Abstract
Autism spectrum disorders (ASDs) are characterized by impaired language and social skills, often with restricted interests and stereotyped behaviors. A previous investigation of blood plasma from children with ASDs (mean age = 5½ years) demonstrated that 21% of samples contained autoantibodies that reacted intensely with GABAergic Golgi neurons of the cerebellum while no samples from non-sibling, typically developing children showed similar staining (Wills et al., 2009). In order to characterize the clinical features of children positive for these autoantibodies, we analyzed plasma samples from children enrolled in the Autism Phenome Project, a multidisciplinary project aimed at identifying subtypes of ASD. Plasma from male and female children (mean age = 3.2 years) was analyzed immunohistochemically for the presence of autoantibodies using histological sections of macaque monkey brain. Immunoreactivity to cerebellar Golgi neurons and other presumed interneurons was observed for some samples but there was no difference in the rate of occurrence of these autoantibodies between children with ASD and their typically developing peers. Staining of neurons, punctate profiles in the molecular layer of the dentate gyrus, and neuronal nuclei were also observed. Taken together, 42% of controls and subjects with ASD demonstrated immunoreactivity to some neural element. Interestingly, children whose plasma reacted to brain tissue had scores on the Child Behavior Checklist (CBCL) that indicated increased behavioral and emotional problems. Children whose plasma was immunoreactive with neuronal cell bodies scored higher on multiple CBCL scales. These studies indicate that additional research into the genesis and prevalence of brain-directed autoantibodies is warranted.
Keywords: Autism, autoantibody, interneurons, immunohistochemistry, Child Behavior Checklist
Introduction
Autism spectrum disorders (ASDs) are characterized by impaired social functioning and language development combined with a restricted or stereotyped set of behaviors that emerge by three years of age. The most recent estimate of their prevalence indicates that one in 110 children in the United States is diagnosed with an ASD (2009). Males are affected about four times more frequently than females (Fombonne, 2005). Higher concordance rates for these disorders in monozygotic twins compared to dizygotic twins suggest that ASDs are highly heritable (Bailey et al., 1995), and multiple different genetic variations are known to be associated with ASDs (Folstein and Rosen-Sheidley, 2001). Currently, ten to twenty percent of cases are associated with defined mutations, genetic syndromes, or de novo copy number variations, and further cases will likely be attributable to genetic variations upon further study (Abrahams and Geschwind, 2008). However, none of the variations discovered so far consistently results in an individual developing an ASD, suggesting other factors must be involved (Abrahams and Geschwind, 2008).
Numerous studies have identified abnormalities in the immune systems of some individuals with an ASD (Ashwood et al., 2006; Pardo et al., 2005). Postmortem studies point to increased microglial activation in the cerebellum, cortex, and white matter of some individuals with ASD compared to typically developing controls (Morgan et al., 2010; Vargas et al., 2005). Brain tissue and cerebrospinal fluid of children and adults with autism display an increased level of the cytokines macrophage chemoattractant protein-1, tumor growth factor-1, tumor necrosis factor , interleukin-6, granulocyte-macrophage colony-stimulating factor, and interferon (Li et al., 2009; Vargas et al., 2005). In a study involving 80 subjects with ASD, a screen for anti-nuclear antibodies in serum found that more children with autism possess anti-nuclear antibodies than typically developing children (Mostafa and Kitchener, 2009). Using western blots, several groups have identified increased levels of autoantibodies to proteins in the central nervous system in the plasma of some children with an ASD (Cabanlit et al., 2007; Singer et al., 2006; Singh et al., 1997; Singh and Rivas, 2004; Wills et al., 2009) and in some mothers of children with ASD (Braunschweig et al., 2008; Croen et al., 2008; Singer et al., 2008; Zimmerman et al., 2007). Immunohistochemical studies, in which plasma from affected children is tested for immunoreactivity to sectioned brain tissue, demonstrate that at least some of these autoantibodies can bind to cells in the brain (Connolly et al., 1999; Dalton et al., 2003; Wills et al., 2009; Wills et al., Submitted).
Our laboratories recently showed that plasma from 21% of a group of 34 children with ASD contained autoantibodies that reacted intensely with GABAergic Golgi neurons in primate cerebellar tissue (Wills et al., 2009). Analysis of the same plasma in different brain regions showed that individuals whose plasma reacted intensely with cerebellar Golgi cells also showed reactivity to interneurons in several other brain regions, including the cerebral cortex and the hippocampus (Wills et al., Submitted). It is currently unknown whether children with interneuron-reactive antibodies can be distinguished behaviorally from children without these antibodies.
The clinical presentation of behaviors varies considerably across individuals diagnosed with an ASD, leading to the suggestion that there may be many different forms of autism and that these forms may arise from different causes (Geschwind and Levitt, 2007). Studies linking biological findings in subjects with ASD to behavioral measures will be instrumental in identifying these multiple forms of ASD. In the cohort for which intense plasma reactivity to cerebellar Golgi neurons was first observed, there was relatively little clinical information to correlate with the presence of autoantibodies. In order to further investigate the clinical characteristics of individuals whose plasma contains autoantibodies to interneurons, we sought to extend our earlier observations by examining the plasma of individuals for which detailed clinical information is available. Thus, we examined autoantibodies in plasma collected as part of the Autism Phenome Project (APP), a multidisciplinary project at the Medical Investigation of Neurodevelopmental Disorders (M.I.N.D) Institute aimed at identifying subtypes of ASD. Subjects participating in the APP undergo a blood draw as part of a comprehensive multidisciplinary analysis that includes diagnostic, behavioral, and neuropsychological assessments, a medical examination, structural magnetic resonance imaging (MRI), electroencephalogram (EEG) analysis, and characterization of immune function. As participants in the APP are mostly in the 24 to 42 month age range, the children investigated in the APP represent a younger subject group than those examined in the initial description by Wills et al. (2009).
We tested plasma from 129 subjects with either typical development or ASD for autoantibodies to Golgi cells of the cerebellum and other interneurons throughout the brain. By enrolling this group of age-matched, preschool-aged children for whom we have high quality multidisciplinary data we aimed to investigate associations between autoantibody status, development, behavior, and other characteristics of ASD.
Methods
Subjects
Subjects were enrolled for participation in the Autism Phenome Project (APP) through the M.I.N.D Institute. The study protocol was approved by the Institutional Review Board for the UC Davis School of Medicine, and parents of each subject provided written informed consent for their child to participate. Subjects included 86 children with ASD (age range = 24 – 67 months, mean age 38.3 months, 71 males and 15 females) who had been previously diagnosed with ASD or suspected of having ASD, and 43 typically developing children (age range = 27 – 56 months, mean age 37.6 months, 29 males and 14 females). Age is reported as age in months at the time of the blood draw (Table 1).
Table 1.
Demographics of study subjects
| Diagnosis | Median Age | Age Range | Male | Female |
|---|---|---|---|---|
| (months) | ||||
| ASD (n=86) | 39 | 24 - 67 | 71 | 15 |
| Typically developing (n=43) | 36 | 27 - 56 | 29 | 14 |
Behavioral Measures
The Autism Diagnostic Observation Schedule (ADOS) and the Autism Diagnostic Interview–Revised (Lord et al., 1994; Lord et al., 2000) were used to re-diagnose all the ASD subjects by clinical psychologists at enrollment into the project. These two measures represent the current gold standard for autism diagnosis. The ADOS involves a semi-structured play based interview with the child and was administered by the diagnosing psychologist. The ADI-R is a detailed parent interview and was administered by a licensed social worker. All administrators of these instruments had been previously trained by Dr. Lord or by a supervising lab psychologist who had been so trained. Inter-rater agreement was monitored and was maintained at 80% or better throughout the study.
Typically developing children were screened for ASD using the Social Communication Questionnaire (SCQ) (Rutter, 2003). Children scoring above 10 on the SCQ, indicating autism risk, received a diagnostic evaluation using the ADOS and ADI-R. Children were included in the ASD group if they (1) met the ADOS cutoff score for ASD, (2) met the autism cutoff score for either the social or communication domain of the ADI-R, (3) were within 2 points of the autism cutoff score for the second of the two ADI-R domains, (4) met all DSM IV clinical diagnostic criteria for an ASD, and (5) and were considered to have autism via clinical judgment by two independent licensed clinical psychologists with expertise in ASD diagnosis. Children were included in the typically developing (TD) group if their score on the SCQ was 10 or below, and if their score on the Mullen Scales of Early Learning was between 70 and 130.
Parents of each subject completed the Child Behavior Checklist, a 100-item checklist that assesses potential behavioral and emotional problems. The CBCL is a very widely used, psychometrically rigorous instrument for assessing a wide range of possible emotional disorders in children and has norms for children 1½ - 5 years of age (Achenbach and Ruffle, 2000), the age group involved in this study. Examples of items included on the CBCL are “Afraid to try new things”, “Doesn’t get along with other children”, and “Too fearful or anxious” (Achenbach and Ruffle, 2000). For each item, the parent circles ‘0’ if the item is not true of their child, ‘1’ if the item is somewhat or sometimes true, and ‘2’ if the item is very true or often true. Thus, a higher total score on the CBCL indicates more behavioral difficulties being reported by the parent. The CBCL provides a number of factor scores as well as a total score: Internalizing and Externalizing factor scores, 7 subscale scores that correspond to symptom domains (Emotionally Reactive, Anxious/Depressed, Somatic Complaints, Withdrawn, Sleep Problems, Attention Problems, and Aggressive Behavior), and 5 DSM-oriented scales (Affective Problems, Anxiety Problems, Pervasive Developmental Problems, Attention Deficit/Hyperactivity Problems, and Oppositional Defiant Problems). Of the 129 subjects whose plasma was assayed, complete CBCLs were available for 116 subjects (77 with ASD, 39 TD). For the remaining 13 subjects, the CBCL was not received from the subjects’ parents within three months of the subject enrolling in the study.
Sample Collection
Blood was collected by clinical pediatric phlebotomists and was coded to conceal the diagnosis of each individual. A total of 30-50 ml of blood was collected from each child as part of the APP, and plasma for this assay was obtained from blood collected into an 8.5 ml BD Vacutainer ACD Yellow Top Tube. Tubes were centrifuged for 10 minutes at 2100 rpm to isolate the plasma, and aliquots of plasma were stored at −80 C until the time of use.
Tissue Preparation
Coronal sections of brain tissue from a rhesus macaque (Macaca mulatta) were used for immunohistochemical analysis. The high degree of cross-reactivity between human and nonhuman primate samples and the ability to obtain multiple sections of a consistently high quality of fixation make nonhuman primate tissue an ideal source of tissue for this assay. Further, the previous detection of autoantibodies to cerebellar Golgi neurons used nonhuman primate tissue for the assay (Wills et al., 2009). All procedures were carried out under an approved University of California Davis Institutional Animal Care and Use Protocol and strictly adhered to National Institutes of Health policies on primate animal subjects. A single healthy adult male (5 ½ year old) rhesus macaque from the colony at the California National Primate Research Center was euthanized with an overdose of sodium pentobarbital and was transcardially perfused with the following: 1% paraformaldehyde (PFA) at 250 ml/min for 2 minutes, 4% PFA at 250 ml/min for 10 minutes, 4% PFA at 100 ml/min for 50 minutes, then 5% sucrose at 100 ml/min for 20 minutes. The brain was removed from the skull, blocked coronally into slabs, cryoprotected for 4 days (overnight in 10% glycerol, 2% DMSO, and for 3 days in 20% glycerol, 2% DMSO), and sectioned at 30 m on a freezing, sliding microtome.
Two sections were immunohistochemically processed for each plasma sample: these included a coronal section of one hemisphere at the level of the hippocampus and a coronal section through the cerebellum. The hemisphere section always included somatosensory cortex as well as many other cortical and subcortical regions (Fig. 1A). To maximize the number of cerebellar sections available for this assay, the cerebellum was detached from the rest of the brain by cutting the brain stem at a level just caudal to the mamillary nuclei and the tissue was cut parallel to this transection resulting in coronal sections (Fig. 1B). Cerebellar sections spanning most of the rostrocaudal extent of the structure were used but the rostralmost and caudalmost sections were excluded because they did not include enough folia to properly analyze the section.
Figure 1.
Representative sections used for immunohistochemical assay. One 30 m coronal section through the cerebrum at the level of the hippocampus (A) and one 30 m section through the cerebellum (B) were used as a source of antigen to test each plasma sample for autoantibodies. Every eighth section of the hemisphere and cerebellum were stained with thionin to identify the cytoarchitectural boundaries within each section. Calibration bar = 5 mm.
Immunohistochemistry (IHC)
Methods for immunohistochemical staining were similar to those previously published, with the addition of an avidin biotin blocking step to reduce background staining (Wills et al., 2009). Tissue was washed three times in 0.1 M PBS for 10 minutes each, pre-incubated in 0.5% H202, washed again, and blocked for 4 hours in 5% normal mouse serum and 0.5% Triton X-100 in PBS. Tissue was further blocked using the Vector avidin biotin blocking kit (SP-2001), then incubated for 40-48 hours in a 1:100 dilution of subject plasma diluted with 2% normal mouse serum and 0.3% Triton X-100 in PBS. Tissue was washed six times in 0.1M PBS with 2% goat serum for 5 minutes, and was then incubated in a 1:500 dilution of biotinylated mouse anti-human IgG (Zymed 05-4240) with 2% normal mouse serum and 0.3% Triton X-100 in PBS for one hour. Tissue was washed again in 0.1 M PBS with 2% goat serum three times for 10 minutes, incubated in ABC peroxidase (Biostain Super ABC/Peroxidase Basic Kit; Biomeda) for 45 minutes, washed in 0.1 M PBS with 2% goat serum three times for 10 minutes, and incubated in secondary solution again for 45 minutes. After an additional three washes with 0.1M PBS, tissue was incubated for a second time in ABC peroxidase for 30 minutes, and was then washed with 0.05M Tris buffer followed by a 30 minute incubation in 3,3-diaminobenzidine (DAB) (Fisher) in 0.05M Tris with 0.04% hydrogen peroxide. After 3 ten minute rinses in 0.05M Tris, tissue was mounted onto gelatin coated slides and dried at 37°C overnight.
Silver Nitrate/Gold Chloride Intensification
The DAB reaction product was intensified by exposing the tissue to silver nitrate followed by gold chloride as described previously (Wills et al., 2009). Briefly, slides were defatted in a mixture of equal parts chloroform and 100% ethanol for 4 hours, hydrated in graded ethanols, and placed in a 37°C incubator overnight. Slides were then rinsed with running deionized water for 10 minutes, incubated in a 1% silver nitrate solution maintained at 56°C for 40 minutes, and rinsed again with running deionized water for 10 minutes. A 10-minute incubation in a 0.2% gold chloride solution with agitation at room temperature was followed with further rinsing and then stabilization in a 5% sodium thiosulfate solution at room temperature for 15 minutes with agitation. A final rinse in running deionized water for 10 minutes was followed by dehydration through graded ethanols and xylenes and coverslipping using DPX mounting medium (Sigma Aldrich).
Nissl Staining
A 1:8 series of sections from the cerebral and cerebellar blocks was stained with thionin to establish the cytoarchitectural boundaries throughout the brain. Sections were collected in PBS, mounted on gelatin-coated slides, and defatted in 1:1 chloroform and 100% ethanol. They were then re-hydrated, placed in 0.25% thionin for 25-45 seconds, rinsed in deionized water and then dehydrated through graded ethanols and xylene. The slides were then coverslipped and dried in the hood for at least one week before viewing.
Analysis of immunoreactivity
This study was designed to further investigate the finding that some children with ASD produce intense reactivity to Golgi neurons of the cerebellum and select other interneurons (Wills et al., 2009). All analyses of immunohistochemical results were carried out blind to the diagnosis of each subject. Sections were scored for the presence of autoantibodies bound to Golgi cells of the cerebellum and cortical interneurons resembling the pattern of immunoreactivity observed in the previous study. Within each batch of immunohistochemically-processed samples, one section was processed using immunoreactive plasma from the Wills et al study. This provided a positive control for the assay, and enabled staining patterns to be compared with those described previously. We did not limit our analysis solely to observations of immunoreactivity to interneurons; any specific labeling of brain tissue in either the cerebral hemisphere or the cerebellar section was noted.
Sections were analyzed by brightfield microscopy using a Nikon Eclipse E600, and were photographed on a Leica Leitz DMRB microscope with a Spot Diagnostic Instruments Digital Camera System and software. Immunolabeled cells were identified using morphological features such as their location, cell body size, and dendritic configuration.
Data Analysis
The number of ASD and control subjects whose plasma demonstrated immunoreactivity to brain tissue was compared using a Chi-square analysis. We report p-values associated with Fisher’s Exact test because in some instances the number of subjects in a given cell of the analysis was less than or equal to five. The pattern of results using Pearson Chi-square (appropriate for larger sample sizes) was the same. P-values less than 0.05 were considered statistically significant. T-tests were used to compare CBCL t-scores for subjects whose plasma reacted to brain tissue to those whose plasma did not. For the Total score, a p-value less than 0.05 was considered significant, and for each of the CBCL subscores the p-value cutoff was divided by the total number of component scores (thus, the Internalizing/Externalizing cutoff was 0.025, the symptom domain cutoff was 0.007, and the DSM-oriented scale cutoff was 0.01). Analyses were conducted using SPSS for Macintosh Version 17.
Results
Were GABAergic autoantibodies observed in this cohort?
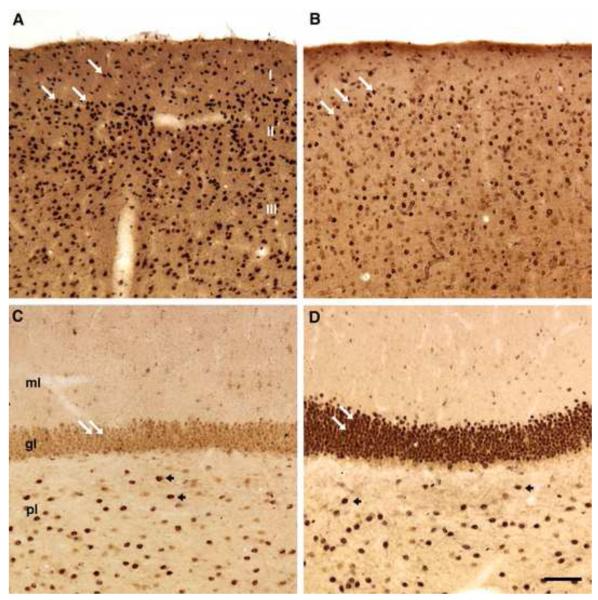
We found thirteen samples that selectively stained interneurons including the cerebellar Golgi cells. The pattern of staining was essentially the same as that observed in Wills et al (2009) (Fig. 2). In the cerebellum, numerous Golgi cells were labeled in the granule cell layer, near its interface with the Purkinje cell layer (Fig. 2A-C,E-F). The position and morphology of labeled cells were strikingly similar across samples that were scored positively in the current study and among sections stained with previously identified immunoreactive plasma. Consistent with the observations made with samples from the cohort analyzed in the Wills et al. (2009) paper, many of the samples that were reactive for cerebellar Golgi cells also labeled interneurons in other brain regions. Several small, round interneurons with radiating dendrites were labeled across multiple regions of the neocortex (Fig. 2G-I). In the dentate gyrus, plasma labeled cells in the polymorphic and molecular layers (Fig. 2J-L) consistent with findings from the earlier cohort (Wills et al, Submitted). We conclude that the interneuron pattern of immunoreactivity identified for 10% of plasma samples from the Autism Phenome Project recapitulates the pattern previously described in Wills et al. (2009).
Figure 2.
Interneuron labeling by plasma samples from the current study recapitulates the immunoreactivity observed in Wills et al. (2009). (A-F) Coronal sections through the macaque cerebellum stained with plasma from the original cohort (A) or from plasma from the current study (B-F, calibration bar = 100 m). (B,C) Two plasma samples from children with ASD in which Golgi cells at the interface between the molecular layer (mol) and granule cell layer (gcl) are specifically labeled, recapitulating the pattern observed in A (arrows). (D) A plasma sample from a TD individual that is negative for interneuron immunoreactivity. (E,F) Two plasma samples from TD children in which the pattern of Golgi cell immunoreactivity shown in panel (A) is observed. (G-I) Coronal sections through macaque somatosensory cortex labeled with plasma from the original study (G) or from the current study (H, I). Multiple interneurons, identified by size and position, are labeled in (G) and the labeling from an individual with ASD (H) and a TD subject (I) from the APP recapitulates this pattern. Calibration bar for panels G-I = 250 m. (J-L) Coronal sections through the macaque dentate gyrus. Panel (J) shows a section processed with interneuron-positive plasma from the original study, in which several plasma-labeled cells are found in the polymorphic layer (pl) and fewer cells are labeled in the granule cell layer (gl) and molecular layer (ml). Panels (K) and (L) show strikingly similar staining with plasma from a subject with ASD (K) and a TD subject (L) from the current cohort. Calibration bar for panels J-L = 100 m.
We next investigated whether this pattern of interneuron immunoreactivity appeared selectively in plasma samples obtained from individuals with ASD. Surprisingly, many of the plasma samples from the typically developing controls expressed the anti-Golgi cell staining that was essentially indistinguishable from the subjects with autism. Interneuron labeling was observed in 10% (9/86) of individuals with ASD (Fig. 2B-C, H, K) and 9% (4/43) of TD individuals (Fig. 2E-F, I, L), a difference that was not significant (p=1.00, Fisher’s exact test).
We also analyzed the intensity of staining for each positively stained section as previously described (Wills et al., 2009). Sections were scored as having “intensely labeled” interneurons, or “++”, if (1) Golgi cells were consistently and darkly labeled across most, or all, of the cerebellum, and (2) numerous cortical interneurons were darkly labeled in multiple regions of cortex. Sections were scored as “moderately labeled”, or “+”, if intense labeling was less consistent across the hemispheric or cerebellar section, and “somewhat labeled” (“−+”) if light labeling of interneurons in the hemisphere or cerebellum was observed. The percentage of individuals given each score was similar for the two groups (Table 2).
Table 2.
Scores for immunolabeling in interneurons.
| Intensity of interneuron immunoreactivity | ||||
|---|---|---|---|---|
| Diagnosis | ++ | + | −+ | − − |
| ASD (n=86) | 3 (3%) | 3 (3%) | 3 (3%) | 77 (90%) |
| TD (n=43) | 2 (5%) | 0 | 2 (5%) | 39 (91%) |
Other patterns of autoantibody immunoreactivity
Several other patterns of immunoreactivity were also observed using this assay (Table 3). The total number of plasma samples for which any type of specific immunoreactive staining was observed was 54 (42%). There was no difference in the occurrence of plasma immunoreactivity to brain tissue among individuals with ASD (36/86, 42%) compared to TD individuals (18/43, 42%, p=1.00, Fisher’s exact test).
Table 3.
Frequency of plasma immunoreac tivity in each category observed.
| Diagnosis | Inter- neurons |
Neurons | Punctate Profiles in DG |
Nuclei | Low Frequency AutoAb |
2 or More Types of AutoAb |
No Immun- oreactivity Observed |
|---|---|---|---|---|---|---|---|
| ASD (n=86) | 9 (10%) | 12 (14%) | 5 (6%) | 3 (3%) | 11 (13%) | 4 (5%) | 50 (58%) |
| TD (n=43) | 4 (9%) | 4 (9%) | 5 (12%) | 2 (5%) | 8 (19%) | 4 (9%) | 25 (58%) |
AutoAb=autoantibody, DG = dentate gyrus
Neuronal staining
Several plasma samples produced immunohistochemical reactivity in most or all cortical neurons. This immunoreactivity did not appear to be restricted to any particular type of neuron. Rather, multiple neurons were labeled in all cortical layers (Fig. 3B). In addition, select neurons in the hippocampal formation were also labeled in these cases, including most, or all, of the neurons in the granule cell layer, many cells in the polymorphic layer, and occasional labeling of cells in the molecular layer of the dentate gyrus (not illustrated). The occurrence of plasma producing this pattern of reactivity in children with ASD (12/86, 14%,) did not differ significantly from that of controls (4/43, 9%, p=0.58). Because this pattern of immunoreactivity and the interneuron pattern of immunoreactivity both involve staining of multiple neurons throughout the brain, we also analyzed the data by grouping together the plasma samples producing a neuronal pattern of staining with those staining interneurons. No difference was found in the occurrence of children with ASD scoring positively for either pattern (19/86, 22%) compared to controls (7/43, 16%, p=0.49, Fisher’s exact test).
Figure 3.
Photomicrographs of coronal sections through the somatosensory cortex showing immunoreactivity to neurons that was observed for some plasma samples. (A) A section that was processed in the absence of plasma in parallel with plasma-processed sections, showing the absence of specific staining within the tissue. The section shown in (B) was processed with plasma from a TD subject in which multiple cortical neurons were labeled (arrows). Calibration bar = 100 m.
Staining in the molecular layer of the dentate gyrus
Multiple plasma-labeled sections displayed intense labeling of small punctate profiles in the outer portion of the molecular layer of the dentate gyrus (Fig. 4B and C). At higher magnification, these profiles look crystalline. For each of the plasma samples that resulted in this pattern of staining, the round profiles were present in a laminar pattern in which staining was restricted to the mid to outer half of the molecular layer of the dentate gyrus. This is the terminal region for the perforant path projection that arises in the entorhinal cortex. This staining pattern has not been observed in our previous neuroanatomical studies of the macaque monkey hippocampal formation. To confirm that this was not a spurious finding related to a particular nonhuman primate, we stained sections from another rhesus macaque and observed similar, albeit less dense, staining in the same regions of the second monkey. Thus, antibodies from several children seem to identify something that is associated with the perforant path distribution in the dentate gyrus but the origin of this profile is currently unknown. This pattern of reactivity in the dentate gyrus was observed in 6% (5/86) of plasma samples from children with ASD and 12% (5/43) of samples from TD children, a difference which was not statistically significant (p=0.30, Fisher’s exact test).
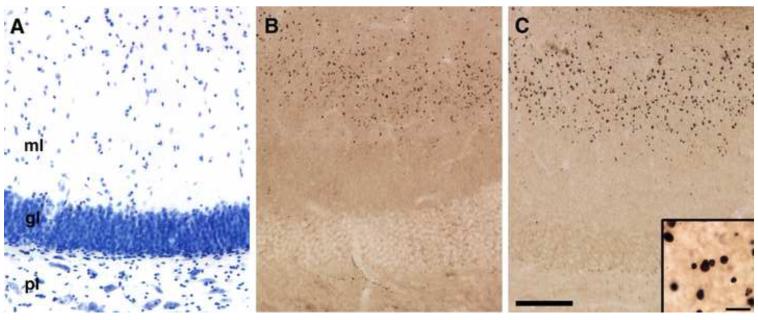
Figure 4.
Photomicrographs of coronal sections through the macaque dentate gyrus showing plasma-immunoreactivity in a laminar pattern in the molecular layer. (A) A Nissl-stained section reveals the three layers of the dentate gyrus: the molecular layer (ml), the granule cell layer (gl), and polymorphic cell layer (pl). The sections in (B) and (C) are representative sections in which processing with plasma resulted in a laminar pattern of staining that was restricted to the outer half of the molecular layer. This pattern of staining was observed with some plasma samples obtained from individuals with ASD (B), and some TD individuals (C). Inset in (C) shows a higher magnification photomicrograph. Calibration bar = 100 m (A-C) and 10 m (inset in C).
Nuclear staining
In five cases, immunohistochemical processing revealed specific labeling of the nuclei in several cells within the brain. Nuclei throughout the cerebral hemisphere were labeled with these five samples and are best illustrated in cortical and hippocampal regions (Fig. 5). In the cortex, nuclei in all layers were labeled by these samples, and within the dentate gyrus, nuclei in the granule cell layer and polymorphic layer were also intensely labeled. Some nuclei in the molecular layer were labeled less intensely. Three of these samples came from individuals with ASD (3%), and two came from TD children (5%). The occurrence of positive reactivity to brain nuclei in the ASD and TD groups did not differ (p=1.00, Fisher’s exact test).
Figure 5.
Plasma labeling of nuclei in the somatosensory cortex and dentate gyrus. (A-B) Coronal sections of macaque somatosensory cortex in which nuclei are labeled (arrows) by plasma from an individual with ASD (A) and a TD individual (B). (C-D) Coronal sections through the dentate gyrus labeled with plasma from two different individuals with ASD in which nuclei in the granule cell layer (gl, white arrows) and polymorphic layer (pl, arrowheads) are intensely labeled. Less intense labeling is also observed in the molecular layer (ml). Calibration bar = 100 m.
Infrequently observed patterns of immunolabeling
Several other patterns of reactivity to sectioned brain tissue were observed at a low frequency (fewer than five of the 129 plasma samples assayed, see Fig. 6 and 7). These patterns included staining of the hilar neurons of the hippocampal formation, which represent subpopulations of GABAergic hippocampal neurons (n=4, 3 from ASD group and 1 from TD group, Fig. 6A), beaded axons (n=3, 1 from ASD group and 2 from TD group, Fig. 6B), other axonal staining (n=3, 2 from ASD group and 1 from TD group, Fig. 6C), blood vessels (n=2, 1 from ASD group and 1 from TD group, Fig. 6D), neurofilaments (n=1, from ASD group, Fig. 6E), Purkinje cells (n=1, from TD group, Fig. 6F), a subset of cortical interneurons distinct from that observed with cerebellar Golgi staining (n=1, from the ASD group), association connections in the dentate gyrus (n=1, from ASD group, Fig. 7A), mossy fibers of the dentate gyrus (n=1, from ASD group, Fig. 7B), proximal dendrites throughout the brain, with specific dendritic labeling found in the neocortex, the CA1 region of the hippocampal formation, and dendrites of the Purkinje cells in the cerebellum (n=1, from the TD group, Fig. 7C-D), and variable diffuse staining (n=1, from TD group). We analyzed these infrequent patterns of staining as a group, to occurrence of one of these patterns of immunoreactivity did not determine whether they occurred more frequently in either the ASD or TD group. The differ significantly between subjects with ASD (11/86, 13%) compared with TD subjects (8/43, 19%, p=0.43, Fisher’s exact test). Of the staining patterns that were produced by more than one sample, no pattern was observed exclusively from samples from children in the ASD or the TD group.
Figure 6.
Photomicrographs of macaque brain sections stained with plasma that produced one of several infrequently observed patterns of immunoreactivity (Part I). (A) A section through the dentate gyrus processed with plasma from an individual with ASD showing specific staining of hilar neurons of the polymorphic layer. (B) Section processed with plasma that produced staining of axons with a beaded morphology in the polymorphic layer of the dentate gyrus (arrows). This section was processed with plasma from a TD subject. (C) A section stained with plasma from a TD subject in which axons are selectively immunolabeled, with several labeled axons in the somatosensory cortex shown here (arrows). (D) A different plasma sample, from a TD subject, produced blood vessel labeling throughout the brain. Labeling (arrows) in the cerebellum is shown here. (E) A section through the cerebellum processed with plasma from a TD subject showing intense immunoreactivity to the cell bodies (*) and dendrites (arrows) of the Purkinje cells. (F) Immunolabeling of the basket plexus (arrows) surrounding Purkinje cells in the cerebellum in the molecular layer. Cell bodies of the Purkinje cells are surrounded by the basket plexus and are denoted by a ‘*’. This tissue was processed with plasma from a subject with ASD that resulted in labeling of neurofilaments throughout the brain. Each calibration bar = 100 m. gl = granule cell layer of dentate gyrus, gcl= granule cell layer of cerebellum, ml = molecular layer of dentate gyrus, mol= molecular layer of cerebellum, pl = polymorphic layer of dentate gyrus. I, II, and III refer to the first, second, and third layers of neocortex.
Figure 7.
Photomicrographs of macaque brain sections stained with plasma that produced one of several infrequently observed patterns of immunoreactivity (Part II). (A) Associational connections in the dentate gyrus (dark band between arrowheads) were labeled with plasma from an individual with ASD. Cells in the polymorphic layer were also labeled (arrows). oml = outer molecular layer, iml = inner molecular layer, gl = granule cell layer, pl = polymorphic cell layer. (B) A section through the CA3 region of the hippocampal formation in which the mossy fibers were labeled by plasma from an individual with ASD. sr = stratum radiatum, sl = stratum lucidum, pcl = pyramidal cell layer, so = stratum oriens. (C,D) Sections labeled with plasma from a TD subject in which dendrites were specifically labeled throughout the brain. Dendrites in the CA1 region of the hippocampal formation (C) and in the molecular layer of the cerebellum (D) are shown here. Abbreviations for layers of the hippocampal formation are noted in (B). gcl= granule cell layer, mol = molecular layer. Each calibration bar = 100 m.
Two or more patterns of autoantibody reactivity
In some cases (n=8/129), a single plasma sample produced positive staining of more than one of the types described above. For example, one sample produced immunolabeling of nuclei as well as blood vessels, and a different sample produced labeling of cortical neurons and punctate profiles in the molecular layer of the dentate gyrus. For each type of immunoreactivity described above, the occurrence of positive staining in the ASD and TD groups was compared, regardless of whether some samples scored positively in another category as well. We also asked whether reactivity in more than one category occurred more frequently in one diagnostic group than the other, and found no statistically significant difference; 5% of subjects with ASD and 9% of TD subjects scored positively in more than one of the above categories (p=0.44, Fisher’s exact test).
Gender and immunoreactivity to brain tissue
Our analyses suggested that the occurrence of immunoreactivity to brain tissue was essentially the same in children with ASD as in TD subjects from the APP cohort. We next asked whether brain immunoreactivity occurred more commonly in either gender. Indeed, immunoreactivity to brain tissue (i.e. any type of positive staining) was significantly more common in females (72%) than in males (33%), p < .01. In looking at each pattern of immunoreactivity separately, we determined that reactivity to neurons occurred significantly more frequently in plasma from females (28%) than from males (8%, p < .01, Fisher’s exact test). There were no significant gender differences in the occurrence of other patterns of immunoreactivity.
Behavior and Emotional Problems
Our original aim included investigating more closely the children diagnosed with an ASD with autoantibodies that react to brain interneurons in order to determine whether they differed behaviorally from other children with an ASD. Because we found no significant differences between the ASD and TD groups with respect to immunoreactivity to brain, we did not pursue an in depth analysis of behavioral differences among the subjects with an ASD. Instead, we conducted an exploratory analysis to determine whether a relationship existed between evidence of autoreactivity to brain tissue and measures of behavior and emotional problems, regardless of diagnosis.
We first compared the CBCL scores of children whose plasma reacted in any way to brain tissue. Children with immunoreactive plasma (n=70) had significantly higher Internalizing t-scores compared to children whose plasma was negative for immunoreactivity (n=46), t(114) = −3.266, p = .001) (Table 4). In addition, children whose plasma showed immunoreactivity to brain tissue scored higher on the Withdrawn scale (t(114) = −3.372, p = .001) and the Pervasive Developmental Disorder scale (t(114) = −3.073, p = .003). When CBCL scores for children with ASD with and without reactivity to brain tissue were compared, children with an ASD with reactivity to brain tissue scored significantly higher on the Internalizing scale (t(75) = −3.442, p = .001), Withdrawn scale (t(75) = −4.076, p < .001), and Pervasive Developmental Disorder scale (t(75) = −3.955, p < .001). When subjects with typical development were analyzed separately, those with reactivity to brain tissue scored higher on the Internalizing scale (t(37) = −2.037, p = .049), but this was not significant after correcting for multiple comparisons. There was no significant difference between CBCL scores for males compared to females, so gender was not controlled for in these analyses.
Table 4.
Mean CBCL t-scores for subjects whose plas ma did and did not react to brain tissue (with the range of scores indicated in parentheses). Only those p values that remained significant after correcting for multiple comparisons are shown.
| Plasma reacted to brain tissue |
Plasma did not react to brain tissue |
p | |
|---|---|---|---|
| Total | 58.76 (37-87) | 54.31 (28-89) | ns |
| Internalizing | 59.20 (33-80) | 52.19 (29-82) | .001 |
| Externalizing | 55.59 (37-86) | 53.67 (28-77) | ns |
| Emotionally Reactive | 57.39 (50-77) | 55.04 (50-83) | ns |
| Anxious/Depressed | 53.74 (50-69) | 52.57 (50-79) | ns |
| Somatic Complaints | 57.61 (50-76) | 54.51 (50-78) | ns |
| Withdrawn | 69.96 (51-94) | 62.21 (50-100) | .001 |
| Sleep Problems | 55.87 (50-82) | 56.56 (50-94) | ns |
| Attention Problems | 61.35 (50-80) | 58.09 (50-80) | ns |
| Aggressive Behavior | 56.13 (50-91) | 56.54 (50-77) | ns |
| Affective Problems | 60.30 (50-77) | 57.13 (50-84) | ns |
| Anxiety Problems | 54.70 (50-75) | 53.81 (50-92) | ns |
| Pervasive Developmental Problems |
68.43 (50-96) | 61.87 (50-88) | .002 |
| Attention Deficit/ Hyperactivity Problems |
57.89 (50-76) | 56.36 (50-76) | ns |
| Oppositional Defiant Problems |
55.61 (50-77) | 56.31 (50-77) | ns |
ns = not significant
We next compared CBCL scores from children whose plasma showed different types of reactivity to brain tissue. Scores from children whose plasma reacted to interneurons (n=11) did not differ from those whose plasma did not (n=105). Similarly, no differences were observed between children with plasma that reacted positively versus negatively to punctate profiles of the molecular layer in the dentate gyrus (n=9 and 107 respectively), nuclei (n=5 and 111), or to an infrequently observed cell type (n=17 and 99).
Children whose plasma reacted to neurons in the brain (n=12) scored higher than those whose plasma did not (n=104) on the Total score (t(114) = −3.874, p < .001), Internalizing Score (t(114) = −3.879, p < .001 ), and Externalizing score (t(114) = −2.704, p = .008). Of the 7 symptom domain scores, subjects with plasma that labeled neurons scored higher on the following scales: Emotionally Reactive (t(114) = −3.502, p = .001), Somatic Complaints (t(114) = −3.584, p < .001), Withdrawn (t(114) = −4.349, p < .001), and Attention Problems (t(114) = −3.467, p = .001). Of the 5 DSM-oriented scales, subjects whose plasma reacted to neurons scored higher on the Affective Problems scale (t(114) = −4.013, p < .001), Pervasive Developmental Disorders scale (t(114) = −4.736, p < .001), and Attention Deficit/Hyperactivity Problems scale (t(114) = −3.200, p = .002) (Table 5). When subjects with ASD were analyzed separately, the Total (t(75) = −3.546, p = .001), Internalizing (t(75) = −3.772, p <.001), Emotional (t(75) = −2.995, p = .004), Withdrawn (t(75) = −3.592, p = .001), Affective Disorder (t(75) = −3.349, p = .001), and Pervasive Developmental Problems (t(75) = −4.550, p <.001) scores were higher for subjects whose plasma reacted to neurons compared to those whose plasma did not. Externalizing (t(75) = −2.274, p = .026), Somatic (t(75) = −2.383, p = .020), Attention Problems scale (t(75) = .2.495, p = .015), and Attention Deficit/Hyperactivity Problems scale (t(75) = −2.473, p = .016) scores were also higher at the p < .05 level, but the difference was not significant after correcting for multiple comparisons. The sample size for typically developing subjects was not large enough to conduct an analysis for these subjects separately.
Table 5.
Mean CBCL t-scores for subjects whose plas ma did and did not react to neurons (with the range of scores indicated in parentheses). Only those p values that remained significant after correcting for multiple comparisons are shown.
| Plasma reacted to neurons |
Plasma did not react to neurons |
p | |
|---|---|---|---|
| Total | 68.25 (46-87) | 54.67 (28-89) | <.001 |
| Internalizing | 66.75 (45-80) | 53.61 (29-82) | <.001 |
| Externalizing | 62.25 (47-86) | 53.53 (28-77) | .007 |
| Emotionally Reactive | 62.42 (50-77) | 55.23 (50-83) | .001 |
| Anxious/Depressed | 55.75 (50-69) | 52.72 (50-79) | ns |
| Somatic Complaints | 62.25 (50-72) | 54.99 (50-78) | <.001 |
| Withdrawn | 79.25 (56-94) | 63.67 (50-100) | <.001 |
| Sleep Problems | 61.92 (50-82) | 55.63 (50-94) | ns |
| Attention Problems | 67.17 (51-77) | 58.48 (50-80) | .001 |
| Aggressive Behavior | 60.92 (50-91) | 55.86 (50-77) | ns |
| Affective Problems | 67.33 (51-77) | 57.36 (50-84) | <.001 |
| Anxiety Problems | 59.17 (50-75) | 53.59 (59-92) | ns |
| Pervasive Developmental Problems |
78.33 (51-96) | 62.88 (50-88) | <.001 |
| Attention Deficit/ Hyperactivity Problems |
62.92 (52-76) | 56.28 (50-76) | .002 |
| Oppositional Defiant Problems |
57.92 (50-77) | 55.82 (50-77) | ns |
ns = not significant
Discussion
This study was initiated to determine whether children with autism spectrum disorders enrolled in the APP study with plasma autoantibodies to neural tissue were phenotypically different from autistic children who did not demonstrate anti-brain antibodies. We confirmed that some children with autism, younger than those that were previously analyzed (Wills et al. 2009), do indeed demonstrate antibodies that are immunoreactive to Golgi neurons in the cerebellum and interneurons distributed throughout the rest of the brain. However, we were surprised to find that age-matched typically developing controls in the APP cohort also demonstrated similar immunoreactivity and with similar prevalence.
We found several distinct patterns of reactivity, suggesting the presence of different autoantibodies, in both the ASD and TD groups. These included labeling of neurons, punctate profiles in the molecular layer of the dentate gyrus, nuclei, and multiple other infrequently observed patterns, suggesting the presence of multiple different brain-directed autoantibodies in our cohort. Our results provide the first detailed description of an immunohistochemical screen of pediatric plasma for antibodies recognizing antigens present in brain tissue.
While the functional implication of brain-reactive antibodies is unknown, the fact that they are present in typically developing children suggests that they may be benign. Diamond and colleagues have recently argued that circulating brain-reactive antibodies may in fact be more common than has previously been recognized (Diamond et al., 2009). They assert that B cells that react with the brain may not be systematically removed from the B cell repertoire, noting previous work that has found that antibodies that are specific for an epitope found on streptococcal bacteria (the N-acetyle--D-glucosamine receptor) can cross-react with neurons (Kirvan et al., 2006). This provides an example in which a brain-recognizing antibody has functional importance in protection against a pathogen. The fact that it also identifies brain proteins may be epiphenomenal.
There is, of course, the possibility that circulating brain directed autoantibodies, while not highly associated with autism may nonetheless be associated with changes in behavior. We were able to assess this since we acquired substantial behavioral data on the children who were evaluated for anti-brain antibodies. We found that subjects from both groups whose plasma contains brain-reactive antibodies had greater emotional - behavioral difficulties, as reported by parents on the CBCL, than did the remaining subjects. In looking at each type of reactivity separately, only the subjects whose plasma showed the neural pattern of reactivity (depicted in Figure 3) scored significantly higher on the CBCL. This was true for Total CBCL scores as well as scores on several subscales, even after correcting for multiple comparisons. This suggests that the subjects whose plasma reacts to neurons in the brain may be distinguishable from their peers behaviorally. While it is too early to make assertions about a possible function of the antibody/ies leading to the neural pattern of reactivity described here, our findings suggest the need for this to be examined in further detail in the future.
Our finding that intense immunoreactivity to interneurons, including Golgi cells of the cerebellum, was not restricted to children with ASD in plasma samples from the APP cohort is in contrast to what was previously reported by Wills et al. (2009), in which intense Golgi neuron immunoreactivity was exclusively observed in children with ASD (and one sibling of a child with autism). One difference between these two studies is the age of the subjects; subjects were younger and fell within a narrower range of ages in the current study (in which subjects were between 24 and 67 months) compared to the previous one (in which subjects were between 30 and 164 months). The current study included a larger number of children in both diagnostic groups (86 children with ASD and 43 TD children) compared to the study by Wills et al. (2009) (34 children with ASD and 23 TD children). In the current study, five percent of plasma samples from typically developing subjects displayed intense immunoreactivity to Golgi neurons. Given that 23 typically developing children were included in the Wills et al. (2009) study, we would only expect one subject to have displayed this pattern of intense staining if the frequency was similar between the two cohorts. Thus, it is perhaps not surprising that none of the control subjects’ plasma reacted intensely to Golgi neurons in the previous study.
In addition to the exclusionary criteria used in the current study (scoring 10 or below on the SCQ and between 70 and 130 on the MSEL), children from the Wills et al. (2009) study were excluded from the pool of typically developing subjects if they did not score within the normal range on the Vineland Adaptive Behavior Scales or were diagnosed with another disorder such as Attention Deficit Hyperactivity Disorder or Tourette’s Syndrome. Perhaps these additional criteria may have contributed to the different findings obtained between the two studies.
Many plasma samples gave rise to patterns of immunoreactivity that were distinct from the anti-GABA neuron pattern observed previously. These patterns included staining of neurons, punctate profiles in the molecular layer of the dentate gyrus, nuclei, and multiple other patterns of staining that were infrequently observed. Some of these additional staining patterns were also observed in the plasma from the Wills et al. (2009) cohort, including staining of nuclei and blood vessels. However, because the sample size was smaller in the previous cohort, fewer such cases were observed and they were not studied in detail.
Antinuclear antibodies have previously been detected in 8-12 year old Egyptian children with autism at a higher percentage than in the healthy control group using indirect immunofluorescence (Mostafa and Kitchener, 2009). In that study, 2.5% of healthy children had anti-nuclear antibodies compared with 20% of children with autism. Our finding that 5% of TD children scored positively for antibodies directed against nuclei roughly agrees with the Mostafa and Kitchener results for healthy children, but we found that a smaller percentage of children with ASD scored positively for anti-nuclear antibodies in our study population. Several differences including the ages of the subjects, differences in the genetic makeup of the study cohort, and very different experimental approaches may account for this discrepancy.
It is unclear whether the endothelial cell staining we observed in two samples is the same as what has been reported by Connoly and colleagues (Connolly et al., 1999). In the present study, the immunoreactivity observed in the two cases for which plasma labeled blood vessels appeared as a labeling of the perimeter of each vessel, with the depth of the vessel being readily observed in each section. In the Connoly et al. (1999) study, the endothelial reactivity appeared morphologically different but the tissue sections used in that study were 8 m thick compared to 30 m sections from the present study, making direct comparisons difficult. Further, in the Connoly et al. (1999) paper, the samples that labeled endothelial cells also labeled glial and neuronal nuclei, a finding that was paired with blood vessel labeling for only one subject in the present study. Nonetheless, we observed blood vessel staining in one individual with ASD and one typically developing subject, representing 1% and 2% of the total sample respectively. This is considerably less common than the 27% of ASD cases for which endothelial cells were labeled in the Connoly et al. (1999) study.
To our knowledge, the current study provides the first description of multiple patterns of specific staining of brain profiles observed in an immunohistochemical screening of plasma from children. While the original goal of our study was to characterize one particular pattern of staining, it is not surprising that we found several different patterns of immunoreactivity. In western blot experiments using human brain tissue as an antigen source to evaluate circulating autoantibodies, several bands indicating immunoreactivity are consistently found (Cabanlit et al., 2007; Singer et al., 2006; Singh and Rivas, 2004; Wills et al., 2009). As is the case in the present immunohistochemical experiment, the specific antigen identified by these immunoreactive bands is often unknown. It is also unsurprising that we found immunoreactivity to brain tissue in some samples from TD children, as immunoreactivity is consistently observed in control samples in the aforementioned western blot experiments; bands that do not discriminate children with autism from their typically developing peers are often present in these studies (Singer et al., 2006; Wills et al., 2009). Further, even those bands that are more prevalent in clinical populations are often present in some samples from control populations. For example, Singer et al. (2006) reported that serum from a significantly higher percentage of children with autism reacted to a 100 kDa protein in the caudate, putamen, and prefrontal cortex compared with controls (Singer et al., 2006). Although reactivity at this molecular weight occurred significantly more frequently in subjects with autism, 69-77% of control serum showed reactivity in the same brain regions. In a recent study aimed at identifying autoantibodies to neurofilament subunits in subjects with Down Syndrome, one third of control subjects were found to possess autoantibodies to the heavy subunit (Talja et al., 2009). For the medium and light subunits, a greater percentage of subjects with Down Syndrome possessed autoantibodies compared to controls. These experiments illustrate that immunoreactivity to brain tissue is frequently found in plasma from control subjects in western blotting experiments.
In a dot blot analysis using human temporal cortex, mean titer of circulating anti-brain antibody in twenty individuals with autism did not differ from those in fifteen control subjects (Todd et al., 1988). However, variation in the titer of anti-brain antibodies was found to be higher in the group of subjects with autism compared to controls. The authors suggest that any role for brain-reactive antibodies in autism would likely be restricted to a few specific antigens. In the current study, the occurrence of reactivity to brain tissue detectable in our immunohistochemical assay was similar in subjects with ASD and in controls, and it is unclear whether the antibodies we detected play a role in ASD.
In summary, this study provides the first published characterization of immunoreactivity to brain tissue using immunohistochemistry in a large group of pediatric samples. By using immunohistochemistry to assay for the presence of autoantibodies, one can simultaneously visualize not only the presence or absence of autoantibodies reacting to brain tissue but also the specific brain profiles to which they bind. Our results provide compelling evidence for multiple brain-reactive antibodies in plasma from children with ASD as well as typically developing toddlers that appear to segregate with behavior rather than diagnosis. Further, these studies highlight the need for future examination of brain-reactive antibodies to determine what, if any, role these autoantibodies may be playing during development.
Research Highligt.
Blood samples from 42% of young children contained brain-directed antibodies, the presence of which was associated with increased report of behavior or emotional problems.
Acknowledgements
This research was supported by NIH grants MH41479, MH089626, MH073124 and by a grant from NARSAD. Primate tissue was acquired from the California National Primate Research Center, RR000169. We would like to thank the laboratory of Dr. Cynthia L. Bethea for helpful advice regarding the use of the Vector Avidin Biotin blocking kit, Jose Rosa for assistance with tissue mounting and immunohistochemical processing, Lou Ann Barnett for coordinating data collected as part of the Autism Phenome Project, and the entire APP team for their contributions toward enrolling the children and collecting the samples analyzed in this study. We also thank the families that are participating in the Autism Phenome Project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors declare that there are no conflicts of interest
References
- Prevalence of autism spectrum disorders - Autism and Developmental Disabilities Monitoring Network, United States, 2006. MMWR Surveill Summ. 2009;58:1–20. [PubMed] [Google Scholar]
- Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet. 2008;9:341–55. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achenbach TM, Ruffle TM. The Child Behavior Checklist and related forms for assessing behavioral/emotional problems and competencies. Pediatr Rev. 2000;21:265–71. doi: 10.1542/pir.21-8-265. [DOI] [PubMed] [Google Scholar]
- Ashwood P, Wills S, Van de Water J. The immune response in autism: a new frontier for autism research. J Leukoc Biol. 2006;80:1–15. doi: 10.1189/jlb.1205707. [DOI] [PubMed] [Google Scholar]
- Bailey A, Couteur A. Le, Gottesman I, Bolton P, Simonoff E, Yuzda E, Rutter M. Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med. 1995;25:63–77. doi: 10.1017/s0033291700028099. [DOI] [PubMed] [Google Scholar]
- Braunschweig D, Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Croen LA, Pessah IN, Van de Water J. Autism: maternally derived antibodies specific for fetal brain proteins. Neurotoxicology. 2008;29:226–31. doi: 10.1016/j.neuro.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabanlit M, Wills S, Goines P, Ashwood P, Van de Water J. Brain-specific autoantibodies in the plasma of subjects with autistic spectrum disorder. Ann N Y Acad Sci. 2007;1107:92–103. doi: 10.1196/annals.1381.010. [DOI] [PubMed] [Google Scholar]
- Connolly AM, Chez MG, Pestronk A, Arnold ST, Mehta S, Deuel RK. Serum autoantibodies to brain in Landau-Kleffner variant, autism, and other neurologic disorders. J Pediatr. 1999;134:607–13. doi: 10.1016/s0022-3476(99)70248-9. [DOI] [PubMed] [Google Scholar]
- Croen LA, Braunschweig D, Haapanen L, Yoshida CK, Fireman B, Grether JK, Kharrazi M, Hansen RL, Ashwood P, Van de Water J. Maternal mid-pregnancy autoantibodies to fetal brain protein: the early markers for autism study. Biol Psychiatry. 2008;64:583–8. doi: 10.1016/j.biopsych.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton P, Deacon R, Blamire A, Pike M, McKinlay I, Stein J, Styles P, Vincent A. Maternal neuronal antibodies associated with autism and a language disorder. Ann Neurol. 2003;53:533–7. doi: 10.1002/ana.10557. [DOI] [PubMed] [Google Scholar]
- Diamond B, Huerta PT, Mina-Osorio P, Kowal C, Volpe BT. Losing your nerves? Maybe it’s the antibodies. Nat Rev Immunol. 2009;9:449–56. doi: 10.1038/nri2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein SE, Rosen-Sheidley B. Genetics of autism: complex aetiology for a heterogeneous disorder. Nat Rev Genet. 2001;2:943–55. doi: 10.1038/35103559. [DOI] [PubMed] [Google Scholar]
- Fombonne E. Epidemiology of autistic disorder and other pervasive developmental disorders. J Clin Psychiatry. 2005;66(Suppl 10):3–8. [PubMed] [Google Scholar]
- Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17:103–11. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Kirvan CA, Swedo SE, Snider LA, Cunningham MW. Antibody-mediated neuronal cell signaling in behavior and movement disorders. J Neuroimmunol. 2006;179:173–9. doi: 10.1016/j.jneuroim.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Li X, Chauhan A, Sheikh AM, Patil S, Chauhan V, Li XM, Ji L, Brown T, Malik M. Elevated immune response in the brain of autistic patients. J Neuroimmunol. 2009;207:111–6. doi: 10.1016/j.jneuroim.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, Couteur A. Le. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–85. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr., Leventhal BL, DiLavore PC, Pickles A, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–23. [PubMed] [Google Scholar]
- Morgan JT, Chana G, Pardo CA, Achim C, Semendeferi K, Buckwalter J, Courchesne E, Everall IP. Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biol Psychiatry. 2010;68:368–76. doi: 10.1016/j.biopsych.2010.05.024. [DOI] [PubMed] [Google Scholar]
- Mostafa GA, Kitchener N. Serum anti-nuclear antibodies as a marker of autoimmunity in Egyptian autistic children. Pediatr Neurol. 2009;40:107–12. doi: 10.1016/j.pediatrneurol.2008.10.017. [DOI] [PubMed] [Google Scholar]
- Pardo CA, Vargas DL, Zimmerman AW. Immunity, neuroglia and neuroinflammation in autism. Int Rev Psychiatry. 2005;17:485–95. doi: 10.1080/02646830500381930. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Berument SK, Conteur A. Le, Lord C. Social Communication Questionnaire (SCQ) manual. Western Psychological Services; Los Angeles: 2003. [Google Scholar]
- Singer HS, Morris CM, Williams PN, Yoon DY, Hong JJ, Zimmerman AW. Antibrain antibodies in children with autism and their unaffected siblings. J Neuroimmunol. 2006;178:149–55. doi: 10.1016/j.jneuroim.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Singer HS, Morris CM, Gause CD, Gillin PK, Crawford S, Zimmerman AW. Antibodies against fetal brain in sera of mothers with autistic children. J Neuroimmunol. 2008;194:165–72. doi: 10.1016/j.jneuroim.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Singh VK, Warren R, Averett R, Ghaziuddin M. Circulating autoantibodies to neuronal and glial filament proteins in autism. Pediatr Neurol. 1997;17:88–90. doi: 10.1016/s0887-8994(97)00045-3. [DOI] [PubMed] [Google Scholar]
- Singh VK, Rivas WH. Prevalence of serum antibodies to caudate nucleus in autistic children. Neurosci Lett. 2004;355:53–6. doi: 10.1016/j.neulet.2003.10.026. [DOI] [PubMed] [Google Scholar]
- Talja I, Reimand T, Uibo O, Reimand K, Aun S, Talvik T, Janmey PA, Uibo R. Antibodies to neurofilaments. Ann N Y Acad Sci. 2009;1173:130–6. doi: 10.1111/j.1749-6632.2009.04624.x. [DOI] [PubMed] [Google Scholar]
- Todd RD, Hickok JM, Anderson GM, Cohen DJ. Antibrain antibodies in infantile autism. Biol Psychiatry. 1988;23:644–7. doi: 10.1016/0006-3223(88)90012-1. [DOI] [PubMed] [Google Scholar]
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- Wills S, Cabanlit M, Bennett J, Ashwood P, Amaral DG, Van de Water J. Detection of autoantibodies to neural cells of the cerebellum in the plasma of subjects with autism spectrum disorders. Brain Behav Immun. 2009;23:64–74. doi: 10.1016/j.bbi.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills S, Rossi CC, Bennett J, Martinez-Cerdeño V, Ashwood P, Amaral DG, Van de Water J. Further Characterization Of Autoantibodies To GABAergic Neurons In The Central Nervous System Produced By A Subset Of Children With Autism. Submitted. [DOI] [PMC free article] [PubMed]
- Zimmerman AW, Connors SL, Matteson KJ, Lee LC, Singer HS, Castaneda JA, Pearce DA. Maternal antibrain antibodies in autism. Brain Behav Immun. 2007;21:351–7. doi: 10.1016/j.bbi.2006.08.005. [DOI] [PubMed] [Google Scholar]