ABSTRACT
FYN kinase is highly expressed in the testis and has been implicated in testis and sperm function, yet specific roles for this kinase in testis somatic and germ cells have not been defined. The purpose of the present investigation was to identify aspects of spermatogenesis, spermiation, or sperm fertilizing capacity that required FYN for normal reproductive function. Matings between Fyn-null males and wild-type females resulted in normal litter sizes, despite the fact that Fyn-null males exhibited reduced epididymal size and sperm count. Morphological analysis revealed a high frequency of abnormal sperm morphology among Fyn-null sperm, and artificial insemination competition studies demonstrated that Fyn-null sperm possessed reduced fertilizing capacity. Fyn-null sperm exhibited nearly normal motility during capacitation in vitro but reduced ability to undergo the acrosome reaction and fertilize oocytes. The typical pattern of capacitation-induced protein tyrosine phosphorylation was slightly modified in Fyn-null sperm, with reduced abundance of several minor phosphoproteins. These findings are consistent with a model in which FYN kinase plays an important role in proper shaping of the head and acrosome within the testis and possibly an additional role in the sperm acrosome reaction, events required for development of full fertilizing capacity in sperm.
Keywords: capacitation, gamete biology, gametogenesis, kinase, spermatogenesis
Fyn tyrosine kinase plays an important role in the morphological development of spermatozoa and in their ability to undergo the acrosome reaction and to fertilize eggs.
INTRODUCTION
The Src protein tyrosine kinase (PTK)-encoding gene family includes nine members that share extensive structural homology, yet with a highly variable N-terminal domain conferring unique character to each family member. The Src gene family kinases have been associated with multiple aspects of sperm and testis function [1–3], and an understanding of their specific actions in the testis and in mature sperm may present unique opportunities for contraceptive development and provide insight into the cause of human male infertility. The Src gene family kinases exhibit distinct expression profiles in the testis, and the four (Src, Yes, Fyn, and Hck) gene family members have been implicated in various aspects of testis or sperm function. In the testis, both the SRC and the structurally similar YES kinases are associated with basal and apical ectoplasmic specializations (ES junctions) of Sertoli cells and are important for junctional dynamics [4–6]. FYN kinase is known to be expressed in two isoforms in the testis: a catalytically active 59-kDa form present in Sertoli cells and spermatozoa and a 22-kDa truncated form (tr-FYN) found in Sertoli cells and spermatids [7]. FYN also is associated with ES junctions in association with actin filaments bundled within these junctions, and structural analysis of the Fyn-null testis has revealed significant ultrastructural defects in apical ES junctions [8], suggesting that FYN may play a role in maintaining junction stability. In addition, expression and localization of tr-FYN in round and elongating spermatids led to the hypothesis that tr-FYN may function in sperm head morphogenesis and acrosomal development [7]. While the above-described functional roles of FYN are supported by circumstantial evidence, no functional evidence has confirmed either role. To date, functional studies of the role of Src gene family kinases during spermatogenesis, sperm capacitation, and acrosome reaction have relied on the use of kinase inhibitors [4, 6, 9–11]. While pharmacological evidence supports a role for the Src gene family PTKs in ES junctions, the limited specificity of these inhibitors does not allow one to distinguish between the specific roles of SRC, YES, or FYN kinases. Single-gene knockout mouse models provide a more specific approach to defining the functions of individual Src gene family members, and, while an earlier study demonstrated that Fyn-null mice exhibit slower testis development and ES junctional defects [8], the fact that simple mating trials failed to detect infertility in Fyn and other Src family knockout males has brought into question whether this kinase family plays an important role in spermatogenesis or in sperm function. The objective of the present study was to apply more definitive tests of sperm function to determine whether sperm from Fyn-null males exhibited normal fertilizing capacity in competitive artificial insemination and in vitro fertilization (IVF) trials. Results showed that while Fyn-null males are technically fertile, a significant fraction of the sperm they produce exhibits morphological and functional defects, resulting in decreased fertilizing capacity.
MATERIALS AND METHODS
Animal Source and Care
CF1 female mice, 6–7 wk old, were obtained from Harlan Sprague Dawley, Inc. (Frederick, MD). Fyn-null mice (B6;129S7-Fyntm1Sor/J strain) [12] and the wild-type (WT) mice used as controls (B6;129SF2/J strain) were obtained from The Jackson Laboratory (Bar Harbor, ME) and maintained as described previously [13]. Housing standards and policies, as well as standard operating procedures regarding handling of mice, were approved by the Institutional Animal Care and Use Committee at the University of Kansas Medical Center and were in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals.
Male Breeding Test
Four Fyn-null or WT males, 8–15 wk old, were each caged with two 8-wk-old CF1 females for a period of 14 days. Females were then euthanized on day 14 after breeding to determine whether they were pregnant and to quantify the number of fetuses produced.
Sperm Competition In Vivo
Sperm competition assays were performed via artificial insemination to evaluate the ability of sperm from Fyn-null males to negotiate the female reproductive tract and fertilize eggs in a way that ruled out other physical and behavioral factors typical of this strain of mice. Cauda epididymal sperm from WT and Fyn-null males (approximately 10 wk old) were released into 0.4 ml of 0.85% sodium chloride by squeezing tubules with a 26-gauge needle. Sperm were incubated in air at 25°C for 10 min and then counted in a hemocytometer. For artificial insemination, a 1:1 mixture of Fyn-null and WT sperm was prepared at a final concentration of 100 × 106 sperm cells/ml. CF1 females were induced to ovulate by intraperitoneal injection of 5 IU of pregnant mare serum gonadotropin (Sigma-Aldrich, St. Louis, MO), followed 48 h later by 5 IU of human chorionic gonadotropin (Sigma-Aldrich, St. Louis, MO). Artificial insemination was carried out using 50 μl of sperm mixture per female as described previously [14, 15]. Sperm mixtures from three pairs of Fyn-null and WT males were used to artificially inseminate a total of 19 WT females, 7 of which became pregnant. At day 15 after artificial insemination, all females were euthanized to determine whether they were pregnant. Any fetuses present were collected for genotyping, which was performed as suggested by the Jackson Laboratory by using the sense primer 5′-CTT GGG TGG AGA GGC TAT TC-3′ (oIMR0013) and antisense primer 5′-AGG TGA GAT GAC AGG AGA TC-3′ (oIMR0014) to amplify a 280-bp fragment of the neomycin gene from Fyn-null sperm-derived fetus samples. As an internal control, the sense primer 5′-TGT GTG TCC TAC TGT GAA ACC C-3′ (oIMR0172) and antisense primer 5′-GCA TCC TTG ACC TAG TTT CAC-3′ (oIMR0173) were used to generate a 103-bp fragment in exon 7 of the Yes1 gene (National Center for Biotechnical Information accession no. NM_009535.2) from all samples. Fetuses containing the neomycin insert were considered derived from Fyn-null sperm and were classified as Fyn-null heterozygotes (Fyn+/−).
Scanning Electronic Microscopy
Cauda epididymal sperm were collected in Tyrode solution [16] without bovine serum albumin (BSA), incubated at 37°C for 10 min, and then diluted and loaded onto a coverslip coated with 0.01% poly-l-lysine (Sigma-Aldrich, St. Louis, MO) and incubated at room temperature for 10 min to allow sperm to attach to the coverslips. After coverslips were washed to remove unbound sperm, 1 ml of potassium simplex optimized medium with amino acids (Millipore Corp., Phillipsburg, NJ) was applied to each coverslip, followed by 1 ml of ice-cold 2% glutaraldehyde in 0.15 M cacodylate buffer (pH7.4). Samples were coded and scored blindly as described previously [17] and then examined using scanning electron microscopy (SEM) as previously described [18].
In Vitro Fertilization
Cauda epididymal sperm from adult Fyn-null or WT mice were collected, capacitated, and used to perform IVF of WT oocytes as described previously [18]. Standard (1 × 105 sperm/ml–3 × 105 sperm/ml) or higher (8-fold) concentrations of Fyn-null sperm were used during IVF to assess the functionality of sperm. Degrees of sperm-zona binding and -zona penetration and rates of fertilization were determined by confocal fluorescence microscopy examination of oocytes recovered from IVF. Meiotic status was monitored by staining chromatin with ethidium homodimer to label DNA. The position of bound sperm heads relative to the egg surface was monitored by staining the eggs with rhodamine-phalloidin (Molecular Probes-Invitrogen, Eugene, OR) to stain the cortical actin layer.
Computer-Assisted Sperm Analysis
Cauda epididymal sperm released from Fyn-null or WT males were suspended in 0.5 ml of noncapacitating medium (Tyrode solution without NaHCO3 and BSA) or capacitating medium (Tyrode solution containing 25 mM Na2HCO3 and 4 mg/ml BSA [Sigma-Aldrich]). Samples were incubated for 90 min at 37°C in a humidified atmosphere of 5% CO2 in air and labeled with 156 nM SYTO 21 live-cell nucleic acid stain (Molecular Probes, Inc., Eugene, OR). To prevent sperm from binding to chambered slides (Leja Products B.V., The Netherlands), 0.2 mg/ml polyvinyl alcohol (average molecular weight, 30 000–70 000 [Sigma-Aldrich]) was added to the samples. Sperm (2 μl) were loaded into the slide chambers and analyzed using the Sperm Vision computer-assisted sperm analysis (CASA) system operated by Sperm Vision PRISM version 3.5 software (Minitube of America, Inc.,Verona, WI). The percentages of sperm (average, 1700 spermatozoa per sample analyzed) displaying total, progressive, local, and hyperactive motility were recorded.
Acrosome Reaction
The capacity of cauda epididymal sperm to undergo spontaneous acrosome reaction was compared with their response to calcium ionophore-induced (A23187; Sigma-Aldrich) acrosome reaction by analysis with a FACSCalibur cytometer (Becton Dickinson) as described previously [19]. Briefly, sperm isolated in Tyrode solution containing 4 mg/ml BSA were incubated for 90 min at 37°C in a humidified atmosphere of 5% CO2 in the presence or absence of 10 μM A23187 to induce acrosome reaction. After incubation, sperm were collected by centrifugation and labeled with 1 μg/ml peanut agglutinin-fluorescein isothiocyanate (PNA-FITC; Sigma-Aldrich) and then with PBS containing 8 μg/ml propidium iodide (PI; Molecular Probes). Percentages of PI-negative but PNA-FITC-positive sperm were recorded as having undergone the acrosome reaction.
Flow Cytometric Analysis of Spermatogenesis
Cytometric analysis was performed as previously described [20]. Briefly, testes from 8- to 9-wk-old Fyn-null and WT males (n = 5) were sequentially digested, first with 1 mg/ml collagenase (type IA; Sigma-Aldrich) and then with 1× trypsin-EDTA solution (Sigma-Aldrich, St. Louis, MO), to dissociate individual cells. After cells were fixed in 70% ethanol, they were stained with 50 μg/ml PI in the presence of 100 μg/ml RNase A (DNase and protease-free; Fermentas, Inc., Glen Burnie, MD) for 30 min at 37°C. Samples were filtered through a 70-μm cell strainer (BD Biosciences Discovery Labware, Bedford, MA), and cell subpopulations in 50 000 events per sample according to DNA content were analyzed with a FACSCalibur cytometer (Becton Dickinson). Quantitation of testicular cells in condensed haploid (the population of elongated spermatids with reduced uptake of stain due to condensation of nuclear DNA), haploid, diploid, S phase, and tetraploid categories was recorded.
Western Blotting
Testis lysates were prepared from Fyn-null and WT males, 8–10 wk old, by homogenization in 2.0 ml of RIPA buffer (containing 10 mM Tris-HCl, pH7.5, 158 mM NaCl, 1% sodium deoxycholate, 0.1% SDS, 1% Triton X-100, 5 mM EDTA, 1mM Na3VO4, 0.1 mM diisopropylfluorophosphate, and a protease inhibitor cocktail [Roche Diagnostics, Indianapolis, IN]). Homogenates were incubated on ice for 30 min and then centrifuged at 25 000 × g for 30 min at 4°C, and supernatants were collected for Western blot analysis. Antibodies used to probe the blots included rabbit anti-FYN (1:1000 dilution; FYN3; Santa Cruz Biotechnology, Inc.), mouse anti-PLZF (1:200 dilution; Calbiochem), rat anti-GCNA1 (1:50 dilution; a kind gift from Prof. George C. Enders, University of Kansas Medical Center), rabbit anti-DDX4 (1 μg/ml; Abcam, Cambridge, MA), mouse antiphosphotyrosine (anti-P-Tyr; 1:1000 dilution; clone 4G10; Millipore, Billerica, MA), mouse anti-beta tubulin (1:1000 dilution; Sigma-Aldrich), and mouse anti-GAPDH (1:2000 dilution; Abcam).
Statistical Analysis
Statistical variance between Fyn-null and WT samples was analyzed by t-test performed with SigmaStat software (Jandel Scientific, San Rafael, CA) to determine whether sample values were significantly different (a P value of ≤0.05 was considered significant).
RESULTS
Effect of Fyn Knockout on the Testis
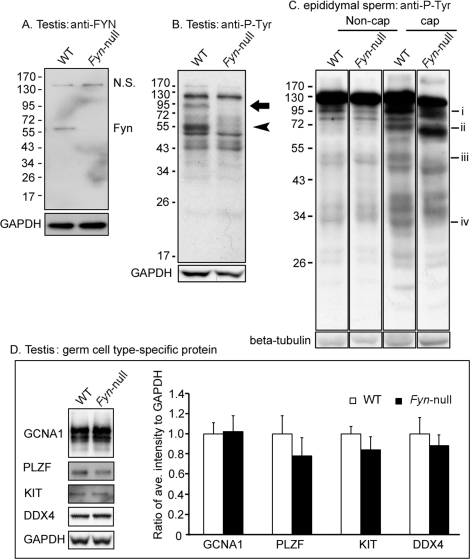
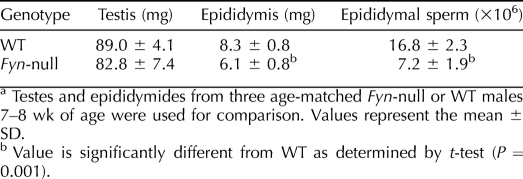
The B6;129S7-Fyntm1Sor/J strain is fertile, allowing continuation of the homozygous Fyn-null mouse line. However, because a detailed analysis of Fyn-null oocytes revealed significant defects in oocyte quality that provided insight into the function of this kinase in the female gamete [13], it was important to identify potential defects in gametogenesis and gamete quality in the Fyn-null male as well. Western blot analysis confirmed that testicular cells from the Fyn-null mice lacked detectable levels of the 59-kDa FYN protein (Fig. 1A), as has been previously demonstrated in other tissues [12]. To characterize the reproductive organs involved in gamete production in Fyn-null males, we analyzed testis and epididymal weight as well as epididymal sperm counts from 7- to 8-wk-old males. Results presented in Table 1 demonstrate the fact that testis weight in Fyn-null males was not significantly different from that in controls; however, epididymal weight and sperm count were significantly lower than those of controls.
FIG. 1.
Western blot analysis of Fyn-null testis and spermatozoa is shown. Testes were homogenized in RIPA buffer, and sperm were lysed in Laemmli sample buffer. After centrifugation, soluble protein was analyzed by Western blotting as described in Materials and Methods. A) The expression levels of FYN kinase in the testis of WT and Fyn-null mice were detected with an anti-FYN antibody (NS, nonspecific band). B) Patterns of P-Tyr-containing proteins in WT and Fyn-null testes were detected with anti-P-Tyr antibody. Anti-GAPDH was used as a loading control. An arrow (close to 95 kDa) and arrowhead (approximately 55 kDa) indicate P-Tyr-containing proteins in the WT but not in the Fyn-null sample. C) Patterns of P-Tyr-containing proteins in WT and Fyn-null sperm were detected in noncapacitating (Non-cap) and capacitating (cap) sperm by using an anti-P-Tyr antibody. P-Tyr-containing proteins at ∼95 kDa (i), ∼72 kDa (ii), ∼50 kDa (iii), or ∼30 kDa (iv) exhibited reduced antibody binding in Fyn-null capacitating sperm relative to those of WT. D) Analysis of the expression levels of different germ cell type-specific proteins in the testis is shown. Testis proteins from WT and Fyn-null males were analyzed by Western blotting with antibodies to GCNA1 (representing germ cells, except for elongating spermatids), PLZF (spermatogonial stem cells or undifferentiated spermatogonia), KIT and DDX4 (differentiating/differentiated germ cells), and GAPDH (a loading control). The bar graph represents the ratios between average intensity of identified protein band and that of GAPDH and WT control. Values represent means ± SD for six testes in a Fyn-null or WT group. Comparison by t-test indicated that individual expression levels of the four analyzed proteins of Fyn-null mice were not significantly different from those of WT mice.
TABLE 1.
Comparison of testis and epididymis weight and epididymal sperm counts.a
Functional evidence that knockout of FYN kinase had biochemical effects on the testis was provided by Western blot analysis of potential targets for tyrosine kinases, as assessed with an anti-P-Tyr antibody. The pattern of P-Tyr-containing testis proteins from Fyn-null males was characterized by the absence of 55- and 95-kDa bands that were easily detectable in the WT testis (Fig. 1B). Similar analysis of epididymal sperm revealed reduced P-Tyr content of a ∼95-kDa band in sperm from Fyn-null males (Fig. 1C). Capacitation of Fyn-null sperm in vitro triggered a general increase in the abundance of P-Tyr in many proteins, as normally seen in WT sperm. However, capacitated Fyn-null sperm did exhibit minor differences such as decreases in P-Tyr levels in bands of approximately 95, 50, and 30 kDa. Surprisingly, the P-Tyr content of a 72-kDa band was higher in capacitated Fyn-null sperm than in WT sperm (Fig. 1C). This result suggests that FYN kinase phosphorylates a select group of sperm proteins during capacitation but that these proteins appear to be targets of other PTKs as well because they contained significant amounts of P-Tyr even in the absence of FYN kinase activity.
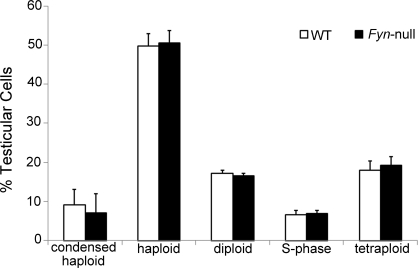
Histological analysis of postpubertal testis by using light microscopy revealed no major structural differences between Fyn-null and WT genotypes. However, to more carefully characterize the possible impact of ablation of FYN kinase on meiosis, the relative abundance of cells representing different stages of spermatogenesis was studied using Western blot analysis with cell type-specific antibodies and DNA flow cytometry. Semiquantitative analysis of Western blots probed with antibodies to GCNA1, representing spermatogonia, spermatocytes, and round spermatids [21]; to PLZF, representing spermatogonial stem cells [22, 23]; to KIT, representing types A1 to A4 spermatogonia and spermatocytes [24, 25]; and to DDX4, representing spermatocytes and round spermatids [26] revealed that the abundance of these proteins in the Fyn-null testis was not significantly different from that in controls (Fig. 1D). Testicular cell composition analysis by DNA flow cytometry also failed to detect significant differences in the relative levels of the five major cell categories that can be differentiated by this technique, including spermatogonia/somatic cells (diploid), S-phase spermatogonia (S phase), primary spermatocytes/G2 spermatogonia (postreplication), round spermatids (haploid), or elongated spermatids (condensed haploid) (Fig. 2).
FIG. 2.
DNA flow cytometric analysis of Fyn-null testicular cell composition is shown. Fyn-null and WT testes (five testes from five mice in each group) were digested to single cells. Testicular cells were then fixed with ethanol and stained with PI. Flow cytometric analysis revealed five populations categorized according to cellular DNA content: condensed haploid (elongated spermatids), haploid (round spermatids), diploid (spermatogonia/somatic cells), S phase (S-phase spermatogonia), and tetraploid (primary spermatocytes/G2 spermatogonia). Percentages of each population (50 000 events per sample analyzed) in the Fyn-null testis were compared with those in the WT testis by t-test and found not to be significantly different (n = 5; P > 0.05). Bars indicate means ± SD.
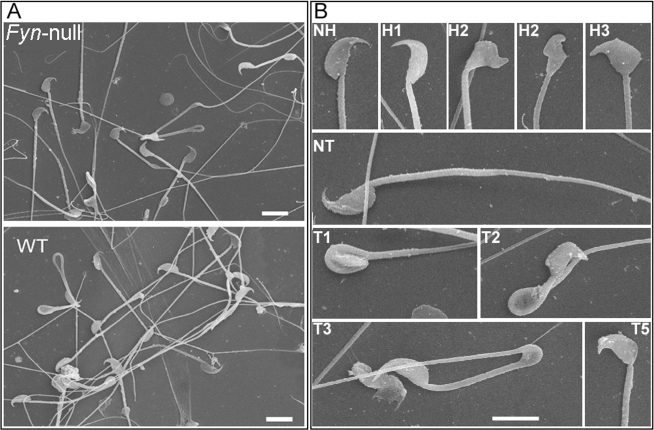
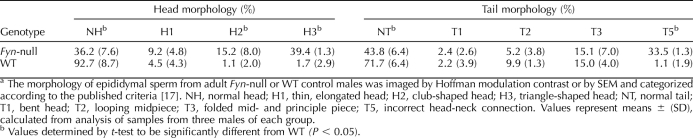
Epididymal sperm morphology was investigated by Hoffman modulation contrast microscopy [27] and by SEM. Sperm from Fyn-null males contained a high frequency of morphological defects that were classified by the morphological assessment system reported previously [17] (Fig. 3). Common defects included club-shaped and triangular head morphologies as well as incorrect head-to-neck connections. Quantitative analysis of these defects based on Hoffman modulation contrast microscopy and SEM revealed that most Fyn-null sperm had morphological defects (Table 2), indicating that the later stages of spermatogenesis in which the physical shaping of the sperm is completed were negatively impacted by the absence of FYN kinase.
FIG. 3.
Characterization of Fyn-null sperm morphology is shown. Cauda epididymal sperm from Fyn-null and WT males were fixed and prepared for SEM as described in Materials and Methods. Normal heads (NH) and tails (NT) and abnormal heads (coded, H1–H3) and tails (coded, T1–T5) were coded as described in Materials and Methods. A) A representative SEM image from samples of Fyn-null or WT sperm is shown. B) Examples of coded groups used to categorize head (H) and tail (T) morphologies are shown. Bar = 6 μm. Percentages of Fyn-null and WT sperm classified within each coding group are summarized in Table 2.
TABLE 2.
Morphological abnormalities of Fyn-null sperm.a
Fertility Analysis of Fyn-Null Male Mice
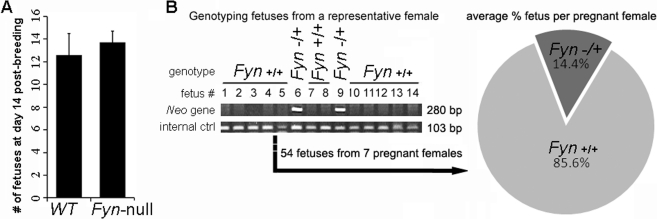
In order to assess the impact of Fyn ablation on male fertility, a breeding study was performed in which the ability of Fyn-null males to produce fetuses when mated with CF1 females was compared with that of WT males. Females caged with either Fyn-null males or WT controls were all pregnant at day 14 after breeding. As shown in Figure 4A, females mated with Fyn-null males produced normal numbers of fetuses that appeared grossly normal, in agreement with previously published results, which indicated that Fyn-null mice were fertile [12]. In order to more precisely assess the ability of Fyn-null sperm to transit the female reproductive tract and fertilize eggs, an in vivo competition assay was performed in which equal numbers of WT and Fyn-null epididymal sperm were mixed and used to artificially inseminate females. Genotype analysis of the resulting fetuses revealed that the percentage of fetuses produced by Fyn-null sperm was significantly lower than that produced by WT sperm because only 14.4% of the total 54 fetuses were derived from Fyn-null sperm (Fig. 4B). This result demonstrates that Fyn-null sperm exhibit impaired fertilizing capacity, which may reflect problems with transiting the female reproductive tract or with fertilizing oocytes. Apparently, the reduced fertilizing capacity of Fyn-null sperm was compensated for during matings by the fact that an excess of sperm is delivered into the female reproductive tract via copulation.
FIG. 4.
In vivo analysis of male fertility and sperm fertilizing capacity is shown. A) A limited mating trial was performed to determine whether Fyn-null males were fertile. Adult Fyn-null or WT males (n = 4) were each caged with two CF1 females, as described in Materials and Methods. The mean ± SD number of fetuses per female is indicated by vertical bars. Analysis by t-test indicated that differences between means were not significant (P > 0.05). B) The relative fertilizing capacities of sperm from three WT and Fyn-null males were tested by artificial insemination of CF1 females by using an equal mixture of Fyn-null and WT sperm. Genotype analysis of fetuses resulting from artificial insemination detected the presence of the neomycin insert in those fetuses produced from Fyn-null sperm (center). The percentage of fetuses per female derived from Fyn-null sperm is presented as dark grey in the pie chart (right). The percentage of fetuses derived from Fyn-null sperm was significantly lower than that derived from WT sperm, as determined by t-test (P < 0.001).
In Vitro Analysis of Sperm Function
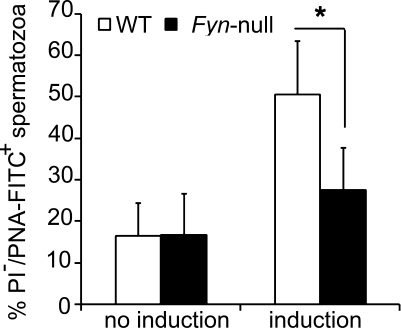
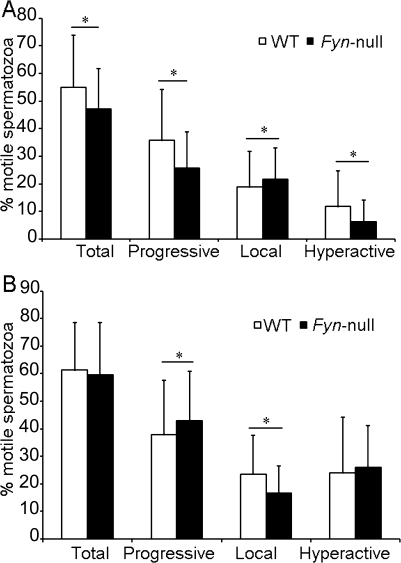
In order to directly test the fertilizing capacity of Fyn-null sperm without complications related to mating and passage through the female reproductive tract, in vitro capacitation and fertilization studies were performed. Fyn-null cauda epididymal sperm exhibited the same low frequency of spontaneous acrosome reactions as WT sperm following capacitation in vitro (Fig. 5). However, the frequency of A23187-induced acrosome reactions in Fyn-null sperm was significantly lower than in WT sperm (Fig. 5). CASA analysis demonstrated that Fyn-null sperm exhibited small decreases in total, progressive, and hyperactive motility and higher local (twitching, nonprogressive) motility before capacitation. After capacitation, Fyn-null sperm demonstrated normal total and hyperactive motility, a slight decrease in progressive motility, and little reduction in local motility (Fig. 6, A and B). Together, these data suggest that the major defects responsible for reduced fertilization capacity of Fyn-null sperm correlate more with lower frequency of acrosome reaction than with flagellar motility of sperm.
FIG. 5.
Percentage of acrosome reactions of Fyn-null sperm following capacitation in vitro is shown. Fyn-null and WT mice (five males in each group) were used to isolate epididymal sperm. Isolated sperm were incubated for 90 min in capacitating medium containing 25 mM NaHCO3 and 4 mg/ml BSA in the absence (no induction) or presence (induction) of 10 μM calcium ionophore A23187 to test sperm acrosome reaction. Flow cytometric analysis (20 000 events per sample) was performed after live sperm were stained with PNA-FITC and PI. The mean ± SD incidence of PNA-FITC-positive but PI-negative (representing those sperm that had successfully undergone acrosome reaction) Fyn-null sperm was compared with that of WT sperm. Asterisk indicates significant difference (P < 0.05).
FIG. 6.
Motility analysis of Fyn-null and WT sperm is shown. CASA motility analysis was performed with cauda epididymal sperm from five different Fyn-null and WT males following incubation under noncapacitating conditions lacking NaHCO3 and BSA (A) or capacitating conditions (B), as described in Materials and Methods. Motility values represent the mean percentage ± SD of sperm within each classification category (1700 spermatozoa ± SD per sample were analyzed). In order to determine whether values for WT and Fyn-null sperm were significantly different, means were compared using a t-test (n = 5). Asterisk indicates significantly different values (P < 0.05).
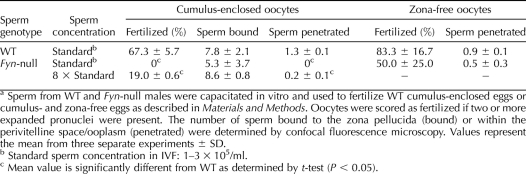
To test the capacity of Fyn-null sperm to penetrate the zona pellucida and fertilize eggs, IVF assays were performed in which capacitated WT or Fyn-null sperm were used to inseminate WT CF1 oocytes in vitro. As shown in Table 3, Fyn-null sperm at concentrations of 1 × 105 to 3 × 105 sperm/ml were unable to fertilize zona-intact oocytes during the 5-h incubation period, while WT sperm at this concentration range fertilized 67.3% of the oocytes. At higher concentrations (8-fold), the Fyn-null sperm were able to fertilize 19.0% of the oocytes, demonstrating that they still retained some fertilizing capability. Microscopic analysis was performed for all oocytes to determine the frequency of sperm bound to the zona pellucida as well as the frequency of sperm in the perivitelline space or in the ooplasm. These observations revealed that Fyn-null sperm were able to bind to the zona pellucida at the same frequency as that of controls but exhibited a significantly reduced ability to penetrate the zona pellucida, as indicated by the lower frequency of sperm retained in the perivitelline space or fused with the ooplasm (Table 3). These results indicate that the reduced fertilizing capacity of Fyn-null sperm in vitro is largely due to reduced ability to undergo the acrosome reaction and penetrate the zona pellucida.
TABLE 3.
In vitro fertilization of oocytes with Fyn-null or WT sperm.a
DISCUSSION
Previous studies have provided evidence that the Src gene family kinases play a role in several aspects of testis and sperm function [1, 3, 8–11, 28]. However, the use of chemical inhibitors that lack absolute specificity and the absence of detailed fertility analysis of mouse models in which Src gene family PTKs have been knocked out have limited the interpretations of the functional studies to date. The present work, based on the use of Fyn-null mice, indicated that Fyn expression is important for development of sperm with normal morphology and normal fertilizing capacity. The lack of Fyn expression did not result in morphological changes in the testis and epididymis, as detected macroscopically or by light microscopy. This finding agrees with that of an earlier study in Fyn-null testes that described only ultrastructural defects consisting of alterations in the basal and apical Sertoli cell junctions [8]. Loss of Fyn expression apparently had little effect on differentiation of the male germ cells, as neither stage-specific protein expression profiles nor DNA flow cytometry analysis detected significant differences between the relative abundance of stages comprising the germ cell compartment of the testis. In contrast, our analysis of sperm showed lower sperm counts in the epididymis of the Fyn knockouts; and associated with this reduction of sperm count was an increase in the frequency of various aberrant sperm morphologies. Most of these defects were localized to the sperm head, suggesting that FYN kinase or the tr-FYN protein is critical for the development of the sperm head structure during spermiogenesis. This finding agrees with a model based on subcellular localization studies in the testis proposed by Kierszenbaum [29] and Kierszenbaum et al. [30], in which FYN and FER would function in acrosome-axoplaxome biogenesis. FYN and FER have been found to function as part of a common pathway, modulating cytoskeletal and cell-cell contacts in other cell types [31], two events that would seem to be integral to the sperm head shaping process.
While functional studies of Src gene family kinases using chemical inhibitors have drawbacks due to limited specificity within the kinase family, single-gene knockout studies suffer from compensation by other Src gene family members that might be expressed in Sertoli cells and/or germ cells (e.g., the Src gene) [32]. The wide range of morphological defects which appeared in Fyn-null sperm may well reflect the modest level of success with which the other Src gene family members can compensate for the loss of FYN. Given the fact that Fyn-null males exhibited normal fertility, it was of particular importance to determine whether the defects resulting from the loss of FYN kinase activity were significant enough to result in reduced sperm fertilizing capacity. Functional defects in sperm from Fyn-null mice were demonstrated most conclusively in competitive artificial insemination studies, where Fyn-null sperm were found to produce embryos at a rate approximately one-sixth that of controls (14.4% vs. 85.6%, respectively). The very significant deficiency in fertilizing capacity detected by using a competitive artificial insemination trial with WT and Fyn-null sperm mixed in an artificial buffer, in contrast to the lack of effect seen with natural matings, highlights the limitations of mating trials in assessing certain parameters that negatively impact sperm function. Most probably, the biology of mating has evolved to provide an oversupply of sperm that can mask significant heterogeneities in sperm function.
Our use of in vitro techniques to characterize the fertility defects in Fyn-null sperm was not ideal in that it exposed the sperm to the stresses of in vitro culture. However, it did allow us to quantify the percentage of acrosome reaction and zona pellucida penetration relative to those of WT sperm, and under identical conditions with adequate statistical power. Fyn-null sperm exhibited only minor differences in motility parameters but exhibited a greatly reduced rate of A23187-induced acrosome reaction following in vitro capacitation. This agrees with previous findings made with chemical inhibitors [10, 11] and suggests that in addition to a potential role in sperm head morphogenesis, FYN kinase may also play a significant role in signaling mechanisms leading to the successful completion of the acrosome reaction.
As mentioned above, the propensity for compensation among Src gene family members can reduce the effectiveness of the single-gene knockout approach. For example, Western blot analysis of P-Ty phosphorylation patterns in Src-null sperm failed to detect differences from WT sperm even after capacitation [1]. Similar Western blot analyses of P-Tyr-containing proteins in Fyn-null sperm reported here demonstrated that WT and Fyn-null epididymal sperm contained nearly identical patterns of P-Tyr labeling. However, Fyn-null sperm consistently exhibited reduced P-Tyr labeling in a 95-kDa band. Following capacitation, Fyn-null sperm exhibited reduced P-Tyr content in several (∼95-, ∼50-, and ∼30-kDa) bands and increased P-Tyr labeling in a ∼72-kDa band. In the absence of data indicating the identity of these P-Tyr-containing bands, it is not possible to be certain whether changes reflect alterations in phosphorylation state or in amounts of each protein expressed intact in the capacitated sperm. However, taken together, these findings suggest that FYN actively phosphorylates multiple proteins during capacitation. Close structural similarities among Src gene family members raise the possibility that FYN could potentially compensate for the loss of SRC kinase, which may explain the demonstrated lack of an effect of Src deletion on sperm phosphorylation patterns [1].
In summary, the loss of FYN kinase in the testis resulted in the development of morphologically defective sperm, leading to structural defects and reduced capacity to undergo the acrosome reaction and penetrate the zona pellucida in vitro. Whether the observed functional defects resulted primarily from the loss of FYN in Sertoli cells, which resulted in defective acrosomal organization and structure, or from the absence of FYN in the sperm, which interrupted a key signaling pathways involved in the acrosome reaction, has not been resolved by this study. Future work with Sertoli or spermatid-targeted Fyn ablation could potentially answer that question. Results presented here do clearly demonstrate that FYN kinase plays an important role(s) in the organization and shaping of sperm and potentially in the acrosome reaction that cannot be fully compensated by other Src gene family members. Further biochemical studies may ultimately identify the specific targets of FYN in the testis and in sperm, which would help to identify the specific pathways in which FYN participate.
Footnotes
Supported by U.S. National Institutes of Health grant HD14846 to W.H.K.
REFERENCES
- Krapf D, Arcelay E, Wertheimer EV, Sanjay A, Pilder SH, Salicioni AM, Visconti PE. Inhibition of Ser/Thr phosphatases induces capacitation-associated signaling in the presence of Src kinase inhibitors. J Biol Chem 2010; 285: 7977 7985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey JL. Factors regulating sperm capacitation. Syst Biol Reprod Med 2010; 56: 334 348 [DOI] [PubMed] [Google Scholar]
- Goupil S, La SS, Trasler JM, Bordeleau LJ, Leclerc P. Developmental expression of SRC-related tyrosine kinases in the mouse testis. J Androl 2011; 32: 95 110 [DOI] [PubMed] [Google Scholar]
- Wong CH, Xia W, Lee NP, Mruk DD, Lee WM, Cheng CY. Regulation of ectoplasmic specialization dynamics in the seminiferous epithelium by focal adhesion-associated proteins in testosterone-suppressed rat testes. Endocrinology 2005; 146: 1192 1204 [DOI] [PubMed] [Google Scholar]
- Wong EW, Mruk DD, Cheng CY. Biology and regulation of ectoplasmic specialization, an atypical adherens junction type, in the testis. Biochim Biophys Acta 2008; 1778: 692 708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Mruk DD, Lee WM. Cheng CY. c-Yes regulates cell adhesion at the blood-testis barrier and the apical ectoplasmic specialization in the seminiferous epithelium of rat testes. Int J Biochem Cell Biol 2011; 43: 651 665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierszenbaum AL, Rivkin E, Talmor-Cohen A, Shalgi R, Tres LL. Expression of full-length and truncated Fyn tyrosine kinase transcripts and encoded proteins during spermatogenesis and localization during acrosome biogenesis and fertilization. Mol Reprod Dev 2009; 76: 832 843 [DOI] [PubMed] [Google Scholar]
- Maekawa M, Toyama Y, Yasuda M, Yagi T, Yuasa S. Fyn tyrosine kinase in Sertoli cells is involved in mouse spermatogenesis. Biol Reprod 2002; 66: 211 221 [DOI] [PubMed] [Google Scholar]
- Baker MA, Hetherington L, Aitken RJ. Identification of SRC as a key PKA-stimulated tyrosine kinase involved in the capacitation-associated hyperactivation of murine spermatozoa. J Cell Sci 2006; 119: 3182 3192 [DOI] [PubMed] [Google Scholar]
- Kumar P, Meizel S. Nicotinic acetylcholine receptor subunits and associated proteins in human sperm. J Biol Chem 2005; 280: 25928 25935 [DOI] [PubMed] [Google Scholar]
- Varano G, Lombardi A, Cantini G, Forti G, Baldi E, Luconi M. Src activation triggers capacitation and acrosome reaction but not motility in human spermatozoa. Hum Reprod 2008; 23: 2652 2662 [DOI] [PubMed] [Google Scholar]
- Stein PL, Lee HM, Rich S. Soriano P. pp59fyn mutant mice display differential signaling in thymocytes and peripheral T cells. Cell 1992; 70: 741 750 [DOI] [PubMed] [Google Scholar]
- Luo J, McGinnis LK, Kinsey WH. Role of Fyn kinase in oocyte developmental potential. Reprod Fertil Dev 2010; 22: 966 976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziuk PJ, Runner MN. Recovery of blastocysts and induction of implantation following artificial insemination of immature mice. J Reprod Fertil 1960; 1: 321 331 [DOI] [PubMed] [Google Scholar]
- Southard JL, Wolfe HG, Russell ES. Artificial insemination of dystrophic mice with mixtures of spermatozoa. Nature 1965; 208: 1126 1127 [DOI] [PubMed] [Google Scholar]
- Tyrode MV. The mode of action of some purgative salts. Arch Intern Pharmacodyn 1910; 17: 205 209 [Google Scholar]
- Ward MA. Intracytoplasmic sperm injection effects in infertile azh mutant mice. Biol Reprod 2005; 73: 193 200 [DOI] [PubMed] [Google Scholar]
- Luo J, McGinnis LK, Kinsey WH. Fyn kinase activity is required for normal organization and functional polarity of the mouse oocyte cortex. Mol Reprod Dev 2009; 76: 819 831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler A, He X, Gordon RE, Wu HS, Gatt S, Schuchman EH. Reproductive pathology and sperm physiology in acid sphingomyelinase-deficient mice. Am J Pathol 2002; 161: 1061 1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter L, Koch E, Bechter R, Bobadilla M. Three-parameter flow cytometric analysis of rat spermatogenesis. Cytometry 1997; 27: 161 168 [DOI] [PubMed] [Google Scholar]
- Enders GC, May JJ. Developmentally regulated expression of a mouse germ cell nuclear antigen examined from embryonic day 11 to adult in male and female mice. Dev Biol 1994; 163: 331 340 [DOI] [PubMed] [Google Scholar]
- Buaas FW, Kirsh AL, Sharma M, McLean DJ, Morris JL, Griswold MD, de Rooij DG, Braun RE. Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet 2004; 36: 647 652 [DOI] [PubMed] [Google Scholar]
- Costoya JA, Hobbs RM, Barna M, Cattoretti G, Manova K, Sukhwani M, Orwig KE, Wolgemuth DJ, Pandolfi PP. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat Genet 2004; 36: 653 659 [DOI] [PubMed] [Google Scholar]
- Dym M, Jia MC, Dirami G, Price JM, Rabin SJ, Mocchetti I, Ravindranath N. Expression of c-kit receptor and its autophosphorylation in immature rat type A spermatogonia. Biol Reprod 1995; 52: 8 19 [DOI] [PubMed] [Google Scholar]
- von Schönfeldt V, Wistuba J, Schlatt S. Notch-1, c-kit and GFRalpha-1 are developmentally regulated markers for premeiotic germ cells. Cytogenet Genome Res 2004; 105: 235 239 [DOI] [PubMed] [Google Scholar]
- Fujiwara Y, Komiya T, Kawabata H, Sato M, Fujimoto H, Furusawa M, Noce T. Isolation of a DEAD-family protein gene that encodes a murine homolog of Drosophila vasa and its specific expression in germ cell lineage. Proc Natl Acad Sci U S A 1994; 91: 12258 12262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman R, Gross L. Modulation contrast microscope. Appl Opt 1975; 14: 1169 1176 [DOI] [PubMed] [Google Scholar]
- Mitchell LA, Nixon B, Baker MA, Aitken RJ. Investigation of the role of SRC in capacitation-associated tyrosine phosphorylation of human spermatozoa. Mol Hum Reprod 2008; 14: 235 243 [DOI] [PubMed] [Google Scholar]
- Kierszenbaum AL. Tyrosine protein kinases and spermatogenesis: truncation matters. Mol Reprod Dev 2006; 73: 399 403 [DOI] [PubMed] [Google Scholar]
- Kierszenbaum AL, Rivkin E, Tres LL. Molecular biology of sperm head shaping. Soc Reprod Fertil Suppl 2007; 65: 33 43 [PubMed] [Google Scholar]
- Kapus A, Di Ciano C, Sun J, Zhan X, Kim L, Wong TW, Rotstein OD. Cell volume-dependent phosphorylation of proteins of the cortical cytoskeleton and cell-cell contact sites. The role of Fyn and FER kinases. J Biol Chem 2000; 275: 32289 32298 [DOI] [PubMed] [Google Scholar]
- Lawson C, Goupil S, Leclerc P. Increased activity of the human sperm tyrosine kinase SRC by the cAMP-dependent pathway in the presence of calcium. Biol Reprod 2008; 79: 657 666 [DOI] [PubMed] [Google Scholar]