Expert panels sponsored by both the World Health Organization and the National Institutes of Health have recommended that obese adults (ie, body mass index ≥30 kg/m2), as well as those who are overweight (body mass index of 25–29.9 kg/m2) and have comorbid conditions, lose 10% of their initial weight.1,2 A comprehensive program of lifestyle modification is considered the first option for achieving this goal.2 Lifestyle modification, also referred to as behavioral weight control, includes 3 primary components: diet, exercise, and behavior therapy.3 This narrative review examines weight losses achieved with this approach, as well as new developments with each of the 3 components.
Overview of Lifestyle Modification
Comprehensive lifestyle modification programs typically provide weekly individual or group treatment sessions designed to modify eating and activity habits.4 This approach is exemplified by the Diabetes Prevention Program (DPP),5 which randomly assigned >3200 participants with impaired glucose tolerance to 1 of 3 groups: placebo, metformin, or lifestyle modification. Participants in the lifestyle intervention were provided 16 individual counseling sessions during the first 24 weeks and at least every-other-month sessions thereafter. They were prescribed a reduced calorie, low-fat diet of conventional foods (1200–2000 kcal/d, depending on body weight) and 150 min/wk of physical activity (typically brisk walking), with the goal of losing 7% of initial weight.5,6 After an average of 2.8 years, participants in the lifestyle intervention lost 5.6 kg in comparison with losses of 2.1 kg and 0.1 kg in the metformin and placebo groups, respectively. The risk in the lifestyle group of developing type 2 diabetes was reduced by 58% in comparison with placebo and by 31% in comparison with metformin.5 Ten years after randomization, lifestyle-treated participants had regained nearly to their baseline weight (with no significant differences in weight loss among groups). However, the incidence of type 2 diabetes was still lowest in the lifestyle group, which had a 34% reduction in risk in comparison with placebo.7 These findings suggest that modest weight loss, even when followed by weight regain, can be beneficial to long-term health.
The Look AHEAD (Action for Health in Diabetes) study8,9 is a 13.5-year randomized trial that was initiated following the DPP to resolve conflicting findings from epidemiological studies concerning the health consequences of intentional weight loss.10,11 More than 5100 overweight participants with type 2 diabetes were randomly assigned to an intensive lifestyle intervention (ILI) or a diabetes support and education (DSE) group. The ILI was designed to induce a mean reduction in initial weight of 7% or more and to increase physical activity to at least 175 min/wk.9 During the first 6 months, participants attended 3 group sessions and 1 individual visit per month and replaced 2 meals per day with a liquid supplement (eg, SlimFast shakes), with a goal of consuming 1200 to 1800 kcal/d (with heavier participants receiving more calories). For months 7 to 12, ILI participants attended 2 group sessions and 1 individual visit each month, and used meal replacements for 1 meal per day. In years 2 to 4, participants were offered 1 individual on-site visit and 1 phone (or e-mail) contact per month, with periodic group sessions to help participants achieve the 7% weight loss goal or to reverse weight regain.12
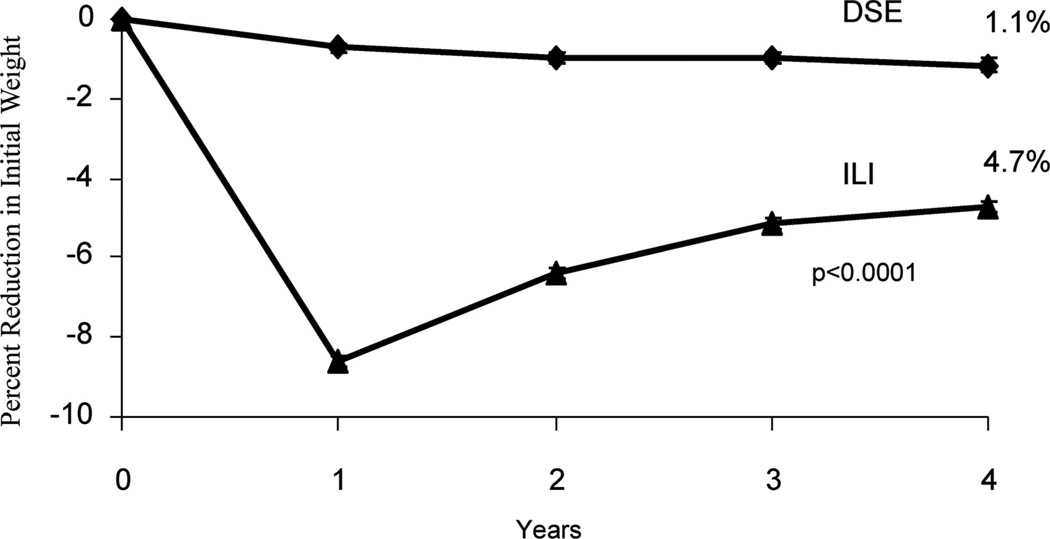
At 1 year, participants in the ILI lost 8.6 kg of initial weight in comparison with 0.7 kg in the DSE group (P<0.001).9 The ILI produced significantly greater improvements in hemoglobin A1c (HbA1c), fitness, and numerous measures of cardiovascular disease (CVD) risk than did DSE. At year 4, participants in the ILI maintained a loss of 4.9 kg in comparison with 1.3 kg in the DSE (P<0.001),12 demonstrating that long-term weight loss can be achieved with continued behavioral treatment. (Figure 1 presents these losses as percentage of reduction in initial weight.) The ILI group also maintained significantly greater improvements than DSE in HbA1c, fitness, high-density lipoprotein cholesterol, and systolic blood pressure.13 The trial is scheduled to conclude in 2014, at which time investigators will determine whether the health improvements observed in the ILI participants translate into a significant reduction in CVD morbidity and mortality.
Figure 1.
Percentage reduction in initial weight over 4 years for participants in the intensive lifestyle intervention and diabetes support and education groups of the Look AHEAD (Action for Health in Diabetes) study. DSE indicates diabetes support and education; ILI, intensive lifestyle intervention. Reprinted from Wadden et al,12 with permission.
Dietary Interventions
Both the DPP and Look AHEAD trials prescribed a traditional low-calorie, low-fat diet. However, the past decade has witnessed a torrent of research on the effects of dietary macronutrient composition on changes in weight and CVD risk factors. Four well-known diets include low-carbohydrate, low-fat, Mediterranean, and low-glycemic load regimens.14–28 Low-carbohydrate approaches, such as the Atkins diet,29 prescribe as few as 20 g/d of carbohydrate and typically are high in protein and fat. Higher protein intake is thought to be associated with better satiety, thus, potentially improving adherence to energy restriction.30,31 Low-fat diets, such as the Ornish diet,32 or as recommended by the American Heart Association,33 provide 10% to 20% of calories from fat and recommend plant-based foods including grains, fruits, and vegetables. Such approaches yield a low-energy-density diet that may improve satiety because of the large volume of food that can be consumed.34,35 Mediterranean-type diets encourage a higher intake of unsaturated fats such as olive oil, nuts, and fish, in lieu of saturated fats (eg, red meat and butter).26,36 The consumption of fruits, vegetables, and whole grains also is encouraged. Low-glycemic load diets are based on the principle that foods with a lower glycemic load—the product of a food’s glycemic index and the amount of carbohydrate in the item—have a more favorable effect on blood sugar.30,37 Portion-controlled diets, as exemplified by meal replacements, offer a fifth dietary option.16,38 They provide a fixed portion of food, often in the form of a high-protein, liquid diet, which simplifies meal preparation and calorie counting. Table 1 presents selected randomized trials (of at least 6 months duration) that compared weight losses achieved with these different approaches.
Table 1.
Weight Losses From Selected Randomized, Controlled Trials That Compared Diets With Varying Macronutrient Compositions
| Study | N | No. Lifestyle Sessions Provided |
Dietary Intervention | Weight Change |
Month | Comment/Other Results |
|---|---|---|---|---|---|---|
| Dansinger et al14 | 160 (51% F) | 4 | Atkins | −2.1 kga | 12 | All participants had hypertension, dyslipidemia, and/or fasting hyperglycemia. |
| 58% completed | Zone | −3.2 kga | There were no significant differences between groups in reductions in CVD risk factors. | |||
| Weight Watchers | −3.0 kga | |||||
| Ornish | −3.3 kga | |||||
| Das et al15* | 34 (% F unknown) | 52 | Low-glycemic load | −7.8%a | 12 | No differences were observed between groups in change in CVD risk factors. |
| 85% completed | High-glycemic load | −8.0%a | ||||
| Ditschuneit et al16* | 100 (79% F) | 27 | MR | −10.4 kgb | 27 | There were significantly greater reductions in total cholesterol and fasting insulin in the group that received meal replacements. |
| 63% completed | Conventional diet for 3 mo, followed by addition of MR | −7.7 kga | ||||
| Fabricatore et al17 | 79 (80% F) | 30 | Low-glycemic load | −4.5%a | 9 | All participants had type 2 diabetes. |
| 63% completed | Low-fat | −6.4%a | Larger reductions in HbA1c in the low-glycemic load group. | |||
| Foster et al18 | 63 (68% F) | 3 | Low-carbohydrate (high protein, high fat) | −4.4%a | 12 | HDL cholesterol increased more and triglycerides decreased more in the low-carbohydrate group. |
| 59% completed | Conventional (high-carbohydrate, low-fat) | −2.5%a | Greater reductions in LDL and total cholesterol in the low-fat group at 3 mo. | |||
| Foster et al19 | 307 (68% F) | 38 | Low-carbohydrate | −6.3 kga | 24 | Greater increase in HDL cholesterol in the low-carbohydrate group. Greater decrease in triglycerides at 3 and 6 mo in the low-carbohydrate group. Greater decrease in VLDL at 3, 6, and 12 mo in the low-carbohydrate group and in LDL at 3 and 6 mo in the low-fat group. |
| 63% completed | Low-fat | −7.4 kga | ||||
| Gardner et al20 | 311 (100% F) | 8 | Atkins (low-carbohydrate) | −4.7 kga | 12 | Greater increase in HDL cholesterol in Atkins than Ornish group and greater decrease in triglyceride levels in Atkins than Zone group. |
| 80% completed | Zone (even distribution) | −1.6 kgb | Systolic blood pressure decreased more in Atkins than in all other groups. Diastolic blood pressure decreased more in Atkins than in Ornish group. | |||
| LEARN (calorie-restricted) | −2.2 kga,b | |||||
| Ornish (low-fat) | −2.6 kga,b | |||||
| Iqbal et al21 | 144 (10% F) | 27 | Low-carbohydrate | −1.5 kga | 24 | All participants had diabetes. |
| 47% completed | Low-fat | −0.2 kga | No differences within or between groups for lipids or glycemic indexes. | |||
| Klemsdal et al22 | 202 (58% F) | 9 | Low-glycemic load | −4.0 kga | 12 | 62% participants with metabolic syndrome. |
| 81% completed | Low-fat | −4.3 kga | Waist circumference decreased more in low-fat diet group. | |||
| Diastolic blood pressure decreased more in the low glycemic load group. | ||||||
| Li et al23* | 104 (% F unknown) | 15 | Individualized diet | −2.4 kga | 12 | |
| 73% completed | Individualized diet+MR | −4.4 kga | HbA1c was significantly lower in MR group with use of repeated measurement analysis. | |||
| Sacks et al24 | 811 (64% F) | 66 | Low-fat, average-protein (highest carbohydrate) | −2.9 kga | 24 | LDL cholesterol decreased significantly more in lowest-fat/highest carbohydrate than in highest-fat/lowest carbohydrate groups. HDL cholesterol increased more with lowest carbohydrate than with the highest carbohydrate diet. |
| 80% completed | Low-fat, high-protein | −3.8 kga | All diets, except the highest carbohydrate, decreased fasting insulin (greater decrease in the high protein vs average protein diets). | |||
| High-fat, average-protein | −3.1 kga | |||||
| High-fat, high-protein (lowest carbohydrate) | −3.5 kga | |||||
| Samaha et al25 | 132 (17% F) | 9 | Low-carbohydrate | −5.8 kga | 6 | Triglyceride levels decreased more in the low-carbohydrate group than in the low-fat group. |
| 60% completed | Conventional (low-fat) | −1.9 kgb | Among diabetic participants, mean fasting glucose level decreased more in the low-carbohydrate group than in the low-fat group. | |||
| Shai et al26 | 322 (14% F) | 24 | Low-fat | −2.9 kga | 24 | HDL cholesterol increased in all groups, significantly more in the low-carbohydrate than low-fat group. |
| 85% completed | Mediterranean | −4.4 kgb | Triglyceride levels decreased more in the low-carbohydrate than in the low-fat group. | |||
| Low-carbohydrate | −4.7 kgb | In diabetic participants, only the Mediterranean diet group had a decrease in fasting glucose. | ||||
| Stern et al27 | 132 (17% F) | 15 | Low-carbohydrate | −5.1 kga | 12 | Triglyceride levels decreased more in the low-carbohydrate group than in the low-fat group. |
| 66% completed | Conventional (low-fat) | −3.1 kga | HDL cholesterol decreased less in the low-carbohydrate group than in the low-fat group. | |||
| Greater improvements in HbA1c in type 2 diabetics in the low-carbohydrate group. | ||||||
| Yancy et al28 | 120 (76% F) | 9 | Low-fat diet | −6.5 kga | 6 | Low-carbohydrate group showed greater decreases in triglycerides and greater increases in HDL. |
| 66% completed | Low-carbohydrate, ketogenic diet with nutritional supplements | −12.0 kgb |
All studies were analyzed by use of an intention-to-treat population, with the exception as indicated by an asterisk (*).
Different letters (in superscript) indicate statistically significant differences in weight loss between groups.
F indicates female; LDL, low-density lipoprotein; HDL, high-density lipoprotein; VLDL, very low density lipoprotein; HbA1c, hemoglobin A1c; MR, meal replacements; CVD, cardiovascular disease.
A completer’s population was examined.
Results reported as “greater,” “larger,” “increased more,” etc represent statistically significant differences between treatment conditions.
Low-Carbohydrate Versus Low-Fat Diets
Numerous trials have examined low-carbohydrate versus more conventional low-fat diets. Samaha et al25 reported that obese participants assigned to a low-carbohydrate diet lost significantly more weight at 6 months than those prescribed a low-calorie, low-fat regimen (−5.8 versus −1.9 kg). (Both groups of participants were provided 9 lifestyle counseling sessions over 6 months, as shown in Table 1.) However, Stern et al,27 in a follow-up of the same participants, found that differences between groups were no longer statistically significant at month 12 (−5.1 versus −3.1 kg). In their first study of this issue, Foster et al18 similarly found that a low-carbohydrate diet induced significantly greater weight loss at 6 months than a low-fat regimen (−7.0% versus −3.2%) when minimal lifestyle counseling was provided. However, differences between groups were not statistically significant at month 12 (−4.4% versus −2.5%). In their second investigation, in which all participants received intensive group lifestyle modification (ie, 33 sessions in the first year), both groups lost ≈11 kg at 1 year and maintained a loss of 6 to 7 kg at 2 years (with no significant differences between groups at any time).19 These findings indicate that a strong lifestyle modification program, which facilitates adherence to prescribed calorie goals, is more important for weight loss than is macronutrient composition per se. The absence of a high-intensity lifestyle intervention probably contributed to the modest weight losses that Iqbal et al21 observed in a 2-year trial of patients with type 2 diabetes who were treated by low-carbohydrate (−1.5 kg) or low-fat (−0.2 kg) diets.
In the largest investigation of this issue (811 participants), Sacks et al24 compared diets that provided either a low or high proportion of calories from fat (20% versus 40%), crossed with a low or high proportion of calories from protein (15% versus 25%). This resulted in 4 diets in which the calories derived from carbohydrate varied from 35% (lowest) to 65% (highest). All participants were instructed to achieve a 750 kcal/d deficit with the diet prescribed, and all received an intensive lifestyle intervention (ie, 22 counseling sessions during the first 6 months). No significant differences in weight loss were observed among the 4 diet groups over 2 years (see Table 1). These findings suggest that macronutrient composition does not contribute to weight loss when the level of caloric restriction is held constant.
Other trials summarized in Table 1 examined the effects of low-carbohydrate versus low-fat diets but included additional dietary comparisons. Shai et al26 reported that participants prescribed a low-fat diet lost significantly less weight (−2.9 kg) at 2 years than those assigned to low-carbohydrate (−4.7 kg) or Mediterranean (−4.4 kg) diets. Gardner et al20 studied women randomly assigned to 1 of 4 diets: low-carbohydrate (Atkins),29 even distribution of macronutrients (Zone),39 balanced calorie-restricted diet (LEARN),40 or low-fat (Ornish). 32 The Zone diet produced significantly less weight loss (−1.6 kg) at 1 year than the Atkins diet (−4.7 kg), whereas participants in the other groups lost ≈2.5 kg.20 Dansinger et al14 compared participants assigned to the Atkins, Zone, Weight Watchers,41 or Ornish diets and found no significant difference in weight loss at 1 year. Instead, only level of adherence to the prescribed diet was associated with weight loss. Weight losses in all 3 of the preceding trials were small (−5 kg), probably because of the limited provision of lifestyle counseling in comparison with that offered by Foster et al19 and Sacks et al.24
The trials summarized in Table 1 suggest that low-carbohydrate diets may produce larger short-term weight losses than low-fat diets, probably by inducing a greater caloric deficit through the near elimination of carbohydrates during the initial phase of dieting. However, this advantage does not typically remain at 1 year or more and can be overridden in the short term by the provision of a strong lifestyle intervention.19 Given the generally equivalent weight losses produced by these different approaches, desired changes in metabolic and cardiovascular risk factors should be considered in selecting a reducing diet. In the preceding trials, low-fat diets generally induced greater reductions in low-density lipoprotein cholesterol,18,19,24 whereas low-carbohydrate regimens were associated with greater improvements in triglycerides14,18–20,25–27 and high-density lipoprotein cholesterol18–20,24,26,27 and, in one study, with greater improvements in HbA1c in patients with type 2 diabetes.27
Glycemic Load Diets
Three trials of glycemic load diets also found no significant differences in weight loss according to diet type. Klemsdal et al22 randomly assigned participants to low-glycemic load or low-fat diets and observed 1-year losses of 4.0 kg and 4.3 kg, respectively. Das et al15 reported 1-year losses of 7.8% and 8.0% for participants assigned to low- versus high-glycemic load diets, respectively. Fabricatore et al17 compared low-glycemic load and low-fat diets in adults with type 2 diabetes. Weight losses did not differ significantly at 20 (−6.7% and −5.7%) or 40 weeks (−6.4% versus −4.5%). However, participants prescribed the low-glycemic load diet had significantly greater improvements in HbA1c than those on the low-fat diet.
Meal Replacements
Ditschuneit et al16 reported that participants who used liquid shakes and meal bars to replace 2 meals and 2 snacks a day lost 7.1 kg in 3 months in comparison with 1.3 kg for those prescribed a diet of conventional foods with the same calorie goal (1200–1500 kcal/d). Participants who were originally assigned to meal replacements, and who continued to replace 1 meal and 1 snack a day during follow-up, maintained a loss of 10.4 kg at 27 months.16 Li et al23 randomly assigned patients with type 2 diabetes to either a diet of soy-based meal replacements or a conventional diet and found that at month 12 the former group lost more weight (−4.4 versus −2.4 kg, P=0.07). A meta-analysis of 6 randomized, controlled trials (which included the 2 previous studies) found that participants who used meal replacements lost ≈2.5 kg more, at both 3 and 12 months, than did individuals prescribed an equivalent calorie diet of conventional foods.42 Additional studies, not included in Table 1, have shown that significantly greater weight loss is achieved by providing dieters portion-controlled servings of conventional foods43,44 or detailed menus of the foods they should consume.45
Summary
Obese individuals can lose weight by following reducing diets that vary widely in macronutrient composition. Caloric restriction, however, rather than macronutrient composition, is the key determinant of weight loss. Because all of the diets reviewed appear to have comparable short- and long-term safety, the choice of the diet can be guided by the desired control of comorbid conditions. Further research is needed to determine the optimal dietary macronutrient composition for improving specific comorbid conditions, such as impaired glycemic control, as investigated in the DPP and Look AHEAD studies. Obese populations selected for specific CVD risk factors will provide greater opportunity to demonstrate the benefits of both weight loss and macronutrient composition than will populations selected on obesity status alone.
The choice of a diet also should address patient preferences, particularly those related to ease of dietary adherence.14 A successful reducing diet is one that an individual can adhere to for several months to lose 5% to 10% of initial weight. Greater weight loss is desirable because it is associated, in a linear manner, with greater improvements in CVD risk factors, including HbA1c, blood pressure, triglycerides, and high-density lipoprotein cholesterol.46 Although dietary interventions for hypertension and other comorbid conditions have shifted to the prescription of dietary patterns, such as the Dietary Approaches to Stop Hypertension diet,47,47,48 such approaches must be combined with calorie restriction to induce clinically meaningful weight loss (as they have in some recent trials).49,50 To achieve long-term weight loss, most obese individuals must consciously restrict their energy intake, whether by reducing portion sizes, decreasing the energy density of the diet, counting calories (or specific macronutrients), or some combination of these approaches.
Physical Activity and Weight Management
Physical activity plays a critical role in improving cardiovascular health in both average-weight and obese individuals. 51–53 In the absence of significant weight loss, regular bouts of aerobic activity have been found to reduce blood pressure54 and lipids,55 as well as visceral fat,56,56,57 the latter which is associated with improved glucose tolerance and insulin sensitivity (in nondiabetic individuals) and glycemic control (in patients with type 2 diabetes).58–61 Leskinen et al62 examined 16 twin pairs with discordant levels of physical activity and found that inactive twins had greater amounts of high-risk fat, including visceral, liver, and intramuscular. In sum, physical activity, independent of weight loss, appears to be associated with improvements in body composition and metabolic conditions.
Physical fitness, which generally increases with increased physical activity, also may attenuate obesity-related mortality. Lee et al63,64 examined >21 000 men in the Aerobic Center Longitudinal Study and found that those who were fat but fit had lower rates of cardiovascular death than those who were average weight but unfit. Subsequent studies, however, with more diverse samples that included both men and women, found that fitness only partially attenuated the risk of mortality associated with excess adiposity.65,66 Fatness and low fitness appear to be independent risk factors for CVD morbidity and mortality, and both should be targeted in comprehensive lifestyle programs.4
Physical Activity and Weight Loss
Physical activity alone is of limited benefit in inducing weight loss, as reported in a recent position article of the American College of Sports Medicine.67 This is because most individuals cannot find the time or motivation to engage in the high volume of activity (eg, 35 miles of walking a week) required to lose even 0.45 kg/wk. This rate of weight loss is more easily achieved by participants’ restricting their food intake by 500 kcal/d. A study by Slentz et al68 underscored the minimal benefit of exercise alone for weight loss. Individuals who jogged/ran the equivalent of 20 miles a week (but were not instructed to restrict their food intake) lost only 3.5 kg at the end of 8 months of training. Individuals who walked 12 miles a week at a moderate intensity (the equivalent of 6 one-half hour bouts of walking a week) lost only 1.1 kg.68 Similarly, the addition of regular exercise to caloric restriction (ie, dieting) only marginally increases short-term weight loss, although it does spare the loss of fat-free mass.69 These findings suggest that obese individuals should be encouraged to exercise, at least in the short term, for the sake of improving their cardiovascular health, rather than for inducing weight loss.
Physical Activity and Weight Loss Maintenance
Physical activity appears to be critical for long-term weight management, as concluded by several investigators.70–75 Jeffery et al71 showed that participants in a 12-month group behavioral weight control program who were prescribed high levels of physical activity (ie, expenditure of 2500 kcal/wk achieved principally with brisk walking) maintained significantly greater weight losses at 12 months and 18 months than participants with a lower, more traditional physical activity goal (ie, 1000 kcal/wk). Losses for the 2 groups at 18 months were 6.7 and 4.1 kg, respectively. By contrast, the greater dose of exercise had no significant effect on weight loss at 6 months (losses of 9.0 and 8.1 kg, respectively).
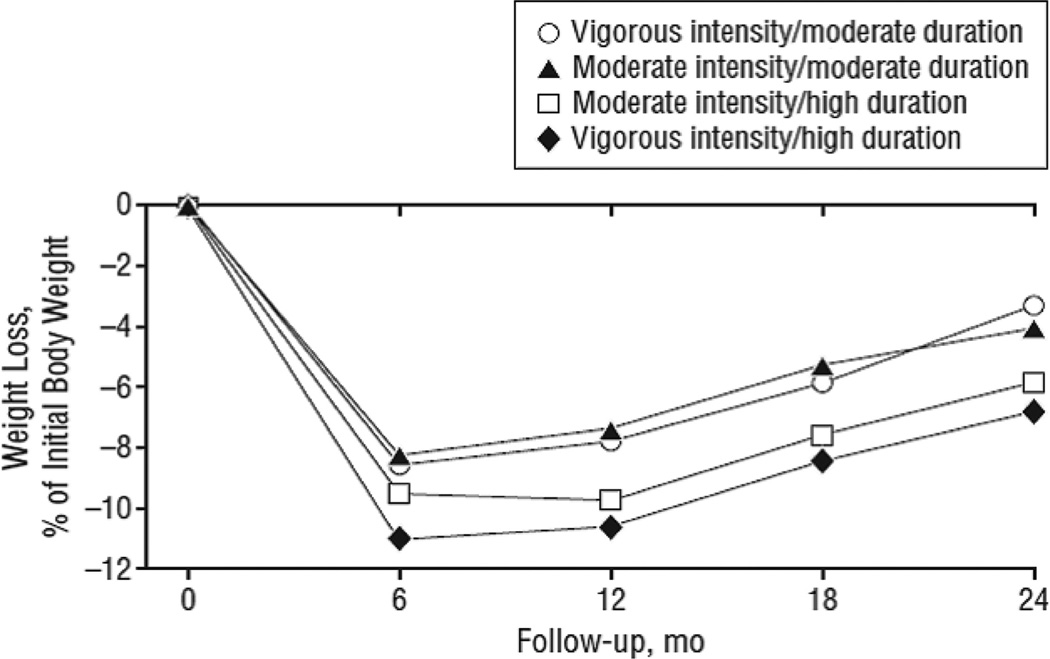
Jakicic et al76 studied the effect of varying both the duration and intensity of physical activity in 4 groups of obese patients, all of whom received group lifestyle modification for 12 months. The moderate exercise duration group was instructed to expend 1000 kcal/wk, whereas the high-duration group had a goal of 2000 kcal/wk. Intensity was determined by use of ratings of perceived exertion.77 All groups were provided motorized treadmills and asked to exercise 5 days a week. At month 12, there were no significant differences in weight loss among the 4 groups, with mean losses ranging from 6.3 kg in the moderate-intensity/moderate-duration group to 8.9 kg in the high-intensity/high-duration condition; the other 2 groups fell in between. At month 24, there again were no significant differences in weight loss among the 4 groups, and all regained weight from month 12 (see Figure 2).78
Figure 2.
Percentage reduction in initial weight for overweight or obese women assigned to 1 of 4 exercise prescriptions (in addition to a 1200–1500 kcal/d diet): moderate-intensity/moderate-energy expenditure (expend 1000 kcal/wk), moderate-intensity/high-energy expenditure (expend 2000 kcal/wk), vigorous-intensity/moderate-energy expenditure, or vigorous-intensity/high-energy expenditure. Reprinted from Jakicic et al,78 with permission of the publisher. Copyright © 2008, American Medical Association.
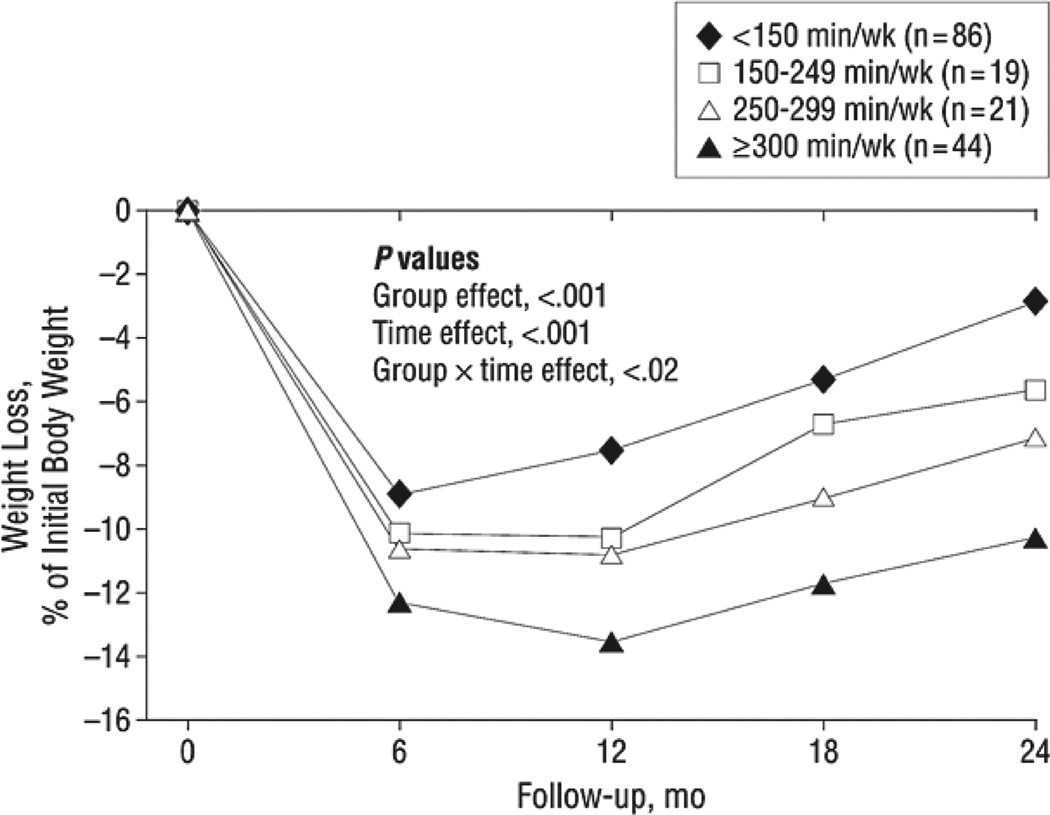
These findings suggest that higher levels of physical activity have no effect on short- or long-term weight loss. This conclusion, however, overlooks the critical issue of participants’ adherence to their exercise prescription. Data were reanalyzed to examine the amount of physical activity participants reported engaging in (regardless of their treatment assignment) and showed that those who exercised ≥300 min/wk (expending ≈2000 kcal/wk) maintained weight losses nearly 3 times as great as participants whose activity was <150 min/wk (who expended <1000 kcal/wk) (see Figure 3). Jakicic et al75 similarly found, in secondary analyses of the results of another randomized trial, that obese individuals who exercised >200 min/wk achieved significantly greater weight losses at 18 months than individuals who were active <150 min/wk.
Figure 3.
Percentage weight loss by minutes of physical activity (kilocalories per week) (n = 170). Reprinted from Jakicic et al,78 with permission of the publisher. Copyright © 2008, American Medical Association.
Two systematic reviews, which examined both randomized trials and observational data, concluded that weight regain (following weight loss) was reduced by engaging in high levels of physical activity, ≈60 min/d of brisk walking.67,79 This is the level of activity displayed by individuals in the National Weight Control Registry, all of whom have lost ≥13.6 kg and maintained the loss for ≥1 year.73,80 A prospective analysis of intentional weight loss in the Nurses’ Health Study II also confirmed the benefits of high levels of activity for maintaining lost weight.81
Mechanisms of Action
Researchers do not fully understand how physical activity facilitates the maintenance of lost weight. The simplest explanation is that increased physical activity helps to maintain energy balance.52 Walking 3 miles a day should offset occasional excess calorie intake that otherwise would be associated with weight regain. Alternatively, high levels of physical activity appear to be necessary to compensate for increased energy efficiency following weight loss. Rosenbaum et al82 studied obese individuals who had lost 10% of initial weight and found that maintenance of their reduced body weight was associated with a decrease in total energy expenditure that was ≈300 to 500 kcal/d greater than that predicted by changes in body mass and composition. The decrease in total energy expenditure was due predominately to reduced energy expended during physical activity (ie, nonresting energy expenditure), reflecting increased work efficiency of skeletal muscle. Thus, paradoxically, successful weight losers may have to approximately double their physical activity to compensate for their increased energy efficiency.
Other possible favorable effects of physical activity, particularly of resistance training, include sparing the loss of fat-free mass, an occurrence that may attenuate the reduction in resting-metabolic rate that accompanies weight loss.52,83–85 Regardless of the mechanisms of action, the message is the same: overweight/obese individuals should increase their physical activity by whatever means possible to keep off the lost weight.
Summary
Overweight and obese individuals, as well as persons of average weight, often report not having time to exercise. Practitioners should emphasize that, for weight control, this activity can be performed at a moderate intensity and in short bouts, as brief as 10 minutes. When included as part of a comprehensive weight loss program, multiple short bouts of activity throughout the day are as effective as 1 long bout (>40 minutes) for achieving weight loss.86,87 Additional studies have shown that lifestyle activity, which involves increasing energy expenditure throughout the day, without concern for the intensity or duration of the activity, is as effective for weight control as more traditional programmed activity (such as jogging, swimming, or biking).88,89 Some investigators believe that further study is needed to identify the types of physical activities that participants in lifestyle interventions engage in to determine that they, in fact, differ from behaviors of individuals assigned to structured exercise programs.90 Pedometers provide one of the most convenient methods of monitoring lifestyle activity.91 Overweight and obese individuals initially should be encouraged to walk an extra 2000 steps per day, which would expend ≈100 kcal more. The goal after losing 10% or more of initial weight is to eventually walk an extra 6000 to 8000 steps daily, which will provide the additional 300 to 400 kcal/d expenditure required for weight maintenance.73,92
Behavior Therapy
As applied to weight control, behavior therapy refers to a set of principles and techniques for helping obese individuals modify eating, activity, and thinking habits that contribute to their excess weight.4,40,93 This approach recognizes that body weight is affected by factors other than behavior, including genetic, metabolic, and hormonal influences, which likely predispose some persons to obesity and set the range of possible weights that a given individual can achieve.4 Key components of behavior therapy include setting specific goals for behavior change that specify what an individual will do, and when, where, how, and for how long he or she will engage in the behavior.94 A behavioral goal for exercising more would take the form of, “I will walk on the track at the local high school for 30 minutes at a brisk pace on Monday to Saturday at 7 am. If it rains, I will exercise in the den using my walking DVD.”
Self-monitoring—recording one’s behavior—is perhaps the most important component of behavioral treatment.4 Patients keep detailed records of their food intake, physical activity, and body weight, which they review with their interventionist to identify areas of success and areas in need of improvement. Record keeping is expanded over time to include information about times, places, thoughts, and feelings associated with eating and physical activity. Frequent self-monitoring is a consistent predictor of both short- and long-term weight loss.95–98
Since its introduction in 1967,99 behavioral treatment has evolved into a package that includes several components in addition to diet, exercise, and self-monitoring. These components include slowing the rate of eating, stimulus control, problem solving, cognitive restructuring, and relapse prevention training. Detailed descriptions of this approach have been provided.6,40
Structure and Efficacy of Behavioral Weight Control
Behavioral weight control traditionally has been provided on site to groups of 10 to 20 individuals by registered dietitians, psychologists, exercise specialists, and other health professionals.4,93 In academic medical centers, intervention is usually delivered weekly for 20 to 26 weeks, followed by every-other-week sessions for an additional 20 to 26 weeks, designed to facilitate the maintenance of lost weight. Treatment sessions usually last 60 to 90 minutes. Group treatment may produce somewhat larger weight loss (about 2 kg) than individual care, as shown by a randomized trial.100 Group sessions are more cost-effective than individual care and provide empathy, competition, and social support.94 Sessions typically begin with a review of participants’ food and activity records and continue with problem solving and the introduction of a new topic.94,101
Short-Term Efficacy
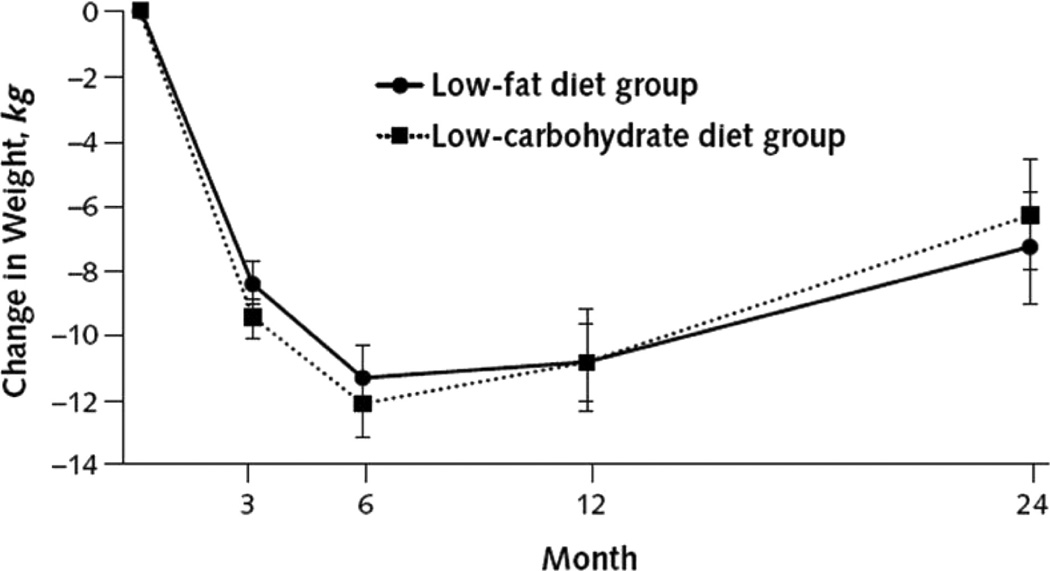
Several reviews have shown that a comprehensive lifestyle modification program, as described above, produces an average weight loss of ≈7 to 10 kg in 6 months, equal to a 7% to 10% reduction in initial weight.2,4,93 This mean loss is generally sustained at 12 months, with the provision of on-site, every-other-week weight maintenance therapy. The previously described results of Foster et al19 are among the best obtained with a state-of-the art lifestyle intervention combined with a traditional low-calorie, low-fat diet. All study participants (including those assigned to a low-carbohydrate diet) were provided 20 initial weekly group sessions, 10 every-other-week maintenance sessions (through week 40), and then every-other-month sessions through year 2. As shown in Figure 4, participants in both diet groups lost ≈11.8 kg at 6 months and maintained a loss of approximately this size at 12 months (10.8 kg for both groups).
Figure 4.
Change in body weight for participants in low-fat and low-carbohydrate diet groups after 24 months, based on random-effects linear model. Reprinted from Foster et al, 19 with permission of the publisher. Copyright © 2010, American College of Physicians.
Long-Term Efficacy
Figure 4 reveals that participants in both the low-fat and low-carbohydrate groups regained ≈3.5 to 4.5 kg from months 12 to 24, equal to a regain of 30% to 40% of their 12-month weight loss. This degree of regain is consistently observed in the year following weight loss in the absence of participants receiving ongoing weight loss maintenance therapy.4,93,101 Foster et al19 provided participants every-other-month group sessions from months 9 to 24, but this frequency of treatment does not appear to be sufficient to prevent weight regain. By contrast, Perri et al102–104 have shown in a series of studies that every-other-week maintenance sessions, provided on site, are associated with excellent long-term weight loss. In a representative study, individuals who attended twice monthly group maintenance sessions for the year following weight reduction maintained 13.0 kg of their 13.2-kg end-of-treatment weight loss, whereas those who did not receive such therapy maintained only 5.7 kg of a 10.8-kg loss.102 In reviewing 13 studies on this topic, Perri and Corsica105 found that patients who received long-term treatment, which averaged 54 weeks, maintained 10.3 kg of their initial 10.7-kg weight loss.
Weight loss maintenance sessions appear to provide participants the support and motivation needed to continue to practice weight control behaviors, which include engaging in ≈60 min/d of physical activity; eating a low-calorie, low-fat diet; monitoring body weight frequently (at least weekly and as often as daily); and periodically recording food intake (particularly in response to weight gain).105 These are the behaviors practiced by participants in the National Weight Control Registry.73 (Table 2 summarizes what the authors believe are the critical components of lifestyle intervention required for the induction and maintenance of weight loss.) Despite concerns that long-term weight control efforts may be associated with depression, eating disorders, and other behavioral complications, these problems generally have not been observed in Registry participants. On the contrary, the great majority of individuals have reported marked improvements in their quality of life with the maintenance of substantial weight loss (≥13.6 kg).73
Table 2.
Key Components of a Comprehensive Lifestyle Modification Program to Achieve and Maintain a 7% to 10% Weight Loss at 1 Year or More
| Components | Weight Loss | Weight Loss Maintenance |
|---|---|---|
| Frequency and duration of treatment contact | Weekly contact, in person or by telephone, for 20–26 wk. (Internet/e-mail contact yields smaller weight loss.) | Every-other-week contact for 52 wk (or longer). (Monthly contact may be adequate.) |
| Group or individual contact. | Group or individual contact. | |
| Dietary prescription | Low-calorie diet (1200–1500 kcal for individuals <250 lb, 1500–1800 kcal for those ≥250 lb). | Consumption of a hypocaloric diet to maintain reduced body weight. |
| Typical macronutrient composition: ≤30% fat (≤7% saturated fat); 15%–25% protein; remainder from carbohydrate. (Diet composition may vary based on individual needs or preferences.) | Typical macronutrient composition: similar to that for weight loss. | |
| Physical activity prescription | 180 min/wk of moderately vigorous aerobic activity (eg, brisk walking); strength training also desirable. | 200 to 300 min/wk of moderately vigorous aerobic activity (eg, brisk walking); strength training also desirable. |
| Behavior therapy prescription | Daily monitoring of food intake and physical activity by use of paper or electronic diaries. | Occasional to daily monitoring of food intake and physical activity by use of similar diaries. |
| Weekly monitoring of weight. | Twice weekly to daily monitoring of weight. | |
| Structured curriculum of behavior change (eg, Diabetes Prevention Program). | Curriculum of behavior change, including relapse prevention and individualized problem solving. | |
| Regular feedback from an interventionist. | Periodic feedback from an interventionist. |
Innovations in the Delivery of Lifestyle Modification
Investigators are examining new modalities for delivering behavioral weight control, given the explosion in electronic means of communication (eg, Internet, e-mail, text messaging, Facebook, and Twitter), with their potentially greater convenience and reduced cost. This section briefly examines efforts to deliver lifestyle modification by telephone and the Internet.
Telephone-Delivered Programs
Participants treated by Donnelly et al106 received a 12-week weight loss program (which included a 1200–1500 kcal/d diet of meal replacements and conventional foods), followed by a 14-week weight maintenance program. Half of the participants received all instruction by way of group conference calls, and the other half attended on-site groups. Median weight losses at 12 weeks were 10.6 kg and 12.7 kg, respectively (P<0.05), and at 26 weeks were 12.8 and 12.5 kg, respectively (and not significantly different). DiGenio et al107 examined the efficacy of telephone versus on-site counseling in participants who also were prescribed 10 mg/d of the weight loss medication sibutramine. At month 6, participants in these 2 groups, each of whom had 18 counseling sessions with a dietitian, lost 7.7% and 8.9% of initial weight, respectively (with no differences between groups). By contrast, participants who received medication but had only monthly (on-site) counseling visits lost 6.4%, whereas those who received no counseling lost 5.2%. Both losses were significantly smaller than those of participants in the first 2 groups. Findings of both Donnelly et al and DiGenio et al suggest that telephone-based counseling is approximately as effective as in-person treatment for inducing weight loss.106,107
Perri et al108 have demonstrated the effectiveness of telephone-based counseling for maintaining lost weight. Obese women who had lost an average of 10 kg during a 6-month run-in period were randomly assigned to receive a twice monthly weight loss maintenance program for 1 year that was delivered by telephone or on site. Women in a third group received newsletters only. Participants in the 2 weight loss maintenance interventions both regained only 1.2 kg during the year in comparison with a significantly greater gain of 3.7 kg for those in the newsletter group. Appel et al50 also reported excellent maintenance of weight loss at 2 years with a principally telephone-delivered intervention. Cost-effective analyses are needed to further demonstrate the benefits of telephone versus on-site interventions.
Internet-Delivered Programs
In an early study of weight loss induction, Tate et al109 randomly assigned participants to one of two 6-month programs delivered by Internet. The educational (control) intervention provided a directory of Internet resources for weight management (but no specific instruction in changing eating and activity habits). The behavior therapy intervention included this directory but also 24 weekly lessons conducted by e-mail in which participants submitted their food and activity records on-line and received feedback from a therapist. Participants in the behavior therapy group lost significantly more weight than those in the educational group (4.1 versus 1.6 kg). In a follow-up study, which lasted 1 year, Tate et al110 randomly assigned individuals at risk of type 2 diabetes to a low-intensity Internet intervention or to the same program with the addition of weekly behavioral counseling, delivered by e-mail. Participants in the latter group lost significantly more weight at 1 year (2.0 versus 4.4 kg). These 2 studies underscore the importance of participants’ monitoring their food intake and physical activity, as well as completing other behavioral assignments. Educational instruction alone (ie, information) is not sufficient to induce clinically meaningful weight loss.111,112
The first controlled comparison of Internet versus on-site interventions for inducing weight loss was recently reported by Harvey-Berino et al,113 who provided obese adults in the 2 groups the same 24-session intervention. Participants in the Internet program lost 5.5 kg in 6 months in comparison with a significantly greater 8.0 kg for those who received on-site treatment. Collectively, these studies suggest that the most successful Internet programs, in which interventionists provide weekly e-mail feedback to participants, will induce weight losses of approximately two-thirds the size of those achieved by traditional on-site behavioral programs.4 The reduced efficacy of Internet programs, however, is offset by the potentially greater accessibility and affordability of this approach in comparison with traditional on-site treatment.
Internet for Maintenance of Lost Weight
Several trials have demonstrated the potential benefit of Internet-delivered programs for improving the maintenance of lost weight.114–116 Two studies compared Internet-delivered programs with those delivered person to person, either on-site or by phone.117,118 Both trials found that person-to-person interventions were slightly more effective in maintaining lost weight than were Internet-based programs. However, the reduced efficacy of the Internet is again potentially mitigated by the increased reach, affordability, and convenience of the latter approach.
Dissemination of Behavioral Treatment
Investigators are exploring additional means, besides electronic interventions, of adapting intensive lifestyle interventions to reach a larger proportion of the overweight and obese individuals who would benefit from them. Behavioral treatment has been provided in community settings and as part of commercial weight loss programs.
Community Settings
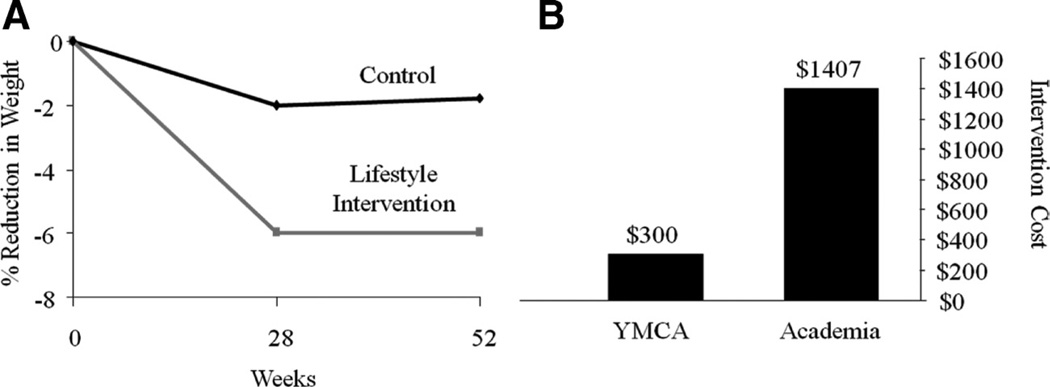
Ackermann et al119 adapted the DPP to be delivered by Young Men’s Christian Association (YMCA) staff. Treated participants received 16 group sessions and lost 6.0% of initial weight at 6 months in comparison with 2.0% for control participants who received advice only. This significant difference between groups was maintained at 12 months. Estimated cost per treated participant was $275 to $325, which was substantially lower than the estimated cost of $1400 per participant for the first year of the original DPP120 (see Figure 5). Although these results are promising, they are limited by the study’s modest sample size (N = 92), high attrition (37% at month 12), and the use of a completers analysis.
Figure 5.
A, Percentage reduction in initial weight for participants who received a version of the (DPP adapted for group-delivery in a YMCA vs participants in a control group. Figure was drawn using data from Ackermann et al.119 B, Cost to implement 1 year of the DPP as adapted for YMCA delivery vs the original DPP program. Figure was draw using data from Ackermann and Marreo.120 DPP indicates Diabetes Prevention Program; YMCA, Young Men’s Christian Association.
Commercial Weight Loss Programs
Commercial weight loss programs vary substantially in the treatment options they offer, as well as in their scientific soundness, efficacy, and cost.121,122 Many include behavioral and dietary counseling (delivered in-person or by telephone or Internet), group support, and prepackaged meals. The 3 largest commercial providers are Weight Watchers, Jenny Craig, and Nutrisystem. Weight Watchers offers a dietary plan based on a point system in combination with group meetings or a Web-based monitoring program.123 Jenny Craig customers consume prepackaged meals and choose from in-person or telephone-delivered individual counseling.124 Nutrisystem also offers prepackaged, home-delivered meals, but typically does not provide counseling; online self-monitoring tools are available on a web site.125
Table 3 presents selected randomized trials (of 6 months or longer) that compared a commercial program with a control group.41,126–131 In the first multisite evaluation of Weight Watchers, Heshka et al randomly assigned participants to Weight Watchers or self-help and found that participants in the former groups lost significantly more weight at 1 year (−4.3 kg versus −1.3 kg) and 2 years (−2.9 kg versus −0.2 kg).41,130 A more recent trial, which included sites from 3 countries, replicated these 1-year weight losses (although far higher attrition was observed).131
Table 3.
Weight Losses From Randomized Trials That Evaluated Selected Commercial Weight Loss Programs
| Program Study | N | Intervention | Mean Weight Change | Mean Weight Change at Long- Term Follow-Up |
Attrition | Attrition at Long-Term Follow-Up |
|---|---|---|---|---|---|---|
| Jenny Craig | ||||||
| Rock et al126 | 70 (100% F) | Center-based Jenny Craig | −7.2 kg at 6 moa | −7.3 kg at 12 moa | 0% at 6 mo | 9% at 12 mo |
| Usual care (2 meetings with dietitian and print materials) | −0.3 kg at 6 mob | −0.7 kg at 12 mob | 0% at 6 mo | 6% at 12 mo | ||
| Rock et al127 | 442 (100% F) | Center-based Jenny Craig | −10.1 kg at 12 moa | −7.4 kg at 24 moa | 5% at 12 mo | 10% at 24 mo |
| Telephone-based Jenny Craig | −8.5 kg at 12 moa | −6.2 kg at 24 moa | 4% at 12 mo | 7% at 24 mo | ||
| Usual care (2 individual counseling sessions and monthly contacts) | −2.4 kg at 12 mob | −2.0 kg at 24 mob | 9% at 12 mo | 7% at 24 mo | ||
| Nutrisystem | ||||||
| Foster et al128 | 69 (71% F) | Nutrisystem D+group behavioral treatment for 24 wk | −7.1% at 12 wka | −9.7% at 24 wk | 14% at 24 wk | N/A |
| 100% T2D | Diabetes Support and Education for 12 wk, then Nutrisystem D+group behavioral treatment for 12 wk | −0.4% at 12 wkb | −5.3% at 24 wk | 18% at 24 wk | ||
| Weight Watchers | ||||||
| Djuric et al129* | 48 (100% F) | Usual care | +0.9 kg at 12 moa | N/A | 19% at 12 mo | N/A |
| Weight Watchers | −2.6 kg at 12 moa | |||||
| Individual counseling | −8.0 kg at 12 mob | |||||
| Weight Watchers+individual counseling | −9.4 kg at 12 mob | |||||
| Heshka et al41,130 | 423 (85% F) | Weight Watchers | −4.3 kg at 12 moa | −2.9 kg at 24 moa | 20% at 12 mo | 25% at 24 mo |
| Self-help (with 2 dietitian visits) | −1.3 kg at 12 mob | −0.2 kg at 24 mob | 17% at 12 mo | 29% at 24 mo | ||
| Jebb et al131 | 772 (87% F) | Weight Watchers | −4.06 kg at 12 moa | N/A | 42% at 12 mo | N/A |
| Usual care | −1.77 kg at 12 mob |
All studies were analyzed using an intention-to-treat population, except as indicated by an asterisk (*), in which case a completers population was examined.
Different letters (in superscript) indicate statistically significant differences between groups.
F indicates female; N/A, not applicable; T2D, type 2 diabetes.
Adapted from Webb et al.122
In the largest assessment of Jenny Craig, Rock et al127 randomly assigned women to usual care (ie, 2 in-person counseling sessions and monthly contacts), Jenny Craig In-Center (ie, face-to-face) or telephone-based Jenny Craig counseling (weekly contact for 2 years). At 1 year, mean losses in the face-to-face and telephone-based groups were 10.1 and 8.5 kg, respectively, in comparison with 2.4 kg for women in usual care. At 2 years, participants maintained losses of 7.4 kg, 6.2 kg, and 2.0 kg, respectively. Both Jenny Craig groups were superior to usual care but did not differ significantly from each other. Nutrisystem has been assessed in a relatively small trial.128 At 3 months, participants who were prescribed the Nutrisystem diet lost significantly more weight than those in a diabetes support and education group (7.1% versus 0.4%). Losses in the Nutrisystem group were maintained at 6 months.
Results from these studies suggest that commercial weight loss programs may be a viable option for overweight and obese individuals who cannot obtain traditional lifestyle modification in community settings or academic medical centers. However, access to these programs, particularly those that provide prepackaged foods, may be limited by cost.
Conclusions and Future Directions
A comprehensive program of lifestyle modification produces a 7% to 10% reduction in initial weight that is associated with clinically meaningful improvements in several CVD risk factors, including the prevention of type 2 diabetes. New technologies, including wi-fi scales (which can automatically transmit weights from a scale to a server), smart phones, and tablets (and dozens of weight loss applications, as well) should make it easier and more convenient for individuals to monitor their food intake, physical activity, and weight—behaviors that are critical for short- and long-term weight control. Moreover, overweight and obese individuals will increasingly participate in programs that are delivered from a distance, whether by call centers, Internet-based programs, text messaging, or social networking sites. These electronic delivery channels should improve the dissemination of intensive lifestyle programs to the millions of individuals who would benefit from them. These developments are anticipated with the full knowledge that efforts to improve the treatment of obesity must be complemented by far greater public health efforts to prevent the development of this disorder.132
Acknowledgments
The authors thank Dr Meghan Butryn for her suggestions for revising the article. We also thank two anonymous reviewers for their valuable suggestions.
Sources of Funding
This work was supported by grant K24-DK065018 from the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Disclosures
Dr Wadden serves on the advisory boards of BMIQ and Free and Clear, each of which provides electronically supported weight reduction programs to the public. He has received grant support from Nutrisystem to participate on a multisite evaluation of this program’s effectiveness. The other authors report no conflicts.
References
- 1.World Health Organization. Obesity: Preventing and Managing the Global Epidemic. Geneva, Switzerland: World Health Organization; 1998. [PubMed] [Google Scholar]
- 2.National Institutes of Health/National Heart, Lung, and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Obes Res. 1998;6:51S–210S. [PubMed] [Google Scholar]
- 3.National Heart, Lung, and Blood Institute (NHLBI) and the North American Association for the Study of Obesity (NAASO) The Practical Guide: Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. Bethesda, MD: National Institutes of Health; 2000. [Google Scholar]
- 4.Wadden TA, Butryn ML, Wilson C. Lifestyle modification for the management of obesity. Gastroenterology. 2007;132:2226–2238. doi: 10.1053/j.gastro.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 5.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Diabetes Prevention Program Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25:2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diabetes Prevention Program Research Group. Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, Brenneman AT, Brown-Friday JO, Goldberg R, Venditti E, Nathan DM. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryan DA, Espeland MA, Foster GD, Haffner SM, Hubbard VS, Johnson KC, Kahn SE, Knowler WC, Yanovski SZ Look AHEAD Research Group. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24:610–628. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 9.Look AHEAD Research Group. Pi-Sunyer X, Blackburn G, Brancati FL, Bray GA, Bright R, Clark JM, Curtis JM, Espeland MA, Foreyt JP, Graves K, Haffner SM, Harrison B, Hill JO, Horton ES, Jakicic J, Jeffery RW, Johnson KC, Kahn S, Kelley DE, Kitabchi AE, Knowler WC, Lewis CE, Maschak-Carey BJ, Montgomery B, Nathan DM, Patricio J, Peters A, Redmon JB, Reeves RS, Ryan DH, Safford M, Van Dorsten B, Wadden TA, Wagenknecht L, Wesche-Thobaben J, Wing RR, Yanovski SZ. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diabetes Care. 2007;30:1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gregg EW, Gerzoff RB, Thompson TJ, Williamson DF. Intentional weight loss and death in overweight and obese U.S. adults 35 years of age and older. Ann Intern Med. 2003;138:383–389. doi: 10.7326/0003-4819-138-5-200303040-00007. [DOI] [PubMed] [Google Scholar]
- 11.Andres R, Muller DC, Sorkin JD. Long-term effects of change in body weight on all-cause mortality. A review. Ann Intern Med. 1993;119:737–743. doi: 10.7326/0003-4819-119-7_part_2-199310011-00022. [DOI] [PubMed] [Google Scholar]
- 12.Wadden TA, Neiberg RH, Wing RR, Clark JM, Delahanty LM, Hill JO, Krakoff J, Otto A, Ryan DH, Vitolins MZ. Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obesity. 2011;19:1987–1998. doi: 10.1038/oby.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Look AHEAD Research Group. Wing RR. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med. 2010;170:1566–1575. doi: 10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA. 2005;293:43–53. doi: 10.1001/jama.293.1.43. [DOI] [PubMed] [Google Scholar]
- 15.Das SK, Gilhooly CH, Golden JK, Pittas AG, Fuss PJ, Cheatham RA, Tyler S, Tsay M, McCrory MA, Lichtenstein AH, Dallal GE, Dutta C, Bhapkar MV, Delany JP, Saltzman E, Roberts SB. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: a 1-y randomized controlled trial. Am J Clin Nutr. 2007;85:1023–1030. doi: 10.1093/ajcn/85.4.1023. [DOI] [PubMed] [Google Scholar]
- 16.Ditschuneit HH, Flechtner-Mors M, Johnson TD, Adler G. Metabolic and weight loss effects of long-term dietary intervention in obese subjects. Am J Clin Nutr. 1999;69:198–204. doi: 10.1093/ajcn/69.2.198. [DOI] [PubMed] [Google Scholar]
- 17.Fabricatore AN, Wadden TA, Ebbeling CB, Thomas JG, Stallings VA, Schwartz S, Ludwig DS. Targeting dietary fat or glycemic load in the treatment of obesity and type 2 diabetes: a randomized controlled trial. Diabetes Res Clin Pract. 2011;92:37–45. doi: 10.1016/j.diabres.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, Szapary PO, Rader DJ, Edman JS, Klein S. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348:2082–2090. doi: 10.1056/NEJMoa022207. [DOI] [PubMed] [Google Scholar]
- 19.Foster GD, Wyatt HR, Hill JO, Makris AP, Rosenbaum DL, Brill C, Stein RI, Mohammed BS, Miller B, Rader DJ, Zemel B, Wadden TA, Tenhave T, Newcomb CW, Klein S. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: a randomized trial. Ann Intern Med. 2010;153:147–157. doi: 10.1059/0003-4819-153-3-201008030-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gardner CD, Kiazand A, Alhassan S, Kim S, Stafford RS, Balise RR, Kraemer HC, King AC. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA. 2007;297:969–977. doi: 10.1001/jama.297.9.969. [DOI] [PubMed] [Google Scholar]
- 21.Iqbal N, Vetter ML, Moore RH, Chittams JL, Dalton-Bakes CV, Dowd M, Williams-Smith C, Cardillo S, Wadden TA. Effects of a low-intensity intervention that prescribed a low-carbohydrate vs. a low-fat diet in obese, diabetic participants. Obesity. 2010;18:1733–1738. doi: 10.1038/oby.2009.460. [DOI] [PubMed] [Google Scholar]
- 22.Klemsdal TO, Holme I, Nerland H, Pedersen TR, Tonstad S. Effects of a low glycemic load diet versus a low-fat diet in subjects with and without the metabolic syndrome. Nutr Metab Cardiovasc Dis. 2010;20:195–201. doi: 10.1016/j.numecd.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Li Z, Hong K, Saltsman P, DeShields S, Bellman M, Thames G, Liu Y, Wang HJ, Elashoff R, Heber D. Long-term efficacy of a soy-based meal replacements vs an individualized diet plan in obese type II DM patients: relative effects on weight loss, metabolic parameters, and C-reactive protein. Eur J Clin Nutr. 2005;59:411–418. doi: 10.1038/sj.ejcn.1602089. [DOI] [PubMed] [Google Scholar]
- 24.Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, McManus K, Champagne CM, Bishop LM, Laranjo N, Leboff MS, Rood JC, de Jonge L, Greenway FL, Loria CM, Obarzanek E, Williamson DA. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859–873. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samaha FF, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, Williams T, Williams M, Gracely EJ, Stern L. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med. 2003;324:2074–2081. doi: 10.1056/NEJMoa022637. [DOI] [PubMed] [Google Scholar]
- 26.Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, Golan R, Fraser D, Bolotin A, Vardi H, Tangi-Rozental O, Zuk-Ramot R, Sarusi B, Brickner D, Schwartz Z, Sheiner E, Marko R, Katorza E, Thiery J, Fiedler GM, Blüher M, Stumvoll M, Stampfer MJ Dietary Intervention Randomized Controlled Trial (DIRECT) Group. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359:229–241. doi: 10.1056/NEJMoa0708681. [DOI] [PubMed] [Google Scholar]
- 27.Stern L, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, Williams M, Gracely EJ, Samaha FF. The effects of a low-carbohydrate versus conventional weight loss diets in severely obese adults: one-year follow-up of a randomized trial. Ann Intern Med. 2004;140:778–785. doi: 10.7326/0003-4819-140-10-200405180-00007. [DOI] [PubMed] [Google Scholar]
- 28.Yancy WS, Jr, Olsen MK, Guyton JR, Bakst RP, Westman EC. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: a randomized, controlled trial. Ann Intern Med. 2004;140:769–777. doi: 10.7326/0003-4819-140-10-200405180-00006. [DOI] [PubMed] [Google Scholar]
- 29.Atkins RC. Dr. Atkins’ New Diet Revolution. New York, NY: Avon; 2002. [Google Scholar]
- 30.Makris AP, Foster GD. Dietary approaches to the treatment of obesity. Psychiatr Clin North Am. 2005;28:117–139. doi: 10.1016/j.psc.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Larsen TM, Dalskov SM, van Baak M, Jebb SA, Papadaki A, Pfeiffer AF, Martinez JA, Handjieva-Darlenska T, Kunešová M, Pihlsgård M, Stender S, Holst C, Saris WH, Astrup A Diet, Obesity, and Genes (Diogenes) Project. Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med. 2010;363:2102–2113. doi: 10.1056/NEJMoa1007137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ornish D. Eat More, Weigh Less. New York, NY: HarperCollins; 2001. [Google Scholar]
- 33.Krauss RM, Eckel RH, Howard B, Appel LJ, Daniels SR, Deckelbaum RJ, Erdman JW, Jr, Kris-Etherton P, Goldberg IJ, Kotchen TA, Lichtenstein AH, Mitch WE, Mullis R, Robinson K, Wylie-Rosett J, St Jeor S, Suttie J, Tribble DL, Bazzarre TL. AHA Dietary Guidelines: revision 2000: a statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation. 2000;102:2284–2299. doi: 10.1161/01.cir.102.18.2284. [DOI] [PubMed] [Google Scholar]
- 34.Rolls BJ, Roe LS, Beach AM, Kris-Etherton PM. Provision of foods differing in energy density affects long-term weight loss. Obes Res. 2005;13:1052–1060. doi: 10.1038/oby.2005.123. [DOI] [PubMed] [Google Scholar]
- 35.Ello-Martin JA, Roe LS, Ledikwe JH, Beach AM, Rolls BJ. Dietary energy density in the treatment of obesity: a year-long trial comparing 2 weight-loss diets. Am J Clin Nutr. 2007;85:1465–1477. doi: 10.1093/ajcn/85.6.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Willett WC, Skerrett PJ. Eat, Drink, and Be Healthy: The Harvard Medical School Guide to Healthy Eating. New York, NY: Simon & Schuster; 2001. [Google Scholar]
- 37.Foster-Powell K, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr. 2002;76:5–56. doi: 10.1093/ajcn/76.1.5. [DOI] [PubMed] [Google Scholar]
- 38.Tsai AG, Wadden TA. The evolution of very-low-calorie diets: an update and meta-analysis. Obesity. 2006;14:1283–1293. doi: 10.1038/oby.2006.146. [DOI] [PubMed] [Google Scholar]
- 39.Sears B. The Zone: a Dietary Road Map. New York, NY: HarperCollins Publishers; 1995. [Google Scholar]
- 40.Brownell KD. The LEARN Program for Weight Management. Dallas TX: American Health Publishing Company; 2004. [Google Scholar]
- 41.Heshka S, Anderson JW, Atkinson RL, Greenway FL, Hill JO, Phinney SD, Kolotkin RL, Miller-Kovach K, Pi-Sunyer FX. Weight loss with self-help compared with a structured commercial program: a randomized trial. JAMA. 2003;289:1792–1798. doi: 10.1001/jama.289.14.1792. [DOI] [PubMed] [Google Scholar]
- 42.Heymsfield SB, van Mierlo CA, van der Knaap HC, Heo M, Frier HI. Weight management using a meal replacement strategy: meta and pooling analysis from six studies. Int J Obes. 2003;27:537–549. doi: 10.1038/sj.ijo.0802258. [DOI] [PubMed] [Google Scholar]
- 43.Jeffery RW, Wing RR, Thorson C, Burton LR, Raether C, Harvey J, Mullen M. Strengthening behavioral incentives for weight loss: a randomized trial of food provision and monetary incentives. J Consult Clin Psychol. 1993;61:1038–1045. doi: 10.1037//0022-006x.61.6.1038. [DOI] [PubMed] [Google Scholar]
- 44.Metz JA, Stern JS, Kris-Etherton P, Reusser ME, Morris CD, Hatton DC, Oparil S, Haynes RB, Resnick LM, Pi-Sunyer FX, Clark S, Chester L, McMahon M, Snyder GW, McCarron DA. A randomized trial of improved weight loss with a prepared meal plan in overweight and obese patients: impact on cardiovascular risk reduction. Arch Intern Med. 2000;160:2150–2158. doi: 10.1001/archinte.160.14.2150. [DOI] [PubMed] [Google Scholar]
- 45.Wing RR, Jeffery RW, Burton LR, Thorson C, Nissinoff KS, Baxter JE. Food provision vs structured meal plans in the behavioral treatment of obesity. Int J Obes Relat Metab Disord. 1996;20:56–62. [PubMed] [Google Scholar]
- 46.Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, Hill JO, Brancati FL, Peters A, Wagenknecht L Look AHEAD Research Group. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481–1486. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Appel LJ, Champagne CM, Harsha DW, Cooper LS, Obarzanek E, Elmer PJ, Stevens VJ, Vollmer WM, Lin PH, Svetkey LP, Stedman SW, Young DR Writing Group of the PREMIER Collaborative Research Group. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA. 2003;289:2083–2093. doi: 10.1001/jama.289.16.2083. [DOI] [PubMed] [Google Scholar]
- 48.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, III, Simons-Morton DG, Karanja N, Lin PH DASH-Sodium Collaborative Research Group. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 49.Hollis JF, Guillon CM, Stevens VJ, Brantley PJ, Appel LJ, Ard JD, Champagne CM, Dalcin A, Erlinger TP, Funk K, Laferriere D, Lin PH, Loria CM, Samuel-Hodge C, Vollmer WM, Svetkey LP Weight Loss Maintenance Trial Research Group. Weight loss during the intensive intervention phase of the weight-loss maintenance trial. Am J Prev Med. 2008;35:118–126. doi: 10.1016/j.amepre.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Appel LJ, Clark JM, Yeh HC, Wang NY, Coughlin JW, Daumit G, Miller ER, III, Dalcin A, Jerome GJ, Geller S, Noronha G, Pozefsky T, Charleston J, Reynolds JB, Durkin N, Rubin RR, Louis TA, Brancati FL. Comparative effectiveness of weight-loss interventions in clinical practice. N Engl J Med. 2011;365:1959–1968. doi: 10.1056/NEJMoa1108660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 52.Blair SN, Leermakers EA. Exercise and weight management. In: Wadden TA, Stunkard AJ, editors. Handbook of Obesity Treatment. New York, NY: Guilford Press; 2002. pp. 283–300. [Google Scholar]
- 53.Mora S, Cook N, Buring JE, Ridker PM, Lee IM. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation. 2007;116:2110–2118. doi: 10.1161/CIRCULATIONAHA.107.729939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med. 2002;136:493–503. doi: 10.7326/0003-4819-136-7-200204020-00006. [DOI] [PubMed] [Google Scholar]
- 55.Kraus WE, Houmard JA, Duscha BD, Knetzger KJ, Wharton MB, McCartney JS, Bales CW, Henes S, Samsa GP, Otvos JD, Kulkami KR, Stentz CA. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002;347:1483–1492. doi: 10.1056/NEJMoa020194. [DOI] [PubMed] [Google Scholar]
- 56.Ross R, Dagnone D, Jones PJ, Smith H, Paddags A, Hudson R, Janssen I. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. Ann Intern Med. 2000;133:92–103. doi: 10.7326/0003-4819-133-2-200007180-00008. [DOI] [PubMed] [Google Scholar]
- 57.Ross R, Janssen I, Dawson J, Kungl A, Kuk J, Wong S, Nguyen-Duy T, Lee S, Kilpatrick K, Hudson R. Exercise-induced reduction in obesity and insulin resistance in women: randomized controlled trial. Obes Res. 2004;12:789–798. doi: 10.1038/oby.2004.95. [DOI] [PubMed] [Google Scholar]
- 58.Hayes C, Kriska A. Role of physical activity in diabetes management and prevention. J Am Diet Assoc. 2008;108:S19–S23. doi: 10.1016/j.jada.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 59.Kay SJ, Fiatarone Singh MA. The influence of physical activity on abdominal fat: a systematic review of the literature. Obes Rev. 2006;7:183–200. doi: 10.1111/j.1467-789X.2006.00250.x. [DOI] [PubMed] [Google Scholar]
- 60.Mourier A, Gautier J, DeKerviler E, Bigard AX, Vilette J, Garnier J, Duvallet A, Guezennec CY, Cathelineau G. Mobilization of visceral adipose tissue related to the improvement in insulin sensitivity in response to physical training in NIDDM. Diabetes Care. 1997;20:385–391. doi: 10.2337/diacare.20.3.385. [DOI] [PubMed] [Google Scholar]
- 61.Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C, White RD. Physical activity/exercise and type 2 diabetes: a consensus statement from the American Diabetes Association. Diabetes Care. 2006;29:1433–1438. doi: 10.2337/dc06-9910. [DOI] [PubMed] [Google Scholar]
- 62.Leskinen T, Sipilä S, Alen M, Cheng S, Pietiläinen KH, Usenius JP, Suominen H, Kovanen V, Kainulainen H, Kaprio J, Kujala UM. Leisure-time physical activity and high-risk fat: a longitudinal population-based twin study. Int J Obes (Lond) 2009;33:1211–1218. doi: 10.1038/ijo.2009.170. [DOI] [PubMed] [Google Scholar]
- 63.Lee CD, Jackson AS, Blair SN. US weight guidelines: is it also important to consider cardiorespiratory fitness? Int J Obes Relat Metab Disord. 1998;22 suppl 2:S2–S7. [PubMed] [Google Scholar]
- 64.Lee CD, Blair SN, Jackson AS. Cardiorespiratory fitness, body composition, and all-cause and cardiovascular disease mortality in men. Am J Clin Nutr. 1999;69:373–380. doi: 10.1093/ajcn/69.3.373. [DOI] [PubMed] [Google Scholar]
- 65.Stevens J, Cai J, Evenson KR, Thomas R. Fitness and fatness as predictors of mortality from all causes and from cardiovascular disease in men and women in the lipid research clinics study. Am J Epidemiol. 2002;156:832–841. doi: 10.1093/aje/kwf114. [DOI] [PubMed] [Google Scholar]
- 66.Hu FB, Willett WC, Li T, Stampfer MJ, Colditz GA, Manson JE. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med. 2004;351:2694–2703. doi: 10.1056/NEJMoa042135. [DOI] [PubMed] [Google Scholar]
- 67.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. American College of Sports Medicine. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459–471. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 68.Slentz CA, Duscha BD, Johnson JL, Ketchum K, Aiken LB, Samsa GP, Houmard JA, Bales CW, Kraus WE. Effects of the amount of exercise on body weight, body composition, and measure of central obesity: STRRIDE–a randomized controlled study. Arch Intern Med. 2004;164:31–39. doi: 10.1001/archinte.164.1.31. [DOI] [PubMed] [Google Scholar]
- 69.Shaw K, Gennat H, O’Rourke P, Del Mar C. Exercise for overweight or obesity. Cochrane Database Syst Rev. 2006;4 doi: 10.1002/14651858.CD003817.pub3. CD003817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cantenacci VA, Wyatt HR. The role of physical activity in producing and maintaining weight loss. Nat Clin Pract Endocrinol Metab. 2007;3:518–529. doi: 10.1038/ncpendmet0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jeffery RW, Wing RR, Sherwood NE, Tate DF. Physical activity and weight loss: does prescribing higher physical activity goals improve outcome? Am J Clin Nutr. 2003;78:684–689. doi: 10.1093/ajcn/78.4.684. [DOI] [PubMed] [Google Scholar]
- 72.Tate DF, Jeffery RW, Sherwood NE, Wing RR. Long-term weight losses associated with prescription of higher physical activity goals. Are higher levels of physical activity protective against weight regain? Am J Clin Nutr. 2007;85:954–959. doi: 10.1093/ajcn/85.4.954. [DOI] [PubMed] [Google Scholar]
- 73.Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr. 2001;21:323–341. doi: 10.1146/annurev.nutr.21.1.323. [DOI] [PubMed] [Google Scholar]
- 74.Wing RR. Physical activity in the treatment of the adulthood overweight and obesity: current evidence and research issues. Med Sci Sports Exerc. 1999;31:S547–S552. doi: 10.1097/00005768-199911001-00010. [DOI] [PubMed] [Google Scholar]
- 75.Jakicic JM, Winters C, Lang W, Wing RR. Effects of intermittent exercise and use of home exercise equipment on adherence, weight loss, and fitness in overweight women: a randomized trial. JAMA. 1999;16:1554–1560. doi: 10.1001/jama.282.16.1554. [DOI] [PubMed] [Google Scholar]
- 76.Jakicic JM, Marcus BH, Gallagher KI, Napolitano M, Lang W. Effect of exercise duration and intensity on weight loss in overweight, sedentary women: a randomized trial. JAMA. 2003;290:1323–1330. doi: 10.1001/jama.290.10.1323. [DOI] [PubMed] [Google Scholar]
- 77.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- 78.Jakicic JM, Marcus BH, Lang W, Janney C. Effect of exercise on 24-month weight loss maintenance in overweight women. Arch Intern Med. 2008;168:1550–1560. doi: 10.1001/archinte.168.14.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fogelholm M, Kujala U, Kaprio J, Sarna S. Predictors of weight change in middle-aged and old men. Obes Res. 2000;8:367–373. doi: 10.1038/oby.2000.44. [DOI] [PubMed] [Google Scholar]
- 80.Catenacci VA, Grunwald GK, Ingebrigtsen JP, Jakicic JM, McDermott MD, Phelan S, Wing RR, Hill JO, Wyatt HR. Physical activity patterns using accelerometry in the national weight control registry. Obesity (Silver Spring) 2011;19:1163–1170. doi: 10.1038/oby.2010.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mekary RA, Feskanich D, Hu FB, Willett WC, Field AE. Physical activity in relation to long-term weight maintenance after intentional weight loss in premenopausal women. Obesity (Silver Spring) 2010;18:167–174. doi: 10.1038/oby.2009.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, Gallagher D, Mayer L, Murphy E, Leibel RL. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005;115:3579–3586. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Arciero PJ, Gentile CL, Martin-Pressman R, Ormsbee MJ, Everett M, Zwicky L, Steele CA. Increased dietary protein and combined high intensity aerobic and resistance exercise improves body fat distribution and cardiovascular risk factors. Int J Sport Nutr Exerc Metab. 2006;16:373–392. doi: 10.1123/ijsnem.16.4.373. [DOI] [PubMed] [Google Scholar]
- 84.Park SK, Park JH, Kwon YC, Kim HS, Yoon MS, Park HT. The effect of combined aerobic and resistance exercise training on abdominal fat in obese middle-aged women. J Physiol Anthropol Appl Human Sci. 2003;22:129–135. doi: 10.2114/jpa.22.129. [DOI] [PubMed] [Google Scholar]
- 85.Ballor DL, Poehlman ET. Exercise-training enhances fat-free mass preservation during diet-induced weight loss: a meta-analytical finding. Int J Obes Relat Metab Disord. 1994;18:35–40. [PubMed] [Google Scholar]
- 86.Murphy MH, Blair SN, Murtagh EM. Accumulated versus continuous exercise for health benefit: a review of empirical studies. Sports Med. 2009;39:29–43. doi: 10.2165/00007256-200939010-00003. [DOI] [PubMed] [Google Scholar]
- 87.Jakicic JM, Donnelly JE, Pronk NP, Jawad AF, Jacobsen DJ. Prescription of exercise intensity for the obese patient: the relationship between heart rate, VO2 and perceived exertion. Int J Obes Relat Metab Disord. 1995;19:382–387. [PubMed] [Google Scholar]
- 88.Andersen RE, Wadden TA, Bartlett SJ, Zemel B, Verde TJ, Franckowiak SC. Effects of lifestyle activity vs. structured aerobic exercise in obese women: a randomized trial. JAMA. 1999;281:335–340. doi: 10.1001/jama.281.4.335. [DOI] [PubMed] [Google Scholar]
- 89.Epstein LH, Wing RR, Koeske R, Valoski A. A comparison of lifestyle exercise, aerobic exercise, and calisthenics on weight loss in obese children. Behav Ther. 1985;16:345–356. [Google Scholar]
- 90.Jakicic JM, Otto AD. Physical activity considerations for the treatment and prevention of obesity. Am J Clin Nutr. 2005;82(1 suppl):226S–229S. doi: 10.1093/ajcn/82.1.226S. [DOI] [PubMed] [Google Scholar]
- 91.Bravata DM, Smith-Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R, Stave CD, Olkin I, Sirard JR. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298:2296–2304. doi: 10.1001/jama.298.19.2296. [DOI] [PubMed] [Google Scholar]
- 92.Villanova N, Pasqui F, Burzacchini S, Forlani G, Manini R, Suppini A, Melchionda N, Marchesini G. A physical activity program to reinforce weight maintenance following a behavior program in overweight/obese subjects. Int J Obes (Lond) 2006;30:697–703. doi: 10.1038/sj.ijo.0803185. [DOI] [PubMed] [Google Scholar]
- 93.Wing RR. Behavioral weight control. In: Wadden TA, Stunkard AJ, editors. Handbook of Obesity Treatment. New York, NY: Guilford Press; 2002. pp. 301–316. [Google Scholar]
- 94.Wadden TA, Foster GD. Behavioral treatment of obesity. Med Clin North Am. 2000;84:441–461. doi: 10.1016/s0025-7125(05)70230-3. [DOI] [PubMed] [Google Scholar]
- 95.Wadden TA, Berkowitz RI, Womble LG, Sarwer DB, Phelan S, Cato RK, Hesson LA, Osei SY, Kaplan R, Stunkard AJ. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med. 2005;353:2111–2120. doi: 10.1056/NEJMoa050156. [DOI] [PubMed] [Google Scholar]
- 96.Berkowitz RI, Wadden TA, Tershakovec AM, Cronquist JL. Behavior therapy and sibutramine for the treatment of adolescent obesity: a randomized controlled trial. JAMA. 2003;289:1805–1812. doi: 10.1001/jama.289.14.1805. [DOI] [PubMed] [Google Scholar]
- 97.Carels RA, Young KM, Coit C, Clayton AM, Spencer A, Hobbs M. Can following the caloric restriction recommendations from the Dietary Guidelines for Americans help individuals lose weight? Eat Behav. 2008;9:328–335. doi: 10.1016/j.eatbeh.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 98.Hesel DL, Jakicic JM, Otto AD. Comparison of techniques for self-monitoring eating and exercise behaviors on weight loss in a correspondence-based intervention. J Am Diet Assoc. 2007;107:1807–1810. doi: 10.1016/j.jada.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 99.Stuart RB. Behavioral control of overeating. Behav Res Ther. 1967;5:357–365. [Google Scholar]
- 100.Renjilian DA, Perri MG, Nezu AM, McKelvey WF, Shermer RL, Anton SD. Individual versus group therapy for obesity: effects of matching participants to their treatment preferences. J Consult Clin Psychol. 2001;69:717–721. [PubMed] [Google Scholar]
- 101.Butryn ML, Webb V, Wadden TA. Behavioral treatment of obesity. Psychiatr Clin North Am. 2011;34:841–859. doi: 10.1016/j.psc.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Perri MG, McAllister DA, Gange JJ, Jordan RC, McAdoo G, Nezu AM. Effects of four maintenance programs on the long-term management of obesity. J Consult Clin Psychol. 1988;56:529–534. doi: 10.1037//0022-006x.56.4.529. [DOI] [PubMed] [Google Scholar]
- 103.Perri MG, Shapiro RM, Ludwig WW, Twentyman CT, McAdoo G. Maintenance strategies for the treatment of obesity: an evaluation of relapse prevention training and posttreatment contact by telephone and mail. J Consult Clin Psychol. 1984;52:404–413. doi: 10.1037//0022-006x.52.3.404. [DOI] [PubMed] [Google Scholar]
- 104.Perri MG, McAdoo WG, McAllister DA, Lauer JB, Yancey DZ. Enhancing the efficacy of behavior therapy for obesity: effects of aerobic exercise and a multicomponent maintenance program. J Consult Clin Psychol. 1986;54:670–675. doi: 10.1037//0022-006x.54.5.670. [DOI] [PubMed] [Google Scholar]
- 105.Perri MG, Corsica JA. Improving the maintenance of weight lost in behavioral treatment of obesity. In: Wadden TA, Stunkard AJ, editors. Handbook of Obesity Treatment. New York, NY: Guilford Press; 2002. pp. 357–379. [Google Scholar]
- 106.Donnelly JE, Smith BK, Dunn L, Mayo MM, Jacobsen DJ, Stewart EE, Gibson C, Sullivan DK. Comparison of a phone vs clinic approach to achieve 10% weight loss. Int J Obes. 2007;31:1270–1276. doi: 10.1038/sj.ijo.0803568. [DOI] [PubMed] [Google Scholar]
- 107.Digenio AG, Mancuso JP, Gerber RA, Dvorak RV. Comparison of methods for delivering a lifestyle modification program for obese patients: a randomized trial. Ann Intern Med. 2009;150:255–262. doi: 10.7326/0003-4819-150-4-200902170-00006. [DOI] [PubMed] [Google Scholar]
- 108.Perri MG, Limacher MC, Durning PE, Janicke DM, Lutes LD, Bobroff LB, Dale MS, Daniels MJ, Radcliff TA, Martin AD. Extended-care programs for weight management in rural communities: the treatment of obesity in underserved rural settings (TOURS) randomized trial. Arch Intern Med. 2008;168:2347–2354. doi: 10.1001/archinte.168.21.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tate D, Wing RR, Winett R. Development and evaluation of an internet behavior therapy program for weight loss. JAMA. 2001;285:1172–1177. doi: 10.1001/jama.285.9.1172. [DOI] [PubMed] [Google Scholar]
- 110.Tate DF, Jackvony EH, Wing RR. Effects of internet behavioral counseling on weight loss in adults at risk for type 2 diabetes. JAMA. 2003;289:1833–1836. doi: 10.1001/jama.289.14.1833. [DOI] [PubMed] [Google Scholar]
- 111.Womble LG, Wadden TA, McGuckin BG, Sargent SL, Rothman RA, Krauthamer-Ewing ES. A randomized controlled trial of a commercial internet weight loss program. Obes Res. 2004;12:1011–1018. doi: 10.1038/oby.2004.124. [DOI] [PubMed] [Google Scholar]
- 112.Rothert K, Strecher VJ, Doyle LA, Caplan WM, Joyce JS, Jimison HB, Karm LM, Mims AD, Roth MA. Web-based weight management programs in an integrated health care setting: a randomized, controlled trial. Obesity. 2006;14:266–272. doi: 10.1038/oby.2006.34. [DOI] [PubMed] [Google Scholar]
- 113.Harvey-Berino J, West D, Krukowski R, Prewitt E, VanBiervliet A, Ashikaga T, Skelly J. Internet delivered behavioral obesity treatment. Prev Med. 2010;51:123–128. doi: 10.1016/j.ypmed.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Harvey-Berino J, Pintauro SJ, Gold EC. The feasibility of using Internet support for the maintenance of weight loss. Behav Modif. 2002;26:103–116. doi: 10.1177/0145445502026001006. [DOI] [PubMed] [Google Scholar]
- 115.Harvey-Berino J, Pintauro S, Buzzell P, DiGiulio M, Casey Gold B, Moldovan C, Ramirez E. Does using the Internet facilitate the maintenance of weight loss? Int J Obes. 2002;26:1254–1260. doi: 10.1038/sj.ijo.0802051. [DOI] [PubMed] [Google Scholar]
- 116.Harvey-Berino J, Pintauro S, Buzzell P, Gold EC. Effect of internet support on the long-term maintenance of weight loss. Obes Res. 2004;12:320–329. doi: 10.1038/oby.2004.40. [DOI] [PubMed] [Google Scholar]
- 117.Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL. A self-regulation program for maintenance of weight loss. N Engl J Med. 2006;355:1563–1571. doi: 10.1056/NEJMoa061883. [DOI] [PubMed] [Google Scholar]
- 118.Svetkey LP, Stevens VJ, Brantley PJ, Appel LJ, Hollis JF, Loria CM, Vollmer WM, Gullion CM, Funk K, Smith P, Samuel-Hodge C, Myers V, Lien LF, Laferriere D, Kennedy B, Jerome GJ, Heinith F, Harsha DW, Evans P, Erlinger TP, Dalcin AT, Coughlin J, Charleston J, Champagne CM, Bauck A, Ard JD, Aicher K Weight Loss Maintenance Collaborative Research Group. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA. 2008;299:1139–1148. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]
- 119.Ackermann RT, Finch EA, Brizendine E, Zhou H, Marrero DG. Translating the Diabetes Prevention Program into the community. The DEPLOY Pilot Study. Am J Prev Med. 2008;35:357–363. doi: 10.1016/j.amepre.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ackermann RT, Marrero DG. Adapting the Diabetes Prevention Program lifestyle intervention for delivery in the community: the YMCA model. Diabetes Educ. 2007;33:69, 74–75, 77–78. doi: 10.1177/0145721706297743. [DOI] [PubMed] [Google Scholar]
- 121.Tsai AG, Wadden TA. Systematic review: an evaluation of major commercial weight loss programs in the United States. Ann Intern Med. 2005;142:56–66. doi: 10.7326/0003-4819-142-1-200501040-00012. [DOI] [PubMed] [Google Scholar]
- 122.Webb VL, Tsai AG, Wadden TA. Commercial weight loss programs and popular diets. Encyclopedia of Body Image and Human Appearance. In press. [Google Scholar]
- 123.Weight Watchers web site. [Accessed July 9, 2011]; Available at: http://www.weightwatchers.com.
- 124.Jenny Craig web site. [Accessed July 9, 2011]; Available at: http://www.jennycraig.com.
- 125.Nutrisystem web site. [Accessed July 9, 2011]; Available at: http://www.nutrisystem.com.
- 126.Rock CL, Pakiz B, Flatt SW, Quintana EL. Randomized trial of a multifaceted commercial weight loss program. Obesity. 2007;15:939–949. doi: 10.1038/oby.2007.614. [DOI] [PubMed] [Google Scholar]
- 127.Rock CL, Flatt SW, Sherwood NE, Karanja N, Pakiz B, Thomson CA. Effect of a free prepared meal and incentivized weight loss program on weight loss and weight loss maintenance in obese and overweight women: a randomized controlled trial. JAMA. 2010;304:1803–1810. doi: 10.1001/jama.2010.1503. [DOI] [PubMed] [Google Scholar]
- 128.Foster GD, Borradaile KE, Vander Veur SS, Leh Shantz K, Dilks RJ, Goldbacher EM, Oliver TL, Lagrotte CA, Homko C, Satz W. The effects of a commercially available weight loss program among obese patients with type 2 diabetes: a randomized study. Postgrad Med. 2009;121:113–118. doi: 10.3810/pgm.2009.09.2046. [DOI] [PubMed] [Google Scholar]
- 129.Djuric Z, DiLaura NM, Jenkins I, Darga L, Jen CK, Mood D, Bradley E, Hryniuk WM. Combining weight-loss counseling with the weight watchers plan for obese breast cancer survivors. Obes Res. 2002;10:657–665. doi: 10.1038/oby.2002.89. [DOI] [PubMed] [Google Scholar]
- 130.Heshka S, Greenway F, Anderson JW, Atkinson RL, Hill JO, Phinney SD, Miller-Kovach K, Xavier Pi-Sunyer F. Self-help weight loss versus a structured commercial program after 26 weeks: a randomized controlled study. Am J Med. 2000;109:282–287. doi: 10.1016/s0002-9343(00)00494-0. [DOI] [PubMed] [Google Scholar]
- 131.Jebb SA, Ahern AL, Olson AD, Aston LM, Holzapfel C, Stoll J, Amann-Gassner U, Simpson AE, Fuller NR, Pearson S, Lau NS, Mander AP, Hauner H, Caterson ID. Primary care referral to a commercial provider for weight loss treatment versus standard care: a randomised controlled trial. Lancet. 2011;378:1485–1492. doi: 10.1016/S0140-6736(11)61344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Novak NL, Brownell KD. Obesity: a public health approach. Psychiatr Clin North Am. 2011;34:895–909. doi: 10.1016/j.psc.2011.08.001. [DOI] [PubMed] [Google Scholar]