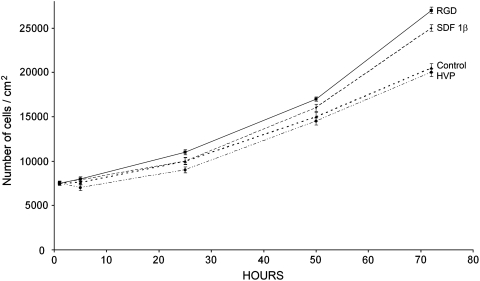
Finally, morphological experiments were carried out by seeding SMCs (7500 cells/cm2) in control conditions (TC plate) and on 2.0 nmoles/cm2, RGD-, SDF-1β, and HVP-coated wells (nTC plate). The experimental time was set at 1, 5, and 72 h, showing no change in cell morphology compared to cells seeded on control wells (Fig. 3). Additionally, an SMC growth curve (Fig. 4), determined in the above-mentioned conditions, confirmed both the ability of SDF 1β and RGD to significantly increase (specifically for the latter) cell proliferation and the similar performance of the HVP-treated wells when compared to the control (TC plate).
FIG. 3.
Morphological aspect of SMCs seeded (at a concentration of 7500 cells/cm2) in control conditions (TC plate) and on 2.0 nmoles/cm2 of RGD-, SDF-1β, and HVP-coated wells (nTC plate). The experimental time frame was settled at 1, 5, and 72 h, showing the absence of any change in cell morphology compared with cells seeded on control wells. Magnification 40×. Bar 40 μm. SDF, stromal cell-derived factor; HVP, human vitronectin protein.
FIG. 4.
Growth curve of SMCs at 5, 24, 50, and 72 h from seeding on each type of well-coated peptide. Each time point for each single sample was performed in triplicate (n=3).
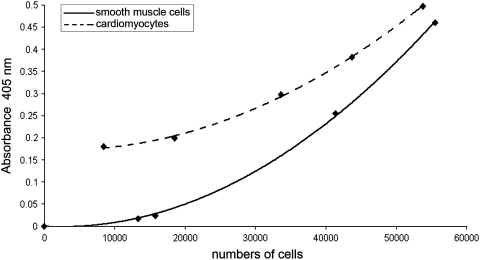
To correlate the absorbance obtained by the Landegren's test and the number of SMCs, different cell concentrations in PBS (as culture medium) were used for the construction of a calibration curve. First, the cells were counted and seeded onto the wells with sudden addition of Landegren's reagents. Graphical interpolation of the absorbance values produced an exponential function (Fig. 5). Especially for small cell quantities (from 10,000 to 30,000), a minimal change in absorbance corresponded to a large increase in the number of cells. This did not provide a precise method to quantify SMCs below a threshold of 50,000 cells.
FIG. 5.
Correlation between absorbance (405 nm) obtained by the Landegren's test and cells number of SMC (solid line) and CM (dotted line). CM, cardiomyocyte.
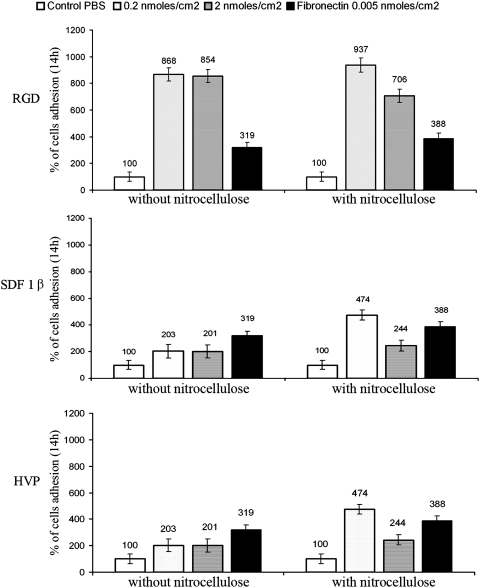
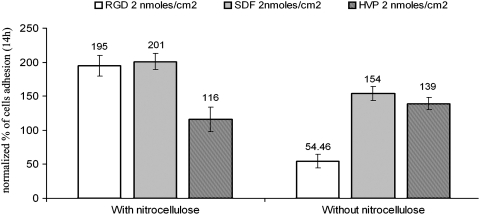
Regarding CMs, in the presence of 0.2 nmoles/cm2 of RGD peptide, the increase in absorbance was significant (p<0.05) compared to those obtained with fibronectin (almost three times lower) and to control (about eight times lower) both in the presence and in the absence of nitrocellulose (Fig. 6). The values of cell adhesion for the two different concentrations of RGD peptide without nitrocellulose were not significantly different (p>0.05); on the contrary, the addition of 2.0 nM nitrocellulose appears to significantly decrease efficiency of cell adhesion to the substrate (Fig. 6).
FIG. 6.
Percentage adhesion of CMs on different concentrations of peptide-pretreated wells after 14 h. The increase in cell adhesion was observed both in the presence and in the absence of nitrocellulose.
SDF-1β at 0.2 nmoles/cm2 increased cell adhesion by two (without nitrocellulose) or threefold (with nitrocellulose) compared to controls, but it was not significantly different (p>0.05) to data achieved in the same conditions with fibronectin. Using a peptide concentration an order of magnitude higher (2.0 nmoles/cm2), the absorbance apparently indicated a lower cell adhesion in the presence of nitrocellulose. This value was not significantly different (p>0.05) compared to that exhibited by fibronectin; however, the 2.0 nmoles/cm2 SDF-1β peptide concentration significantly (p<0.05) doubled cell adhesion compared to controls (Fig. 6).
The general behavior of 0.2 nmoles/cm2 HVP showed an increase in cell adhesion comparable to that of fibronectin and four times higher than that of control, only in the presence of nitrocellulose. This improvement of adhesion was reduced to half for 2.0 nmoles/cm2 of HVP. The absence of nitrocellulose did not provide significant differences (p>0.05) in absorbance between the two different concentrations of HVP (Fig. 6). Despite this, the cell adherence was twice as high as the control, but not significantly different from that obtained by fibronectin.
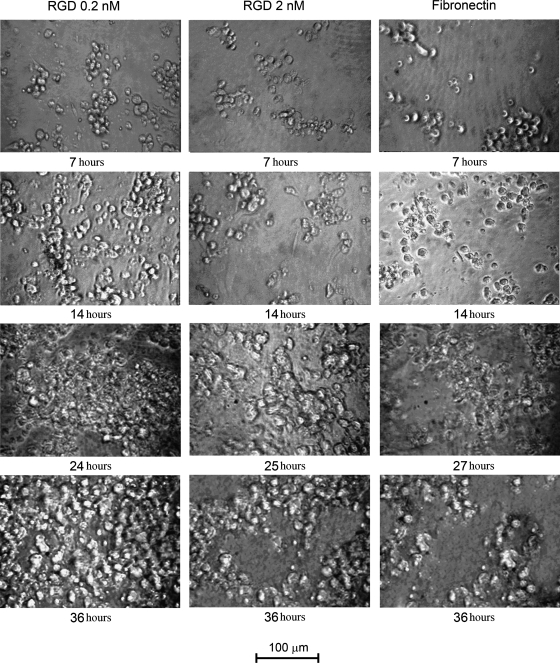
An experiment of CM seeding was also carried out on surfaces coated with fibronectin+nitrocellulose or with 0.2 nmoles/cm2 RGD or 2.0 nmoles/cm2 RGD, alternately. At 24 h postseeding, the 0.2 nmoles/cm2 RGD developed contractile activity (as visualized by inspection through optical microscope) displaying asynchronous beating of small clusters of cells (see Supplementary Video S1; Supplementary Data are available online at www.liebertonline.com/tea).
Contractile activity for 2.0 nmoles/cm2 RGD-functionalized well appeared at 25 h. Finally, only after 27 h the same phenomenon was observed on the fibronectin+nitrocellulose–coated wells.
After seeding on 0.2 nmoles/cm2 RGD-functionalized wells, cells were first synchronously organized through a contractile functional syncytium at 36 h, forming a continuous cell carpet (Fig. 7) covering the whole well surface. Similar behavior was also demonstrated with CMs seeded on 2.0 nmoles/cm2 RGD, although the activity of sustained contraction was lower compared to the conditions previously described. Finally, cells seeded on a fibronectin+nitrocellulose–coated surface were organized in functional isolated clusters (Fig. 7), which, although retaining asynchronous contractile activity, did not uniformly cover the culture plate. As indicated both in literature and in the instructions provided by Lonza, CMs seeded on 2.0 nmoles/cm2 RGD or fibronectin+nitrocellulose wells retain their viability and contractile capacity for no longer than 7 days.27,35,36 On the contrary, on 0.2 nmoles/cm2 RGD-functionalized wells, optical microscope inspection revealed viable CMs exhibiting contractility over 14 days (see Supplementary Video S2).
FIG. 7.
Morphological aspect of CMs seeded in fibronectin and 0.2/2.0 nmoles/cm2 RGD-coated wells at different time frames. Magnification 20×. Bar 100 μm.
Additionally, a calibration curve was obtained for CMs using the same protocol as for SMCs. In contrast to the SMCs, the CMs were seeded at a concentration of 64,000 cells/well. After 14 h of culture, each well was gently washed with PBS to remove the unattached elements and the cells in suspension were automatically counted. Then, adherent cells were trypsinized, harvested, and counted and the obtained calibration curve is shown in Figure 5. The sum of suspended cells and adherent cells corresponded to the original amount before seeding on the wells. Since SMCs and CMs differ in phenotype and morphology, the result obtained by the Landegren's test was not comparable between the two populations.
Cell attachment on the RGD, HVP, and SDF-1β sequences was also analyzed by normalizing adsorption of the three peptides to the lowest value achieved (Fig. 8 for SMCs and Fig. 9 for CMs). The lowest value belonged to the SDF-1β sequence either with or without nitrocellulose (Fig. 1). If all peptides were adsorbed at the same percentage, the HVP sequence would have exhibited the greatest ability to promote cell adhesion. It is important to point out that the RGD peptide, despite being adsorbed to the plastic surface to the greatest extent, yielded the lowest value of cell adhesion compared to HVP and SDF-1β either in the presence or in the absence of nitrocellulose.
FIG. 8.
SMCs attachment on the RGD, HVP, and SDF-1β sequences analyzed by normalizing adsorption of the three peptides to the lowest value achieved. The lowest value belonged to the SDF sequence either with or without nitrocellulose (see Fig. 1).
FIG. 9.
CM attachment on peptide-pretreated wells. Data have been normalized to the lowest achieved value of adsorbed peptide. The lowest value belonged to the SDF-1β sequence either with or without nitrocellulose (see Fig. 1).
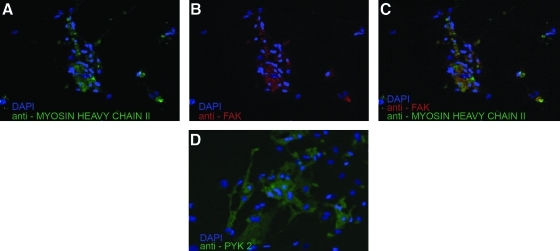
Immunofluorescence labeling indicates the presence of both Pyk-2 (cytoplasmic tyrosyn kinase related to FAK complex) and formation of FAK complexes in CMs after 14 h of culture in 0.2 nmoles/cm2 of RGD-pretreated wells (Fig. 10).
FIG. 10.
Immunofluorescence labeling of focal adhesion complexes formed by CMs after 14 h of culture on 2.0 nmoles/cm2 RGD. DAPI staining for nuclei (blue) and fluorescence antibody (green) against sarcomeric myosin heavy chain II (A); DAPI staining for nuclei (blue) and fluorescence antibody (red) against FAK (B); merge of A and B (C); DAPI staining for nuclei (blue) and fluorescence antibody (green) against Pyk-2 (D). All images are magnified 20×. DAPI, 4,6-diamidino-2-phenylindole; FAK, focal adhesion kinase.
Less intense images of SDF 1β-coated wells were otherwise comparable with those resulting from RGD pretreatment; finally, HVP peptide showed no difference compared to control TC wells (data not shown).
Discussion
We suggest that the mechanism of RGD binding to integrins is the same as that exploited in 2002 by Hynes,37 and Schwartz and Ginsberg,38 which results in the recruitment of FAK and Src kinase and their subsequent activation in focal adhesion complexes.
The HVP peptide possesses the heparin-binding sequence of human vitronectin that interacts with cell membrane HSPGs,39 which is very important for the formation of an organized cell cytoskeleton and focal adhesion spots.40
The SDF 1β peptide is the most novel sequence among the three proposed. The mechanism of action of SDF 1β is still not well known, but it has been reported to act as a mediator of retention and release of neutrophils from bone marrow via a specific integrin.41
The cell adhesion experiments were conducted on nTC plates in order to verify the real contribution and interaction of RGD, HVP, SDF-1β, fibronectin, and nitrocellulose to CMs and SMCs, and it was verified that the presence of these compounds did not influence the development of the yellow color provided by the Landegren's test. Since SMCs and CMs have different physiological roles and hence differing number of lysosomes,42,43 absolute data obtained from these two populations cannot be compared. Nevertheless, it was verified that the number of lysosomes in both CMs and SMCs remained unaltered over the culture time (with or without FBS and HS). The Landegren's reaction is hence a powerful and simple tool to determine qualitatively whether or not a peptide enhances cell adhesion on bidimensional substrates. Thus, we attempted to correlate cell absorbance to actual cell number, performing many consistent tests in order to minimize possible variability.
Despite their importance, such results should not be used as an absolute reference for cell types other than those studied. In fact, activated cells of any type could display an increased number of lysosomes, not consistent with data presented herein.
It is important to note that nitrocellulose and fibronectin are commonly known as pro-adhesive molecules, used in vitro, respectively, for CMs cultivation and as a coating molecule for cells of mesenchymal origin. As such, in CM adhesion assays, the use of nitrocellulose showed a synergistic effect in combination with each of the three peptides. On the other hand, the addition of serum to the medium for SMCs inhibited the pro-adhesive effect of the peptides especially in the case of pretreatment with 2.0 nmoles/cm2 SDF-1β. Although the percentage increase of SMC adhesion promoted by the RGD peptide was lower in the presence of serum, the difference with respect to the control remains significant in both conditions.44
For both CMs and SMCs, the RGD peptide proved to be the best candidate for enhancing early cell adhesion as it binds more effectively and in greater quantity on the described culture substrates. However, RGD was not the most potent peptide at increasing adhesion; in fact, at equal levels of peptide adsorption, both SDF-1β and HVP demonstrated a higher potency.
It is likely that CMs adhered to the substrates through two different mechanisms: RGD/integrin interaction and heparin binding site/cell surface GAG interaction. HVP and SDF-1β were responsible for promoting the latter mechanism. In particular, the HVP peptide is able to modify osteoblast gene expression, increasing Glipican 6 (Gpc6) expression: Gpc6 belongs to the family of membrane-associated heparin sulfate proteoglycans known to be involved in the interactions with ECM components.45 The used SDF peptide, mapped on SDF-1 β isoform, includes a consensus sequence BBXB and comprehends the four terminal additional residues distinguishing SDF-1β from SDF-1α. Dettin et al.24 demonstrated that the interaction between this SDF peptide and GAGs happens through the above-mentioned consensus sequence on heparin and chondroitin sulfate.
The two different cell types (SMC and CM) are both stimulated by SDF peptide, whereas HVP increases CMs adhesion but is ineffective for SMCs. This occurrence is likely due to the presence of different types and quantities of GAGs on cell membranes related not only to different cell types, but also to variation and conditioning in cell state. Boateng et al.30 proved that RGD and YIGSR peptides promote the same degree of neonatal CMs adhesion as their native proteins (fibronectin and vitronectin); however, they are unable to promote the signaling required for normal FAK expression and complete sarcomere formation and exhibit significant morphological differences at 48 h after seeding with respect to native proteins.
Our morphological analysis at 14 h after seeding was not consistent with this observation: the morphology of cells seeded on peptide-pretreated surfaces and on fibronectin-pretreated surfaces was the same. The strength of the adhesive peptide approach is related to the versatility of synthetic sequences in covalent binding strategies and their capacity to promote a stronger cell adhesion that can resist mechanical stress30; the same effect cannot be attributed to physio-adsorbed proteins.
Additionally, unlike Boateng et al.,30 the RGD peptide apparently enabled stronger cell adhesion, allowing the development of FAK complex, parallel to the presence of FAK-related tyrosyn kinase Pyk-2.
The failure of cell adhesion promotion, consequent to loss of proteins, would result in the cancellation of all the above-described effects.
Conclusions
This article assesses the adhesive properties of three synthetic peptides adsorbed to culture plate wells; the detected bioactivity could be stressed by a covalent immobilization approach ensuring optimal molecular orientation and by the simultaneous use of different peptides able to promote complementary adhesive mechanisms.46
Especially for CMs, evaluation of the RGD sequence's ability to promote cell adhesion allowed it to be earmarked as a possible in vitro substitute for nitrocellulose. We demonstrated for the first time that our peptide, carrying four RGD motifs, maintained cell viability and contractility over a considerably long period on in vitro culture. Hence, RGD sequence could potentially also be used as an in vivo preconditioning agent (e.g., in combination with self-assembling sequences) in ischemic hearts before the therapeutic injection of the patient's autologous stem cells47,48 feasibly providing a properly bio-compatible substrate for cell homing and engraftment. Our RGD peptide proved to work well for SMCs too; the incorporation of each of the proposed peptides into customized synthetic matrices is likely to promote cell engraftment,45 leading possibly to novel secretion and turn-over of ECM. The modification of two-dimensional surface of polymeric films with RGD peptides49,50 has already highlighted their ability to enhance cell attachment33,34 and they are thus suitable for use on decellularized biological scaffold for the construction of cardiac valves (using porcine aortic root or bovine pericardium) and vascular grafts.51,52 One of the main problems in developing a fully compatible graft for clinical use, avoiding the use of glutaraldehyde in xenogeneic bioprostheses, is the engraftment of the host cells in vitro, during dynamic cultivation, before implantation in an animal model.
Enhancing cell attachment would result in a promising perspective of an effective colonization of the 3D biological substrate.34–36 To date, we are testing the ability to covalently crosslink these three peptides, via UV light irradiation, to decellularized ECM derived from bovine and porcine pericardia, seeded with CMs, for the creation of regenerative myocardial patches for ischemic cardiac tissue.
Besides the pro-adhesive sequences application for tissue engineering and regenerative medicine, these results could be particularly promising for their potential to increase the yield of in vitro cell cultures53 particularly for biotechnology and pharmaceuticals companies aimed at the extraction of metabolites and active ingredients.
Supplementary Material
Acknowledgments
This research has been supported by the Regione Veneto Grant RSF 286/08 and Azione Biotech III.
Disclosure Statement
The authors do not have any conflicts to disclose. No competing financial interests exist.
Authors' Contributions
Gerosa, G., and Dettin, M., are equally responsible for concept and design of this study.
References
- 1.Radisic M. Park H. Genecht S. Cannizzaro C. Langer R. Vunjak- Novakovic G. Biomimetic approach to cardiac tissue engineering. Philos Trans R Soc Lond B Biol Sci. 2007;362:1357. doi: 10.1098/rstb.2007.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kyle S. Aggeli A. Ingham E. McPherson M.J. Recombinant self-assembling peptides as biomaterials for tissue engineering. Biomaterials. 2010;31:9395. doi: 10.1016/j.biomaterials.2010.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Von der Mark K. Park J. Bauer S. Schmuki P. Nanoscale engineering of biomimetic surfaces: cues from the extracellular matrix. Cell Tissue Res. 2010;339:1531. doi: 10.1007/s00441-009-0896-5. [DOI] [PubMed] [Google Scholar]
- 4.Holmes T.C. Novel peptide-based biomaterial scaffolds for tissue engineering. Trends Biotech. 2002;20:16. doi: 10.1016/s0167-7799(01)01840-6. [DOI] [PubMed] [Google Scholar]
- 5.Ingber D.E. Mow V.C. Butler D. Niklason L. Huard J. Mao J. Yannas I. Kaplan D. Vunjak-Novakovic G. Tissue engineering and developmental biology: going biomimetic. Tissue Eng. 2006;12:3265. doi: 10.1089/ten.2006.12.3265. [DOI] [PubMed] [Google Scholar]
- 6.Shi J. Votruba A.R. Farokhzad O.C. Langer R. Nanotechnology in drug delivery and tissue engineering: from discovery to applications. Nano Lett. 2010;10:3223. doi: 10.1021/nl102184c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romano N.H. Sengupta D. Chung C. Heilshorn S.C. Protein-engineered biomaterials: nanoscale mimics of the extracellular matrix. Biochim Biophys Acta. 2011;1810:339. doi: 10.1016/j.bbagen.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Comisar W.A. Mooney D.J. Linderman J.J. Integrin organization: linking adhesion ligand nanopatterns with altered cell responses. J Theor Biol. 2011;274:120. doi: 10.1016/j.jtbi.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Humphries M.J. Travis M.A. Clark K. Mould A.P. Mechanisms of integration of cells and extracellular matrices by integrins. Mould Biochem Soc Trans. 2004;32:822. doi: 10.1042/BST0320822. [DOI] [PubMed] [Google Scholar]
- 10.Jeon W.B. Park B.H. Wei J. Park R.W. Stimulation of fibroblasts and neuroblasts on a biomimetic extracellular matrix consisting of tandem repeats of the elastic VGVPG domain and RGD motif. J Biomed Mater Res A. 2011;97:152. doi: 10.1002/jbm.a.33041. [DOI] [PubMed] [Google Scholar]
- 11.Shekaran A. Garcia A.J. Nanoscale engineering of extracellular matrix-mimetic bioadhesive surfaces and implants for tissue engineering. Biochim Biophys Acta. 2011;1810:350. doi: 10.1016/j.bbagen.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pierschbacher M.D. Ruoslahti E. Variants of the cell recognition site of fibronectin that retain attachment-promoting activity. Proc Natl Acad Sci U S A. 1984;1:5985. doi: 10.1073/pnas.81.19.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pytela R. Pierschbacher M.D. Ruoslahti E. Identification and isolation of a 140 kDa cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985;40:191. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- 14.Leiss M. Beckmann K. Girós A. Costell M. Fässler R. The role of integrin binding sites in fibronectin matrix assembly in vivo. Curr Opin Cell Biol. 2008;20:502. doi: 10.1016/j.ceb.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Bacakova L. Filova E. Kubies D. Machova L. Proks V. Malinova V. Lisa V. Rypacek F. Adhesion and growth of vascular smooth muscle cells in cultures on bioactive RGD peptide-carrying polylactides. J Mater Sci Mater Med. 2007;18:1317. doi: 10.1007/s10856-006-0074-1. [DOI] [PubMed] [Google Scholar]
- 16.Mann B.K. Gobin A.S. Tsai A.T. Schmedlen R.H. West J.L. Smooth muscle cell growth in photopolymerized hydrogels with cell adhesive and protolytically degradable domains: synthetic ECM analogs for tissue engineering. Biomaterials. 2001;22:3045. doi: 10.1016/s0142-9612(01)00051-5. [DOI] [PubMed] [Google Scholar]
- 17.Shachar M. Tsur-Gang O. Dvir T. Leor J. Cohen S. The effect of immobilized RGD peptide in alginate scaffolds on cardiac tissue engineering. Acta Biomater. 2010;7:152. doi: 10.1016/j.actbio.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 18.Dettin M. Conconi M.T. Gambaretto R. Bagno A. Di Bello C. Menti A.M. Grandi C. Parnigotto P.P. Effect of synthetic peptides on osteoblast adhesion. Biomaterials. 2005;26:4507. doi: 10.1016/j.biomaterials.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 19.Schvartz I. Seger D. Shaltiel S. Vitronectin. Int J Biochem Cell Biol. 1999;31:539. doi: 10.1016/s1357-2725(99)00005-9. [DOI] [PubMed] [Google Scholar]
- 20.Dettin M. Bagno A. Morpurgo M. Cacchioli A. Conconi M.T. Di Bello C. Gabbi C. Gambaretto R. Parnigotto P.P. Pizzinato S. Ravanetti F. Guglielmi M. Evaluation of silicon dioxide-based coating enriched with bioactive peptides mapped on human vitronectin and fibronectin: in vitro and in vivo assays. Tissue Eng. 2006;12:3509. doi: 10.1089/ten.2006.12.3509. [DOI] [PubMed] [Google Scholar]
- 21.Michel J.B. Anoikis in the cardiovascular system: known and unknown extracellular mediators. Arterioscler Thromb Vasc Biol. 2003;23:2146. doi: 10.1161/01.ATV.0000099882.52647.E4. [DOI] [PubMed] [Google Scholar]
- 22.Hu X. Dai S. Wu W.J. Tan W. Zhu X. Mu J. Guo Y. Bolli R. Rokosh G. Stromal cell-derived factor-1α confers protection against myocardial ischemia/reperfusion injury. Circulation. 2007;116:654. doi: 10.1161/CIRCULATIONAHA.106.672451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orbach R. Adler-Abramovich L. Zigerson S. Mironi-Harpaz I. Seliktar D. Gazit E. Self-assembled Fmoc-peptides as a platform for the formation of nanostructures and hydrogels. Biomacromolecules. 2009;10:2646. doi: 10.1021/bm900584m. [DOI] [PubMed] [Google Scholar]
- 24.Dettin M. Pasquato A. Scarinci C. Zanchetta M. De Rossi A. Di Bello C. Anti-HIV activity and conformational studies of peptides derived from the C-terminal sequenze of SDF-1. J Med Chem. 2004;47:3058. doi: 10.1021/jm031067a. [DOI] [PubMed] [Google Scholar]
- 25.Oakes B.W. Batty A.C. Handley C.J. Sandberg L.B. The synthesis of elastin, collagen, and glycosaminoglycans by high density primary cultures of neonatal rat aortic smooth muscle. An ultrastructural and biochemical study. Eur J Cell Biol. 1982;27:34. [PubMed] [Google Scholar]
- 26.Rapuano B.E. Wu C. MacDonald D.E. Osteoblast-like cell adhesion to bone sialoprotein peptides. J Orthop Res. 2004;22:353. doi: 10.1016/S0736-0266(03)00180-3. [DOI] [PubMed] [Google Scholar]
- 27.Wallukat G. Nissen E. Neichel D. Harris J. Spontaneously beating neonatal rat heart myocyte culture-a model to characterize angiotensin II at(1) receptor autoantibodies in patients with preeclampsia. In Vitro Cell Dev Biol Anim. 2002;38:376. doi: 10.1290/1071-2690(2002)038<0376:SBNRHM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 28.Weikert C. Eppenberger-Eberhardt M. Eppenberger H.M. Cellular engineering of ventricular adult rat cardiomyocytes. Cardiovasc Res. 2003;59:874. doi: 10.1016/s0008-6363(03)00508-x. [DOI] [PubMed] [Google Scholar]
- 29.Landegren U. Measurement of cell numbers by means of the endogenous enzyme hexosaminidase. Applications to detection of lymphokines and cell surface antigens. J Immunol Methods. 1984;67:379. doi: 10.1016/0022-1759(84)90477-0. [DOI] [PubMed] [Google Scholar]
- 30.Boateng S.Y. Lateef S.S. Mosley W. Hartman T.J. Hanley L. Russell B. RGD and YIGSR synthetic peptides facilitate cellular adhesion identical to that of laminin and fibronectin but alter the physiology of neonatal cardiac myocytes. Am J Physiol Cell Physiol. 2005;288:C30. doi: 10.1152/ajpcell.00199.2004. [DOI] [PubMed] [Google Scholar]
- 31.Cushing M.C. Jaeggli M.P. Masters K.S. Leinwand L.A. Anseth K.S. Serum deprivation improves seeding and repopulation of acellular matrices with valvular interstitial cells. J Biomed Mater Res A. 2005;75:232. doi: 10.1002/jbm.a.30412. [DOI] [PubMed] [Google Scholar]
- 32.Mann B.K. West J.L. Cell adhesion peptides alter smooth muscle cell adhesion, proliferation, migration, and matrix protein synthesis on modified surfaces and in polymer scaffolds. J Biomed Mater Res. 2002;60:86. doi: 10.1002/jbm.10042. [DOI] [PubMed] [Google Scholar]
- 33.Carragher N.O. Levkau B. Ross R. Raines E.W. Degraded collagen fragments promote rapid disassembly of smooth muscle focal adhesions that correlates with cleavage of pp125(FAK), paxillin, and talin. J Cell Biol. 1999;147:619. doi: 10.1083/jcb.147.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sandeman S.R. Lloyd A.W. Tighe B.J. Franklin V. Li J. Lydon F. Liu C.S. Mann D.J. James S.E. Martin R. A model for the preliminary biological screening of potential keratoprosthetic biomaterials. Biomaterials. 2003;24:4729. doi: 10.1016/s0142-9612(03)00370-3. [DOI] [PubMed] [Google Scholar]
- 35.Sekine H. Shimizu T. Yang J. Kobayashi E. Okano T. Pulsatile myocardial tubes fabricated with cell sheet engineering. Circulation. 2006;114:I87. doi: 10.1161/CIRCULATIONAHA.105.000273. [DOI] [PubMed] [Google Scholar]
- 36.Jin S. Novel method for the establishment of cardiomyocytes derived from rat embryonic stem cells in vitro. Hum Cell. 2007;20:11. doi: 10.1111/j.1749-0774.2007.00038.x. [DOI] [PubMed] [Google Scholar]
- 37.Hynes R.O. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 38.Schwartz M.A. Ginsberg M.H. Networks and crosstalk: integrin signalling spreads. Nat Cell Biol. 2002;4:E65. doi: 10.1038/ncb0402-e65. [DOI] [PubMed] [Google Scholar]
- 39.Conconi M.T. Ghezzo F. Dettin M. Urbani L. Grandi C. Guidolin D. Nico B. Di Bello C. Ribatti D. Parnigotto P.P. Effects on in vitro and in vivo angiogenesis induced by small peptides carrying adhesion sequences. J Pept Sci. 2010;16:349. doi: 10.1002/psc.1251. [DOI] [PubMed] [Google Scholar]
- 40.Chillakuri C.R. Jones C. Mardon H.J. Heparin binding domain in vitronectin is required for oligomerization and thus enhances integrin mediated cell adhesion and spreading. FEBS Lett. 2010;584:3287. doi: 10.1016/j.febslet.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 41.Petty J.M. Lenox C.C. Weiss D.J. Poynter M.E. Suratt B.T. Crosstalk between CXCR4/stromal derived factor-1 and VLA-4/VCAM-1 pathways regulates neutrophil retention in the bone marrow. J Immunol. 2009;182:604. doi: 10.4049/jimmunol.182.1.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iwai-Kanai E. Yuan H. Huang C. Sayen M.R. Perry-Garza C.N. Kim L. Gottlieb R.A. A method to measure cardiac autophagic flux in vivo. Autophagy. 2008;4:322. doi: 10.4161/auto.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thyberg J. Caveolae and cholesterol distribution in vascular smooth muscle cells of different phenotypes. J Histochem Cytochem. 2002;50:185. doi: 10.1177/002215540205000206. [DOI] [PubMed] [Google Scholar]
- 44.Dong Y. Li P. Chen C.B. Wang Z.H. Ma P. Chen G.Q. The improvement of fibroblast growth on hydrophobic biopolyesters by coating with polyhydroxyalkanoate granule binding protein PhaP fused with cell adhesion motif RGD. Biomaterials. 2010;31:8921. doi: 10.1016/j.biomaterials.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 45.Luchini A. Bioengineering, University of Padova; 2005. Transcriptional analysis of osteoblast-biomaterial interaction [Ph.D. thesis] [Google Scholar]
- 46.Kao W.J. Lee D. Schense J.C. Hubbell J.A. Fibronectin modulates macrophage adhesion and FBGC formation: the role of RGD, PHSRN, and PRRARV domains. J Biomed Mater Res. 2001;55:79. doi: 10.1002/1097-4636(200104)55:1<79::aid-jbm110>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 47.Hilfiker A. Kasper C. Hass R. Haverich A. Mesenchymal stem cells and progenitor cells in connective tissue engineering and regenerative medicine: is there a future for transplantation? Langenbecks Arch Surg. 2011;396:489. doi: 10.1007/s00423-011-0762-2. [DOI] [PubMed] [Google Scholar]
- 48.Heldman A.W. Zambrano J.P. Hare J.M. Cell therapy for heart disease: where are we in 2011? J Am Coll Cardiol. 2011;57:466. doi: 10.1016/j.jacc.2010.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gabriel M. Amerongen G.P. Van Hinsbergh V.W. Amerongen A.V. Zentner A. Direct grafting of RGD-motif-containing peptide on the surface of polycaprolactone films. J Biomater Sci Polym Ed. 2006;17:567. doi: 10.1163/156856206776986288. [DOI] [PubMed] [Google Scholar]
- 50.Blit P.H. Shen Y.H. Ernsting M.J. Woodhouse K.A. Santerre J.P. Bioactivation of porous polyurethane scaffolds using fluorinated RGD surface modifiers. J Biomed Mater Res A. 2010;94:1226. doi: 10.1002/jbm.a.32804. [DOI] [PubMed] [Google Scholar]
- 51.Massia S.P. Hubbell J.A. Covalently attached GRGD on polymer surfaces promotes biospecific adhesion of mammalian cells. Ann N Y Acad Sci. 1990;589:261. doi: 10.1111/j.1749-6632.1990.tb24251.x. [DOI] [PubMed] [Google Scholar]
- 52.Li J. Ding M. Fu Q. Tan H. Xie X. Zhong Y. A novel strategy to graft RGD peptide on biomaterials surfaces for endothelization of small-diamater vascular grafts and tissue engineering blood vessel. J Mater Sci Mater Med. 2008;19:2595. doi: 10.1007/s10856-007-3354-5. [DOI] [PubMed] [Google Scholar]
- 53.Kumar P. Pillay V. Modi G. Choonara Y.E. du Toit L.C. Naidoo D. Self-assembling peptides: implications for patenting in drug delivery and tissue engineering. Recent Pat Drug Deliv Formul. 2011;5:24. doi: 10.2174/187221111794109510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.