ABSTRACT
Chromosome segregation errors in female meiosis lead to aneuploidy in the resulting egg and embryo, making them one of the leading genetic causes of spontaneous abortions and developmental disabilities in humans. It is known that aneuploidy of meiotic origin increases dramatically as women age, and current evidence suggests that most errors occur in meiosis I. Several hypotheses regarding the cause of maternal age-related aneuploidy have been proposed, including recombination errors in early meiosis, a defective spindle assembly checkpoint in meiosis I, and deterioration of sister chromatid cohesion with age. This review discusses findings in each area, and focuses especially on recent studies suggesting that deterioration of cohesion with increasing maternal age is a leading cause of age-related aneuploidy.
Keywords: aging, aneuploidy, female infertility, meiosis, oocyte
This review discusses maternal age-related aneuploidy and focuses especially on recent studies suggesting that deterioration of cohesion is a leading cause.
INTRODUCTION
The relationship between Down syndrome and advanced maternal age was first described more than 70 yr ago, well before the association with trisomy 21 was observed [1, 2]. It is now well established that aneuploidy dramatically increases as women age: the incidence of trisomy in clinically recognized pregnancies remains low in a woman in her 20s, around 2%–3%, but dramatically increases to about 35% in a woman in her 40s [3]. All autosomal monosomies and most trisomies are inviable, and the few viable trisomies (e.g., trisomies 13 and 21, sex chromosome trisomies) lead to severe mental and health disabilities [4]. Aneuploidy of the egg is the leading genetic cause of spontaneous abortions and developmental disabilities, but the molecular basis for these chromosome segregation errors has only recently become clearer.
Most human aneuploidies found in embryos originate from the egg and not sperm [5–9], likely because of one critical difference in the meiotic process between males and females. Meiosis begins with an initial step of DNA replication followed by two rounds of cell divisions to generate haploid gametes. In prophase I, homologous chromosomes synapse and recombination occurs. These homologous chromosomes then separate at the end of meiosis I (MI), whereas sister chromatids separate in meiosis II (MII). In males this process initiates throughout lifetime after puberty. In contrast, in females meiosis initiates during fetal development, arrests at prophase I before birth, and does not resume until prior to ovulation in adulthood, up to 50 yr later in humans. The long time interval between meiotic arrest in the fetus and each ovulation cycle in the adult allows maternal age to affect aneuploidy incidence. Furthermore, standard in vitro fertilization procedures show that whereas an older patient (acting as both donor and recipient) has more difficulty reaching successful fertilization and embryo implantation, an oocyte donated from a young woman to an older recipient can restore implantation and pregnancy rates. Thus, the aging oocyte, and not the uterus, is responsible for problems associated with maternal age [10, 11].
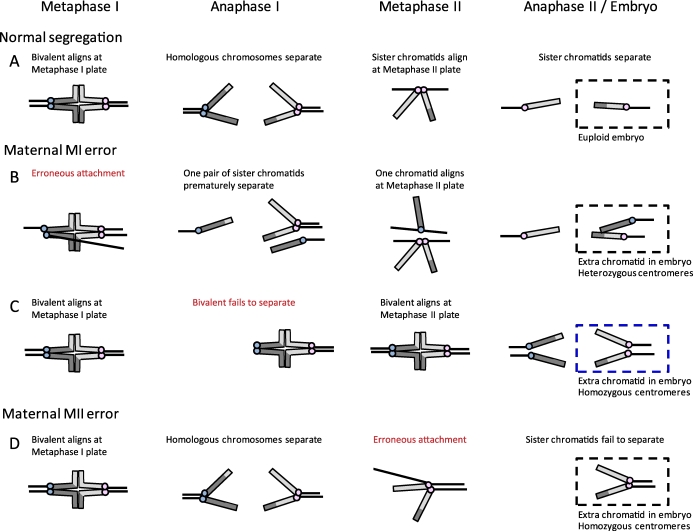
To understand the origin of aneuploidy in oocytes, it is important to know when chromosome segregation errors occur. A recent report shows that even in phenotypically normal fetuses, a proportion of ovarian cells, presumably oocytes, are trisomic for chromosome 21 (oocyte mosaicism) [12]. This finding led to a new hypothesis, the oocyte mosaicism selection model, which suggests that mitotic errors occur before entry into meiosis, leading to aneuploid oocytes in primordial follicles that are preferentially recruited with increased maternal age [13, 14]. However, whether there is preferential recruitment of trisomy 21 oocytes remains to be tested. The rest of this review will focus on the assumption that most errors occur in meiosis, which is how the data have generally been interpreted. Using pericentromeric markers, homozygosity or heterozygosity of centromeres on the extra chromosome in a trisomy can be used to determine whether a segregation error occurred in MI or MII (Fig. 1) [7, 15, 16]. Homologous chromosomes separate at the end of MI, so if centromeres in the trisomic individual are heterozygous, then an error occurred in MI where the homologous chromosomes failed to segregate from each other (Fig. 1B). On the other hand, homozygous centromere markers indicate that sister chromatids failed to separate during MII (Fig. 1D). With this analysis, for example, almost all trisomy 16 cases are assumed to result from MI errors, and over 50% of trisomy 18 cases from MII errors [7, 17]. This categorization is problematic, however, because an MI error can also produce homozygous centromere markers (Fig. 1C). For example, if a bivalent fails to separate in MI, reductional division may occur at MII instead so that sister chromatids are pulled toward the same pole at MII. In this case, the centromeres would be homozygous even though the initial error occurred in MI. Regardless of potential misclassifications, most trisomies of maternal origin are due to errors in MI (e.g., trisomies 13, 15, 16, 21) [3, 6, 18–20].
FIG. 1.
Analysis of centromeres to determine the origin of maternal errors in trisomies. Schematic of chromosome segregation in MI and MII. Chromosomes are depicted as telocentric (as in mice) for simplicity, and centromeres of sister chromatids are colored alike (e.g., blue or pink). A) Normal segregation at both MI and MII, with a euploid embryo. B) Example of an error at MI, where a pair of sister kinetochores erroneously biorients at metaphase I and prematurely separates at anaphase I; the resulting chromatids in the embryo have heterozygous centromeres. Note that there are several possible segregation errors that can occur at MI (e.g., because of failure of recombination or loss of cohesion), of which one example is shown, but heterozygous centromeres in the embryo indicate that an MI error occurred. C) Example of an error at MI, where a bivalent fails to separate at anaphase I and instead separates at anaphase II. Here the centromeres in the embryo are homozygous and would be problematically classified as a MII error, even though the initial error occurred in MI. D) Example of an MII error, where sister chromatids fail to separate at anaphase II; the resulting chromatids in the embryo are homozygous.
Several hypotheses regarding the causes of maternal age-related aneuploidy have been proposed, including recombination errors in early meiosis, a defective spindle assembly checkpoint (SAC) at MI, and deterioration of sister chromatid cohesion with age. Perturbations made in animal models to test each of these ideas reveal processes that may be susceptible to age, and it is clear that many things can go wrong to increase aneuploidy incidence. However, it is also important to determine what occurs in the natural aging process, without any experimental perturbations. The mouse is a good model for studying age-related aneuploidy because, as in humans, advanced maternal age is associated with an increase in aneuploidy incidence in naturally aged mice, although such an increase has not been observed in pig [21–24]. The combination of genetically altered animals and natural aging models helps us understand the age-dependent increase of chromosome segregation errors in female meiosis.
MEIOTIC RECOMBINATION AND SYNAPSIS
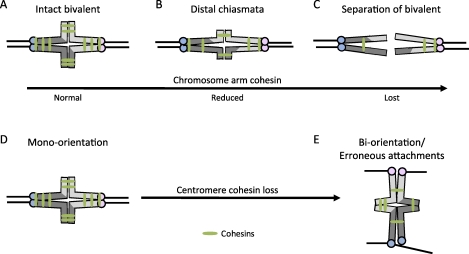
Synapsis and recombination of homologous chromosomes are two of the first critical steps of meiosis that occur in prophase I during fetal development in female mammals. Synapsis, the physical pairing of homologous chromosomes mediated by the synaptonemal complex, is important for recombination. Meiotic recombination results in the exchange of genetic material between chromatids of homologous chromosomes and allows for formation of bivalents. Crossovers form at sites of recombination so that cohesion between the original sister chromatids, distal to chiasmata, holds bivalents together (Fig. 2A) [25].
FIG. 2.
The effects of reduced chromosome cohesion in MI. A–C) Chromosome arm cohesion distal to crossover sites holds bivalents together in MI (A). Loss of arm cohesion leads to a shift of chiasmata toward the distal end of chromosomes (B), and complete loss of arm cohesion results in premature complete separation of a bivalent (C). D–E) Centromere cohesion holds sister kinetochores together to facilitate mono-orientation (D), and a reduction of centromere cohesion promotes sister kinetochores to erroneously biorient at MI (E).
Studies of human trisomies suggest that different recombination patterns can lead to aneuploidy. Reduced recombination along chromosome 21, estimated using DNA polymorphic markers, is associated with trisomy 21 [19, 26]. Similar reductions in recombination have also been found in maternally MI-derived trisomies 15, 16, and 18, as well as sex-chromosome trisomies [18, 20, 27, 28]. Furthermore, studies of recombination positions show that exchanges too close to either the telomere (distal) or the centromere (proximal) are associated with trisomy 21 [15, 19, 29]. In mice deficient of the synaptonemal complex component SYCP3, reduced recombination is associated with an elevated incidence of aneuploidy, with significantly more single chromatids in MI and MII eggs compared to wild-type controls [30, 31]. In addition, a mouse model for reduced recombination was created by mating two closely related species with an estimated sequence divergence of ∼1%. In these mice, the aneuploidy incidence increased from less than 1% in control mice to about 10% in the crossed progeny at 4 wk of age, and further increased to 20% by 8–11 mo of age [32]. Together, results from both mouse and human studies suggest that recombination errors, either in the frequency or positions of exchanges, affect aneuploidy incidence.
To determine whether recombination problems contribute to age-related aneuploidy, maps were created for chromosome 21 in individuals with mothers of varying ages. The long arm of chromosome 21 was divided into different intervals to examine recombination frequency in each interval. An increase of either more proximal or distal recombination events was observed in trisomic individuals with young mothers, but not in individuals with older mothers [33, 34]. In another study of trisomy 21, the presence of a single pericentromeric exchange correlated with increasing maternal age [35]. Thus, whether different recombination patterns contribute to age-related aneuploidy remains unclear. Because recombination occurs at the onset of meiosis and is not age dependent, it is not obvious how recombination errors would lead to age-dependent aneuploidy. A possible explanation is that reduced frequency or problematic recombination positions make certain chromosomes more vulnerable to an age-dependent deterioration of another process years later [3, 19, 36].
THE SAC
Aneuploidy is the direct result of chromosome segregation errors. Because most errors originate from MI, many studies have focused on this first cell division—including spindle assembly, microtubule-kinetochore interactions, and the SAC. In the first 4 h after germinal vesicle breakdown, the bipolar spindle forms and bivalents congress at the metaphase plate [37, 38]. The SAC functions in MI as in mitosis, delaying anaphase until proper kinetochore-microtubule attachments are formed. In the presence of nocodazole, which depolymerizes microtubules, oocytes arrest at MI, which depends on known SAC proteins such as MAD2L1, BUB1B, and TTK (also known as MAD2, BUBR1, and MPS1, respectively) [39–43]. All of these SAC proteins localize to unattached kinetochores in MI, and come off once kinetochore-microtubule attachments form [39, 40, 42]. Disrupting MAD2L1, BUB1, BUB1B, or TTK in mice results in both an earlier anaphase and an increased aneuploidy frequency [39, 41, 44, 45]. Despite these similarities, the SAC may be less effective in MI than in mitosis. Although a single unattached kinetochore in mitosis can effectively delay anaphase, oocytes from XO mice with an unaligned X univalent go through MI with similar timing as control oocytes [46, 47]. In addition, oocytes lacking functional MLH1—a protein involved in recombination—have multiple univalent chromosomes, but can exit MI before all chromosomes align at the metaphase plate [48]. Together, these results suggest that the SAC in oocytes might be more permissive of errors or may deteriorate with age, leading to increased aneuploidy. Consistent with this hypothesis, disrupted spindles, misaligned chromosomes, and a decreased expression of SAC components Mad2l1 and Bub1 have all been observed in aged human oocytes [49–51]. Similarly, in naturally aged mice, several SAC genes are misexpressed in old oocytes compared to young counterparts [21].
The idea that the SAC deteriorates with age has been investigated in the CBA/Ca mouse and the senescence-accelerated mouse (SAM), two engineered strains that exhibit a premature decline in fertility [52, 53]. In both strains, problems in chromosome congression and premature exit from MI correlate with increasing age, suggesting that errors in SAC function contribute to age-related aneuploidy. However, these mice may not be suitable reproductive aging models because the SAM phenotype is due to mitochondrial dysfunction and oxidative damage, and the origin of the fertility phenotypes in CBA/Ca mice is not known. It remains unclear whether these events contribute to meiotic defects during natural aging.
To address what occurs during the natural aging process, SAC function has been tested in oocytes from old mice. Because early anaphase onset is a hallmark of a disrupted SAC, the duration of MI was used as a readout for SAC function. In two different strains of mice, no significant differences were found in MI duration between oocytes from young and old mice (young and old oocytes), suggesting that SAC function is intact [54, 55]. Most importantly, there was no correlation between early anaphase onset and aneuploidy in individual cells [54], which suggests that aneuploid MII eggs are not results of a defective SAC. Furthermore, when oocytes were treated with nocodazole as another way to assess SAC function, both young and old oocytes had an equally robust metaphase arrest [54, 55]. Taken together, the results suggest that the SAC is similarly functional in young and old oocytes, and there appears to be no gross perturbation in the SAC with increasing age.
SISTER CHROMATID COHESION
A leading hypothesis to explain maternal age-related aneuploidy is a deterioration of sister chromatid cohesion. Experiments in yeast show that sister chromatids can only be effectively held together when the mitotic cohesin protein SCC1 is expressed before S phase, indicating that cohesion is established in S phase [56]. Chromosome cohesion is lost at anaphase onset when the protease separase cleaves SCC1. A mutant form of SCC1, which cannot be cleaved by separase, blocked chromosome segregation only when expressed before S phase [57]. In mammalian cells expressing GFP-tagged cohesin proteins, results of fluorescence recovery after photobleaching experiments showed that chromatin-bound cohesins are stable from S phase until anaphase [58]. Together these results show that mitotic cohesion is established in S phase, and once loaded, cohesin proteins remain stably bound to chromosomes until cleavage by separase at anaphase. Meiotic cohesion is similarly established in S phase in yeast [59, 60]. Because cohesion must remain functional for up to 50 yr until meiosis resumes in human oocytes, cohesion is a good candidate for a process that might fail with maternal age and lead to increased aneuploidy.
Cohesion along chromosome arms keeps bivalents intact in MI (Fig. 2A), and centromere cohesion holds sister chromatids together in MII (Fig. 2D). A defect in cohesion distal to crossover sites may result in a shift of chiasmata placement (chiasmata slippage; Fig. 2B) or even premature bivalent separation in MI (Fig. 2C), whereas reduced centromere cohesion may result in premature separation of sister chromatids in MII. The relationship between premature chromosome separation, different positions of chiasmata, and maternal age was first documented in mice in 1968 [61]. The distal movement of chiasmata is now recognized as chiasmata slippage, suggesting that loss of cohesion occurs with age. In mice and Drosophila deficient in the meiotic cohesin protein SMC1B, chiasmata slippage and premature chromosome separation in oocytes were also observed. In both cases, the loss of cohesion phenotype worsened with maternal age [62, 63], consistent with the idea that cohesion defects may contribute to age-related aneuploidy.
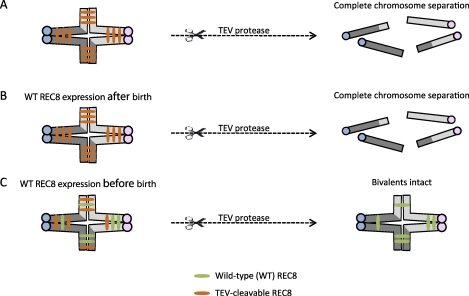
Transgenic mice have been engineered to test two critical parts of the cohesion hypothesis: 1) whether new cohesion can be established after S phase, and 2) the stability of cohesins with age. To address the first question, cleavage sites specific for TEV protease were inserted into the endogenous locus of REC8, the meiotic counterpart of SCC1 [64]. The engineered sites allow TEV protease to mimic separase by cleaving REC8 and releasing cohesion. Microinjection of TEV protease into these oocytes thus triggers chromosome separation in the absence of endogenous wild-type REC8. To determine when cohesion can be established, an additional transgene encoding wild-type REC8 was conditionally activated. Wild-type REC8 expression in the fetus, before the onset of meiosis, prevented TEV-mediated chromosome separation (Fig. 3). In contrast, when wild-type REC8 expression was driven after birth, exposure to TEV protease led to prematurely separated chromosomes. These results show that cohesion can only be established in S phase before birth, and once loaded, cohesin proteins do not exchange after birth. The second part of the hypothesis—stability of cohesin proteins—was tested in mice by conditionally deleting the Smc1b gene [65]. When Smc1b inactivation occurred shortly after birth, the initial amount of SMC1B was sufficient to hold bivalents together and maintain chiasmata placement for at least 8 mo in aging mice [65]. Together, these results suggest that cohesins load onto chromosomes and establish cohesion only during fetal development, and the cohesin complexes, once loaded, remain functional until meiosis resumes.
FIG. 3.
Schematic of experiments to test when functional chromosome cohesion is established. Transgenic mice with cleavage sites specific for TEV protease on REC8 were engineered [64]. A) Microinjecting TEV protease into oocytes where bivalents were held together by TEV-cleavable REC8 resulted in complete bivalent and chromatid separation. B) When a transgene encoding Myc-tagged wild-type REC8 (WT REC8) was expressed after birth, the introduction of TEV protease also led to complete chromosome separation. C) When WT REC8 was expressed in oocytes before birth, bivalents remained intact even after microinjection of TEV protease.
Results from genetically perturbed models are consistent with the cohesion hypothesis, but do not address what occurs during the natural aging process. Staining for either SMC1B or REC8 in naturally aged oocytes showed an age-dependent decrease of chromosome-associated cohesins [55, 66, 67]. For example, chromosome-associated REC8 levels gradually decreased with age, by at least 90% by 12 mo of age [67]. Interestingly, aneuploidy incidence remained low at 12 mo old, but increased dramatically by 15 mo. These results suggest that cohesins are originally loaded in excess, and aneuploidy occurs only when REC8 levels fall below a threshold. In addition, total REC8 protein was similar between young and old oocytes, further supporting the idea that once REC8 is lost from chromosomes, it cannot be effectively reloaded. Although a shift of chiasmata toward the distal end was also observed in old oocytes when compared to their young counterparts, complete separation of bivalents at MI was rare, suggesting that the observed aneuploidies are not due to complete loss of cohesins from chromosome arms [55, 67].
Centromere cohesion, on the other hand, has an important function at MI: to physically link sister kinetochores to promote attachment to one pole, or mono-orientation, so reductional segregation of homologous chromosomes can take place [59, 68]. Loss of cohesion at the core centromere in fission yeast led to increased separation of sister kinetochores and thus promoted erroneous biorientation in MI (Fig. 2B) [68]. Similarly, in plants centromere cohesion is required for mono-orientation, and increased separation of sister kinetochores resulted in lagging chromosomes and chromosome segregation errors at anaphase [69, 70]. In mouse, an increased separation of sister kinetochores at MI in old oocytes suggests that centromere cohesion is indeed weakened with maternal age. In addition, live imaging of individual young and old oocytes through anaphase I showed a higher incidence of lagging chromosomes in old oocytes, consistent with erroneous MI attachments. Chromosome counts in the resulting eggs at metaphase II often contained aneuploidies of single chromatids, indicating a premature loss of centromere cohesion [55, 67].
Although the results in natural aging models are consistent with the cohesion hypothesis, whether chromosome cohesion is actually reduced in naturally aged oocytes was not directly tested by any experimental perturbation. To directly target cohesion, separase was prematurely activated by depleting securin and preventing separase phosphorylation by CDK1 at S1121 and T1342 [71–73]. Chromosome cohesion in old oocytes was more susceptible to premature separase activation compared to chromosome cohesion in young oocytes, as shown by premature chromosome separation, demonstrating that cohesion is indeed reduced with natural aging [74]. Together, all of these results in naturally aged mice indicate that a deterioration of cohesion, especially at the centromere, occurs with increasing age and leads to increased aneuploidy.
In humans, there are also clues that cohesion becomes defective with age. When aneuploidy types were characterized in oocytes from women of different ages by cytogenetic approaches, aneuploidies due to single chromatids generally exceeded whole chromosome aneuploidies [75–79]. This result argues that defective cohesion must make a major contribution to age-related aneuploidy. A recent study of human oocytes found Smc1b mRNA levels to be similar between young and old oocytes, but it is unclear whether SMC1B protein is made in the mature oocyte [80]. Studies in mouse oocytes suggest that cohesin proteins can load onto chromosomes only during S phase, but what occurs in human oocytes remains unknown.
CONCLUSIONS
It is clear that a gradual loss of cohesin proteins from chromosomes is a major factor in maternal age-related aneuploidy in mouse models. Mouse is currently the established animal model for studying age-related aneuploidy, but we do not know how closely it mimics humans. Deterioration of cohesion is likely a contributing factor in humans because it is a highly conserved process, and the prevalence of prematurely separated sister chromatids in human oocytes suggests that cohesion is weakened or lost in aged women. Other factors may also contribute to maternal age-related aneuploidy in humans [3]. For example, the chances of segregation errors seem to vary for different chromosomes, so there may be chromosome-specific causes that have not been thoroughly investigated [81]. Furthermore, the timescales for the two organisms are very different: whereas mouse cohesin proteins may be stable in complex and maintained for up to 2 yr, can the same be true in humans, where the proteins would then need to be stable for over 40 yr? What mechanisms are in place to maintain cohesin complexes and what determines the timescale (e.g., 2 yr in mice vs. 40 yr in humans) are still open questions. Intriguingly, when adult mice are put on moderate calorie-restrictive diets, female reproductive lifespan is significantly extended, fertility and offspring survival are improved even at very advanced ages (over 20 mo old), and aneuploidy is reduced [24, 82]. Given the recent findings linking cohesion loss to maternal age, diet may affect the timescale of this important process.
Footnotes
Supported by grants from the National Institutes of Health (NIH) (HD 058730 to R.M.S. and M.A.L.) and a Searle Scholar award to M.A.L.
REFERENCES
- Penrose LS. The relative effects of paternal and maternal age in mongolism. J Genet 1933; 88: 9 14 [DOI] [PubMed] [Google Scholar]
- Lejeune J, Gautier M, Turpin R. [Study of somatic chromosomes from 9 mongoloid children]. C R Hebd Seances Acad Sci 1959; 248: 1721 1722 [PubMed] [Google Scholar]
- Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet 2001; 2: 280 291 [DOI] [PubMed] [Google Scholar]
- Hassold T, Hall H, Hunt P. The origin of human aneuploidy: where we have been, where we are going. Hum Mol Genet 2007; 16 (spec no. 2): R203 R208 [DOI] [PubMed] [Google Scholar]
- May KM, Jacobs PA, Lee M, Ratcliffe S, Robinson A, Nielsen J, Hassold TJ. The parental origin of the extra X chromosome in 47, XXX females. Am J Hum Genet 1990; 46: 754 761 [PMC free article] [PubMed] [Google Scholar]
- Hassold T, Jacobs PA, Leppert M, Sheldon M. Cytogenetic and molecular studies of trisomy 13. J Med Genet 1987; 24: 725 732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassold TJ, Pettay D, Freeman SB, Grantham M, Takaesu N. Molecular studies of non-disjunction in trisomy 16. J Med Genet 1991; 28: 159 162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaesu N, Jacobs PA, Cockwell A, Blackston RD, Freeman S, Nuccio J, Kurnit DM, Uchida I, Freeman V, Hassold T. Nondisjunction of chromosome 21. Am J Med Genet Suppl 1990; 7: 175 181 [DOI] [PubMed] [Google Scholar]
- Martin RH, Rademaker AW. The effect of age on the frequency of sperm chromosomal abnormalities in normal men. Am J Hum Genet 1987; 41: 484 492 [PMC free article] [PubMed] [Google Scholar]
- Sauer MV. The impact of age on reproductive potential: lessons learned from oocyte donation. Maturitas 1998; 30: 221 225 [DOI] [PubMed] [Google Scholar]
- Stolwijk AM, Zielhuis GA, Sauer MV, Hamilton CJ, Paulson RJ. The impact of the woman's age on the success of standard and donor in vitro fertilization. Fertil Steril 1997; 67: 702 710 [DOI] [PubMed] [Google Scholar]
- Hulten MA, Patel SD, Tankimanova M, Westgren M, Papadogiannakis N, Jonsson AM, Iwarsson E. On the origin of trisomy 21 Down syndrome. Mol Cytogenet 2008; 1: 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulten MA, Patel S, Jonasson J, Iwarsson E. On the origin of the maternal age effect in trisomy 21 Down syndrome: the Oocyte Mosaicism Selection model. Reproduction 2010; 139: 1 9 [DOI] [PubMed] [Google Scholar]
- Obradors A, Rius M, Cuzzi J, Daina G, Gutierrez-Mateo C, Pujol A, Marina F, Marquez C, Benet J, Navarro J. Errors at mitotic segregation early in oogenesis and at first meiotic division in oocytes from donor females: comparative genomic hybridization analyses in metaphase II oocytes and their first polar body. Fertil Steril 2010; 93: 675 679 [DOI] [PubMed] [Google Scholar]
- Lamb NE, Feingold E, Savage A, Avramopoulos D, Freeman S, Gu Y, Hallberg A, Hersey J, Karadima G, Pettay D, Saker D, Shen J. et al. Characterization of susceptible chiasma configurations that increase the risk for maternal nondisjunction of chromosome 21. Hum Mol Genet 1997; 6: 1391 1399 [DOI] [PubMed] [Google Scholar]
- Allen EG, Freeman SB, Druschel C, Hobbs CA, O'Leary LA, Romitti PA, Royle MH, Torfs CP, Sherman SL. Maternal age and risk for trisomy 21 assessed by the origin of chromosome nondisjunction: a report from the Atlanta and National Down Syndrome Projects. Hum Genet 2009; 125: 41 52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JM, Harvey JF, Morton NE, Jacobs PA. Trisomy 18: studies of the parent and cell division of origin and the effect of aberrant recombination on nondisjunction. Am J Hum Genet 1995; 56: 669 675 [PMC free article] [PubMed] [Google Scholar]
- Hassold T, Merrill M, Adkins K, Freeman S, Recombination Sherman S. and maternal age-dependent nondisjunction: molecular studies of trisomy 16. Am J Hum Genet 1995; 57: 867 874 [PMC free article] [PubMed] [Google Scholar]
- Lamb NE, Freeman SB, Savage-Austin A, Pettay D, Taft L, Hersey J, Gu Y, Shen J, Saker D, May KM, Avramopoulos D, Petersen MB. et al. Susceptible chiasmate configurations of chromosome 21 predispose to non-disjunction in both maternal meiosis I and meiosis II. Nat Genet 1996; 14: 400 405 [DOI] [PubMed] [Google Scholar]
- Robinson WP, Kuchinka BD, Bernasconi F, Petersen MB, Schulze A, Brondum-Nielsen K, Christian SL, Ledbetter DH, Schinzel AA, Horsthemke B, Schuffenhauer S, Michaelis RC. et al. Maternal meiosis I non-disjunction of chromosome 15: dependence of the maternal age effect on level of recombination. Hum Mol Genet 1998; 7: 1011 1019 [DOI] [PubMed] [Google Scholar]
- Pan H, Ma P, Zhu W, Schultz RM. Age-associated increase in aneuploidy and changes in gene expression in mouse eggs. Dev Biol 2008; 316: 397 407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccotti M, Boiani M, Garagna S, Redi CA. Analysis of aneuploidy rate in antral and ovulated mouse oocytes during female aging. Mol Reprod Dev 1998; 50: 305 312 [DOI] [PubMed] [Google Scholar]
- Hornak M, Jeseta M, Musilova P, Pavlok A, Kubelka M, Motlik J, Rubes J, Anger M. Frequency of aneuploidy related to age in porcine oocytes. PLoS One 2011; 6: e18892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selesniemi K, Lee HJ, Muhlhauser A, Tilly JL. Prevention of maternal aging-associated oocyte aneuploidy and meiotic spindle defects in mice by dietary and genetic strategies. Proc Natl Acad Sci U S A 2011; 108: 12319 12324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanowitz J. Meiosis: making a break for it. Curr Opin Cell Biol 2010; 22: 744 751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren AC, Chakravarti A, Wong C, Slaugenhaupt SA, Halloran SL, Watkins PC, Metaxotou C, Antonarakis SE. Evidence for reduced recombination on the nondisjoined chromosomes 21 in Down syndrome. Science 1987; 237: 652 654 [DOI] [PubMed] [Google Scholar]
- Bugge M, Collins A, Petersen MB, Fisher J, Brandt C, Hertz JM, Tranebjaerg L, de Lozier-Blanchet C, Nicolaides P, Brondum-Nielsen K, Morton N, Mikkelsen M. Non-disjunction of chromosome 18. Hum Mol Genet 1998; 7: 661 669 [DOI] [PubMed] [Google Scholar]
- Thomas NS, Ennis S, Sharp AJ, Durkie M, Hassold TJ, Collins AR, Jacobs PA. Maternal sex chromosome non-disjunction: evidence for X chromosome-specific risk factors. Hum Mol Genet 2001; 10: 243 250 [DOI] [PubMed] [Google Scholar]
- Hassold T, Sherman S. Down syndrome: genetic recombination and the origin of the extra chromosome 21. Clin Genet 2000; 57: 95 100 [DOI] [PubMed] [Google Scholar]
- Yuan L, Liu JG, Hoja MR, Wilbertz J, Nordqvist K, Hoog C. Female germ cell aneuploidy and embryo death in mice lacking the meiosis-specific protein SCP3. Science 2002; 296: 1115 1118 [DOI] [PubMed] [Google Scholar]
- Kouznetsova A, Lister L, Nordenskjold M, Herbert M, Hoog C. Bi-orientation of achiasmatic chromosomes in meiosis I oocytes contributes to aneuploidy in mice. Nat Genet 2007; 39: 966 968 [DOI] [PubMed] [Google Scholar]
- Koehler KE, Schrump SE, Cherry JP, Hassold TJ, Hunt PA. Near-human aneuploidy levels in female mice with homeologous chromosomes. Curr Biol 2006; 16: R579 R580 [DOI] [PubMed] [Google Scholar]
- Lamb NE, Yu K, Shaffer J, Feingold E, Sherman SL. Association between maternal age and meiotic recombination for trisomy 21. Am J Hum Genet 2005; 76: 91 99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Feingold E, Dey SK. Etiology of Down syndrome: evidence for consistent association among altered meiotic recombination, nondisjunction, and maternal age across populations. Am J Med Genet A 2009; 149A: 1415 1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver TR, Feingold E, Yu K, Cheung V, Tinker S, Yadav-Shah M, Masse N, Sherman SL. New insights into human nondisjunction of chromosome 21 in oocytes. PLoS Genet 2008; 4: e1000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KT. Meiosis in oocytes: predisposition to aneuploidy and its increased incidence with age. Hum Reprod Update 2008; 14: 143 158 [DOI] [PubMed] [Google Scholar]
- Schuh M, Ellenberg J. Self-organization of MTOCs replaces centrosome function during acentrosomal spindle assembly in live mouse oocytes. Cell 2007; 130: 484 498 [DOI] [PubMed] [Google Scholar]
- Brunet S, Maria AS, Guillaud P, Dujardin D, Kubiak JZ, Maro B. Kinetochore fibers are not involved in the formation of the first meiotic spindle in mouse oocytes, but control the exit from the first meiotic M phase. J Cell Biol 1999; 146: 1 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hached K, Xie SZ, Buffin E, Cladiere D, Rachez C, Sacras M, Sorger PK, Wassmann K. Mps1 at kinetochores is essential for female mouse meiosis I. Development 2011; 138: 2261 2271 [DOI] [PubMed] [Google Scholar]
- Wassmann K, Niault T, Maro B. Metaphase I arrest upon activation of the Mad2-dependent spindle checkpoint in mouse oocytes. Curr Biol 2003; 13: 1596 1608 [DOI] [PubMed] [Google Scholar]
- Niault T, Hached K, Sotillo R, Sorger PK, Maro B, Benezra R, Wassmann K. Changing Mad2 levels affects chromosome segregation and spindle assembly checkpoint control in female mouse meiosis I. PLoS One 2007; 2: e1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane SI, Chang HY, Jennings PC, Jones KT. The Aurora kinase inhibitor ZM447439 accelerates first meiosis in mouse oocytes by overriding the spindle assembly checkpoint. Reproduction 2010; 140: 521 530 [DOI] [PubMed] [Google Scholar]
- Brunet S, Pahlavan G, Taylor S, Maro B. Functionality of the spindle checkpoint during the first meiotic division of mammalian oocytes. Reproduction 2003; 126: 443 450 [DOI] [PubMed] [Google Scholar]
- Homer HA, McDougall A, Levasseur M, Yallop K, Murdoch AP, Herbert M. Mad2 prevents aneuploidy and premature proteolysis of cyclin B and securin during meiosis I in mouse oocytes. Genes Dev 2005; 19: 202 207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinness BE, Anger M, Kouznetsova A, Gil-Bernabe AM, Helmhart W, Kudo NR, Wuensche A, Taylor S, Hoog C, Novak B, Nasmyth K. Regulation of APC/C activity in oocytes by a Bub1-dependent spindle assembly checkpoint. Curr Biol 2009; 19: 369 380 [DOI] [PubMed] [Google Scholar]
- LeMaire-Adkins R, Radke K, Hunt PA. Lack of checkpoint control at the metaphase/anaphase transition: a mechanism of meiotic nondisjunction in mammalian females. J Cell Biol 1997; 139: 1611 1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder CL, Cole RW, Khodjakov A, Sluder G. The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal produced by unattached kinetochores. J Cell Biol 1995; 130: 941 948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka SI, Hodges CA, Albertini DF, Hunt PA. Oocyte-specific differences in cell-cycle control create an innate susceptibility to meiotic errors. Curr Biol 2011; 21: 651 657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia DE, Goodwin P, Klein NA, Soules MR. Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Hum Reprod 1996; 11: 2217 2222 [DOI] [PubMed] [Google Scholar]
- Volarcik K, Sheean L, Goldfarb J, Woods L, Abdul-Karim FW, Hunt P. The meiotic competence of in-vitro matured human oocytes is influenced by donor age: evidence that folliculogenesis is compromised in the reproductively aged ovary. Hum Reprod 1998; 13: 154 160 [DOI] [PubMed] [Google Scholar]
- Steuerwald N, Cohen J, Herrera RJ, Sandalinas M, Brenner CA. Association between spindle assembly checkpoint expression and maternal age in human oocytes. Mol Hum Reprod 2001; 7: 49 55 [DOI] [PubMed] [Google Scholar]
- Eichenlaub-Ritter U, Boll I. Age-related non-disjunction, spindle formation and progression through maturation of mammalian oocytes. Prog Clin Biol Res 1989; 318: 259 269 [PubMed] [Google Scholar]
- Liu L, Keefe DL. Ageing-associated aberration in meiosis of oocytes from senescence-accelerated mice. Hum Reprod 2002; 17: 2678 2685 [DOI] [PubMed] [Google Scholar]
- Duncan FE, Chiang T, Schultz RM, Lampson MA. Evidence that a defective spindle assembly checkpoint is not the primary cause of maternal age-associated aneuploidy in mouse eggs. Biol Reprod 2009; 81: 768 776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister LM, Kouznetsova A, Hyslop LA, Kalleas D, Pace SL, Barel JC, Nathan A, Floros V, Adelfalk C, Watanabe Y, Jessberger R, Kirkwood TB. et al. Age-related meiotic segregation errors in mammalian oocytes are preceded by depletion of cohesin and Sgo2. Curr Biol 2010; 20: 1511 1521 [DOI] [PubMed] [Google Scholar]
- Uhlmann F, Nasmyth K. Cohesion between sister chromatids must be established during DNA replication. Curr Biol 1998; 8: 1095 1101 [DOI] [PubMed] [Google Scholar]
- Haering CH, Schoffnegger D, Nishino T, Helmhart W, Nasmyth K, Structure Lowe J. and stability of cohesin's Smc1-kleisin interaction. Mol Cell 2004; 15: 951 964 [DOI] [PubMed] [Google Scholar]
- Gerlich D, Koch B, Dupeux F, Peters JM, Ellenberg J. Live-cell imaging reveals a stable cohesin-chromatin interaction after but not before DNA replication. Curr Biol 2006; 16: 1571 1578 [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Nurse P. Cohesin Rec8 is required for reductional chromosome segregation at meiosis. Nature 1999; 400: 461 464 [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Yokobayashi S, Yamamoto M, Nurse P. Pre-meiotic S phase is linked to reductional chromosome segregation and recombination. Nature 2001; 409: 359 363 [DOI] [PubMed] [Google Scholar]
- Henderson SA, Edwards RG. Chiasma frequency and maternal age in mammals. Nature 1968; 218: 22 28 [DOI] [PubMed] [Google Scholar]
- Hodges CA, Revenkova E, Jessberger R, Hassold TJ, Hunt PA. SMC1beta-deficient female mice provide evidence that cohesins are a missing link in age-related nondisjunction. Nat Genet 2005; 37: 1351 1355 [DOI] [PubMed] [Google Scholar]
- Subramanian VV, Bickel SE. Aging predisposes oocytes to meiotic nondisjunction when the cohesin subunit SMC1 is reduced. PLoS Genet 2008; 4: e1000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana-Konwalski K, Godwin J, van der Weyden L, Champion L, Kudo NR, Adams DJ, Nasmyth K. Rec8-containing cohesin maintains bivalents without turnover during the growing phase of mouse oocytes. Genes Dev 2010; 24: 2505 2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revenkova E, Herrmann K, Adelfalk C, Jessberger R. Oocyte cohesin expression restricted to predictyate stages provides full fertility and prevents aneuploidy. Curr Biol 2010; 20: 1529 1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Keefe DL. Defective cohesin is associated with age-dependent misaligned chromosomes in oocytes. Reprod Biomed Online 2008; 16: 103 112 [DOI] [PubMed] [Google Scholar]
- Chiang T, Duncan FE, Schindler K, Schultz RM, Lampson MA. Evidence that weakened centromere cohesion is a leading cause of age-related aneuploidy in oocytes. Curr Biol 2010; 20: 1522 1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuno T, Tada K, Watanabe Y. Kinetochore geometry defined by cohesion within the centromere. Nature 2009; 458: 852 858 [DOI] [PubMed] [Google Scholar]
- Li X, Dawe RK. Fused sister kinetochores initiate the reductional division in meiosis I. Nat Cell Biol 2009; 11: 1103 1108 [DOI] [PubMed] [Google Scholar]
- Chelysheva L, Diallo S, Vezon D, Gendrot G, Vrielynck N, Belcram K, Rocques N, Marquez-Lema A, Bhatt AM, Horlow C, Mercier R, Mezard C. et al. AtREC8 and AtSCC3 are essential to the monopolar orientation of the kinetochores during meiosis. J Cell Sci 2005; 118: 4621 4632 [DOI] [PubMed] [Google Scholar]
- Stemmann O, Zou H, Gerber SA, Gygi SP, Kirschner MW. Dual inhibition of sister chromatid separation at metaphase. Cell 2001; 107: 715 726 [DOI] [PubMed] [Google Scholar]
- Herbert M, Levasseur M, Homer H, Yallop K, Murdoch A, McDougall A. Homologue disjunction in mouse oocytes requires proteolysis of securin and cyclin B1. Nat Cell Biol 2003; 5: 1023 1025 [DOI] [PubMed] [Google Scholar]
- Nabti I, Reis A, Levasseur M, Stemmann O, Jones KT. Securin and not CDK1/cyclin B1 regulates sister chromatid disjunction during meiosis II in mouse eggs. Dev Biol 2008; 321: 379 386 [DOI] [PubMed] [Google Scholar]
- Chiang T, Schultz RM, Lampson MA. Age-dependent susceptibility of chromosome cohesion to premature separase activation in mouse oocytes. Biol Reprod 2011; 85: 1279 1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellestor F, Andreo B, Arnal F, Humeau C, Demaille J. Mechanisms of non-disjunction in human female meiosis: the co-existence of two modes of malsegregation evidenced by the karyotyping of 1397 in-vitro unfertilized oocytes. Hum Reprod 2002; 17: 2134 2145 [DOI] [PubMed] [Google Scholar]
- Pellestor F, Andreo B, Arnal F, Humeau C, Demaille J. Maternal aging and chromosomal abnormalities: new data drawn from in vitro unfertilized human oocytes. Hum Genet 2003; 112: 195 203 [DOI] [PubMed] [Google Scholar]
- Vialard F, Petit C, Bergere M, Gomes DM, Martel-Petit V, Lombroso R, Ville Y, Gerard H, Selva J. Evidence of a high proportion of premature unbalanced separation of sister chromatids in the first polar bodies of women of advanced age. Hum Reprod 2006; 21: 1172 1178 [DOI] [PubMed] [Google Scholar]
- Angell R. First-meiotic-division nondisjunction in human oocytes. Am J Hum Genet 1997; 61: 23 32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialard F, Lombroso R, Bergere M, Gomes DM, Hammoud I, Bailly M, Selva J. Oocyte aneuploidy mechanisms are different in two situations of increased chromosomal risk: older patients and patients with recurrent implantation failure after in vitro fertilization. Fertil Steril 2007; 87: 1333 1339 [DOI] [PubMed] [Google Scholar]
- Garcia-Cruz R, Brieno MA, Roig I, Grossmann M, Velilla E, Pujol A, Cabero L, Pessarrodona A, Barbero JL, Garcia Caldes M. Dynamics of cohesin proteins REC8, STAG3, SMC1 beta and SMC3 are consistent with a role in sister chromatid cohesion during meiosis in human oocytes. Hum Reprod 2010; 25: 2316 2327 [DOI] [PubMed] [Google Scholar]
- Risch N, Stein Z, Kline J, Warburton D. The relationship between maternal age and chromosome size in autosomal trisomy. Am J Hum Genet 1986; 39: 68 78 [PMC free article] [PubMed] [Google Scholar]
- Selesniemi K, Lee HJ, Tilly JL. Moderate caloric restriction initiated in rodents during adulthood sustains function of the female reproductive axis into advanced chronological age. Aging Cell 2008; 7: 622 629 [DOI] [PMC free article] [PubMed] [Google Scholar]