ABSTRACT
Primary Sertoli cells isolated from mouse testes survive when transplanted across immunological barriers and protect cotransplanted allogeneic and xenogeneic cells from rejection in rodent models. In contrast, the mouse Sertoli cell line (MSC-1) lacks immunoprotective properties associated with primary Sertoli cells. In this study, enriched primary Sertoli cells or MSC-1 cells were transplanted as allografts into the renal subcapsular area of naive BALB/c mice, and their survival in graft sites was compared. While Sertoli cells were detected within the grafts with 100% graft survival throughout the 20-day study, MSC-1 cells were rejected between 11 and 14 days, with 0% graft survival at 20 days posttransplantation. Nonetheless, the mechanism for primary Sertoli cell survival and immunoprotection remains unresolved. To identify immune factors or functional pathways potentially responsible for immune privilege, gene expression profiles of enriched primary Sertoli cells were compared with those of MSC-1 cells. Microarray analysis identified 2369 genes in enriched primary Sertoli cells that were differentially expressed at ±4-fold or higher levels than in MSC-1 cells. Ontological analyses identified multiple immune pathways, which were used to generate a list of 340 immune-related genes. Three functions were identified in primary Sertoli cells as potentially important for establishing immune privilege: suppression of inflammation by specific cytokines and prostanoid molecules, slowing of leukocyte migration by controlled cell junctions and actin polymerization, and inhibition of complement activation and membrane-associated cell lysis. These results increase our understanding of testicular immune privilege and, in the long-term, could lead to improvements in transplantation success.
Keywords: immune privilege, microarray, MSC-1 cells, Sertoli cells, testis, transplantation
Sertoli cells are immunologically privileged and survive allogeneic transplantation. They express immunoregulatory factors, which can be grouped into functional pathways that may be responsible for their immunologically privileged properties.
INTRODUCTION
Immunologically privileged sites are places where autoantigens or foreign antigens are tolerated without evoking detrimental inflammatory immune responses. The testis is an immunologically privileged site that not only provides the tolerogenic environment for protection of maturing autoimmunogenic male germ cells but also allows for prolonged survival of foreign (allogeneic and xenogeneic) tissues when they are transplanted into the testis without any immunosuppressive drugs [1]. Primary Sertoli cells play an important role in creating the immunologically privileged environment in the testis, as grafts have been found to be protected from rejection after most of the Leydig cells and germ cells, the other main testicular components, were removed [2, 3]. Further confirmation of the immunologically privileged abilities of primary Sertoli cells was provided by transplantation studies. Primary Sertoli cells (enriched primary Sertoli cells) prolonged the survival of cotransplanted islets or neuronal cells when they were transplanted across immunological barriers as allografts [4, 5] or xenografts [6–8]. In contrast, when the immunoprotective abilities of enriched primary Sertoli cells were compared with those of an immortalized mouse Sertoli cell line, MSC-1, it was found that MSC-1 cells were unable to protect cotransplanted allogeneic islet grafts [9], suggesting that MSC-1 cells are not immunologically privileged and lack some factors that confer immunoprotection to transplants.
A typical immune response to grafted tissue consists of activation of humoral and cell-mediated immunity. The humoral response involves antibody-mediated activation of the complement cascade that results in formation of the cytolytic membrane attack complex (MAC), a transmembrane channel that promotes osmotic lysis of cells. One mechanism of immune privilege uses molecules that inhibit complement activation [10]. It was shown previously that neonatal porcine Sertoli cells resist complement-mediated lysis by inhibiting the complement cascade and MAC formation [11].
Active suppression of inflammation and alteration of cell-mediated immune responses are other mechanisms that establish tolerance at immunologically privileged sites, such as the testis, and promote survival of allogeneic cells after transplantation [12–15]. Typically, this can be accomplished by promoting production of cytokines that favor a type 2 over a type 1 immune response [16]. Type 2 anti-inflammatory responses favor immature dendritic cells and regulatory T cells (Treg), which are important for creating and maintaining immunological tolerance [17]. On the other hand, proinflammatory type 1 responses promote cytotoxic killer cell activation and graft destruction [18].
In the current study, we used the MSC-1 cell line and enriched primary Sertoli cells as model systems for nonimmunologically privileged and immunologically privileged cells, respectively. We compared the survival of MSC-1 cells with that of enriched primary Sertoli cells cultured as aggregates after allotransplantation into naïve mice and conducted a global approach, using microarray and ontological analyses, to identify genes and immune-related functional pathways differentially regulated in these cells. We hypothesized that a comparison of genes expressed by immunologically privileged cells (enriched primary Sertoli cells) with those expressed by nonimmunologically privileged (MSC-1) cells could identify functional pathways important for the survival and protection of transplanted cells. Our results indicated that the mechanisms responsible for immune privilege are complex, orchestrated by a combination of cytokines, chemokines, anti-inflammatory modulators, complement activation inhibitors, and adhesion molecules that favor a type 2 immune response, immature dendritic cells, and Treg cells.
MATERIALS AND METHODS
Animals
Male C57BL/6 ×129 mice (The Jackson Laboratory, Bar Harbor, ME), 19–20 days old, were used as primary Sertoli cell donors. Male BALB/c mice (Charles Rivers Laboratories, Wilmington, MA), 6–8 wk old, were used as recipients. Care and maintenance of animals was carried out in accordance with protocols, following the NIH guidelines, approved by the Institutional Animal Care and Use Committee of Texas Tech University.
Cell Preparation and Transplantation
Primary Sertoli cells were isolated from mouse testes by collagenase and trypsin digestion and cultured on nontreated Petri dishes containing Ham F10 medium with supplements as described previously [9] and 10% fetal bovine serum for 48 h at 37°C to allow formation of enriched primary Sertoli cell aggregates (apSC) (see Supplemental Fig. S1, A and C, available at www.biolreprod.org) [9]. The MSC-1 cell line was derived from a Sertoli cell tumor in transgenic C57BL/6×SJL mixed hybrid mice carrying a transgene containing DNA encoding both small and large T antigens of the SV40 virus fused to the promoter for human Müllerian inhibiting substance [19]. Prior to transplantation, MSC-1 cells were cultured and aggregated (aMSC-1) as described above for apSC (see Supplemental Fig. S1, B and D). Numbers of aMSC-1 and apSC were estimated using a DNA assay [20], and 4 million cells were transplanted into the left renal subcapsular space of naïve isoflurane-anesthetized BALB/c mice [9].
To determine the cell composition of apSC after 2 days of culture (i.e., at the time of transplantation and collection of RNA for microarray analysis), apSC were dissociated and immunostained as described previously [21], using mouse monoclonal anti-human GATA4 (1:50 dilution; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit polyclonal anti-rat cytochrome P450 side chain cleavage enzyme (1:500 dilution; Chemicon International, Temecula, CA), and mouse monoclonal anti-human smooth muscle alpha actin (1:50 dilution; DAKO, Carpinteria, CA) to detect Sertoli, Leydig, and myoid cells, respectively [21]. A nonfixed aliquot of cells was immunostained with rat anti-mouse CD11B (1:25 dilution; Becton Dickinson Pharmingen, San Jose, CA), which detects macrophages, dendritic cells, granulocytes, and natural killer cells. A minimum of 500 cells was counted in each preparation. The apSC, after 2 days of culture, were primarily Sertoli cells (91% Sertoli cells, 0.5% Leydig cells, 0.8% myoid cells, and less than 0.7% macrophages). Remaining cells (i.e., ∼7%) were most likely germ cells.
Graft Characterization
Grafts were removed for histological analysis at 1, 2, 5, 8, 11, 14, and 20 days posttransplantation, and tissue sections were immunostained to identify MSC-1, using mouse monoclonal anti-SV40 large T antigen (1:100 dilution; BD Biosciences, San Jose, CA), or to identify primary Sertoli cells, using mouse monoclonal anti-human GATA4 (1:50 dilution) as described previously [9]. The presence of immune cell infiltrate was assessed based on the morphological characteristic of immune cells (small cells with blue nuclei) and CD3 (T-cell marker) immunohistochemistry on frozen graft sections, using rabbit monoclonal anti-CD3 primary antibody (1:75 dilution; Cell Marque Corporation, Rocklin, CA), as described previously [20]. Diaminobenzidine (Biogenex Laboratories, San Ramon, CA) was used as the chromogen. All sections were counterstained with hematoxylin. Negative controls put through the same procedure without primary antibody lacked a positive reaction.
Relative numbers of GATA4-positive Sertoli cells or large T antigen-positive MSC-1 cells and amounts of immune cell infiltrate were scored semiquantitatively. The percentage of graft survival indicated the percentage of grafts that contained GATA4- or large T antigen-positive cells of the total number of transplants collected at each time point.
Apoptosis Assay
Terminal deoxynucleotidyl transferase (TdT)-mediated TUNEL assay was performed with graft tissue sections to determine apoptosis according to the manufacturer's protocol (Promega Biotech Corp., Madison, WI). Sections were counterstained with 2 μM bisBenzimide Hoechst 33342 (Sigma Chemical Corp., St. Louis, MO). Negative control sections incubated without TdT enzyme lacked a positive signal.
To determine the relative fold increase in TUNEL-positive cells, we counted the number of TUNEL-positive cells within two separate tissue sections from each graft (n ≥ 3). These values were averaged and multiplied by the average size of an apoptotic Sertoli cell to determine the area of TUNEL-positive cells. To control for graft size, the area of TUNEL-positive cells was divided by the total area of the graft in each tissue section. These values were normalized to Day 1 values for aMSC-1, and the relative fold change in apoptosis was graphed. Significant differences between aMSC-1 and apSC were calculated by one-way ANOVA followed by pairwise comparisons of the means at a P value of ≤0.05, using JMP IN version 5.1 software (SAS Institute Inc., Cary, NC).
RNA Extraction, Microarray Array Processing, and Data Analysis
aMSC-1 or apSC (n ≥ 3) were lysed in 1 ml of Trizol reagent, and RNA was extracted according to the manufacturer's protocol (Invitrogen Corp., Carlsbad, CA). The quality of RNAs was verified by formaldehyde agarose gel electrophoresis (data not shown). Transcriptome profiling was performed using Mouse Expression 430 2.0 microarrays containing 45 101 total probes (23 843 genes; Affymetrix, Santa Clara, CA), using one chip per RNA sample for aMSC-1 (n = 3) and apSC (n = 3). Briefly, the double-stranded cDNA template synthesized from 10 μg of total RNA was used to generate antisense biotin-labeled cRNA. Fifteen micrograms of biotin-labeled target cRNA was fragmented and hybridized with the GeneChip probe array, followed by incubation with a streptavidin-phycoerythrin conjugate. Resulting image files were detected with an Affymetrix Genechip model 3000 scanner and analyzed with Affymetrix GenChip Operating Software to determine the raw signal intensity.
Raw intensity data sets were first normalized using default normalization parameters of GeneSpring version 7.3 software (Agilent Technologies, Foster City, CA). These parameters included data transformation (setting signal values from <0.01–0.01), normalization of each chip to the 50th percentile, and, for each probe, setting the normalization to the median value of the probe for all chips. Normalized data were filtered for at least one sample having a raw signal of ≥50 and, for both samples, having a normalized signal of ≥0.025 to remove any potential noise. Then, ANOVA at a P value of ≤0.05 for each probe was conducted to compare all experimental samples to each other, assuming variances were not equal, and including calculations made from the Cross-Gene error model of GeneSpring version 7.3 software (Agilent Technologies). After ANOVA testing, the significant probes were filtered to obtain a list of probes that had expression levels that were ±4.0-fold or higher in apSC than in aMSC-1. A 4-fold cutoff difference was used because this was previously shown to be a good cutoff for determining differential gene expression changes between different cell types [22].
To conduct ontological analyses, gene symbol identifiers of probes were imported into Pathway Express (http://vortex.cs.wayne.edu/ontoexpress/), KEGG (http://www.genome.jp/kegg/), and DAVID (http://david.abcc.ncifcrf.gov/) to annotate functional pathways. Pathway Express analysis is most stringent in the calculation of statistical significance and provides the overall significance of pathways, indicated by gamma P value, based on positive or negative fold differences for each probe [23]. Functional clusters from DAVID analysis are associated with geometric medians, which can be used to rank the significance of functional clusters [24].
Real-Time PCR Assays
Real-time PCR primers (Table 1) were designed using Primer Express version 2.0 software (Applied Biosystems Technology, Foster City, CA). cDNA synthesized from 500 ng of RNA by using iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA) was used as the template for real-time PCR assays with a 7500 Fast real-time PCR system (Applied Biosystems Technology). Typically, a 25-μl reaction mixture contained 12.5 μl of 2× Power SYBR Green PCR Master Mix (Applied Biosystems Technology), 500 nM of forward and reverse primers, 5 μl of diluted cDNA (diluted 1:20), and distilled water. The PCR run was 1 cycle at 50°C for 2 min, 1 cycle at 95°C for 10 min, and 40 cycles at 95°C for 15 sec and 60°C for 1 min. Real-time PCR was conducted in triplicate with three biological samples.
TABLE 1.
Primers for real-time RT-PCR.
The expression level of the gene of interest was evaluated using the 2−(ΔΔCt) method [25]. Cycle threshold values (Ct) for the gene of interest and the ribosomal S2 protein (Rps2) gene, a housekeeping gene, were determined using Prism SDS version 1.1 software (Applied Biosystems Technology). Ct values for the gene of interest were normalized to those of Rps2 values in each sample, and then the fold change for the gene of interest was calculated relative to the level in the reference sample. Examination of the dissociation curve indicated that there was only a single PCR product for each primer set. Amplification plots were logarithmic and similar to each other, indicating that PCR efficiencies were equivalent for primer sets and close to 1. One-way ANOVA was performed, followed by pairwise comparisons of the means at a P value of ≤0.01, using JMP IN version 5.1 software (SAS Institute Inc.).
Protein Isolation and Western Blot Analysis
Cellular proteins (n = 3) were isolated from aMSC-1 and apSC by lysis with radioimmunoprecipitation assay (RIPA) buffer (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1% nonidet P-40 [NP-40], 0.1% SDS, 1% deoxycholic acid, 5 mM NaF, 1 mM ethylenediaminetetraacetic acid [DTA]) containing phenylmethylsulfonyl fluoride and protease inhibitor cocktail (Sigma Chemical Corp.) for 30 min on ice, followed by centrifugation at 12 000 × g for 5 min. Protein concentrations were determined by the Bradford assay (Bio-Rad Laboratories). For Western blot analyses, membranes were incubated with the primary antibodies mouse antiserine protease inhibitor B9 (SERPINB9; 1:2000 dilution; Axxora, LLC, San Diego, CA), rabbit anti-transferrin (1:1000 dilution; Cappel Laboratories, Aurora, OH), and rabbit antisteroidogenic acute regulatory (STAR) protein (1:15 000 dilution) [26]. To determine equal protein loading, we incubated membranes with mouse anti-actin (1:15 000 dilution; Millipore Corporation, Bedford, MA).
RESULTS
Survival of Primary Sertoli Cells
apSC were transplanted into naïve mice as allografts (see Supplemental Fig. S1, A and C, available online at www.biolreprod.org). After transplantation, tissue sections from apSC grafts were analyzed for the percentage of graft survival, the relative number of Sertoli cells, and the presence of immune cell infiltrate (Fig. 1 and Table 2 and see Supplemental Table S1A). At all time points throughout the 20-Day study period, large clusters of GATA4-positive Sertoli cells were detected in 100% of the grafts (Table 2 and Fig. 1, A, C, E, and G). Sertoli cell survival at Day 20 was also confirmed by immunostaining with another Sertoli cell marker, Wilms tumor 1 (data not shown) [27]. At Day 1, virtually no immune cell infiltrate was detected within the grafts (Table 2). At Days 2 and 5, little to no infiltration of immune cells was found within the grafts (Table 2). The amount of infiltrating cells increased at Day 8, and grafts were flooded with infiltrating immune cells that surrounded Sertoli cell clusters by days 11 and 14 (Table 2, Fig. 1E). By Day 20, amounts of infiltrating cells had decreased, but Sertoli cells were still present in abundance (Fig. 1G). Specific immunostaining to identify CD3-positive T cells showed a similar pattern of cellular infiltrate in the grafts. T cells began to enter grafts at Days 5 and 8, grafts were flooded with T cells at Day 14, and numbers of T cells within the grafts decreased by Day 20 (see Supplemental Table S1A).
FIG. 1.
Immunohistochemical analysis of Sertoli cell grafts is shown. Four million apSC were transplanted underneath the kidney capsule of BALB/c mice. Grafts were collected on Days 2 (A, B), 5 (C, D), 14 (E, F), and 20 (G, H) and immunostained for the Sertoli cell marker GATA4 (brown color). Inset shows higher magnification than that in B. The graft is separated from the kidney (K) by a dotted line (A, C, E, G). Sections were counterstained with hematoxylin (blue color). Note that GATA4 also stains kidney epithelial cells; however, grafts and Sertoli cells within the grafts are clearly distinguishable from kidney cells. Arrow, Sertoli cells; I, cellular infiltrate.
TABLE 2.
Survival of primary Sertoli cells or MSC-1 cells transplanted into BALB/c mice.
Examination of the grafts at higher magnification (Fig. 1, B, D, F, and H) revealed that Sertoli cells had begun to cluster and organize into tubule-like structures with Sertoli cell nuclei localized along the basal edge and their cytoplasm extended toward the center of tubules within 2 days (Fig. 1B, inset). After 5 days, most Sertoli cells were located within tubule-like structures or were clustered as cellular aggregates (Fig. 1D). Tubules were disrupted, with some Sertoli cell nuclei detected within the center of the tubule-like structures by 11 days (data not shown) and 14 days posttransplantation (Fig. 1F), which correlated with the level of immune cell infiltration within the grafts (Table 2). However, at 20 days postengraftment, infiltrating cells decreased and Sertoli cells reorganized, again into tubule-like structures in two of the five grafts (Fig. 1H).
Survival of MSC-1 Cells
After aMSC-1 cells (see Supplemental Fig. S1, B and D) were transplanted as allografts into the renal subcapsular space of naïve mice, tissue sections from aMSC-1 grafts were analyzed (Fig. 2 and Table 2 and see Supplemental Table S1B). At 1, 2, and 5 days posttransplantation, the percentage of graft survival was 100%, with a large number of large T antigen-positive MSC-1 cells present within all grafts (Fig. 2, A–F), except for one. By Day 8, the percentage of graft survival was still 100%, but the numbers of MSC-1 cells within the grafts began to decrease, ranging from grafts that contained many MSC-1 cells to grafts with less than 50 cells per section (Table 2). By Days 11 and 14, grafts contained less than 50 MSC-1 cells per section in 67% and 33% of grafts, respectively (Fig. 2, G and H, and Table 2). At Day 20, all grafts had been rejected (Table 2), and no large T antigen-positive MSC-1 cells remained in the tissue sections (Fig. 2, I and J). Unlike transplanted primary Sertoli cells, MSC-1 cells were randomly arranged, with no tubule-like structures (Fig. 2B, inset).
FIG. 2.
Immunohistochemical analysis of MSC-1 cell grafts is shown. Four million aMSC-1 cells were transplanted underneath the kidney capsule of BALB/c mice. Grafts were collected on Days 1 (A, B), 2 (C, D), 5 (E, F), 11 (G, H), and 20 (I, J) and immunostained for the MSC-1 cell marker large T-antigen (brown color). Inset is a higher magnification of B. The graft is separated from the kidney (K) by a dotted line (A, C, E, G, I). All sections were counterstained with hematoxylin (blue color). Arrow, MSC-1 cells; C, connective tissue; I, cellular infiltrate.
Infiltrating leukocytes were first detected in one aMSC-1 graft, 1 day after transplantation (Table 2). The number of grafts with infiltrating immune cells further increased at Day 2 (Fig. 2C), and at days 5 and 8, infiltrating cells began to surround MSC-1 cells (Fig. 2, E and F). By 11 days, most of the graft consisted of infiltrating immune cells and connective tissue (Fig. 2, G and H). After 20 days, MSC-1 cells had been rejected, and the graft site consisted of immune cell infiltrate and connective tissue (Fig. 2, I and J). Immunostaining for T cells with anti-CD3 antibody confirmed that T cells first entered the grafts at Day 2, and the number of T cells increased by Days 8 and 11, filling the grafts by Day 14 (see Supplemental Table S1B).
Induction of Apoptosis Within the Grafts
Numbers of apoptotic cells in the aMSC-1 grafts were similar to those in apSC grafts at Day 1 posttransplantation, with an average of 33 and 36 TUNEL-positive cells, respectively. For the apSC grafts, the relative number of apoptotic cells remained consistent (average, 5–32 apoptotic cells/section) with no significant change throughout the study (Fig. 3, A and E). These results are consistent with the observed survival of apSC grafts shown in Table 2 and Figure 1.
FIG. 3.
TUNEL analyses of Sertoli cell and MSC-1 cell grafts are shown. Graft-bearing kidneys were removed 8 days after transplantation of apSC (A, B) or aMSC-1 (C, D), and tissue sections were analyzed for apoptotic cells by TUNEL assay (green color [A, C]). Sections were counterstained with bisBenzimide (Hoechst 33342; blue color [B, D]). A dotted line separates grafts from kidney (K). Insets in A and C show superimposed images of TUNEL-positive cells among Hoechst dye-positive cells at a higher magnification. E) Relative fold changes in the number of apoptotic cells in the SC (grey bar) or MSC-1 cell (black bar) grafts are shown. Data shown are means ± SEM for at least three different experiments per time point. Different letters denote significant differences in means determined by ANOVA followed by the Student t-test method at a P value of ≤0.05.
In contrast, the relative number of TUNEL-positive cells in aMSC-1 grafts at Day 2 posttransplantation were 3.3-fold increased compared to those at Day 1 posttransplantation and remained increased at Days 5 and 8 (Fig. 3E). At Days 11 and 14 posttransplantation, the number of apoptotic cells in aMSC-1 grafts decreased significantly (Fig. 3E), which is consistent with rejection of aMSC-1 grafts by Day 14 (Table 2 and Fig. 2). At Day 20, the number of apoptotic cells was 2.6-fold increased (Fig. 3E). These apoptotic cells could be T cells, which die by apoptosis after eliminating the foreign antigen (in this case, aMSC-1).
Microarray Hybridization, GeneSpring Analysis, and Expression Verification by Real-Time RT-PCR and Western Blot Analysis
Biotin-labeled cRNA targets prepared from RNAs from three independent preparations of each cell type were hybridized to Affymetrix Mouse Expression Array 430 version 2.0 to determine gene expression. Raw signals were processed by computational filtering and ANOVA calculation at a P of ≤0.05, as described in Materials and Methods. A total of 3198 probes with ±4.0-fold or higher expression were submitted to Gene Expression Omnibus (GEO; accession no. GSE23200), and of these, 1902 probes were higher and 1296 probes were lower in apSC than in aMSC-1; 3198 probes corresponded to 2369 genes, and 50 expressed sequence tags (ESTs).
To verify microarray data, some immune-related genes were selected for real-time RT-PCR. The signal transducer CD24 (Cd24), granulocyte colony-stimulating factor 3 (Csf3), interleukin 11 (Il11), serping1 (Serping1), complement decay-accelerating factor transmembrane isoform (Daf2/Cd55b), complement decay-accelerating factor (Cd55), interleukin 6 (Il6), and serine protease inhibitor B9 (Serpinb9) genes had higher mRNA expression in apSC than in aMSC-1 (Fig. 4, A–C). On the other hand, the CD59 glycoprotein (Cd59) gene had higher mRNA expression in aMSC-1 than in apSC (Fig. 4), while the transforming growth factor beta 1 (Tgfb1) gene was found to have expression levels that were similar in apSC and aMSC-1 (Fig. 4A). These results were consistent with microarray data (GEO accession no. GSE23200), except for the Tgfb1 gene, which did not reach the P cutoff of ≤0.05 to be included in the microarray database.
FIG. 4.
Real-time RT-PCR results show normalized signal expression and fold difference between immune-related mRNAs in aMSC1 and those in apSC. The y axis shows the average normalized means ± SEM of the transcript level for aMSC1 (white bar) and apSC (black bar) normalized to those of Rps2 for Cd59, Tgfb1, Il6, Serpinb9, Il11, Csf3, and Serping1 (A), Cd55 and Daf2 (B), and Cd24 (C) transcripts. Real-time RT-PCR was conducted in triplicate using three biological samples per cell type. Relative fold difference between apSC and aMSC1 are designated above each gene. *, significant differences at P ≤ 0.01.
Real-time RT-PCR was also performed for genes known to be expressed in Sertoli cells. The follicle-stimulating hormone receptor (Fshr), inhibin alpha (Inha), clusterin (Clu), steroidogenic acute regulatory protein (Star), cAMP-responsive element modulator (Crem), and lecithin-retinol acyltransferase (Lrat) genes were found to have higher mRNA expression in apSC than in aMSC-1 (Fig. 5, A and B). These results indicate that expression of several genes, particularly the Crem, Star, Clu, and Inha genes normally expressed in primary Sertoli cells, is virtually undetected in aMSC-1. These results were consistent with the microarray data findings (GEO accession no. GSE23200), except for Fshr and Lrat expression levels, which were low and did not reach the P cutoff of ≤0.05 for the microarray database.
FIG. 5.
Real-time RT-PCR results show normalized signal expression and fold difference between known Sertoli cells genes in aMSC1 and those in apSC. The y axis shows the average normalized means ± SEM of transcript level for aMSC1 (white bar) and apSC (black bar) normalized to Rps2 for Lrat, Inha, Fshr, Star, and Clu (A), and Crem (B) transcript levels. Real-time RT-PCR was conducted in triplicate using three biological samples per cell type. Relative fold differences in apSC compared to those in aMSC1 are designated above each gene. *, significant differences at P ≤ 0.01.
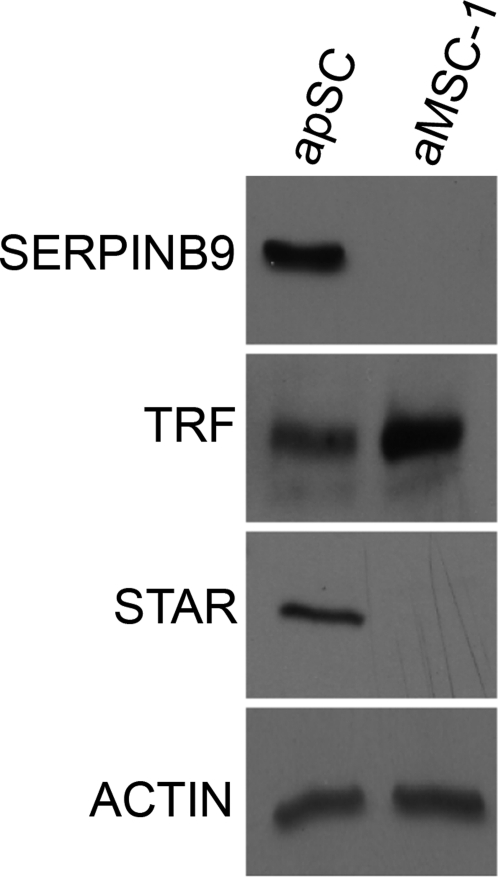
Additionally, Western blot analysis was performed for STAR, SERPINB9, and transferrin (Fig. 6). SERPINB9 and STAR were both expressed at higher levels in apSC than in aMSC-1. In contrast, the transferrin level was higher in aMSC-1 than in apSC. These results were consistent with the microarray data findings (GEO accession no. GSE23200).
FIG. 6.
Western blot analysis of SERPINB9, TRF (transferrin), and STAR are shown in apSC and in aMSC-1. Proteins were isolated from apSC and aMSC-1. Equal protein loading was confirmed by immunoblotting the same membranes with an anti-actin antibody. Blots representing three separate experiments are shown.
Functional Pathway Analyses of Immune- Related Genes
Ontology analyses with Pathway Express, KEGG, and DAVID were conducted with 2369 differentially expressed genes. Common functional pathways found between Pathway Express and KEGG analyses were ranked by their statistical significance, according to their gamma P values (see Supplemental Table S2). DAVID analysis ordered the functional pathways by Geo median (see Supplemental Table S3). Then, to focus on genes related to immune functions, we compiled a list of 340 immune-related genes (see Supplemental Table S4), guided by the Immunome database (http://bioinf.uta.fi/immunome/), which contains 893 immune-related genes [28] and genes in immune-related pathways identified by the three ontology analyses (see Supplemental Tables S2 and S3). Arrows were inserted in front of the gene name in Table 3 and Supplemental Tables S2 and S3 to indicate whether expression was increased (up) or decreased (down) in apSC compared to aMSC-1, by microarray analysis.
Immune-related genes were further annotated using Pathway Express and KEGG (Table 3). Functional pathways identified were cytokine–cytokine receptor interactions, chemokine signaling, arachidonic acid and eicosanoid metabolism, complement and coagulation cascades, extracellular matrix-receptor interaction, tight junction, regulation of actin cytoskeleton, cell adhesion molecules, leukocyte transendothelial migration, and adherens junction (Table 3). In addition, hematopoietic cell lineage, Jak-Stat signaling, TGF beta signaling, natural killer cell-mediated cytotoxicity, Toll-like receptor, B-cell receptor signaling, T-cell receptor signaling, mTOR signaling, and antigen processing and presentation pathways were found (Table 3).
TABLE 3.
Functional pathways from KEGG and Pathway Express analyses of immune-related genes.*
DISCUSSION
The testis is an immunologically privileged site that provides appropriate immune regulation to support survival of autoantigenic germ cells [12, 13]. Primary Sertoli cells play an important role in creating this immunologically privileged environment [2, 3]. To increase our understanding of immune privilege, the survival of apSC was compared with that of aMSC-1 after transplantation into naïve BALB/c mice. All aMSC-1 grafts were rejected by 20 days posttransplantation, while primary Sertoli cells survived for at least 20 days posttransplantation in 100% of the apSC grafts. These survival results demonstrate that apSC transplanted into naïve BALB/c mice confer immune privilege to the graft site, while the MSC-1 cell line lacks some properties necessary for graft survival after transplantation. Thus, we used apSC and aMCS-1 as model systems with which to identify immune factors that could be responsible for immune privilege. Ontological analyses of microarray data revealed several major functional pathways in apSC that were significantly different from those in aMSC-1. These functional pathways were further categorized into three biological clusters for antimigration of leukocytes involving regulation of cell junctions and actin dynamics, anti-inflammation involving cytokines and eicosanoids, and inhibition of complement activation and membrane-associated cell lysis. These clusters are compatible with inhibition of humoral immunity and modification of cell-mediated response, both of which are postulated to be important for creating immune privilege [10].
Increased Cell Adhesion (Adherens and Tight Junctions) and Decreased Leukocyte Migration
Cell junctional molecules, together with molecules that regulate actin cytoskeletal organization, modulate lymphocyte interactions with antigen-presenting cells, allowing T cells to respond appropriately [29, 30]. Moreover, these same molecules control leukocyte interactions with vascular endothelial cells in the recruitment of circulating leukocytes to sites of inflammation [29, 30]. Thus, it is significant that genes for cell junctions, leukocyte transendothelial migration, and actin cytoskeletal organization were identified as immune-related genes expressed in apSC (Table 3, boldface type). Higher Icam1, Vcam1, and Itgae mRNA expression in apSC indicated that apSC can interact strongly with other cells (Fig. 7A), for example, T cells (Fig. 8). Previously, it was shown that ICAM1 and VCAM1 interacting with integrin alpha/beta slowed leukocyte migration [29] and increased lymphocyte adhesion to Sertoli cells [31] and mesenchymal stem cells [32], another immunologically privileged cell type. More interestingly, this increased cell adhesion function in mesenchymal stem cells resulted in decreased T-cell activation and decreased inflammation [32].
FIG. 7.
Selected immune-related functional pathways are shown. Immune-related functional pathways for cell adhesion and tight junction molecules (A), cytokine–cytokine receptor interactions (B), arachidonic acid and eicosanoid metabolism (C), and classical pathway of the complement cascade (D) are shown. Genes in red are higher and genes in green are lower expression in apSC than in aMSC-1. Boxes touching indicate they are in a complex; black line indicates binding; black arrow indicates direct stimulatory regulation or modification; T indicates inhibitory regulation; thick lines in B represent inner and outer leaflets of membrane.
FIG. 8.
A summary diagram is shown. Primary Sertoli cells form tubules with tight junctions (thick red lines) and increase adhesion to lymphocytes, limiting leukocyte migration. A) Under this condition, the Sertoli cell secretory factors (e.g., cytokines and prostanoids) modify the cell-mediated immune response to a type 2 response and suppress inflammation, resulting in immune tolerance and immune privilege. B) Humorally, primary Sertoli cells express complement inhibitors that inhibit activation of the complement cascade and cell lysis, decreasing anaphylotoxin production, leukocyte recruitment, and inflammation and, thus, contribute to immune privilege. Green line, adhesion molecules; green and brown dots, anti-inflammatory molecules secreted by Treg and immature dendritic cells that further promote a type 2 immune response; Y, immunoglobulin.
Additionally, lower α-catenin (CTNNA2) and JAM2 expression levels in apSC are expected to decrease leukocyte migration (Fig. 7A), as higher α-catenin [33] and JAM2 [34] levels have been demonstrated to promote migration of leukocytes. Furthermore, lower SIPA1 and RAP1GAP expression levels and higher GNAI1 expression levels in apSC (Fig. 7A) enhance RAP1 activation, resulting in decreased actin dynamics. Decrease in actin dynamics has been shown to increase interaction between actin and the catenin/cadherin complex, leading to increased stability of adherens junctions and decreased migration of leukocytes [35–37]. Thus, the expression patterns of cell adhesion and actin organization molecules in apSC suggest that apSCs strongly interact with leukocytes, limiting leukocyte migration and allowing for modification of T cells in a way that is compatible with immune privilege (Fig. 8).
In support of the above-described gene expression results, histological and immunological results revealed that formation of tubule-like structures after transplantation, indicative of enhanced cell adhesion and junction formation, was inversely correlated to leukocyte infiltration. Transplanted apSC formed tubule-like structures within 2 days of transplantation and then again by Day 20, at which point the apSC appeared to overcome the effect of leukocyte infiltration. In comparison, MSC-1 cells were randomly arranged, tubule-like structures were not observed, and uncontrolled leukocyte infiltration resulted in MSC-1 cell apoptosis. Consistently, it was shown recently that Sertoli cell-targeted deletion of androgen receptor, which led to a decreased blood–testis barrier function, resulted in loss of testicular immune privilege [38]. Androgen receptor mRNA expression was 17-fold higher in apSC than in aMSC-1 (see Supplemental Table S4). Moreover, Cd24 mRNA was higher in apSC than in aMSC-1 cells. CD24 (alias, heat-stable antigen [HSA]) enhanced the intercellular barrier function of tight junctional proteins and decreased immune response following acute tissue damage [39, 40]. In contrast, CD24 deficiency caused excessive T-cell proliferation and killing of CD24-deficient recipients [41]. Although it is accepted that the blood–testis barrier alone cannot account for testicular immune privilege [13, 14], our results suggest that increased interactions of primary Sertoli cells, such as Sertoli cell–Sertoli cell interactions and Sertoli cell–T-cell interactions, are important for limiting leukocyte trafficking and modulating T cells to create immune privilege (Fig. 8).
Anti-Inflammatory Response Involving Cytokines and Eicosanoids
It is known that transplantation tolerance in graft sites can be accomplished by promoting production of cytokines that favor a type 2 over a type 1 immune response [16]. Cytokine immune modulators act as chemoattractants to guide migration of immune cells to antigen-presenting cells, regulate appropriate T-cell response, and modulate inflammation [14, 15]. Thus, it is significant that microarray and oncological analyses identified functional pathways for cytokine–cytokine receptor interaction, chemokine signaling, and other molecules (Table 3) that could suppress inflammation and promote a type 2, tolerogenic immune response (Fig. 8). These cytokines and chemokines may be important for the immune response observed in the apSC grafts. Initially, the immune response in the apSC grafts appeared similar to that in the aMSC-1 grafts. However, while the aMSC-1 grafts followed the classical proinflammatory rejection response with infiltrating immune cells entering the graft site and killing aMSC-1 cells in 11 to 14 days, the same time frame reported for rejection of allogeneic islet cells [42], the apSC cells modified the typical immune response, induced a tolerogenic environment, and were not rejected.
Some of the cytokines with higher expressions in apSC can activate a proinflammatory response (e.g., interleukin 1R [IL1R], IL6, and IL33), but the pattern of expression for their interacting proteins (IL1RAP, IL11, and CSF3) suggests that inflammation may be curtailed at the apSC graft site (Fig. 7B). Specifically, IL1RAP expression, required for the proinflammatory IL1/IL1R [43] and IL33 functions [44], is 18-fold downregulated in apSC (see Supplemental Table S4). The implication is that it is not one but a combination of cytokines and their interacting partners that creates an immunotolerant environment. For example, IL6, which was more highly expressed in apSC than in aMSC-1, is usually considered proinflammatory. However, it can induce Th2 T-cell differentiation and promote immature dendritic cells, which in turn induces Treg associated with a type 2 response, in the presence of IL4 and IL11 or CSF [17, 45]. Both IL11 and CSF expression levels are high in apSC. These complex responses of IL6 may be possible because IL6 shares IL6ST (gp130) with other cytokines including IL11 and LIF (Fig. 7B) [46].
One molecule consistently implicated in modifying immune response is TGFB [47, 48]. TGFB has been shown to prolong islet graft survival when islets were cotransplanted with primary Sertoli cells by promoting Th2 over Th1 T cell differentiation [47] and inducing Treg production associated with immune tolerance during pregnancy [48]. Thus, it is interesting that the expression levels of thrombospondin 1 (Thbs1) and Thbs2 mRNAs were higher in apSC than in aMSC-1. THBS1 and THBS2 are known to convert latent TGFB into active TGFB (Fig. 7B). THBS1 and THBS2 may be important for activation of TGFB at a specific time and place, when it is necessary for immune privilege but not constitutively. Consistently, THBS1 has been shown to function in retinal pigment epithelial cells that line the immunologically privileged subretinal space in the posterior segment of the eye [49].
Other notable examples of Th2 T-cell induction or Treg production in apSC occur with TNFRSF21 (alias, death receptor 6 [DR6]) (Fig. 7B), which directs naïve T cells to differentiate to Th2 T cells [50], and IRAK3 (IRAK-M), which increases Treg [51] (see Supplemental Table S4). Conversely, Irak3−/− (null) mice exhibited increased proinflammatory cytokine production in response to bacterial infection [52]. LIF also plays an important role in Treg cell-induced tolerance during implantation [48] and regulates Plac8 mRNA levels, which were 112-fold higher in apSC than in aMSC-1 (see Supplemental Table S4). PLAC8 has a role in anti-inflammation as a targeted deletion of the Plac8 gene increased inflammation and impaired host defense [53]. In contrast, IL18 induces interferon gamma (IFNG) production in T cells and promotes type 1 activities leading to severe inflammation [18], and a receptor for tumor necrosis factor (TNF) TNFRSF11B, alias osteoprotegerin, elevates LPS-induced production of proinflammatory cytokines in osteoclasts [54]. However, both IL18 and osteoprotegerin expression were low in apSC.
Other important classes of molecules for the resolution of inflammation are arachidonic acid and eicosanoids, precursors to prostanoids, leukotrienes, and hydroxy and epoxy acids [55] (arachidonic acid and eicosanoid metabolism pathways are shown in Table 3). apSC had higher levels of the phospholipase A enzymes (PLA2G2C, PLA2G4A, and PLA2G6) that control the production of arachidonic acid (Fig. 7C). The expressions of enzymes that produce anti-inflammatory prostanoids were higher and those of proinflammatory prostanoids were lower in apSC. PTGES and PTGIS can convert prostaglandin H2 (PGH2) to anti-inflammatory PGE2 and PGI2, respectively, whereas PTGDS can covert PGH2 to proinflammatory PGD2 (Fig. 7C). PGE2 plays an immunosuppressive role, associated with dendritic cells and Treg, and inhibits T-cell activation and expansion and decreases the production of IL2 [56]. Similarly, PGI2 (prostacyclin) has an anti-inflammatory role and decreases proinflammatory IL1, increases anti-inflammatory IL10, and inhibits platelet-activating factor (PAF) [57]. PAF is an inflammatory molecule that promotes eosinophil chemotaxis and neutrophil recruitment. On the other hand, PGD2 is proinflammatory, a potent chemoattractant of eosinophils [58].
Inhibition of Complement Activation
It is well known that complement activation leads to hyperacute, acute, and chronic graft rejection. On the other hand, inhibiting complement activation can lead to decreased leukocyte recruitment and activation, decreased inflammation, and increased immune privilege [10]. In the current study, microarray analyses identified a functional pathway for complement and coagulation cascades (Table 3) that includes serine protease inhibitor G1 (SERPING1), serine protease inhibitor E1 (SERPINE1), decay-accelerating factor 2 (DAF2/CD55B), and CD55, all of which are increased in apSC. They are early inhibitors of complement activation and anaphylatoxin (C4a, C3a, and C5a) production (Fig. 7D). SERPING1 inactivates C1 proteinases in the C1 complex, preventing formation of C3 convertase [59]. CD55 inhibits C3 and C5 convertases and inhibits T-cell activation. Increased expression of CD55 is the hallmark of ocular immune privilege and of corneal transplantation survival [60]. In contrast, Cd55−/− mice produced an elevated level of proinflammatory IFNG and IL2 and a reduced level of immunosuppressive IL10 [61]. Moreover, thrombomodulin (THBD) inactivated proinflammatory C3a and C5a anaphylatoxins, inhibiting leukocyte recruitment and inflammation (Fig. 7D) [62]. Additionally, clusterin blocks MAC-mediated cell lysis [63] (Fig. 7D). These results indicate that control of both the early part of complement cascade amplification and anaphylatoxin production and the later part of complement cascade, the prevention of MAC-mediated cell lysis, may be important for promotion of immune privilege in the graft site (Fig. 8B). In contrast, although CD59 is known to block MAC formation, CD59, which is high in MSC-1 cells, by itself in MSC-1 cells could not provide immunoprotection.
In conclusion, transplantation experiments confirmed that apSC possess unique immunologically privileged abilities that allow their survival when cells are transplanted as allografts, while aMSC-1 are lacking these properties. We used a global approach to identify a number of immune-related genes and pathways that were differentially expressed in apSC versus those in aMSC-1. Patterns of expression for cytokines, chemokines, complement inhibitors, adhesion molecules, and other immune modulators support the notion that apSC contribute to the generation of a unique immunologically privileged milieu by attenuating the immune response at three functional levels, that is, increasing cell–cell interactions and decreasing leukocyte migration, increasing a type 2 immune response, and complement inhibition, all of which lead to an anti-inflammatory response (Fig. 8). These clusters of immune-related functions in primary Sertoli cells may be important for graft survival and immune privilege or tolerance. Observations made here indicate that immune cells infiltrate both apSC and aMSC-1 grafts, but based on the immune-related molecular context, the phenotype of the infiltrating cells may be altered and a tolerogenic environment created that favors Treg, immature dendritic cells, and a type 2 anti-inflammatory responses within the apSC graft site (Fig. 8). Our results have the potential to generate important hypotheses to test concerning immune privilege for many investigators in the near future.
Supplementary Material
ACKNOWLEDGMENT
The authors would like to thank Dr. D.B. Hales (Department of Physiology and Biophysics, University of Illinois, Chicago, IL) for generous use of the StAR antibody.
Footnotes
These authors contributed equally to this work.
Supported in part by National Institutes of Health, National Institute of Child Health and Human Development grant HD067400 (to J.M.D.) and the American Diabetes Association, Laura W. Bush Institute for Women's Health (LWBIWH) and University Medical Center. W.T.M. is a recipient of a LWBIWH grant and a Texas Tech University Health Sciences Center, School of Medicine Summer Research Fellowship.
REFERENCES
- Barker CF, Billingham RE. Immunologically privileged sites. Adv Immunol 1977; 25: 1 54 [PubMed] [Google Scholar]
- Whitmore WF, III, Karsh L, Gittes RF. The role of germinal epithelium and spermatogenesis in the privileged survival of intratesticular grafts. J Urol 1985; 134: 782 786 [DOI] [PubMed] [Google Scholar]
- Cameron DF, Whittington K, Schultz RE, Selawry HP. Successful islet/abdominal testis transplantation does not require Leydig cells. Transplantation 1990; 50: 649 653 [DOI] [PubMed] [Google Scholar]
- Selawry HP, Cameron DF. Sertoli cell-enriched fractions in successful islet cell transplantation. Cell Transplant 1993; 2: 123 129 [DOI] [PubMed] [Google Scholar]
- Korbutt GS, Elliott JF, Rajotte RV. Cotransplantation of allogeneic islets with allogeneic testicular cell aggregates allows long-term graft survival without systemic immunosuppression. Diabetes 1997; 46: 317 322 [DOI] [PubMed] [Google Scholar]
- Sanberg PR, Borlongan CV, Saporta S, Cameron DF. Testis-derived Sertoli cells survive and provide localized immunoprotection for xenografts in rat brain. Nat Biotechnol 1996; 14: 1692 1695 [DOI] [PubMed] [Google Scholar]
- Yang H, Wright JR., Jr Co-encapsulation of Sertoli enriched testicular cell fractions further prolongs fish-to-mouse islet xenograft survival. Transplantation 1999; 67: 815 820 [DOI] [PubMed] [Google Scholar]
- Dufour JM, Rajotte RV, Kin T, Korbutt GS. Immunoprotection of rat islet xenografts by cotransplantation with sertoli cells and a single injection of antilymphocyte serum. Transplantation 2003; 75: 1594 1596 [DOI] [PubMed] [Google Scholar]
- Dufour JM, Dass B, Halley KR, Korbutt GS, Dixon DE, Rajotte RV. Sertoli cell line lacks the immunoprotective properties associated with primary Sertoli cells. Cell Transplant 2008; 17: 525 534 [DOI] [PubMed] [Google Scholar]
- Asgari E, Zhou W, Sacks S. Complement in organ transplantation. Curr Opin Organ Transplant 2010; 15: 486 491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour JM, Hamilton M, Rajotte RV, Korbutt GS. Neonatal porcine Sertoli cells inhibit human natural antibody-mediated lysis. Biol Reprod 2005; 72: 1224 1231 [DOI] [PubMed] [Google Scholar]
- Mital P, Kaur G, Dufour JM. Immunoprotective sertoli cells: making allogeneic and xenogeneic transplantation feasible. Reproduction 2010; 139: 495 504 [DOI] [PubMed] [Google Scholar]
- Meinhardt A, Hedger MP. Immunological, paracrine and endocrine aspects of testicular immune privilege. Mol Cell Endocrinol 2011; 335: 60 68 [DOI] [PubMed] [Google Scholar]
- Hedger MP, Meinhardt A. Cytokines and the immune-testicular axis. J Reprod Immunol 2003; 58: 1 26 [DOI] [PubMed] [Google Scholar]
- Guazzone VA, Jacobo P, Theas MS, Lustig L. Cytokines and chemokines in testicular inflammation: a brief review. Microsc Res Tech 2009; 72: 620 628 [DOI] [PubMed] [Google Scholar]
- Cobbold SP. Regulatory T cells and transplantation tolerance. J Nephrol 2008; 21: 485 496 [PubMed] [Google Scholar]
- Hegde S, Pahne J, Smola-Hess S. Novel immunosuppressive properties of interleukin-6 in dendritic cells: inhibition of NF-kappaB binding activity and CCR7 expression. FASEB J 2004; 18: 1439 1441 [DOI] [PubMed] [Google Scholar]
- Fujimori Y, Takatsuka H, Takemoto Y, Hara H, Okamura H, Nakanishi K, Kakishita E. Elevated interleukin (IL)-18 levels during acute graft-versus-host disease after allogeneic bone marrow transplantation. Br J Haematol 2000; 109: 652 657 [DOI] [PubMed] [Google Scholar]
- Peschon JJ, Behringer RR, Cate RL, Harwood KA, Idzerda RL, Brinster RL, Palmiter RD. Directed expression of an oncogene to Sertoli cells in transgenic mice using Mullerian inhibiting substance regulatory sequences. Mol Endocrinol 1992; 6: 1403 1411 [DOI] [PubMed] [Google Scholar]
- Dufour JM, Lord SJ, Kin T, Rayat GR, Dixon DE, Bleackley RC, Korbutt GS, Rajotte RV. Comparison of successful and unsuccessful islet/Sertoli cell cotransplant grafts in streptozotocin-induced diabetic mice. Cell Transplant 2008; 16: 1029 1038 [PubMed] [Google Scholar]
- Dufour JM, Hemendinger R, Halberstadt CR, Gores P, Emerich DF, Korbutt GS, Rajotte RV. Genetically engineered Sertoli cells are able to survive allogeneic transplantation. Gene Ther 2004; 11: 694 700 [DOI] [PubMed] [Google Scholar]
- Johnston DS, Wright WW, Dicandeloro P, Wilson E, Kopf GS, Jelinsky SA. Stage-specific gene expression is a fundamental characteristic of rat spermatogenic cells and Sertoli cells. Proc Natl Acad Sci U S A 2008; 105: 8315 8320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draghici S, Khatri P, Tarca AL, Amin K, Done A, Voichita C, Georgescu C, Romero R. A systems biology approach for pathway level analysis. Genome Res 2007; 17: 1537 1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 2009; 4: 44 57 [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 2008; 3: 1101 1108 [DOI] [PubMed] [Google Scholar]
- Hales KH, Diemer T, Ginde S, Shankar BK, Roberts M, Bosmann HB, Hales DB. Diametric effects of bacterial endotoxin lipopolysaccharide on adrenal and Leydig cell steroidogenic acute regulatory protein. Endocrinology 2000; 141: 4000 4012 [DOI] [PubMed] [Google Scholar]
- Mundlos S, Pelletier J, Darveau A, Bachmann M, Winterpacht A, Zabel B. Nuclear localization of the protein encoded by the Wilms' tumor gene WT1 in embryonic and adult tissues. Development 1993; 119: 1329 1341 [DOI] [PubMed] [Google Scholar]
- Ortutay C, Vihinen M. Immunome knowledge base (IKB): an integrated service for immunome research. BMC Immunol 2009; 10: 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P, Weigelin B. Interstitial leukocyte migration and immune function. Nat Immunol 2008; 9: 960 969 [DOI] [PubMed] [Google Scholar]
- Muller WA. Mechanisms of leukocyte transendothelial migration. Annu Rev Pathol 2011; 6: 323 344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccioli A, Filippini A, De Cesaris P, Barbacci E, Stefanini M, Starace G, Ziparo E. Inflammatory mediators increase surface expression of integrin ligands, adhesion to lymphocytes, and secretion of interleukin 6 in mouse Sertoli cells. Proc Natl Acad Sci U S A 1995; 92: 5808 5812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren G, Zhao X, Zhang L, Zhang J, L'Huillier A, Ling W, Roberts AI, Le AD, Shi S, Shao C, Shi Y. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J Immunol 2010; 184: 2321 2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Buul JD, van Alphen FP, Hordijk PL. The presence of alpha-catenin in the VE-cadherin complex is required for efficient transendothelial migration of leukocytes. Int J Biol Sci 2009; 5: 695 705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Leger CA, Aurrand-Lions M, Beltraminelli N, Fasel N, Imhof BA. Junctional adhesion molecule-2 (JAM-2) promotes lymphocyte transendothelial migration. Blood 2002; 100: 2479 2486 [DOI] [PubMed] [Google Scholar]
- Kometani K, Ishida D, Hattori M, Minato N. Rap1 and SPA-1 in hematologic malignancy. Trends Mol Med 2004; 10: 401 408 [DOI] [PubMed] [Google Scholar]
- Katayama K, Melendez J, Baumann JM, Leslie JR, Chauhan BK, Nemkul N, Lang RA, Kuan CY, Zheng Y, Yoshida Y. Loss of RhoA in neural progenitor cells causes the disruption of adherens junctions and hyperproliferation. Proc Natl Acad Sci U S A 2011; 108: 7607 7612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler V, Schlegel N, Waschke J. Role of GTPases in control of microvascular permeability. Cardiovasc Res 2010; 87: 243 253 [DOI] [PubMed] [Google Scholar]
- Meng J, Greenlee AR, Taub CJ, Braun RE. Sertoli Cell-specific deletion of the androgen receptor compromises testicular immune privilege in mice. Biol Reprod 2011; 85: 254 260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye P, Nadkarni MA, Simonian M, Hunter N. CD24 regulated gene expression and distribution of tight junction proteins is associated with altered barrier function in oral epithelial monolayers. BMC Cell Biol 2009; 10: 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GY, Tang J, Zheng P, Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science 2009; 323: 1722 1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li O, Chang X, Zhang H, Kocak E, Ding C, Zheng P, Liu Y. Massive and destructive T cell response to homeostatic cue in CD24-deficient lymphopenic hosts. J Exp Med 2006; 203: 1713 1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallgren AC, Karlsson-Parra A, Korsgren O. The main infiltrating cell in xenograft rejection is a CD4+ macrophage and not a T lymphocyte. Transplantation 1995; 60: 594 601 [DOI] [PubMed] [Google Scholar]
- Volpe F, Clatworthy J, Kaptein A, Maschera B, Griffin AM, Ray K. The IL1 receptor accessory protein is responsible for the recruitment of the interleukin-1 receptor associated kinase to the IL1/IL1 receptor I complex. FEBS Lett 1997; 419: 41 44 [DOI] [PubMed] [Google Scholar]
- Palmer G, Lipsky BP, Smithgall MD, Meininger D, Siu S, Talabot-Ayer D, Gabay C, Smith DE. The IL-1 receptor accessory protein (AcP) is required for IL-33 signaling and soluble AcP enhances the ability of soluble ST2 to inhibit IL-33. Cytokine 2008; 42: 358 364 [DOI] [PubMed] [Google Scholar]
- Diehl S, Rincon M. The two faces of IL-6 on Th1/Th2 differentiation. Mol Immunol 2002; 39: 531 536 [DOI] [PubMed] [Google Scholar]
- Boulanger MJ, Garcia KC. Shared cytokine signaling receptors: structural insights from the gp130 system. Adv Protein Chem 2004; 68: 107 146 [DOI] [PubMed] [Google Scholar]
- Suarez-Pinzon W, Korbutt GS, Power R, Hooton J, Rajotte RV, Rabinovitch A. Testicular sertoli cells protect islet beta-cells from autoimmune destruction in NOD mice by a transforming growth factor-beta1-dependent mechanism. Diabetes 2000; 49: 1810 1818 [DOI] [PubMed] [Google Scholar]
- Zenclussen AC, Gerlof K, Zenclussen ML, Ritschel S. Zambon Bertoja A, Fest S, Hontsu S, Ueha S, Matsushima K, Leber J, Volk HD. Regulatory T cells induce a privileged tolerant microenvironment at the fetal-maternal interface. Eur J Immunol 2006; 36: 82 94 [DOI] [PubMed] [Google Scholar]
- Zamiri P, Masli S, Kitaichi N, Taylor AW, Streilein JW. Thrombospondin plays a vital role in the immune privilege of the eye. Invest Ophthalmol Vis Sci 2005; 46: 908 919 [DOI] [PubMed] [Google Scholar]
- DeRosa DC, Ryan PJ, Okragly A, Witcher DR, Benschop RJ. Tumor-derived death receptor 6 modulates dendritic cell development. Cancer Immunol Immunother 2008; 57: 777 787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringwood L, Li L. The involvement of the interleukin-1 receptor-associated kinases (IRAKs) in cellular signaling networks controlling inflammation. Cytokine 2008; 42: 1 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Hernandez LD, Galan JE, Janeway CA, Jr, Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell 2002; 110: 191 202 [DOI] [PubMed] [Google Scholar]
- Ledford JG, Kovarova M, Koller BH. Impaired host defense in mice lacking ONZIN. J Immunol 2007; 178: 5132 5143 [DOI] [PubMed] [Google Scholar]
- Maruyama K, Takada Y, Ray N, Kishimoto Y, Penninger JM, Yasuda H, Matsuo K. Receptor activator of NF-kappa B ligand and osteoprotegerin regulate proinflammatory cytokine production in mice. J Immunol 2006; 177: 3799 3805 [DOI] [PubMed] [Google Scholar]
- Gilroy DW, Newson J, Sawmynaden P, Willoughby DA, Croxtall JD. A novel role for phospholipase A2 isoforms in the checkpoint control of acute inflammation. FASEB J 2004; 18: 489 498 [DOI] [PubMed] [Google Scholar]
- Lee SY, Choi HK, Lee KJ, Jung JY, Hur GY, Jung KH, Kim JH, Shin C, Shim JJ, In KH, Kang KH, Yoo SH. The immune tolerance of cancer is mediated by IDO that is inhibited by COX-2 inhibitors through regulatory T cells. J Immunother 2009; 32: 22 28 [DOI] [PubMed] [Google Scholar]
- McManus LM, Pinckard RN. PAF. a putative mediator of oral inflammation. Crit Rev Oral Biol Med 2000; 11: 240 258 [DOI] [PubMed] [Google Scholar]
- Powell WS. A novel PGD(2) receptor expressed in eosinophils. Prostaglandins Leukot Essent Fatty Acids 2003; 69: 179 185 [DOI] [PubMed] [Google Scholar]
- Murray-Rust TA, Kerr FK, Thomas AR, Wu T, Yongqing T, Ong PC, Quinsey NS, Whisstock JC, Wagenaar-Bos IC, Freeman C, Pike RN. Modulation of the proteolytic activity of the complement protease C1s by polyanions: implications for polyanion-mediated acceleration of interaction between C1s and SERPING1. Biochem J 2009; 422: 295 303 [DOI] [PubMed] [Google Scholar]
- Esposito A, Suedekum B, Liu J, An F, Lass J, Strainic MG, Lin F, Heeger P, Medof ME. Decay accelerating factor is essential for successful corneal engraftment. Am J Transplant 2010; 10: 527 534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Fukuoka Y, Spicer A, Ohta R, Okada N, Harris CL, Emancipator SN, Medof ME. Tissue distribution of products of the mouse decay-accelerating factor (DAF) genes. Exploitation of a Daf1 knock-out mouse and site-specific monoclonal antibodies. Immunology 2001; 104: 215 225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delvaeye M, Noris M, De Vriese A, Esmon CT, Esmon NL, Ferrell G, Del-Favero J, Plaisance S, Claes B, Lambrechts D, Zoja C, Remuzzi G. et al. Thrombomodulin mutations in atypical hemolytic-uremic syndrome. N Engl J Med 2009; 361: 345 357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenne DE, Tschopp J. Molecular structure and functional characterization of a human complement cytolysis inhibitor found in blood and seminal plasma: identity to sulfated glycoprotein 2, a constituent of rat testis fluid. Proc Natl Acad Sci U S A 1989; 86: 7123 7127 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.