Abstract
Rationale
Electrogram-based catheter ablation, targeting complex fractionated atrial electrograms (CFAEs), is empirically known to be effective in halting persistent/permanent atrial fibrillation (AF). However, the mechanisms underlying CFAEs and electrogram-based ablation remain unclear.
Objective
Because atrial fibrosis is associated with persistent/permanent AF, we hypothesized that electrotonic interactions between atrial myocytes and fibroblasts play an important role in CFAE genesis and electrogram-based catheter ablation.
Methods and Results
We used a human atrial tissue model in heart failure and simulated propagation and spiral wave reentry with and without regionally proliferated fibroblasts. Coupling of fibroblasts to atrial myocytes resulted in shorter action potential duration, slower conduction velocity, and lower excitability. Consequently, heterogeneous fibroblast proliferation in the myocardial sheet resulted in frequent spiral wave breakups, and the bipolar electrograms recorded at the fibroblast proliferation area exhibited CFAEs. The simulations demonstrated that ablation targeting such fibroblast-derived CFAEs terminated AF, resulting from the ablation site transiently pinning the spiral wave and then pushing it out of the fibroblast proliferation area. CFAEs could not be attributed to collagen accumulation alone.
Conclusions
Fibroblast proliferation in atria might be responsible for the genesis of CFAEs during persistent/ permanent AF. Our findings could contribute to better understanding of the mechanisms underlying CFAE-targeted AF ablation.
Keywords: atrial fibrillation, complex fractionated atrial electrograms, catheter ablation, spiral wave reentry, fibroblasts
Electrogram-based catheter ablation, targeting areas of complex fractionated atrial electrograms (CFAEs), is empirically known to be effective in halting atrial fibrillation (AF). CFAEs were first defined by Nademanee et al1 as fractionated atrial electrograms with continuous prolonged activation and/or electrograms with a very short cycle length, less than 120 ms. In patients with persistent and permanent AF, CFAE-targeted ablation, combined with anatomic approach to catheter ablation (pulmonary vein isolation), is superior to pulmonary vein isolation alone in both terminating AF during ablation and in diminishing AF recurrence after ablation.2,3 In contrast, the additional benefits from CFAE-targeted ablation are not apparent in patients with paroxysmal AF.3
Recent studies4,5 have linked CFAEs to the activity of ganglionated plexi in the atria. However, an anatomic study6 found that the number of neurons in the ganglionated plexi of the human heart decreases with age. This suggests that the ganglionated plexi may not be responsible for CFAE formation in aging patients with persistent/permanent AF, raising the following questions:
What is the mechanism underlying CFAEs in patients with persistent/permanent AF?
How does CFAE-targeted ablation terminate AF?
Myocardial tissue is mainly composed of myocytes and fibroblasts, which produce collagen.7–9 Under pathological conditions, such as persistent/permanent AF associated with heart failure, fibroblasts proliferate and play an important role in myocardial remodeling, often differentiating into larger myofibroblasts.7–9 Electric coupling between myocytes and fibroblasts has been demonstrated in cell cultures.10,11 Recent experimental observations12–14 have shown that the nonexcitable fibroblasts (including myofibroblasts) form electric coupling with myocytes through gap junctions, exerting electrotonic influences.
On the basis of these facts, we hypothesized that the electrotonic interactions between atrial myocytes and coupled fibroblasts play an important role in the genesis of CFAEs and that catheter ablation targeting such fibroblast-derived CFAE sites could terminate AF. The goal of the present study was to address the questions above and to test the proposed hypotheses in computer simulations of atrial excitation propagation under the conditions of heart failure.
Methods
An expanded Methods section is available in the Online Data Supplement at http://circres.ahajournals.org.
Atrial Myocyte and Fibroblast Models
The action potential was represented by the Courtemanche human atrial model.15 Each myocyte was electrically connected to a number of MacCannell model16 fibroblasts (Figure 1A). The fibroblast model16 includes time- and voltage-dependent K+ current (IKv), inward-rectifying K+ current (IK1), Na+-K+ pump current (INaK), and Na+ background current (INab). Fibroblasts and myofibroblasts were represented by the same ionic model but had different capacitances, 6.3 and 50.4 pF, respectively. Consistent with results by MacCannell et al,16 we used the same gap junctional conductance (3.0 nS) for both fibroblast and myofibroblast coupling to a myocyte.
Figure 1. Human atrial tissue model with regionally proliferated fibroblasts.
A, Schematic representation of the Courte-manche human atrial myocyte model16 connecting to the Mac-Cannell fibroblast model17 (Fb). B, Homogeneous myocardial sheet (bottom) and the automated CFAE detection algorithm (top). Curved white lines inside the sheet represent trajectories of the spiral wave phase singularities (PS) during the last 500 ms of activity. Fb-Area represents (myo)fibroblast proliferation area. Blue circles denote local bipolar electrogram (EG) recording sites. Catheter ablation was also applied to these blue circles. C, Low-density and high-density fibroblast distributions (LD-Fbs and HD-Fbs). Green dots represent myocytes connected to fibroblasts.
To represent atrial electric remodeling under heart failure, we incorporated modifications in ion channel conductances in the atrial myocyte model.17 The maximum conductances of the L-type Ca2+ current (ICaL), the slow component of delayed rectifier K+ current (IKs), the transient outward K+ current (Ito), and the Na+/Ca2+ exchanger current (INaCa) were assumed to be 70%, 70%, 50%, and 145%, respectively, of the original values. With these modifications, the action potential duration (APD) of the human atrial myocyte decreased (eg, from 243–214 ms for APD70 at 2 Hz).
To represent gap junctional remodeling in persistent/permanent AF,18,19 we used 0.384 and 0.126 mS/cm as the longitudinal and transverse intracellular conductivities, respectively, and 6.25 and 2.36 mS/cm as longitudinal and transverse extracellular conductivities, respectively. These values achieve the marked decrease in the longitudinal-to-transverse ratio (≈2.2) of conduction velocity (CV) under persistent/permanent AF.20
Myofiber Model
We simulated a 1-dimensional myofiber of length 4.5 cm. Fibroblasts or myofibroblasts, with side connections to myocytes, proliferated at the right half of the myofiber. Pacing stimuli of 1000-ms basic cycle length were applied to the left myofiber end.
Myocardial Sheet Model
The model was extended to a 2-dimensional (2-D) myocardial sheet of size 4.5×4.5 cm (Figure 1B, bottom) and simulations of spiral wave reentry conducted. The justification for the use of a 2-D model in the study of CFAEs in persistent/permanent AF is based on a recent experimental study21 demonstrating dissociation of activity between epicardial and endocardial layers in persistent/permanent AF but not in paroxysmal AF.
In Figure 1B, parabolic gray curves represent fiber direction. The red circle of 3.0 cm diameter represents the (myo)fibroblast proliferation area (Fb-Area). Local bipolar electrograms were recorded simultaneously at the center of each blue circle in Figure 1B, and a 5-second-long bipolar electrogram window was analyzed. Catheter ablation was modeled as a circular insulating area (without myocytes or fibroblasts) of 0.5 cm diameter (same size as blue circles).
CFAE Detection
The top panel in Figure 1B presents the CFAE detection algorithm that we used; a bipolar electrogram is shown. To detect excitation timings, the peaks of the first derivative of the bipolar electrogram were used. The tags above the electrogram mark excitation intervals > 120 ms (L, black tags) and in the interval 70–120 ms (S, red tags). An electrogram was labeled CFAE when S/(L+S) (termed CFAE ratio) was ≥25%. This criterion reflects the occurrence of spiral wave breakup (and thus AF maintenance) under these conditions, as found in a preliminary simulation study. The rationale here is that if CFAEs are the targets for catheter ablation, CFAEs should be linked to the mechanisms of AF maintenance.1
Fibroblast Proliferation in the Myocardial Sheet
Figure 1C portrays the distributions of low-density and high-density fibroblasts, LD-Fbs and HD-Fbs, respectively, in the myocardial sheet. The average fibroblast cluster sizes for the LD-Fbs and HD-Fbs cases were 0.56 and 2.25 mm2, respectively. These values are within the reported range.22 For LD-Fbs and HD-Fbs cases, a 100-pF atrial myocyte connecting with four 6.3-pF fibroblasts (+4 Fbs) accounts for 12.5% and 50.0%, respectively, of the Fb-Area. We also implemented, in additional simulations, HD-Fbs (50.0% area) but with two 50.4-pF myofibroblasts per myocyte (+2 MFbs). The choice of the number of fibroblasts and myofibroblasts was based on preliminary simulation results, which showed that spiral wave behavior in the +2 MFbs case was similar to that in the +4 Fbs case.
Computation
The numeric approach, including methods for integration and solution of the linear system, has been described elsewhere.23 The spatial discretization for the myocardial fiber and sheet was 150 μm, and the time discretization was 5 μs. The method for calculating electrograms has also been described previously.24 We used 8.0 mS/cm as the bath conductivity. The ECG was simulated as being recorded by a unipolar electrode located 3 cm above the center of the myocardial sheet, and each local bipolar electrogram resulted from 2 unipolar electrodes, separated by a distance of 8 mm.
Results
Electrotonic Effects of Fibroblasts on Propagation
The effects of myocyte coupling to 6.3-pF fibroblasts on propagation in the myofiber model are shown in Figure 2A. As the result of the fibroblast electrotonic influence, 4 or more fibroblasts coupled to a 100-pF myocyte decreased CV (white dashed arrows in Figure 2A), and more than 10 fibroblasts caused conduction block (white double short line in Figure 2A).
Figure 2.
A, Effect of myocyte coupling to 6.3-pF fibroblasts on the excitation propagation in a myofiber model The vertical axis represents time and the horizontal axis indicates location along the myofiber (blue bar). Pacing stimuli were applied at the left end of the myofiber. Fb-Area represents fibroblast proliferation area. The number of fibroblasts connected to a myocyte (+1 to +10 Fbs) in the right half of the myofiber is indicated in each transmembrane potential map. White solid arrows represent direction of wave propagation in control (without fibroblasts); white dashed arrows and the double short line represent slowed propagation and conduction block, respectively. B, Effect of fibroblast coupling on myocyte action potential morphology. The action potentials were recorded from a myocyte located at the middle of the Fb-Area in the myofiber. C and D, Action potential duration at 70% repolarization, APD70 (C) and conduction velocity, CV (D) as a function of diastolic interval, DI, in cases of control, +1 Fb, +2 Fbs, and +4 Fbs.
Figure 2B shows action potentials of a myocyte located at the middle of the Fb-Area in the myofiber. As previously shown in a single atrial myocyte model study by Maleckar et al,25 the increase in fibroblast number shortened APD; similar behavior was found here. Furthermore, because the resting membrane potential of the uncoupled fibroblast was around −50 mV,26 the increase in the fibroblast number changed the diastolic potential of the myocyte from −79 to −54 mV (compare the control with the +10 Fbs case in Figure 2B).
As shown in Figure 2C and 2D, both APD shortening and CV decrease by fibroblast proliferation were more pronounced at shorter diastolic intervals (DI).
Electrotonic Effects of Fibroblasts on Spiral Wave Behavior
As shown in Figure 3A (Online Movie I, top left), a spiral wave reentry (as a model of AF) was induced by an S1–S2 cross-field protocol, with onset of S2 assumed as time zero (0-ms panel). In this control case, that is, without fibroblasts, spiral wave breakups arising from wave front–tail interaction were rarely observed. The spiral wave meandered (500- to 1250-ms panels) and terminated shortly thereafter (see simulated ECG at the bottom) because of collision with the sheet boundary (2500-ms panel).
Figure 3. Examples demonstrating the effect of myocyte-fibroblast coupling on spiral wave behavior in a myocardial sheet of size 4.5×4.5 cm.
A, Control case without fibroblasts. B and C, Low-density and high-density fibroblast proliferation (LD-Fbs and HD-Fbs) models, respectively (refer to Figure 1C). In the LD-Fbs and HD-Fbs models, atrial myocytes (100 pF), each connecting to 4 fibroblasts (6.3 pF) (+4 Fbs) within the Fb-Area, account for 12.5% and 50.0% of that area, respectively. The simulated ECG in each case is shown at the bottom.
In contrast, for the LD-Fbs case, as shown in Figure 3B (Online Movie I, top right), the spiral wave induced by the same protocol (0-ms panel) meandered within or around the Fb-Area (500- to 3850-ms panels) and was sustained longer than in control. Spiral wave breakup rarely occurred. The simulated ECG (bottom) showed coarse f waves, similar to typical clinical ECGs during paroxysmal AF.
Implementation of HD-Fbs instead resulted in transition, as shown in Figure 3C (Online Movie I, bottom left), from spiral wave reentry (0- to 500-ms panels) to multiple wavelets (1000- to 3850-ms panels) because of frequent wave breakups. In this case, the simulated ECG (bottom) shows fine f waves, similar to typical clinical ECGs in patients with persistent/permanent AF.
Bipolar Electrograms and CFAEs
Figure 4 shows simulated ECGs and typical examples of bipolar electrograms recorded inside and outside the Fb-Area in the LD-Fbs case. CFAEs were not recorded regardless of the bipolar electrode location, with the exception of the bipolar electrogram in red, which satisfied the CFAE criteria.
Figure 4. Simulated ECG and examples of bipolar electrograms in the LD-Fbs myocardial sheet, based on the same data as Figure 3B.
Each bipolar electrogram was classified as either non-CFAE or CFAE according to the value of S/(L+S) (CFAE ratio), where S and L stand for the numbers of red tags (excitation interval, 70–120 ms) and black tags (excitation interval >120 ms), respectively. A bipolar electrogram in red is CFAE.
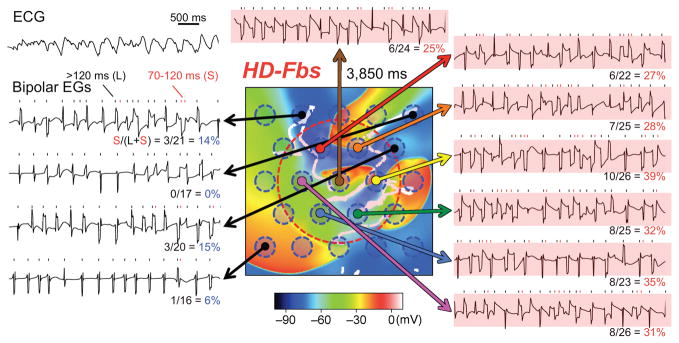
In contrast, Figure 5 shows simulated ECGs and typical examples of bipolar electrograms in the HD-Fbs case. In this case, the bipolar electrograms classified as CFAEs were recorded within the Fb-Area (6 bipolar electrograms in red satisfied the CFAE criteria).
Figure 5. Simulated ECG and examples of bipolar electrograms in the HD-Fbs myocardial sheet, based on the same data as Figure 3C.
Each bipolar electrogram was classified as either non-CFAE or CFAE according to the value of the CFAE ratio. Bipolar electrograms in red are CFAEs.
CFAE-Targeted Ablation
If the hypothesis that fibroblasts are responsible for CFAE is valid, then catheter ablation targeting CFAE sites must terminate AF. To ascertain whether this is true, we conducted simulations of spiral wave ablation in the HD-Fbs sheet, administered at 150 ms after the S2 onset, as shown in Figure 6A. To mimic CFAE-targeted ablation, ablation was modeled as 1, 2, 4, or 7 nonexcitable small black circles of diameter 0.5 cm, depending on the CFAE ratio. For each simulation with a given number of ablation sites, the target sites were chosen to be those with the highest CFAE ratios (Figure 5).
Figure 6. CFAE-targeted ablation in the HD-Fbs myocardial sheet.
A, Consecutive snapshots of transmembrane potential maps (every 500 ms except for the last panel, which is just before spiral wave termination). Ablation takes place 150 ms after S2 onset; +ABL-0, +ABL-1, +ABL-2, +ABL-4, and +ABL-7 denote the numbers of ablation sites, represented by the small black circles. The number displayed on the right of each row is the time of spiral wave termination. B through E, Examples demonstrating the effect of CFAE-targeted ablation on spiral wave behavior, corresponding to timings indicated by red (800 ms), green (2250 ms), blue (2250 ms), and black triangles (1080 ms), respectively, in A. White arrows and double short line indicate direction of wave front propagation and collision between wave fronts, respectively. Red arrows represent the directions of spiral wave drift before termination.
Spiral wave reentry terminated earlier as the number of ablation sites increased; all spiral wave activity was annihilated at 3480 ms, 2860 ms, 2620 ms, and 1380 ms in the cases of 1, 2, 4, and 7 ablation sites, respectively (2nd to 5th rows in Figure 6A) (Online Movie II). In contrast, in the case of no ablation, spiral waves continued for over 5000 ms (top row in Figure 6A). In another case of a single ablation site (Online Figure I), where AF was not terminated, excitation intervals at the ablated site were prolonged and the excitation complexity decreased. This phenomenon is consistent with what we commonly observe in clinical practice.
Examples demonstrating the effect of CFAE-targeted ablation on spiral wave behavior are presented in Figure 6B through 6E, corresponding to the timings indicated by red, green, blue, and black triangles, respectively, in Figure 6A. The CFAE-targeted ablation site transiently pinned the spiral wave and its phase singularity (compare panels in Figure 6B), preventing wave breakup by blocking the shortcut of the reentry. The spiral wave drifted between the ablation sites and finally collided with the sheet boundary (red arrows in Figure 6C through 6E), resulting in AF termination.
To demonstrate the robustness of our simulation results regarding CFAE-targeted ablation, we conducted an additional simulation of ablation, at 7 sites, of AF induced by a cross-stimulation protocol of different timing (S1–S2 interval 20 ms shorter than in Figure 3C). Although the multiple wavelets dynamics sustaining AF was different, AF was again terminated shortly after ablation (Online Figure II), very similar to results in Figure 6A, 5th row.
To further test the validity of the proposed CFAE mechanism, we simulated the effect of ibutilide, blocker of the fast component of the delayed rectifier K+ current (IKr), on CFAEs as in a recent clinical study.27 In the HD-Fbs case, we found that a 50% decrease in IKr led to organization of atrial activity, decrease in CFAEs, and to a tendency toward AF termination (Online Figure III), consistent with the clinical study on patients with persistent AF.27
Similarities Between Electrotonic Effects of Myofibroblasts and Fibroblasts on Excitation Waves
As shown in Figure 7, when 50.4-pF myofibroblasts were modeled instead of 6.3-pF fibroblasts (Figure 2B through 2D), similar results were obtained. Four or more coupled 50.4-pF myofibroblasts per myocyte diminished CV (white dashed arrows in Figure 7A), led to shorter APD and more depolarized myocyte diastolic potential (Figure 7B), and caused shifts in APD and CV restitution curves (Figure 7C and 7D, respectively).
Figure 7.
A, The effect of myocyte coupling to 50.4-pF myofibroblasts on excitation propagation in a myofiber model The number of myofibroblasts connecting to a myocyte (+1 to +8 MFbs) in the right half of the myofiber is shown in each transmembrane potential map. Other representations and symbols are as in Figure 2A. B, Effect of myofibroblast coupling on myocyte action potential morphology. C and D, APD70 (C) and CV (D) as a function of DI in cases of control, +1 MFb, +2 MFbs, and +4 MFbs. E, Example demonstrating effect of the myocyte-myofibroblast coupling on spiral wave behavior in the same HD-Fbs myocardial sheet as in Figure 3C but with +2 MFbs. Other representations as in Figure 3.
However, because the electrotonic effects of a 50.4-pF myofibroblast on a myocyte are more pronounced than those of a 6.3-pF fibroblast, 8 coupled myofibroblasts (instead of 10) per myocyte were sufficient for conduction block (white double short line in Figure 7A). Moreover, both APD shortening (Figure 7C) and CV decrease (Figure 7D) were exacerbated at shorter DIs, as compared with the fibroblast case (compare with Figure 2C and 2D, respectively).
As shown in Figure 7E (Online Movie I, bottom right), in the case of HD-Fbs but with +2 MFbs instead of +4 Fbs, frequent wave breakups were observed, similar to the case of 6.3-pF fibroblasts shown in Figure 3C. The simulated ECG also exhibited fine f waves.
Effects of Collagen Accumulation on Spiral Wave Behavior
Additional simulations we conducted to examine the effect of collagen accumulation on spiral wave reentry induced by the same protocol.
Figure 8A (Online Movie III, top left) shows the spiral wave behavior in the myocardial sheet, in which 18.8% of the Fb-Area was replaced by nonexcitable and nonconductive tissue (black dots in 0-ms panel) to represent collagen accumulation at low density (LD-Collagen). The spiral wave meandered around the collagen accumulation region, surviving for >5000 ms (500- to 3750-ms panels); the simulated ECG shows coarse f waves, similar to Figure 3B.
Figure 8.
A and B, Examples demonstrating the effect of low-density (A) and high-density (B) collagen accumulation (LD-Collagen and HD-Collagen, respectively) on spiral wave behavior in the myocardial sheet. Black dots indicate atrial myocytes replaced by collagen. Curved white arrows represent direction of wave front propagation during a stationary reentry. C, Consecutive snapshots of transmembrane potential maps (every 500 ms) in the LD-Collagen and HD-Collagen myocardial sheets, incorporating ablation 150 ms after the S2 onset (0 ms). Seven small black circles indicate the ablation sites (+ABL-7).
In Figure 8B (Online Movie III, bottom left) the density of the collagen is high (HD-Collagen, 37.5% of Fb-Area). The spiral wave immediately attached to the clustered collagen (500-ms panel), resulting in a stationary reentry (curved white arrows in 2000- to 3750-ms panels). The simulated ECG (bottom) resembles typical clinical ECG during atrial tachycardia or flutter.
In both cases, collagen accumulation itself caused neither frequent wave breakups nor CFAEs in the bipolar electrograms recorded around the collagen accumulation region (data not shown). As shown in Figure 8C (Online Movie III, top and bottom right), catheter ablation targeting the collagen accumulation region (same ablation sites as in Figure 6A, +ABL-7 case) could not terminate the spiral wave.
Discussion
This study focused on the poorly understood mechanisms of CFAEs during persistent/permanent AF and CFAE-targeted ablation. To address these issues, we modeled human atrial tissue under heart failure conditions and conducted simulations of propagation and spiral wave reentry with and without fibroblast proliferation. The main findings are:
The (myo)fibroblast proliferation results in frequent spiral wave breakups, accompanied by fine f waves in the ECG.
Bipolar electrograms recorded from the Fb-Area during spiral wave reentry show CFAEs.
Ablation targeting such fibroblast-derived CFAEs terminates spiral wave reentry.
CFAEs are not attributed to collagen accumulation itself.
Fibroblast Proliferation as a Mechanism of CFAEs
To the best of our knowledge, the present study is the first to focus on the relationship between fibroblast proliferation and CFAE genesis. The faithful reproduction of CFAEs by our AF model suggests that the electrotonic interactions between myocytes and fibroblasts are an important mechanism of CFAEs during persistent/permanent AF (Figure 3C and Figure 5). Furthermore, the present study demonstrates that the differentiation of fibroblasts into myofibroblasts of larger capacitance did not alter the basic CFAE mechanism (Figure 7). We also simulated the effect of IKr blocker on CFAEs (Online Figure III), which is consistent with the results of a recent clinical study.27
The present study suggests that decreases in APD (Figure 2A through 2C), CV (Figure 2A and 2D), and myocardial excitability (Figure 2A and 3C) are requirements for CFAEs. Fibroblast proliferation was found to satisfy these 3 requirements simultaneously, since the resting membrane potential of the nonexcitable fibroblasts (around −50 mV26) electrotonically decreased the action potential plateau level while elevating the resting membrane potential of the coupled atrial myocytes.
Evidence Supporting the Fibroblast CFAE Hypothesis
The fibroblast CFAE hypothesis is supported by previous observational evidence that fibrosis plays a role in atrial structural remodeling under persistent/permanent AF28,29 and that (myo)fibroblast proliferation is involved in the process of fibrosis.8–10 Furthermore, an experimental study14 using optical mapping of cocultured cardiac monolayers demonstrated direct evidence for the propensity to reentrant arrhythmia under (myo)fibroblast proliferation, consistent with our results.
Experimental and clinical studies have demonstrated that conduction delay/block leading to fibrillatory conduction was observed predominantly in long-term rather than short-term AF,30 and that it occurred in areas where structural remodeling, including fibrosis, was confirmed by histology30 and where electrograms consistently exhibited CFAEs during AF.31,32 Our simulation results are consistent with these findings: as seen in Figure 2A and 3C, fibroblast proliferation causes conduction delay/block, resulting in CFAEs (Figure 5).
Clinically, CFAE sites are found in clusters over the atria and exhibit temporal and spatial stability1,2,5; such clustered distribution is very similar to the distribution of fibrotic remodeling areas in patients with persistent/permanent AF.33,34 Indeed, probably because of the fibrotic remodeling, the distribution of CFAE sites during persistent/permanent AF is different from that during paroxysmal AF, with the number of CFAE sites being larger in persistent/permanent AF than in paroxysmal AF.1,5,35 Furthermore, the addition of CFAE-targeted ablation to pulmonary vein isolation provides great benefit in the treatment of persistent/permanent AF,2,3 which is not the case for paroxysmal AF.3 These findings are consistent with our hypothesis that CFAEs under persistent/ permanent AF are attributable, at least partially, to structural remodeling (fibroblast proliferation and/or fibrosis) and that such CFAE sites are important in the maintenance of persistent/permanent AF.
Hunter et al36 classified CFAEs into 5 categories according to their complexity: Grade 1 (uninterrupted fractionated), Grade 2 (interrupted fractionated), Grade 3 (intermittent fractionated), Grade 4 (complex), and Grade 5 (normal). Following this classification, CFAEs in our study are Grade 2 and/or 4, both of which were found to be effective ablation targets.36 Our results regarding CFAE-targeted ablation are also supported by the clinical observation that temporal and frequency gradients of activations can be used to guide effective ablation of AF sources.37 Our simulated CFAEs (Figure 5) showed both the temporally alternating potentials between adjacent bipoles (temporal gradient of activation) and the distribution of activation cycle length, which decays from the Fb-Area to the surrounding tissue (frequency gradient).
Other Proposed CFAE Mechanisms
The activity of ganglionated plexi has been proposed as a mechanism of CFAEs.4,5 However, as mentioned in the Introduction, the number of neurons in the ganglionated plexi decreases with age6 and thus the contribution of the plexi to CFAEs in aged patients with persistent/permanent AF could be lower than expected. Indeed, the ganglionated plexi-targeted ablation has been shown to be effective for paroxysmal AF38 but not for persistent AF.39
Another possible CFAE mechanism, at least contributing to CFAE complexity, could be pulmonary vein triggers. Indeed, CFAE sites tend to locate at pulmonary vein ostia, thus pulmonary vein triggers could play an important role in CFAE formation. However, the contribution of pulmonary vein triggers in AF maintenance seems to be less important regarding maintenance of persistent/permanent AF based on the fact that pulmonary vein isolation alone fails in most cases to restore stable sinus rhythm in these types AF patients.35,40,41
Additional proposed mechanism underlying CFAEs is endo-epicardial breakthroughs.21,30 Experimental studies using a multi-electrode mapping system have demonstrated that 3-dimensional intramural reentry, resulting in breakthroughs, can occur in the thin atrial wall,42 and that the emergence of breakthroughs could cause CFAE-like electrograms.30
Finally, CFAEs have been proposed to arise at the periphery of high-frequency rotors, where frequent wave breakups and fibrillatory conduction occur because of regional ion-channel changes causing electrophysiological heterogeneity.41,43 In this case, CFAE sites should encircle the dominant frequency area, with the frequency at the CFAE sites being lower than that at the surrounding tissue. However, according to clinical studies,1,2,5 CFAE sites exhibit shorter cycle lengths than the surrounding tissue and have been found to arise in clusters over the atria, which is consistent with our simulation results.
Why Is CFAE-Targeted Ablation Effective for Persistent/Permanent AF?
On the basis of the hypothesis1 that CFAE sites are critical in perpetuating AF, these sites have become important targets for AF ablation, especially in patients with persistent/permanent AF.2,3 However, previous research has not provided a direct evidence that CFAEs reflect AF substrates and that CFAE-targeted ablation can terminate AF.
Effective catheter ablation for tachyarrhythmias is commonly achieved by (1) linear ablation crossing anatomic reentrant circuits, (2) focal ablation targeting abnormal automaticities, or (3) circumferential ablation encircling abnormal automaticities and/or rotors. What we found here is that CFAE-targeted ablation is different from the above 3 well-known approaches.
As demonstrated by our simulations, spiral wave reentry was transiently pinned at the ablation site (Figure 6A and Online Movie II) because the ablated circle (0.5 cm in diameter) was too small to permanently attach the spiral wave. Thus, CFAE-targeted ablation suppressed the generation of new wave fronts by blocking the shortcut of the reentry (Figure 6B) and pushed the spiral wave out of the Fb-Area (Figure 6C through 6E), resulting in AF termination.
However, in the case of collagen accumulation such scattered ablation sites converted AF, manifested as a meandering spiral wave reentry (Figure 8A), into a sustained stationary reentry (Figure 8C, top), for example, atrial tachycardia. The collagen accumulation region provided a better anchor for the spiral wave than the fibroblast proliferation area, and ablation at the multiple sites could not push the spiral wave reentry out of the collagen accumulation region.
Although clustered collagen represents nonexcitable regions (Figure 8A and 8B) similar to those of ablation lesions (Figure 6A), the former leads to sustained reentry whereas the latter results in reentry termination. Our simulation results demonstrate that spiral wave reentry is eliminated if the wave transiently attaches to the nonexcitable ablation lesion (Figure 6C through 6E). The ablation lesion in this study was 5 mm in diameter, which corresponds to the critical size of a nonexcitable anatomic obstacle required for spiral wave attachment.44 Our simulations also demonstrate that a meandering spiral wave becomes stationary when small nonexcitable areas were distributed throughout the collagen accumulation region (Figure 8A and 8B). A single small nonexcitable area of diameter <5 mm does not change spiral wave behavior44; however, our results show that many small nonexcitable areas scattered throughout the collagen accumulation region prevent the spiral wave from leaving that region. Indeed, the reason why ablation applied at the collagen accumulation region causes sustained atrial tachycardia rather than AF termination may be explained by the fact that the total area of nonexcitable clustered collagen plus nonexcitable ablation lesions far exceeds the critical size for spiral wave attachment.44
Clinical Implications
The significance of CFAEs and the efficacy of CFAE-targeted ablation remain not well understood. The proposed fibroblast CFAE hypothesis provides a systematic explanation of the mechanisms contributing not only to CFAEs but also to CFAE-targeted ablation during persistent/permanent AF.
Based on our simulation results, CFAE-targeted ablation may be effective for AF under fibroblast proliferation but not when accompanied by significant collagen accumulation. This suggests that CFAE-targeted ablation is more effective for patients in earlier stages of persistent AF, where collagen accumulation in the atria is severe. This speculation is consistent with a clinical study demonstrating that longer AF duration is one of the independent predictors of AF recurrence after CFAE-targeted ablation combined with pulmonary vein isolation for patients with persistent AF.45
Study Limitations
For CFAE detection, we did not use voltage thresholds but rather excitation intervals in the bipolar electrograms. CFAE detection algorithms vary among studies,1,35,46–48 with the very short cycle length, below 120 ms, which we used in our criterion, being the most fundamental characteristic of CFAEs.35,47
We used a 2-D atrial sheet model in this study. Thus, we could not investigate the contribution of epi-endocardial breakthroughs to CFAEs. Moreover, each fibroblast was connected to 1 myocyte only, and we did not consider fibroblast-fibroblast electrotonic interactions. Despite the simplified model, our simulation results are consistent with clinical observations in many aspects as discussed above.
In vivo myocyte-fibroblast electric coupling has been found in the atrium but only in the sinoatrial node, where fibroblasts are most abundant under physiological conditions.49 However, because fibroblast proliferation is mechanically and chemically enhanced by the remodeled myocardium7–9 and the myocyte-fibroblast interactions are enhanced by cardiac injury,50 it is possible that (myo)fibroblasts are abundant in the atria in persistent/permanent AF under heart failure conditions. Moreover, because the (myo)fibroblasts produce collagen fibers in the process of myocardial remodeling,7–9 it is possible that (myo)fibroblasts act homeostatically as an electric bridge between different regions of myocytes isolated by collagen fibers. Myocyte-fibroblast electric coupling has been widely demonstrated in cell cultures,10–14 suggesting that myocyte-fibroblast electric coupling in the remodeled atria is a possible pathophysiological outcome. However, direct demonstration of the fibroblast’s ability to couple to a remodeled atrial myocyte under the conditions of fibrosis associated with persistent/permanent AF is currently lacking. This is due to the fibroblast’s nature to constantly adapt to its environment,51,52 making it very difficult to electrically identify the fibroblast in situ.
Finally, the model does not incorporate heterogeneity of ion channel distribution or other electrophysiological and functional changes associated with persistent/permanent AF, as well as pulmonary vein triggers, which might also be contributing to CFAE generation, as described in the Discussion.
Despite these limitations, the present study captures essential features of CFAEs during persistent/permanent AF, and the fibroblast CFAE hypothesis provides a possible mechanism of CFAE-targeted ablation. Further studies are needed to fully elucidate the mechanisms of CFAEs and CFAE-targeted ablation.
Supplementary Material
Novelty and Significance.
What Is Known?
Electrogram-based catheter ablation, targeting complex fractionated atrial electrograms (CFAEs), is empirically known to be effective in halting persistent/permanent atrial fibrillation (AF).
Myocardial tissue is mainly composed of myocytes and collagen-producing fibroblasts. Fibroblasts proliferate as part of the atrial structural remodeling associated with persistent/permanent AF.
What New Information Does This Article Contribute?
Fibroblast proliferation and electrotonic interactions between atrial myocytes and fibroblasts in fibrotic regions lead to the formation of CFAEs during persistent/permanent AF.
Catheter ablation targeting fibroblast-derived CFAEs can terminate AF by suppressing spiral wave breakup and pushing spiral waves out of the fibroblast proliferation areas.
Targeting CFAEs by electrogram-based catheter ablation is empirically known to be effective in halting persistent/permanent AF. However, the mechanisms underlying CFAEs during persistent/permanent AF and CFAE-targeted ablation are poorly understood. Fibrosis plays an important role in atrial structural remodeling during persistent/permanent AF. In the present study, we conducted computer simulations of spiral wave reentry as a representation of AF and demonstrated for the first time that fibroblast proliferation, which is involved in the process of fibrotic remodeling, is responsible for the genesis of CFAEs. Because electric coupling between atrial myocytes and fibroblasts shortens action potential duration electrotonically, slows conduction velocity, and lowers excitability, regionally proliferated fibroblasts cause frequent spiral wave breakups, and the bipolar electrograms recorded at the fibroblast proliferation area during spiral wave reentry exhibit CFAEs. The CFAEs could not be attributed to collagen accumulation. We further found that the fibroblast proliferation area is critical in perpetuating AF and that ablation targeting fibroblast-derived CFAEs can terminate AF by suppressing spiral wave breakup and pushing the spiral wave out of the fibroblast proliferation area. The proposed fibroblast CFAE hypothesis provides a systematic explanation of the mechanisms that underlie the success of CFAE-targeted ablation during persistent/permanent AF.
Acknowledgments
Sources of Funding
This work was supported by grant-in-aid 21790717 (to T.A.), 22136011 (to T.A., R.H., and K.N.), and 21500420 (to T.A., R.H., K.N., T.N., and T.I.) from the MEXT of Japan, and by National Institutes of Health grants R01 HL082729 and R01 HL103428, and NSF grant CBET-0933029 (to N.T.).
Non-standard Abbreviations and Acronyms
- 2-D
2-dimensional
- AF
atrial fibrillation
- APD
action potential duration
- APD70
action potential duration at 70% of repolarization
- CFAE
complex fractionated atrial electrogram
- CV
conduction velocity
- DI
diastolic interval
- Fb-Area
fibroblast proliferation area
- Fbs
fibroblasts
- HD-Collagen
collagen accumulation at high density
- HD-Fbs
fibroblasts of high density
- LD-Collagen
collagen accumulation at low density
- LD-Fbs
fibroblasts of low density
- MFbs
myofibroblasts
Footnotes
Disclosures
None.
References
- 1.Nademanee K, McKenzie J, Kosar E, Schwab M, Sunsaneewitayakul B, Vasavakul T, Khunnawat C, Ngarmukos T. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004;43:2044–2053. doi: 10.1016/j.jacc.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 2.Elayi CS, Verma A, Di Biase L, Ching CK, Patel D, Barrett C, Martin D, Rong B, Fahmy TS, Khaykin Y, Hongo R, Hao S, Pelargonio G, Dello Russo A, Casella M, Santarelli P, Potenza D, Fanelli R, Massaro R, Arruda M, Schweikert RA, Natale A. Ablation for longstanding permanent atrial fibrillation: results from a randomized study comparing three different strategies. Heart Rhythm. 2008;5:1658–1664. doi: 10.1016/j.hrthm.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 3.Verma A, Mantovan R, Macle L, De Martino G, Chen J, Morillo CA, Novak P, Calzolari V, Guerra PG, Nair G, Torrecilla EG, Khaykin Y. Substrate and Trigger Ablation for Reduction of Atrial Fibrillation (STAR AF): a randomized, multicentre, international trial. Eur Heart J. 2010;31:1344–1356. doi: 10.1093/eurheartj/ehq041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin J, Scherlag BJ, Zhou J, Lu Z, Patterson E, Jackman WM, Lazzara R, Po SS. Autonomic mechanism to explain complex fractionated atrial electrograms (CFAE) J Cardiovasc Electrophysiol. 2007;18:1197–1205. doi: 10.1111/j.1540-8167.2007.00976.x. [DOI] [PubMed] [Google Scholar]
- 5.Nademanee K, Lockwood E, Oketani N, Gidney B. Catheter ablation of atrial fibrillation guided by complex fractionated atrial electrogram mapping of atrial fibrillation substrate. J Cardiol. 2010;55:1–12. doi: 10.1016/j.jjcc.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Pauza DH, Skripka V, Pauziene N, Stropus R. Morphology, distribution, and variability of the epicardiac neural ganglionated subplexuses in the human heart. Anat Rec. 2000;259:353–382. doi: 10.1002/1097-0185(20000801)259:4<353::AID-AR10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 7.Baudino TA, Carver W, Giles W, Borg TK. Cardiac fibroblasts: friend or foe? Am J Physiol Heart Circ Physiol. 2006;291:H1015–H1026. doi: 10.1152/ajpheart.00023.2006. [DOI] [PubMed] [Google Scholar]
- 8.Souders CA, Bowers SL, Baudino TA. Cardiac fibroblast: the renaissance cell. Circ Res. 2009;105:1164–1176. doi: 10.1161/CIRCRESAHA.109.209809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kakkar R, Lee RT. Intramyocardial fibroblast myocyte communication. Circ Res. 2010;106:47–57. doi: 10.1161/CIRCRESAHA.109.207456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goshima K. Synchronized beating of and electrotonic transmission between myocardial cells mediated by heterotypic strain cells in monolayer culture. Exp Cell Res. 1969;58:420–426. doi: 10.1016/0014-4827(69)90523-0. [DOI] [PubMed] [Google Scholar]
- 11.Hyde A, Blondel B, Matter A, Cheneval JP, Filloux B, Girardier L. Homo- and heterocellular junctions in cell cultures: an electrophysiological and morphological study. Prog Brain Res. 1969;31:283–311. doi: 10.1016/S0079-6123(08)63247-1. [DOI] [PubMed] [Google Scholar]
- 12.Gaudesius G, Miragoli M, Thomas SP, Rohr S. Coupling of cardiac electrical activity over extended distances by fibroblasts of cardiac origin. Circ Res. 2003;93:421–428. doi: 10.1161/01.RES.0000089258.40661.0C. [DOI] [PubMed] [Google Scholar]
- 13.Camelliti P, Borg TK, Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc Res. 2005;65:40–51. doi: 10.1016/j.cardiores.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 14.Zlochiver S, Munoz V, Vikstrom KL, Taffet SM, Berenfeld O, Jalife J. Electrotonic myofibroblast-to-myocyte coupling increases propensity to reentrant arrhythmias in two-dimensional cardiac monolayers. Biophys J. 2008;95:4469–4480. doi: 10.1529/biophysj.108.136473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Courtemanche M, Ramirez RJ, Nattel S. Ionic mechanisms underlying human atrial action potential properties: insights from a mathematical model. Am J Physiol. 1998;275:H301–H321. doi: 10.1152/ajpheart.1998.275.1.H301. [DOI] [PubMed] [Google Scholar]
- 16.MacCannell KA, Bazzazi H, Chilton L, Shibukawa Y, Clark RB, Giles WR. A mathematical model of electrotonic interactions between ventricular myocytes and fibroblasts. Biophys J. 2007;92:4121–4132. doi: 10.1529/biophysj.106.101410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li D, Melnyk P, Feng J, Wang Z, Petrecca K, Shrier A, Nattel S. Effects of experimental heart failure on atrial cellular and ionic electrophysiology. Circulation. 2000;101:2631–2638. doi: 10.1161/01.cir.101.22.2631. [DOI] [PubMed] [Google Scholar]
- 18.Kostin S, Klein G, Szalay Z, Hein S, Bauer EP, Schaper J. Structural correlate of atrial fibrillation in human patients. Cardiovasc Res. 2002;54:361–379. doi: 10.1016/s0008-6363(02)00273-0. [DOI] [PubMed] [Google Scholar]
- 19.Nao T, Ohkusa T, Hisamatsu Y, Inoue N, Matsumoto T, Yamada J, Shimizu A, Yoshiga Y, Yamagata T, Kobayashi S, Yano M, Hamano K, Matsuzaki M. Comparison of expression of connexin in right atrial myocardium in patients with chronic atrial fibrillation versus those in sinus rhythm. Am J Cardiol. 2003;91:678–683. doi: 10.1016/s0002-9149(02)03403-3. [DOI] [PubMed] [Google Scholar]
- 20.Polontchouk L, Haefliger JA, Ebelt B, Schaefer T, Stuhlmann D, Mehlhorn U, Kuhn-Regnier F, De Vivie ER, Dhein S. Effects of chronic atrial fibrillation on gap junction distribution in human and rat atria. J Am Coll Cardiol. 2001;38:883–891. doi: 10.1016/s0735-1097(01)01443-7. [DOI] [PubMed] [Google Scholar]
- 21.Eckstein J, Maesen B, Linz D, Zeemering S, van Hunnik A, Verheule S, Allessie M, Schotten U. Time course and mechanisms of endo-epicardial electrical dissociation during atrial fibrillation in the goat. Cardiovasc Res. 2011;89:816–824. doi: 10.1093/cvr/cvq336. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka K, Zlochiver S, Vikstrom KL, Yamazaki M, Moreno J, Klos M, Zaitsev AV, Vaidyanathan R, Auerbach DS, Landas S, Guiraudon G, Jalife J, Berenfeld O, Kalifa J. Spatial distribution of fibrosis governs fibrillation wave dynamics in the posterior left atrium during heart failure. Circ Res. 2007;101:839–847. doi: 10.1161/CIRCRESAHA.107.153858. [DOI] [PubMed] [Google Scholar]
- 23.Ashihara T, Namba T, Yao T, Ozawa T, Kawase A, Ikeda T, Nakazawa K, Ito M. Vortex cordis as a mechanism of postshock activation: arrhythmia induction study using a bidomain model. J Cardiovasc Electrophysiol. 2003;14:295–302. doi: 10.1046/j.1540-8167.2003.02408.x. [DOI] [PubMed] [Google Scholar]
- 24.Ashihara T, Namba T, Ikeda T, Ito M, Kinoshita M, Nakazawa K. Breakthrough waves during ventricular fibrillation depend on the degree of rotational anisotropy and the boundary conditions: a simulation study. J Cardiovasc Electrophysiol. 2001;12:312–322. doi: 10.1046/j.1540-8167.2001.00312.x. [DOI] [PubMed] [Google Scholar]
- 25.Maleckar MM, Greenstein JL, Giles WR, Trayanova NA. Electrotonic coupling between human atrial myocytes and fibroblasts alters myocyte excitability and repolarization. Biophys J. 2009;97:2179–2190. doi: 10.1016/j.bpj.2009.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamkin A, Kiseleva I, Wagner KD, Bohm J, Theres H, Gunther J, Scholz H. Characterization of stretch-activated ion currents in isolated atrial myocytes from human hearts. Pflugers Arch. 2003;446:339–346. doi: 10.1007/s00424-002-0948-0. [DOI] [PubMed] [Google Scholar]
- 27.Singh SM, D’Avila A, Kim SJ, Houghtaling C, Dukkipati SR, Reddy VY. Intraprocedural use of ibutilide to organize and guide ablation of complex fractionated atrial electrograms. J Cardiovasc Electrophysiol. 2010;21:608–616. doi: 10.1111/j.1540-8167.2009.01671.x. [DOI] [PubMed] [Google Scholar]
- 28.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation: a study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 29.Everett TH, IV, Wilson EE, Verheule S, Guerra JM, Foreman S, Olgin JE. Structural atrial remodeling alters the substrate and spatiotemporal organization of atrial fibrillation: a comparison in canine models of structural and electrical atrial remodeling. Am J Physiol Heart Circ Physiol. 2006;291:H2911–H2923. doi: 10.1152/ajpheart.01128.2005.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verheule S, Tuyls E, van Hunnik A, Kuiper M, Schotten U, Allessie M. Fibrillatory conduction in the atrial free walls of goats in persistent and permanent atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:590–599. doi: 10.1161/CIRCEP.109.931634. [DOI] [PubMed] [Google Scholar]
- 31.Konings KT, Smeets JL, Penn OC, Wellens HJ, Allessie MA. Configuration of unipolar atrial electrograms during electrically induced atrial fibrillation in humans. Circulation. 1997;95:1231–1241. doi: 10.1161/01.cir.95.5.1231. [DOI] [PubMed] [Google Scholar]
- 32.Roberts-Thomson KC, Stevenson I, Kistler PM, Haqqani HM, Spence SJ, Goldblatt JC, Sanders P, Kalman JM. The role of chronic atrial stretch and atrial fibrillation on posterior left atrial wall conduction. Heart Rhythm. 2009;6:1109–1117. doi: 10.1016/j.hrthm.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 33.Verma A, Wazni OM, Marrouche NF, Martin DO, Kilicaslan F, Minor S, Schweikert RA, Saliba W, Cummings J, Burkhardt JD, Bhargava M, Belden WA, Abdul-Karim A, Natale A. Pre-existent left atrial scarring in patients undergoing pulmonary vein antrum isolation: an independent predictor of procedural failure. J Am Coll Cardiol. 2005;45:285–292. doi: 10.1016/j.jacc.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 34.Oakes RS, Badger TJ, Kholmovski EG, Akoum N, Burgon NS, Fish EN, Blauer JJ, Rao SN, DiBella EV, Segerson NM, Daccarett M, Windfelder J, McGann CJ, Parker D, MacLeod RS, Marrouche NF. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009;119:1758–1767. doi: 10.1161/CIRCULATIONAHA.108.811877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scherr D, Dalal D, Cheema A, Cheng A, Henrikson CA, Spragg D, Marine JE, Berger RD, Calkins H, Dong J. Automated detection and characterization of complex fractionated atrial electrograms in human left atrium during atrial fibrillation. Heart Rhythm. 2009;4:1013–1020. doi: 10.1016/j.hrthm.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 36.Hunter RJ, Diab I, Tayebjee M, Richmond L, Sporton S, Earley MJ, Schilling RJ. Characterization of fractionated atrial electrograms critical for maintenance of AF: a randomized controlled trial of ablation strategies (the CFAE AF Trial) Circ Arrhythm Electrophysiol. 2011;4:622–629. doi: 10.1161/CIRCEP.111.962928. [DOI] [PubMed] [Google Scholar]
- 37.Lim KT, Knecht S, Wright M, Haissaguerre M. Atrial substrate ablation in atrial fibrillation. In: Zipes DP, Jalife J, editors. Cardiac Electrophysiology: From Cell to Bedside. 5. Philadelphia, PA: Saunders Elsevier; 2009. pp. 1059–1070. [Google Scholar]
- 38.Pappone C, Santinelli V, Manguso F, Vicedomini G, Gugliotta F, Augello G, Mazzone P, Tortoriello V, Landoni G, Zangrillo A, Lang C, Tomita T, Mesas C, Mastella E, Alfieri O. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation. 2004;109:327–334. doi: 10.1161/01.CIR.0000112641.16340.C7. [DOI] [PubMed] [Google Scholar]
- 39.Pokushalov E, Romanov A, Artyomenko S, Turov A, Shugayev P, Shirokova N, Katritsis DG. Ganglionated plexi ablation for longstanding persistent atrial fibrillation. Europace. 2010;12:342–346. doi: 10.1093/europace/euq014. [DOI] [PubMed] [Google Scholar]
- 40.Oral H, Knight BP, Tada H, Ozaydin M, Chugh A, Hassan S, Scharf C, Lai SW, Greenstein R, Pelosi F, Jr, Strickberger SA, Morady F. Pulmonary vein isolation for paroxysmal and persistent atrial fibrillation. Circulation. 2002;105:1077–1081. doi: 10.1161/hc0902.104712. [DOI] [PubMed] [Google Scholar]
- 41.Atienza F, Almendral J, Moreno J, Vaidyanathan R, Talkachou A, Kalifa J, Arenal A, Villacastín JP, Torrecilla EG, Sanchez A, Ploutz-Snyder R, Jalife J, Berenfeld O. Activation of inward rectifier potassium channels accelerates atrial fibrillation in humans: evidence for a reentrant mechanism. Circulation. 2006;114:2434–2442. doi: 10.1161/CIRCULATIONAHA.106.633735. [DOI] [PubMed] [Google Scholar]
- 42.Schuessler RB, Kawamoto T, Hand DE, Mitsuno M, Bromberg BI, Cox JL, Boineau JP. Simultaneous epicardial and endocardial activation sequence mapping in the isolated canine right atrium. Circulation. 1993;88:250–263. doi: 10.1161/01.cir.88.1.250. [DOI] [PubMed] [Google Scholar]
- 43.Kalifa J, Tanaka K, Zaitsev AV, Warren M, Vaidyanathan R, Auerbach D, Pandit S, Vikstrom KL, Ploutz-Snyder R, Talkachou A, Atienza F, Guiraudon G, Jalife J, Berenfeld O. Mechanisms of wave fractionation at boundaries of high-frequency excitation in the posterior left atrium of the isolated sheep heart during atrial fibrillation. Circulation. 2006;113:626–633. doi: 10.1161/CIRCULATIONAHA.105.575340. [DOI] [PubMed] [Google Scholar]
- 44.Ikeda T, Yashima M, Uchida T, Hough D, Fishbein MC, Mandel WJ, Chen PS, Karagueuzian HS. Attachment of meandering reentrant wave fronts to anatomic obstacles in the atrium: role of the obstacle size. Circ Res. 1997;81:753–764. doi: 10.1161/01.res.81.5.753. [DOI] [PubMed] [Google Scholar]
- 45.McCready JW, Smedley T, Lambiase PD, Ahsan SY, Segal OR, Rowland E, Lowe MD, Chow AW. Predictors of recurrence following radiofrequency ablation for persistent atrial fibrillation. Europace. 2011;13:355–361. doi: 10.1093/europace/euq434. [DOI] [PubMed] [Google Scholar]
- 46.Porter M, Spear W, Akar JG, Helms R, Brysiewicz N, Santucci P, Wilber DJ. Prospective study of atrial fibrillation termination during ablation guided by automated detection of fractionated electrograms. J Cardiovasc Electrophysiol. 2008;19:613–620. doi: 10.1111/j.1540-8167.2008.01189.x. [DOI] [PubMed] [Google Scholar]
- 47.Bencsik G, Martinek M, Hassanein S, Aichinger J, Nesser HJ, Purerfellner H. Acute effects of complex fractionated atrial electrogram ablation on dominant frequency and regulatory index for the fibrillatory process. Europace. 2009;11:1011–1017. doi: 10.1093/europace/eup113. [DOI] [PubMed] [Google Scholar]
- 48.Tsai WC, Lin YJ, Tsao HM, Chang SL, Lo LW, Hu YF, Chang CJ, Tang WH, Tuan TC, Udyavar AR, Wang JH, Chen SA. The optimal automatic algorithm for the mapping of complex fractionated atrial electrograms in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2010;21:21–26. doi: 10.1111/j.1540-8167.2009.01567.x. [DOI] [PubMed] [Google Scholar]
- 49.Camelliti P, Green CR, LeGrice I, Kohl P. Fibroblast network in rabbit sinoatrial node: structural and functional identification of homogeneous and heterogeneous cell coupling. Circ Res. 2004;94:828–835. doi: 10.1161/01.RES.0000122382.19400.14. [DOI] [PubMed] [Google Scholar]
- 50.Vasquez C, Mohandas P, Louie KL, Benamer N, Bapat AC, Morley GE. Enhanced fibroblast-myocyte interactions in response to cardiac injury. Circ Res. 2010;107:1011–1020. doi: 10.1161/CIRCRESAHA.110.227421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Camelliti P, Green CR, Kohl P. Structural and functional coupling of cardiac myocytes and fibroblasts. Adv Cardiol. 2006;42:132–149. doi: 10.1159/000092566. [DOI] [PubMed] [Google Scholar]
- 52.McDowell KS, Trayanova N, Kohl P. Fibroblasts Cardiac Electrophysiology. In: Turner NA, editor. The Cardiac Fibroblast. Kerala, India: Research Signpost; 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.