Abstract
Hendra virus (HeV) is a recently emerged zoonotic paramyxovirus that can cause a severe and often fatal disease in horses and humans. HeV is categorized as a biosafety level 4 agent, which has made the development of animal models and testing of potential therapeutics and vaccines challenging. Infection of African Green monkeys (AGMs) with HeV was recently demonstrated and disease mirrored fatal HeV infection in humans, manifesting as a multisystemic vasculitis with widespread virus replication in vascular tissues and severe pathologic manifestations in the lung, spleen and brain. Here, we demonstrate that m102.4, a potent HeV neutralizing human monoclonal antibody (hmAb), can protect AGMs from disease post infection (p.i.) with HeV. Fourteen AGMs were challenged intratracheally with a lethal dose of HeV and twelve subjects were infused twice with a 100 mg dose of m102.4 beginning at either 10 hr, 24 hr or 72 hr p.i. and again approximately 48 hrs later. The presence of viral RNA, infectious virus and HeV-specific immune responses demonstrated that all subjects were infected following challenge. All twelve AGMs that received m102.4 survived infection; whereas the untreated control subjects succumbed to disease on day 8 p.i.. Animals in the 72 hr treatment group exhibited neurological signs of disease but all animals started to recover by day 16 p.i.. These results represent successful post-exposure in vivo efficacy by an investigational drug against HeV and highlight the potential impact a hmAb can have on human disease.
Introduction
In the middle to late 1990’s, two new paramyxoviruses capable of causing severe lethal disease in both animals and humans were identified, Hendra virus (HeV) and Nipah virus (NiV). The first two outbreaks of HeV occurred in Queensland, Australia in 1994 and were associated with fatalities in horses and humans. In total, fifteen horses and two of three infected humans succumbed to fatal HeV disease (1). Infection manifested as a severe respiratory disease in horses; whereas in humans, one fatality was associated with respiratory failure and the other developed encephalitic complications that manifested some 13 months following a recovery from a mild meningitic illness that was later found to have been caused by HeV. NiV appeared a few years later in peninsular Malaysia in 1998 causing a wide-spread outbreak among farmed pigs along with numerous cases of human infection. By mid-1999 over 265 human cases of encephalitis, including 105 deaths, had been reported in Malaysia and 11 cases of either encephalitis or respiratory illness with one fatality were reported in Singapore (1). More than one million pigs were culled to control the disease outbreak which caused significant economic and social impacts which are still felt to this day. Upon further biological, molecular and serological characterization, HeV and NiV were discovered to be closely related viruses that had emerged independently and are now grouped together in the new genus Henipavirus (1, 2), and both are classified as select viral agents in the United States by the Centers for Disease Control and Prevention and require biological safety level 4 (BSL-4) containment worldwide.
Pteropus fruit bats, commonly known as flying foxes, are the natural reservoirs for both viruses and as a group they are wide-ranging and can be found throughout Asia-Pacific, and as far West as Africa and as far North as India, Pakistan and the Philippines (3, 4). The persistence of HeV and NiV in an animal reservoir, their broad species tropism (5) and the severe disease they cause in a wide variety of mammalian hosts including humans distinguish them from all other known paramyxoviruses. NiV outbreaks have occurred nearly every year since its initial discovery (6–9) and in all outbreaks severe disease in humans has occurred with fatality rates ranging from 40–75%. Of significance, from 2001–2007, more than half of the identified NiV cases resulted from person-to-person transmission (7). Conversely, HeV initially appeared more sporadically in Australia since its initial emergence, with horse fatalities recorded in 1999, 2004 and 2006 and one mildly ill, sero-converting, human case reported in 2004 (10, 11). However, since 2006 HeV has appeared in horses annually along with two severe human cases, one fatal, in 2008 and another fatality in 2009 (12, 13). A spillover of HeV occurred in May 2010 (14), with one horse fatality and 11 humans with potential virus exposure (15). Just prior to the 2010 episode, unusual large scale flying fox movements were reported and a HeV warning was issued (16). Most recently there has been a flurry of HeV spillovers in Queensland and New South Wales, Australia which began on June 26, 2011 (17). Seventeen separate occurrences have been reported as of August 30, 2011 including numerous horse deaths and cases of human exposure with multiple properties quarantined and under surveillance.
Currently, there are no approved vaccines or therapeutics against HeV or NiV (18). However, in the 2010 HeV outbreak, an experimental human monoclonal antibody (hmAb) was used to treat two individuals who had a significant exposure risk (19). To date, both of these individuals have no evidence of HeV infection. The experimental hmAb, m102.4, which targets the ephrin-B2 and -B3 receptor binding domain of the henipavirus G envelope glycoprotein (20–23), is a potent cross-reactive neutralizing antibody in vitro (24, 25) and had been shown capable of protecting ferrets from a lethal NiV challenge (26). Recently, we reported on the development of successful nonhuman primate (NHP) models of NiV and HeV infection and disease in the African green monkey (AGM) (27, 28). For both viruses, infection of AGMs is uniformly lethal and disease essentially mirrors the severe clinical symptoms seen in humans; with wide-spread systemic vasculitis and disease manifestations observed in multiple organ systems. The lungs and brain were the main targets of infection and development of clinical signs was directly associated with damage of these organs. Here, we report the pharmacokinetic characteristics and efficacy of hmAb m102.4 using the lethal HeV infection AGM model. We present data demonstrating that m102.4 is highly efficacious in preventing lethal disease in HeV-infected AGMs when administered 10, 24 or 72 hours following virus challenge. The exceptional potency and half-life of m102.4 in AGMs demonstrate its tremendous post-exposure therapeutic potential for future use in humans. Indeed, the recent decision to administer m102.4 to two humans with high risk HeV exposure (19) was significantly influenced by some elements of the data now reported here.
Results
In vivo pharmacokinetics of human monoclonal antibody m102.4 in African green monkeys
The hmAb m102.4 was originally isolated from a bacteriophage recombinant antibody library derived from a pool of naive human peripheral blood mononuclear cells (24). Biochemical analysis indicated that m102.4 bound the receptor binding domain of the HeV and NiV attachment glycoprotein (G) (24). Following conversion to an IgG1 format, m102.4 demonstrated exceptional cross-reactive neutralizing capability in vitro (25) and could also completely protect ferrets from lethal NiV-mediated disease when administered 10 hrs after virus challenge (26). The first objective of the present study was to determine the half-life of m102.4 in AGMs. As AGMs are approximately 2–3 times the weight of ferrets, absolute doses of the antibody were doubled as compared to what was done previously (26); however, the approximate dose per kg was similar. Four subjects were given m102.4 intravenously, either 50 mg per subject (AGM 1 and AGM 2) or 10 mg (AGM 3 and AGM 4). The subjects were monitored physically for respiration, allergic reaction, eating/drinking abnormalities or lethargy, during and following the antibody infusion and no adverse reactions were observed in any subject. Serum was collected at various time points following infusion and m102.4 concentrations were determined as previously described (26). As indicated in Table 1, the serum levels of m102.4 were above 0.5 mg/ml in the two high dose subjects and approximately 0.1 mg/ml in the low dose subjects immediately following antibody infusion. Serum m102.4 concentration decreased over time and concentrations correlated with antibody dosage. On day 12, the high dose subjects had over 50 μg/ml serum m102.4 and the low dose subjects had over 10 μg/ml serum m102.4. A distribution half-time (t11/2) of ~1 day and the elimination half-time (t21/2) of ~11 days of m102.4 were calculated and are typical for human IgG in NHPs (29). The area under curve (AUC), which is a measure for the exposure to the hmAb, is also shown. As demonstrated, the average AUC values for the two treatment groups are approximately 5 fold different, reflecting the difference in the m102.4 dose.
Table 1.
m102.4 concentrations and half-life in African green monkeys.
| AGM 11 | AGM 2 | AGM 3 | AGM 41 | |
|---|---|---|---|---|
| Total | 50 mg | 50 mg | 10 mg | 10 mg |
| Dose | 11.1 mg/kg | 11.0 mg/kg | 2.5 mg/kg | 2.6 mg/kg |
| Day | μg/ml | |||
| 02,3 | 501.1 | 513.7 | 117.6 | 88.9 |
| 1 | 222.3 | 351.7 | 57.1 | 22.5 |
| 3 | 180.7 | 356.3 | 47.3 | 36.7 |
| 6 | 90.0 | 85.2 | 27.1 | 21.8 |
| 12 | 61.8 | 50.3 | 17.5 | 12.2 |
| 24 | 25.4 | 29.1 | 7.6 | 7.6 |
| 36 | 6.8 | 9.4 | 2.8 | 3.9 |
| 48 | 6.8 | 5.1 | 2.7 | 1.9 |
| Half-life | Days | |||
| t1½ | .85 | 1.83 | .96 | .50 |
| t2½ | 10.2 | 10.3 | 11.7 | 12.5 |
| mg*h/L | ||||
| AUC | 59910 | 73828 | 17230 | 13503 |
| Mean AUC | 66,869 | 15,367 | ||
male;
m102.4 infusion;
serum collected post-infusion;
Abbreviations: distribution half-life time (t11/2), elimination half-life time (t21/2), area under curve (AUC).
Post-exposure protection of African green monkeys from lethal HeV-mediated disease by m102.4
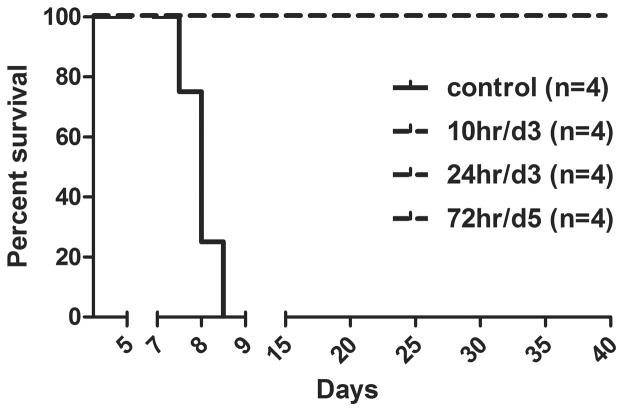
Previously, we demonstrated that intratracheal inoculation of AGMs with 4×105 TCID50 of HeV caused a uniformly lethal outcome (28). Rapidly progressive clinical illness was noted in these studies, clinical signs included severe depression, respiratory disease leading to acute respiratory distress, severely reduced mobility and time to reach approved humane endpoint criteria for euthanasia ranged from 7 to 9 days. Here we sought to evaluate the therapeutic benefit of m102.4 using this HeV AGM model. The first m102.4 efficacy study conducted in ferrets demonstrated complete protection against a lethal NiV challenge when a single 50 mg dose was administered intravenously, at ~25 mg/kg, 10 hrs following oral-nasal NiV challenge (26). Notably, surviving ferrets had the highest levels of serum m102.4 on day 3 p.i. suggesting that day 3 was a potentially important therapeutic window. Here in the AGM model, we chose to use a 100 mg dose of m102.4 administered intravenously (~20 mg/kg) twice, with the first dose given 10 hrs p.i. and the second dose 3 days following virus challenge. To determine if the m102.4 therapeutic window could be extended, we also included two additional treatment groups, one group received m102.4 (100 mg/dose) beginning at 24 hrs p.i. and again at 3 days p.i. and the third treatment group receiving m102.4 (100 mg/dose) at 72 hrs p.i. and again at 5 days following virus challenge. In total, fourteen subjects were challenged with a uniformly lethal dose of HeV (4×105 TCID50) by intratracheal inoculation. Following challenge, two control subjects received infusions of saline and for each treatment group, 4 subjects received m102.4 intravenously. No adverse reactions were observed upon m102.4 infusion in any animal. The control subjects (AGM 13, AGM 17), consistent with historical controls, showed elevated temperatures (102.2–104°F), severe sustained behavior changes (depression, decreased activity), a gradual increase in respiratory rate (>50/min with open mouth breathing) and a significant decrease in platelet count at end stage disease (Table S1). Both subjects developed respiratory disease and were euthanized with respiratory distress on day 8 p.i.. AGM 17 showed radiological changes consistent with interstitial pneumonia. In contrast, subjects treated with m102.4 at10 hrs or 24 hrs p.i. and again 3 days later (10hr/d3 and 24hr/d3) (AGM 14, 15, 16, 18, 19, 20, 21, 22) showed either mild (mildly depressed) or no clinical signs of disease (Table S1), no radiological changes were noted (Fig. S1) and hematology and clinical chemistry assays were normal. One of the four subjects (AGM 23) that received m102.4 therapy at 72 hrs p.i. and again on day 5 p.i. (72hr/d5) started to show radiological changes on day 6 in the lung fields (mild interstitial pneumonia); however, changes were transient and not as severe as those noted in the control subject AGM 17. Between days 6–13, all four subjects in this group (AGM 23, 24, 25, 26) showed a transient decrease in platelet counts and from days 7–11 they developed temporal moderate to severe neurological signs including depression, imbalance, tremor/twitching (mainly in legs), head tilting, and seizure (only AGM 23) (Table S1). Remarkably, by days 17–18, all subjects in the 72 hr/day5 treatment group began to improve clinically and between days 24–27 all subjects appeared healthy and neurological signs were no longer present. A Kaplan-Meier survival graph with two additional historic control subjects is shown in Fig. 1.
Figure 1. Survival curve of HeV-infected monkeys.
Data from control subjects and m102.4-treated subjects were used to generate the Kaplan-Meier survival curve. Control included data from two additional historical control subjects (28). Subjects received m102.4 infusions at 10 hours and 3 days p.i. (10hr/d3), 24 hrs and 3 days p.i. (24hr/d3) or 72 hrs and 5 days p.i. (72hr/d5). Each group contained 4 subjects (n=4). Average time to end stage disease was 8 days in control subjects whereas all m102.4 treated subjects survived until euthanasia at the end of the study.
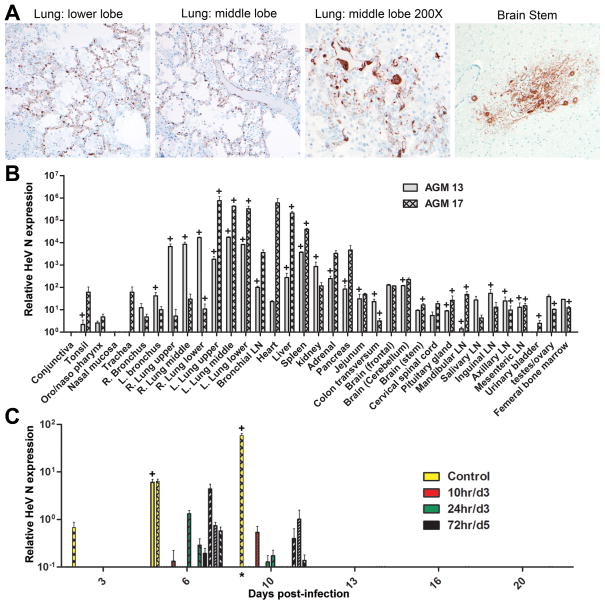
Upon necropsy, gross pathological changes in the control subjects were consistent with those demonstrated in historical controls (28), whereas HeV-specific pathological changes were not observed in any of the m102.4-treated animals (Table S2). Immunohistochemistry revealed high levels of HeV antigen in the lung and brain of the control subjects (Fig. 2A). Further molecular analysis revealed a tissue tropism and HeV RNA load that was similar to historic controls (28), with significant evidence of virus in the lung, spleen, lymph nodes and brain (Fig. 2B) Virus isolation was attempted from all tissues collected from the control subjects and positive tissues are indicated with a (+) in Fig. 2B. As expected, HeV was recovered from numerous tissues, highlighting the extensive dissemination of HeV within the body. In previous studies, low amounts of viral RNA were detected and HeV was isolated from AGM blood following HeV infection and viral RNA loads increased as disease manifested (28). Here, blood samples collected over the course of infection were assayed for infectious HeV and HeV RNA and positive samples are shown in Fig. 3. Consistent with previous findings, a gradual rise in viral RNA over time was evident in the control subjects and HeV was isolated from blood of both control subjects as indicated (+). Only very low levels of viral RNA could be detected on days 6 and 10 in some m102.4-treated subjects and all blood samples from all m102.4-treated subjects were negative for HeV isolation. Tissue samples were collected from all m102.4-treated subjects upon necropsy and assayed for the presence of HeV RNA and infectious virus. Occasionally, TaqMan PCR detected only very low levels of viral RNA in some tissues (spleen, lung and brain), the major target organs of HeV and NiV infection in vivo and predominantly only in the late treatment cohort (72hr/d5), unlike the control subjects where significant levels of HeV RNA were detected in every sampled tissue (Fig. 2B). Importantly, infectious HeV could not be recovered from any of the tissues collected from the m102.4-treated subjects. Together these data demonstrate that m102.4 therapy prevented wide-spread HeV dissemination in the challenged subjects. Spleen, lung and brain tissues from surviving subjects were also assayed for the presence of HeV antigen. All tissue architecture appeared normal and all survivor tissues examined were negative for HeV antigen (Fig. S2) and control subject tissues showed significant HeV antigen staining. In the spleen, occasional cells were found to be antigen-positive in two subjects, AGM 18 (10hr/d3) and AGM 19 (24hr/d3) (Fig. S3), but this was dramatically less in comparison to the antigen staining in the control animal (Fig. S3C) and most likely represents residual viral antigen.
Figure 2. HeV antigen and viral RNA in untreated control AGM tissues and viral RNA in blood from m102.4 efficacy trial.
(A) Localization of HeV antigen by immunohistochemical stain in the lung and brainstem of AGM 13. Sections were stained with a NiV nucleoprotein (N) specific rabbit polyclonal antibody and images were obtained at an original magnification of at 40X; however one panel was photographed at 200X as indicated. (B) Detection of HeV RNA in tissues collected from AGM 13 and AGM 17. (C) Detection of HeV RNA in blood samples. RNA samples were assayed in triplicate using TaqMan PCR. Blood and tissue Ct values were analyzed against Ct values generated from a standard curve of HeV RNA, as described in the methods, and a relative HeV N expression value was calculated for each blood replicate. Results are shown as average relative HeV N gene expression levels. The data are mean +/− SD. In panel C data from individual subjects are shown and indicated as none or different hatched patterns, and bar coloration (inset legend) indicates the different treatment groups. Virus isolation was attempted on all tissue and blood samples, and positive samples are indicated by (+). The blood sample (*) from one control subject was a terminal sample taken on day 8; lymph node.
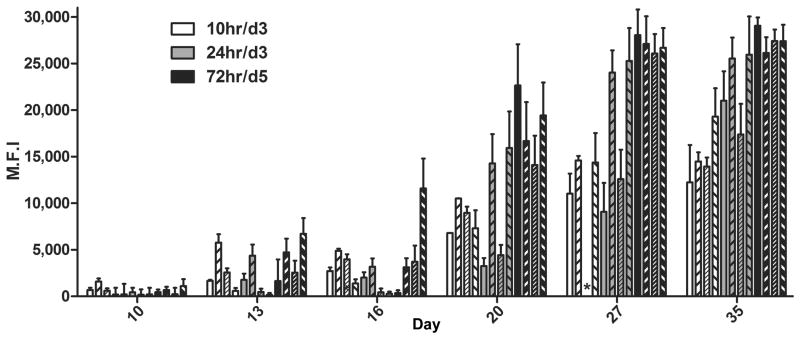
Figure 3. Detection of F-specific antibodies in m102.4 treated AGMs.
Median fluorescence intensities (M.F.I.) are shown on the Y-axis and represent binding to soluble F. Error bars represent the standard deviation of fluorescence intensity across 100 beads for each sample. Three of 4 subjects in the 10hr/d3 group were sampled on days 24 and 30 instead of days 27 and 35; the remaining animal (
 ) in this group was sampled on day 27 and 35. No sample (*) was collected for one animal (
) in this group was sampled on day 27 and 35. No sample (*) was collected for one animal (
 ) on day 24.
) on day 24.
To evaluate whether the host immune response contributed to the mechanism of protection from disease, plasma samples from HeV-infected AGMs plasma were assayed for the presence of antibodies directed against the HeV fusion glycoprotein (F), the other major antigenic target of virus-neutralizing antibodies, and results are shown in Fig. 3. Anti-F antibodies were detected in m102.4-treated subjects on day 13 p.i. and gradually rose over a three week period in all treated subjects. The control subjects did not seroconvert, which is not unexpected given the severe disease observed and that the subjects had to be euthanized due to irreversible clinical signs on day 8 p.i.. However, for the treated subjects, seroconversion to HeV F and a subsequent rise in titer over time, suggests that all treated subjects became infected with HeV, including the subjects in groups 10hr/d3 and 24hr/d3 where no clinical signs were evident. Additionally, anti-F antibodies increased dramatically on day 20 p.i. and likely played a role in recovery from disease in the 72hr/d5 treated animals. Plasma m102.4 levels were measured in HeV-infected AGMs and results are shown in Table 2. As expected, in the 10hr/d3 and 24hr/d3 groups, m102.4 levels increased on day 6, following the second dose of m102.4 infused on day 3. All subjects in the 72hr/d5 group had high levels of m102.4 by day 6 following the two doses administered on day 3 and day 5. Although m102.4 levels remained high on day 10 p.i., the 72hr/d5 subjects presented with the first signs of neurological disease as early as day 7 p.i. which progressed to moderate/severe disease over the following days. HeV-mediated disease began to abate around day 17/18 and started to resolve around day 20, coinciding with the significant rise in anti-F antibody titer demonstrated in Fig. 3. Taken together these data strongly suggest that m102.4 therapy did not prevent dissemination but instead significantly reduced viral loads in vivo which in effect allowed the host an extended period to mount a protective immune response. Finally, data generated from both the uninfected and the HeV-infected AGMs demonstrate that the half-life of m102.4 in AGMs of approximately 10–12 days (Table 1) is far superior to the m102.4 half-life previously observed in NiV-infected ferrets (26) which is not unexpected as m102.4 is human in origin and hypothesized to be more homologous to antibodies from NHPs.
Table 2.
m102.4 plasma levels (μg/ml) in Hendra virus-challenged African green monkeys.
| AGM # | day 0a | day 3a | day 6 | day 10 | day 13 | day 16 |
|---|---|---|---|---|---|---|
| Control | ||||||
| 13b | 0.0 | 0.0 | 0.0 | * | * | * |
| 17b | 0.0 | 0.0 | 0.1 | * | * | * |
| 10 hr/d3d | ||||||
| 14 | 3.0 | 934.8 | 1152.4 | 802.5 | 586.3 | 484.0 |
| 15 | 0.0 | 642. | 903.2 | 604.9 | 583.3 | 310.6 |
| 16 | 2.5 | 957.4 | 1344.1 | 830.3 | 600.1 | 458.5 |
| 18 | 0.0 | 381.3 | 372.0 | 406.8 | 123.8 | 84.7 |
| 24hr/d3d | ||||||
| 19 | 0.0 | 423.5 | 406.9 | 345.0 | 252.8 | 178.4 |
| 20 | 0.0 | 519.1 | 612.9 | 351.6 | 230.2 | 106.4 |
| 21 | 2.1 | 543.7 | 647.7 | 299.9 | 42.9 | 72.6 |
| 22 | 0.0 | 528.9 | 690.2 | 406.7 | 27.4 | 11.2 |
| 72hr/d5d | ||||||
| 23 | 0 | 0 | 1581.9 | 695.2 | 118.2 | 11.7 |
| 24 | 9.4 | 0 | 624.8 | 575.0 | 239.2 | 96.7 |
| 25 | 0.0 | 2.3 | 828.7 | 527.7 | 197.5 | 158.7 |
| 26 | 0.0 | 3.5 | 951.0 | 534.7 | 305.1 | 316.6 |
sample collected before infusion;
succumbed to disease on day 8;
each animal received two 100 mg doses as indicated;
not sampled.
Our findings demonstrate that m102.4, a henipavirus neutralizing hmAb that is specific for the ephrin receptor binding site of the viral attachment G glycoprotein, is capable of preventing lethal disease in HeV-infected NHPs. The exceptional antiviral potency and pharmacological properties of m102.4 highlight its therapeutic potential for future approved use in humans.
Discussion
Human case fatality rates are approximately 60% for HeV and as high as 75% for NiV, and during past decade nearly annual spillover occurrences of both viruses have been recorded. Because of these repeated episodes together with an ever increasing number of research facilities working with infectious HeV and NiV, the development of effective therapies against these pathogens has become a critical need. An increasing amount of scientific evidence supports the notion that antibody can be sufficient to protect against lethal HeV- and NiV-mediated disease (26, 30–33) following virus exposure. With the exception of the m102.4 trial in ferrets, all previous experiments that have examined the effectiveness of antibody therapy against henipaviruses have used either polyclonal hamster serum or mouse monoclonal antibody (mAb) neither of which would be suitable for use in humans. The goal of the present study was to evaluate the protective efficacy of m102.4 in a model system in which both the disease pathogenic processes and the animal host closely reflected the human condition. For these reasons, we examined the efficacy of m102.4, a recombinant hmAb, using a lethal NHP model of HeV infection. In these studies, a high dose of HeV was inoculated by an intratracheal route and hmAb was administered by intravenous infusion, mimicking a mucosal challenge and paralleling a systemic treatment scenario. We found that HeV-mediated disease and its associated pathogenic processes in AGMs essentially mirrored the outcomes observed in HeV infected humans. In addition, the pharmacokinetics of m102.4 in the AGM was similar to previously published human immunoglobulin half-life data generated in NHPs. Of the utmost importance and significance, we were able to demonstrate that m102.4 protected all subjects from illness (10hr/d3 and 24hr/d3) and fatal disease (72hr/d5) when administered following a lethal HeV challenge. The data presented here were generated from experiments that were designed in a way to reflect as close as possible those circumstances that might be expected to be encountered in the management of human cases of HeV exposure and possible infection and we believe the results are highly relevant to the potential post-exposure treatment of human cases.
In August 2009, a limited amount of m102.4 was administered on a compassionate use basis to treat a HeV-infected individual while in a coma (Playford, E.G., personal communication). At that time, only the potent in vitro neutralization activity of m102.4 and its pharmacokinetic properties in ferrets had been known. Unfortunately, the available antibody dose was low (100mg corresponding to about 1–2 mg/kg) and intravenous administration of the hmAb occurred well-after the onset of encephalitis, and the individual died shortly thereafter. The efficacy of m102.4 in NiV-infected ferrets has since been reported, demonstrating a viable therapeutic treatment option against NiV (26). During the 2010 HeV spillover occurrence, 11 people had potential exposure and two of those individuals were considered to be at high risk for HeV exposure (15). In this recent episode, m102.4 was requested by Australian health authorities early as a compassionate use therapeutic option even though no human safety testing has been carried out and it was not recommended for use in humans. In addition however, a preliminary m102.4 pharmacokinetic and efficacy study in HeV-infected AGMs had been recently completed and a portion of those findings now included here were available at that time. In this instance, m102.4 was administered to two individuals prior to any HeV diagnosis or onset of clinical disease (19) with doses (~19mg/kg) sufficient to achieve a high serum concentration, and to date both individuals remain healthy and no evidence of HeV infection was ever detected (Playford, E.G., personal communication). The antibody appeared well tolerated when administered which was not unexpected since m102.4 is a fully human mAb. As we continue to evaluate the efficacy of m102.4 in NHPs, it will be critical to establish acceptable therapeutic guidelines pertinent to either accidental or natural exposure to HeV. In addition, both the therapeutic window and appropriate dose of m102.4 need to be defined and its efficacy evaluated in NiV-infected AGMs as well.
Taken together, the results presented here strongly support the further development of m102.4 as a post-exposure therapeutic modality for HeV and NiV-infected humans. Importantly, m102.4 is a recombinant human mAb and the only one available to demonstrate potent in vivo efficacy against a highly lethal emerging infectious agent in a relevant NHP model of disease. Its success represents a significant technological milestone in the fight against an important emerging viral disease. HeV and NiV cause similar disease in AGMs and an important next step will be to evaluate the efficacy of m102.4 in NiV-infected AGMs. The importance of such studies are highlighted by the continuing appearance of NiV and HeV with the most recent outbreaks occurring in March (9) and June-July 2011 (17), respectively.
Materials and Methods
Statistics
Conducting animal studies in biosafety level 4 (BSL-4) severely restricts the number of animal subjects, the volume of biological samples that can be obtained and the ability to repeat assays independently and thus limit statistical analysis. Consequently, data are presented as the mean or median calculated from replicate samples, not replicate assays, and error bars represent the standard deviation across replicates.
Viruses and cells
Hendra virus (HeV) was propagated and titered on Vero cells as previously described (28). All infectious virus work was performed in a BSL-4 at the Integrated Research Facility, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health of the Rocky Mountain Laboratories Hamilton, MT.
Animals
The m102.4 pharmacokinetic studies
Four young adult African green monkeys (AGM) (Chlorocebus aethiops), weighing 4–6kg (Three Springs Scientific Inc.) were caged individually. Purified m102.4 was prepared as previously described (26). Prior to antibody infusion or phlebotomy monkeys were sedated using ketamine (5–20 mg/kg) injected intramuscularly. Antibody was infused using either the saphenous or cephalic vein. Antibody dose was 10 mg or 50 mg m102.4, and each animal was administered m102.4 as a single intravenous infusion at ~2.5 mg/kg or ~11 mg/kg, respectively. Subjects were monitored for adverse effects during and after the antibody infusion and during blood withdrawal. Blood was collected in serum tubes from the femoral, saphenous, or cephalic veins on days 0, 1, 3, 6, 12, 24, 36 and 48 post-infusion. Serum was frozen at −20°C. Approval for animal experiments was obtained from the Boston University Institutional Animal Care and Use Committee (IACUC).
The m102.4 efficacy trials
Fourteen young adult AGMs weighing 4–6kg (Three Springs Scientific Inc.) were caged individually. Subjects were anesthetized by intramuscular injection of ketamine (10–15 mg/kg) and inoculated intratracheally (it) with 4×105 TCID50 of HeV in 4 ml of Dulbecco’s minimal essential medium (DMEM) (Sigma-Aldrich). Four subjects were infused with m102.4 beginning 10 hr after challenge and again 3 days after challenge (10hr/d3); four subjects were infused with m102.4 beginning 24 hr after challenge and again 3 days after challenge (24hr/d3); four subjects were infused with m102.4 beginning 72 hr after challenge and again 5 days after challenge (72hr/d5). Control subjects (AGM 13 and AGM 17) were infused with saline. Each dose of m102.4 was 100 mg administered intravenously ~25 mg/kg. Subjects were anesthetized for antibody infusion and clinical examination including temperature, respiration rate, chest radiographs, blood draw and swabs of nasal, oral and rectal mucosa on days 0, 1, 3, 6, 9, 13, 16, 20, 27 and 35 post-infection (p.i.). Subjects were euthanized on day 40 p.i. except 3 subjects in the 10hr/d3 group where euthanasia was done 88 days post-challenge. Both control subjects had to be euthanized according to approved humane end points on day 8 p.i.. All other subjects survived until the end of the study. Upon necropsy, various tissues were collected for virology and histopathology. Tissues sampled include: conjunctiva, tonsil, oro/naso pharynx, nasal mucosa, trachea, right bronchus, left bronchus, right lung upper lobe, right lung middle lobe, right lung lower lobe, light lung upper lobe, light lung middle lobe, light lung lower lobe, bronchial lymph node (LN), heart, liver, spleen, kidney, adrenal gland, pancreas, jejunum, colon transversum, brain (frontal), brain (cerebellum), brain stem, cervical spinal cord, pituitary gland, mandibular LN, salivary LN, inguinal LN, axillary LN, mesenteric LN, urinary bladder, testes or ovaries, femoral bone marrow. Experiments were conducted under BSL-4 conditions, and approval for animal experiments was obtained from the Rocky Mountain Laboratories IACUC. All animal work was performed by certified staff in an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) approved facility at Boston University and Rocky Mountain Laboratories.
Measurement of serum or plasma m102.4 and F glycoprotein specific antibodies
The m102.4 levels were determined using previously published multiplexed microsphere assays (26). Antibodies to the fusion (F) glycoprotein were measured in HeV-infected subjects simultaneously by including a recombinant soluble F (sF) glycoprotein (34) coupled microsphere in the assay. Coupling of sF to microsphere #43 (Luminex Corporation was done as described previously (35). Plasma from HeV-infected AGMs was inactivated by gamma irradiation (5 mrad) prior to testing. Sera and plasma were assayed in at 1:5,000 and 1:10,000 dilutions. Assays were performed on a Luminex® 200 IS™ machine equipped with Bio-Plex Manager Software (v 5.0) (Bio-Rad Laboratories). Median fluorescent intensity (M.F.I.) and the standard deviation of fluorescence intensity across 100 beads were determined for each sample. For m102.4 concentrations, unknowns were extrapolated from an m102.4 standard curve using nonlinear regression analysis (Bio-Plex Manager Software (v 5.0)). Each pharmacokinetic data set in Table 1, plotted as ln[C] vs t, was interpolated by two linear regressions: ln[C] = k1*t + A1 for t ≤ 1 day and ln[C] = k2*t + A2 for t ≥ 3 days, describing respectively the antibody distribution and elimination phases. Here, k1 is the distribution rate constant; k2 is the elimination rate constant, A1 and A2 are measures for the volume of the distribution. Distribution half-life time t11/2 = ln2/k1 and elimination half-life time t21/2 = ln2/k2. The area under curve (AUC), i.e., the integral of the plasma concentration on time, was calculated by the trapezoid method for the time ≤ 48 days. A correction for the time > 48 days was calculated using the formula: exp(A2)*exp(48*k2)/k2, which was obtained by extrapolation of the elimination phase regression to infinity. AUC values are measure for the antibody presence in vivo, i.e., exposure of body to m102.4, and also account for the bioavailability of m102.4.
Specimen collection and processing in HeV-infected AGMs
Blood was collected in EDTA, sodium citrate or serum Vacutainers (Beckman Dickinson). Immediately following sampling, 140 μl of blood was added to 560 μl of AVL viral lysis buffer (Qiagen Inc.) for RNA extraction. Serum was frozen for chemistry and serological assays. For tissues, approximately 100 mg was stored in 1ml RNAlater (Qiagen Inc.) for a minimum of 24 hr to stabilize RNA and approximately 100 mg was stored for virus isolation. For tissues stored in RNAlater, RNAlater was completely removed and tissues were homogenized in 600 μl RLT buffer in a 2 ml cryovial using QIAGEN tissue lyser and stainless steel beads. An aliquot representing approximately 30 mg was added to fresh RLT buffer (600 μl final volume) (Qiagen Inc.) for RNA extraction. Blood samples in AVL viral lysis buffer and tissue samples in RLT buffer were removed from the BSL-4 laboratory using approved standard operating protocols. RNA was isolated from blood and swabs using the QIAamp viral RNA kit (Qiagen Inc.) and from tissues using the RNeasy Mini kit (Qiagen Inc.) according to manufacturer’s instructions supplied with each kit.
Virus Isolation
Vero cells were seeded in 24 well plates in DMEM containing 10% fetal calf serum (FCS), 100 U/ml penicillin and 100 mg/ml streptomycin. Tissues were weighed and homogenized (1:10 weight/volume in PBS) in a 2 ml cryovial for 8 min using QIAGEN tissue lyser and stainless steel beads. Homogenates were clarified by centrifugation and diluted 1:10 using DMEM containing 1% FCS, 100 U/ml penicillin and 100 mg/ml streptomycin (DMEM-1). Duplicate wells were inoculated with 200μl of a 1% tissue homogenate and incubated at 37°C. One well was incubated 30 minutes, and one well was incubated overnight, both were washed once with PBS and cultured in 1ml DMEM-1. Cultures were examined for the presence or absence of syncytia/cytopathic effect (CPE) for 5 days. Negative samples were passaged twice onto new cells before being deemed negative.
Histopathology and immunohistochemistry
Necropsy was performed on all subjects. Tissue samples of all major organs were collected for histopathologic and immunohistochemical examination and were immersion-fixed in 10% neutral buffered formalin for at least 7 days in BSL-4. Subsequently, formalin was changed; specimens were removed from BSL-4 under approved standard operating protocols, were processed in BSL-2 by conventional methods and embedded in paraffin and sectioned at 5 μm thickness. Tissues for immunohistochemistry were stained on the Discovery XT automated stainer (Ventana Medical Systems, Inc., Oro Valley, AZ) using an anti-Nipah-nucleoprotein antibody (1:5,000) and the DAB map detection kit (Ventana Medical Systems, Inc.). Non-immune rabbit IgG was used as a negative staining control.
HeV Taqman PCR
The HeV Taqman PCR assay has been described previously (28). All reactions contained 2 μl of RNA, master mixes were set up following the manufacturer’s protocols and each reaction was done in a total volume of 25 μl. For blood, 2 μl RNA represented 4.7 μl of whole blood and for tissues 2 μl of RNA represented 1.2 mg of tissue. All samples were assayed in triplicate. To account for plate to plate variation, for each TaqMan plate, RNA representing 4.7, 47 and 470 TCID50 HeV, derived from the inoculum, were assayed in triplicate and the average Ct was set to a relative N expression value of 1, 10 and 100, respectively. Sample Ct values were analyzed against Ct values generated from the standard curve of HeV RNA and a relative HeV N expression value was calculated for each replicate. Ct value analysis was done using Rotor Gene 6000 software and data are shown as the mean relative HeV N gene expression levels +/− standard deviation (SD).
Supplementary Material
Fig. S1. Radiographic images of lung fields on day 6 post Hendra virus infection of African green monkeys.
Fig. S2. Absence of Hendra virus antigen in the lung and brain of m102.4 treated African green monkeys.
Fig. S3. Hendra virus antigen detection in spleens of African green monkeys that received m102.4 therapy.
Table S1. Clinical scoring and outcome of Hendra virus-infected African green monkeys.
Table S2. Gross Pathology summary of Hendra-infected African green monkeys.
Acknowledgments
We thank the staff of the Rocky Mountain Veterinary Branch for animal care and veterinary support. We thank Yang Feng for help with the m102.4 production.
Funding: These studies were supported in part by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health and in part by the Department of Health and Human Services, National Institutes of Health, grants AI082121 and AI057159 to TWG; AI054715 and AI077995 to CCB; and the Intramural Biodefense Program of the National Institute of Allergy and Infectious Diseases, the Intramural Program of the National Cancer Institute, Center for Cancer Research, and by federal funds from the National Cancer Institute, NIH, under contract N01-CO-12400.
Footnotes
Author contributions: K.N.B., B.R., H.F., C.C.B. and T.W.G. designed the studies. B.R., H.F., T.W.G., F.F., D.B. and J.B.G. contributed to performance of experiments and data collection and analysis. B.R., H.F., T.W.G. carried out gross pathological analysis. B.R., K.N.B., A.C.H and J.C. performed serological and nucleic acid analysis experiments and data collection. Y-P.C. provided critical reagents and contributed to assay design. L.Y., Y-R.F., and Y.W. produced, purified and quality controlled the m102.4 monoclonal antibody. Z.Z. and D.S.D provided critical reagents, contributed to study design and reviewed and edited the manuscript. D.S. carried out histological analysis and J.B.G. and D.B. facilitated the conduct of the in vivo animal studies. A.S.D. provided the analysis of the antibody pharmacokinetics studies. K.N.B., C.C.B., B.R., H.F. and T.W.G. wrote and edited the manuscript. C.C.B. prepared the final versions of manuscript and supplementary material.
Competing interests: C.C.B. and D.S.D. are United States federal employees, and D.S.D., Z.Z. and C.C.B. are coinventors on United States patent 7,988,971, Human monoclonal antibodies against Hendra and Nipah viruses; assignees are The United States of America as represented by the Department of Health and Human Services (Washington, DC), Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. (Bethesda, MD). All other authors declare no competing interests. The opinions or assertions contained herein are the private ones of the author(s) and are not to be construed as official or reflecting the views of the Department of Defense, the Uniformed Services University of Health Sciences, and the National Institutes of Allergy and Infectious Diseases, National Institutes of Health.
References
- 1.Eaton BT, Broder CC, Middleton D, Wang LF. Hendra and Nipah viruses: different and dangerous. Nat Rev Microbiol. 2006;4:23. doi: 10.1038/nrmicro1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayo MA. A summary of taxonomic changes recently approved by ICTV. Arch Virol. 2002;147:1655. doi: 10.1007/s007050200039. [DOI] [PubMed] [Google Scholar]
- 3.Field H, Young P, Yob JM, Mills J, Hall L, Mackenzie J. The natural history of Hendra and Nipah viruses. Microbes Infect. 2001;3:307. doi: 10.1016/s1286-4579(01)01384-3. [DOI] [PubMed] [Google Scholar]
- 4.Iehle C, Razafitrimo G, Razainirina J, Andriaholinirina N, Goodman SM, Faure C, Georges-Courbot MC, Rousset D, Reynes JM. Henipavirus and Tioman virus antibodies in pteropodid bats, Madagascar. Emerg Infect Dis. 2007;13:159. doi: 10.3201/eid1301.060791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bossart KN, Tachedjian M, McEachern JA, Crameri G, Zhu Z, Dimitrov DS, Broder CC, Wang LF. Functional studies of host-specific ephrin-B ligands as Henipavirus receptors. Virology. 2008;372:357. doi: 10.1016/j.virol.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Bishop KA, Broder CC. In: Emerging Infections. Scheld WM, Hammer SM, Hughes JM, editors. American Society for Microbiology; Washington, D.C: 2008. pp. 155–187. [Google Scholar]
- 7.Luby SP, Hossain MJ, Gurley ES, Ahmed BN, Banu S, Khan SU, Homaira N, Rota PA, Rollin PE, Comer JA, Kenah E, Ksiazek TG, Rahman M. Recurrent zoonotic transmission of Nipah virus into humans, Bangladesh, 2001–2007. Emerg Infect Dis. 2009;15:1229. doi: 10.3201/eid1508.081237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anonymous. Pro-MED-mail. International Society for Infectious Diseases; Jan 22, 2010. Nipah virus, fatal - Bangladesh: Faridpur. archive no. 20100122.0250 Available at www.promedmail.org. [Google Scholar]
- 9.Anonymous. Pro-MED-mail. International Society for Infectious Diseases; Mar 8, 2011. Nipah encephalitis, human - Bangladesh: Rangpur (05) archive no. 20110308.0756 Available at www.promedmail.org. [Google Scholar]
- 10.Field HE, Barratt PC, Hughes RJ, Shield J, Sullivan ND. A fatal case of Hendra virus infection in a horse in north Queensland: clinical and epidemiological features. Aust Vet J. 2000;78:279. doi: 10.1111/j.1751-0813.2000.tb11758.x. [DOI] [PubMed] [Google Scholar]
- 11.Hanna JN, McBride WJ, Brookes DL, Shield J, Taylor CT, Smith IL, Craig SB, Smith GA. Hendra virus infection in a veterinarian. Med J Aust. 2006;185:562. doi: 10.5694/j.1326-5377.2006.tb00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anonymous. Pro-MED-mail. International Society for Infectious Diseases; Aug 20, 2009. Hendra virus, equine - Australia (03): Queensland, human exposure. archive no. 20090821.2963 Available at www.promedmail.org. [Google Scholar]
- 13.Playford EG, McCall B, Smith G, Slinko V, Allen G, Smith I, Moore F, Taylor C, Kung YH, Field H. Human Hendra virus encephalitis associated with equine outbreak, Australia, 2008. Emerg Infect Dis. 2010;16:219. doi: 10.3201/eid1602.090552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anonymous. Pro-MED-mail. International Society for Infectious Diseases; May 22, 2010. Hendra virus, equine - Australia (03): Queensland, human exposure. archive no. 20100522.1699). Available at www.promedmail.org. [Google Scholar]
- 15.Anonymous. Pro-MED-mail. International Society for Infectious Diseases; May 24, 2010. Hendra virus, equine - Australia (04): Queensland, human exposure. archive no. 20100522.1724). Available at www.promedmail.org. [Google Scholar]
- 16.Anonymous. Melton Weekly, Express telegraph. Melton/Moorabool, Queensland; Australia: May 18, 2010. Vets warn on spread of hendra virus. Available at www.meltonweekly.com.au. [Google Scholar]
- 17.Anonymous. Pro-MED-mail. International Society for Infectious Diseases; Aug 30, 2011. Hendra Virus, Equine - Australia (26): New South Wales. archive no. 20100527.1761). Available at www.promedmail.org. [Google Scholar]
- 18.Bossart KN, Bingham J, Middleton D. Targeted strategies for Henipavirus therapeutics. Open Virology Journal. 2007;1:14. doi: 10.2174/1874357900701010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anonymous. Pro-MED-mail. International Society for Infectious Diseases; May 27, 2010. Hendra virus, equine - Australia (05): Queensland, human exposure. archive no. 20100527.1761). Available at www.promedmail.org. [Google Scholar]
- 20.Bonaparte MI, Dimitrov AS, Bossart KN, Crameri G, Mungall BA, Bishop KA, Choudhry V, Dimitrov DS, Wang LF, Eaton BT, Broder CC. Ephrin-B2 ligand is a functional receptor for Hendra virus and Nipah virus. Proc Natl Acad Sci U S A. 2005;102:10652. doi: 10.1073/pnas.0504887102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Negrete OA, Levroney EL, Aguilar HC, Bertolotti-Ciarlet A, Nazarian R, Tajyar S, Lee B. EphrinB2 is the entry receptor for Nipah virus, an emergent deadly paramyxovirus. Nature. 2005;436:401. doi: 10.1038/nature03838. [DOI] [PubMed] [Google Scholar]
- 22.Negrete OA, Wolf MC, Aguilar HC, Enterlein S, Wang W, Muhlberger E, Su SV, Bertolotti-Ciarlet A, Flick R, Lee B. Two Key Residues in EphrinB3 Are Critical for Its Use as an Alternative Receptor for Nipah Virus. PLoS Pathog. 2006;2:e7. doi: 10.1371/journal.ppat.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bishop KA, Stantchev TS, Hickey AC, Khetawat D, Bossart KN, Krasnoperov V, Gill P, Feng YR, Wang L, Eaton BT, Wang LF, Broder CC. Identification of Hendra virus G glycoprotein residues that are critical for receptor binding. J Virol. 2007;81:5893. doi: 10.1128/JVI.02022-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu Z, Dimitrov AS, Bossart KN, Crameri G, Bishop KA, Choudhry V, Mungall BA, Feng YR, Choudhary A, Zhang MY, Feng Y, Wang LF, Xiao X, Eaton BT, Broder CC, Dimitrov DS. Potent neutralization of Hendra and Nipah viruses by human monoclonal antibodies. J Virol. 2006;80:891. doi: 10.1128/JVI.80.2.891-899.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu Z, Bossart KN, Bishop KA, Crameri G, Dimitrov AS, McEachern JA, Feng Y, Middleton D, Wang LF, Broder CC, Dimitrov DS. Exceptionally potent cross-reactive neutralization of Nipah and Hendra viruses by a human monoclonal antibody. J Infect Dis. 2008;197:846. doi: 10.1086/528801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bossart KN, Zhu Z, Middleton D, Klippel J, Crameri G, Bingham J, McEachern JA, Green D, Hancock TJ, Chan YP, Hickey AC, Dimitrov DS, Wang LF, Broder CC. A neutralizing human monoclonal antibody protects against lethal disease in a new ferret model of acute nipah virus infection. PLoS Pathog. 2009;5:e1000642. doi: 10.1371/journal.ppat.1000642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geisbert TW, Daddario-DiCaprio KM, Hickey AC, Smith MA, Chan YP, Wang LF, Mattapallil JJ, Geisbert JB, Bossart KN, Broder CC. Development of an acute and highly pathogenic nonhuman primate model of Nipah virus infection. PLoS One. 2010;5:e10690. doi: 10.1371/journal.pone.0010690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rockx B, Bossart KN, Feldmann F, Geisbert JB, Hickey AC, Brining D, Callison J, Safronetz D, Marzi A, Kercher L, Long D, Broder CC, Feldmann H, Geisbert TW. A novel model of lethal Hendra virus infection in African green monkeys and the effectiveness of ribavirin treatment. J Virol. 2010;84:9831. doi: 10.1128/JVI.01163-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, Lanigan CM, Landucci G, Forthal DN, Parren PW, Marx PA, Burton DR. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 30.McEachern JA, Bingham J, Crameri G, Green DJ, Hancock TJ, Middleton D, Feng YR, Broder CC, Wang LF, Bossart KN. A recombinant subunit vaccine formulation protects against lethal Nipah virus challenge in cats. Vaccine. 2008;26:3842. doi: 10.1016/j.vaccine.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pallister J, Middleton D, Wang LF, Klein R, Haining J, Robinson R, Yamada M, White J, Payne J, Feng YR, Chan YP, Broder CC. A recombinant Hendra virus G glycoprotein-based subunit vaccine protects ferrets from lethal Hendra virus challenge. Vaccine. 2011;29:5623. doi: 10.1016/j.vaccine.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guillaume V, Contamin H, Loth P, Grosjean I, Courbot MC, Deubel V, Buckland R, Wild TF. Antibody prophylaxis and therapy against Nipah virus infection in hamsters. J Virol. 2006;80:1972. doi: 10.1128/JVI.80.4.1972-1978.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guillaume V, Wong KT, Looi RY, Georges-Courbot MC, Barrot L, Buckland R, Wild TF, Horvat B. Acute Hendra virus infection: Analysis of the pathogenesis and passive antibody protection in the hamster model. Virology. 2009;387:459. doi: 10.1016/j.virol.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Chan YP, Yan L, Feng YR, Broder CC. Preparation of recombinant viral glycoproteins for novel and therapeutic antibody discovery. Methods Mol Biol. 2009;525:31. doi: 10.1007/978-1-59745-554-1_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bossart KN, McEachern JA, Hickey AC, Choudhry V, Dimitrov DS, Eaton BT, Wang LF. Neutralization assays for differential henipavirus serology using Bio-Plex Protein Array Systems. J Virol Methods. 2007;142:29. doi: 10.1016/j.jviromet.2007.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Radiographic images of lung fields on day 6 post Hendra virus infection of African green monkeys.
Fig. S2. Absence of Hendra virus antigen in the lung and brain of m102.4 treated African green monkeys.
Fig. S3. Hendra virus antigen detection in spleens of African green monkeys that received m102.4 therapy.
Table S1. Clinical scoring and outcome of Hendra virus-infected African green monkeys.
Table S2. Gross Pathology summary of Hendra-infected African green monkeys.