Abstract
We describe the use of Co(III) Schiff base-DNA conjugates, a versatile class of research tools that target C2H2 transcription factors, to inhibit the Hedgehog (Hh) pathway. In developing mammalian embryos, Hh signaling is critical for the formation and development of many tissues and organs. Inappropriate activation of the Hedgehog (Hh) pathway has been implicated in a variety of cancers including medulloblastomas and basal cell carcinomas. It is well known that Hh regulates the activity of the Gli family of C2H2 zinc finger transcription factors in mammals. In Drosophila the function of the Gli proteins is performed by a single transcription factor with an identical DNA binding consensus sequence, Cubitus Interruptus (Ci). We have demonstrated previously that conjugation of a specific 17 base-pair oligonucleotide to a Co(III) Schiff base complex results in a targeted inhibitor of the Snail family C2H2 zinc finger transcription factors. Modification of the oligonucleotide sequence in the Co(III) Schiff base-DNA conjugate to that of Ci’s consensus sequence (Co(III)-Ci) generates an equally selective inhibitor of Ci. Co(III)-Ci irreversibly binds the Ci zinc finger domain and prevents it from binding DNA in vitro. In a Ci responsive tissue culture reporter gene assay, Co(III)-Ci reduces the transcriptional activity of Ci in a concentration dependent manner. In addition, injection of wild-type Drosophila embryos with Co(III)-Ci phenocopies a Ci loss of function phenotype, demonstrating effectiveness in vivo. This study provides evidence that Co(III) Schiff base-DNA conjugates are a versatile class of specific and potent tools for studying zinc finger domain proteins and have potential applications as customizable anti-cancer therapeutics.
Keywords: Basal Cell Carcinoma, Cobalt chelate/Schiff base, Development, Drosophila, Hedgehog Signaling, Cubitus interruptus, Transcription Factor, Zinc Fingers
Introduction
Zinc finger domains are compact globular protein structures in which Cys and/or His residues are coordinated to a Zn(II) ion, which is necessary for structure and function 1. Many zinc finger domains bind with DNA and RNA and are essential for the function and activity of the corresponding transcription factors 2–4. Human Cys2His2 (C2H2) zinc finger domain proteins comprise the largest motif-containing-family with a predicted 4500 C2H2 zinc finger domains spread amongst 564 different proteins 5. Current approaches to understand these proteins by blocking their function utilize antibodies and model organism mutant collections. However, mutant collections are incomplete and antibodies are not always functional both in vivo and in vitro. Therefore, new tools designed to investigate these proteins are highly desired.
The Co(III) containing Co(III) Schiff base complex (Co(III)-sb) (Figure 1) binds histidine residues via a dissociative ligand exchange of the labile axial ligands 6–10. Co(III)-sb non-specifically and irreversibly inhibits protein activity by coordinating to important histidine residues in active sites and those critical to structure 6, 8, 10. In the context of a DNA binding C2H2 zinc finger, histidine binding by Co(III)-sb displaces the zinc ion and disrupts the structure of the zinc finger domain, preventing DNA binding 7.
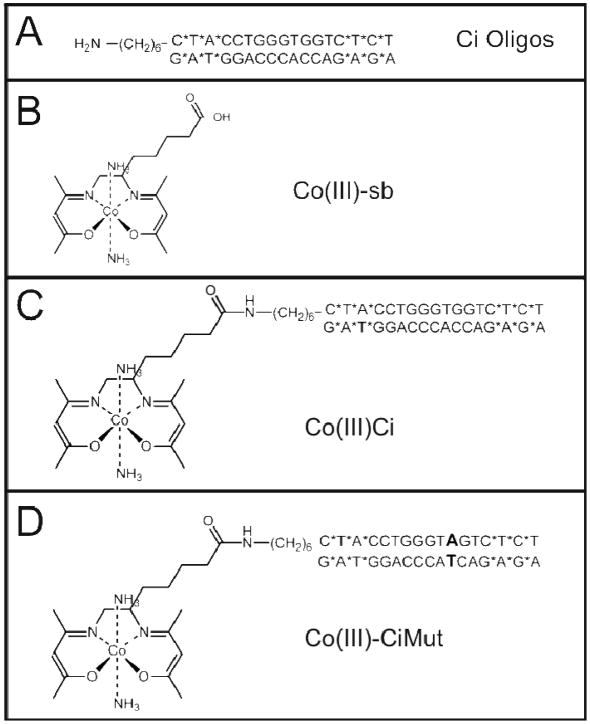
Figure 1.
Chemical structures of Co(III) Schiff base-DNA complexes utilized in the experiments. (A) Ci Oligos. (B) Co(III)-sb. (C) Co(III)-Ci. (D) Co(III)-CiMut.
While the inhibitory action of unconjugated Co(III)-sb is not specific, the addition of a targeting moiety by conjugating DNA to Co(III)-sb significantly increased the potency and specificity for the transcription factor Sp1 in vitro 7. This approach was extended to in vivo by conjugating Co(III)-sb to a 17-bp DNA sequence containing the Ebox consensus sequence of the Snail family of transcription factors (Co(III)-Ebox). Co(III)-Ebox is a specific and potent inhibitor of Snail family transcription factors in Xenopus laevis10. Co(III)-Ebox inhibition was 150 fold more effective than Co(III)-sb and was dependent upon the presence of both the Ebox consensus sequence as well as the Co(III)-sb 10. We hypothesized that by substituting the DNA sequence conjugated to Co(III)-sb, it would be possible to change the target of the Co(III) Schiff base. The focus of this work was to vary the DNA targeting moiety and develop a specific and potent inhibitor of the Ci/Gli family of proteins, C2H2 zinc finger containing transcription factors regulated by the Hedgehog (Hh) signaling pathway 11.
Hedgehog signaling is essential for the growth and patterning of multiple tissues and organs during mammalian embryonic development 12–15. Aberrant activation of Hh signaling drives the establishment and progression of a variety of tumors including basal cell carcinomas and medulloblastomas 16–18. In mammals, Hh performs these functions through the regulation of the Gli family of C2H2 zinc finger transcription factors; in Drosophila the function is performed by a single C2H2 zinc finger transcription factor Cubitus Interruptus (Ci).
During tumorigenesis, Hh signaling can act in multiple manners. In medulloblastomas and basal cell carcinomas, activation of the Gli transcription factors directly drives tumor growth. In pancreatic and colon cancer, paracrine Hh signaling from tumor cells leads to activation of Hh target genes in the surrounding stroma 19, 20. Gli activation in the stroma leads to a proliferative signal sent back to the tumor, similar to what occurs in development 21, 22. While promising Hh anti-cancer therapeutics exist, (most notably GDC-0449 and HhAntag 23, 24), a number of limitations persist. These drugs are ineffective on tumors arising as a result of mutations in the Hh pathway downstream of the transmembrane protein Smo 25, 26 and patient relapse has been observed by spontaneous mutation in Smo rendering the drug unable to bind and inhibit signaling 27. Young mice treated with HhAntag have permanent defects in bone growth including loss of proliferation in chondrocytes and premature fusion of the growth plate resulting in mice with truncated limbs 28. It is important to develop inhibitors that target additional components of the Hh pathway. The Gli proteins are particularly appropriate as they represent the terminal step in the pathway and work identifying GANT61, an inhibitor of the Glis has shown promising results in xenograft tumor models 29.
Here, we show that changing the DNA targeting sequence of Co(III) Schiff base-DNA conjugates creates a specific and potent inhibitor of Ci. Drosophila was used as a model to thoroughly investigate the mechanism of Co(III) Schiff base-DNA conjugate action in vitro as well as in vivo. The optimal Gli binding consensus sequence (TGGG[T/A]GGTC) is known and 100% conserved with the Drosophila homologue Ci and therefore, results for Ci should directly translate to the Gli’s 30–33. This study demonstrates the synthetic ease and versatility for creating an entire class of specific and potent Co(III) Schiff base-DNA conjugates. These conjugates can be utilized as experimental tools to study C2H2 zinc finger proteins and have potential applications as personalized anti-cancer therapeutics.
Materials and Methods
Co(III)-Oligonucleotide Conjugates
Co(III)-Ci and Co(III)-CiMut were synthesized by coupling an oligonucleotide containing 3 phosphorothioate linkages (indicated by *) at both the 3′ and 5′ ends of both strands (Integrated DNA Technologies) to a Co(III)-Schiff base complex via a 6-carbon amino-terminated linker at the 5′ end of one of the strands. To improve yields, the synthesis of Co(III)-sb as previously described was modified and verified (See Supporting Information Figures 1–4) 10. The oligonucleotide sequences used are as follows:
Co(III)-Ci: 5′-C*T*A*CCTGGGTGGTC*T*C*T-3′
Co(III)-CiMut: 5′-C*T*A*CCTGGGTAGTC*T*C*T-3′
CiZn and KrZn Protein Extract
Drosophila Schneider 2 (S2) cells were obtained from the Drosophila Genomics Resource Center and grown as directed. Plasmids used for transfection were UAS-CiZn, which consists of amino acids 440–684 of Ci (the zinc finger domain includes 453–603)34, UAS-KrZn in which the zinc finger domain of Kr (amino acids 214–363) was cloned out of the DGRC cDNA clone RE30918 using the primers: 5′-CATGAATTCATGAAGGATCCATCTCGCGACAAAA – 3′ and CATCTCGAGCGCGGGAGTAGGCGGCGACTGGA, digested and ligated into the EcoRI/XhoI sites of pUAST, and pMT-Gal4 35, 36. S2 cells were seeded at 4.5 × 106 cells/25cm2 flask dish and transfected using the Qiagen Effectene Transfection Reagent Kit (Qiagen) with a total of 1 μg of DNA (0.5 μg UAS construct 0.5 μg of pMT-Gal4) following the manufacturer’s protocol. 12 hours later the cells were induced by adding CuSO4 to a final concentration of 1mM. After 18 hours, the cells were centrifuged and resuspended in 330 μL EMSA Binding Buffer (20 mM Hepes, ph 7.6, 150 mM KCl, 3 mM MgCl2, 0.5 mg/ml BSA, 10% glycerol and 0.2 mM ZnSO4). The cells were lysed by freezing overnight at −80C, thawed on ice, aliquoted and stored at −20C.
Radiolabeled Probe Reactions
10 pmol of annealed oligonucleotide probes (IDT DNA) or Co(III)-Oligonucleotide Conjugates were radiolabeled on their 5′ end(s) with 32P ATP by T4 polynucleotide kinase (New England Biolabs) according to the manufacturer’s protocol. Unincorporated nucleotides were removed using ProbeQuant G-50 Micro Columns (GE Healthcare Life Sciences). To create a single labeled probe, one strand was phosphorylated before being annealed to the unlabeled reverse complement. The oligonucleotide sequences are as follows:
Ci: 5′-CTACCTGGGTGGTCTCT-3′
CiMut: 5′-CTACCTGGGTAGTCTCT-3′
Kr: 5′-GGCGAGAACGGGTTAAGATC-3′
KrMut: 5′-GGCGAGAACAAATTAAGATC-3′
Electrophoretic Mobility Shift Assay
For the irreversibility assays, 5 μL of protein extract, 1 μL of salmon sperm DNA (1 mg/ml), and 12 μL of binding buffer were mixed with 1 μL 0.2 μM radiolabeled Co(III)-Ci and incubated at room temperature for 3 hours. Either 1 μL of binding buffer or 1 μL 30 μM cold competitor was added and incubated for 15 minutes. For standard assays, 5 μL of protein extract, 1 μL of salmon sperm DNA (1 mg/ml), and 12 μL of binding buffer were mixed with 1 μL 0.2 μM Co(III)-Ci (or other cold competitor) and incubated at room temperature for 3 hours. 1 μL of 0.2 μM radiolabeled oligo probe was then added and allowed to bind for 15 minutes. Reactions were subsequently resolved on a TBE/acrylamide gel and imaged/quantified on a Storm 680 (GE Healthcare) phosphorimager using ImageQuant software. Shift intensities were corrected for individual lane background and normalized with no competitor shifts set to 100%. Statistical analysis was performed using a t-test.
Transfection and Luciferase Assay
Drosophila Schneider 2 R+ (S2R+) cells were obtained from the Drosophila Genomics Resource Center, seeded at 105 cells/well of a 24 well plate and transfected using the Qiagen Effectene Transfection Reagent Kit (Qiagen) according to the manufacturer’s protocol using 30 ng pACT-RL (Renilla control), 150 ng or 75 ng Co(III) Schiff base-DNA conjugate, 100 ng pPAC-HACi(m1-4) and 100 ng of ptcΔ136-FLuc or ptcΔ136mut-FLuc 36, 37. Transfected cells were incubated for 15 hours, lysed and analyzed with the Dual-Luciferase® Reporter Assay System (Promega) according to the manufacturer’s protocol. Results were normalized by dividing by Renilla expression and are reported as fold inductions over pPAC-HACi(m1-4) + ptcΔ136mut-FLuc levels. Statistical analysis was performed on results using a t-test.
Drosophila Injections
Drosophila embryos, 0–45 minutes old, were dechorionated with bleach, desiccated for 10 minutes, lined up on a glue-coated microscope slide, covered in halocarbon oil and microinjected according standard Drosophila injection protocols 38. Injections were through the posterior end of the embryo and the injected material was deposited along the ventral surface of the embryo. Injected embryos were left on the slides in oil, placed in an 18 °C incubator and given 48 hours to recover and develop before washing in heptane to remove the halocarbon oil, squashing in lactic acid and imaging denticle patterning by phase contrast light microscopy. Statistical analysis was performed using Fisher’s Exact Probability Test.
Results
Co(III)-Ci binds specifically and irreversibly to the Ci zinc finger domain
The Ci zinc finger domain (CiZn) is known to bind a nine bp consensus sequence with high affinity (Supporting Information Figure 5). To determine the effectiveness of a modified Co(III)-sb targeted to Ci, we coupled an oligonucleotide (Figure 1A) containing the Ci binding sequence (TGGGTGGTC) flanked by phosphorothioate linkages at the terminal ends (indicated by a *, Figure 1A, C–D) to a Co(III)-sb complex (Figure 1B) to prepare Co(III)-Ci (Figure 1C). The resulting conjugate was radiolabeled with 32P and incubated with extracts from S2 cells overexpressing CiZn. Reactions were analyzed by electromobility shift assays. Three biological replicates were performed; a representative gel is shown (Figure 2A) with combined results quantified (Figure 2B).
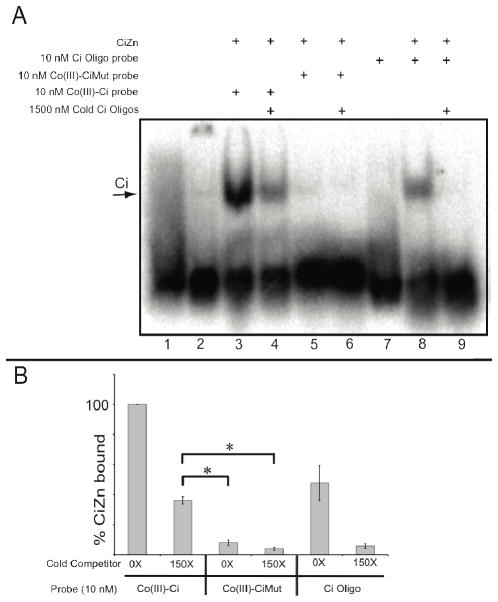
Figure 2.
Co(III)-Ci irreversibly binds to the Ci zinc finger domain. (A) Lane 1. 10 nM 32P radiolabeled Co(III)-Ci. Lane 2. 10 nM 32P radiolabeled Co(III)-Ci incubated with control S2 extracts not expressing the Ci zinc finger domain. The Ci zinc finger domain was overexpressed in S2 cells, cells were lysed and incubated for 3 hours with either 1X 32P radiolabeled Co(III)-Ci (lanes 3 and 4), 10 nM 32P radiolabeled Co(III)-CiMut (lanes 5 and 6) or 32P radiolabeled Ci probe (lanes 8 and 9). Then either buffer (A, lanes 3, 5 and 8) or 150X cold competitor (lanes 4, 6 and 9) was added to reactions for 15 minutes. Reactions were analyzed by EMSA on a 4% TBE/acrylamide gel and performed in triplicate. Representative gels are shown. (B) EMSAs were quantified using ImageQuant software. Error bars are one standard deviation. Final reaction concentrations are given.
10 nM radiolabeled Co(III)-Ci was able to bind to CiZn (Figure 2A, Lane 3). With addition of 1500 nM (150X) cold competitor, 36 +/− 2.4% of radiolabeled Co(III)-Ci remained bound (Figure 2A, lane 4), whereas the same concentration of cold competitor was able to completely eliminate binding of a 10 nM radiolabeled Ci binding site DNA probe (Figure 2A lanes 7–9). More Co(III)-Ci remained bound to CiZn than the Ci binding site probe (Figure 2B, 100% versus 48 +/− 12%, p = 0.016) even in the absence of competitor. These divergent results show that coupling Co(III)-sb to the Ci binding site oligo has resulted in a transition metal conjugate capable of irreversibly binding to CiZn.
To determine whether Co(III)-Ci binding was acting in a sequence-dependent manner, we coupled a single base-pair mutation of the Ci binding sequence (TGGGTAGTC) to Co(III)-sb to create Co(III)-CiMut (Figure 1D). This single base-pair differences abrogated Co(III)-CiMut binding to CiZn (Figure 2A, lane 5), showing a sequence specificity requirement in the oligo to target Co(III)-sb to a zinc finger domain and providing support for a direct interaction between CiZn and the DNA moiety of Co(III)-Ci.
Co(III)-Ci prevents the Ci zinc finger domain from binding to DNA
Since Co(III)-Ci binds irreversibly to the CiZn domain, the ability of Co(III)-Ci to prevent CiZn binding to DNA was assayed. Co(III)-Ci was incubated with a CiZn-containing cell extract and a radiolabeled DNA probe containing the Ci consensus sequence (CTACCTGGGTGGTCTCT) was added to allow CiZn binding. The DNA-protein complex was analyzed by EMSA, imaged and quantified. Co(III)-Ci reduced the amount of CiZn bound to the radiolabeled DNA probe to 34.5 +/− 6.8%, a significant reduction compared to baseline (Figure 3A, lanes 3 and 4, p = 0.0035). At the same concentration, neither unmodified Co(III)-sb (Figure 3A, lane 7) nor Co(III)-CiMut (Fig 3A, lane 5) were able to significantly inhibit CiZn from binding DNA. Additionally, the same concentration of the Ci binding site oligo alone had no effect on CiZn’s ability to bind the radiolabeled DNA probe (Figure 3, lane 8). To obtain inhibition similar to that of Co(III)-Ci (Figure 3B) required a 100 fold excess concentration of cold Ci binding site oligo (Figure 3A, lane 9).
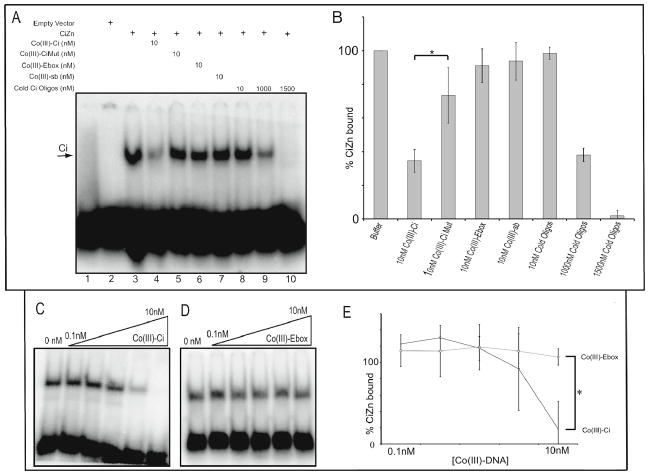
Figure 3.
Co(III)-Ci prevents the Ci zinc finger domain from binding DNA in a specific and concentration-dependent manner. (A) Lane 1. 10 nM of a 32P radiolabeled Ci probe. Lane 2. 10 nM of a 32P radiolabeled Ci probe incubated for 15 minutes in an S2 cell extract not expressing the Ci zinc finger domain. The Ci zinc finger domain was overexpressed in S2 cells, cells were lysed and incubated for 3 hours with buffer (lane 3), 1X Co(III)-Ci (lane 4), 10 nM Co(III)- CiMut (lane 5), 10 nM Co(III)-Ebox (lane 6), or 10 nM Co(III)-sb (lanes 3–7) and 1X, 100X or 150X cold oligo competitor (lanes 8–10). Then 10 nM of a 32P radiolabeled Ci probe was added to reactions for 15 minutes. Reactions were performed in triplicate (representative gel is shown) and the reactions were analyzed by EMSA as previously described. (C) Varying concentrations ranging from 0.1 nM to 10 nM of Co(III)-Ci or (D) Co(III)-Ebox were analyzed for their ability to prevent the Ci zinc finger domain from binding to 10 nM 32P radiolabeled Ci probe as in (A). (B) and (E) Triplicate EMSAs were quantified using ImageQuant software. Error bars are one standard deviation, (* = p < 0.05). Final reaction concentrations are given.
To continue the analysis of specificity, a Co(III) Schiff base-DNA conjugate targeting another transcription factor was studied. Co-(III)-Ebox has an oligo coupled to Co(III)-sb that targets the Snail family of transcription factors and is able to prevent their binding to DNA 10. If Co(III)-Ebox targets only the Ebox-binding transcription factors then it should be ineffective at preventing CiZn from binding DNA. Indeed, Co(III)-Ci was able to inhibit CiZn binding in a concentration dependent manner, whereas Co(III)-Ebox had no effect (Figure 3D). Co(III)-Ci inhibited CiZn’s DNA binding to 18.5+/− 14.7%, a significant reduction compared to Co(III)-Ebox that had no effect (p < 0.05).
Co(III)-Ci does not prevent the highly related transcription factor Krüppel from binding to DNA
The sequence of the oligo attached to Co(III)-Ci targets it specifically to the Ci zinc finger domain. We expected Co(III)-Ci would have no effect on the DNA binding ability of transcription factors that bind different sequences. The closely related protein Krüppel (Kr) is a transcription factor in the same family as Ci and has a known consensus sequence (AACGGGTTAA) that is distinct from that of Ci 39–41. The Kr zinc finger domain (KrZn) was overexpressed in S2 cells and a lysate was prepared. The KrZn lysate was incubated for 3 hours at room temperature with buffer or competitor and the radiolabeled Kr probe (GGCGAGAACGGGTTAAGATC) added. DNA-protein complexes were analyzed by EMSA. KrZn bound to the Kr probe and binding was abrogated by addition of the unlabeled Kr binding site oligo (Supporting Information Figure 6). Further, KrZn did not bind a radiolabeled KrMut probe (GGCGAGAACAAATTAAGATC) and was not hindered by addition of 150X cold KrMut oligos (Supporting Information Figure 6). A non-specific band and was identified and not used in further analysis (Supporting Information Figure 6).
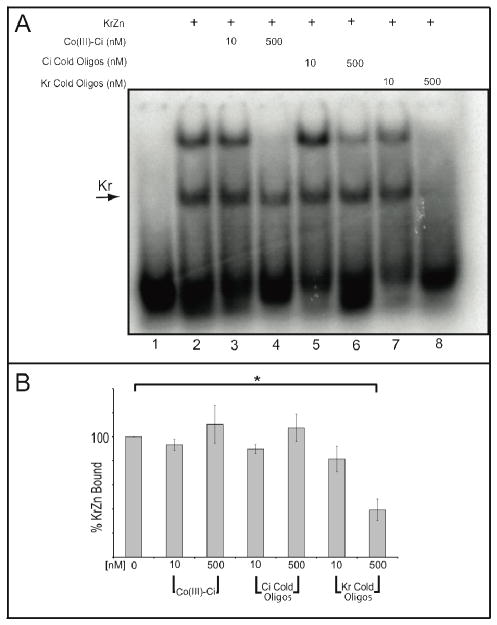
Importantly, Co(III)-Ci had no effect on KrZn’s ability to bind its target probe. Cold competitors were incubated with KrZn extract for 3 hours, radiolabeled Kr probe was added for 15 minutes and DNA-protein complexes were analyzed by EMSA, imaged and quantified. 10 nM and 500 nM Co(III)-Ci had no significant effect on KrZn’s ability to bind radiolabeled Kr probe (Figure 4A, lanes 2–4), nor did 10 nM and 500 nM cold Ci oligos (Figure 4A, lanes 5–6). Comparatively, only 39 +/− 9% of probe remained bound when competed with 500 nM cold Kr oligos, representing a significant reduction relative to buffer control (p = 0.007). These results confirm that Co(III)-Ci does not prevent a highly related transcription factor from binding DNA. The oligo directs the Co(III) Schiff base-DNA conjugates to the target DNA-binding protein, providing specificity.
Figure 4.
Co(III)-Ci is unable to inhibit the Krüppel zinc finger domain from binding DNA. (A) Lane 1. 10 nM of a 32P radiolabeled Kr probe. The Kr zinc finger domain was overexpressed in S2 cells, cells were lysed and incubated for 3 hours with buffer (lane 2), 1X or 50X Co(III)-Ci (lanes 3 and 4), 1X or 50X Ci Cold Oligos (lanes 5 and 6) or 1X or 50X Kr Cold Oligos (lanes 7 and 8). Then 10 nM of a 32P radiolabeled Kr probe was added to the reactions for 15 minutes and the reactions were analyzed by EMSA as previously described. Reactions were performed in triplicate (representative gel is shown). The relevant Kr specific shift is indicated by an arrow and (B) was quantified using ImageQuant software. The higher, non-specific band (see Supporting Information Fig. 6) was left out of the analysis. Error bars are one standard deviation. Final reaction concentrations are given.
Co(III)-Ci reduces the activity of full length Ci
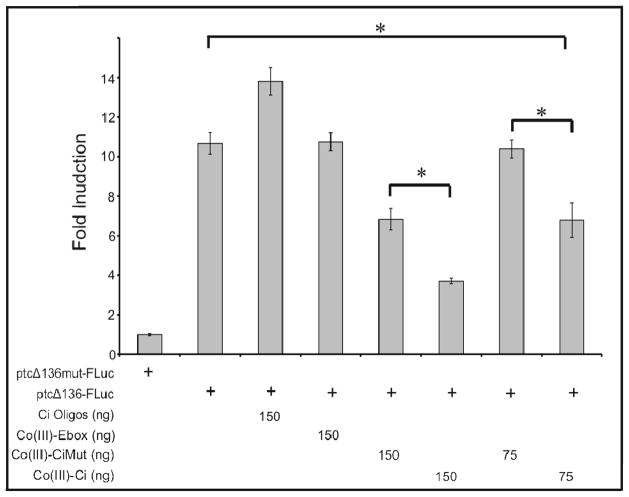
Co(III)-Ci irreversibly binds to Ci and prevents it from binding DNA. This action should abolish the transcriptional activity of full length Ci. To confirm this, we used a luciferase reporter gene assay in S2R+ cells to probe transcriptional activity of Ci. The ptc gene is an endogenous target of Hh signaling and fusing the ptc promoter to luciferase creates ptcΔ136-FLuc, a Hh responsive luciferase readout of Ci activity in cell culture 36, 37, 42.
S2R+ cells do not express Ci and do not have a Ci-dependent transcriptional response to Hh. However, they retain many of the intracellular pathway components that respond to Hh 43. To generate a constitutively-active luciferase response that is dependent on Ci, we overexpressed Ci(m1-4), a constitutively-active mutant form of Ci 36. Expression of Ci(m1-4) in the presence of ptcΔ136mut-FLuc, a ptc promoter with mutated Ci binding sites, had no effect on luciferase reporter gene activity (Figure 5A, lane 1). However, co-transfection of Ci(m1-4) with ptcΔ136- FLuc induced a significant increase in reporter expression as compared to either ptcΔ136-FLuc alone or ptcΔ136mut-FLuc + Ci(m1-4) (Figure 5A). Both ptcΔ136-FLuc and ptcΔ136mut-FLuc without Ci(m1-4) had similar basal levels of expression (Supporting Information Figure 7). Taken together, these results demonstrate that ptcΔ136-FLuc expression is dependent on the binding and activity of Ci(m1-4) in S2R+ cells.
Figure 5.
Co(III)-Ci prevents target gene activation by a full length, constitutively active Ci. S2R+ cells were transfected with a constitutively active form of Ci, Ci(m1-4) (all columns), either the Ci responsive ptcΔ136-Fluc luciferase vector (columns 2–8) or ptcΔ136mut-Fluc in which the Ci binding sites have been mutated (column 1), and 150ng of either Ci binding site oligos (column 3), Co(III)-Ebox (column 4), Co(III)-CiMut (column 5), Co(III)-Ci (column 6) or 75 ng of Co(III)-CiMut (column 7) or Co(III)-Ci (column 8). Cells were then incubated for 12 hours, lysed and assayed for luciferase activity. Experiments were performed in triplicate and error bars represent one standard deviation.
Co(III)-Ci irreversibly binds and inhibits Ci, attenuating Ci(m1-4) activation of ptcΔ-136-FLuc. Co-transfection of 150 ng Co(III)-Ci reduced the signal to 35% compared to buffer only (Figure 5, p < 0.005), whereas co-transfection of 150 ng Co(III)-Ebox, or Ci oligos did not result in significant reduction. Co-transfection of Co(III)-CiMut reduced the luciferase signal to 64% of buffer alone; however Co(III)-Ci reduction was significantly greater when compared to Co(III)- CiMut (p = 0.039). Co(III)-CiMut addition did not effect Ci(m1-4) activation of ptcΔ136-FLuc when the amount co-transfected was reduced to 75 ng, whereas 75 ng Co(III)-Ci was still able to significantly reduce the signal to 64% (p < 0.005).
Co(III)-Ci specifically inhibits Ci in vivo
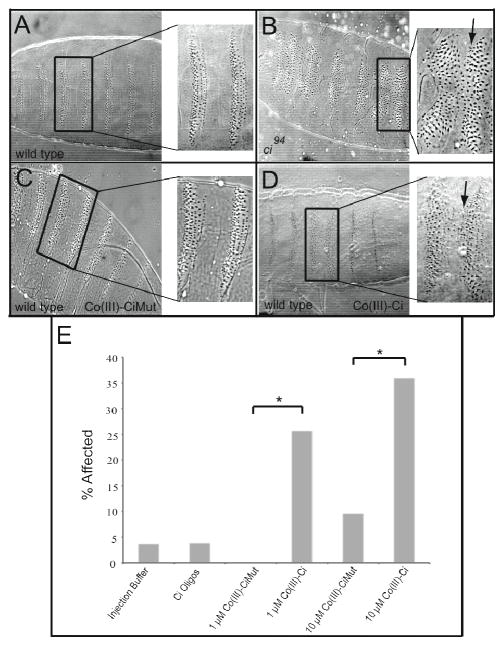
We have shown that Co(III)-Ci has high specificity for Ci and provided evidence for minimal off-target effects, however these results were observed in vitro. Ci plays a major role in Drosophila development and helps pattern the segmentally repeating denticle belts on a developing Drosophila embryo (Figure 6A) 32, 44, 45. Genetic removal of Ci in ci94 null mutant embryos causes a specific denticle patterning phenotype – the fusion of some of the denticle belts (Figure 6B, arrow). If Co(III)-Ci is able to bind specifically to Ci and inhibit Ci’s ability to bind DNA, we expect treatment of Drosophila embryos with Co(III)-Ci will phenocopy a ci null. This was investigated by injecting embryos with Co(III)-Ci at the syncytical blastoderm stage prior to cellularization and allowing the embryos to develop for 48 hours. Embryos were imaged and scored for Ci phenotypes. Injection of 1 μM Co(III)-Ci caused an elimination of naked cuticle and the merging of adjacent denticle belts, creating a localized Ci null phenotype (Figure 6D, arrow) in the region immediately surrounding the site of injection in 26% of embryos (8 of 31) whereas injection of 1 μM Co(III)-CiMut had no effect (0 of 26) (Figure 6C). Co(III)-Ci caused a statistically significant phenotype versus buffer alone (p = 0.03) and versus Co(III)- CiMut (p < 0.005) (Figure 6E).
Figure 6.
Injection of Co(III)-Ci but not Co(III)-CiMut is able to phenocopy loss of ci function in vivo. Drosophila cuticle mounts showing denticle belt patterning of 48 hour old (A) wild-type and (B) ci94 (null) embryos. Wild-type embryos between 0 and 45 minutes old were microinjected with 1 μM (C) Co(III)-CiMut or (D) Co(III)-Ci, allowed to develop for 48 hours, mounted and imaged by phase contrast light microscopy. Arrow points to denticle belt fusion characteristic of a ci mutant (B) and (D). (E) Wild-type Drosophila embryos were injected with buffer (column 1), Ci oligos (column 2), 1 μM Co(III)-CiMut (column 3), 1 μM Co(III)-Ci (column 4), 10 μM Co(III)-CiMut or 10 μM Co(III)-Ci and allowed develop and prepared as previously described. Bars represent the percent of embryos that showed a denticle belt fusion. No other phenotypes were seen. (* = p < 0.05) Injected concentrations are given.
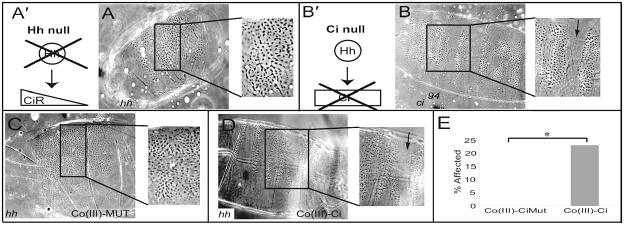
Embryos injected with Co(III)-Ci showed a localized Ci null phenotype. The question arises if this disruption in denticle belt patterning is due to specific inhibition of the Hh pathway, or could be explained by non-specific effects of Co(III)-Ci or perhaps even injection artifacts. To address this we turned to Hh null embryos. In hh null embryos, full length Ci is processed into the Ci repressor, which actively represses target genes, and consequentially, hh null embryos have a lawn of denticles on their ventral surface with no indication of segmentation, a phenotype much more severe than a ci null (Figure 7, A and B). Ci repressor is a truncation of full length Ci, with the same zinc finger domain, and binds the same DNA sequence as full length Ci and therefore should be targeted by Co(III)-Ci. Injection of Co(III)-Ci into a hh null mutant embryo should inactivate the Ci repressor, partially rescue the segmentation defect, and phenocopy a ci null. Injection of 1 μM Co(III)-Ci was indeed able to restore hh−/− embryos to a ci null phenotype (Figure 7C, 4 of 12 embryos) whereas 1 μM Co(III)-CiMut was not (Figure 7D, n = 18), a significant difference (Figure 7E, p = 0.018).
Figure 7.
Injection of Co(III)-Ci but not Co(III)-CiMut is able to partially rescue the effects of a genetic hh null in vivo. hh null embryos create Ci repressor (diagram A′) whereas a ci null will not (B′). These different genotypes result in distinct phenotypes. (A) Drosophila cuticle mounts showing the denticle belt patterning of 48 hour old (A) hh null and (B) ci94 (null) embryos. hh null embryos between 0 and 45 minutes old were microinjected with 1 μM (C) Co(III)-CiMut or (D) Co(III)-Ci, allowed to develop, mounted and imaged as previously. Arrow points to separation and patterning between denticle belts present in the ci null but never present in a hh null. (E) Percent of embryos that showed a rescue of denticle belt patterning. (* = p < 0.05)
Discussion
Coupling the Ci consensus binding sequence to Co(III)-sb resulted in a potent complex able to specifically inhibit Ci protein from binding DNA 7, 10. The Co(III)-Ci complex was able to inhibit Ci’s DNA significantly better than either Co(III)-sb or Co(III)-Ebox. Mutating a single base-pair in the oligo component of Co(III)-Ci abrogated its ability to inhibit binding. Co(III)-Ci effects were shown to be specific, as it was not able to inhibit the DNA binding ability of a highly related C2H2 zinc finger transcription factor, Krüppel, presumably due to differences in the DNA binding consensus sequence. These results demonstrate that very slight modification of the attached oligo can have profound effects on the targeting of a Co(III) Schiff base-DNA conjugate.
We have demonstrated the effectiveness of the Co(III)-Ci complex both in vitro and in vivo. Drosophila denticle patterning is a well-described process governed by a wide variety of proteins, many of which are C2H2 zinc finger proteins and altering the function of any of these results in a characteristic patterning defect 44. Injection of Co(III)-Ci showed a merging of the denticle belts at the site of injection, a localized phenocopy of a ci null. This patterning defect is distinct from what would be expected if Co(III)-Ci were inhibiting a different DNA binding protein, such as Even-skipped or Krüppel 44. At the highest concentration of Co(III)-Ci injected (10 uM), 36% of embryos showed a phenotype. We believe two major technical issues prevent us from increasing the percent affected. First, the injection location is critical yet precise injection remains technically difficult. Indeed, multiple preliminary injections not directly at the ventral surface failed to induce a phenotype. Second, the timing of the experiment adds another technical issue. Embryos must be injected prior to cellularization. Yet in wild type embryos, denticle belt patterning does not occur until stage 11, nearly 5 hours later 46. Therefore, Co(III)-Ci has the potential to nonspecifically interact with proteins for 5 hours before carrying out its specific function.
It remained possible that the phenotype could be explained by a localized lethality caused by the Co(III)-Ci complex. hh null embryos have a more severe patterning phenotype than ci null embryos, due to constitutive Ci repressor formation. Injection of hh null embryos with Co(III)- Ci should rescue the hh null to that of a ci null and indeed that is what we found. Were the phenotype due solely to lethality or injection artifacts we would not expect injection of Co(III)- Ci to improve the phenotype. These experiments indicate that injection of Co(III)-Ci specifically abrogates Ci function in vivo and does so in a manner dependent on the sequence of the oligo attached to the Co(III) Schiff base-DNA conjugate.
Previous studies have shown that the mechanism of Co(III) Schiff base-DNA conjugate inhibition of zinc fingers involve the replacement of the zinc(II) ion with Co(III)-sb through histidine coordination at the axial sites of the complex 7, 10. This in turn disrupts the structure of the C2H2 zinc finger and irreversibly inhibits the zinc finger from binding DNA 7. Co(III)-sb will non-specifically bind and inhibit all zinc finger containing proteins in vivo. Conjugation of a binding site DNA moiety to Co(III)-sb targets it towards a specific zinc finger protein that binds to the DNA. C2H2 zinc finger domains are particularly appropriate targets where the cobalt can displace the zinc(II) ion and collapse the zinc finger 10.
The exact molecular interaction between Co(III)-sb and the zinc finger domain and the mechanism of structural perturbation remains elusive. Mass spectrometry studies are not straightforward; in the gas phase and ionizing conditions, Co(III)-sb is unstable due to redox propensities of the metal center. We are actively studying the interaction at the molecular level with structural investigations including extensive NMR, circular dichroism, and tandem mass spectrometry analysis. Further, we are using model peptides to obtain thermodynamic and kinetic parameters of the Co(III)-sb/His interaction with and without a targeting domain. A complete understanding of the inhibitory mechanism will allow us to develop and tune the Co(III)-Ci probe.
While the Co(III) Schiff base-DNA conjugates bind in a sequence specific manner, only a fraction (36%) are irreversibly bound after three hours (Figure 2A). This result is consistent with what has been previously seen with Co(III) Schiff base-DNA conjugates 10. We believe this shows that the conjugate exhibits at least two binding modes. First, the attached oligo maintains a quick, reversible binding interaction to zinc finger domains, localizing the compound near the specific zinc finger domain. Second, we believe the Co(III)-sb portion of the conjugate retains a slower irreversible binding interaction with the zinc finger domain. It seems unlikely due to physical constraints the oligo and cobalt are simultaneously bound. The size and identity of the linker may be important and work is currently in progress investigating this as well as identifying the binding mode(s) of Co(III) Schiff base-DNA conjugates.
By altering the oligonucleotide sequence of the DNA targeting moiety in the Co(III) Schiff base-DNA conjugate, we have created an irreversible inhibitor of Ci. Utilizing their inhibitory action, Co(III) Schiff base-DNA conjugates can be applied as tools to the study the functions of zinc finger proteins. It should be possible to extend their range of application by using commercially available modifications to the ends of DNA, such as the attachment of a fluorophore or biotin. Addition of a fluorophore could provide for in vivo imaging opportunities. Further, addition of biotin would allow for affinity chromatography, western blots and other immunoanalytical methods. This work demonstrates Co(III) Schiff base-DNA conjugates represent a versatile class of specific and potent tools available for studying zinc finger domain proteins.
Supplementary Material
Acknowledgments
We are grateful to Dr. Richard Carthew (Northwestern University) and Dr. Sarah Smolik (Oregon Health & Science University) for providing DNA constructs. We thank Lisa Manus and Tulay Atesin for contribution to the synthesis and purification of Co(III)-Ci and Co(III)-CiMut. We thank Sara Hurtado and Dr. Vince Gerbasi for helpful discussion and technical advice. Finally we thank Maggie Sledd and Erin Schrader for their helpful advice on the manuscript. This work was supported by the National Institute of Health under Award P50CA090386 and the Center for Cancer Nanotechnology Excellence (CCNE) initiative of the National Institutes of Health’s National Cancer Institute under Award U54CA119341.
Footnotes
The supporting information contains detailed synthesis information for Co(III)-Ci and Co(III)- CiMut. It also contains control experiments verifying the identity of EMSA shifted bands CiZn and KrZn. Finally, it contains verification experiments PtcΔ136mut-Fluc is a Ci dependent luciferase readout of Ci activity. This information is available free of charge via the Internet at http://pubs.acs.org
References
- 1.Miller J, McLachlan AD, Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985;4(6):1609–14. doi: 10.1002/j.1460-2075.1985.tb03825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henikoff S, Greene EA, Pietrokovski S, Bork P, Attwood TK, Hood L. Gene families: the taxonomy of protein paralogs and chimeras. Science. 1997;278(5338):609–14. doi: 10.1126/science.278.5338.609. [DOI] [PubMed] [Google Scholar]
- 3.Berg JM, Shi Y. The galvanization of biology: a growing appreciation for the roles of zinc. Science. 1996;271(5252):1081–5. doi: 10.1126/science.271.5252.1081. [DOI] [PubMed] [Google Scholar]
- 4.Joho KE, Darby MK, Crawford ET, Brown DD. A finger protein structurally similar to TFIIIA that binds exclusively to 5S RNA in Xenopus. Cell. 1990;61(2):293–300. doi: 10.1016/0092-8674(90)90809-s. [DOI] [PubMed] [Google Scholar]
- 5.Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, Smith HO, Yandell M, Evans CA, Holt RA, Gocayne JD, Amanatides P, Ballew RM, Huson DH, Wortman JR, Zhang Q, Kodira CD, Zheng XH, Chen L, Skupski M, Subramanian G, Thomas PD, Zhang J, Gabor Miklos GL, Nelson C, Broder S, Clark AG, Nadeau J, McKusick VA, Zinder N, Levine AJ, Roberts RJ, Simon M, Slayman C, Hunkapiller M, Bolanos R, Delcher A, Dew I, Fasulo D, Flanigan M, Florea L, Halpern A, Hannenhalli S, Kravitz S, Levy S, Mobarry C, Reinert K, Remington K, Abu-Threideh J, Beasley E, Biddick K, Bonazzi V, Brandon R, Cargill M, Chandramouliswaran I, Charlab R, Chaturvedi K, Deng Z, Di Francesco V, Dunn P, Eilbeck K, Evangelista C, Gabrielian AE, Gan W, Ge W, Gong F, Gu Z, Guan P, Heiman TJ, Higgins ME, Ji RR, Ke Z, Ketchum KA, Lai Z, Lei Y, Li Z, Li J, Liang Y, Lin X, Lu F, Merkulov GV, Milshina N, Moore HM, Naik AK, Narayan VA, Neelam B, Nusskern D, Rusch DB, Salzberg S, Shao W, Shue B, Sun J, Wang Z, Wang A, Wang X, Wang J, Wei M, Wides R, Xiao C, Yan C, Yao A, Ye J, Zhan M, Zhang W, Zhang H, Zhao Q, Zheng L, Zhong F, Zhong W, Zhu S, Zhao S, Gilbert D, Baumhueter S, Spier G, Carter C, Cravchik A, Woodage T, Ali F, An H, Awe A, Baldwin D, Baden H, Barnstead M, Barrow I, Beeson K, Busam D, Carver A, Center A, Cheng ML, Curry L, Danaher S, Davenport L, Desilets R, Dietz S, Dodson K, Doup L, Ferriera S, Garg N, Gluecksmann A, Hart B, Haynes J, Haynes C, Heiner C, Hladun S, Hostin D, Houck J, Howland T, Ibegwam C, Johnson J, Kalush F, Kline L, Koduru S, Love A, Mann F, May D, McCawley S, McIntosh T, McMullen I, Moy M, Moy L, Murphy B, Nelson K, Pfannkoch C, Pratts E, Puri V, Qureshi H, Reardon M, Rodriguez R, Rogers YH, Romblad D, Ruhfel B, Scott R, Sitter C, Smallwood M, Stewart E, Strong R, Suh E, Thomas R, Tint NN, Tse S, Vech C, Wang G, Wetter J, Williams S, Williams M, Windsor S, Winn-Deen E, Wolfe K, Zaveri J, Zaveri K, Abril JF, Guigo R, Campbell MJ, Sjolander KV, Karlak B, Kejariwal A, Mi H, Lazareva B, Hatton T, Narechania A, Diemer K, Muruganujan A, Guo N, Sato S, Bafna V, Istrail S, Lippert R, Schwartz R, Walenz B, Yooseph S, Allen D, Basu A, Baxendale J, Blick L, Caminha M, Carnes-Stine J, Caulk P, Chiang YH, Coyne M, Dahlke C, Mays A, Dombroski M, Donnelly M, Ely D, Esparham S, Fosler C, Gire H, Glanowski S, Glasser K, Glodek A, Gorokhov M, Graham K, Gropman B, Harris M, Heil J, Henderson S, Hoover J, Jennings D, Jordan C, Jordan J, Kasha J, Kagan L, Kraft C, Levitsky A, Lewis M, Liu X, Lopez J, Ma D, Majoros W, McDaniel J, Murphy S, Newman M, Nguyen T, Nguyen N, Nodell M, Pan S, Peck J, Peterson M, Rowe W, Sanders R, Scott J, Simpson M, Smith T, Sprague A, Stockwell T, Turner R, Venter E, Wang M, Wen M, Wu D, Wu M, Xia A, Zandieh A, Zhu X. The sequence of the human genome. Science. 2001;291(5507):1304–51. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 6.Blum O, Haiek A, Cwikel D, Dori Z, Meade TJ, Gray HB. Isolation of a myoglobin molten globule by selective cobalt(III)-induced unfolding. Proc Natl Acad Sci U S A. 1998;95(12):6659–62. doi: 10.1073/pnas.95.12.6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louie AY, Meade TJ. A cobalt complex that selectively disrupts the structure and function of zinc fingers. Proc Natl Acad Sci U S A. 1998;95(12):6663–8. doi: 10.1073/pnas.95.12.6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takeuchi T, Bottcher A, Quezada CM, Meade TJ, Gray HB. Inhibition of thermolysin and human alpha-thrombin by cobalt(III) Schiff base complexes. Bioorg Med Chem. 1999;7(5):815–9. doi: 10.1016/s0968-0896(98)00272-7. [DOI] [PubMed] [Google Scholar]
- 9.Takeuchi T, Bottcher A, Quezada CM, Simon MI, Meade TJ, Gray HB. Selective inhibition of human alpha-thrombin by cobalt(III) Schiff base complexes. J Am Chem Soc. 1998;120(33):8555–8556. [Google Scholar]
- 10.Harney AS, Lee J, Manus LM, Wang P, Ballweg DM, LaBonne C, Meade TJ. Targeted inhibition of Snail family zinc finger transcription factors by oligonucleotide-Co(III) Schiff base conjugate. Proc Natl Acad Sci U S A. 2009;106(33):13667–72. doi: 10.1073/pnas.0906423106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pavletich NP, Pabo CO. Crystal structure of a five-finger GLI-DNA complex: new perspectives on zinc fingers. Science. 1993;261(5129):1701–7. doi: 10.1126/science.8378770. [DOI] [PubMed] [Google Scholar]
- 12.Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383(6599):407–13. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- 13.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15(23):3059–87. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 14.McMahon AP, Ingham PW, Tabin CJ. Developmental roles and clinical significance of hedgehog signaling. Curr Top Dev Biol. 2003;53:1–114. doi: 10.1016/s0070-2153(03)53002-2. [DOI] [PubMed] [Google Scholar]
- 15.Hooper JE, Scott MP. Communicating with Hedgehogs. Nat Rev Mol Cell Biol. 2005;6(4):306–17. doi: 10.1038/nrm1622. [DOI] [PubMed] [Google Scholar]
- 16.Kinzler KW, Bigner SH, Bigner DD, Trent JM, Law ML, O’Brien SJ, Wong AJ, Vogelstein B. Identification of an amplified, highly expressed gene in a human glioma. Science. 1987;236(4797):70–3. doi: 10.1126/science.3563490. [DOI] [PubMed] [Google Scholar]
- 17.Gailani MR, Stahle-Backdahl M, Leffell DJ, Glynn M, Zaphiropoulos PG, Pressman C, Unden AB, Dean M, Brash DE, Bale AE, Toftgard R. The role of the human homologue of Drosophila patched in sporadic basal cell carcinomas. Nat Genet. 1996;14(1):78–81. doi: 10.1038/ng0996-78. [DOI] [PubMed] [Google Scholar]
- 18.Goodrich LV, Milenkovic L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277(5329):1109–13. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- 19.Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, Marshall D, Fu L, Januario T, Kallop D, Nannini-Pepe M, Kotkow K, Marsters JC, Rubin LL, de Sauvage FJ. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455(7211):406–10. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 20.Fan L, Pepicelli CV, Dibble CC, Catbagan W, Zarycki JL, Laciak R, Gipp J, Shaw A, Lamm ML, Munoz A, Lipinski R, Thrasher JB, Bushman W. Hedgehog signaling promotes prostate xenograft tumor growth. Endocrinology. 2004;145(8):3961–70. doi: 10.1210/en.2004-0079. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Lipinski R, Shaw A, Gipp J, Bushman W. Lack of demonstrable autocrine hedgehog signaling in human prostate cancer cell lines. J Urol. 2007;177(3):1179–85. doi: 10.1016/j.juro.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 22.Shaw A, Bushman W. Hedgehog signaling in the prostate. J Urol. 2007;177(3):832–8. doi: 10.1016/j.juro.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 23.Rudin CM, Hann CL, Laterra J, Yauch RL, Callahan CA, Fu L, Holcomb T, Stinson J, Gould SE, Coleman B, LoRusso PM, Von Hoff DD, de Sauvage FJ, Low JA. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N Engl J Med. 2009;361(12):1173–8. doi: 10.1056/NEJMoa0902903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romer JT, Kimura H, Magdaleno S, Sasai K, Fuller C, Baines H, Connelly M, Stewart CF, Gould S, Rubin LL, Curran T. Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1(+/−)p53(−/−) mice. Cancer Cell. 2004;6(3):229–40. doi: 10.1016/j.ccr.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 25.Taylor MD, Liu L, Raffel C, Hui CC, Mainprize TG, Zhang X, Agatep R, Chiappa S, Gao L, Lowrance A, Hao A, Goldstein AM, Stavrou T, Scherer SW, Dura WT, Wainwright B, Squire JA, Rutka JT, Hogg D. Mutations in SUFU predispose to medulloblastoma. Nat Genet. 2002;31(3):306–10. doi: 10.1038/ng916. [DOI] [PubMed] [Google Scholar]
- 26.Lee Y, Kawagoe R, Sasai K, Li Y, Russell HR, Curran T, McKinnon PJ. Loss of suppressor-of-fused function promotes tumorigenesis. Oncogene. 2007;26(44):6442–7. doi: 10.1038/sj.onc.1210467. [DOI] [PubMed] [Google Scholar]
- 27.Yauch RL, Dijkgraaf GJ, Alicke B, Januario T, Ahn CP, Holcomb T, Pujara K, Stinson J, Callahan CA, Tang T, Bazan JF, Kan Z, Seshagiri S, Hann CL, Gould SE, Low JA, Rudin CM, de Sauvage FJ. Smoothened mutation confers resistance to a Hedgehog pathway inhibitor in medulloblastoma. Science. 2009;326(5952):572–4. doi: 10.1126/science.1179386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimura H, Ng JM, Curran T. Transient inhibition of the Hedgehog pathway in young mice causes permanent defects in bone structure. Cancer Cell. 2008;13(3):249–60. doi: 10.1016/j.ccr.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 29.Lauth M, Bergstrom A, Shimokawa T, Toftgard R. Inhibition of GLI-mediated transcription and tumor cell growth by small-molecule antagonists. Proc Natl Acad Sci U S A. 2007;104(20):8455–60. doi: 10.1073/pnas.0609699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Von Ohlen T, Hooper JE. Hedgehog signaling regulates transcription through Gli/Ci binding sites in the wingless enhancer. Mech Dev. 1997;68(1–2):149–56. doi: 10.1016/s0925-4773(97)00150-0. [DOI] [PubMed] [Google Scholar]
- 31.Kinzler KW, Vogelstein B. The GLI gene encodes a nuclear protein which binds specific sequences in the human genome. Mol Cell Biol. 1990;10(2):634–42. doi: 10.1128/mcb.10.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orenic TV, Slusarski DC, Kroll KL, Holmgren RA. Cloning and characterization of the segment polarity gene cubitus interruptus Dominant of Drosophila. Genes Dev. 1990;4(6):1053–67. doi: 10.1101/gad.4.6.1053. [DOI] [PubMed] [Google Scholar]
- 33.Vokes SA, Ji H, McCuine S, Tenzen T, Giles S, Zhong S, Longabaugh WJ, Davidson EH, Wong WH, McMahon AP. Genomic characterization of Gli-activator targets in sonic hedgehog-mediated neural patterning. Development. 2007;134(10):1977–89. doi: 10.1242/dev.001966. [DOI] [PubMed] [Google Scholar]
- 34.Hepker J, Wang QT, Motzny CK, Holmgren R, Orenic TV. Drosophila cubitus interruptus forms a negative feedback loop with patched and regulates expression of Hedgehog target genes. Development. 1997;124(2):549–58. doi: 10.1242/dev.124.2.549. [DOI] [PubMed] [Google Scholar]
- 35.Klueg KM, Alvarado D, Muskavitch MA, Duffy JB. Creation of a GAL4/UAS-coupled inducible gene expression system for use in Drosophila cultured cell lines. Genesis. 2002;34(1–2):119–22. doi: 10.1002/gene.10148. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y, Cardinaux JR, Goodman RH, Smolik SM. Mutants of cubitus interruptus that are independent of PKA regulation are independent of hedgehog signaling. Development. 1999;126(16):3607–16. doi: 10.1242/dev.126.16.3607. [DOI] [PubMed] [Google Scholar]
- 37.Fukumoto T, Watanabe-Fukunaga R, Fujisawa K, Nagata S, Fukunaga R. The fused protein kinase regulates Hedgehog-stimulated transcriptional activation in Drosophila Schneider 2 cells. J Biol Chem. 2001;276(42):38441–8. doi: 10.1074/jbc.M105871200. [DOI] [PubMed] [Google Scholar]
- 38.Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, Couto A, Marra V, Keleman K, Dickson BJ. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448(7150):151–6. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- 39.Kinzler KW, Ruppert JM, Bigner SH, Vogelstein B. The GLI gene is a member of the Kruppel family of zinc finger proteins. Nature. 1988;332(6162):371–4. doi: 10.1038/332371a0. [DOI] [PubMed] [Google Scholar]
- 40.Stanojevic D, Hoey T, Levine M. Sequence-specific DNA-binding activities of the gap proteins encoded by hunchback and Kruppel in Drosophila. Nature. 1989;341(6240):331–5. doi: 10.1038/341331a0. [DOI] [PubMed] [Google Scholar]
- 41.Sauer F, Jackle H. Concentration-dependent transcriptional activation or repression by Kruppel from a single binding site. Nature. 1991;353(6344):563–6. doi: 10.1038/353563a0. [DOI] [PubMed] [Google Scholar]
- 42.Chen CH, von Kessler DP, Park W, Wang B, Ma Y, Beachy PA. Nuclear trafficking of Cubitus interruptus in the transcriptional regulation of Hedgehog target gene expression. Cell. 1999;98(3):305–16. doi: 10.1016/s0092-8674(00)81960-1. [DOI] [PubMed] [Google Scholar]
- 43.Lum L, Zhang C, Oh S, Mann RK, von Kessler DP, Taipale J, Weis-Garcia F, Gong R, Wang B, Beachy PA. Hedgehog signal transduction via Smoothened association with a cytoplasmic complex scaffolded by the atypical kinesin, Costal-2. Mol Cell. 2003;12(5):1261–74. doi: 10.1016/s1097-2765(03)00426-x. [DOI] [PubMed] [Google Scholar]
- 44.Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287(5785):795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 45.Slusarski DC, Motzny CK, Holmgren R. Mutations that alter the timing and pattern of cubitus interruptus gene expression in Drosophila melanogaster. Genetics. 1995;139(1):229–40. doi: 10.1093/genetics/139.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alexandre C, Lecourtois M, Vincent J. Wingless and Hedgehog pattern Drosophila denticle belts by regulating the production of short-range signals. Development. 1999;126(24):5689–98. doi: 10.1242/dev.126.24.5689. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.