Abstract
A series of dithiocarbamates was prepared by reaction of primary/secondary amines with carbon disulfide in the presence of bases. These compounds were tested for the inhibition of 4 human (h) isoforms of the zinc enzyme carbonic anhydrase, CA (EC 4.2.1.1), hCA I, II, IX and XII, involved in pathologies such as glaucoma (CA II and XII) or cancer (CA IX). Several low nanomolar inhibitors targeting these CAs were detected. X-ray crystal structure of hCA II adduct with morpholine dithiocarbamate evidenced the inhibition mechanism of these compounds, which coordinate to the metal ion through a sulfur atom from the dithiocarbamate zinc-binding function. Some dithiocarbamates showed effective intraocular pressure lowering activity in an animal model of glucoma.
Introduction
Carbonic anhydrases (CAs, EC 4.2.1.1) are widespread zinc metalloenzymes found in higher vertebrates including humans.1–3 16 isozymes have been characterized to date, many of which are involved in critical physiological processes. They catalyze the following reaction: CO2+H2O ↔H++HCO3−.1–3 In humans, CAs are present in a large variety of tissues including the gastrointestinal tract, the reproductive tract, the nervous system, kidneys, lungs, skin and eyes.2,3 The different isozymes are localized in different parts of the cell with CA I and CA II, important isozymes in normal cells, being localized in the cytosol. 1–4
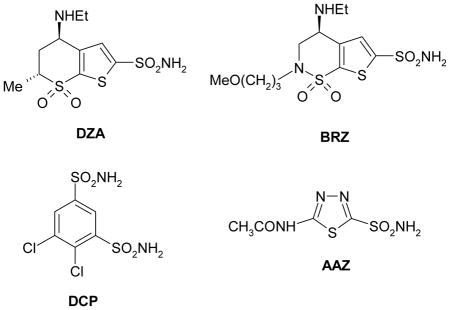
Many of the CA isozymes are important therapeutic targets with the potential to be inhibited to treat a range of disorders.1–7 CA II plays a role in bicarbonate production in the eye and is therefore a target for therapy of eye disease such as glaucoma.7–9 Indeed, CA inhibitors (CAIs) of the sulfonamide type such as dorzolamide DZA or brinzolamide BRZ are topically used antiglaucoma agents,7–10 whereas the older drugs, such as acetazolamide AAZ or dichlorophenamide DCP show the same action through systemic administration, which however leads to a wide range of side effects due to inhibition of the enzyme from other organs than the target one, i.e., the eye.11 CA XII, a transmembrane isoform with an extracellular active site, was shown to be overexpressed in glaucomatous patients eyes.12
As some solid tumors grow in cancer patients, hypoxic regions are formed, particularly in the interior of the tumor.13 The gene expression profile of a hypoxic cancer cell is different from that of other cancer cells in a normally-oxygenated environment, i.e., in normoxic conditions.13–15 Under hypoxia, the distribution of CA isoforms is altered as compared with normoxic cells.13,14 As a result, CA isozymes IX and XII are overexpressed in hypoxic tumor cells, in a variety of solid tumors.13–15 Unlike other CAs, CA IX and CA XII are both extracellularly localized on hypoxic tumor cells. 13–15 These enzymes play various roles in tumorigenesis, by regulating pH inside and outside the tumor cell,15 interfering with phosphorylation of various proteins16 or by playing a role in the cell-cell adhesion.13–15 They therefore provide a target for cancer therapy because they are relatively specific to the hypoxic tumor cells and appear to be important in their survival and proliferation.15 Indeed, several antibodies targeting CA IX are in Phase III clinical development for the treatment of solid tumors (or for their imaging)17 whereas some small molecule inhibitors are also in advanced preclinical evaluation.15,18,19
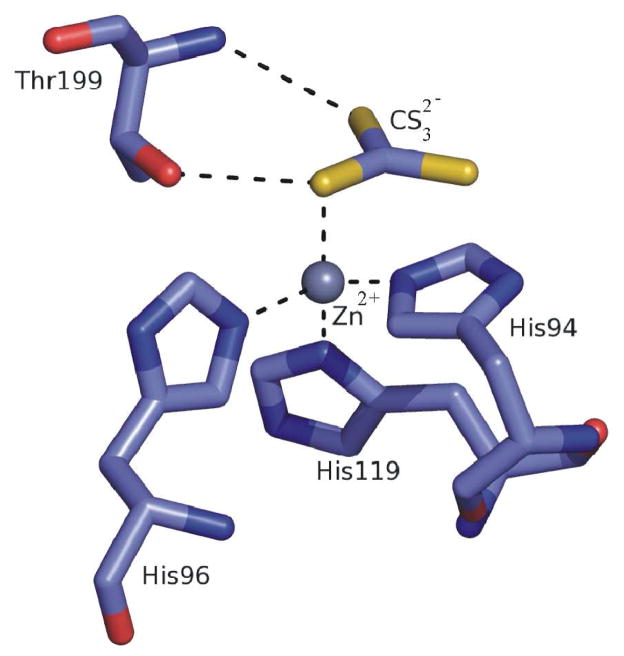
The classical carbonic anhydrase inhibitors (CAIs) are the sulfonamides and their isosteres (sulfamates, sulfamides, etc).1–4 However, most of these compounds indiscriminately inhibit many of the 16 CA isoforms known to date in mammals.1–3 Thus, efforts have been made to find different CAIs, from the sulfonamide, sulfamate and sulfamide ones. Indeed, recently the coumarins were discovered as mechanism-based inhibitors which act as prodrugs and bind in a very different mode compared to sulfonamides and their isosteres,20 whereas some polyamines (such as spermine),21 as well as a range of phenols22 were also investigated and showed interesting such properties and novel mechanisms of inhibition. Among CAIs investigated to date there are also the inorganic anions, which coordinate to the zinc ion from the enzyme active site.1,23,24 Indeed, trithiocarbonate (CS32−) an anion similar to carbonate, has recently been investigated and shown to constitute a “lead” for novel CAIs.23 The X-ray crystal structure for the adduct of trithiocarbonate (CS32−), bound to hCA II has recently been reported (Fig. 1).23 The inhibitor binds to the Zn2+ in the hCA II active site in a slightly distorted tetrahedral geometry of the metal ion, occupying a position similar to that observed in the case of hCA II-bicarbonate complex.23 Trithiocarbonate was monocoordinated to the Zn(II) ion by means of one of the sulfur atoms. The same sulfur made a hydrogen bond to the OH of Thr199 whereas a second sulfur atom participated to another hydrogen bond with the NH group of the same amino acid residues, Thr199. This binding mode explains the low micromolar affinity of this inhibitor to many of the CA isoforms investigated to date.23 Based on this binding mode of a millimolar inhibitor, trithiocarbonate (CS32−), we hypothesized that compounds incorporating this new zinc-binding function, CS2−, may act as even stronger CAIs. Indeed, we have recently demonstrated in a preliminary communication that dithiocarbamates (DTCs), compounds possessing the general formula R1R2N-CS2−M+ act as highly efficient CAIs.25 Here we report the first detailed study of the DTCs as a class of potent CAIs, with a mechanism of action different of that of the sulfonamides. Furthermore, we prove that some of these highly water soluble compounds possess excellent intraocular pressure (IOP) lowering properties in an animal model of glaucoma, making them interesting candidates for developing antiglaucoma drugs.
Fig 1.
Trithiocarbonate (CS32−), a recently investigated low micromolar CAI, binds to the Zn2+ in the hCA II active site in a slightly distorted tetrahedral geometry of the metal ion, occupying a position similar to that observed in the case of hCA II-bicarbonate complex. The protein zinc ligands (His94, 96 and 119) and Thr199 are shown (PDB code 3K7K).24
Results and Discussion
Chemistry
DTCs are well known metal complexing agents and they also possess interesting biomedical and agricultural applications.26–29 Although this class of compounds (and their metal complexes) started to be used as fungicides more than 50 years ago,29 few studies investigated their interactions with metalloenzymes.27 Apart studies of DTCs as inhibitors of tyrosinase, a copper enzyme,27 only one such work investigated the inhibition of N,N-diethyl-DTC with bovine CA (bCA).28 By using Co(II)-substituted CA, Morpurgo et al.28 showed that the inhibitor does not extrude the metal ion from the enzyme active site (as it does with the copper ion from the tyrosinase active site)27 and that it binds to it, probably in a trigonal-bipyramidal geometry of Co(II). However, no other DTC were subsequently investigated for their interaction with CAs till our group reported that trithiocarbonate and related compounds containing the new zinc binding group (ZBG) found in it, i.e., CS2−, inhibit several CA isoforms in the low micromolar or submicromolar range.23 Here we extend those findings, showing that a wide range of DTCs incorporating various aliphatic and/or aromatic moieties at the nitrogen atom, acts as low nanomolar and even subnanomolar CAIs.
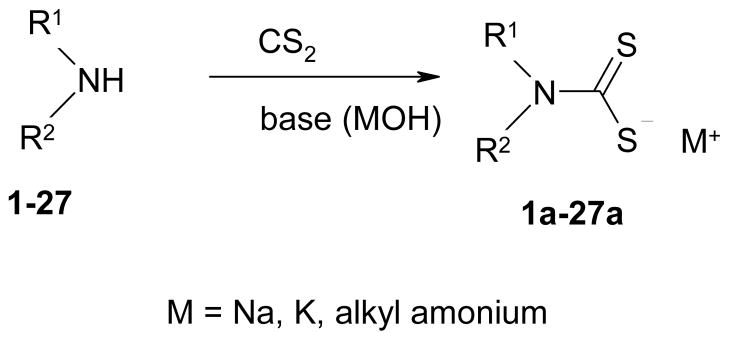
We prepared a series of 27 DTCs, of types 1a-27a, by the classical reaction27,30,31 of dithiocarbamoylation between primary/secondary amines 1-27, and CS2, in the presence of a base, which most of the time was NaOH, KOH but in the case of more basic amines, the amine itself can act as base (Scheme 1). As shown in Table 1, a large variety of R1 and R2 moieties are present in DTCs reported here, i.e., hydrogen, alkyl, aryl, aralkyl, hetaryl and cyclic such moieties, which lead to the generation of a wide chemical diversity in these compounds. Presumably, this should be reflected also in varied physico-chemical and biological properties of these DTCs. Compounds 1a-27a were characterized by physico-chemical standard procedures (IR, 1H- an 13C-NMR spectroscopy, MS) and were > 99 % pure, as determined by HPLC (see Experimental protocols for details)
Scheme 1.
Preparation of DTCs 1a-27a by reaction of amines 1-27 with carbon disulfide in the presence of bases.
Table 1.
CA I, II, IX and XII inhibition data with dithiocarbamates 1a-27a by a stopped-flow, CO2 hydrase assay.32
| R1R2N-CSS−M+ 1a-27a
| ||||||
|---|---|---|---|---|---|---|
| No*,# | R1 | R2 | KI (nM)# | |||
| hCA I | hCA II | hCA IX | hCA XII | |||
| 1a | H | Ph | 4.8 | 4.5 | 4.2 | 4.3 |
| 2a | H | O[(CH2CH2)]2N | 4.8 | 3.6 | 29.1 | 9.2 |
| 3a | H | MeN[(CH2CH2)]2N | 33.5 | 33.0 | 22.1 | 17.5 |
| 4a | H | 2-butyl | 21.1 | 29.4 | 4.6 | 31.7 |
| 5a | H | O[(CH2CH2)]2N(CH2)2 | 31.8 | 36.3 | 4.5 | 4.2 |
| 6a | H | N[(CH2CH2)N]3 | 31.9 | 13.5 | 27.4 | 9.3 |
| 7a | H | PhCH2 | 4.1 | 0.70 | 19.2 | 11.5 |
| 8a | H | 4-PyridylCH2 | 3.5 | 16.6 | 26.0 | 24.1 |
| 9a | H | [(CH2)5N]CH2CH2 | 4.5 | 20.3 | 3.6 | 20.5 |
| 10a | H | 2-thiazolyl | 3.9 | 4.6 | 12.6 | 22.0 |
| 11a | H | KOOCCH2 | 13.1 | 325 | 57.1 | 6.7 |
| 12a | H | imidazol-1-yl-(CH2)3 | 8.6 | 24.7 | 4.3 | 6.5 |
| 13a | Me | Me | 699 | 6910 | 714 | 798 |
| 14a | Et | Et | 790 | 3100 | 1413 | 1105 |
| 15a | (CH2)5 | 0.96 | 27.5 | 70.4 | 46.1 | |
| 16a | iso-Bu | iso-Bu | 0.97 | 0.95 | 4.5 | 0.99 |
| 17a | n-Pr | n-Pr | 1838 | 55.5 | 53.8 | 7.0 |
| 18a | n-Bu | n-Bu | 43.1 | 50.9 | 50.3 | 5.8 |
| 19a | n-Hex | n-Hex | 48.0 | 51.3 | 27.4 | 16.1 |
| 20a | Et | n-Bu | 157 | 27.8 | 25.9 | 7.5 |
| 21a | HOCH2CH2 | HOCH2CH2 | 9.2 | 4.0 | 4.3 | 4.2 |
| 22a | Me | Ph | 39.6 | 21.5 | 28.2 | 7.7 |
| 23a | Me | PhCH2 | 69.9 | 25.4 | 53.0 | 3.0 |
| 24a | O[(CH2CH2)]2 | 0.88 | 0.95 | 6.2 | 3.4 | |
| 25a | NaS(S=C)N[(CH2CH2)]2 | 12.6 | 0.92 | 37.5 | 0.78 | |
| 26a | (NC)(Ph)C(CH2CH2)2 | 48.4 | 40.8 | 757 | 169 | |
| 27a | (S)-[CH2CH2CH2CH(COONa)] | 2.5 | 17.3 | 4.1 | 4.0 | |
| AAZ | - | 250 | 12 | 25 | 5.7 | |
Compounds 1a, 8a and 10a were thriethylamonium salts; 2a-6a, 9a, 11a and 12a were potasium salts, and the remaining ones were sodium salts.
Mean from 3 different assays. Errors were within ± 5–10 % of the reported values (data not shown).
Inhibition data against hCA I, II and IX for 9 DTCs, i.e. compounds 13a, 14a, 16–18a, 20a, 22a, 23a and 26a, were reported in ref.25
Carbonic anhydrase inhibition
Compounds 1a-27a were assayed32 for the inhibition of four physiologically relevant CA isoforms, hCA I, II, IX and XII. All of them are drug targets: hCA I, II and XII for ophthalmologic diseases, mainly glaucoma,1,10 whereas CA IX and XII for antitumor drugs/tumor imaging agents.1,15,17–19 Inhibition data with the sulfonamide, clinically used agent acetazolamide (AAZ) are also reported in Table 1, for comparison reasons.
The following structure-activity relationship (SAR) can be observed for the CA inhibition data with DTCs 1a-27a investigated here:
The cytosolic isoform hCA I was strongly inhibited by DTCs 1a-27a investigated here, with KIs in the range of 0.88 – 1838 nM. It may be observed that irrespective of the nature of R2, the DTCs prepared from primary amines (R1 = H) 1a-12a were highly effective hCA I inhibitors, with inhibition constants in the low nanomolar range (3.5 – 33.5 nM). On the contrary, the compounds prepared from secondary amines showed a more varied biological activity. Thus, the simple dimethyl- and diethyl-DTCs 13a and 14a were weak hCA I inhibitors, with KIs in the range of 699–790 nM. The same is true for the di-n-propyl derivative 17a (KI of 1838 nM). However, the cyclic derivative 15a, which differs from 14a by the cyclic structure and an extra carbon atom present in its molecule, is a subnanomolar hCA I inhibitor, showing a dramatic increase of potency of 823 times compared to 14a. It is also interesting to compare the potencies of 16a and 18a, which incorporate iso-butyl and n-butyl moieties (and are isomers), and differ by a factor of 44, unexpectedly, in favor of the compound with a branched scaffold. Increasing the length of the aliphatic chains to C6, as in 19a leads to a slight loss of potency compared to the most active compounds in the aliphatic series, which are 15a and 16a. Furthermore, the presence of hydroxyethyl moieties (instead of the ethyl ones) as in 21a, lead to a steady increase of potency, 21a being 85.9 times a better hCA I inhibitor compared to 14a. The compounds incorporating one alkyl and one aryl moiety at the nitrogen atom of the DTC function, such as 22a and 23a were also effective hCA I inhibitors (KIs of 39.6–69.9 nM), as were also the heterocyclic derivatives 24a-27a, some of which showed subnanomolar activity (the morpholine DTC 24a had a KI of 0.88 nM and was the best DTC hCA I inhibitor and also the best hCA I inhibitor ever described, as far as we know). Thus, many of these chemotypes explored here show excellent hCA I inhibitory activity, which range from the subnanomolar to the micromolar. Furthemore, many of the DTCs are much more effective as hCA I inhibitors compared to the sulfonamide AAZ, which has a KI of 250 nM against this isoform (Table 1).
The physiologically dominant cytosolic isoform hCA II also showed an interesting inhibition profile with DTCs 1a-27a. Thus, several primary DTCS (1a, 2a, 7a, and 10a) and several secondary ones (16a, 21a, 24a and 25a) were excellent hCA II inhibitors, with KIs in the range of 0.70 – 4.6 nM, being more effective (even one order of magnitude) than the clinically used sulfonamide AAZ (Table 1). It may be observed that these compounds incorporate aromatic, arylakyl, hetaryl, alkyl and hydroxyalkyl moieties substituting the nitrogen atom from the DTC moiety. Another rather large group of derivatives, such as 3a-6a, 8a, 9a, 12a, 15a, 17a-20a, 22a, 23a, 26a and 27a, were slightly less effective hCA II inhibitors, but still possessed a high efficacy, with KIs in the range of 13.5 - 55.5 nM. Again both primary and secondary DTCs are present in this subgroup. They incorporate various types of substituents, such as alkyl, aryl, aralkyl, and hetaryl ones. It is obvious that small structural changes in the DTC scaffold influence dramatically the biological activity. For example, for the aliphatic secondary DTCs, the isomeric pair 16a – 18a, which differ only by the nature of the aliphatic chain (iso-Bu moieties in the first compound and n-Bu in the second derivative) have KIs which differ by a factor of 53.6. The length of the alkyl chain also strongly influence activity, with compounds possessing a medium chain (e.g., 15a-20a) being more effective than the ones with shorter chains, such as 13a and 14a, which are rather ineffective as hCA II inhibitors (KIs of 3.1 – 6.9 μM). Also the glycine DTC 11a was a medium potency hCA II inhibitor, with a KI of 325 nM.
The tumor-associated isoform hCA IX was highly inhibited by the DTCs investigated here, with KIs in the range of 3.6 – 1413 nM. The simple aliphatic secondary DTCs 13a/14a and the bulky cyclic derivative 26a, were the least effective inhibitors (KIs of 0.714–1.413 μM), and four other compounds (11a, 15a, 17a and 18a) were effective, medium potency inhibitors, with KIs in the range of 50.3 – 70.4 nM. They incorporate the carboxyalkyl moiety present in glycine (11a), the five-membered aliphatic ring (from 15a) and 3- or 4-carbon atom n-alkyl chains (17a and 18a). All the remaining derivatives showed highly effective hCA IX inhibitory properties, with KIs < 30 nM. Thus, a rather high structural diversity (aliphatic, aromatic, aralkyl, hetaryl moieties) present in primary/secondary DTCs lead to highly effective hCA IX inhibitors, with minor structural changes drastically affecting enzyme inhibition. Many DTCs were more effective hCA IX inhibitors compared to acetazolamide (Table 1).
A rather similar SAR as the one discussed above for hCA IX, was observed for the inhibition of the second transmembrane isoform, hCA XII, with DTCs 1a-27a. Thus, 13a/14a and 26a, were the least effective inhibitors (KIs in the range of 169 – 1105 nM), whereas the remaining DTCs were highly effective hCA XII inhibitors, with KIs in the range of 0.78 – 31.7 nM (Table 1). Among the best hCA XII inhibitors (subnanomolar inhibition constants) were the di-isobutyl-DTC 16a and the piparazine-bis-DTC 25a. Again the main conclusion is that a large number of substitution patterns, incorporating varied moieties, lead to highly effective hCA XII inhibitors.
The DTCs investigated here showed a rather promiscuous inhibitory activity against all four CA isoforms described here, although each of these CAs had a different inhibition profile with all these compounds. For example, 15a was a subnanomolar inhibitor of hCA I and inhibited the remaining 3 isoforms with KIs in the range of 27.5–70.4 nM, having thus an acceptable selectivity ratio for the inhibition of hCA I over the remaining three CAs. 25a was a subnanomolar inhibitor of hCA II and XII, and inhibited hCA I and IX with higher KIs, of 12.6–37.5 nM. Compound 23a showed a rather good selectivity for inhibiting hCA XII over hCA I, II and IX (Table 1). It should be also mentioned that being negatively charged, these compounds show membrane impermeability33 which may be a favorable pharmacological property in vivo.
X-ray crystallography
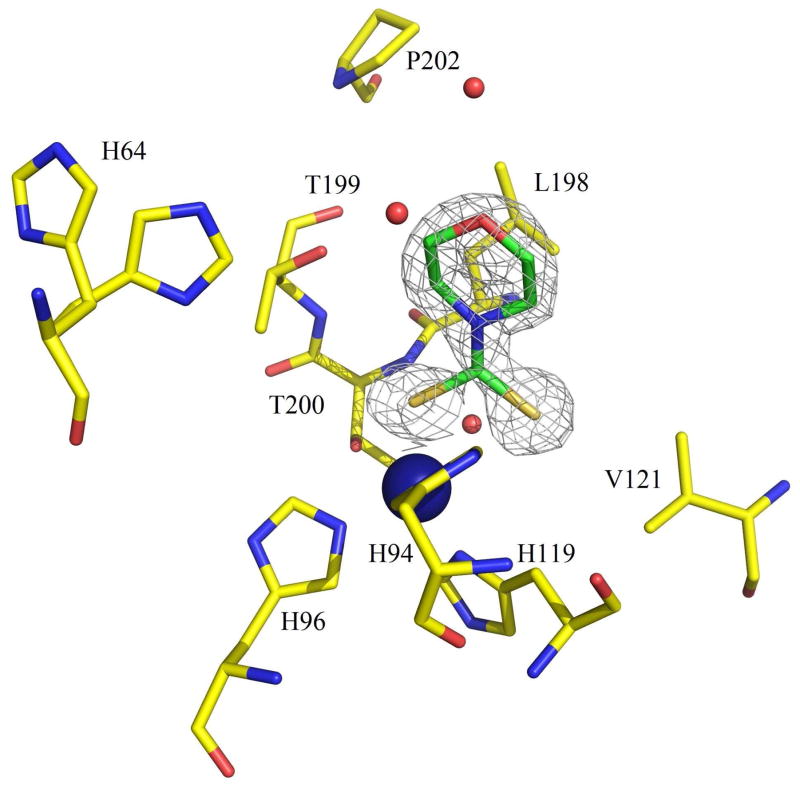
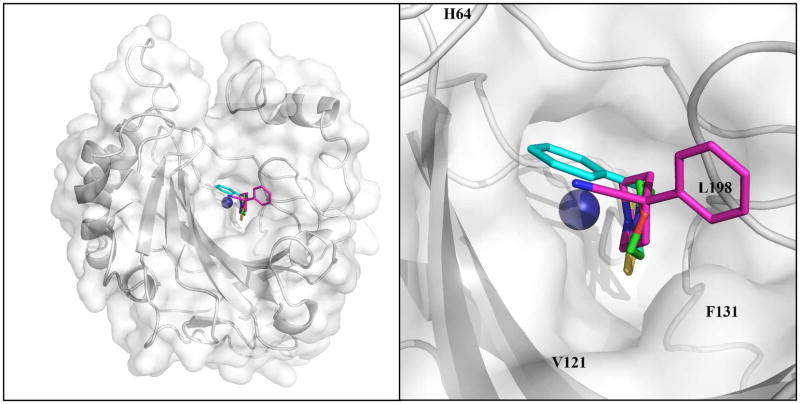
In order to explain the potent CA inhibitory properties of the DTCs, which are a new class of CAIs, we resolved the X-ray crystal structure of hCA II in complex with the very potent inhibitor 24a (KI of 0.95 nM). Compound 24a was well ordered and refined with an occupancy of 1.0 with B-factors that were comparable to the solvent within the active site (Table 2). The compounds is buried deep into the active site, displacing the catalytic zinc-bound solvent, such that one of the sulfur atoms coordinates directly to the zinc ion of the enzyme. The overall zinc coordination (3N from the coordinating histidine residues His94, 96 and 119, and 1S from the inhibitor ligand) can be described as a distorted tetrahedron (Fig. 2). The details of this tetrahedral geometry are provided in Table 3. The zinc bound sulfur also interacts with the O atom of Thr199 in a similar manner to that observed in the more classical clinically used sulfonamides and sulfamates CAIs.34–36 Compound 24a possesses a puckered ring and binds with a slightly higher B-factor of 23.9 Å2, compared to the two other DTCs for which the X-ray structure in complex with hCA II were reported earlier,25 i.e., 23a and 26a. For a structural comparison the three compounds were superposed onto each other (Fig. 3). For unbound hCA II the side chain conformation of His64 has been shown to be dependent upon the buffer pH, which effects the protonation state of the imidazole ring. It is widely believed that this side chain flips from an “in” to “out” conformation as part of the proton transfer mechanism in hCA II, hence two conformations of the residue are often observed in crystal structures.34–36 His64 in the hCA II structure in complex with compound 26a (PDB ID 3P5L) and 24a (PDB ID 3P5A) has a dual conformtion. In the case of 26a, the terminal six-membered hydrophobic ring sites close to F131, V135 and P202 at the rim of the active site in a hydrophobic pocket of hCA II. Whereas, for 24a, the six-membered ring does not extend far enough out of the active site to either reach this hydrophobic pocket or close enough to the in-conformation of His64. Hence, compounds 24 and 26a are 5.6 and 5.0 Å respectively from His64 and therefore do not affect its conformation. Whereas in the hCA II – 23a structure (PDB ID 3P58) the six-membered planar ring forms a T-shaped π-stacking with the imidzole ring of His64 and stabilizes this amino acid in the “in” conformation.25 In addition 24a is positioned 3.2 Å from Thr200 but does not form hydrogen bonds with either the protein main- or side-chain. However, the endocyclic oxygen atom in the tail ring of 24a was within hydrogen bond distance of 3.4 and 3.2 Å from water393 and water540, respectively (Fig. 2) which probably also contribute to its high affinity to hCA II.
Table 2.
Crystallographic data refinement and model quality statistics for thr hCA II-24a complex.
| PDB accession number | 3P5A |
|
| |
| Data-collection statistics | |
|
| |
| Temperature (K) | 100 |
|
| |
| Wavelength (Å) | 1.5418 |
|
| |
| Space group | P21 |
|
| |
| Unit-cell parameters (Å,°) | a = 42.3; b = 41.2 |
| c = 72.1; β = 104.2 | |
|
| |
| Total theoretical reflections | 39632 |
|
| |
| Total measured reflections | 39395 |
|
| |
| Resolution (Å) | 50.0-1.5 (1.54-1.50) |
| a Rsym (%); I/σ(I) | 6.4 (18.1); 17.5 (5.8) |
| Completeness; Redundancy | 99.4 (95.7); 3.7 (3.4) |
|
| |
| Final Model Statistics | |
|
| |
| b Rcryst (%); c Rfree(%) | 0.148; 0.169 |
|
| |
| Residue Nos. | 4–261 |
|
| |
| d No. of protein atoms | 2078 |
|
| |
| No. of compound atoms | 9 |
|
| |
| No. of H2O molecules | 300 |
|
| |
| R.M.S.D. bond lengths (Å) | 0.012 |
| bond angles (°) | 1.488 |
|
| |
| Ramachandran statistics (%) | 89.4, 10.7, 0.0 |
|
| |
| Average B factors (Å2) Main-, side-chain, compound, solvent | 13.6, 18.1, 23.9, 29.4 |
Rsym = Σ |I − <I>|/Σ <I>.
Rcryst = (Σ |Fo| − |Fc|/Σ |Fobs|) × 100.
Rfree is calculated in same manner as Rcryst, except that it uses 5% of the reflection data omitted from refinement.
Includes alternate conformations.
Values in parenthesis represent highest resolution bin.
Fig. 2.
Stick representation of hCA II active site with compound 24a (green) complexed within it. The active-site zinc is depicted as a blue sphere. Water molecules are depicted as red spheres. The electron density is represented by a Σ-weighted 2Fo − Fc Fourier map (gray mesh). Amino acids involved in binding of inhibitor are also shown. Figure made using PyMOL (DeLano Scientific).
Table 3.
Geometry of the Zn(II) ion and bound S atom (of the DTCs) or compounds 23a, 24a and 26a complexced within the hCA II active site (Angle defined as His-Zn-S).
| 23a | 24a | 26a | |
|---|---|---|---|
| Distance (Å) | 2.3 | 2.3 | 2.3 |
| Angle (°) with His94 | 111.3 | 107.3 | 108.8 |
| Angle (°) with His96 | 114.2 | 112.2 | 112.9 |
| Angle (°) with His119 | 119.4 | 128.3 | 126.1 |
Fig. 3.
View of compounds 23a (cyan), 24a (green), 26a (magenta), superposed in the active site of hCA II. Left and right panel depict overall and zoomed active site view, respectively. hCA II is depicted as a grey surface representation. The active-site zinc is depicted as a blue sphere. Figure made using PyMOL (DeLano Scientific).
Intraocular pressure lowering in hypertensive rabbits
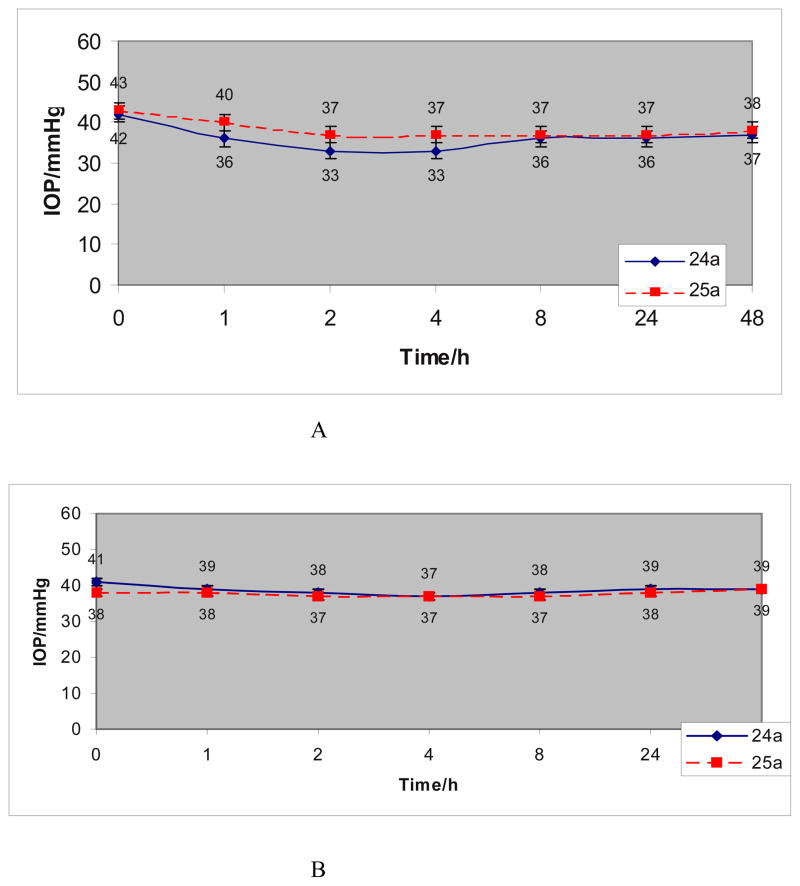
Two of the new CAIs investigated here, compounds 24a and 25a, which show excellent hCA II and XII inhibitory properties (Table 1) were investigated in vivo, for their ability to lower intraocular pressure (IOP) in carbomer-induced glaucoma in rabbits.10c Normal IOP in rabbits, like in humans is of around 15–20 mm Hg. In this model, the IOP is quite elevated, mimicking thus the pathologic situation observed in the human disease.10 Clinically used drugs such as dorzolamide, induce a maximal IOP lowering of 4–5 mm Hg, as reported recently by us.10 The two dithiocarbamates investigated here in detail were chosen both due to their excellent enzyme inhibitory activity in vitro and also because they show very good water solubility, being formulated at 2 % eye drop solution at neutral pH (due to their salt character, whereas DZA is formulated at pH 5.5, as hydrochloride salt, and induces eye irritation).10 In fact, water solubility of eye drugs is a significant problem,8–10 with many classes of drugs achieving an acceptable solubility only as salts with strong acids, such as HCl, which leads to acidic pH values causing eye irritation (dorzolamide DZA is a well known such case).10
Rabbits were treated with 2 % solutions of DTCs 24a and 25a and their IOP was monitored for 48 h (Fig. 4a). The contralateral eye was treated with vehicle and was used as control (Fig. 4B). As observed from Figure 4, both compounds were effective in reducing elevated IOP time dependently, for a rather long period. The maximal effect (of –6–10 mm Hg) has been observed after 2 hours post administration, and it lasted for up to 4–8 hours, being almost double that reported for DZA (of 4–5 mm Hg, and lasting only for about 3–4 hours).10 DTC 24a was slightly more effective than 25a as an IOP lowering agent. The IOP in vehicle-treated eyes was rather constant during the entire duration of the experiments, varying between 37–39 mm Hg for animals treated with 25a and between 37–40 mm Hg for animals treated with 24a (the data of the Figures are the mean for 3 different animals and the error range is shown in Fig. 4).
Fig. 4.
A: IOP lowering versus time, of glaucomatous rabbits treated with one drop (50 μL) of a 2 % water solution of DTC 24a and 25a; B: IOP in the eyes treated with vehicle (IOP are the mean from 3 different animals). Error bars are shown in both figures, accounting for an average of 0.8 – 1.5 mm Hg.
Conclusions
We report here that DTCs represent a novel class of highly effective CAIs. DTCs are easy to prepare from simple starting materials, they can incorporate a very high chemical diversity, and act as inhibitors of several physiologically relevant CA isoforms, with potencies from the subnanomolar to the micromolar. SAR for the inhibition of isoforms hCA I, II, IX and XII were straightforward and slightly different, with small modifications in the backbone of the compound leading to dramatic changes of biological activity. The inhibition mechanism of the DTCs was also explained, by resolving the X-ray crystal structure for hCA II complexed with a heterocyclic DTC. The CS2− moiety present in DTCs represents a new zinc-binding function. It is directly coordinated to the Zn(II) ion from the enzyme active site and also participates in an interaction (hydrogen bond) with the OH moiety of Thr199, an amino acid essential for the binding of many classes of CAIs (and of the substrates). The organic scaffold of the DTC is deeply buried within the enzyme active site and also participates in favorable interactions with it, which leads to a high stabilization of the enzyme-inhibitor adduct. Some of the most potent CAIs detected here showed favorable IOP lowering effects in an animal model of glaucoma. Being water soluble, with pH of the solution in the neutral range, and with duration of action lasting up to 4–8 h, this new class of CAIs may constitute interesting candidates for developing novel antiglaucoma therapies, a field in which no new drug emerged in the last 15 years.
Experimental Protocols
Chemistry
1H, 13C, DEPT, COSY, HMQC and HMBC spectra were recorded using a Bruker Advance III 400 MHz spectrometer. The chemical shifts are reported in parts per million (ppm) and the coupling constants (J) are expressed in Hertz (Hz). For all new compounds DEPT, COSY, HMQC and HMBC were routinely used to definitely assign the signals of 1H and 13C. Infrared spectra were recorded on a Perkin Elmer Spectrum R XI spectrometer as solids on KBr plates. Melting points (m.p.) were measured in open capillary tubes, unless otherwise stated, using a Büchi Melting Point B-540 melting point apparatus and are uncorrected. Thin layer chromatography (TLC) was carried out on Merck silica gel 60 F254 aluminium backed plates. Elution of the plates was carried out using ethyl acetate/n-hexane or MeOH/DCM systems. Visualization was achieved with UV light at 254 nm, by dipping into a 0.5 % aqueous potassium permanganate solution, by Hanessian’s Stain solution and heating with a hot air gun or by exposure to iodine.
All other solvents and chemicals were used as supplied from Aldrich Chemical Co., Acros, Fisher, Alfa Aesar or Lancaster Synthesis. Aniline 1, morpholin-4-amine 2, 4-methylpiperazin-1-amine 3, (±) sec-Butylamine 4, 2-Morpholinoethanamine 5, N1,N1-bis(2-Aminoethyl)ethane-1,2-diamine 6 (Tris), Benzylamine 7 (CAS 100-46-9), Pyridin-4-ylmethanamine 8 (CAS 3731-53-1), 2′-(Piperidin-1-yl)ethanamine 9, 2-Aminothiazole 10, Glycine 11, 3-(1H-Imidazol-1-yl)propan-1-amine 12, sodium dimethyldithiocarbamate 13a, sodium diethyldithiocarbamate 14a, Pyrrolidine 15, Diisobutylamine 16, Dipropylamine 17, Dibutylamine 18, Dihexylamine 19, Ethylbutyamine 20, Diethanolamine 21, N-methylbenzenamine 22, N,N-Benzylmethylamine 23, Morpholine 24, Piperazine 25, 4-Cyano-4-phenylpiperidine hydrochloride 26, L-Proline 27 were purchased from Sigma-Aldrich (Milan, Italy) and were of the highest available purity. Purity of the prepared DTCS has been determined by HPLC and was > 99 %.
General procedure for the synthesis of compounds 1a-27a.30
Secondary/primary amines 1-27 (1.0 g, 1.0 eq) were treated with a NaOH, KOH or Et3N (1.0 - 2.2 eq), 4.0 ml of MeOH as co-solvent was used, and the solutions were stirred at 0°C for 20 min (Scheme 1). Then carbon disulfide (1.2 – 2.4 eq) was added dropwise and the mixture was stirred at r.t. until starting material was consumed (TLC monitoring). The solvents were removed under vacuo at r.t. and the residues obtained were dissolved in MeOH, filtered off trough Celite and the filtrate was concentrated in vacuo not exceeding 20 °C.
Synthesis of triethylammonium phenylcarbamodithioate 1a
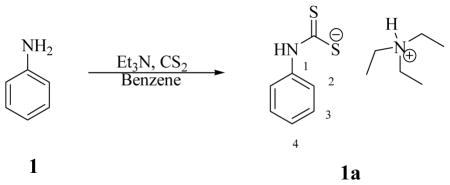
Aniline 1 (0.5 g, 1.0 eq) was treated with triethylamine (1.0 eq) in benzene (0.5 ml) followed by addition of carbon disulfide (1.0 eq) at 0°C. The mixture was warmed to r.t. and stirred O.N. at r.t.. The solid formed was washed with diethyl ether and dried under vacuo to afford the titled compound as a light yellow solid in 51% yield.
Triethylammonium phenylcarbamodithioate 1a: νmax (KBr) cm−1, 2960, 2886, 1648, 1599, 1520, 1451; δH (400 MHz, DMSO-d6) 1.13 (9H, t, J 6.8, 3x CH2CH3), 2.98 (6H, brs, 3x CH2CH3), 6.97 (1H, t, J 8.0, 4-H), 7.22 (2H, dd, J 8.3, 8.0, 2x 3-H), 7.93 (2H, d, J 8.3, 2x 2-H), 9.00 (1H, brs, exchange with D2O, (CH3CH2)3N+-H), 10.10 (1H, brs, exchange with D2O, N-H); δC (100 MHz, DMSO-d6) 10.0, 46.6, 114.8, 122.9, 128.4, 143.2, 215.5 (C=S); m/z (ESI), 168 [M-Na]−.
Diisobutylcarbodithioic acid sodium salt 16a: m.p. 220 °C with dec; νmax (KBr) cm−1, 2961, 2933, 2867, 1640, 1601, 1520, 1480, 1090; δH (400 MHz, DMSO-d6) 0.84 (12H, d, J 6.8, 4 x CH3), 2.43 (4H, m, 2 x CH), 3.86 (4H, d, J 7.2, 2 x CH2); δC (100 MHz, DMSO-d6) 21.2, 27.4, 61.5, 215.4 (C=S); m/z (ESI), 204 [M-Na]−. Data are in agreement with reported data.30
Synthesis of morpholinecarbamodithioate sodium salt 24a.
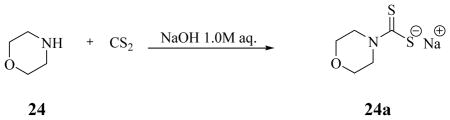
Morpholine 24 (1.0 g, 1.0 eq) was treated according to the general procedure with 1.0 M aqueous solution of NaOH (1.0 eq) followed by addition of carbon disulfide (1.2 eq). The title compound was obtained as a white solid in quantitative yield.
Morpholinecarbamodithioate sodium salt 24a: m.p. 320 °C with dec; νmax (KBr) cm−1, 2966, 2901, 2854, 1625, 1520, 1416, 1215; δH (400 MHz, DMSO-d6) 3.52 (4H, t, J 8.0 CH2), 4.33 (2H, t, J 8.0, CH2); δC (100 MHz, DMSO-d6) 50.6, 67.1, 215.4 (C=S); m/z (ESI), 162 [M-Na]−.
CA inhibition
An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalysed CO2 hydration activity. Phenol red (at a concentration of 0.2 mM) has been used as indicator, working at the absorbance maximum of 557 nm, with 20 mM Hepes (pH 7.5) as buffer, and 20 mM Na2SO4 (for maintaining constant the ionic strength), following the initial rates of the CA-catalyzed CO2 hydration reaction for a period of 10–100 s.32 The CO2 concentrations ranged from 1.7 to 17 mM for the determination of the kinetic parameters and inhibition constants. For each inhibitor at least six traces of the initial 5–10% of the reaction have been used for determining the initial velocity. The uncatalyzed rates were determined in the same manner and subtracted from the total observed rates. Stock solutions of inhibitor (0.1 mM) were prepared in distilled-deionized water and dilutions up to 0.01 nM were done thereafter with distilled-deionized water. Inhibitor and enzyme solutions were preincubated together for 15 min at room temperature prior to assay, in order to allow for the formation of the E-I complex. The inhibition constants were obtained by non-linear least-squares methods using PRISM 3, as reported earlier,33 and represent the mean from at least three different determinations. All CA isofoms were recombinant ones obtained in house as reported earlier.9,10
Co-crystallization and X-ray data collection of CA II complex
Co-crystals for the hCA II – 24a complex were obtained using the hanging drop vapor diffusion method.37 Drops of 10 μL (0.3 mM hCA II; 0.7 mM DTC 24a; 0.1 % dimethyl sulfoxide; 0.8 M sodium citrate; 50 mM Tris-HCl; pH 8.0) were equilibrated against the precipitant solution (1.6 M sodium citrate; 50 mM Tris-HCl; pH 8.0) at room temperature (~20 °C). Crystals were observed after 5 days. Based on visual selection a crystal of the complex was cryoprotected by quick immersion into 20% glycerol precipitant solution and flash-cooled by exposing to a gaseous stream of nitrogen at 100 K. The X-ray diffraction data was collected using an R-AXIS IV++ image plate system on a Rigaku RU-H3R Cu rotating anode operating at 50 kV and 22 mA, using Osmic Varimax HR optics. The detector-crystal distance was set to 80 mm. The oscillation steps were 1° with a 5 min exposure per image. Indexing, integration, and scaling were performed using HKL2000.38
Structure determination of CA II drug complex
Starting phases were calculated from Protein Data Bank (PDB) entry 3KS339 with waters removed. Refinement using Phenix package,40 with 5% of the unique reflections selected randomly and excluded from the refinement data set for the purpose of Rfree calculations,41 was alternated with manual refitting of the model in Coot.42 The validity of the final model was assessed by PROCHECK.43 Complete refinement statistics and model quality are included in Table 2.
Animals and glaucoma induction
Adult male New Zealand Albino rabbits weighing 2–2.5 Kg were employed in this study. The animals were utilized in groups of eight for each of the chosen specific treatments. The experimental procedures were conform to those of the Declaration of Helsinki and with the Guideline for the Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National Institute of Health, and were conducted upon authorization of the Italian Regulations on Protection of Animals used for experimental and other scientific purpose (DM 116/1992) as well as with the European Union Regulations (OJ of ECL 358/1, 12/12/1986), and the experimental protocol was approved by the local animal care committee of the University of Florence (Florence, Italy). The rabbits were kept in individual cages; food and water were provided ad libitum. The animals were identified with a tattoo on the ear, numbered consecutively and maintained on a 12–12h light/dark cycle in a temperature controlled room (22°–23°C). All selected animals were examined before the beginning of the study and were determined to be normal on ophthalmic and general examinations. Glaucoma was induced by injection of 0.1 ml 0.25% carbomer (Siccafluid, FarMila - THEA Pharmaceuticals) into anterior eye-chamber bilaterally in New Zeeland albino rabbits] anesthetized with tiletamine and zolazepam (Zoletil 100, 0.05 mg/Kg b.w.) plus xilazine (Xilor 2%, 0.05 ml/Kg b.w.) i.m., by the procedure previously reported.10c IOP was measured before carbomer injection and after 1, 2 and 4 hours the first day and three times a day until stabilization and then every 24 hours. All rabbits treated with carbomer presented a net increase in IOP. One drop of 0.2% oxybuprocaine hydrochloride (Novesine, Sandoz) diluted 1:1 with sterile saline was instilled in each eye immediately before each set of pressure measurements. IOP was measured using a Tono-Pen XL tonometer (Medtronic Solan, USA) as reported by earlier.10 The pressure readings were matched with two-point standard pressure measurements at 1, 2, 4 and 8 hours after the instillation of the drug and once a day for the following days using a Digilab calibration verifier. All IOP measurements were done by the same investigators using the same tonometer. As soon as a stable IOP increase was obtained, the animals were treated with the drugs in study. The efficacy of the different drugs in lowering IOP was evaluated after drug administration over four hours, with the following schedule: before and after 30, 60, 90, 120 and 240 minutes after drug administration. The treatment was performed in three animals per drug in one eye and compared to the contralateral eye treated with vehicle. A group of 4 non glaucomatous albino rabbits was treated with the drugs of this study and used as control. At the end of the experiments the animals were killed with a lethal dose of Pentothal (Abbott S.p.A., Campoverde di Aprilia, LT).
Supplementary Material
Acknowledgments
This research was financed by an FP7 EU project (Metoxia) and a grant from the NIH GM 25154. We thank Dr. G. Formicola and Dr. T. Somma for their collaboration in intraocular pressure measurements.
Abbreviations
- CA
carbonic anhydrase
- CAI
carbonic anhydrase inhibitor
- DTC
dithiocarbamate
Footnotes
The atomic coordinates of the hCA II-dithiocarbmate adduct (code 3P5A), have been deposited in the Protein Data Base.
Supporting information available: The complete characterization of compunds 1a-27a is described in detail. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.a) Krishnamurthy VM, Kaufman GK, Urbach AR, Gitlin I, Gudiksen KL, Weibel DB, Whitesides GM. Carbonic Anhydrase as a Model for Biophysical and Physical-Organic Studies of Proteins and Protein-Ligand Binding. Chem Rev. 2008;108:946–1051. doi: 10.1021/cr050262p. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zatovicova M, Jelenska L, Hulikova A, Csaderova L, Ditte Z, Ditte P, Goliasova T, Pastorek J, Pastorekova S. Carbonic anhydrase IX as an anticancer therapy target: preclinical evaluation of internalizing monoclonal antibody directed to catalytic domain. Curr Pharm Des. 2010;16:3255–3263. doi: 10.2174/138161210793429832. [DOI] [PubMed] [Google Scholar]; c) Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov. 2008;7:168–181. doi: 10.1038/nrd2467. [DOI] [PubMed] [Google Scholar]; d) Supuran CT, Scozzafava A, Casini A. Development of sulfonamide carbonic anhydrase inhibitors (CAIs) In: Supuran CT, Scozzafava A, Conway J, editors. Carbonic anhydrase – Its Inhibitors and Activators. CRC Press; Boca Raton (FL): 2004. pp. 67–147. [Google Scholar]
- 2.a) Supuran CT, Scozzafava A, Casini A. Carbonic anhydrase inhibitors. Med Res Rev. 2003;23:146. doi: 10.1002/med.10025. [DOI] [PubMed] [Google Scholar]; b) Pastorekova Parkkila S, Pastorek J, Supuran CT. Carbonic anhydrases: current state of the art, therapeutic applications and future prospects. J Enzyme Inhib Med Chem. 2004;19:199–229. doi: 10.1080/14756360410001689540. [DOI] [PubMed] [Google Scholar]; c) Thiry A, Dogné JM, Masereel B, Supuran CT. Targeting tumor-associated carbonic anhydrase IX in cancer therapy. Trends Pharmacol Sci. 2006;27:566–573. doi: 10.1016/j.tips.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 3.a) Supuran CT. Carbonic anhydrase inhibitors. Bioorg Med Chem Lett. 2010;20:3467–3474. doi: 10.1016/j.bmcl.2010.05.009. [DOI] [PubMed] [Google Scholar]; b) Supuran CT. Carbonic anhydrase inhibitors and activators for novel therapeutic applications. Future Med Chem. 2011;3:1165–1180. doi: 10.4155/fmc.11.69. [DOI] [PubMed] [Google Scholar]; c) Nishimori I, Minakuchi T, Maresca A, Carta F, Scozzafava A, Supuran CT. The β-carbonic anhydrases from Mycobacterium tuberculosis as drug targets. Curr Pharm Des. 2010;16:3300–3309. doi: 10.2174/138161210793429814. [DOI] [PubMed] [Google Scholar]
- 4.a) Supuran CT. Bacterial carbonic anhydrases as drug targets: towards novel antibiotics ? Front Pharmacol. 2011;2:34. doi: 10.3389/fphar.2011.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Winum JY, Kohler S, Supuran CT. Brucella carbonic anhydrases: New targets for designing anti-infective agents. Curr Pharm Des. 2010;16:3310–3316. doi: 10.2174/138161210793429850. [DOI] [PubMed] [Google Scholar]; c) Lopez M, Bornaghi LF, Innocenti A, Vullo D, Charman SA, Supuran CT, Poulsen SA. Sulfonamide Linked Neoglycoconjugates - A New Class of Inhibitors for Cancer-Associated Carbonic Anhydrases. J Med Chem. 2010;53:2913–2926. doi: 10.1021/jm901888x. [DOI] [PubMed] [Google Scholar]
- 5.a) Švastová E, Hulıeková A, Rafajová M, Zat’ovičová M, Gibadulinová A, Casini A, Cecchi A, Scozzafava A, Supuran C, Pastorek J. Hypoxia activates the capacity of tumor-associated carbonic anhydrase IX to acidify extracellular pH. FEBS Letters. 2004;577:439–444. doi: 10.1016/j.febslet.2004.10.043. [DOI] [PubMed] [Google Scholar]; b) Ebbesen P, Pettersen EO, Gorr TA, Jobst G, Williams K, Kienninger J, Wenger RH, Pastorekova S, Dubois L, Lambin P, Wouters BG, Supuran CT, Poellinger L, Ratcliffe P, Kanopka A, Görlach A, Gasmann M, Harris AL, Maxwell P, Scozzafava A. Taking advantage of tumor cell adaptations to hypoxia for developing new tumor markers and treatment strategies. J Enzyme Inhib Med Chem. 2009;24(S1):1–39. doi: 10.1080/14756360902784425. [DOI] [PubMed] [Google Scholar]
- 6.a) Dubois L, Lieuwes NG, Maresca A, Thiry A, Supuran CT, Scozzafava A, Wouters BG, Lambin P. Imaging of CA IX with fluorescent labelled sulfonamides distinguishes hypoxic and (re)-oxygenated cells in a xenograft tumour model. Radiother Oncol. 2009;92:423–428. doi: 10.1016/j.radonc.2009.06.019. [DOI] [PubMed] [Google Scholar]; b) Ahlskog JKJ, Dumelin CE, Trüssel S, Marlind J, Neri D. In vivo targeting of tumor-associated carbonic anhydrases using acetazolamide derivatives. Bioorg Med Chem Lett. 2009;19:4851–4856. doi: 10.1016/j.bmcl.2009.06.022. [DOI] [PubMed] [Google Scholar]; c) Buller F, Steiner M, Frey K, Mircsof D, Scheuermann J, Kalisch M, Bühlmann P, Supuran CT, Neri D. Selection of Carbonic Anhydrase IX Inhibitors from One Million DNA-Encoded Compounds. ACS Chem Biol. 2011;6:336–344. doi: 10.1021/cb1003477. [DOI] [PubMed] [Google Scholar]; d) Chiche J, Ilc K, Laferrière J, Trottier E, Dayan F, Mazure NM, Brahimi-Horn MC, Pouysségur J. Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res. 2009;69:358–368. doi: 10.1158/0008-5472.CAN-08-2470. [DOI] [PubMed] [Google Scholar]; e) Swietach P, Wigfield S, Cobden P, Supuran CT, Harris AL, Vaughan-Jones RD. Tumor-associated carbonic anhydrase 9 spatially coordinates intracellular pH in three-dimensional multicellular growth. J Biol Chem. 2008;283:20473–20483. doi: 10.1074/jbc.M801330200. [DOI] [PubMed] [Google Scholar]
- 7.a) Supuran CT. Diuretics: From classical carbonic anhydrase inhibitors to novel applications of the sulfonamides. Curr Pharm Des. 2008;14:641–648. doi: 10.2174/138161208783877947. [DOI] [PubMed] [Google Scholar]; b) De Simone G, Di Fiore A, Supuran CT. Are carbonic anhydrase inhibitors suitable for obtaining antiobesity drugs ? Curr Pharm Des. 2008;14:655–660. doi: 10.2174/138161208783877820. [DOI] [PubMed] [Google Scholar]; c) de Leval X, Ilies M, Casini A, Dogné JM, Scozzafava A, Masini E, Mincione F, Starnotti M, Supuran CT. Carbonic anhydrase inhibitors: Synthesis and topical intraocular pressure lowering effects of fluorine-containing inhibitors devoid of enhanced reactivity. J Med Chem. 2004;4:2796–2804. doi: 10.1021/jm031116j. [DOI] [PubMed] [Google Scholar]
- 8.a) Sugrue MF. The pharmacology of antiglaucoma drugs. Pharmacol Ther. 1999;43:91–138. doi: 10.1016/0163-7258(89)90049-1. [DOI] [PubMed] [Google Scholar]; b) Carta F, Supuran CT, Scozzafava A. Novel therapies for glaucoma. A patent review 2007–2011. Expert Opin Ther Pat. 2012;22:79–88. doi: 10.1517/13543776.2012.649006. [DOI] [PubMed] [Google Scholar]
- 9.a) Mincione F, Scozzafava A, Supuran CT. The development of topically acting carbonic anhydrase inhibitors as antiglaucoma agents. Curr Pharm Des. 2008;14:649–654. doi: 10.2174/138161208783877866. [DOI] [PubMed] [Google Scholar]; b) Mincione F, Scozzafava A, Supuran CT. Antiglaucoma carbonic anhydrase inhibitors as ophthalomologic drugs. In: Supuran CT, Winum JY, editors. In Drug Design of Zinc-Enzyme Inhibitors: Functional, Structural, and Disease Applications. Wiley; Hoboken (NJ): 2009. pp. 139–154. [Google Scholar]
- 10.a) Steele RM, Batugo MR, Benedini F, Biondi S, Borghi V, Carzaniga L, Impagnatiello F, Maglietta D, Chong WKM, Rajapakse R, Cecchi A, Temperini C, Supuran CT. Nitric oxide-donating carbonic anhydrase inhibitors for the treatment of open-angle glaucoma. Bioorg Med Chem Lett. 2009;19:6565–6570. doi: 10.1016/j.bmcl.2009.10.036. [DOI] [PubMed] [Google Scholar]; b) Mincione F, Benedini F, Biondi S, Cecchi A, Temperini C, Formicola G, Pacileo I, Scozzafava A, Masini E, Supuran CT. Synthesis and crystallographic analysis of new sulfonamides incorporating NO-donating moieties with potent antiglaucoma action. Bioorg Med Chem Lett. 2011;21:3216–3221. doi: 10.1016/j.bmcl.2011.04.046. [DOI] [PubMed] [Google Scholar]; c) Fabrizi F, Mincione F, Somma T, Scozzafava G, Galassi F, Masini E, Impagnatiello F, Supuran CT. A new approach to antiglaucoma drugs: carbonic anhydrase inhibitors with or without NO donating moieties. Mechanism of action and preliminary pharmacology. J Enzyme Inhib Med Chem. 2012;27:138–147. doi: 10.3109/14756366.2011.597749. [DOI] [PubMed] [Google Scholar]
- 11.Supuran CT, Scozzafava A. Carbonic anhydrase inhibitors and their therapeutic potential. Expert Opin Ther Pat. 2000;10:575–600. [Google Scholar]
- 12.Liao SY, Ivanov S, Ivanova A, Ghosh S, Cote MA, Keefe K, Coca-Prados M, Stanbridge EJ, Lerman MI. Expression of cell surface transmembrane carbonic anhydrase genes CA9 and CA12 in the human eye: overexpression of CA12 (CAXII) in glaucoma. J Med Genet. 2003;40:257–261. doi: 10.1136/jmg.40.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.a) Kivelä AJ, Parkkila S, Saarnio J, Karttunen TJ, Kivelä J, Parkkila AK, Pastoreková S, Pastorek J, Waheed A, Sly WS, Rajaniemi H. Expression of the membrane-associated carbonic anhydrase isozyme XII in the human kidney and renal tumors. Histochem Cell Biol. 2000;114:197–204. doi: 10.1007/s004180000181. [DOI] [PubMed] [Google Scholar]; b) Hussain SA, Ganesan R, Reynolds G, Gross L, Stevens A, Pastorek J, Murray PG, Perunovic B, Anwar MS, Billingham L, James ND, Spooner D, Poole CJ, Rea DW, Palmer DH. Hypoxia-regulated carbonic anhydrase IX expression is associated with poor survival in patients with invasive breast cancer. Br J Cancer. 2007;96:104–109. doi: 10.1038/sj.bjc.6603530. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Chen CL, Chu JS, Su WC, Huang SC, Lee WY. Hypoxia and metabolic phenotypes during breast carcinogenesis: expression of HIF-1alpha, GLUT1, and CAIX. Virchows Arch. 2010;457:53–61. doi: 10.1007/s00428-010-0938-0. [DOI] [PubMed] [Google Scholar]
- 14.a) Chia SK, Wykoff CC, Watson PH, Han C, Leek RD, Pastorek J. Prognostic significance of a novel hypoxia-regulated marker, carbonic anhydrase IX, in invasive breast carcinoma. J Clin Oncol. 2001;19:3660–8. doi: 10.1200/JCO.2001.19.16.3660. [DOI] [PubMed] [Google Scholar]; b) Wykoff CC, Beasley NJ, Watson PH, Turner KJ, Pastorek J, Sibtain A, Wilson GD, Turley H, Talks KL, MaxwellPHPugh CW, Ratcliffe PJ, Harris AL. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 2000;60:7075–7083. [PubMed] [Google Scholar]; c) Bartosova M, Parkkila S, Pohlodek K, Karttunen TJ, Galbavy S, Mucha V, Harris AL, Pastorek J, Pastorekova S. Expression of carbonic anhydrase IX in breast is associated with malignant tissues and is related to overexpression of c-erbB2. J Pathol. 2002;197:1–8. doi: 10.1002/path.1120. [DOI] [PubMed] [Google Scholar]
- 15.Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov. 2011;10:767–777. doi: 10.1038/nrd3554. [DOI] [PubMed] [Google Scholar]
- 16.Ditte P, Dequiedt F, Svastova E, Hulikova A, Ohradanova-Repic A, Zatovicova M, Csaderova L, Kopacek J, Supuran CT, Pastorekova S, Pastorek J. Phosphorylation of carbonic anhydrase IX controls its ability to mediate extracellular acidification in hypoxic tumors. Cancer Res. 2011;71:7558–7767. doi: 10.1158/0008-5472.CAN-11-2520. [DOI] [PubMed] [Google Scholar]
- 17.a) Stillebroer AB, Mulders PF, Boerman OC, Oyen WJ, Oosterwijk E. Carbonic anhydrase IX in renal cell carcinoma: implications for prognosis, diagnosis, and therapy. Eur Urol. 2010;58:75–83. doi: 10.1016/j.eururo.2010.03.015. [DOI] [PubMed] [Google Scholar]; b) Siebels M, Rohrmann K, Oberneder R, Stahler M, Haseke N, Beck J. A clinical phase I/II trial with the monoclonal antibody cG250 (RENCAREXR) and interferon-alpha-2a in metastatic renal cell carcinoma patients. World J Urol. 2011;29:121–126. doi: 10.1007/s00345-010-0570-2. [DOI] [PubMed] [Google Scholar]; c) van Schaijk FG, Oosterwijk E, Soede AC, Broekema M, Frielink C, McBride WJ. Pretargeting with bispecific anti-renal cell carcinoma x anti-DTPA(In) antibody in 3 RCC models. J Nucl Med. 2005;46:495–501. [PubMed] [Google Scholar]
- 18.a) Lou Y, McDonald PC, Oloumi A, Chia SK, Ostlund C, Ahmadi A, Kyle A, Auf dem Keller U, Leung S, Huntsman DG, Clarke B, Sutherland BW, Waterhouse D, Bally MB, Roskelley CD, Overall CM, Minchinton A, Pacchiano F, Carta F, Scozzafava A, Touisni N, Winum JY, Supuran CT, Dedhar S. Targeting Tumor Hypoxia: Suppression of Breast Tumor Growth and Metastasis by Novel Carbonic Anhydrase IX Inhibitors. Cancer Res. 2011;71:3364–3376. doi: 10.1158/0008-5472.CAN-10-4261. [DOI] [PubMed] [Google Scholar]; b) Pacchiano F, Carta F, McDonald PC, Lou Y, Vullo D, Scozzafava A, Dedhar S, Supuran CT. Ureido-substituted benzenesulfonamides potently inhibit carbonic anhydrase IX and show antimetastatic activity in a model of breast cancer metastasis. J Med Chem. 2011;54:1896–1902. doi: 10.1021/jm101541x. [DOI] [PubMed] [Google Scholar]
- 19.a) Groves K, Bao B, Zhang J, Handy E, Kennedy P, Cuneo G, Supuran CT, Yared W, Peterson JD, Rajopadhye M. Synthesis and evaluation of near-infrared fluorescent sulfonamide derivatives for imaging of hypoxia-induced carbonic anhydrase IX expression in tumors. Bioorg Med Chem Lett. 2012;22 doi: 10.1016/j.bmcl.2011.10.058. in press. [DOI] [PubMed] [Google Scholar]; b) Touisni N, Maresca A, McDonald PC, Lou Y, Scozzafava A, Dedhar S, Winum JY, Supuran CT. Glycosyl coumarin carbonic anhydrase IX and XII inhibitors strongly attenuate the growth of primary breast tumors. J Med Chem. 2012;54:8271–8277. doi: 10.1021/jm200983e. [DOI] [PubMed] [Google Scholar]
- 20.a) Maresca A, Temperini C, Vu H, Pham NB, Poulsen SA, Scozzafava A, Quinn RJ, Supuran CT. Non-zinc mediated inhibition of carbonic anhydrases: coumarins are a new class of suicide inhibitors. J Am Chem Soc. 2009;131:3057–3062. doi: 10.1021/ja809683v. [DOI] [PubMed] [Google Scholar]; b) Maresca A, Temperini C, Pochet L, Masereel B, Scozzafava A, Supuran CT. Deciphering the mechanism of carbonic anhydrase inhibition with coumarins and thiocoumarins. J Med Chem. 2010;53:335–344. doi: 10.1021/jm901287j. [DOI] [PubMed] [Google Scholar]
- 21.Carta F, Temperini C, Innocenti A, Scozzafava A, Kaila K, Supuran CT. Polyamines inhibit carbonic anhydrases by anchoring to the zinc-coordinated water molecule. J Med Chem. 2010;53:5511–5522. doi: 10.1021/jm1003667. [DOI] [PubMed] [Google Scholar]
- 22.a) Innocenti A, Vullo D, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. Interactions of phenols with the 12 catalytically active mammalian isoforms (CA I – XIV) Bioorg Med Chem Lett. 2008;18:1583–1587. doi: 10.1016/j.bmcl.2008.01.077. [DOI] [PubMed] [Google Scholar]; b) Innocenti A, Vullo D, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of mammalian isoforms I – XIV with a series of substituted phenols including paracetamol and salicylic acid. Bioorg Med Chem. 2008;16:7424–742. doi: 10.1016/j.bmc.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 23.a) Innocenti A, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of cytosolic isoforms I, II, III, VII and XIII with less investigated inorganic anions. Bioorg Med Chem Lett. 2009;19:1855–1857. doi: 10.1016/j.bmcl.2009.02.088. [DOI] [PubMed] [Google Scholar]; b) Innocenti A, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of transmembrane isoforms IX, XII and XIV with less investigated inorganic anions. Bioorg Med Chem Lett. 2010;20:1548–1550. doi: 10.1016/j.bmcl.2010.01.081. [DOI] [PubMed] [Google Scholar]
- 24.Temperini C, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. X-Ray crystal studies of the carbonic anhydrase II – trithiocarbonate adduct – An inhibitor mimicking the sulfonamide and urea binding to the enzyme. Bioorg Med Chem Lett. 2010;20:474–478. doi: 10.1016/j.bmcl.2009.11.124. [DOI] [PubMed] [Google Scholar]
- 25.Carta F, Aggarwal M, Maresca A, Scozzafava A, McKenna R, Supuran CT. Dithiocarbamates: a new class of carbonic anhydrase inhibitors. Crystallographic and kinetic investigations. Chem Commun (Cambridge) 2012;48:1868–1870. doi: 10.1039/c2cc16395k. [DOI] [PubMed] [Google Scholar]
- 26.Marzano C, Ronconi L, Chiara F, Giron MC, Faustinelli I, Cristofori P, Trevisan A, Fregona D. Gold(III)-dithiocarbamato anticancer agents: activity, toxicology and histopathological studies in rodents. Int J Cancer. 2011;129:487–496. doi: 10.1002/ijc.25684. [DOI] [PubMed] [Google Scholar]
- 27.Amin E, Saboury AA, Mansuri-Torshizi H, Moosavi-Movahedi AA. Potent inhibitory effects of benzyl and p-xylidine-bis dithiocarbamate sodium salts on activities of mushroom tyrosinase. J Enzyme Inhib Med Chem. 2010;25:272–281. doi: 10.1080/14756360903179351. [DOI] [PubMed] [Google Scholar]
- 28.Morpurgo L, Desideri A, Rigo A, Viglino P, Rotilio G. Reaction of N,N-diethyl-dithiocarbamate and other bidentate ligands with Zn, Co and Cu bovine carbonic anhydrases. Inhibition of the enzyme activity and evidence for stable ternary enzyme-metal-ligand complexes. Biochim Biophys Acta. 1983;746:168–175. doi: 10.1016/0167-4838(83)90071-7. [DOI] [PubMed] [Google Scholar]
- 29.Kolayli S, Karahalil F, Sahin H, Dincer B, Supuran CT. Characterization and inhibition studies of an α-carbonic anhydrase from the endangered sturgeon species Acipenser gueldenstaedti. J Enzyme Inhib Med Chem. 2011;26:895–900. doi: 10.3109/14756366.2011.554415. [DOI] [PubMed] [Google Scholar]
- 30.a) Kiran Kumar STVS, Kumar L, Sharma VL, Jain A, Jain RK, Maikhuri JP, Kumar M, Shukla PK, Gupta G. Carbodithioic acid esters of fluoxetine, a novel class of dual-function spermicides. Eur J Med Chem. 2008;43:2247–256. doi: 10.1016/j.ejmech.2007.10.024. [DOI] [PubMed] [Google Scholar]; b) Maatschappij BV. Organic molibdenum compounds, use thereof as friction modifiers and lubricating compositions. 113814 A1 WO Pat. 2008; c) Ivanov AV, Korneeva EV, Gerasimenko AV, Forsling W. Structural Organization of Nickel(II), Zinc(II), and Copper(II) Complexes with Diisobutyldithiocarbamate: EPR, 13C and 15N CP/MAS NMR, and X-Ray Diffraction Studies. Russ J Coord Chem. 2005;31:695–707. [Google Scholar]; d) Rodina TA, Ivanov AV, Gerasimenko AV, Ivanov MA, Zaeva AS, Philippova TS, Antzutkin ON. A pyridine adduct of bis(di-iso-butyldithiocarbamato-S,S′)cadmium(II): Multinuclear (13C, 15N, 113Cd) CP/MAS NMR spectroscopy, crystal and molecular structure, and thermal behavior. Inorg Chim Acta. 2011;368:263–270. [Google Scholar]; e) Treasurywala AAM, Bagli J, Baker H. Substituted pyrimidones with antifungal properties. 1232904. CA Pat. 1996; f) Soliman R. Dithiocarbamate esters. Part III: Synthesis and spectra of tetrahydroquinoline dithiocarbamate and xanthate esters. Egypt J Chem. 1990;31:175–186. [Google Scholar]
- 31.Ivanov AV, Pakusina AP, Ivanov MA, Sharutin VV, Gerasimenko AV, Antzutkin ON, Groebner G, Forsling W. Synthesis and single-crystal X-ray diffraction and CP/MAS 13C and 15N NMR study of tetraphenylantimony N,N-dialkyldithiocarbamate complexes: A manifestation of conformational isomerism. Phys Chem. 2005;401:44–48. [Google Scholar]
- 32.Khalifah RG. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem. 1971;246:2561–2573. [PubMed] [Google Scholar]
- 33.a) Scozzafava A, Briganti F, Ilies MA, Supuran CT. Carbonic anhydrase inhibitors Synthesis of membrane-impermeant low molecular weight sulfonamides possessing in vivo selectivity for the membrane-bound versus the cytosolic isozymes. J Med Chem. 2000;43:292–300. doi: 10.1021/jm990479+. [DOI] [PubMed] [Google Scholar]; b) Supuran CT, Scozzafava A, Ilies MA, Briganti F. Carbonic anhydrase inhibitors. Synthesis of sulfonamides incorporating 2,4,6-trisubstituted-pyridinium-ethylcarboxamido moieties possessing membrane-impermeability and in vivo selectivity for the membrane-bound (CA IV) versus the cytosolic (CA I and CA II) isozymes. J Enzyme Inhib. 2000;15:381–401. doi: 10.1080/14756360009040695. [DOI] [PubMed] [Google Scholar]
- 34.(a) Alterio V, Di Fiore A, D’Ambrosio K, Supuran CT, De Simone G. X-Ray crystallography of CA inhibitors and its importance in drug design. In: Supuran CT, Winum JY, editors. Drug Design of Zinc-Enzyme Inhibitors: Functional, Structural, and Disease Applications. Wiley; Hoboken: 2009. pp. 73–138. [Google Scholar]; (b) Alterio V, Hilvo M, Di Fiore A, Supuran CT, Pan P, Parkkila S, Scaloni A, Pastorek J, Pastorekova S, Pedone C, Scozzafava A, Monti SM, De Simone G. Crystal structure of the extracellular catalytic domain of the tumor-associated human carbonic anhydrase IX. Proc Natl Acad Sci USA. 2009;106:16233– 16238. doi: 10.1073/pnas.0908301106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.a) Casini A, Antel J, Abbate F, Scozzafava A, David S, Waldeck H, Schafer S, Supuran CT. Carbonic anhydrase inhibitors: SAR and X-ray crystallographic study for the interaction of sugar sulfamates/sulfamides with isozymes I, II and IV. Bioorg Med Chem Lett. 2003;13:841–845. doi: 10.1016/s0960-894x(03)00029-5. [DOI] [PubMed] [Google Scholar]; b) De Simone G, Di Fiore A, Menchise V, Pedone C, Antel J, Casini A, Scozzafava A, Wurl M, Supuran CT. Carbonic anhydrase inhibitors. Zonisamide is an effective inhibitor of the cytosolic isozyme II and mitochondrial isozyme V: solution and X-ray crystallographic studies. Bioorg Med Chem Lett. 2005;15:2315–2320. doi: 10.1016/j.bmcl.2005.03.032. [DOI] [PubMed] [Google Scholar]; c) Temperini C, Innocenti A, Scozzafava A, Parkkila S, Supuran CT. The coumarin-binding site in carbonic anhydrase accommodates structurally diverse inhibitors: the antiepileptic lacosamide as an example. J Med Chem. 2010;53:850– 854. doi: 10.1021/jm901524f. [DOI] [PubMed] [Google Scholar]
- 36.a) Avvaru BS, Wagner JM, Maresca A, Scozzafava A, Robbins AH, Supuran CT, McKenna R. Carbonic anthydarse inhibitors. The X-ray crystal structure of human isoform II in adduct with adamantyl analogue of acetazolamide resides in a less utilized binding pocket than most hydrophobic inhibitors. Bioorg Med Chem Lett. 2010;20:4376–4381. doi: 10.1016/j.bmcl.2010.06.082. [DOI] [PubMed] [Google Scholar]; b) Wagner JM, Avvaru BS, Robbins AH, Scozzafava A, Supuran CT, McKenna R. Cumarinyl-substituted sulfonamides strongly inhibit several human carbonic anhydrase isoforms: solution and cryatallographic investigations. Bioorg Med Chem. 2010;18:4873–4878. doi: 10.1016/j.bmc.2010.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Pacchiano F, Aggarwal M, Avvaru BS, Robbins AH, Scozzafava A, McKenna R, Supuran CT. Selective hydrophobic pocket binding observed within the carbonic anhydrase II active site accommodate different 4-substituted-ureido-benzenesulfonamides and correlate to inhibitor potency. Chem Commun (Camb) 2010;46:8371–8373. doi: 10.1039/c0cc02707c. [DOI] [PubMed] [Google Scholar]
- 37.McPherson A. Preparation and Analysis of Protein Crystals. 1. Wiley; New York: 1982. [Google Scholar]
- 38.Otwinowski Z, Minor W. Processing of x-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 39.Avvaru BS, Kim CU, Sippel KH, Gruner SM, Agbandje-McKenna M, Silverman DN, McKenna R. A short, strong hydrogen bond in the active site of human carbonic anhydrase II. Biochemistry. 2010;49:249–251. doi: 10.1021/bi902007b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart P. H PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Cryst. 2010;D66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brunger AT. Free R value: a novel statistical quantity for assessing the accuracy of crystal structures. Nature. 1992;355:472–475. doi: 10.1038/355472a0. [DOI] [PubMed] [Google Scholar]
- 42.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr Sect. 2004;D60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 43.Laskowski RA, MacArthur MW, Moss DS, Thornton JM. PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Cryst. 1993;26:283–291. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.