ABSTRACT
Polycystic ovary syndrome (PCOS) is characterized by ovarian enlargement, theca-interstitial hyperplasia, and increased androgen production by theca cells. Previously, our group has demonstrated that statins (competitive inhibitors of 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase, a rate-limiting step of the mevalonate pathway) reduce proliferation of theca-interstitial cells in vitro and decrease serum androgen levels in women with PCOS. The present study evaluated the effect of simvastatin on rat ovarian theca-interstitial cell steroidogenesis. Because actions of statins may be due to reduced cholesterol availability and/or isoprenylation of proteins, the present study also investigated whether steroidogenesis was affected by cell- and mitochondrion-permeable 22-hydroxycholesterol, isoprenylation substrates (farnesyl-pyrophosphate [FPP] and geranylgeranyl-pyrophosphate [GGPP]), as well as selective inhibitors of farnesyltransferase (FTI) and geranylgeranyltransferase (GGTI). Theca-interstitial cells were cultured for 12, 24, and 48 h with or without simvastatin, GGPP, FPP, FTI, GGTI, and/or 22-hydroxycholesterol. Simvastatin decreased androgen levels in a time- and concentration-dependent fashion. This inhibitory effect correlated with a decrease in mRNA levels of Cyp17a1, the gene encoding the key enzyme regulating androgen biosynthesis. After 48 h, GGPP alone and FPP alone had no effect on Cyp17a1 mRNA expression; however, the inhibitory action of simvastatin was partly abrogated by both GGPP and FPP. The present findings indicate that statin-induced reduction of androgen levels is likely due, at least in part, to the inhibition of isoprenylation, resulting in decreased expression of CYP17A1.
Keywords: androgens/androgen receptor, cell culture, CYP17A1, ovarian theca-interstitial cells, ovary, simvastatin, steroid hormones/steroid hormone receptors, steroidogenesis, theca cells
Simvastatin induces a dose- and time-dependent decrease in Cyp17a1 gene expression mediated, at least in part, by decreased isoprenylation.
INTRODUCTION
Simvastatin belongs to the family of statins, which are competitive inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, the rate-limiting enzyme in cholesterol synthesis. In addition to the beneficial effects of statins on cardiovascular diseases by decreasing the biosynthesis of cholesterol [1, 2], these agents possess a broad range of biological properties, including anti-inflammatory and antiproliferative effects on various tissues [3–5]. In vitro studies have shown that one of the statins, mevastatin, inhibits proliferation and steroidogenesis of rat ovarian theca-interstitial cells in a concentration-dependent fashion [6]. Another statin, lovastatin, induces a dose-dependent inhibition of androstenedione production by porcine theca cells [7]. These actions of statins may have clinical relevance to conditions associated with thecal hyperplasia and hyperandrogenism, such as polycystic ovary syndrome (PCOS), whereby statins may potentially reduce theca-interstitial growth and steroidogenesis. Indeed, several studies have evaluated the effects of statins on the endocrine profile of women with PCOS. In the first randomized clinical trial, simvastatin induced a significant reduction of total and free testosterone and normalization of gonadotropin levels [8]. In a subsequent randomized trial, other investigators found that another statin, atorvastatin, also reduced androgen levels and free androgen index in women with PCOS [9].
Rat ovarian theca cell androgen production is regulated, in part, by the modulation of the expression of several key enzymes involved in individual steps of steroidogenesis. The steroidogenic acute regulatory protein (STAR) initiates the process of steroidogenesis by transporting cholesterol from the outer to the inner mitochondrial membranes of the cell [10]. Subsequently, cholesterol is converted to pregnenolone through a reaction catalyzed by the cytochrome P450 side-chain cleavage (P450scc) enzyme encoded by the Cyp11a1 gene, which is located on the matrix side of the inner mitochondrial membrane [11]. Then, pregnenolone is transported to the smooth reticulum, where it is converted to progesterone by the action of 3β-hydroxysteroid dehydrogenase type 1 encoded by the Hsd3b1 gene. The conversions of pregnenolone to 17-hydroxypregnenolone and dehydroepiandrosterone and of progesterone to 17-hydroxyprogesterone and androstenedione are mediated by a single enzyme with dual activity: 17α-hydroxylase/17,20-lyase. This enzyme is encoded by the Cyp17a1 gene and is the key enzyme regulating androgen biosynthesis. Previous studies have shown that theca cells of women with PCOS are characterized by excessive expression of several genes involved in the regulation of steroidogenesis, including STAR, CYP11A1, HSD3B2, and CYP17A1 [12–14]. In particular, the overexpression of CYP17A1 explains the increased circulating levels of 17-hydroxyprogesterone in response to gonadotropin stimulation in these women [15].
Simvastatin, like other statins, is thought to act primarily by competitive inhibition of HMG-CoA reductase, an early step of mevalonate pathway; thus, the effects of statins may be related to a decreased availability of several downstream products of this pathway, such as substrates of isoprenylation (farnesyl-pyrophosphate [FPP] and geranylgeranyl-pyrophosphate [GGPP]), as well as to a reduction of cholesterol.
In view of these considerations, the present study was designed to evaluate the effects of simvastatin on rat theca-interstitial cell steroidogenesis and to determine the role of substrates of isoprenylation and cholesterol in this process.
MATERIALS AND METHODS
Animals
Female Sprague Dawley rats were obtained at 22 days of age from Charles River Laboratories and housed in an air-conditioned environment with a 12L:12D photoperiod. All animals received standard rat chow and water ad libitum. At the ages of 27, 28, and 29 days, the rats were injected with 17β-estradiol (1 mg per 0.3 ml of sesame oil s.c.) to stimulate ovarian development and growth of antral follicles. Twenty-four hours after the last injection, the animals were anesthetized using ketamine and xylazine (i.p.) and euthanized by intracardiac perfusion using 0.9% saline. All treatments and procedures were carried out in accordance with accepted standards of humane animal care as outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and a protocol approved by the Institutional Animal Care and Use Committee at the University of California, Davis.
Cell Culture and Reagents
The collection and purification of ovarian theca-interstitial cells were performed as described previously [16, 17]. Briefly, the ovaries were removed from the animals and dissected free of oviducts and fat under a dissecting microscope. After a 60-min collagenase digestion, theca-interstitial cells were purified using discontinuous Percoll gradient centrifugation. The cells were counted, and viability, as assessed by the trypan blue exclusion test, was routinely found to be in the 90%–95% range. Theca-interstitial cells were incubated in 24-well, fibronectin-coated plates at a density of 400 000 cells/well. The cultures were carried out for 12, 24, and 48 h at 37°C in an atmosphere of 5% CO2 in humidified air in serum-free McCoy 5A culture medium supplemented with 1% antibiotic/antimycotic mix, 0.1% bovine serum albumin, and 2 mM l-glutamine. The cells were incubated in the absence (control) or in the presence of simvastatin (1–10 μM), FPP (30 μM), GGPP (30 μM), farnesyltransferase inhibitor 277 (FTI; 10 μM), geranylgeranyltransferase inhibitor 298 (GGTI; 10 μM), and/or cell membrane- and mitochondrion-permeable 22-hydroxycholesterol (10 μM). The concentrations of these compounds were selected based on our previous studies evaluating effects of isoprenylation on growth of ovarian theca cells [18]. All cultures were carried out in the presence of luteinizing hormone (LH; 5 ng/ml). All the above-mentioned chemicals were purchased from Sigma Chemical Co. except for LH, which was obtained from the National Hormone & Pituitary Program at the Harbor-UCLA Medical Center.
Androstenedione, androsterone, progesterone, and the deuterated derivative of androsteneione-d7 were obtained from Steraloids, whereas testosterone-d3 was obtained from Cerillient. Acetonitrile and methanol were high-performance liquid chromatography (HPLC) grade and obtained from Burdick and Jackson. Acetone, isopropanol, and ammonium hydroxide were Optima grade and obtained from Fisher. Formic acid was ACS grade and obtained from EMD.
Total RNA Isolation and Quantitative Real-Time PCR
Total RNA was isolated using the MagMAX-96 Total RNA Isolation Kit (Applied Biosystems) and the KingFisher robot (Thermo Scientific). Reverse transcription of total RNA to cDNA was performed using High Capacity cDNA Reverse Transcription Kit for RT-PCR (Applied Biosystems). PCR runs were set up in 28-μl volumes, consisting of 5 μl of cDNA, 4.5 μl of forward and 4.5 μl of reverse 900 nM primers, and 14 μl of 2× SYBR Green PCR Master Mix (Applied Biosystems).
Quantitative real-time PCR reactions were performed in triplicate using the ABI 7300 Real-Time PCR System (Applied Biosystems). Separate cDNA dilutions were included in each PCR run to generate standard curves. Data were analyzed using SDS 1.4 software (Applied Biosystems). The relative amount of target mRNA was expressed as a ratio normalized to hypoxanthine phosphoribosyltransferase (Hprt). The primer sequences were as follows: for rat Star: forward, 5′-GCC TGA GCA AAG CGG TGT C-3′; reverse, 5′-CTG GCG AAC TCT ATC TGG GTC TGT-3′; for rat Cyp11a1: forward, 5′-GCT GGA AGG TGT AGC TCA GG-3′; reverse, 5′-CAC TGG TGT GGA ACA TCT GG-3′; for rat Hsd3b1: forward, 5′-CCA GAA ACC AAG GAG GAA T-3′; reverse, 5′-CCA GAA ACC AAG GAG GAA T-3′; for rat Cyp17a1: forward, 5′-ACT GAG GGT ATC GTG GAT GC-3′; reverse, 5′-CCG TCA GGC TGG AGA TAG AC-3′; and for rat Hprt: forward, 5′-TTG TTG GAT ATG CCC TTG ACT-3′; reverse, 5′-CCG CTG TCT TTT AGG CTT TG-3′.
Sample Preparation and Processing for Quantification of Steroids
Each sample was directly assayed; the following extraction procedure was applied to each specimen. Each sample aliquot (300 μl) was placed in a 2.0-ml autosampler vial and spiked with 150 μl of internal standard solution (i.e., androsteneione-d7 and testosterone-d3). Detection and quantitation of all analytes was accomplished using selective reaction monitoring (SRM).
Mass Spectrometry
We developed a novel turbulent flow chromatography (TFC) HPLC-tandem mass spectrometry (MS/MS) method that allowed the simultaneous detection of androstenedione, androsterone, and progesterone. It consists of an HPLC instrument configuration multiplexing Thermo Aria TLX-2 TFC (two loading pumps and two eluting pumps; LC-10AD; Shimadzu) system and an autosampler outfitted with a 300-position Peltier tray, coupled to a Thermo Scientific TSQ Vantage triple-quadrupole mass spectrometer, equipped with a heated electrospray ionization source. The instrument was controlled using Aria software (Version 1.6.1; Thermo Scientific). A Thermo Cyclone P extraction column (0.5 × 50 mm, 60-μm particle size; Thermo Scientific) was utilized for online sample extraction of diluted serum, and HPLC separation was carried out by a 2.1- × 100-mm, 3-μm particle size, ACE C18 column protected by a reverse-phase guard cartridge (Mac-Mod) contained within a Hot Pocket column heater (Thermo Scientific).
Precursor and product ions for each target analyte were chosen for SRM transitions, and the related parameters for the different analytes were isolated by HPLC separation based on the following mobile-phase gradient: solvent A, water containing 0.1% formic acid; solvent B, methanol; solvent C, acetonitrile/isopropyl alcohol/acetone (60:30:10 v/v/v); and solvent D, water/acetonitrile (98:2 v/v) with 0.1% ammonium hydroxide.
Detection and quantification employed SRM liquid chromatography (LC)-MS/MS transitions of initial precursor ions for androstenedione, androsterone, and progesterone mass-to-charge ratios (m/z) of 287.2, 291.4, and 315.2, respectively. The response for the major product ions for each of the analytes were plotted, and peaks at the proper retention time were integrated using LCquan software (Version 2.5; Thermo Scientific). This software was used to generate calibration curves and quantitate the analytes in all samples. The concentration of androstenedione, androsterone, and progesterone in each sample (e.g., calibrators, quality control, and unknowns) were determined by an internal standard method using the peak area ratio and linear regression analysis. The response for androstenedione, androsterone, and progesterone were linear and gave correlation coefficients (R2) of 0.99 or better.
Statistical Analysis
Statistical analysis was performed using JMP 8.0 software (SAS Institute). Data are presented as the mean ± SEM. Means were compared by analysis of variance followed by post-hoc testing using Tukey honestly significant difference test. When appropriate, data were logarithmically transformed. A value of P < 0.05 was considered to be statistically significant.
RESULTS
Effect of Simvastatin on Gene Expression of Steroidogenic Enzymes
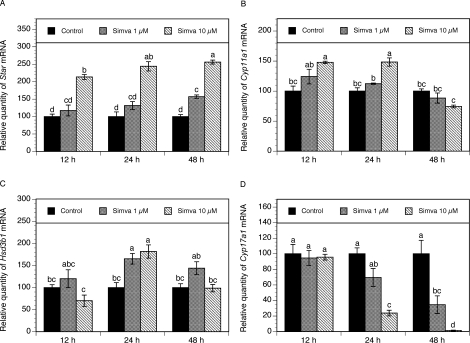
To determine whether simvastatin affects the expression of the key genes involved in the regulation of steroidogenesis, theca-interstitial cells were cultured for 12, 24, and 48 h in the absence or presence of simvastatin (1 and 10 μM). As presented in Figure 1A, simvastatin exerted a significant stimulatory effect on Star mRNA expression in a time- and dose-dependent manner. Simvastatin at 1 and 10 μM induced an approximately 1.5- and 2.5-fold increase of Star mRNA levels above the control level (P < 0.001) after 48 h of treatment. In the same experiments, simvastatin (1–10 μM) induced a modest increase in Cyp11a1 transcripts at 12 and 24 h, although statistical significance was noted only at the dose of 10 μM (P < 0.001). At 48 h, simvastatin no longer induced a significant effect on Cyp11a1 mRNA expression (Fig. 1B).
FIG. 1.
Time course of the effect of simvastatin (1 and 10 μM) on mRNA expression of Star (A), Cyp11a1 (B), Hsd3b1 (C), and Cyp17a1 (D). Theca-interstitial cells were cultured in chemically defined media supplemented with LH (5 ng/ml) for 12, 24, and 48 h in the absence (control) or in the presence of simvastatin. Total cellular RNA was isolated, and mRNA expression was determined using quantitative real-time PCR reactions and normalized to Hprt mRNA levels. Results are presented as a percentage of control. Each bar represents the mean ± SEM. Means with no superscripts in common are significantly different (P < 0.05).
Simvastatin had no significant effect on Hsd3b1 mRNA expression after 12 and 48 h. However, at 24 h, simvastatin at the doses of 1 and 10 μM induced a significant increase in Hsd3b1 transcripts of 65% (P < 0.01) and 81% (P < 0.05), respectively, above the control level (Fig. 1C).
Exposure to simvastatin at the doses of 1 and 10 μM led to profound decrease of Cyp17a1 mRNA expression in a dose- and time-dependent fashion. At the highest concentration (10 μM), simvastatin, at 24 and 48 h, induced a significant decrease in Cyp17a1 mRNA levels by 76% (P < 0.001) and 99% (P < 0.001), respectively, compared with control levels (Fig. 1D).
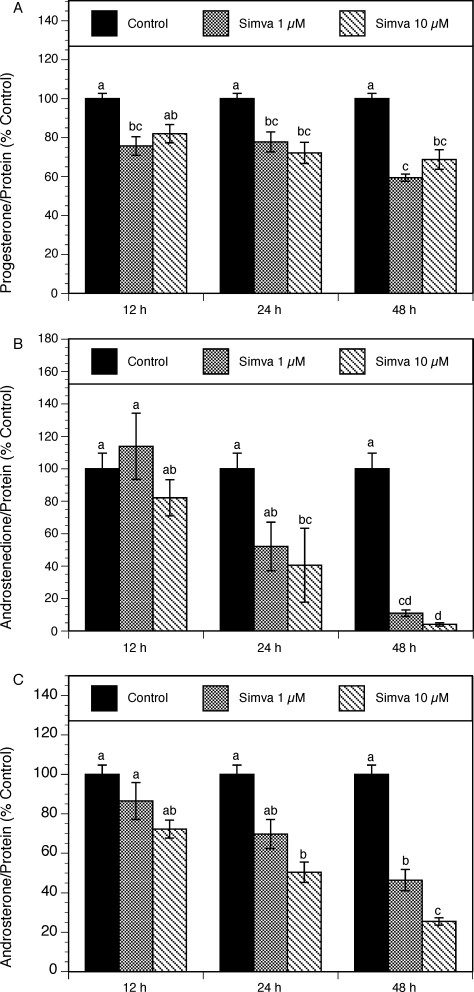
To evaluate the effects of simvastatin on individual steroids, the levels of progesterone, androstenedione, and androsterone were evaluated in spent media using LC-MS/MS. To account for simvastatin-related effects on the cell number, steroid production was expressed as percentage of control, per unit of protein content, in individual cultures. As shown in Figure 2A, in the presence of 10 μM simvastatin, progesterone levels decreased by 28% (P < 0.001) and 31% (P < 0.001) at 24 and 48 h, respectively.
FIG. 2.
Time course of the effect of simvastatin (1 and 10 μM) on steroid production by theca-interstitial cell cultures: progesterone (A), androstenedione (B), and androsterone (C). The cells were cultured in chemically defined media supplemented with LH (5 ng/ml) for 12, 24, and 48 h in the absence (control) or in the presence of simvastatin. Steroid levels were determined using LC-MS/MS. Results are presented as a percentage of control. Each bar represents the mean ± SEM. Means with no superscripts in common are significantly different (P < 0.05).
Simvastatin had the most profound effect on the reduction of androgen levels measured in spent culture media. Androstenedione levels declined in a time-dependent manner (Fig. 2B). After 48 h, simvastatin at 1 and 10 μM decreased androstenedione production by 89% (P < 0.05) and 96% (P < 0.001), respectively. Similarly, simvastatin at the doses of 1–10 μM induced a dose- and time-dependent decrease in androsterone levels (Fig. 2C). At 24 and 48 h, simvastatin at the highest concentration (10 μM) induced a significant inhibitory effect on androsterone production by 50% (P < 0.01) and 75% (P < 0.01), respectively.
Effect of Substrates of Isoprenylation
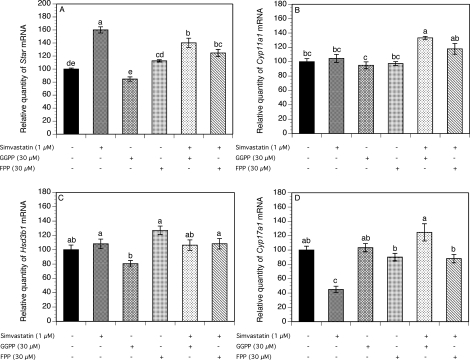
To test whether the effect of simvastatin on steroidogenesis is related to decreased isoprenylation, theca-interstitial cells were cultured for 48 h without (control) or with simvastatin (1 μM), GGPP (30 μM), and/or FPP (30 μM) (Fig. 3).
FIG. 3.
Effect of simvastatin (1 μM), GGPP (30 μM), and FPP (30 μM) on mRNA expression of Star (A), Cyp11a1 (B), Hsd3b1 (C), and Cyp17a1 (D). Theca-interstitial cells were cultured in chemically defined media supplemented with LH (5 ng/ml) for 48 h in the absence (control) or in the presence of simvastatin and/or GGPP or FPP. The mRNA expression was determined as described in Figure 1. Results are presented as a percentage of control. Each bar represents the mean ± SEM. Means with no superscripts in common are significantly different (P < 0.05).
Simvastatin increased Star mRNA expression by 60% above the control level (P < 0.001). Neither GGPP nor FPP had any significant effect on Star mRNA expression. However, the addition of GGPP and FPP to simvastatin-treated cultures resulted in a significant downregulation of Star mRNA levels by 20% (P < 0.001) and 36% (P < 0.001), respectively, below the level observed in the presence of simvastatin alone (Fig. 3A).
As shown in Figure 3, B and C, the addition of simvastatin, GGPP, or FPP alone had no effect on either Cyp11a1 or Hsd3b1 mRNA expression. However, the combination of simvastatin and GGPP increased Cyp11a1 transcripts by 33% (P < 0.001).
The exposure of cells to simvastatin significantly decreased the Cyp17a1 mRNA expression by 55% (P < 0.001). In contrast, GGPP alone and FPP alone had no significant effect on Cyp17a1 gene expression. However, in the presence of simvastatin, the addition of GGPP and FPP resulted in a reversal of the inhibitory effect of simvastatin on Cyp17a1 mRNA levels by 80% (P < 0.001) and 43% (P < 0.001), respectively, above the level detected in the presence of simvastatin alone (Fig. 3D).
As presented in Figure 4A, simvastatin induced a significant decrease in progesterone levels by 52% (P < 0.001), whereas GGPP alone modestly increased progesterone levels by 22% (P < 0.05). The addition of either GGPP or FPP to simvastatin-treated cultures resulted in significant reversal of the inhibitory effect of simvastatin on progesterone levels by 43% (P < 0.001) and 24% (P < 0.01), respectively, above the level detected in the presence of simvastatin alone.
FIG. 4.
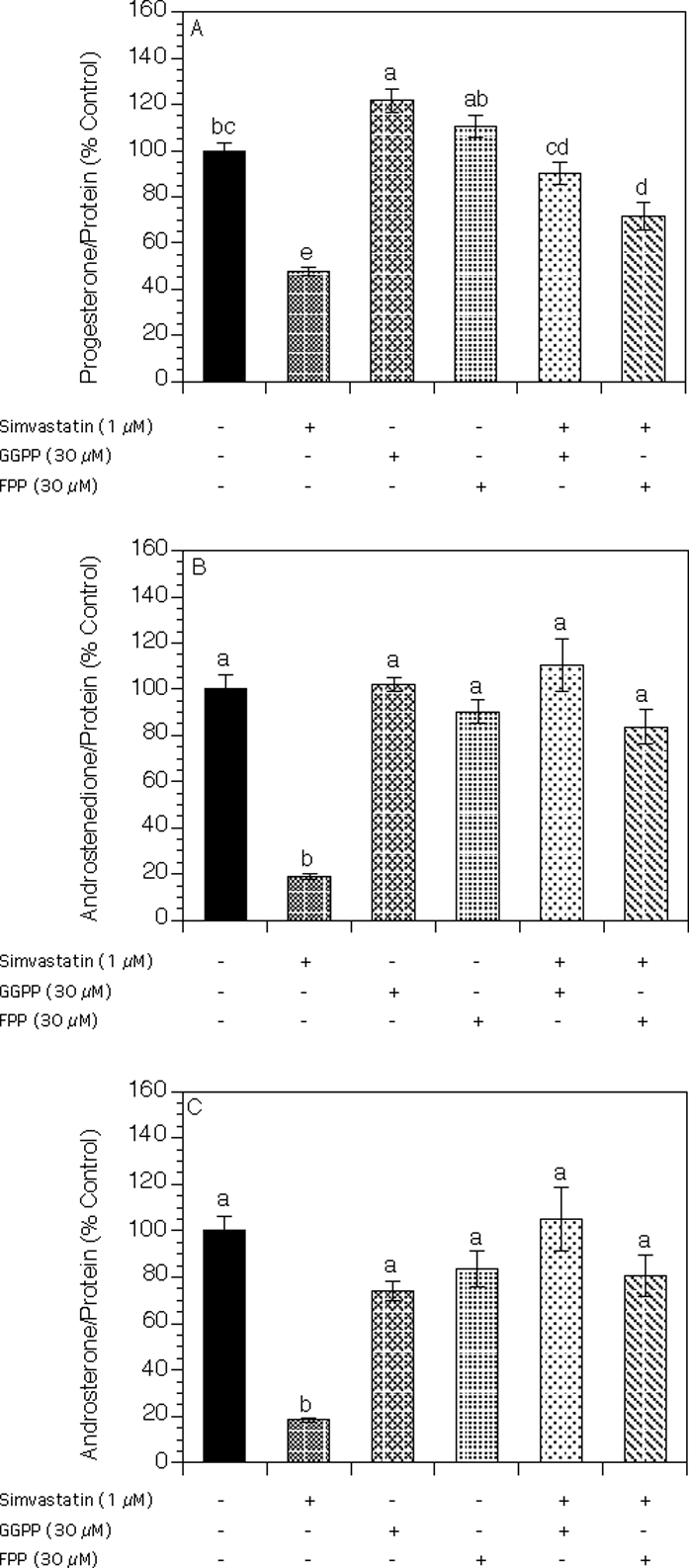
Effect of simvastatin (1 μM), GGPP (30 μM), and FPP (30 μM) on steroid production by theca-interstitial cells: progesterone (A), androstenedione (B), and androsterone (C). The cells were cultured in chemically defined media supplemented with LH (5 ng/ml) for 48 h in the absence (control) or in the presence of simvastatin and/or GGPP or FPP. Steroid levels were determined as described in Figure 2. Results are presented as a percentage of control. Each bar represents the mean ± SEM. Means with no superscripts in common are significantly different (P < 0.05).
Androstenedione and androsterone levels in the spent media from the same experiment are shown in Figure 4, B and C. Simvastatin significantly decreased androstenedione and androsterone levels by 81% (P < 0.001 for both). This inhibitory effect was reversed by the addition of either GGPP or FPP by 92% (P < 0.001) and 65% (P < 0.001), respectively, for androstenedione and by 87% (P < 0.001) and 62% (P < 0.001), respectively, for androsterone above the levels detected in the presence of simvastatin alone. GGPP or FPP alone had no significant effect on androgen levels.
Effects of Inhibitors of Isoprenylation and 22-Hydroxycholesterol
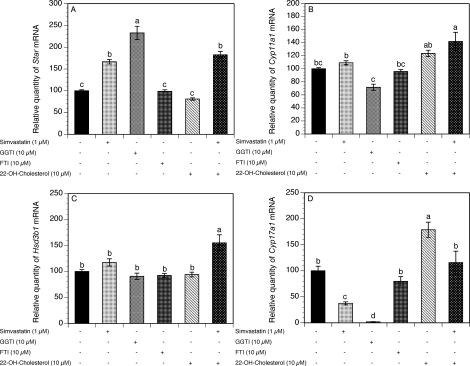
The inhibition of the mevalonate pathway by statins leads to decreased production of cholesterol as well as substrates for isoprenylation: FPP, which is required for farnesylation, and GGPP, which is required for geranylgeranylation. Because the effect of simvastatin on steroidogenesis may be due to a depletion of either of these downstream compounds, additional experiments were carried out to determine the effects of inhibitors of isoprenylation (GGTI and FTI) on the key genes regulating ovarian steroidogenesis. In the same set of experiments, we also tested the effects of cell- and mitochondrion permeable 22-hydroxycholesterol. Theca-interstitial cells were cultured for 48 h without additives (control) or with simvastatin (1 μM), GGTI (10 μM), FTI (10 μM), and/or 22-hydroxycholesterol (10 μM).
As shown in Figure 5A, both simvastatin and GGTI induced a significant stimulatory effect on Star mRNA expression by 66% (P < 0.001) and 133% (P < 0.001), respectively, above the control values. Addition of FTI or 22-hydroxycholesterol alone had no effect on Star mRNA expression, whereas the combination of 22-hydroxycholesterol and simvastatin significantly increased Star mRNA levels by 82% (P < 0.001).
FIG. 5.
Effects of simvastatin (1 μM), an inhibitor of farnesylation (FTI; 10 μM), an inhibitor of geranylgeranylation (GGTI; 10 μM), and 22-hydroxycholesterol (10 μM) on the mRNA expression of Star (A), Cyp11a1 (B), Hsd3b1 (C), and Cyp17a1 (D). The cells were cultured for 48 h in chemically defined media supplemented with LH (5 ng/ml) in the absence (control) or in the presence of simvastatin, GGTI, FTI, and/or 22-hydroxycholesterol. The mRNA expression was determined as described in Figure 1. Results are presented as a percentage of control. Each bar represents the mean ± SEM. Means with no superscripts in common are significantly different (P < 0.05).
The exposure of theca-interstitial cells to simvastatin, GGTI, FTI, or 22-hydroxycholesterol had no effect on either Cyp11a1 or Hsd3b1 mRNA expression (Fig. 5, B and C). Interestingly, the combination of simvastatin and 22-hydroxycholesterol induced a significant increase in both Cyp11a1 and Hsd3b1 transcripts by 42% (P < 0.001) and 55% (P < 0.001), respectively, above the control levels.
In the presence of either simvastatin or GGTI, Cyp17a1 mRNA expression was profoundly inhibited by 63% (P < 0.01) and 98% (P < 0.001), respectively, below the control values. The exposure of theca-interstitial cells to FTI had no significant effect on Cyp17a1 mRNA levels. In contrast, 22-hydroxycholesterol alone significantly induced an 80% increase in Cyp17a1 transcripts above the control level (P < 0.001); this effect was reversed by simvastatin by 63% (P < 0.01) below the level attained in the presence of 22-hydroxycholesterol alone (Fig. 5D).
As illustrated in Figure 6A, the addition of either simvastatin or GGTI to theca cell cultures significantly decreased progesterone levels by 45% (P < 0.001) and 25% (P < 0.05), respectively, whereas FTI had no significant effect on progesterone production. Interestingly, 22-hydroxycholesterol induced an 8-fold increase in progesterone levels (P < 0.001), whereas the addition of simvastatin had no effect compared to 22-hydroxycholesterol alone.
FIG. 6.
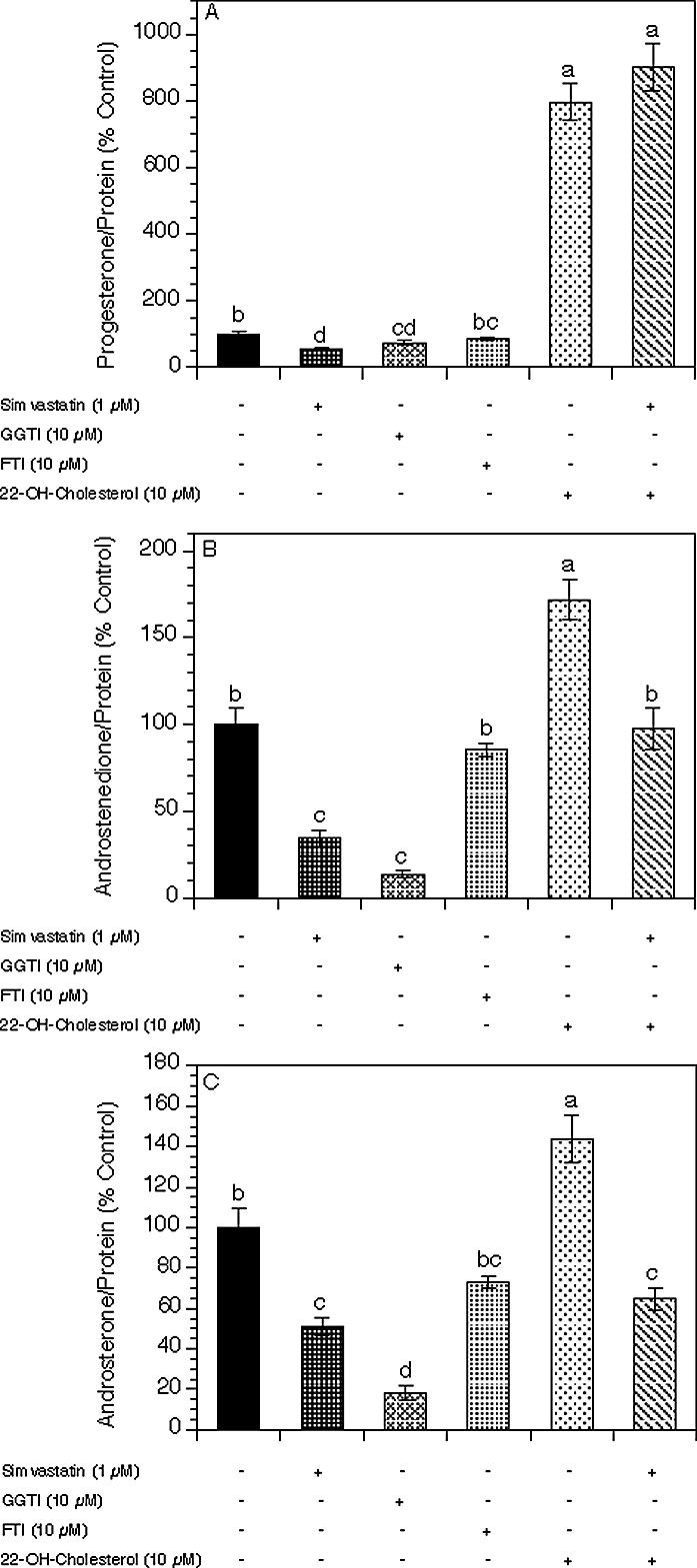
Effects of simvastatin (1 μM), an inhibitor of farnesylation (FTI; 10 μM), an inhibitor of geranylgeranylation (GGTI; 10 μM), and 22-hydroxycholesterol (10 μM) on production of progesterone (A), androstenedione (B), and androsterone (C) by theca-interstitial cells. The cells were cultured for 48 h in chemically defined media supplemented with LH (5 ng/ml) in the absence (control) or in the presence of simvastatin, GGTI, FTI, and/or 22-hydroxycholesterol. Steroid levels were determined as described in Figure 2. Results are presented as a percentage of control. Each bar represents the mean ± SEM. Means with no superscripts in common are significantly different (P < 0.05).
In the presence of simvastatin alone or GGTI alone, the androgen levels were significantly decreased (Fig. 6, B and C): Androstenedione levels declined by 65% (P < 0.001) and 86% (P < 0.001), respectively, whereas androsterone levels were decreased by 49% (P < 0.001) and 82% (P < 0.001), respectively. Whereas FTI had no effect on androgen production, 22-hydroxycholesterol increased both androstenedione and androsterone levels by 72% (P < 0.001) and 44% (P < 0.001), respectively. The addition of simvastatin abrogated the stimulatory effect of 22-hydroxycholesterol on androstenedione and androsterone production by 74% (P < 0.001) and 79% (P < 0.001), respectively, below the value attained in the presence of 22-hydroxycholesterol alone.
DISCUSSION
In the present study, we have demonstrated the following in cultures of theca-interstitial cells: 1) Simvastatin inhibits theca-interstitial cell production of androgens and, to a lesser degree, progesterone in a time- and dose-dependent manner; 2) the suppressive effect of simvastatin on androgen production is mediated by inhibition of Cyp17a1 mRNA expression; 3) simvastatin-induced inhibition of Cyp17a1 mRNA expression is partly abrogated by the addition of the precursors of isoprenylation and cholesterol; 4) Cyp17a1 mRNA levels are decreased by the inhibitor of isoprenylation GGTI; and 5) simvastatin inhibits progesterone production by reducing the supply of cholesterol.
Two principal factors influence the total amount of steroids secreted by the ovary: the total number of steroid producing cells in the ovary and the steroidogenic capacity of each cell. Our present and previous findings [6, 18–20] indicate that statins might alter ovarian steroidogenesis by reducing steroidogenic activity as well as decreasing the growth and, hence, the number of theca-interstitial cells. Our previous studies have shown that statins inhibit the proliferation of both rat and human theca-interstitial cells irrespective of the availability of cholesterol in both normal and PCOS ovaries [6, 20]. Furthermore, in clinical trials, statins, including simvastatin, have been shown to decrease ovarian androgen levels in patients with PCOS [21–23].
The present study provides new insights regarding the mechanisms underlying the inhibitory effects of statins on rat ovarian steroidogenesis and, especially, androgen production. The primary finding pertains to the demonstration of a simvastatin-induced decrease of androgen production and inhibition of the expression of a key regulator of the androgen biosynthesis pathway: the gene encoding CYP17A1. These inhibitory actions of simvastatin may be mediated by several mechanisms related to the inhibition of HMG-CoA reductase and, hence, a decreased production of several biologically active downstream products of the mevalonate pathway (Fig. 7), including cholesterol and substrates for isoprenylation (FPP and GGPP). Because the availability of cholesterol is essential for androgen production, the simvastatin-induced reduction of cholesterol level may explain, at least in part, the reduced levels of both androstenedione and androsterone in cultures of theca-interstitial cells. Indeed, in the present study, the addition of 22-hydroxycholesterol (a membrane- and mitochondrion-permeable analog of cholesterol) increased androgen levels as well as Cyp17a1 mRNA expression and abrogated the inhibitory effects of simvastatin.
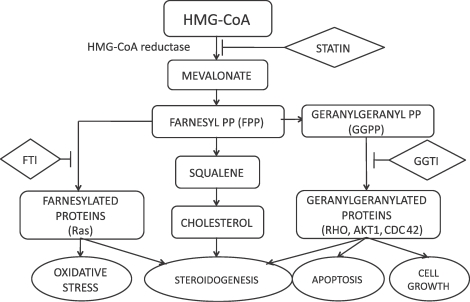
FIG. 7.
Outline of the mevalonate pathway. Conversion of acetoacetyl-coenzyme A to HMG-CoA to mevalonic acid is regulated by HMG-CoA reductase, the rate-limiting step of cholesterol synthesis. Statins competitively inhibit HMG-CoA reductase. FPP and GGPP are the intermediate downstream products of mevalonic acid. FPP is involved in farnesylation of proteins and is inhibited by FTI. GGPP is involved in geranylgeranylation of proteins and is inhibited by GGTI.
Another possible mechanism of action of simvastatin on the inhibition of steroidogenesis may be related to a decrease in isoprenylation substrates. This concept is consistent with the present findings, whereby both FPP and GGPP significantly reversed simvastatin-induced suppression of Cyp17a1 mRNA expression and androgen levels. Furthermore, these observations are verified by the observation that GGTI also decreased Cyp17a1 transcripts and androgen levels.
It should be noted, however, that the addition of FPP or GGPP alone had no significant effect on androgens and expression of Cyp17a1. We hypothesize that under basal conditions in the absence of inhibition by simvastatin, theca-interstitial cells may have a sufficient supply of isoprenylation substrates and, therefore, that a further increase in the supply of these compounds may have no significant effect on theca-interstitial steroidogenesis.
Isoprenylation consists of the attachment of lipophilic FPP (farnesylation) or GGPP (geranylgeranylation) to the carboxyl terminus of proteins [24]. These posttranslational modifications facilitate the membrane attachment and function of several families of proteins, including RAS, Ras-related GTP-binding proteins, and protein kinases [25]. These small GTPases modulate cell biological functions, such as proliferation, apoptosis, and oxidative stress. Nevertheless, the role of isoprenylation in steroidogenesis remains poorly characterized.
The blockade of farnesylation by simvastatin leads to a reduction of posttranslational modifications of several RAS family proteins and a subsequent decrease in the activity of the RAS-mitogen-activated protein kinase (MAPK) signaling pathway. Previously, the Kras gene has been shown to be associated with cell growth, synthesis, and secretion of cortisol in mutant KRAS-transfected human adrenocortical cells [26]. In that study, the upregulation of mRNA expression for Cyp11a1, Cyp17a1, and Hsd3b was reversed by lovastatin, a pharmacologic inhibitor of KRAS (p21ras) function. Furthermore, the same group demonstrated that KRAS influences the regulation of steroidogenesis in adrenocortical cells through the RAF-MEK-MAPK pathway [27]. Thus, it is tempting to speculate that in ovarian theca cells, the simvastatin-induced inhibition of farnesylation might affect Cyp17a1 mRNA expression through the RAS-MAPK pathway.
Another process that may play a key role in the regulation of ovarian androgen production is geranylgeranylation. In the present study, GGTI profoundly decreased Cyp17a1 mRNA expression and androgen levels compared to the decrease observed with simvastatin or FTI. Consequently, we hypothesize that geranylgeranylated proteins may be more relevant than farnesylated proteins in the modulation of theca-interstitial steroidogenesis. ROCK1, a serine/threonine kinase that phosphorylates the small G-protein RHOA, is involved in an increased androgen biosynthetic capacity of adrenocortical cells by phosphorylation of P450c17 [28], indicating that the RHO pathway plays a prominent role in the regulation of a key enzyme of steroidogenesis.
The present observations may be clinically relevant to situations associated with thecal hyperplasia and hyperandrogenism, such as PCOS. Because several studies have indicated that the increased activity of 17α-hydroxylase/17,20-lyase likely plays a significant role in PCOS-related androgen excess [13, 29], a simvastatin-induced decrease in CYP17A1 mRNA expression may be important in reducing the total androgen output secreted by the ovary in women with PCOS.
The present study demonstrated that simvastatin also decreased theca-interstitial cell progesterone production; however, this decrease was approximately one third of the decrease of androgen production. The relatively modest decline of progesterone may be due to the observed simvastatin-induced increase in Star mRNA expression. We propose that the simvastatin-induced inhibition of progesterone levels is related primarily to a reduced supply of cholesterol and, to a lesser degree, to changes in isoprenylation. In support of this concept, we observed that the exposure of simvastatin-treated theca-interstitial cells to 22-hydroxycholesterol induced an 8-fold increase of progesterone levels above the level measured in the presence of simvastatin alone, whereas addition of GGPP and FPP induced a relatively minor increase of progesterone (by 42% and 24%, respectively). In contrast, changes in isoprenylation appear to have a more important role than the availability of cholesterol in the regulation of androgen production. Indeed, in theca-interstitial cell cultures, the addition of GGTI led to a significant decrease in both androstenedione and androsterone levels (by 86% and 82%, respectively), whereas 22-hydroxycholesterol significantly increased androstenedione and androsterone levels (by 72% and 44%, respectively). These findings indicate that different mechanisms regulate androgen and progesterone production by theca-interstitial cells. Overall, a relatively modest inhibitory effect of simvastatin on progesterone level, when compared to a large effect on androgens, may be related to a combination of simvastatin-induced reduction of cholesterol as well as to an increase in mRNA expression of Cyp11a1 and Hsd3b1 (observed at 24 h), leading to increased conversion of pregnenolone to progesterone. However, it should be noted that relative effects of agents such as GGTI or 22-hydroxycholesterol should be interpreted with caution, in particular because only a single dose of each of these compounds was tested in the present study.
Steroidogenic acute regulatory protein is synthesized in response to tropic hormones and mediates the transfer of cholesterol from the outer to the inner mitochondrial membrane, the rate-limiting step in steroidogenesis [30]. In the present study, simvastatin increased Star mRNA expression in a dose- and time-dependent manner. We speculate that simvastatin-induced upregulation of Star transcripts might be due to mechanisms involving the sterol regulatory element (SRE)-binding protein (SREBP) pathway. The members of this transcription factor family (SREPB-1A, -1c, and -2) are released under conditions of sterol depletion and play a prominent role in the regulation of the expression of sterol-sensitive genes closely related to cholesterol metabolism [31]. Previously, rat Star promoter has been shown to contain five SRE sites, which demonstrated specific binding with recombinant SREBP-1a [32]. Therefore, simvastatin-induced depletion of intracellular cholesterol might trigger the release of mature SREBP and the subsequent activation of Star promoter, increasing Star mRNA levels.
In summary, the present results suggest that simvastatin inhibits theca-interstitial steroidogenesis primarily by inhibiting Cyp17a1 mRNA expression in a dose- and time-dependent fashion and that this suppressive effect of simvastatin on steroidogenesis is mediated, at least in part, by mechanisms involving decreased isoprenylation. Furthermore, the present study suggests novel mechanisms of differentially controlled androgen and progesterone production in theca cells.
Footnotes
Supported by grant R01-HD050656 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (to A.J.D.).
REFERENCES
- Arca M. Atorvastatin efficacy in the prevention of cardiovascular events in patients with diabetes mellitus and/or metabolic syndrome. Drugs 2007; 67 (suppl 1): 43 54 [DOI] [PubMed] [Google Scholar]
- Matoba T, Egashira K. Statins in the management of acute coronary syndrome [in Japanese]. Nippon Rinsho 2010; 68: 692 698 [PubMed] [Google Scholar]
- Sokalska A, Wong DH, Cress A, Piotrowski PC, Rzepczynska I, Villanueva J, Duleba AJ. Simvastatin induces apoptosis and alters cytoskeleton in endometrial stromal cells. J Clin Endocrinol Metab 2010; 95: 3453 3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassan M, Montero MJ, Sevilla MA. In vitro antioxidant activity of pravastatin provides vascular protection. Eur J Pharmacol 2010; 630: 107 111 [DOI] [PubMed] [Google Scholar]
- Leung BP, Sattar N, Crilly A, Prach M, McCarey DW, Payne H, Madhok R, Campbell C, Gracie JA, Liew FY, McInnes IB. A novel anti-inflammatory role for simvastatin in inflammatory arthritis. J Immunol 2003; 170: 1524 1530 [DOI] [PubMed] [Google Scholar]
- Izquierdo D, Foyouzi N, Kwintkiewicz J, Duleba AJ. Mevastatin inhibits ovarian theca-interstitial cell proliferation and steroidogenesis. Fertil Steril 2004; 82 (suppl 3): 1193 1197 [DOI] [PubMed] [Google Scholar]
- Engelhardt H, Gore-Langton RE, Armstrong DT. Mevinolin (lovastatin) inhibits androstenedione production by porcine ovarian theca cells at the level of the 17alpha-hydroxylase:C-17,20-lyase complex. Endocrinology 1989; 124: 2297 2304 [DOI] [PubMed] [Google Scholar]
- Duleba AJ, Banaszewska B, Spaczynski RZ, Pawelczyk L. Simvastatin improves biochemical parameters in women with polycystic ovary syndrome: results of a prospective, randomized trial. Fertil Steril 2006; 85: 996 1001 [DOI] [PubMed] [Google Scholar]
- Sathyapalan T, Kilpatrick ES, Coady AM, Atkin SL. The effect of atorvastatin in patients with polycystic ovary syndrome: a randomized double-blind placebo-controlled study. J Clin Endocrinol Metab 2009; 94: 103 108 [DOI] [PubMed] [Google Scholar]
- Kahsar-Miller MD, Conway-Myers BA, Boots LR, Azziz R. Steroidogenic acute regulatory protein (StAR) in the ovaries of healthy women and those with polycystic ovary syndrome. Am J Obstet Gynecol 2001; 185: 1381 1387 [DOI] [PubMed] [Google Scholar]
- Lambeth JD, Xu XX, Glover M. Cholesterol sulfate inhibits adrenal mitochondrial cholesterol side chain cleavage at a site distinct from cytochrome P-450scc. Evidence for an intramitochondrial cholesterol translocator. J Biol Chem 1987; 262: 9181 9188 [PubMed] [Google Scholar]
- McAllister JM, Kerin JF, Trant JM, Estabrook RW, Mason JI, Waterman MR, Simpson ER. Regulation of cholesterol side-chain cleavage and 17alpha-hydroxylase/lyase activities in proliferating human theca interna cells in long term monolayer culture. Endocrinology 1989; 125: 1959 1966 [DOI] [PubMed] [Google Scholar]
- Wickenheisser JK, Nelson-Degrave VL, McAllister JM. Dysregulation of cytochrome P450 17alpha-hydroxylase messenger ribonucleic acid stability in theca cells isolated from women with polycystic ovary syndrome. J Clin Endocrinol Metab 2005; 90: 1720 1727 [DOI] [PubMed] [Google Scholar]
- Jakimiuk AJ, Weitsman SR, Navab A, Magoffin DA. Luteinizing hormone receptor, steroidogenesis acute regulatory protein, and steroidogenic enzyme messenger ribonucleic acids are overexpressed in thecal and granulosa cells from polycystic ovaries. J Clin Endocrinol Metab 2001; 86: 1318 1323 [DOI] [PubMed] [Google Scholar]
- Wachs DS, Coffler MS, Malcom PJ, Shimasaki S, Chang RJ. Increased androgen response to follicle-stimulating hormone administration in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2008; 93: 1827 1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magoffin DA, Erickson GF. Purification of ovarian theca-interstitial cells by density gradient centrifugation. Endocrinology 1988; 122: 2345 2347 [DOI] [PubMed] [Google Scholar]
- Duleba AJ, Spaczynski RZ, Olive DL, Behrman HR. Effects of insulin and insulin-like growth factors on proliferation of rat ovarian theca-interstitial cells. Biol Reprod 1997; 56: 891 897 [DOI] [PubMed] [Google Scholar]
- Rzepczynska IJ, Piotrowski PC, Wong DH, Cress AB, Villanueva J, Duleba AJ. Role of isoprenylation in simvastatin-induced inhibition of ovarian theca-interstitial growth in the rat. Biol Reprod 2009; 81: 850 855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwintkiewicz J, Foyouzi N, Piotrowski P, Rzepczynska I, Duleba AJ. Mevastatin inhibits proliferation of rat ovarian theca-interstitial cells by blocking the mitogen-activated protein kinase pathway. Fertil Steril 2006; 86: 1053 1058 [DOI] [PubMed] [Google Scholar]
- Sokalska A, Piotrowski PC, Rzepczynska IJ, Cress A, Duleba AJ. Statins inhibit growth of human theca-interstitial cells in PCOS and non-PCOS tissues independently of cholesterol availability. J Clin Endocrinol Metab 2010; 95: 5390 5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banaszewska B, Pawelczyk L, Spaczynski RZ, Duleba AJ. Comparison of simvastatin and metformin in treatment of polycystic ovary syndrome: prospective randomized trial. J Clin Endocrinol Metab 2009; 94: 4938 4945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya C, Pabuccu R, Cengiz SD, Dunder I. Comparison of the effects of atorvastatin and simvastatin in women with polycystic ovary syndrome: a prospective, randomized study. Exp Clin Endocrinol Diabetes 2010; 118: 161 166 [DOI] [PubMed] [Google Scholar]
- Banaszewska B, Pawelczyk L, Spaczynski RZ, Dziura J, Duleba AJ. Effects of simvastatin and oral contraceptive agent on polycystic ovary syndrome: prospective, randomized, crossover trial. J Clin Endocrinol Metab 2007; 92: 456 461 [DOI] [PubMed] [Google Scholar]
- Zhang FL, Casey PJ. Protein prenylation: molecular mechanisms and functional consequences. Annu Rev Biochem 1996; 65: 241 269 [DOI] [PubMed] [Google Scholar]
- Zhang FL, Casey PJ. Influence of metal ions on substrate binding and catalytic activity of mammalian protein geranylgeranyltransferase type-I. Biochem J 1996; 320 (pt 3): 925 932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CH, Lee SC, Chiu HH, Yang YC, Lian ST, Shin SJ, Lin SR. Morphologic change and elevation of cortisol secretion in cultured human normal adrenocortical cells caused by mutant p21K-ras protein. DNA Cell Biol 2002; 21: 21 29 [DOI] [PubMed] [Google Scholar]
- Wu CH, Chen YF, Wang JY, Hsieh MC, Yeh CS, Lian ST, Shin SJ, Lin SR. Mutant K-ras oncogene regulates steroidogenesis of normal human adrenocortical cells by the RAF-MEK-MAPK pathway. Br J Cancer 2002; 87: 1000 1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tee MK, Dong Q, Miller WL. Pathways leading to phosphorylation of p450c17 and to the posttranslational regulation of androgen biosynthesis. Endocrinology 2008; 149: 2667 2677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JM, Lee EJ, Ramakrishna S, Cha DH, Baek KH. Association study for single nucleotide polymorphisms in the CYP17A1 gene and polycystic ovary syndrome. Int J Mol Med 2008; 22: 249 254 [PubMed] [Google Scholar]
- Manna PR, Dyson MT, Stocco DM. Regulation of the steroidogenic acute regulatory protein gene expression: present and future perspectives. Mol Hum Reprod 2009; 15: 321 333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato R. Sterol metabolism and SREBP activation. Arch Biochem Biophys 2010; 501: 177 181 [DOI] [PubMed] [Google Scholar]
- Shea-Eaton WK, Trinidad MJ, Lopez D, Nackley A, McLean MP. Sterol regulatory element binding protein-1a regulation of the steroidogenic acute regulatory protein gene. Endocrinology 2001; 142: 1525 1533 [DOI] [PubMed] [Google Scholar]