Abstract
BACKGROUND:
Data are limited on the impact of methicillin-resistant Staphylococcus aureus (MRSA) on morbidity and mortality among very low birth weight (VLBW) infants with S aureus (SA) bacteremia and/or meningitis (B/M).
METHODS:
Neonatal data for VLBW infants (birth weight 401–1500 g) born January 1, 2006, to December 31, 2008, who received care at centers of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network were collected prospectively. Early-onset (≤72 hours after birth) and late-onset (>72 hours) infections were defined by blood or cerebrospinal fluid cultures and antibiotic treatment of ≥5 days (or death <5 days with intent to treat). Outcomes were compared for infants with MRSA versus methicillin-susceptible S aureus (MSSA) B/M.
RESULTS:
Of 8444 infants who survived >3 days, 316 (3.7%) had SA B/M. Eighty-eight had MRSA (1% of all infants, 28% of infants with SA); 228 had MSSA (2.7% of all infants, 72% of infants with SA). No infant had both MRSA and MSSA B/M. Ninety-nine percent of MRSA infections were late-onset. The percent of infants with MRSA varied by center (P < .001) with 9 of 20 centers reporting no cases. Need for mechanical ventilation, diagnosis of respiratory distress syndrome, necrotizing enterocolitis, and other morbidities did not differ between infants with MRSA and MSSA. Mortality was high with both MRSA (23 of 88, 26%) and MSSA (55 of 228, 24%).
CONCLUSIONS:
Few VLBW infants had SA B/M. The 1% with MRSA had morbidity and mortality rates similar to infants with MSSA. Practices should provide equal focus on prevention and management of both MRSA and MSSA infections among VLBW infants.
KEY WORDS: Staphylococcus aureus, methicillin resistant, infant, newborn
What’s Known on This Subject:
There is a perception among clinicians that methicillin-resistant Staphylococcus aureus (MRSA) bacteremia and/or meningitis result in a greater burden of disease than invasive infections attributed to methicillin-susceptible Staphylococcus aureus (MSSA) among very low birth weight (VLBW) infants.
What This Study Adds:
VLBW infants with MRSA and MSSA bacteremia and/or meningitis have equivalent morbidity and mortality. These findings suggest that allocation of resources for prevention and treatment of both MRSA and MSSA infections among VLBW infants should be comparable.
Contact with environmental sources and endogenous colonization with Staphylococcus aureus (SA) may result in life-threatening infections. Among preterm neonates, invasive staphylococcal infections result in substantial morbidity and mortality.1,2 Very low birth weight (VLBW) infants have an increased risk of infection due to exposure to nosocomial pathogens during extended hospitalizations. Most publications highlighting the epidemiology of infections with methicillin-resistant S aureus (MRSA) and methicillin-sensitive S aureus (MSSA) among VLBW neonates have described clusters of infections attributed to nosocomial outbreaks.3–6 There is a perception among clinicians and policy makers that a greater severity of illness results from MRSA compared with MSSA infections. As a result, infection control policies at many centers mandate screening and isolation for individuals colonized or infected with MRSA but not for those colonized or infected with MSSA.7 We hypothesized that among VLBW infants hospitalized at academic centers of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network (NRN) morbidity and mortality among those with bacteremia and/or meningitis (B/M) would be similar for those infected with MRSA and MSSA.
Methods
Population and Clinical Outcomes
We studied VLBW infants (401–1500 g birth weight [BW]) born between January 1, 2006, and December 31, 2008, who were cared for at 1 of the 20 study centers participating in the NICHD NRN and included in a registry of VLBW infants maintained by the NRN. We limited this analysis to infants who survived >3 days, a cohort surviving long enough to develop late-onset culture-proven B/M. Infections were defined by the isolation of a bacterial or fungal organism from blood or cerebrospinal fluid (CSF) cultures obtained ≤72 hours (early-onset) or >72 hours (late-onset) after birth and antimicrobial treatment of ≥5 days or death <5 days with intent to treat.1,8,9 All VLBW infants born 2006–2007 who were admitted to NRN centers before 14 days of age (inborn and outborn) were eligible for the registry. Eligibility was limited in January 2008 to inborn infants with BW 401–1000 g or gestational age (GA) 22 through 28 completed weeks or infants enrolled in another NRN study to focus on the most vulnerable preterm infants.
Trained research personnel prospectively collected maternal and infant data from birth until hospital discharge, death, or 120 days. Neonatal information included BW, GA, gender, race, ethnicity, mode of delivery, final status (discharged or died), and cause of death, if applicable. Neonatal morbidities included respiratory distress syndrome (RDS), patent ductus arteriosus, necrotizing enterocolitis (NEC), intraventricular hemorrhage (IVH), periventricular leukomalacia (PVL), retinopathy of prematurity (ROP), and bronchopulmonary dysplasia (BPD). Each of the participating NRN study centers was queried regarding surveillance screening for MRSA and infection control practices related to SA at their nurseries during the study period.
Growth charts10 were used to classify infants as small for GA (SGA) at birth, defined by a BW <10th percentile for gender and GA. Neonates were considered to have RDS if they required oxygen or positive pressure support for >6 hours within the first 24 hours after birth. Infants were given a diagnosis of NEC based on the modified Bell’s criteria (Stage IIA or greater).11,12 IVH grade was defined based on the most severe findings on a cranial sonogram obtained within 28 days of birth by using the criteria of Papile13; grades 3 and 4 were considered severe. PVL was determined by cranial sonogram obtained within the first 28 days after birth or an imaging study performed after 28 days and closest to 36 weeks’ postmenstrual age (PMA). ROP was defined for infants hospitalized at 28 days of age who underwent an ophthalmologic examination. BPD was defined as the need for supplemental oxygen at 36 weeks’ PMA and was not defined for infants who died before 36 weeks’ PMA.
Participation in the VLBW registry was approved by the institutional review boards at each of the participating centers. Informed consent was obtained from participants at 3 centers; a waiver of consent was granted by the institutional review boards at the other centers.
Statistical Analysis
Infection rates were expressed as the proportion of infants infected during their birth hospitalization and as the number of infants infected per 1000 hospital days. Statistical significance for unadjusted comparisons was determined by Fisher’s exact or χ2 tests for categorical variables and the Kruskal-Wallis test for continuous variables. Kaplan-Meier survival curves were used to estimate median length of hospital stay (time from birth to discharge with deaths treated as censored observations) with statistical significance between groups determined by the log-rank test.
Baseline risk factors for MRSA and for MSSA were examined among all infants by using Poisson regression. Separate models were fit to each binary outcome. Risk factors for MRSA were also examined in the subgroup of infants with SA B/M. Because of the small number of MRSA and MSSA cases at some centers, study center could not be included as a fixed effect and was instead treated as a random effect in these models. Poisson regression models with robust variance estimators14 and fixed effects only were used to examine the risk of morbidities and mortality for infants with MRSA compared with infants with MSSA. Models examining outcomes for infants with SA were based on data from all infants and included an infection group indicator (MRSA B/M, MSSA B/M, non-SA B/M, no B/M), which allowed for estimation of relative risks (RRs) comparing infants with MRSA and MSSA. Morbidity RRs were adjusted for GA, male gender, race/ethnicity, and study center. Adjusted RRs, 95% confidence intervals (CIs), and F, Score, or Wald χ2 tests were reported based on parameter and variance estimates from each model.
Results
Study Population
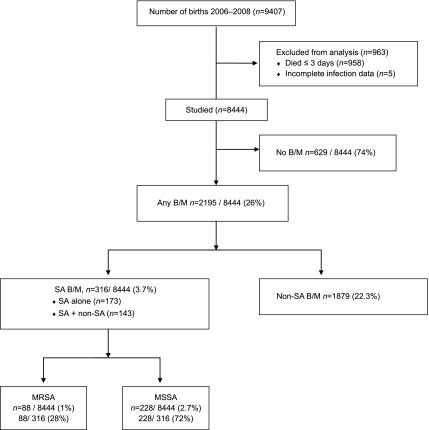
Between January 1, 2006, and December 31, 2008, 9407 infants with BWs 401 to 1500 g were born and/or admitted to 1 of 20 NRN study centers. Of these, 730 (8%) died within 12 hours of birth and 228 (2%) died between 12 hours and 3 days after birth; no B/M due to SA was reported in these infants. After excluding the 958 infants who died in the first 3 days and 5 infants with incomplete infection information, 8444 infants were included in the analysis (Fig 1). Median length of hospitalization was 68 days overall (25th–75th percentiles: 46–97). During the hospital stay, 2195 (26%) infants had B/M due to any pathogen. The majority, 2052 (24% of all infants), had late-onset infections alone, 103 (1.2%) had early-onset infections alone, and 40 (0.5%) had both early- and late-onset infections. Most infants with B/M were of lower GA and were hospitalized longer than those without infections (median 101 days versus 60 days, P < .001).
FIGURE 1.
VLBW cohort characteristics and rates of SA.
Rate of SA B/M
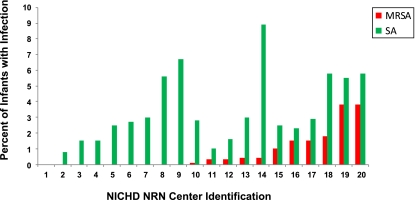
B/M due to SA was diagnosed in 316 (3.7%) infants (Fig 1). Of these, 173 infants had 1 or more episodes of B/M due to SA (97% had 1 episode) and no episodes of B/M due to other pathogens, and 143 had B/M attributed to both SA and non-SA pathogens (multiple episodes and/or at least 1 polymicrobial episode involving SA and non-SA pathogens; Table 1). One center that participated in the NRN for only 3 months of the study period reported no infants with SA. Rates of SA ranged from 0.8% to 8.9% across the other centers, P < .001 (Fig 2). Of those infected with SA B/M, 311 (98%) had late-onset SA B/M only, 4 (1%) had early-onset SA B/M only, and 1 infant had both early- and late-onset SA B/M. SA was isolated from blood of 5 infants with early-onset infections, from blood alone for 301 of the late-onset infections, from blood and CSF for 8, and from CSF alone for 3 late-onset SA infections.
TABLE 1.
Rates of SA B/M Among VLBW Infants Who Survived >3 Days, Overall and by BW: NICHD NRN 2006–2008
| Infants With SA B/M Onlya | Infants With Both SA and Non-SA B/Ma | Infants With MRSA B/Ma | Infants With MSSA B/Ma | Total Infants or HDsa,b | |
|---|---|---|---|---|---|
| Overall | |||||
| N infants with B/M (%) | 173 (2.0) | 143 (1.7) | 88 (1.0) | 228 (2.7) | 8444 |
| Infants infected/1000 HDsc | 0.29 | 0.24 | 0.15 | 0.38 | 596 442 |
| BW 401–750 g | |||||
| N infants with B/M (%) | 76 (4.1) | 63 (3.4) | 39 (2.1) | 100 (5.4) | 1854 |
| Infants infected/1000 HDs | 0.44 | 0.37 | 0.23 | 0.58 | 172 038 |
| BW 751–1000 g | |||||
| N infants with B/M (%) | 50 (2.2) | 53 (2.3) | 33 (1.4) | 70 (3.1) | 2295 |
| Infants infected/1000 HDs | 0.26 | 0.27 | 0.17 | 0.36 | 193 136 |
| BW 1001–1250 g | |||||
| N infants with B/M (%) | 36 (1.7) | 19 (0.9) | 8 (0.4) | 47 (2.2) | 2122 |
| Infants infected/1000 HDs | 0.27 | 0.14 | 0.06 | 0.35 | 133 724 |
| BW 1251–1500 g | |||||
| N infants with B/M (%) | 11 (0.5) | 8 (0.4) | 8 (0.4) | 11 (0.5) | 2173 |
| Infants infected/1000 HDs | 0.11 | 0.08 | 0.08 | 0.11 | 97 544 |
Numbers of infants infected and total HDs are listed to facilitate calculation of additional rates.
HD, hospital day.
Rates of SA are listed per 1000 HDs.
FIGURE 2.
Rates of MRSA and SA 2006–2008 by the NRN Center. Center 1 participated in the NRN for only 3 months during the study period.
Rates of MRSA and MSSA B/M
Eighty-eight infants had MRSA B/M, representing 1% of all infants or a rate of 0.15 infants infected per 1000 hospital days (Table 1) and 28% of those with SA (Fig 1); 228 infants had MSSA B/M, 2.7% of all infants or a rate of 0.38 infected per 1000 hospital days. No infant had both MRSA and MSSA infections. No infants with MRSA were reported from 8 centers with SA infections. The remaining 11 centers with SA infections reported MRSA among 0.1% to 3.8% of all infants at their center (P < .001) or 3.8% to 69% of the infants with SA (Fig 2).
Risk of MRSA and MSSA B/M
The risk of B/M due to either MRSA or MSSA increased with decreasing BW and GA, P < .001 (Tables 1 and 2). Among all infants who survived >3 days, infants born at <25 weeks’ GA had over 5 times the risk of having either type of B/M compared with infants born at ≥29 weeks (adjusted RR for MRSA: 5.60, 95% CI: 2.80–11.18; adjusted RR for MSSA: 6.24, 95% CI: 3.94–9.91). Infants born between 25 and 28 weeks had over 3 times the risk for either infection. After accounting for center variation in MRSA rates, differences in risk of MRSA B/M were not found for infants with the other baseline characteristics examined. Among 8246 infants with available information, no association was detected between the type of maternal antibiotic given within 72 hours before delivery, including ampicillin, erythromycin, and penicillin, and risk of infant MRSA B/M. Statistically significant reductions in risk of MSSA B/M were observed in infants whose mothers had chorioamnionitis recorded in the medical record and in those infants whose mothers received antibiotics before their delivery. Potential risk factors for MRSA were also examined in the subset of 316 infants with SA B/M. Differences in the risk of MRSA compared with MSSA were not found by GA in this subset and were not detected by infant gender, race/ethnicity, congenital birth defect status, maternal steroid or antibiotic use (adjusted RR: 1.28, 95% CI: 0.76–2.16, P = .3), or maternal chorioamnionitis.
TABLE 2.
Risk of MRSA and MSSA B/M Among 8444 VLBW Infants Who Survived >3 Days: NICHD NRN 2006–2008
| Characteristicsa | Total No. | Infants With MRSA, No. (%) | Adjusted RR for MRSA (95% CI)b | Infants With MSSA, No. (%) | Adjusted RR for MSSA (95% CI)b |
|---|---|---|---|---|---|
| Maternal antibiotic use during delivery admission | |||||
| Yes | 4916 | 56 (1.1) | 0.94 (0.58–1.53) | 118 (2.4) | 0.65 (0.49–0.87)d |
| No | 3395 | 32 (0.9) | Referencec | 106 (3.1) | Reference |
| Maternal steroid use before delivery | |||||
| Yes | 6806 | 74 (1.1) | 1.38 (0.72–2.64) | 187 (2.7) | 1.37 (0.95–1.99) |
| No | 1566 | 12 (0.8) | Reference | 41 (2.6) | Reference |
| Chorioamnionitis noted in mother’s medical record | |||||
| Yes | 1028 | 7 (0.7) | 0.50 (0.23–1.11) | 11 (1.1) | 0.31 (0.17–0.57)e |
| No | 7313 | 81 (1.1) | Reference | 216 (3.0) | Reference |
| Infant gender | |||||
| Boy | 4219 | 45 (1.1) | 1.05 (0.69–1.61) | 127 (3.0) | 1.21 (0.93–1.57) |
| Girl | 4225 | 43 (1.0) | Reference | 101 (2.4) | Reference |
| GA (wk) | |||||
| <25 | 1027 | 23 (2.2) | 5.60 (2.80–11.18)e | 52 (5.1) | 6.24 (3.94–9.91)e |
| 25–28 | 4162 | 52 (1.3) | 3.20 (1.73–5.93) | 145 (3.5) | 4.17 (2.80–6.20) |
| 29+ | 3253 | 13 (0.4) | Reference | 31 (1.0) | Reference |
| Race/ethnicity | |||||
| Non-Hispanic black | 3070 | 49 (1.6) | 1.08 (0.65–1.78) | 85 (2.8) | 1.17 (0.85–1.63) |
| Hispanic | 1518 | 9 (0.6) | 1·01 (0.45–2.27) | 48 (3.2) | 1.16 (0.79–1.70) |
| Other | 434 | 2 (0.5) | 0.84 (0.19–3.68) | 10 (2.3) | 1.03 (0.52–2.02) |
| Non-Hispanic white | 3391 | 28 (0.8) | Reference | 85 (2.5) | Reference |
| Congenital birth defect | |||||
| Yes | 395 | 5 (1.3) | 1.59 (0.64–3.95) | 11 (2.8) | 1.52 (0.83–2.80) |
| No | 8049 | 83 (1.0) | Reference | 217 (2.7) | Reference |
Information was missing for maternal antibiotic use: 133 infants; maternal steroid use: 72; chorioamnionitis: 103; GA: 2; race/ethnicity: 31.
RRs, CIs, and P values by the F test from Poisson regression models that included the variables shown and used data from the full cohort. One model was fit to the binary outcome MRSA, yes/no (for RRs associated with MRSA), and another to the binary outcome MSSA, yes/no (for RRs associated with MSSA). Variation between study centers was accounted for by treating center as a random effect.
All instances of reference refer to the comparison group.
P ≤ .01.
P ≤ .001.
Characteristics of Infants With MRSA and MSSA
The median BWs and GAs of infants with MRSA and MSSA B/M were similar (Table 3). A higher proportion of infants with MRSA were non-Hispanic black compared with infants with MSSA (56% vs 37%, P = .02). Infant race/ethnicity differed at the study centers, with large numbers of African American infants at the 2 centers with highest MRSA B/M rates. At the 3 centers with the largest number of cases, MRSA rates were similar among African American and white infants. After controlling for study center, differences in race/ethnicity were no longer found, P = .9, indicating that center and race/ethnicity were confounded in the cohort. Statistically significant differences were not found between the infants with MRSA and MSSA on the other characteristics examined (Table 3).
TABLE 3.
Maternal and Neonatal Characteristics of VLBW Infants Who Survived >3 Days: Infants With MRSA Compared With Infants With MSSA B/M, NICHD NRN 2006–2008
| Characteristica | MRSA N = 88 | MSSA N = 228 | Pb |
|---|---|---|---|
| Mother’s age, y | |||
| Median (25th–75th percentile) | 25 (22–32) | 26 (21–32) | 0.7 |
| Antenatal antibiotic use during delivery admission, n (%) | 56 (64) | 118 (53) | 0.1 |
| Antenatal steroid use before delivery, n (%) | 74 (86) | 187 (82) | 0.5 |
| Chorioamnionitis noted in mother’s medical record, n (%) | 7 (8) | 11 (5) | 0.3 |
| Placental pathology performed, n (%) | 72 (83) | 163 (74) | 0.1 |
| Histologic chorioamnionitis,c n (%) | 22 (31) | 47 (29) | 0.8 |
| Cesarean section delivery, n (%) | 65 (74) | 159 (70) | 0.5 |
| Multiple births, n (%) | 24 (27) | 52 (23) | 0.5 |
| BW, g | |||
| Median (25th–75th percentile) | 780 (636–935) | 783 (646–1009) | 0.6 |
| Category, n (%) | |||
| 401–750 | 39 (44) | 100 (44) | |
| 751–1000 | 33 (38) | 70 (31) | |
| 1001–1250 | 8 (9) | 47 (21) | |
| 1251–1500 | 8 (9) | 11 (5) | |
| GA, wk | |||
| Median (25th–75th percentile) | 26 (24–28) | 26 (25–28) | 0.7 |
| Category, n (%) | |||
| <25 | 23 (26) | 52 (23) | |
| 25–28 | 52 (59) | 145 (64) | |
| 29+ | 13 (15) | 31 (14) | |
| SGA, n (%) | 14 (16) | 38 (17) | 1.0 |
| Boy, n (%) | 45 (51) | 127 (56) | 0.5 |
| Race/ethnicity, n (%) | 0.02d | ||
| Non-Hispanic black | 49 (56) | 85 (37) | |
| Non-Hispanic white | 28 (32) | 85 (37) | |
| Hispanic | 9 (10) | 48 (21) | |
| Other | 2 (2) | 10 (4) | |
| Major birth defect, n (%) | 5 (6) | 11 (5) | 0.8 |
| Total episodes of B/M,e n (%) | |||
| 1 | 52 (59) | 156 (68) | 0.1 |
| 2 | 26 (30) | 54 (24) | |
| 3 or more | 10 (11) | 18 (8) | |
| Any non-SA B/M,f n (%) | 45 (51) | 98 (43) | 0.3 |
| Timing of SA B/M, n (%) | 0.8 | ||
| Early onset | 1 (1) | 3 (1) | |
| Late onset | 87 (99) | 224 (98) | |
| Both early and late | 0 (0) | 1 (<1) | |
| SA infection site, n (%) | 0.1 | ||
| Blood | 82 (93) | 223 (98) | |
| CSF | 2 (2) | 1 (<1) | |
| Both blood and CSF | 4 (5) | 4 (2) |
Median values or the number and percent of infants with MRSA and of infants with MSSA who had each characteristic are shown.
Information was missing for antenatal antibiotics: from the mothers of 4 infants; antenatal steroids: 2; chorioamnionitis: 1; placental pathology performed: 8; histologic chorioamnionitis: 3.
P value for a test between infants with MRSA versus infants with MSSA by the Kruskal-Wallis test (median mother’s age, BW, GA), Fisher’s exact test, the row mean score χ2 test (number of episodes), or the general association χ2 test.
Percents are among infants with placental pathology performed.
P = .9 by the Cochran-Mantel-Haenszel χ2 test after controlling for study center.
Episodes were defined by positive cultures taken 5 or more days apart. Positive cultures taken within 4 days were considered indicative of the same episode. The 316 infants with SA had a total of 463 episodes of B/M.
B/M due to a pathogen other than SA on 1 or more episodes separate from B/M due to SA and/or a polymicrobial infection involving SA and a non-SA pathogen. Polymicrobial infections were reported for 73 infants (76 episodes).
One infant with MRSA had early-onset B/M; the remaining 87 infants (99%) had late-onset B/M. MRSA was isolated from blood and CSF of 4 infants (5%), from blood alone in 82 infants (93%), and from CSF alone in 2 infants (2%; Table 3). Of the 87 infants with late-onset B/M, 76 (87%) had 1 episode of MRSA B/M only, whereas 11 (13%) had 2 episodes; none had more than 2. Most infants with MSSA had late-onset B/M: 224 (98%) had late-onset only, 3 (1%) had early-onset only, and 1 infant (<1%) had both early- and late-onset MSSA B/M. MRSA caused the first B/M episode for 70 of the 88 infants (80%), and MSSA caused the first B/M for 193 of 228 infants (85%). Age at first SA B/M was similar in the 2 groups, P = .7 (median age, 25th–75th percentile, MRSA: 23 days, 11–46 days; MSSA: 21 days, 13–35 days).
Morbidity Among Infants With MRSA and MSSA
Statistically significant differences were not found between infants with MRSA compared with MSSA with regard to ventilator use, feeding outcomes, or any of the in-hospital morbidities examined, including RDS, patent ductus arteriosus, IVH, PVL, NEC, BPD, or ROP (data not shown).
Mortality, Survival, and Length of Hospitalization
Among the 88 infants with MRSA B/M, 64 (73%) were discharged from the hospital, 1 (1%) was hospitalized at 1 year of age, and 23 died (26%). Final status was similar for the 228 infants with MSSA, of whom 172 (75%) were discharged from the hospital, 1 (0.4%) was hospitalized at 1 year of age, and 55 (24%) died. No statistically significant differences in in-hospital mortality were found for infants with MRSA compared with MSSA B/M when stratified by BW (Table 4). In-hospital mortality was higher for VLBW infants with SA B/M (78 of 316, 25%) than for VLBW infants with no B/M (555 of 6249, 9%; P < .001) but similar to mortality among infants with non-SA B/M (404 of 1879, 22%; P = .21). Coded cause of death for infants with SA included infection for 16 of 23 (70%) infants with MRSA and for 42 of 55 (76%) of those with MSSA. The proportions of infants who died within 1, 3, and 7 days of the first culture with growth of SA was similar for infants with MRSA and MSSA (1 day: 5 of 88 [6%] vs 13 of 228 [6%], P = 1.0; 3 days: 7 of 88 [8%] vs 22 of 228 [10%], P = .8; 7 days: 10 of 88 [11%] vs 31 of 228 [14%], P = .7). Infection was the coded cause of death in 6 of 7 (86%) infants who died within 3 days of a culture with MRSA and in 20 of 22 (91%) of infants with MSSA. Length of hospitalization was similar for infants with MRSA and MSSA B/M (median, 25th–75th percentile, MRSA: 100 days, 79–152; MSSA: 108 days, 84–139, P = .63).
TABLE 4.
Mortality and Survival to Hospital Discharge Among VLBW Infants Who Survived >3 Days: Infants With MRSA Compared With Infants With MSSA B/M, NICHD NRN 2006–2008
| N (%) | MRSA | MSSA | Adjusted RR for Death, MRSA Versus MSSA (95% CI)a |
|---|---|---|---|
| Overall | |||
| Number of infants | 88 | 228 | |
| Survived | 65 (74) | 173 (76) | |
| Died | 23 (26) | 55 (24) | 0.96 (0.63–1.46) |
| 401–750 g BW | |||
| Number of infants | 39 | 100 | |
| Survived | 25 (64) | 66 (66) | |
| Died | 14 (36) | 34 (34) | 0.96 (0.58–1.59) |
| 751–1000 g BW | |||
| Number of infants | 33 | 70 | |
| Survived | 27 (82) | 56 (80) | |
| Died | 6 (18) | 14 (20) | 0.81 (0.34–1.91) |
| 1001–1250 g BW | |||
| Number of infants | 8 | 47 | |
| Survived | 7 (88) | 42 (89) | |
| Died | 1 (13) | 5 (11) | 1.09 (0.16–7.23) |
| 1251–1500 g BW | |||
| Number of infants | 8 | 11 | |
| Survived | 6 (75) | 9 (82) | |
| Died | 2 (25) | 2 (18) | 1.22 (0.23–6.47) |
RR and CI from a modified Poisson regression model utilizing data from all infants in the cohort (N = 8444). Covariates included study center, GA (<25, 25–28, 29+ weeks), BW (401–750, 751–1000, 1001–1250, 1251–1500), boy, race/ethnicity (non-Hispanic black, non-Hispanic white, Hispanic, other), and an infection group indicator (MRSA B/M, MSSA B/M, non-SA B/M, no B/M). RRs and CIs in each BW group were from a model that included the interaction between BW and infection group in addition to the covariates listed. No RRs were significantly different from 1.0.
MRSA Surveillance Screening at NRN
Of the 16 NRN centers for which information was available, 12 (75%) conducted surveillance screening for MRSA during the study period. All 16 centers reported infants with SA B/M, but the proportion of infants with SA B/M was generally higher at the 12 centers that conducted MRSA surveillance screening than at the 4 that did not (4.1% vs 2.8%, P = .01). However, the percent of MRSA among infants with SA B/M varied widely across centers, regardless of screening practices. Among the 12 NRN centers that conducted MRSA surveillance screening, 3 had no cases of MRSA B/M, and the proportion of infants infected with SA who had MRSA ranged from 3.8% to 69% at the other 9 centers. Among the 4 NRN centers that did not report MRSA surveillance, 2 had no cases of MRSA B/M, and the percent of MRSA among infants with SA B/M was ∼20% to 30% at the other 2 centers. Reports from all 16 NRN centers indicated that infants identified as infected with MRSA were placed on contact precautions; only 2 centers indicated that MSSA infected infants were isolated. Fourteen of 16 centers placed infants found to be colonized but not infected with MRSA in contact precautions, but only 1 center isolated those colonized with MSSA.
Discussion
Staphylococcal organisms are an uncommon yet significant cause of infection among VLBW infants, accounting for notable mortality and morbidity. Although fewer than 4% of the 8444 VLBW neonates in this cohort developed culture proven SA B/M, one-quarter of those with MRSA and MSSA B/M died. Despite the rare occurrence of meningitis without bacteremia in this clinical cohort, the 3 cases identified remind clinicians that isolated central nervous system manifestations of MRSA and MSSA do exist. Consistent with other reports, extreme prematurity and low BW were the greatest risk factors for both MRSA and MSSA B/M.1,8 The prolonged length of stay associated with extreme preterm birth likely increased the opportunity for acquisition of a late-onset infection. Comparing the characteristics of infants with MRSA and MSSA B/M both with and without assessing risk yielded similar results in both analyses. Including only VLBW infants with blood or CSF culture proven infection likely under represents the true burden of disease from SA, as infections resulting from skin and soft tissue abscesses, surgical wounds, pneumonia, and metastatic dissemination that were not accompanied by isolation of SA from blood or CSF were not included in our analyses.
The use of intrapartum antibiotic prophylaxis has been assessed in relationship to the rising rates of antimicrobial resistance.15,16 These evaluations have not demonstrated a direct relationship between the use of intrapartum prophylaxis and the emergence of resistant organisms.17–21 As MRSA has gained notoriety, the impact of resistant gram-positive infections in the neonate has been assessed.22,23 In our cohort of VLBW neonates who survived >3 days, we did not detect an increased risk of MRSA among infants whose mothers received intrapartum antibiotics compared with those whose mothers did not. As expected, maternal chorioamnionitis was not found to be a risk factor for the predominantly late-onset SA B/M infections.
The proportion of infants diagnosed with SA B/M varied across the NRN study centers, with no MRSA cases at some centers and with more than half of the SA B/M resistant at others. There was no evidence of temporal clustering or outbreaks of MRSA infections. Surveillance screening for MRSA was conducted at a majority of centers; information regarding legal mandates and screening practices was not available. Isolation practices after identification of infants infected or colonized with MRSA or MSSA varied. Our finding that the proportion of infants with SA B/M was generally higher at the centers that conducted MRSA surveillance screening compared with those that did not may suggest that surveillance screening is less efficacious in preventing SA B/M. Furthermore, this supports the important contribution of MSSA to SA B/M. Notably, only 2 of the 16 centers with available information reported placing infants infected with MSSA on contact precautions, and only 1 center isolated infants colonized with MSSA, consistent with the perception that MSSA infections are responsible for a lower burden of disease than MRSA infections. Future investigations should examine surveillance and isolation practices in relation to baseline rates of MRSA and MSSA infections at individual centers to understand and optimize best practices.
Although MRSA is perceived as a pathogen of greater consequence, our findings suggest that MSSA is a pathogen of similar significance with a comparable severity of illness in this cohort of VLBW infants. These results support reconsideration of isolation policies and practices among VLBW infants that have traditionally focused only on MRSA and broadening their scope to include MSSA.
Our ability to draw conclusions about early-onset SA B/M was limited by the small number of infants who fit this classification. Centers did not collect vaginal or rectal surveillance swabs from mothers at the time of birth. Colonization status of infants before the development of infection was also not collected. Information regarding antimicrobial susceptibility and molecular characteristics of the SA isolates and antimicrobial management of the infants was not available and therefore could not be included in our analyses. Consistent with other reports of late-onset sepsis, coagulase-negative Staphylococcus (CoNS) was the most frequent non-SA pathogen isolated. Klebsiella spp, Enterobacter spp, and Serratia spp were the most frequent gram-negative isolates, and Candida albicans was the most frequently isolated fungal organism. We were unable to correct rates of infections for catheter-days because we did not have access to information regarding vascular catheter-associated practices and utilization from each of the sites. The presence of CSF shunts and the characteristics of CSF collected were similarly unavailable.
Conclusions
There is a perception that MRSA infections result in greater morbidity and mortality than infections with MSSA. However, we found no significant differences in the risk for morbidity or mortality during the birth hospitalization of VLBW infants with MRSA compared with those with MSSA B/M. Overall mortality was high for VLBW infants with MRSA and MSSA B/M (∼25%), including death within 7 days after isolation of SA (11% and 14% for MRSA and MSSA, respectively). Continued surveillance to monitor the rates of MRSA and MSSA B/M provides an appreciation of the clinical significance of staphylococcal sepsis in extremely premature neonates.
Acknowledgments
Data collected at participating sites of the NICHD NRN were transmitted to RTI International, the data coordinating center (DCC) for the network, which stored, managed, and analyzed the data for this study. On behalf of the NRN, Dr Abhik Das (DCC principal investigator) and Ms. Nellie Hansen (DCC statistician) had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
The following investigators, in addition to those listed as authors, participated in this study: NRN Steering Committee Chair: Michael S. Caplan, MD, University of Chicago, Pritzker School of Medicine; Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island (U10 HD27904): William Oh, MD, Angelita M. Hensman, RN, BSN, Dawn Andrews, RN, MS, and Kristen Angela, RN; Case Western Reserve University, Rainbow Babies & Children’s Hospital (U10 HD21364, M01 RR80): Avroy A. Fanaroff, MD, and Bonnie S. Siner, RN; Cincinnati Children’s Hospital Medical Center, University Hospital and Good Samaritan Hospital (U10 HD27853, M01 RR8084): Kurt Schibler, MD, Edward F. Donovan, MD, Kate Bridges, MD, Barbara Alexander, RN, Cathy Grisby, BSN, CCRC, Holly L. Mincey, RN, BSN, Jody Hessling, RN, and Lenora Jackson; Duke University School of Medicine University Hospital, Alamance Regional Medical Center, and Durham Regional Hospital (U10 HD40492, GCRC M01 RR30): Ronald N. Goldberg, MD, C. Michael Cotten, MD, MHS, Kathy J. Auten, MSHS, Kimberley A. Fisher, PhD, FNP-BC, IBCLC, and Katherine A. Foy, RN; Emory University, Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory University Hospital Midtown (U10 HD27851, M01 RR39): David P. Carlton, MD, and Ann M. Blackwelder, RNC, BS, MS; Eunice Kennedy Shriver National Institute of Child Health and Human Development: Stephanie Wilson Archer, MA; Floating Hospital for Children at Tufts Medical Center (U10 HD53119, GCRC M01 RR54): Ivan D. Frantz III, MD, Brenda L. MacKinnon, RNC, and Ellen Nylen, RN, BSN; Indiana University, University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services (U10 HD27856, M01 RR750): Brenda B. Poindexter, MD, MS, James A. Lemons, MD, Dianne E. Herron, RN, Lucy C. Miller, RN, BSN, CCRC, and Leslie Dawn Wilson, BSN, CCRC; RTI International (U10 HD36790): W. Kenneth Poole, PhD, Dennis Wallace, PhD, Jeanette O’Donnell Auman, BS, Margaret Cunningham, BS, Carolyn M. Petrie Huitema, MS, James W. Pickett II, BS, Scott E. Schaefer, MS, and Kristin M. Zaterka-Baxter, RN, BSN; Stanford University, Dominican Hospital, El Camino Hospital, and Lucile Packard Children’s Hospital (U10 HD27880, M01 RR70): Krisa P. Van Meurs, MD, David K. Stevenson, MD, Marian M. Adams, MD, M. Bethany Ball, BS, CCRC, Melinda S. Proud, RCP, and Andrew W. Palmquist, RN, BSN; University of Alabama at Birmingham Health System and Children’s Hospital of Alabama (U10 HD34216, M01 RR32): Waldemar A. Carlo, MD, Namasivayam Ambalavanan, MD, Monica V. Collins, RN, BSN, MaEd, and Shirley S. Cosby, RN, BSN; University of California–San Diego Medical Center and Sharp Mary Birch Hospital for Women and Newborns (U10 HD40461): Neil N. Finer, MD, Maynard R. Rasmussen, MD, Paul R. Wozniak, MD, Kathy Arnell, RNC, Renee Bridge, RN, Clarence Demetrio, RN, and Wade Rich, BSHS, RRT; University of Iowa Children’s Hospital (U10 HD53109, M01 RR59): John A. Widness, MD, Karen J. Johnson, RN, BSN, and Nancy J. Krutzfield, RN, MA; University of Miami, Holtz Children’s Hospital (U10 HD21397): Shahnaz Duara, MD, and Ruth Everett-Thomas, RN, MSN; University of New Mexico Health Sciences Center (U10 HD53089, M01 RR997): Kristi L. Watterberg, MD, Lu-Ann Papile, MD, Robin K. Ohls, MD, Conra Backstrom Lacy, RN, Rebecca Montman, BSN, and Carol Hartenberger, BSN, MPH; University of Rochester Medical Center, Golisano Children’s Hospital (U10 HD40521, M01 RR44): Dale L. Phelps, MD, Linda J. Reubens, RN, CCRC, and Rosemary Jensen; University of Texas Southwestern Medical Center at Dallas, Parkland Health & Hospital System, and Children’s Medical Center Dallas (U10 HD40689, M01 RR633): Charles R. Rosenfeld, MD, Walid A. Salhab, MD, Alicia Guzman, Gaynelle Hensley, RN, Melissa H. Leps, RN, Melissa H. Leps, RN, and Nancy A. Miller, RN; University of Texas Health Science Center at Houston, Medical School, Children’s Memorial Hermann Hospital, and Lyndon Baines Johnson General Hospital/Harris County Hospital District (U10 HD21373): Kathleen A. Kennedy, MD, MPH, Jon E. Tyson, MD, MPH, Beverly Foley Harris, RN, BSN, Anna E. Lis, RN, BSN, Sarah Martin, RN, BSN, Georgia E. McDavid, RN, Patti L. Pierce Tate, RCP, and Maegan C. Simmons, RN; University of Utah Medical Center, Intermountain Medical Center, LDS Hospital, and Primary Children’s Medical Center (U10 HD53124, M01 RR64): Roger G. Faix, MD, Bradley A. Yoder, MD, Karen A. Osborne, RN, BSN, CCRC, Jennifer J. Jensen, RN, BSN, Cynthia Spencer, RNC, and Kimberlee Weaver-Lewis, RN, BSN; Wake Forest University, Baptist Medical Center, Brenner Children’s Hospital, and Forsyth Medical Center (U10 HD40498, M01 RR7122): T. Michael O’Shea, MD, MPH, and Nancy J. Peters, RN, CCRP; Wayne State University, Hutzel Women’s Hospital, and Children’s Hospital of Michigan (U10 HD21385): Rebecca Bara, RN, BSN; Yale University, Yale-New Haven Children’s Hospital, and Bridgeport Hospital (U10 HD27871, UL1 RR24139, M01 RR125): Richard A. Ehrenkranz, MD, Harris Jacobs, MD, Patricia Cervone, RN, Monica Konstantino, RN, BSN, JoAnn Poulsen, RN, and Janet Taft, RN, BSN.
We thank our medical and nursing colleagues, the infants, and their parents who agreed to take part in this study.
Glossary
- B/M
bacteremia and/or meningitis
- BPD
bronchopulmonary dysplasia
- BW
birth weight
- CI
confidence interval
- CSF
cerebrospinal fluid
- GA
gestational age
- IVH
intraventricular hemorrhage
- MRSA
methicillin-resistant Staphylococcus aureus
- MSSA
methicillin-susceptible Staphylococcus aureus
- NEC
necrotizing enterocolitis
- NICHD
Eunice Kennedy Shriver National Institute of Child Health and Human Development
- NRN
Neonatal Research Network
- PMA
postmenstrual age
- PVL
periventricular leukomalacia
- RDS
respiratory distress syndrome
- ROP
retinopathy of prematurity
- RR
relative risk
- SA
Staphylococcus aureus
- SGA
small for gestational age
- VLBW
very low birth weight
Footnotes
Drs Shane, Stoll, Bell, Sánchez, Shankaran, Laptook, Das, and Walsh, Ms Hale, Ms Newman, and Drs Schrag and Higgins planned this study, oversaw its design, and contributed to writing the article; Dr Stoll chairs the Neonatal Research Network subcommittee with responsibility for the database and data collection process; Ms Hansen, on behalf of the Data Coordinating Center at RTI International, analyzed all data; Dr Shane, Ms Hansen, and Dr Stoll drafted the article; all Centers mentioned in the acknowledgment section contributed subjects to the database and had oversight for data collection at their Neonatal Research Network Center; all authors critically reviewed the article and approved the final version submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: The National Institutes of Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) provided grant support for the Neonatal Research Network’s (NRN) Generic Database Study. The funding source had no direct involvement in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. Funded by the National Institutes of Health (NIH).
References
- 1.Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110(2 pt 1):285–291 [DOI] [PubMed] [Google Scholar]
- 2.Carey AJ, Duchon J, Della-Latta P, Saiman L. The epidemiology of methicillin-susceptible and methicillin-resistant Staphylococcus aureus in a neonatal intensive care unit, 2000-2007. J Perinatol. 2010;30(2):135–139 [DOI] [PubMed] [Google Scholar]
- 3.Saiman L, Cronquist A, Wu F, et al. An outbreak of methicillin-resistant Staphylococcus aureus in a neonatal intensive care unit. Infect Control Hosp Epidemiol. 2003;24(5):317–321 [DOI] [PubMed] [Google Scholar]
- 4.Morel AS, Wu F, Della-Latta P, Cronquist A, Rubenstein D, Saiman L. Nosocomial transmission of methicillin-resistant Staphylococcus aureus from a mother to her preterm quadruplet infants. Am J Infect Control. 2002;30(3):170–173 [DOI] [PubMed] [Google Scholar]
- 5.Regev-Yochay G, Rubinstein E, Barzilai A, et al. Methicillin-resistant Staphylococcus aureus in neonatal intensive care unit. Emerg Infect Dis. 2005;11(3):453–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haley RW, Cushion NB, Tenover FC, et al. Eradication of endemic methicillin-resistant Staphylococcus aureus infections from a neonatal intensive care unit. J Infect Dis. 1995;171(3):614–624 [DOI] [PubMed] [Google Scholar]
- 7.Siegel JD, Rhinehart E, Jackson M, Chiarello L, Healthcare Infection Control Practices Advisory Committee . Management of multidrug-resistant organisms in health care settings, 2006. Am J Infect Control. 2007;35(10 suppl 2):S165–S193 [DOI] [PubMed] [Google Scholar]
- 8.Stoll BJ, Hansen N, Fanaroff AA, et al. Changes in pathogens causing early-onset sepsis in very-low-birth-weight infants. N Engl J Med. 2002;347(4):240–247 [DOI] [PubMed] [Google Scholar]
- 9.Stoll BJ, Hansen NI, Higgins RD, et al. National Institute of Child Health and Human Development . Very low birth weight preterm infants with early onset neonatal sepsis: the predominance of gram-negative infections continues in the National Institute of Child Health and Human Development Neonatal Research Network, 2002-2003. Pediatr Infect Dis J. 2005;24(7):635–639 [DOI] [PubMed] [Google Scholar]
- 10.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87(2):163–168 [DOI] [PubMed] [Google Scholar]
- 11.Bell MJ, Ternberg JL, Feigin RD, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187(1):1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986;33(1):179–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529–534 [DOI] [PubMed] [Google Scholar]
- 14.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706 [DOI] [PubMed] [Google Scholar]
- 15.Chen KT, Huard RC, Della-Latta P, Saiman L. Prevalence of methicillin-sensitive and methicillin-resistant Staphylococcus aureus in pregnant women. Obstet Gynecol. 2006;108(3 pt 1):482–487 [DOI] [PubMed] [Google Scholar]
- 16.Andrews WW, Schelonka R, Waites K, Stamm A, Cliver SP, Moser S. Genital tract methicillin-resistant Staphylococcus aureus: risk of vertical transmission in pregnant women. Obstet Gynecol. 2008;111(1):113–118 [DOI] [PubMed] [Google Scholar]
- 17.Baltimore RS, Huie SM, Meek JI, Schuchat A, O’Brien KL. Early-onset neonatal sepsis in the era of group B streptococcal prevention. Pediatrics. 2001;108(5):1094–1098 [DOI] [PubMed] [Google Scholar]
- 18.Baltimore RS. Consequences of prophylaxis for group B streptococcal infections of the neonate. Semin Perinatol. 2007;31(1):33–38 [DOI] [PubMed] [Google Scholar]
- 19.Bizzarro MJ, Dembry LM, Baltimore RS, Gallagher PG. Changing patterns in neonatal Escherichia coli sepsis and ampicillin resistance in the era of intrapartum antibiotic prophylaxis. Pediatrics. 2008;121(4):689–696 [DOI] [PubMed] [Google Scholar]
- 20.Freedman RM, Ingram DL, Gross I, Ehrenkranz RA, Warshaw JB, Baltimore RS. A half century of neonatal sepsis at Yale: 1928 to 1978. Am J Dis Child. 1981;135(2):140–144 [DOI] [PubMed] [Google Scholar]
- 21.Gladstone IM, Ehrenkranz RA, Edberg SC, Baltimore RS. A ten-year review of neonatal sepsis and comparison with the previous fifty-year experience. Pediatr Infect Dis J. 1990;9(11):819–825 [DOI] [PubMed] [Google Scholar]
- 22.Cohen-Wolkowiez M, Benjamin DK, Jr, Fowler VG, Jr, et al. Mortality and neurodevelopmental outcome after Staphylococcus aureus bacteremia in infants. Pediatr Infect Dis J. 2007;26(12):1159–1161 [DOI] [PubMed] [Google Scholar]
- 23.Deville JG, Adler S, Azimi PH, et al. Linezolid versus vancomycin in the treatment of known or suspected resistant gram-positive infections in neonates. Pediatr Infect Dis J. 2003;22(suppl 9):S158–S163 [DOI] [PubMed] [Google Scholar]