Abstract
OBJECTIVES:
Examine statistical effects of sleep-disordered breathing (SDB) symptom trajectories from 6 months to 7 years on subsequent behavior.
METHODS:
Parents in the Avon Longitudinal Study of Parents and Children reported on children’s snoring, mouth breathing, and witnessed apnea at ≥2 surveys at 6, 18, 30, 42, 57, and 69 months, and completed the Strengths and Difficulties Questionnaire at 4 (n = 9140) and 7 (n = 8098) years. Cluster analysis produced 5 “Early” (6–42 months) and “Later” (6–69 months) symptom trajectories (“clusters”). Adverse behavioral outcomes were defined by top 10th percentiles on Strengths and Difficulties Questionnaire total and subscales, at 4 and 7 years, in multivariable logistic regression models.
RESULTS:
The SDB clusters predicted ≈20% to 100% increased odds of problematic behavior, controlling for 15 potential confounders. Early trajectories predicted problematic behavior at 7 years equally well as at 4 years. In Later trajectories, the “Worst Case” cluster, with peak symptoms at 30 months that abated thereafter, nonetheless at 7 years predicted hyperactivity (1.85 [1.30–2.63]), and conduct (1.60 [1.18–2.16]) and peer difficulties (1.37 [1.04–1.80]), whereas a “Later Symptom” cluster predicted emotional difficulties (1.65 [1.21–2.07]) and hyperactivity (1.88 [1.42–2.49]) . The 2 clusters with peak symptoms before 18 months that resolve thereafter still predicted 40% to 50% increased odds of behavior problems at 7 years.
CONCLUSIONS:
In this large, population-based, longitudinal study, early-life SDB symptoms had strong, persistent statistical effects on subsequent behavior in childhood. Findings suggest that SDB symptoms may require attention as early as the first year of life.
KEY WORDS: sleep-disordered breathing, behavior, longitudinal
What's Known on This Subject:
Sleep-disordered breathing is associated with neurobehavioral morbidity in children. Prior related research has generally been cross-sectional or short (ie, 1–2 years) follow-up studies of a single symptom (ie, snoring, obstructive sleep apnea, mouth breathing), with limited control for confounders.
What This Study Adds:
Sleep-disordered breathing was assessed as a trajectory of combined symptoms from 6 months to 69 months, in more than 11 000 children. Sleep-disordered breathing was associated with 40% and 60% more behavioral difficulties at 4 and 7 years, respectively.
Neurobehavioral morbidity is common in childhood sleep-disordered breathing (SDB) that can range from snoring to obstructive sleep apnea. Mouth breathing is another frequent clinical finding.1,2 SDB causes abnormal gas exchange, interferes with sleep’s restorative processes, and disrupts cellular and chemical homeostasis.3 The supposed resultant dysfunction of the prefrontal cortex impairs attention, executive functioning, behavioral inhibition, self-regulation of affect and arousal, and other socio-emotional behaviors.4 Behavioral manifestations include both externalizing (eg, hyperactivity, aggression, impulsivity) and internalizing (eg, somatic complaints, social withdrawal) behaviors.5 SDB reportedly peaks from 2 to 6 years of age,6 but also occurs in younger children.7 SDB’s neurologic effects may be irreversible,8 highlighting the saliency of underdetection.
SDB presents as a heterogeneous disorder in children. Understanding how and when SDB symptom patterns in early life affect neurobehavioral outcomes has clinical implications for deciding whether, how, and in whom to intervene.9 Yet, existing studies of SDB’s neurobehavioral effects in children are primarily cross-sectional, and limited by poor sampling, insufficient consideration of confounders, and imprecise use of statistical tools.10,11 The few longitudinal studies are either before or after tonsillectomy or follow children for ≤2 years.
This study describes the combined trajectory of 3 hallmark SDB symptoms (snoring, mouth breathing, and witnessed apnea) and their longitudinal statistical effects on behavior. Our research questions were (1) What effect do early SDB trajectories, from 6 through 42 months of life, have on social-emotional behavior at 4 and 7 years? and (2) What effect do SDB trajectories from 6 months through 69 months have on behavior at 7 years of age? We analyzed previously collected observational data from a critical period in SDB development, from 6 months through nearly 7 years of age in a prospective, population-based cohort.
Methods
Population
The Avon Longitudinal Study of Parents and Children (ALSPAC), a geographically based cohort study of children, enrolled pregnant women residing in a defined part of the former county of Avon in southwest England with an expected date of delivery between April 1991 and December 1992. A total of 14 541 pregnant women were enrolled. The cohort, described in detail elsewhere,12 is broadly representative of the UK population in terms of socioeconomic status (SES), although with a slight underrepresentation of ethnic minority families, and overrepresentation of wealthier families. Our analyses excluded twin, triplet, and quadruplet births; children who did not survive to 1 year; and children with conditions, such as major congenital disorders, that are likely to affect SDB or behavioral assessment. The resulting base sample, used to derive SDB clusters and behavioral outcomes, was 13 467infants.
ALSPAC’s internal law and ethics committee reviews all proposals for secondary analyses and approves policies for data handling and analysis. Ethical approval for this analysis was obtained from the ALSPAC Law and Ethics Committee and UK Local Research Ethics Committee. All participants provided informed consent.
SDB Assessment
Questionnaires, designed and mailed as part of the original ALSPAC study when children were 6, 18, 30, 42, 57, and 69 months of age, asked parents to report on their child’s snoring, apnea, and mouth breathing. These items were as follows: (1) Mouth breathing: “Does he or she breathe through the mouth rather than the nose?” At 57 months and older, parents were asked to report separately for mouth breathing when awake versus asleep, although only the latter was used in analyses. (2) Snoring: “Does he or she snore for more than a few minutes at a time?” (3) Apnea: “When asleep, does he or she seem to stop breathing or hold breath for several seconds at a time?” The ALSPAC parent-reported SDB measures are similar or identical to items validated against polysomnographic data from sleep laboratories. Some validated questionnaires have included parent report of all 3 SDB symptoms,13–17 whereas others have included only snoring and apnea.18,19
Responses were categorized along ordinal scales of 3, 4, or 5 levels. Given this inconsistency in (preexisting) response categories, we extrapolated the values to a common scale (0–100) with the “Always” responses anchored at one end and the “Never” or “Rarely/Never” responses anchored at the other, and proportionate spacing in-between (ie, a 4-category scale was recoded as 0, 33, 66, 100). Variables were then transformed to z scores, with higher scores indicating greater symptom burden.
Behavior Assessment
The Strengths and Difficulties Questionnaire (SDQ),20 a widely used behavioral screen, was completed by mothers when children were ∼4 and 7 years old. The 25-item SDQ has 5 scales: inattention/hyperactivity, emotional symptoms (anxiety and depression), peer problems, conduct problems (aggressiveness and rule breaking), and a pro-social scale (sharing, helpfulness, and so forth). A total difficulties (range = 0–40) score is generated by summing all but the latter scale because the absence of pro-social behavior is conceptually different from the presence of psychological difficulties. Higher scores denote more problems. Missing data were prorated according to SDQ instructions.21 The SDQ scores were dichotomized at the upper 10% based on psychometric testing,22 ALSPAC,23,24 and other UK cohort studies.25
Covariate Assessment
Initial covariate selection was guided by previous ALSPAC studies of SDQ outcomes,24,26–32 and non-ALSPAC studies of sleep problem effects on SDQ outcomes.33,34 Based on this literature review, and exploratory analyses, 15 potential confounders were incorporated into analyses.
SES was measured by paternal employment (manual versus professional), maternal education (higher versus lower), and housing inadequacy (if either >1 person/room or homeless). Family adversity was measured by an 18-item index of stressors (eg, maternal psychopathology, crime, financial insecurity) used in other ALSPAC analyses35; higher values signify more adversity. Intrauterine exposures of maternal smoking or alcohol use in the first trimester (yes/no) and fish intake at 32 weeks’ gestation (servings/week) were assessed, as was whether the child was ever breastfed and the mother’s age at delivery. Household variables included family size (0, 1, or ≥2 children in household at 6-month interview) and the Home Observation for Measurement of the Environment (HOME) Inventory36 to assess the quality of parenting and home environment. Child demographics included race (white versus other) and gender, low birth weight (<2500 g) and prematurity (<37 weeks). Analyses of the subsample with BMI z score data are in Supplemental Tables.
To predict behavior at 4 years, “Early” clusters were derived from the 11 049 participants in the base sample with SDB data for ≥2 of the first 4 time points (ie, 6, 18, 30, and/or 42 months). To predict behavior at 7 years, “Later” clusters were derived for the 11 235 participants in the base sample with SDB data for ≥2 of the first 6 time points (ie, 6, 18, 30, 42, 57, and/or 69 months). Through a process described in detail elsewhere,37 we produced 5 conceptually and statistically distinct Early clusters (6–42 months), and 5 comparable Later clusters (6–69 months) that were extensions of Early clusters.
Statistical Analysis
For SDQ scores at 4 and 7 years, we calculated the mean (SD) and proportions above and below the 10% cutoff for the base sample, and their associations with putative covariates either from χ2 test or analysis of variance. We describe the association between SDQ mean (SD) total scores at 4 and 7 years and the Early and Later clusters, by analysis of variance. Only participants not missing SDQ data are included in analyses of behavioral outcomes.
Multivariate logistic regression analyses examined adjusted and unadjusted relationships between clusters and SDQ total and subscales at 4 and 7 years. To streamline presentation, unadjusted analyses are in the Supplemental Tables. Initial models included all putative covariates. Only those variables that were significant (P ≤ .05) were retained in multivariate models. Odds ratios (ORs) and 95% confidence intervals (95% CIs) represent the odds of being in the top 10% versus the remaining 90% of SDQ scores. To address multicollinearity, variance inflation factors were derived to assess the effects of individual independent variables on variance. A conservative variance inflation factor threshold of 10 was used in model testing.38 Analyses were conducted by using SAS version 9.1 (SAS Institute, Inc, Cary, NC).
Results
Data Completion and Attrition
SDB longitudinal data were relatively complete. Early cluster analyses of the 7996 participants with SDQ 7-year outcomes included 7716 (96%) with SDB data for ≥3/4 time points. Likewise, Later cluster analyses (SDQ 7-year data) for 8064 participants, included 7383 (92%) with SDB data for ≥5/6 time points. Missing SDQ or SDB data were significantly associated with nonwhite race, prematurity, low birth weight, manual (versus professional) paternal employment, lower (versus higher) maternal educational status, housing inadequacy, not being breastfed, and higher levels of wheezing (not shown).
Sample Characteristics and Association With Top 10% of SDQ Total Scores
Characteristics of the base sample and associations with behavioral outcomes are shown in Table 1. Children in the upper 10% of SDQ scores had significantly more adverse characteristics (eg, higher maternal smoking, older delivery age, lower maternal education, higher Family Adversity Index scores, lower HOME scores, housing inadequate, prematurity, low birth weight, and being male) than the remaining 90% at 4 and 7 years, but there were no differences by race, maternal alcohol intake during pregnancy, or tonsils removal.
TABLE 1.
Demographics for Total Sample and by SDQ Scores at 4 and 7 Years
| Total Samplea n = 13 467 | SDQ Total Score, 4 y | SDQ Total Score, 7 y | |||
|---|---|---|---|---|---|
| Top 10% n = 1218 | Lower 90% n = 7922 | Top 10% n = 889 | Lower 90% n = 7055 | ||
| Maternal | |||||
| Smoked during pregnancy, any | 25.0% | 30.5%b | 20.0% | 30.9%b | 18.7% |
| Alcohol during pregnancy, any | 54.6% | 55.6% | 55.5% | 58.3% | 55.6% |
| Fish intake during pregnancy, mean (SD) | 1.87 (1.75) | 1.94 (1.76) | 1.88 (1.75) | 1.96 (1.86) | 1.88 (1.73) |
| Age at delivery, mean years (SD) | 27.96 (4.98) | 27.47 (4.80)b | 28.86 (4.65) | 28.08 (4.76)b | 29.02 (4.57) |
| Breastfed this child, ever | 75.5% | 73.0%b | 78.6% | 76.5% | 79.3% |
| Child | |||||
| Gender, male | 51.5% | 55.1%c | 51.1% | 59.5%b | 50.6% |
| Race, white | 97.4% | 98.1% | 98.4% | 98.1% | 98.3% |
| Premature, <37 wk | 4.9% | 5.6%c | 4.3% | 6.1%c | 4.1% |
| Low birth weight, <2500 g | 4.3% | 4.7%c | 3.5% | 5.0%b | 3.3% |
| Adenoids removed, ever | 7.4% | —– | —– | 9.4%b | 6.2% |
| Tonsils removed, ever | 4.5% | —– | —– | 5.0% | 3.8% |
| BMI z score at 42 mo | 0.27 (1.06) | 0.24 (1.10) | 0.27 (1.04) | 0.29 (1.12) | 0.26 (1.03) |
| Socioeconomic and family | |||||
| Maternal education,d lower (%) | 64.6% | 71.5%c | 58.9% | 69.0%b | 57.8% |
| Paternal employment, manual (%) | 44.0% | 52.4%b | 39.2% | 48.5%b | 39.0% |
| Housing, inadequate, (%) | 12.3% | 18.9%b | 11.6% | 18.3%b | 10.9% |
| Family Adversity Index, mean (range: 0–18) | 1.77 (1.98) | 2.65 (2.28)b | 1.73 (1.87) | 2.87 (2.39)b | 1.70 (1.85) |
| HOME score, mean (range: 0–8) | 5.74 (1.66) | 5.60 (1.77)b | 5.80 (1.62) | 5.48 (1.74)b | 5.84 (1.61) |
| Parity, ≥1 | 55.1% | 52.3% | 54.3% | 50.8%c | 54.5% |
P values are calculated from χ2 test for categorical variables and analysis of variance for continuous variables. HOME, Home Observation for Measurement of the Environment.
These 13 467 constitute the base sample used to derive the clusters and SDQ outcomes.
P < .01 for difference between top 10% versus lower 90%.
P < .05 for difference between top 10% versus lower 90%.
“Lower defined as “O” level education or less (equivalent to school-leaving certificate at 16 in the UK), from 5 original groupings.
Cluster Description and Association With Sample Characteristics
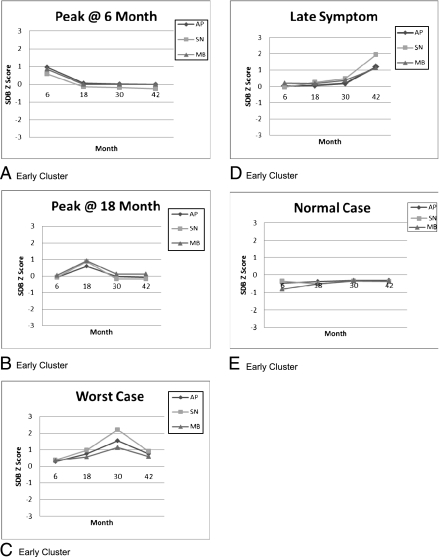
Cluster analyses yielded 1 asymptomatic (“Normals,” 45% of sample) and 4 symptomatic (55% of sample) trajectories. Early clusters, shown in Fig 1, can be summarized as (1) symptoms “Peak @ 6” and then abate, (2) symptoms “Peak @ 18” months and then abate, (3) symptoms peak at 30 months and then persist (“Worst Case”), (4) symptoms emerge at 42 months and then persist (“Late Symptom”) and (5) “Normals” who are asymptomatic throughout. Snoring levels were nearly double those of apnea or mouth breathing in Worst Case and Late Symptom versus comparable symptom levels in other clusters. Whether assessed as a continuous or dichotomous (10% vs 90%) variable, SDQ total scores differed significantly across the 4 symptomatic clusters, and in combined symptomatic clusters versus Normals (Table 2). Early clusters’ descriptive characteristics are shown in Supplemental Table 6.
FIGURE 1.
Early clusters.
TABLE 2.
Association Between Early Clusters (n = 11c049) and SDQ outcome
| Peak at 6 n = 2277 (20.6%) (1) | Peak at 18 n = 1881 (17.0%) (2) | Worst Case n = 878 (8.0%) (3) | Late Symptom n = 1023 (9.3%) (4) | Normals n = 4990 (45.2%) (5) | |
|---|---|---|---|---|---|
| Outcome SDQ | |||||
| SDQ top 10% at 4 ya | 13.7% | 15.0% | 18.8% | 19.5% | 10.1% |
| SDQ top 10% at 7 ya | 12.1% | 12.9% | 17.7% | 14.2% | 8.4% |
| SDQ continuous at 4 y, mean (SD)a | 14.59 (3.59) | 14.66 (3.70) | 15.32 (3.96) | 15.35 (3.91) | 13.88 (3.44) |
| SDQ continuous at 7 y, mean (SD)a | 8.13 (4.98) | 7.94 (4.93) | 8.83 (5.31) | 8.36 (4.91) | 6.76 (4.49) |
| SDQ top 10% at 4 ya | 15.74% | 10.07% | |||
| SDQ top 10% at 7 ya | 13.46% | 8.41% | |||
| SDQ continuous at 4 y, mean (SD)a | 14.84 (3.74) | 13.88 (3.44) | |||
| SDQ continuous at 7 y, mean (SD)a | 8.20 (5.01) | 6.76 (4.49) | |||
P values are calculated from χ2 test for categorical variables and analysis of variance for continuous variables.
P < .01 for difference between top 10% versus lower 90%.
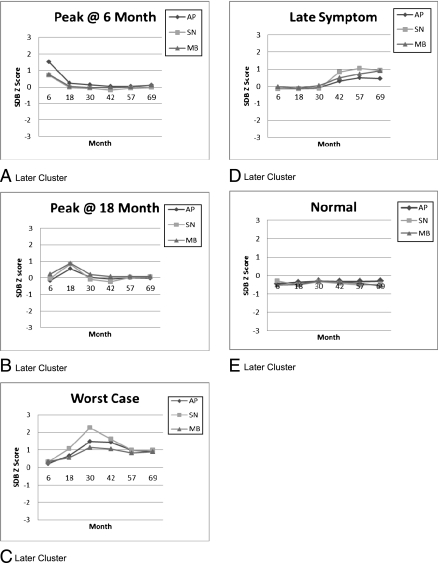
Five comparable Later clusters are illustrated in Fig 2. Patterns are similar to the Early clusters, except that in this Late Symptom cluster, snoring and mouth breathing peak together at lower levels at 57 months with no marked apnea, and the Peak @ 6 apnea levels are nearly double those of the Early clusters.
FIGURE 2.
Later clusters.
SDQ Total Score
The SDB clusters significantly predict SDQ total scores at 4 and 7 years (Table 3). Early cluster effects are 20% to 70% in multivariate analyses. The strongest and most persistent is for Worst Case, with comparable outcomes at 4 (OR = 1.49, 95% CI = 1.11–1.99) and 7 years (OR = 1.72, 95% CI = 1.31–2.25). Later clusters’ effects are 40% to 100% in multivariate analyses, including a ≈40% effect for Peak @ 6 and ≈50% effect for Peak @ 18. Membership in any symptomatic cluster is associated with being in the upper 10% of total SDQ scores (versus Normals), an effect that increases slightly from 4 (OR = 1.33, 95% CI = 1.14–1.56) to 7 years (OR = 1.52, 95% CI = 1.24–1.85: Later clusters). Multivariate effects are strongly attenuated from unadjusted effects (Supplemental Table 7).
TABLE 3.
Cluster Effects on SDQ Total Scores at 4 and 7 Years
| Early Cluster Models | Later Cluster Models | ||
|---|---|---|---|
| Top 10% vs Lower 90% OR (95% CI) | 4 ya n = 9007 | 7 ya,b n = 7996 | 7 ya,b n = 8064 |
| Adjusted without BMIc | |||
| Peak at 6 (1) | 1.23 (1.00–1.51)e | 1.25 (1.02–1.53)e | 1.39 (1.11–1.74)d |
| Peak at 18 mo (2) | 1.26 (1.01–1.57)e | 1.39 (1.12–1.72)d | 1.50 (1.21–1.86)d |
| Worst Case (3) | 1.49 (1.11–1.99)d | 1.72 (1.31–2.25)d | 2.00 (1.53–2.62)d |
| Late symptom (4) | 1.56 (1.19–2.03)d | 1.46 (1.12–1.91)d | 1.68 (1.35–2.10)d |
| Smoking during pregnancy | N.S. | 1.21 (1.01–1.45)e | NS |
| Gender, male | 1.18 (1.01–1.37)e | 1.50 (1.29–1.76)d | NS |
| Maternal education, lower | 1.40 (1.18–1.66)d | 1.31 (1.11–1.55)d | 1.33 (1.13–1.57)d |
| Paternal employment, manual | 1.28 (1.08–1.51)d | NS | NS |
| Family Adversity Index | 1.20 (1.15–1.24)d | 1.25 (1.20–1.30)d | 1.25 (1.21–1.30)d |
| HOME score | NS | 0.91 (0.87–0.95)d | 0.91 (0.87–0.96)d |
| Parity 1 vs 0 ≥2 vs 0 | 1.00 (0.85–1.19) 0.66 (0.53–0.83)d | 0.72 (0.61–0.86)d 0.58 (0.46–0.72)d | 0.72 (0.61–0.86)d 0.59 (0.47–0.74)d |
| Clusters 1, 2, 3 and 4 vs Normals (5) | 1.33 (1.14–1.56)d | 1.24 (1.02–1.51)e | 1.52 (1.24–1.85)d |
HOME, Home Observation for Measurement of the Environment; NS, not significant.
Adjusted for fish intake, Family Adversity Index, mother and home score, smoke during pregnancy, alcohol during pregnancy, race, breastfeeding ever, housing inadequacy, parity, gestation age, paternal social, maternal education, birth weight, maternal age, gender.
Additional adjusted for tonsils or adenoids removed.
Covariates shown are only those that were significant (P < .05) in reduced models with each of the 4 symptomatic models incorporated as a separate variable (versus combined clusters 1, 2, 3, and 4).
P < .01.
P < .05.
SDB effects exceeded those of maternal smoking, maternal education, and paternal employment in multivariate analyses (Table 3). Race, prematurity, low birth weight, maternal fish intake, or maternal age were not significant in any multivariate analyses, total or subscale (not shown). Only male gender and not being the second or later-born child had greater adverse effects. Greater family adversity was associated with poorer behavioral outcomes, whereas higher home environment scores were associated with improved outcomes. Gender-cluster interactions were not significant for any total or subscore analyses.
SDQ Subscores
In adjusted analyses, nearly every cluster-subscale association was significant (Table 4), with effects of 20% to 100% (see Supplemental Table 7 for unadjusted effects.) Details are in the following paragraphs.
TABLE 4.
Adjusted Clusters Effects on SDQ Subscales at 4 and 7 Years
| Top 10% vs Lower 90% OR (95% CI) | Early Cluster Models | Later Cluster Models | |
|---|---|---|---|
| 4 ya | 7 yb | 7 yb | |
| Pro-social | |||
| Peak at 6 | 1.28 (1.05–1.55)c | 1.26 (1.02–1.54)c | 1.29 (1.04–1.60)d |
| Peak at 18 mo | 1.18 (0.95–1.46) | 1.25 (0.99–1.56) | 1.21 (0.99–1.49) |
| Worst Case | 1.14 (0.85–1.52) | 1.24 (0.91–1.68) | 1.05 (0.78–1.41) |
| Late Symptom | 1.01 (0.76–1.34) | 1.01 (0.74–1.37) | 0.91 (0.71–1.16) |
| Hyperactivity | |||
| Peak at 6 | 1.19 (1.00–1.42) d | 1.50 (1.23–1.83) c | 148 (1.12–1.97) d |
| Peak at 18 mo | 1.12 (0.92–1.35) | 1.40 (1.13–1.74) c | 1.51 (1.15–1.98) c |
| Worst Case | 1.56 (1.23–1.98) c | 1.98 (1.52–2.58) c | 1.85 (1.30–2.63) c |
| Late Symptom | 1.51 (1.21–1.89) c | 1.57 (1.20–2.05) c | 1.88 (1.42–2.49) c |
| Emotional | |||
| Peak at 6 | 1.20 (0.99–1.44) | 1.38 (1.15–1.66) c | 1.45 (1.18–1.78) c |
| Peak at 18 mo | 1.24 (1.02–1.52) d | 1.32 (1.09–1.61) c | 1.47 (1.21–1.78) c |
| Worst Case | 1.47 (1.14–1.89) c | 1.41 (1.08–1.85) ‡ | 1.58 (1.21–2.07) c |
| Late Symptom | 1.50 (1.19–1.91) c | 1.62 (1.27–2.06) c | 1.65 (1.35–2.02) c |
| Conduct | |||
| Peak at 6 | 1.47 (1.24–1.73) c | 1.31 (1.06–1.63)d | 1.29 (1.01–1.65) d |
| Peak at 18 mo | 1.26 (1.04–1.52) d | 1.32 (1.05–1.67) d | 1.42 (1.13–1.78) c |
| Worst Case | 1.52 (1.20–1.92) c | 1.53 (1.13–2.08) c | 1.60 (1.18–2.16) c |
| Late Symptom | 1.68 (1.34–2.09) c | 1.13 (0.83–1.54) | 1.40 (1.09–1.79) c |
| Peer | |||
| Peak at 6 | 1.27 (1.04–1.56)d | 1.14 (0.94–1.38) | 1.03 (0.82–1.29) |
| Peak at 18 mo | 1.29 (1.04–1.60) d | 1.18 (0.96–1.46) | 1.22 (0.99–1.49) d |
| Worst Case | 1.33 (1.01–1.77) d | 1.48 (1.13–1.93) c | 1.37 (1.04–1.80) d |
| Late Symptom | 1.21 (0.92–1.59) | 1.19 (0.91–1.55) | 1.17 (0.93–1.46) |
Adjusted for fish intake, Family Adversity Index, mother and home score, smoke during pregnancy, alcohol during pregnancy, race, breastfeeding ever, housing inadequacy, parity, gestation age, paternal social, maternal education, birth weight, maternal age, gender.
Additional adjusted for tonsils or adenoids removed.
P < .01.
P < .05.
Pro-social
Peak @ 6 was associated with ≈30% greater odds of being in the lowest decile across outcomes at 4 and 7 years. All of the remaining associations were not significant.
Hyperactivity
With 1 exception, all effects (≈20%–100%) were significant, and increased from 4 to 7 years. Furthermore, Early cluster effects at 4 years for Worst Case and Late Symptom equaled or increased at 7 years.
Emotional
With 1 exception at 4 years, all effects were significant (range ≈20%–65%), and most increased from 4 to 7 years. Late Symptom had the strongest effect at 4 and 7 years based on Early cluster models, with effects persisting to 7 years in Later cluster models.
Conduct
With 1 exception, all effects were significant (range ≈30%–70%). Both Worst Case and Peak@18 effects increased from 4 to 7 years, whereas Late Symptom and Peak @ 6 effects attenuated over that time.
Peer
Half of the cluster-subscale associations were significant, and SDB effects were more modest (range ≈30%–50%) and stable over time, compared with the other subscales. Effects were strongest for Worst Case.
Discussion
We examined the effects of snoring, apnea, and mouth-breathing patterns (clusters) on behavior, from infancy through 7 years in more than 11 000 children. By 4 years, children in the symptomatic clusters were ≈20% to 60% more likely to exhibit behavioral difficulties consistent with a clinical diagnosis; by 7 years, they were ≈40% to 100% more likely. These effects, in a population-based cohort that controlled for 15 putative confounders, exceeded those of any measured prenatal (ie, maternal age, smoking or alcohol use), gestational age, birth weight, breastfeeding, SES, family adversity, or home environment exposure. Furthermore, SDB effects at 4 years were as predictive of behavioral difficulties at 7 years. The worst symptoms were associated with the worst behavioral outcomes. Among the neuroehavioral domains assessed, hyperactivity was most affected.
Compared with previous parent-reported effects of SDB on Later behavior, our findings are conservative. In a study of ≈1000 third graders, snoring at baseline was associated with 2- to 10-fold increases in SDQ-assessed hyperactivity, and emotional, conduct, and peer difficulties at 1-year follow-up in age- and gender-adjusted analyses.39 A pediatric clinic sample of 229 2- to 13-year-olds found that baseline SDB symptoms predicted fourfold increases in hyperactive behaviors at 4-year follow-up, after adjustment for age, gender, and baseline hyperactivity.40 These studies differed from ours, with smaller, less representative samples, lack of data from the earliest years, use of other (non-SDQ) behavioral measures, and limited control for confounders. Alternatively, several cross-sectional studies that used different operational definitions of SDB and SDQ outcomes found no effects. One, a large nationally representative cross-sectional sample of ≈5000 Australian 4- to 5-year-olds, found that neither SDQ total, nor the Hyperactivity, Peer, or Emotional scale (actual) scores were greater among children with “snoring and/or breathing difficulties” during sleep ≥4 times per week.33 Likewise, among 635 6- to 8-year-olds, snoring ≥1 time per week in the past 6 months was not associated with high (upper 10th percentile) SDQ scores.34
This is the first study to assess SDB as a trajectory of combined symptoms, across a key period of SDB development from 6 months to 81 months, in a large sample. Previous studies had smaller samples that were often cross-sectional, or had shorter follow-up. Many were not population-based, involved school-age children only, or did not adjust for as wide a range of confounders.8,41 The potential impact of confounders is illustrated by the fact that several covariates independently associated with the clusters were not significant in multivariate analyses, and most unadjusted effects of SDB attenuated in controlled analyses. Although residual confounding is possible, covariates were selected based on previous ALSPAC analyses of SDQ outcomes, and non-ALSPAC studies of sleep problem effects on SDQ outcomes.
The current study has several limitations. First, SDB data were derived from parent report, rather than objective testing; however, the symptom-items used reflect widely accepted and well-validated SDB risk factors. Second, because parents may hear snoring from another room, they may be more likely to report it than either apnea or mouth breathing. Third, observed apnea during infancy is difficult to distinguish from the more common central apnea; however, our finding that observed apnea in infancy tracked with more clearly obstructive symptoms through 7 years suggests that observed apneas of infancy may not always have a central etiology. Fourth, missing SDB and SDQ data were associated with identified SDB risk factors (eg, maternal smoking, lower SES). Although biases that involve selective dropout may alter prevalence estimates, other ALSPAC analyses found only marginal effects on regression models predicting behavioral outcomes.42 This is likely the case in our study, in which such biases would render our findings more conservative.
These findings, from the largest-ever cohort study of SDB exposure and neurobehavioral morbidity, provide epidemiologic evidence that early childhood SDB effects may only become apparent years later. The most significant long-term effects occurred in children with the greatest overall levels of snoring, apnea, and mouth breathing throughout, peaking at 30 months. Even very early symptom peaks at 6 and 18 months are associated with ≈40% and 50%, respectively, increased behavioral morbidity at 7 years of age. This may be because of the increased vulnerability to SDB effects during this early critical period of brain development,43 when there is the greatest need for sleep.44
SDB is relatively common in childhood. In previous analyses of this cohort, the prevalence of habitual snoring ranged from 10% to 21%, from 6 months to 81 months.45 The potential clinical and educational implications of untreated SDB, therefore, are notable. As an example, we found significant (nearly twofold), and sustained effects on hyperactivity. In national survey data, children with attention-deficit/hyperactivity disorder had increased adjusted risks of comorbid learning disability (eightfold), anxiety (eightfold), and low social competence (threefold).46 Further, 40% to 80% of the nation’s 3 million 6- to 21-year-olds who receive special education for a developmental disability or delay also have attention-deficit/hyperactivity.47
Conclusions
These population-based data found a strong and persistent association between SDB symptoms and behavior. This has clinical implications for screening and treatment. A 2009 consensus statement by UK pediatricians and pediatric specialists noted that “the natural history of SDB, where a child changes from normality to abnormality, and where the risks of developing complications of the condition outweigh the risks of the surgical intervention, has not been established.”48 Although data from multicenter, randomized controlled trials, such as the current National Institutes of Health–funded Childhood Adenotonsillectomy study, will provide some evidence of cause-and-effect relationships, our findings provide further epidemiologic evidence to support attention to SDB symptoms beginning as early as the first year of life.
Supplementary Material
Supplemental Information
Acknowledgments
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting the families, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses. In addition, we thank the following members of the study’s advisory group: Dr Raanan Arens (Montefiore Medical Center/Albert Einstein College of Medicine), Dr John Bent (Montefiore Medical Center/Albert Einstein College of Medicine), Dr Peter Blair (University of Bristol), Dr Pauline Emmett (University of Bristol), Dr Peter Fleming (University of Bristol), Dr Jon Heron (University of Bristol), Dr Carole Marcus (Children’s Hospital of Philadelphia), Dr Kenneth Ong (Cambridge University), Dr Sanjay Parikh (Montefiore Medical Center/Albert Einstein College of Medicine), and Dr Susan Redline (Case Western Reserve University).
Glossary
- ALSPAC
Avon Longitudinal Study of Parents and Children
- CI
confidence interval
- HOME
Home Observation for Measurement of the Environment
- OR
odds ratio
- SDB
sleep-disordered breathing
- SDQ
Strengths and Difficulties Questionnaire
- SES
socioeconomic status
Footnotes
All authors meet the criteria for authorship; Dr Bonuck conceptualized and designed the study, drafted the initial manuscript, reviewed and modified the analyses in collaboration with Drs Freeman and Xu, and incorporated coauthor feedback into the final manuscript; Dr Freeman worked to develop the methods, carried out initial analyses, supervised final analyses of Dr Xu, and reviewed and revised the final manuscript; Dr Chervin advised on study design and analyses, and carefully reviewed and revised multiple versions of the manuscript; Dr Xu collaborated on statistical design issues, completed final analyses, and reviewed and revised the final version of the article.
Dr Xu's current affiliation is Department of Pediatrics, Baylor College of Medicine. Dr Freeman is currently semi-retired.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by grants from the National Heart, Lung, and Blood Institute (R21HL091241 and R21HL091241-01A1). Funded by the National Institutes of Health (NIH).
References
- 1.Li HY, Lee LA. Sleep-disordered breathing in children. Chang Gung Med J. 2009;32(3):247–257 [PubMed] [Google Scholar]
- 2.Sahin U, Ozturk O, Ozturk M, Songur N, Bircan A, Akkaya A. Habitual snoring in primary school children: prevalence and association with sleep-related disorders and school performance. Med Princ Pract. 2009;18(6):458–465 [DOI] [PubMed] [Google Scholar]
- 3.Beebe DW, Gozal D. Obstructive sleep apnea and the prefrontal cortex: towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res. 2002;11(1):1–16 [DOI] [PubMed] [Google Scholar]
- 4.Beebe DW. Neurobehavioral effects of obstructive sleep apnea: an overview and heuristic model. Curr Opin Pulm Med. 2005;11(6):494–500 [DOI] [PubMed] [Google Scholar]
- 5.Owens JA. Neurocognitive and behavioral impact of sleep disordered breathing in children. Pediatr Pulmonol. 2009;44(5):417–422 [DOI] [PubMed] [Google Scholar]
- 6.Halbower AC, Marcus CL. Sleep disorders in children. Curr Opin Pulm Med. 2003;9(6):471–476 [DOI] [PubMed] [Google Scholar]
- 7.Section on Pediatric Pulmonology, Subcommittee on Obstructive Sleep Apnea Syndrome. American Academy of Pediatrics . Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002;109(4):704–712 [DOI] [PubMed] [Google Scholar]
- 8.Simmons MS, Clark GT. The potentially harmful medical consequences of untreated sleep-disordered breathing: the evidence supporting brain damage. J Am Dent Assoc. 2009;140(5):536–542 [DOI] [PubMed] [Google Scholar]
- 9.Marcus CL. Childhood obstructive sleep apnoea: to treat or not to treat, that is the question. Thorax. 2010;65(1):4–5 [DOI] [PubMed] [Google Scholar]
- 10.Ebert CS, Jr, Drake AF. The impact of sleep-disordered breathing on cognition and behavior in children: a review and meta-synthesis of the literature. Otolaryngol Head Neck Surg. 2004;131(6):814–826 [DOI] [PubMed] [Google Scholar]
- 11.Mitchell RB, Kelly J. Behavior, neurocognition and quality-of-life in children with sleep-disordered breathing. Int J Pediatr Otorhinolaryngol. 2006;70(3):395–406 [DOI] [PubMed] [Google Scholar]
- 12.Golding J, ALSPAC Study Team . The Avon Longitudinal Study of Parents and Children (ALSPAC)—study design and collaborative opportunities. Eur J Endocrinol. 2004;151(suppl 3):U119–U123 [DOI] [PubMed] [Google Scholar]
- 13.Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000;1(1):21–32 [DOI] [PubMed] [Google Scholar]
- 14.Chervin RD, Weatherly RA, Garetz SL, et al. Pediatric sleep questionnaire: prediction of sleep apnea and outcomes. Arch Otolaryngol Head Neck Surg. 2007;133(3):216–222 [DOI] [PubMed] [Google Scholar]
- 15.Franco RA, Jr, Rosenfeld RM, Rao M. First place—resident clinical science award 1999. Quality of life for children with obstructive sleep apnea. Otolaryngol Head Neck Surg. 2000;123(1 pt 1):9–16 [DOI] [PubMed] [Google Scholar]
- 16.Li AM, Cheung A, Chan D, et al. Validation of a questionnaire instrument for prediction of obstructive sleep apnea in Hong Kong Chinese children. Pediatr Pulmonol. 2006;41(12):1153–1160 [DOI] [PubMed] [Google Scholar]
- 17.Brouillette RT, Fernbach SK, Hunt CE. Obstructive sleep apnea in infants and children. J Pediatr. 1982;100(1):31–40 [DOI] [PubMed] [Google Scholar]
- 18.Bruni O, Ottaviano S, Guidetti V, et al. The Sleep Disturbance Scale for Children (SDSC). Construction and validation of an instrument to evaluate sleep disturbances in childhood and adolescence. J Sleep Res. 1996;5(4):251–261 [DOI] [PubMed] [Google Scholar]
- 19.Ferreira VR, Carvalho LB, Ruotolo F, de Morais JF, Prado LB, Prado GF. Sleep disturbance scale for children: translation, cultural adaptation, and validation. Sleep Med. 2009;10(4):457–463 [DOI] [PubMed] [Google Scholar]
- 20.Goodman R. The strengths and difficulties questionnaire: a research note. J Child Psychol Psychiatry. 1997;38(5):581–586 [DOI] [PubMed] [Google Scholar]
- 21.Scoring the informant-rated strengths and difficulties questionnaire. Available at: http://brightfutures.aap.org/pdfs/Other%203/SDQ%20Scoring%20Instructions%20(Parent,%20Teacher).pdf. Accessed February 2, 2012
- 22.Goodman R. Psychometric properties of the strengths and difficulties questionnaire. J Am Acad Child Adolesc Psychiatry. 2001;40(11):1337–1345 [DOI] [PubMed] [Google Scholar]
- 23.Ramchandani P, Psychogiou L. Paternal psychiatric disorders and children’s psychosocial development. Lancet. 2009;374(9690):646–653 [DOI] [PubMed] [Google Scholar]
- 24.Hibbeln JR, Davis JM, Steer C, et al. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study. Lancet. 2007;369(9561):578–585 [DOI] [PubMed] [Google Scholar]
- 25.Kelly Y, Sacker A, Gray R, Kelly J, Wolke D, Quigley MA. Light drinking in pregnancy, a risk for behavioural problems and cognitive deficits at 3 years of age? Int J Epidemiol. 2009;38(1):129–140 [DOI] [PubMed] [Google Scholar]
- 26.Wiles NJ, Northstone K, Emmett P, Lewis G. “Junk food” diet and childhood behavioural problems: results from the ALSPAC cohort. Eur J Clin Nutr. 2009; Apr 63(4):491–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brion MJ, Victora C, Matijasevich A, et al. Maternal smoking and child psychological problems: disentangling causal and noncausal effects. Pediatrics. 2010;126(1). Available at: www.pediatrics.org/cgi/content/full/126/1/e57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Enoch MA, Steer CD, Newman TK, Gibson N, Goldman D. Early life stress, MAOA, and gene-environment interactions predict behavioral disinhibition in children. Genes Brain Behav. 2010;9(1):65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawson DW, Mace R. Siblings and childhood mental health: evidence for a later-born advantage. Soc Sci Med. 2010;70(12):2061–2069 [DOI] [PubMed] [Google Scholar]
- 30.Huisman M, Araya R, Lawlor DA, Ormel J, Verhulst FC, Oldehinkel AJ. Cognitive ability, parental socioeconomic position and internalising and externalising problems in adolescence: findings from two European cohort studies. Eur J Epidemiol. 2010;25(8):569–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiles NJ, Peters TJ, Heron J, Gunnell D, Emond A, Lewis G. Fetal growth and childhood behavioral problems: results from the ALSPAC cohort. Am J Epidemiol. 2006;163(9):829–837 [DOI] [PubMed] [Google Scholar]
- 32.Brion MJ, Zeegers M, Jaddoe V, et al. Intrauterine effects of maternal prepregnancy overweight on child cognition and behavior in 2 cohorts. Pediatrics. 2011;127(1). Available at: www.pediatrics.org/cgi/content/full/127/1/e202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hiscock H, Canterford L, Ukoumunne OC, Wake M. Adverse associations of sleep problems in Australian preschoolers: national population study. Pediatrics. 2007;119(1):86–93 [DOI] [PubMed] [Google Scholar]
- 34.Smedje H, Broman JE, Hetta J. Associations between disturbed sleep and behavioural difficulties in 635 children aged six to eight years: a study based on parents’ perceptions. Eur Child Adolesc Psychiatry. 2001;10(1):1–9 [DOI] [PubMed] [Google Scholar]
- 35.Bowen E, Heron J, Waylen A, Wolke D, ALSPAC Study Team . Domestic violence risk during and after pregnancy: findings from a British longitudinal study. BJOG. 2005;112(8):1083–1089 [DOI] [PubMed] [Google Scholar]
- 36.Mundfrom DJ, Bradley RH, Whiteside L. A factor-analytic study of the infant-toddler and early-childhood versions of the HOME inventory. Educ Psychol Meas. 1993;53(2):479–489 [DOI] [PubMed] [Google Scholar]
- 37.Freeman K, Bonuck K. Snoring, mouth-breathing, and apnea trajectories in a population-based cohort followed from infancy to 81 months: a cluster analysis. Int J Pediatr Otorhinolaryngol 2012;76:122–130 [DOI] [PubMed] [Google Scholar]
- 38.O'Brien RM. A caution regarding rules of thumb for variance inflation factors. Qual Quant. 2007;41(5):673–690 [Google Scholar]
- 39.Urschitz MS, Eitner S, Guenther A, et al. Habitual snoring, intermittent hypoxia, and impaired behavior in primary school children. Pediatrics. 2004;114(4):1041–1048 [DOI] [PubMed] [Google Scholar]
- 40.Chervin RD, Ruzicka DL, Archbold KH, Dillon JE. Snoring predicts hyperactivity four years later. Sleep. 2005;28(7):885–890 [DOI] [PubMed] [Google Scholar]
- 41.Kuehni CE, Strippoli MPF, Chauliac ES, Silverman M. Snoring in preschool children: prevalence, severity and risk factors. Eur Respir J. 2008;31(2):326–333 [DOI] [PubMed] [Google Scholar]
- 42.Wolke D, Waylen A, Samara M, et al. Selective drop-out in longitudinal studies and non-biased prediction of behaviour disorders. Br J Psychiatry. 2009;195(3):249–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jan JE, Reiter RJ, Bax MCO, Ribary U, Freeman RD, Wasdell MB. Long-term sleep disturbances in children: a cause of neuronal loss. Eur J Paediatr Neurol. 2010;14(5):380–390 [DOI] [PubMed] [Google Scholar]
- 44.Galland BC, Taylor BJ, Elder DE, Herbison P. Normal sleep patterns in infants and children: a systematic review of observational studies [published online ahead of print July 22, 2011]. Sleep Med Rev. 2011 [DOI] [PubMed]
- 45.Bonuck KA, Chervin RD, Cole TJ, et al. Prevalence and persistence of sleep disordered breathing symptoms in young children: a 6-year population-based cohort study. Sleep. 2011;34(7):875–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larson K, Russ SA, Kahn RS, Halfon N. Patterns of comorbidity, functioning, and service use for US Children with ADHD, 2007. Pediatrics. 2011;127(3):462–470 [DOI] [PMC free article] [PubMed]
- 47.Boulet SL, Boyle CA, Schieve LA. Health care use and health and functional impact of developmental disabilities among US children, 1997-2005. Arch Pediatr Adolesc Med. 2009;163(1):19–26 [DOI] [PubMed] [Google Scholar]
- 48.Robb PJ, Bew S, Kubba H, et al. Tonsillectomy and adenoidectomy in children with sleep related breathing disorders: consensus statement of a UK multidisciplinary working party. Clin Otolaryngol. 2009;34(1):61–63 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Information