Abstract
The nonhuman primate entorhinal cortex is the primary interface for information flow between the neocortex and the hippocampal formation. Based on previous retrograde tracer studies, neocortical afferents to the macaque monkey entorhinal cortex originate largely in polysensory cortical association areas. However, the topographical and laminar distributions of cortical inputs to the entorhinal cortex have not yet been comprehensively described. The present study examines the regional and laminar termination of projections within the entorhinal cortex arising from different cortical areas. The study is based on a library of fifty-one 3H-amino acid injections that involve most of the afferent regions of the entorhinal cortex. The range of termination patterns was broad. Some areas, such as the medial portion of orbitofrontal area 13 and parahippocampal areas TF and TH, project widely within the entorhinal cortex. Other areas have a more focal and regionally selective termination. The lateral orbitofrontal, insular, anterior cingulate and perirhinal cortices, for example, project only to rostral levels of the entorhinal cortex. The upper bank of the superior temporal sulcus projects mainly to intermediate levels of the entorhinal cortex and the parietal and retrosplenial cortices project to caudal levels. The projections from some of these cortical regions preferentially terminate in the superficial layers (I–III) of the entorhinal cortex whereas others project more heavily to the deep layers (V–VI). Thus, some of the cortical inputs may be more influential on the cortically directed outputs of the hippocampal formation or on gating neocortical information flow into the other fields of the hippocampal formation rather than contributing to the perforant path inputs to other hippocampal fields.
Keywords: hippocampal formation, parahippocampal gyrus, topography, anterograde tracers, macaque monkey, memory, consolidation
INTRODUCTION
There is now ample evidence that the hippocampal formation, and the regions with which it is connected, form the major neural substrate for declarative memory processing (Squire and Zola 1996; Squire and Zola 1998). A number of early studies provided evidence that the nonhuman primate hippocampal formation receives inputs from a variety of neocortical regions (Whitlock and Nauta 1956; Jones and Powell 1970; Leichnetz and Astruc 1975; Van Hoesen, Pandya et al. 1975; Van Hoesen and Pandya 1975a; Van Hoesen and Pandya 1975b). We have carried out a series of studies designed to ascertain the major inputs and outputs of the macaque monkey hippocampal formation. The initial studies focused on the entorhinal cortex, which was thought to be the major interface for cortical-hippocampal interconnections. The first retrograde tracer study (Insausti, Amaral et al. 1987a) was designed to determine the totality of cortical and subcortical regions that project to the entorhinal cortex. This study established that almost two-thirds of the cortical neurons projecting to the monkey entorhinal cortex are located in the adjacent temporopolar, perirhinal and parahipocampal cortices. Another cortical region that was found to have robust projections to the entorhinal cortex included the cingulate cortex and the medial frontal cortex. Projections arose from rostral (areas 24, 32 and 25) as well as from retrosplenial (areas 29 and 30) regions while area 23 showed only moderate projections. Areas 13 and 12 of the orbitofrontal cortex also gave rise to dense projections to the entorhinal cortex while projections from more medial and rostral parts of the orbitofrontal cortex (areas 11, 14 and 10) were less prominent. Another region identified as a source of substantial input to the entorhinal cortex is located along the dorsal bank of the superior temporal sulcus (Amaral, Insausti et al. 1983; Insausti, Amaral et al. 1987a). Minor projections were found to arise in the parainsular cortex and in the inferior parietal lobule (area 7), and even lighter projections arose in the dorsomedial frontal cortex (areas 8 and 9). While this study provided suggestive evidence for a topographic organization of the cortical inputs to the entorhinal cortex, the large size of the retrograde tracer injections that involved all layers, plus the potential for fiber-of-passage involvement by the injection, precluded definitive statements on this issue.
The topographic organization of interconnections between the perirhinal and parahippocampal cortices and the entorhinal cortex was described in more detail in an anterograde and retrograde tracer study (Suzuki and Amaral 1994) from our laboratory. Here there was clear evidence that the perirhinal cortex (areas 35 and 36) projects more heavily to rostral levels of the entorhinal cortex while the parahippocampal cortex (areas TF and TH) projects to more caudal levels. Subsequent studies, using more discrete anterograde tracers, have provided additional details about the projections of the parahippocampal cortex and the parietal cortex to the entorhinal cortex in the nonhuman primate (Wellman and Rockland 1997; Ding, Van Hoesen et al. 2000).
Other recent studies have described the relationship of the posterior cingulate and retrosplenial cortices with the entorhinal cortex (Kobayashi and Amaral 2003; Kobayashi and Amaral 2007). In comparison to the parahippocampal cortex, which gives rise to very extensive projections to the entorhinal cortex, the cingulate and retrosplenial cortices have much more circumscribed projection patterns. In the process of conducting the previous studies, it became clear that the cortical inputs to the macaque monkey entorhinal cortex demonstrate a fairly heterogenous pattern of regional and laminar organization. This paper thus represents an attempt to provide a general overview of the termination pattern of cortical projections to the nonhuman primate entorhinal cortex. It is based, in part, on conclusions from previous studies as well as entirely new analyses of experimental cases with injections involving afferent cortical areas that have not previously been studied. We address a number of questions pertaining to the organization of cortical inputs to the entorhinal cortex. These include: 1. How extensive is the terminal field of a given cortical area within the entorhinal cortex? 2. Is there a topographic relationship between different cortical areas and the fields of the entorhinal cortex? 3. Is there a common pattern of laminar termination within the entorhinal cortex? We have produced and evaluated a library of 3H-amino acid injection cases in the macaque monkey to provide answers to these questions.
MATERIALS AND METHODS
This study was based on a series of experiments carried out in fifty-one Macaca fascicularis monkeys of both sexes ranging in weight between 3 and 5 Kg at the time of surgery. All procedures were carried out in accordance with guidelines for animal use published by the NIH and were approved by Institutional Animal Use and Care Committees. Each animal subject received 3H-amino acid injections aimed at a single cortical region. The anterograde tracer injections were designed to involve each of the cortical regions that we had previously established project to the entorhinal cortex (Insausti, Amaral et al. 1987a). We also prepared control cases with injections that involved the inferior temporal gyrus (area TE); this area did not demonstrate entorhinal projections in the previous study. A list of the experimental cases is presented in Table 1.
Table 1.
Library of Injection Sites Analyzed in This Study
| CASE NUMBER | INJECTION SITE (CORTICAL AREA) |
|---|---|
| FRONTAL CORTEX | |
| Frontal Cortex (orbitofrontal) | |
| M10–87 | A 13 (rostral and medial) |
| M8–94 | A 13 (rostral and central) |
| IM-12 | A 13 (caudal and medial) |
| OM8E | A 13 (caudal and central) |
| M3–86 | A 13 (caudal and lateral) |
| M1–86 | A 12 (lateral) |
| M2–94 | A 12 (medial) |
| Frontal Cortex (medial frontal) | |
| M2–83 | A 25/14 |
| M11–88 | A 10 (caudal)/Area 32 |
| INSULAR CORTEX | |
| M1–89 | Ia (rostral) |
| M6–93 | Ia (caudal) |
| M7–93 | Ia/Id |
| M9–93 | Parainsular |
| TEMPORAL LOBE | |
| Temporal lobe – dorsal STS | |
| M6–88 | (rostral/mid rostrocaudal) |
| IM-9L | (mid rostrocaudal) |
| M9–88 | (caudal) |
| Temporal lobe – inferotemporal cortex | |
| M5–93 | ATE |
| M10–94 | ATE |
| M8–87 | ATE |
| M7–88 | ATE |
| Temporal lobe – perirhinal/parahippocampal cortex | |
| DM-46 | A 36rv (temporopolar cortex) |
| M22–91 | A 36rv |
| M7–83 | A 36rv |
| M7–92 | A 36rl |
| M5–83 | A 36rl |
| M6–83 | A 36rv |
| M11–87 | A 36rl |
| M30–92 | A 36 |
| DM-42 | A 35/EC |
| M1–88 | A 36m |
| M6–92 | A 36l |
| DM-22 | A 36c |
| M7–91 | A 36c |
| M2–93 | TF/TH |
| M13–91 | TF/TH |
| DM-30 | TF |
| M4–94 | TH |
| MF-2 | TF |
| M2–90 | TF |
| M4–88 | TF |
| M14–91 | TF |
| M15–91 | TF |
| M3–93 | TFc |
| M9–91 | TF |
| CINGULATE CORTEX | |
| Anterior Cingulate Cortex | |
| M8–88 | A 24 |
| Posterior Cingulate/Retrosplenial Cortices | |
| M3–95 | A 29l |
| M9–87 | A 29m/l |
| M10–88 | A 29/30 |
| M13–87 | A 30 |
| M5–95 | A 30 |
| PARIETAL CORTEX | |
| M12–87 | A7 |
All of the cases (except for one kindly provided by Dr. Joseph L. Price of the Washington University School of Medicine, Saint Louis) were prepared according to the following experimental protocol. For experiments carried out prior to 1990, animals were sedated with ketamine hydrochloride (Ketolar, Parke-Davis, USA, 8mg/kg i.m.) and brought to an anesthetic level with sodium pentobarbital injected intraperitoneally (Nembutal, Abbott, USA) at a dose of 25 mg/kg, and supplemented as necessary, either through the same route or intravenously. For experiments carried out after 1990, animals were sedated with Ketamine (Ketolar, Parke-Davis, USA, 8mg/kg i.m.) intubated with a tracheal cannula, and placed under mechanical ventilation, while anesthesia was maintained with isoflurane (1–3%).
Regardless of the anesthetic procedure, animals were placed in a Kopf stereotaxic apparatus. Using aseptic procedures, burr holes were drilled in the skull to expose the intended injection target. Some of the injections were made using stereotaxic coordinates based on the atlas of Szabo and Cowan (Szabo and Cowan 1984). In these cases, the dorsoventral coordinates were corrected after extracellular electrophysiological recordings using a tungsten microelectrode. The extracellular unit responses provided guidance about the depth of certain cortical areas and the bottom of the brain. In those locations in which a direct visual approach was made to the selected cortical region, the initial burr hole was enlarged with rongeurs. After exposure of the brain surface and proper identification based on the landmarks provided by the cortical sulci, a glass micropipette mounted in a special holder (Amaral and Price 1983) and filled with 3H-amino acids was lowered into the desired target. The micropipette was left in place for 5 minutes before injecting the tracer. The anterograde tracer employed in all cases was a cocktail containing equal amounts of 3H-leucine and 3H-proline (New England Nuclear, now Perkin Elmer Life and Analytical Sciences, Inc. Wellesley, MA USA), vacuum evaporated and reconstituted to a final concentration of 100 μC/μl. Either single or multiple injections of the 3H-amino acid solution was made by means of air pulses (Picospritzer, General Valve, USA) into the targeted cortical area. The total amount injected ranged between 50 nl and 1 μl, in 50–150 nl steps. When the intended amount of tracer was completely dispensed, the micropipette was raised 200 μm, left in place for an additional period of 5 minutes, and slowly withdrawn to prevent leakage of the tracer along the pipette track. Following the completion of the injection, the wound was closed in layers. Postoperatively, prophylactic doses of antibiotic (Ampicillin, 250 mg/day or 50 mg/Kg of Clarofan, three times daily) were administered. Analgesics (0.15 mg/kg of oxymorphone given three times daily for two days) were also administered post-surgically.
Survival time was generally two weeks, except for two cases (M1-88 and M4-88 which were three and two days, respectively). In these cases, there was also an injection of wheat germ agglutinin conjugated to horseradish peroxidase requiring the shorter survival period. At the end of the survival period, animals were deeply anesthetized with an overdose of sodium pentobarbital and perfused transcardially. The perfusion solutions consisted typically of a 1–2 minute flush with saline followed by at least 4 liters of 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Perfusion parameters varied somewhat depending on the types of injections in a particular experiment. At the end of the perfusion, the head was placed in a Kopf stereotaxic apparatus, the brain was exposed by completely removing the dorsal calvarium and the dura mater was removed. The brain was blocked in the coronal stereotactic plane into 1.5–2 cm slabs.
The brain was removed, photographed and transferred to a container with 2 liters of cryoprotectant solution (10% glycerol in 0.1 M phosphate buffer (pH 7.4) and 2% dimethylsulfoxide) for 24 h, followed by 20% glycerol in 0.1 M phosphate buffer (pH 7.4)plus 2% dimethysulfoxide for 24–48 h (Rosene et al. (1986). The brain slabs were frozen in 2-Methylbutane (isopentane) cooled at −70°C in a bath of alcohol and dry ice for a period of approximately 20 minutes and stored at −70°C until sectioning.
The cases were cut in the coronal plane using a freezing, sliding microtome at 30 μm, or, in selected cases, at 50 μm. Every eighth section (1-in-8 series) or every fifth section (1-in-5 series) was immediately mounted and processed according to the protocol described by Cowan et al. (Cowan, Gottlieb et al. 1972) for the autoradiographic demonstration of anterogradely transported tracer. Exposure times varied between 4 and 10 weeks. At the end of the exposure, the slides were developed and stained with thionin through the emulsion to reveal cytoarchitectonic features.
Sections were analyzed with a Leitz Dialux-20 microscope under bright and dark field illumination. Drawings of the entorhinal cortex as well as the injection sites and the course of the fibers from the injection site to the entorhinal cortex were drawn with a camera lucida attached to a Nikon stereomicroscope. The distribution and density of labeling was plotted onto the drawings and ultimately the cytoarchitectonic boundaries were added. In about one-half of the cases, the distribution of the labeling was plotted with the help of an X-Y recorder (Minnesota Datametrics, now AccuStage) mounted onto the stage of the microscope.
We used criteria reported earlier (Amaral 1987; Insausti, Amaral et al. 1987a) to define the cytoarchitectonic borders for the different entorhinal subfields and to define the location and extent of the injection sites in the neocortex. In order to see the overall distribution of labeling, a two dimensional, unfolded map of the entorhinal cortex (Amaral 1987) in each case was prepared and the distribution of labeling in layers I–III and layers V–VI was represented.
Photomicrographs were taken using a LEITZ DMRD microscope with a SPOT microscope digital camera using SPOT software (v. 4.5). Images were then imported in Adobe Photoshop (v. 8.0) where contrast, color and brightest were adjusted for optimal viewing and panels were composed into figures and labeled.
RESULTS
Summary of injection sites
The approximate locations of all of the injections analyzed for this study (Table 1) are plotted on the surface drawings of the monkey brain shown in Figure 1. Virtually all of the regions that we had previously demonstrated to project to the entorhinal cortex were involved in one or more injections. Areas that demonstrated very few retrogradely labeled cells, such as the dorsolateral prefrontal cortex, did not receive anterograde tracer injections. Brightfield photomicrographs of representative injection sites are illustrated in Figure 2. The injections of 3H-amino acids were generally large enough to involve all cortical layers at the site of the injection (Figure 2). Figures 3 and 4 are photomicrographs of representative examples of the terminal labeling in the EC.
Figure 1.
Line drawings of the lateral (A), ventral (B), medial (C), and frontal (D) views of the Macaca fascicularis monkey brain. The dots indicate the location of the injection sites followed by arrows indicating the case name.
Figure 2.
Photomicrographs showing examples of the tracer injections in selected experimental cases. Panel A shows the injection site in case IM-12 located in the medial part of caudal A13 (caudo-medial orbitofrontal cortex). Panel B illustrates the injection site in case M-6-93 located in the agranular insular cortex. Panel C illustrates the injection site in case M-6-88, in the dorsal bank of the superior temporal sulcus. Panel D shows an injection into area TF of the parahippocampal cortex (case M-4-88).
Figure 3.
Dark-field photomicrographs showing examples of labeled fibers and terminals in the entorhinal cortex. Borders among layers are represented as broken while lines. Case IM-12 (medial and caudal A13 of the orbitofrontal cortex, see also Fig. 5) is presented in A; case M-2-83 (A25/14 of the medial frontal cortex) is presented in B. Note the density of the projection in layers V and VI and the adjacent white matter. The projection arising in the anterior insular cortex is presented in C (case M1-89, agranular insula, see also Fig. 11). The projection extends as a continuous band in the deep layers of the rostral art of the entorhinal cortex, extending into deep layer III; columns of labeled fibers and terminals would run towards layer I, where they form a continuous band of labeling. Panel D corresponds to the projection from parahippocampal cortex (case M2-93, see also Fig. 14). Note the density of the projection throughout all layers except layer VI where density is lighter. Scale bar for all panels is 200μm.
Figure 4.
Dark-field photomicrographs showing examples of the distribution in the entorhinal cortex of the projection arising in caudal perirhinal cortex (panel A, case M7–91, see also Fig. 17) and anterior cingulate cortex (panel B, M8–88, see also Fig. 19). Scale bar is 200μm.
General summary of projections
Figure 5 presents a prototype unfolded map of the entorhinal cortex that we have used previously to provide a global view of the distribution of projections to the entorhinal cortex. The distribution of labeled fibers and terminals for a selected number of cases is represented in Figure 6. This figure provides a general summary of the diversity of projection patterns. While the individual panels of this figure could be organized in a variety of fashions, we have elected to place the individual cases from rostral (A) to caudal (P). In general, cases are arranged from those that have projections to more rostral levels of the entorhinal cortex (Fig. 6A–D) to cases that have projections to more caudal levels of the entorhinal cortex (Fig. 6O and 6P). Please note that the maps have different shapes because they are created using sections from each individual brain. Most of the injections gave rise to a relatively limited terminal field within the entorhinal cortex. Notable exceptions are the injections in area 13 of the orbitofrontal cortex (i.e. Fig. 6H), and in the parahippocampal cortex (Fig. 6M and 6N).
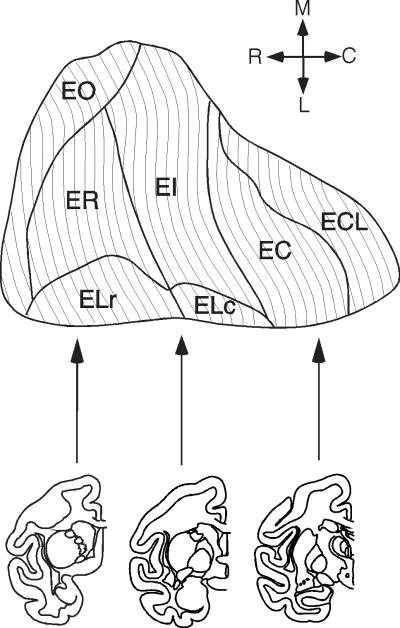
Figure 5.
Line drawings depicting a prototype two-dimensional unfolded map of the entorhinal cortex The location and areal extent of the seven cytoarchitectonic subfields that we have recognized in the entorhinal cortex are demarcated. The line drawings below the map illustrate coronal sections through the Macaca fascicularis monkey brain at approximately the three rostrocaudal levels illustrated with arrows. Upper arrows indicate the general orientation of the unfolded map.
Figure 6.
Unfolded map line drawings of representative cases (A to P) of the entorhinal cortex of the present experimental series. The 16 (out of 51) maps present as grayed areas the approximate extent of the termination fields in the entorhinal cortex (only dense and moderate labeling have been represented; light or very light labeling has been omitted for simplicity). Cases have been arranged from rostral (A), to caudal termination field in the EC (P). The identification of the case, as well as the area of the injection site, is indicated in the upper right-hand side on each unfolded map. Horizontal hatching represents terminal fields in superficial (layers I–III); in the deep (layers V–VI) is presented as gray shading. All cases present an overlap of both superficial and deep pattern of terminations, although the superficial one is predominant, except in two cases: orbitofrontal cortex, namely caudomedial area 13 (Fig. 4A, panel H) and Area 25/14 of the medial frontal cortex (I in figure 4B). Note also the similar extent of the termination in both superficial and deep layers of the projections from the temporal pole (area 36rv) in case DM-46 (C in figure 4B).
The injections led to different patterns of laminar terminal labeling. The most common pattern was of more extensive labeling of the superficial layers (I–III). However, there were other cases where the densest fiber and terminal labeling was over the deep layers (V and VI). And in many cases, labeling was distributed throughout all layers, particularly in the zones of heaviest innervation. We have attempted to demonstrate these different patterns through the use of two shading patterns in Figure 6.
Projections from the frontal cortex
Orbitofrontal projections
Experimental Cases
Projections from orbitofrontal cortex were analyzed in a total of seven cases (Table I – Fig. 1). Two injections involved rostral area 13 (cases M-8-94 and M-10-87), three injections involved caudal area 13 (cases M-3-86, OM8E, IM-12) and two injections involved area 12 (cases M-1-86 and M-2-94).
Fiber Trajectories
Fibers labeled following the area 13 injections were observed traveling in the uncinate fasciculus (Fig. 7). Medially located injections gave rise to labeled fibers that ran laterally into the temporal lobe and reached the ventral aspect of the external capsule (Fig. 7A and B). Labeled fibers formed a loose bundle that continued caudally for some distance behind the frontotemporal junction (limen insulae) before turning ventrally towards the ventromedial temporal lobe (Fig. 7C). Fibers turned ventrally in the uncinate fasciculus for about 1 mm. A topographic organization within the uncinate fasciculus was observed: fibers from medial and caudal portions of the orbitofrontal cortex turned ventrally approximately 1.2 mm behind the limen insulae, while fibers originating at more rostral and lateral portions of the orbitofrontal cortex turned ventrally at a more caudal level, approximately 2 mm behind the limen insulae. Fibers originating in area 12 descended even more caudally (approximately 3 mm) to the limen insulae. Therefore, fibers originating from caudal portions of orbitofrontal cortex occupied rostral portions in the uncinate fasciculus on their way to the ventromedial temporal lobe, while fibers originating from more rostral and lateral parts of the orbitofrontal cortex occupied progressively more caudal portions of the uncinate fasciculus (i.e. resembling an onion-layered concentric arrangement of fibers). Once the fibers reached the entorhinal cortex, they gathered in the white matter deep to the rhinal sulcus (Fig. 7E–I).
Figure 7.
Representation of the fiber trajectories followed by the projection from the medial portion of Area 13 (case IM-12, see also Fig. 9) to the entorhinal cortex. The course of the fibers is presented as black dots and lines in a series of coronal sections of one cerebral hemisphere of the monkey brain. Fibers leaving the Injection site (panel A) travel laterally and caudally to reach the uncinate fascicle (panels B and C), from where thy run in a caudal direction (panels D, E) to occupy the white matter at the fundus of the rhinal sulcus at the level of the rostral amygdala. The collection of fibers travels caudalwards at that location as far as the caudal pole of the entorhinal cortex.
A number of cases had injections that involved rostral portions of the orbitofrontal cortex. All of them gave rise to projections that were limited to the rostral one-half of the entorhinal cortex (ELr and ER) with termination in layers I, III and V. Projections from injections that were located more caudally and laterally demonstrated a similar pattern of projections. An example of this is case M-3-86 (Fig. 8). Labeling was observed mainly in the rostral and lateral portions of the entorhinal cortex. Labeled fibers from this injection concentrated in the deep portion of layer III and in layers V and VI. In some areas, labeled fibers and terminals adopted a column-like appearance that reached layer I, but tended to avoid the clusters of cells that populate layer III. Terminals in layer I formed dense and discontinuous patches. There was little or no labeling over the cell islands of layer II.
Figure 8.
Line drawings of eight representative levels through the entorhinal cortex in case M-3-86. Sections are arranged from rostral (A) to caudal (H) in the entorhinal cortex, and the injection site is represented in the upper left corner. The limit of the entorhinal cortex subfields are indicated by arrows. Each of the layers of the entorhinal cortex is indicated as continuous lines, with the exception of layer II, where the outline of layer II islands has been represented. Note the termination field restricted to the rostral part of the entorhinal cortex. Similar representation applies for the following Figures 9–12, 14–19 and 21.
Injections of the more central and medial portions of area 13 gave rise to somewhat more expansive projections to the entorhinal cortex. The heaviest labeling was observed in case IM-12 with a medially located injection (Figs. 2A, 9). In this case, the labeled fibers in the fundus of the rhinal sulcus contributed projections to layer VI in all subfields of the entorhinal cortex. It was striking that virtually all of the labeling was limited to layer VI (Fig 3A) although layer V also had some labeled fibers, particularly in sublayer Vc, immediately adjacent to layer VI. There was also some moderate labeling within layer III that extended into the anterior part of subfield EC (Fig. 9F). Layer III labeling had a patchy appearance rostrally, often surrounding the characteristic cell clusters of ER. Layer I contained patches of light labeling, especially in ER. Experiment OM8E presented a similar pattern though less dense labeling.
Figure 9.
Distribution of the projection from caudomedial area 13 in case IM-12 (injection site is represented in the upper left corner). Eight rostrocaudal levels of the entorhinal cortex have been represented (A–H). Note the continuous labeling in the deep layers that enters into the white matter border at all levels, from rostral (A) to the caudalmost level (H) of the entorhinal cortex. In contrast, little or no labeling is present in superficial layers I–III. Conventions are as in Figure 8.
The two-dimensional representation of the extent of the labeling in experiments M-3-86 and IM-12 are illustrated in Figures 6 G and 6H. The projections from the caudal orbitofrontal cortex are both more extensively distributed rostrocaudally and more limited to the deep layers. The rostral projections are more limited in their area of distribution and terminate more evenly throughout the entorhinal layers.
Medial frontal projections
Experimental Cases
There were two injections that involved the medial portions of the frontal lobe. Case M-11-88 had an injection located at the transition of caudal area 10 and the most rostral portion of area 32, while case M-2-83 had an injection that was focused at the transition of areas 25 and 14.
Fiber Trajectories
The injection in case M-11-88 gave rise to labeled fibers that concentrated in the white matter deep to the medial orbital sulcus and traveled caudally for about 0.5 mm. This fascicle of fibers then turned laterally beneath the head of the caudate nucleus to attain a position in the deep part of the external capsule, where it continued caudally to the level of the limen insulae. At a point approximately 2 mm caudal to the limen insulae, the fibers turned ventrally in the uncinate fasciculus to reach the entorhinal cortex. In case M-2-83, with an injection of area 25, labeled fibers essentially followed the same trajectory to the entorhinal cortex. However, they descended in the uncinate fasciculus approximately 1.2 mm caudal to the limen insulae rather than 2 mm as in case M-11-88.
Terminal fields
In case M-2-83, the terminal field in the entorhinal cortex resembled the pattern observed in the orbitofrontal cortex injections. Labeled fibers and terminals were denser in rostral parts of the entorhinal cortex but also included the most rostral portion of EI (Fig.6 I). A very weak terminal field continued as far as the rostral portion of EC (Fig. 10G). Labeled fibers and terminals were mainly observed over layer VI, extending into adjacent layer V (Figs. 3B and 10B–G). Patches of labeling were also present in layer III at the transition between subfields ER and EI. Very light labeling was also observed in layers II and I, only in subfield ER (Fig. 7B). The injection in M-11-88 gave rise to only light projections to much of the extent of the entorhinal cortex except the rostral and caudal extremes (EO and ECL). Labeling terminated only in layers V and VI.
Figure 10.
Line drawings showing the distribution of the medial frontal cortex (Areas 25/14) projection to different rostrocaudal levels of the entorhinal cortex. Note the overall paucity of the projection to the superficial layers of the entorhinal cortex; in contrast, the deep layers (layers V and VI) contained labeled fibers and terminals that extended for almost two- thirds of the entorhinal cortex extent. In this respect, this case resembles the projections from the medial portion of caudal A13 injection (case IM-12). Conventions are as in Figure 8.
Projections from the insular cortex
Experimental Cases
Three injections involved the insular cortex (Fig. 1 and Fig.2B). They involved different rostrocaudal portions of the insular cortex (from rostral to caudal: M-1-89 (Ia), M-6-93 (Ia), M-7-93 (Ia/Id). One additional case involved the parainsular cortex (M-9-93).
Fiber Trajectories
Fibers emanating from the insular cortex concentrated in a thick fascicle just behind the level of the limen insulae and descended vertically in the white matter located lateral to the amygdala towards the ventral aspect of the temporal lobe. These fibers intermingled with fibers of the uncinate fasciculus before entering the white matter deep to the entorhinal cortex. When the injection was located more posteriorly in the insular cortex, labeled fibers took a directly ventral course, traversing the amygdaloid complex, and terminating in the entorhinal cortex.
Terminal fields
The pattern of projections following an agranular insular injection is represented in case M-1-89. The densest terminal labeling was located rostrally (Fig. 11A and B), encompassing EO, ELr and rostral ER. Moderately dense labeling was also observed in the caudal part of ER (Fig. 11C) though there was little or no labeling of the remainder of the entorhinal cortex (Fig. 11E–H). The fiber and terminal labeling was densest in layers VI and V. However, the labeling tended to have a columnar appearance and the columns extended from the deep layers towards the superficial layers, terminating particularly heavily in layer III, but continuing as far as layer I (Fig 3C, Fig 11B). The labeled fibers and terminals tended to avoid the cell clusters in layer III. There was also a dense band of labeled fibers and terminals in layer I that extended across the full transverse extent of the rostral entorhinal cortex (Fig. 11A and B). At progressively more caudal levels, the band broke up into patches that often surrounded the layer II cell islands.
Figure 11.
Distribution of the projections originated in the anterior part of the agranular insular cortex (Ia). The density of labelled fibers and terminals in layers I and II of the rostral portion of the entorhinal cortex can be appreciated in sections A and B, and in particular in EO. The remainder of the entorhinal cortex does not present significant amount of labelled fibers and terminals. Conventions are as in Figure 8.
Labeling following a more caudal injection of the insular cortex is demonstrated in case M-6-93 (Fig. 12). This injection involved both the agranular and the disgranular insular cortex (Jones and Burton 1976). Topographically, the terminal field was restricted to the rostral part of the entorhinal cortex. Terminals were dense in EO throughout all layers, although the superficial portion of layer III had a lower density of labeled terminals. As in the previous case, terminals in layer III formed columns that surrounded, rather than overlapped, the cell clusters present at this level. Columns of labeled fibers and terminals extended into layer I. Subfields ER and ELr also contained dense terminal labeling, albeit at a somewhat lower density than in EO. This was true except in layer I which was as dense as in EO. At more caudal levels, labeled fibers and terminals were confined almost exclusively to EO (Fig 12, C and D). And, at these levels, labeling was mainly restricted to layer I. An unfolded, two-dimensional representation of the labeling in this case is illustrated in Figure 6A
Figure 12.
Series of line drawings at different rostrocaudal levels of the entorhinal cortex showing the projection from the posterior portion of the agranular insular cortex. Note the similitude with the projection from more anterior parts of the agranular insular cortex shown in the previous figure. The densest labelling distributes in the anterior portion of the entorhinal cortex, in particular layer I throughout the whole extent of EO (panels A–D). Also, it is worthwhile noting that the terminal field hardly reaches the dorsal aspect of EI (panels D–E). Conventions are as in Figure 8.
Case M-9-93 had an injection that involved the cortex deep to the inferior limiting sulcus, i.e. the parainsular cortex. The pattern of projections was much like that resulting from other insular cortex injections (Fig. 6A, panel B). The terminal field was located in rostral levels of the entorhinal cortex, particularly EO, ER and rostral ELr. Rostrally, terminals were distributed to both deep (V–VI) and superficial (I–III) layers, though more caudally they were limited to superficial layers (Fig. 6A, panel B).
Projections from the temporal cortex
The temporal neocortex contains several functionally distinct areas that project to the entorhinal cortex. We will present the results of our injections into each of these regions separately. We will first present results of the injections focused in the polymodal association cortex along the dorsal bank of the superior temporal sulcus. Second, we will describe the rather meager projections to the entorhinal cortex arising from unimodal visual association cortex (area TE). Finally, we will describe projections arising from the perihinal and parahippocampal cortices.
Dorsal bank of superior temporal sulcus projections
Experimental Cases
Three rostrocaudal levels of the cortex lining the upper bank of the superior temporal sulcus were involved in injections in our library of cases (rostral, case M-6-88; middle, case IM-9L, and caudal, case M-9-88 (Fig. 2 C).
Fiber Trajectories
Case M-9-88 provides a good representation of the fiber labeling resulting from all of these cases (Figure 13). Fibers leaving the injection site formed a very dense bundle in the white matter of the superior temporal gyrus (Fig. 13 F–I). Labeled fibers traveled rostrally and ventrally, either to reach the white matter deep to the fundus of the anterior middle temporal sulcus (Fig. 13 G and H), or to continue ventromedially to the white matter deep to the entorhinal cortex. A component of the labeled fibers located in the white matter deep to the anterior middle temporal sulcus (Fig. 13 F–H) turned ventromedially to reach the white matter deep to the rhinal sulcus. From this point, the bundle of fibers entered the lateral part of the entorhinal cortex at a mid rostrocaudal level (Fig. 13 E). Labeled fibers located deep to the entorhinal cortex extended in both rostral and caudal directions for about 10 mm, to terminate in the entorhinal cortex.
Figure 13.
Representation of the fiber trajectories followed by the projection from the cortex at the upper bank of the superior temporal sulcus to the entorhinal cortex in case M-9-88 (see also Fig. 14). The injection site is at the level of the end of the uncal hippocampus (panels H–I), from where thick fascicles of labeled fibers course rostrally (solid black fascicles, panels G–I) in the ventral part of the external capsule as far as the anterior commissure (panels A–B). Fibers bound to the entorhinal cortex course in the white matter of the inferior temporal gyrus and sweep medially to reach the angular bundle before terminating in the entorhinal cortex.
Terminal fields
Projections from the upper bank of the superior temporal gyrus terminated most heavily in the mid to caudal levels of the entorhinal cortex (Fig. 6B, panel L, Fig. 14). Based on the three cases available for analysis, it appears that there is a rostrocaudal topography to these projections with the rostral injection leading to a more rostrally located terminal field and the caudal injection leading to a more caudally located terminal field. Terminal labeling was most conspicuous in layer I (M-9-88, Fig. 14). Only ELc had labeling in all layers (Fig. 14E). At the transition between EI and the rostral part of EC there were patches of labeled terminals that extended throughout the layers. Interestingly, this was the only case in which some fiber and terminal labeling was observed in lamina dissecans (Fig 14E, asterisk). There was some additional fiber and terminal labeling in the caudal part of ELr that extended very lightly into adjacent ER
Figure 14.
Projection to the entorhinal cortex of the polysensory cortex at the upper bank of the superior temporal sulcus, represented in a series of line drawings from rostral (A) to caudal (H). Labeled terminals are present in layer I of the caudal half of the entorhinal cortex. Note the relatively selective termination in ELc (panels D) and the specific termination in lamina dissecans in the middle third of EC (panel E, asterisk). Labeled fibers and terminals extend in layer I as far as the caudal extreme of the entorhinal cortex. Conventions are as in Figure 8.
Projections arising from the two other injections were very comparable to those described for case M-9-88. In case M-6-88, for example, the heaviest fiber and terminal labeling was again observed in layer I of areas EI, EC and ECL. There was also lighter labeling of layer III in ER, ELr and ELc.
Area TE projections
In the Insausti et al (1987) paper, only a few retrogradely labeled cells were located in area TE following entorhinal injections. There have been reports, however, that the most anterior and ventral portions of TE project to the most lateral aspect of the entorhinal cortex (Saleem and Tanaka 1996). In order to avoid contamination of the adjacent area 35, the retrograde tracer injections in Insausti et al (1987) purposely avoided the most lateral aspects of the entorhinal cortex. We therefore decided to reevaluate cases from our library that contained anterograde tracer injections into area TE. Four cases had injections of 3H-amino acids in different parts of the inferior temporal cortex (cases M-10-94, M-7-88, M-8-87 and M-5-93, Fig.1). None of these cases clearly demonstrated above background levels of labeling. In cases M-5-93 and M-10-94 there appeared to be a slight increase in the number of silver grains over the most lateral aspect of the rostral entorhinal cortex. It was difficult to conclude, however, that this actually constituted a very minor level of fiber and terminal labeling. In both of these cases, there were clearly labeled projections and terminal fields within other parts of the temporal lobe, within the amygdala and within the striatum. While we have not extensively sampled all regions of area TE, these four anterograde cases have confirmed our impression from the previous retrograde tracer study. While there may be a minor projection arising from the ventromedial portion of area TE much of area TE does not project at all to the entorhinal cortex. In this respect, area TE is very different from the perirhinal and parahippocampal portions of the inferior temporal lobe that we turn to next.
Perirhinal and Parahippocampal Cortices
A total of 23 cases had injections in either the perirhinal or parahippocampal cortices. Most of these cases have been described previously in relation to the general organization of connections to the entorhinal cortex or to other cortical regions (Suzuki and Amaral 1994; Suzuki and Amaral 1994; Lavenex, Suzuki et al. 2002). Here we will briefly review the topographic organization of the projections to the entorhinal cortex. We also present a more detailed account of the laminar organization of these projections. We have elected to present the projections from this region in two sections: perirhinal cortex (areas 35 and 36, including the temporopolar region) and posterior parahippocampal cortex (areas TF and TH). Representative cases of this group are presented in Figures 15–18 and in Figure 6D–F and K, M, and N).
Figure 15.
Temporal pole projection to the entorhinal cortex depicted in a series of sections from rostral (A) to caudal (H). Labeled fibers and terminals concentrate in the deep layers at rostral levels, extending to the superficial layers among the cell clusters of layer III (panels A and B). Note the density of the termination field in ELr and ELc that reaches the most lateral part of rostral EC (panels C–G). Conventions are as in Figure 8.
Figure 18.
Projection from the parahippocampal cortex (Area TF) to the entorhinal cortex showing the distribution of labeled fibers and terminals. Note the relatively light projection to the rostral parts of the entorhinal cortex compared to more caudal levels. In this particular case, the terminal field becomes dense at the level of the caudal part of ER, and throughout much of EI, to distribute, in a somewhat lighter way, to ECL and EC subfields. Also note the lack of significant projection to ELr and ELc. Conventions are as in Figure 8.
Perirhinal Cortex (area 35 and 36) projections
Experimental Cases
There were 13 cases in which the injection involved the perirhinal cortex. The most rostrally located injections were in the temporal polar portions of perirhinal cortex. Case DM-46 had a large, multiple injection that involved the ventromedial part of the temporal pole. Cases M-5-83, M-11-87 and M-7-92 involved the lateral part of temporopolar cortex, while cases M-6-83, M-7-83 and M-22-91 were located in the medial part (Fig. 1). Six additional cases (M-30-92, DM-42, M-1-88, M-6-92, DM-22 and M-7-91) involved more caudal portions of the perirhinal cortex (Fig.1). The injection in case DM-42 also involved area 35. Because of its difficult position in the fundus of the rhinal sulcus, we do not have an injection that discretely involves area 35.
Fiber Trajectories
Cases with injections in the temporal polar portion of the perirhinal cortex gave rise to labeled fibers that were concentrated in the white matter that makes up the core of the temporal pole. These fibers continued caudally as loose bundles in the white matter located deep to the fundus of the superior temporal sulcus. Fascicles of fibers from these bundles swept ventromedially to reach the white matter deep to the rhinal sulcus. These fibers took a rostrocaudal trajectory and labeled fibers peeled off medially to innervate the entorhinal cortex. For the perirhinal injections located at more caudal levels, labeled fibers ran medially under the amygdala and entered the angular bundle. They continued caudally and then turned ventrally and ventrolaterally to reach the entorhinal cortex.
Terminal fields
We will first describe the projections from the temporal polar portion of area 36. Case DM-46 provides a good example of the regional and laminar projection patterns in these cases. Despite the large size of this injection, the projection from this case terminated mainly in the rostral half of the entorhinal cortex. Subfields ER, ELr and ELc (but not EO) contained a dense concentration of fiber and terminal labeling (Fig. 15 B–D). A similar density of labeling extended into a narrow, more caudally located region that included parts of EI and EC (Fig 15, E and F). A two-dimensional representation of the densest labeling in this case is presented in Figure 6K).
Fiber and terminal labeling was densest in the superficial layers, especially the deep portion of layer III (Fig. 15 C and D). At rostral levels, where the terminal field was densest, labeling had a columnar appearance that extended from the deep aspect of layer III into layer I (Fig. 15 B–D). In the caudal half of the entorhinal cortex, light labeling was seen in the lateral half of layer I but was progressively reduced from layer III (Fig. 15 G and H).
The other cases with injections at these rostral or polar levels of area 36 demonstrated very similar topographic and laminar patterns of termination even though the injections tended to be much more restricted. While there was little labeling in EO in case DM-46, other cases such as M-22-91 (Fig. 6 E) exhibited denser labeling in this subfield, predominantly in layers I and III.
Injections that involved area 36r gave rise to labeling that was located exclusively in the rostral half of the entorhinal cortex, namely subfields EO, ER and ELr (Fig. 6A, panels D and E). As observed in case M-30-92 (Fig. 16) layer III demonstrated the highest density of labeled fibers and terminals (Fig. 16 A–D). The whole thickness of the layer was labeled rostrally while labeling was more restricted to the upper half of layer III further caudally. The terminal field extended into the cell islands of layer II and into layer I for most of the transverse extent of the rostral entorhinal cortex. Some labeled fibers and terminals were also observed in layers V and VI at very rostral levels (Fig. 16 A–D), while what appeared to be fibers-of-passage were observed further caudally. A two-dimensional representation of the extent of the terminal field in case M-30-92 is presented in Figure 6 D).
Figure 16.
Distribution of the terminal field of perirhinal cortex projection in a series of line drawings throughout the entorhinal cortex. Labeled fibers and terminals are present in the upper layers at rostral levels of the entorhinal cortex throughout the mediolateral extent, from EO to ELr and ELc. The terminal field extends caudally mainly in layer I. Conventions are as in Figure 8.
The laminar projection pattern for cases with area 36c injection was generally similar to that described above, although with some modifications. However, the projection did extend further caudally in the entorhinal cortex (Figs. 4A, 6 F and 17). Thus, area 36c injections gave rise to some significant labeling in the lateral portion of EC and ECL (Fig. 17 G and H). The laminar termination of cases with perirhinal injections, however, was always quite similar (higher density of the projection to layers IIII, (cf. Figs. 16E and 17D). For additional details on the topographic organization of connections between the entorhinal cortex and the perirhinal and parahippocampal cortices see Suzuki and Amaral (1994).
Figure 17.
Distribution of the terminal field in the entorhinal cortex of the projection arising in perirhinal cortex at a level caudal and lateral to the case presented in Figure 16. The terminal field in this case is located more laterally (panels C–E) and caudally as far as ECL (panels G–H). Conventions are as in Figure 8.
Parahippocampal cortex (areas TH and TF) projections
Experimental Cases
There were 11 cases in which the injection involved either area TF or area TH. The rostrocaudal and mediolateral distribution of injections in area TF was broad and sampled essentially all rostrocaudal regions of this cortical area. There were three injections of area TH and at least two of these also involved the medial portion of area TF (Figs. 1 and 2D). Only case M4-94 involved primarily area TH.
Fiber Trajectories
Labeled fibers from injections into the parahippocampal cortex were observed to run medially towards the angular bundle where they formed a compact fascicle in the lateral part of the white matter deep to the fundus of the rhinal sulcus. This bundle extended rostrally as far as the rostral pole of the amygdala (Fig. 18). Fascicles of fibers from this bundle peeled off medially to innervate the entorhinal cortex.
Terminal fields
Projections from the parahippocampal cortex tended to be more extensive than those from the perirhinal cortex. While labeling was generally heaviest at caudal or mid rostrocaudal levels of the entorhinal cortex, it extended nearly to the rostral pole (Fig. 6 M and N), at least in the lateral aspect of the entorhinal cortex. Labeling tended also to be heavier at mid mediolateral levels in all cases (Fig. 18). However, as noted in Suzuki and Amaral (1994) the injection sites in our library tended to be focused at mid mediolateral portions of area TF. Therefore, a different pattern may have obtained if the injection was either more medially or more laterally situated within area TF. In general, the superficial layers (I–III) received the densest projection.
As illustrated for case M-2-93 (Fig. 18), particularly dense patches of labeling were observed in EI. Fiber and terminal labeling was very dense throughout layers V to I (Fig. 3D). Labeling in layer III was a little denser in the superficial (close to layer II) and deep (closer to layer V) portions of the layer, and lighter in the middle of the layer. Layer VI received a lighter projection. In this case, labeling was not columnar but was distributed rather homogeneously throughout the medial two-thirds of the entorhinal cortex. Labeling was either lighter or non-existent in the lateral third of the cortex (Fig. 18 D–E). Rostrally, there were patches of lighter labeling in ER (Fig. 18 A–C). In the caudal part of the entorhinal cortex, dense labeling was mainly confined to layers I and II though there was a narrow band of labeling in layer Vc (Fig. 18 F–H). A two-dimensional representation of the projection in this case is presented in Figure 6 M.
Other cases had injections at more rostral or caudal levels. In cases where the injection was located more rostrally, the density of the labeling was higher in the lateral part of the entorhinal cortex (ELr and ELc) but the pattern of laminar termination was similar to that in case M-2-93. An example of the terminal field distribution from a more rostrally placed injection into the parahippocampal cortex (case M-13-91) is shown in Fig. 6 N. Cases with a more caudal injection had a much more limited projection that terminated exclusively in subfields EC and ECL, although the laminar distribution was again very similar.
Projections from the Cingulate and Retrosplenial Cortices
Experimental Cases
Six experimental cases contained 3H-amino acid injections that involved some portion of the cingulate cortex (Fig 1). One of these, M-8-88 was focused in area 24. Five cases had injections in either the posterior cingulate cortex (case M-9-87) or in the retrosplenial cortex (area 30, cases M-13-87 and M-5-95; area 29 and 30, case M-10-88; area 29m and 29l, cases M-3-95 and M-9-87). Fiber trajectories and terminal fields will be described separately for injections involving anterior cingulate cortex and posterior cingulate/retrosplenial cortices. Overall projections from the posterior cingulate and retrosplenial cortices have recently been extensively described in Kobayashi and Amaral (2007).
Anterior cingulate cortex projections
Fiber Trajectories
Case M-8-88 had an injection in area 24 (Figs. 1 and 19). A thick bundle of labeled fibers descended ventrally from the injection site through the anterior portion of the rostrum of the corpus callosum, to reach the ventral part of the external capsule and the uncinate fasciculus. Other fibers turned laterally to descend as far as the external capsule where they also joined the uncinate fasciculus. One other component reached the most rostral extreme of the internal capsule to also descend and join the uncinate fasciculus. The fibers thus collected in a single bundle, turned ventrally in the white matter deep to the limen insulae, and traveled for about 2 mm caudally, to enter the entorhinal cortex through the white matter deep to it.
Figure 19.
Terminal field of the anterior cingulate cortex (A24) projection to the entorhinal cortex. The distribution of labeled fibers and terminals is mainly directed to the rostral half of the entorhinal cortex, especially in layer I. Note that medial portions of ER and EI do not receive projection in this case. Conventions are as in Figure 8.
Terminal fields
The injection in case M-8-88 generated a terminal field that most heavily innervated central portions of the entorhinal cortex within ER, ELr, EI, ELc and EC. There were no labeled fibers and terminals in ECL or EO (Fig. 6 J). Where this projection terminated most heavily (Fig 19 C and D), labeled fibers and terminals encompassed all layers over the lateral two-thirds of the transverse extent of the entorhinal cortex. Further caudally, the density of the projection decreased in layers V and VI but continued to be moderate in layer I (Fig. 19 F–H).
Posterior cingulate/retrosplenial cortex projections
Fiber Trajectories
The trajectory of fibers from the posterior cingulate/retrosplenial region is illustrated in Figure 20 (case M-9-87). Labeled fibers from the injection coursed caudally around the splenium of the corpus callosum, then traveled ventrolaterally and collected in the white matter along the dorsal bank of the calcarine fissure. Labeled fibers then traveled rostrally in the angular bundle, from where they distributed within the entorhinal cortex.
Figure 20.
Representation of the fiber trajectories followed by the projection from the cortex at the dorsal part of retrosplenial cortex (Case M-9-87, A29m-l) to the entorhinal cortex. Injection site is shown in panels D and E, from where they enter the caudalmost part of the cingulum bundle. Labeled fibers arch over the dorsal part the lateral ventricle (panel F) and turn ventromedially to the dorsal bank of the calcarine sulcus, where they travel in a rostral direction in the white matter of the parahippocampal cortex (panels B–F) as far as the caudal part of the entorhinal cortex (panel A).
Terminal fields
There were both commonalities and differences in the pattern of termination in the cases in this group. Case M-13-87 (Fig. 21) provides a fairly representative picture of the pattern of termination. In all cases (Fig 6 O and P), as in M-13-87 (Fig. 21), the heaviest distribution of labeled fibers and terminals was at caudal levels of the entorhinal cortex (EI, EC, and ECL). Terminal labeling was often densest in layer I. However, when the injection was more dorsally placed than the one in M-13-87, there was also substantial labeling in layer VI and sometimes layer V.
Figure 21.
Projection of the retrosplenial cortex (A30) to the entorhinal cortex represented in a series of coronal sections from rostral (A) to caudal (H). Note the total absence of labeled fibers and terminals in the rostral half of the entorhinal cortex, as well as the specificity of the projection to layer I at the caudal subfields (EC and ECL) of the entorhinal cortex. Conventions are as in Figure 8.
Projections from Parietal Cortex
There was one case in our library that had an injection of 3H-amino acids in area 7 of the inferior parietal lobe (Fig. 1). The pattern of labeling resembled that of projections originating in the posterior cingulate and retrosplenial cortices. Terminal fields were limited to EC and ECL. The projection gave rise to low levels of labeled fibers and terminals in layer I of the entorhinal cortex and to even a lower density of labeling in layer III.
DISCUSSION
This study presents a comprehensive overview of the topography and laminar distribution of neocortical inputs to the macaque monkey entorhinal cortex. A unique aspect of this study is that most of the known entorhinal cortical afferent regions have been involved by one or more anterograde tracer injections. Despite the fairly extensive literature on entorhinal projections to the neocortex based on retrograde tracer studies (Mesulam, Van Hoesen et al. 1977; Kosel, Van Hoesen et al. 1982; Goldman-Rakic, Selemon et al. 1984; Cavada and Goldman-Rakic 1989; Morecraft, Geula et al. 1992; Carmichael and Price 1995; Barbas, Ghashghaei et al. 1999; Cavada, Company et al. 2000; Petrides and Pandya 2002; Kobayashi and Amaral 2003; Morecraft, Cipolloni et al. 2004; Munoz and Insausti 2005), there is only limited data on entorhinal inputs from the neocortex in the monkey (Leichnetz and Astruc 1975; Leichnetz and Astruc 1976; Mesulam and Mufson 1982; Mufson and Pandya 1984; Morris, Petrides et al. 1999; Rempel-Clower and Barbas 2000). In most of these papers, projections to the entorhinal cortex were presented as an incidental finding and neither the topographic organization nor the laminar termination were described in detail. More detailed studies have, however, been carried out in nonprimate species (Room and Groenewegen 1986).
A number of general conclusions can be drawn from the experiments presented in the current paper. First, all of the regions that were previously demonstrated to project to the macaque monkey entorhinal cortex using retrograde tracing techniques (Insausti, Amaral et al. 1987a) have been confirmed in this anterograde tracing study. This is of some importance since the retrograde tracers that were used previously are susceptible to the fiber-of-passage problem. Second, projections arising from different cortical areas generally have a spatially restricted terminal field within the entorhinal cortex. Third, while the projections arising from each cortical region generally terminate in more than one layer of the entorhinal cortex, the layer with the heaviest density of fiber and terminal labeling varied substantially from cortical region to cortical region. Some cortical areas terminate predominantly in layers I–III that contain most of the neurons that give rise to the perforant path projections to other regions within the hippocampal formation. Other cortical areas terminate predominantly within layers V and VI that give rise to the major cortical and subcortical projections of the entorhinal cortex. Of course, there is also a very substantial network of intrinsic connections within the entorhinal cortex (Chrobak and Amaral, 2007) that has the potential of further integrating inputs between deep and superficial layers. Nonetheless, it seems likely that projections that terminate mainly in the superficial layers of the entorhinal cortex will have a distinctly different effect on the synaptic economy of the hippocampal formation than those that terminate mainly on the deep layers We will discuss these issues in more detail in the remainder of the discussion and will refer to figures 22 and 23 that present schematic summaries of the topographic and laminar patterns of cortical inputs to the entorhinal cortex.
Figure 22.
Summary diagram of the laminar organization of cortical inputs to the entorhinal cortex. The subfields of the entorhinal cortex are indicated on top and the layers of the cortex are indicated at left. Each block indicates the presence of substantial input from one of eight cortical regions (Of – orbitofrontal cortex, MF – medial prefrontal (infralimbic) cortex, I – insular cortex, STS – dorsal bank of the superior temporal sulcus, PR – perirhinal cortex, PH – parahippocampal cortex, AC, anterior cingulate cortex, RSP, retrosplenial cortex. The coding is generally an integration of findings across several experiments. Some cortical projections, such as from the retrosplenial cortex are directly primarily to the superficial layers of the entorhinal cortex. Other projections, such as from the orbitofrontal cortex, are directed primarily to the deep layers.
Figure 23.

Schematic diagram of the entorhinal cortex illustrating implications of topographical and laminar organization of cortical inputs to the entorhinal cortex. Simplified neurons in layers II, III, V and VI are illustrated and their predominant projection either to the perforant path (I and II) to other hippocampal fields or (V. VI) to other cortical and subcortical regions are indicated. Legend below indicates the location of a subset of entorhinal inputs.
Topographic Organization
The most widespread projections to the entorhinal cortex arise in the orbitofrontal cortex and the perirhinal and parahippocampal cortices (Figs. 6 and 22). The caudomedial portion of orbitofrontal cortex gives rise to prominent projections that terminate throughout the entire rostrocaudal extent of the entorhinal cortex with heavier labeling located rostrally and laterally. Other portions of the orbitofrontal and medial frontal cortices that project to the entorhinal cortex terminate mainly in rostral levels of the entorhinal cortex. Nearly as extensive as the frontal projections are those from the parahippocampal cortex (areas TF and TH). Projections from these areas terminate in all regions except for EO, though the density of termination tends to be far heavier caudally. All portions of the perirhinal cortex terminate heavily within the entorhinal cortex but these projections are limited to EO, ER and the rostral part of EI. A detailed description of the topographic organization of the perirhinal and parahippocampal projections to the entorhinal cortex has been presented previously (Suzuki and Amaral 1994).
The remainder of the cortical inputs to the entorhinal cortex tend to have a more limited areal distribution. The insular and peri-insular cortices, for example, project most heavily to EO and rostral ER. The retrosplenial cortex, in contrast, projects mainly to EC and ECL. This general topographic organization is consistent with previous retrograde tracer studies (Insausti, Amaral et al. 1987a) as well as the few studies that have demonstrated cortical inputs to the macaque monkey entorhinal cortex (Mesulam and Mufson 1982; Morris, Petrides et al. 1999; Kobayashi and Amaral 2007). While the topographic organization that we have described is consistent across a number of experimental cases, we should point out that it is based on a sampling of projections from a restricted portion of each of the entorhinal cortical afferents. We have not attempted to saturate any particular projection region with an anterograde tracer. Thus, there may be more overlap of terminal fields from different cortical areas than is portrayed in Figure 6 and the description above.
Finally, we have confirmed our previous finding that, for the most part, area TE does not project to the entorhinal cortex. Our preparations do suggest the possibility for a light projection from the most ventromedial portion of area TE to the most lateral aspect of the entorhinal cortex. This finding would seem to be in contrast to the paper by Saleem and Tanaka (1996) which reported area TE projections both to the perirhinal cortex and to the entorhinal cortex. However, they demonstrated that the more dorsally located regions of area TE actually do not project to entorhinal cortex. The projections they report arise in their area TEav which forms the lateral border of (and partially overlaps with) the perirhinal cortex. If one surveys their Figure 9, it becomes clear that even projections from this region are extremely modest into the entorhinal cortex and mainly confined to the lateral border of the entorhinal cortex. This is in contrast to the massive projection to area 36 which is demonstrated in the same figure. Thus, while it does appear that some fibers from a circumscribed region of the most ventromedial portion of area TE do reach the entorhinal cortex, this is a far less significant projection than any of the others that we have described in this paper.
FUNCTIONAL IMPLICATIONS - TOPOGRAPHY
Before discussing some of the functional implications of the topography of cortical inputs to the entorhinal cortex, it is important to briefly review some features of the relationship between the entorhinal cortex and the other fields of the hippocampal formation (Figure 23). Neurons in layers II and III of the entorhinal cortex give rise to the major portion of the perforant path projection (Witter, Van Hoesen et al. 1989), although a few cells in the deep layers also contribute to this projection. Layer II cells preferentially project to the dentate gyrus and CA3 field of the hippocampus whereas layer III cells project to CA1 and the subiculum. Layer II cells located rostrally tend to project to the outer portion of the molecular layer of the dentate gyrus and stratum lacunosum-moleculare of CA3 whereas more caudally situated layer II cells project to middle portions of the molecular layer and to the deeper portion of stratum lacunosum-moleculare. Caudally situated layer III cells project to the proximal portion of CA1 (closer to CA3) and to the distal portion of the subiculum (closer to the presubiculum) whereas rostrally located layer III cells tend to terminate near the CA1/subiculum border. There are at least three rostrocaudally oriented bands of cells in the entorhinal cortex that project to different rostrocaudal levels of the other hippocampal fields. Cells located laterally project to caudal levels of the dentate gyrus, hippocampus and subiculum whereas more medially situated neurons terminate at more rostral levels of these fields (Witter and Amaral 1991). We have also demonstrated that intrinsic connections of the entorhinal cortex respect both the mediolateral band organization as well as the rostrocaudal segregation of information (Chrobak and Amaral 2007).
A somewhat simplistic implication of this organization is that if a cortical projection terminates in a selected rostrocaudal and/or mediolateral portion of the entorhinal cortex, information would be conveyed via the perforant path to a restricted rostrocaudal portion of the remainder of the hippocampal formation. For example, the insular cortex has a relatively restricted projection to EO and ER but is distributed throughout the mediolateral extent of the entorhinal cortex. This information would be expected to go to all rostrocaudal levels of the dentate gyrus but would terminate on the distal portion of the granule cell dendrites. The information would also preferentially terminate on the CA1 and subicular cells located at the border of these two fields. Conversely, input from the retrosplenial cortex terminates at caudal and lateral levels of the entorhinal cortex. This information would tend to have more influence on caudal levels of the dentate gyrus and on the more proximal portions of the dendrites of the granule cells. At the same caudal levels, CA1 cells located closer to CA3 and subicular cells located closer to the presubiculum would be more influenced by this input. While a further refinement of any segmentation of information processing within the primate and human hippocampal formation will need additional functional analyses, it is clear that the organization of projections to the entorhinal cortex predicts that there may be transverse domains of specialization within the dentate gyrus and hippocampus (Figure 22). Thus, the retrosplenial and parahippocampal cortices, both of which project to caudal levels of the entorhinal cortex, would tend to have greater influence on proximal CA1 cells and distal subicular cells, inputs from the perirhinal cortex and the insular cortex, which mainly terminate rostrally, would tend to have greater influence on the cells at the CA1/subiculum border. This transverse information processing is further emphasized by the fact that the rostral and caudal levels of layer III of the entorhinal cortex receive quite distinct inputs. Rostral levels of layer III receive a substantial input from the amygdaloid complex, particularly the most ventral and polysensory portion of the lateral nucleus (Pitkänen 2002). In contrast, layer III of caudal levels, but not rostral levels, of the entorhinal cortex receives a massive input from the presubiculum (Kohler 1984); Amaral and Witter, unpublished observations). It is likely that these two inputs are providing additional forms of information that are then distributed to CA1 and the subiculum. The amygdala could be providing information relevant to the emotional salience of perceived stimuli whereas the presubiculum, through its inputs from the anterior thalamus, could be providing navigational information. Unfortunately, the modest electrophysiological analysis that has been carried out in the nonhuman primate hippocampus has not attended to response properties related to transverse location. This will be an interesting issue to address in future single cell or functional imaging studies.
We did not see enormous evidence for a strictly mediolateral pattern of termination of cortical inputs to the entorhinal cortex similar to what has been reported in the rat (Burwell and Amaral 1998). In the rat, the bulk of neocortical input to the entorhinal cortex terminates in the lateral third. Since the lateral third of the entorhinal cortex preferentially terminates in the dorsal or septal third of the dentate gyrus and hippocampus, one can claim that this region of the hippocampus gets more sensory input than, for example, the temporal third. The cortical projections to the nonhuman primate entorhinal cortex are distributed throughout its mediolateral extent providing less justification for a rostrocaudal difference in information processing.
LAMINAR DISTRIBUTION
In virtually all of the experimental cases, the region of highest fiber and terminal labeling within the entorhinal cortex demonstrated tracer over all layers. Away from this “hot zone”, some layers demonstrate much reduced or completely eliminated fiber and terminal labeling. Again, for most of the experimental cases, the remaining labeling was most dense in the superficial layers (I–III) with a predominance within layer I. However, when the injection site involved the caudal and medial portions of the orbitofrontal or the infralimbic cortices, the peripheral projection continued within layers V and VI (Figure 22). Projections to the deep layers of the entorhinal cortex from frontal lobe areas have been demonstrated previously both for the macaque monkey (Rempel-Clower and Barbas 2000) and for the squirrel monkey (Leichnetz and Astruc 1975; Leichnetz and Astruc 1976). A recent paper by Jones and Witter (Jones and Witter 2007) has emphasized that the retrospenial cortex terminates predominantly in the deep layers of that rat medial entorhinal cortex. It will be interesting to determine whether this result reflects the fact that the retrosplenial projections that we demonstrated here are due, inpart, to cells located in area 30 or a true species difference of the projections of area 29.
FUNCTIONAL IMPLICATIONS – LAMINAR DISTRIBUTION
As noted above and illustrated in Figure 23, neurons in layers II and III largely give rise to the perforant path projection. Neurons in layers V and VI, in contrast, are the recipients of return projections from the CA1 field of the hippocampus and the subiculum (Rosene and Van Hoesen 1977; Saunders and Rosene 1988) and give rise, in turn, to the major cortical and subcortical efferent projections of the entorhinal cortex (Munoz and Insausti 2005). Thus, layers II and III can be thought of as the “input” layers of the entorhinal cortex whereas layers V and VI can be considered the “output” layers of the entorhinal cortex. An implication of the laminar organization of the entorhinal cortex is that inputs that terminate preferentially on layers II and III, such as the perirhinal, parahippocampal and retrosplenial cortices, will tend to have an influence on the other hippocampal fields via the perforant pathway. Projections that terminate preferentially on the deep layers of the entorhinal cortex, such as the orbital and infralimbic cortices, may have more influence on the output of the entorhinal cortex. Although, this conclusion must be made with caution since intrinsic deep to superficial projections (Chrobak and Amaral, 2007) decidedly complicate this simple interpretation. Projections to the deep layers, for example, could inhibit information flow entering through the superficial layers via a deep to superficial inhibitory projection. And even those projections that most heavily innervate the superficial layers appear to have at least focal termination within deep layers. Moreover, the deep layers also give rise to at least a minor component of the perforant path projections to other regions of the hippocampal formation (Steward and Scoville 1976).
NOT ALL ENTORHINAL CORTICAL INPUTS ARE ALIKE
It is widely accepted that the hippocampal formation is critical for memory processing both in humans and non-human primates (Squire and Zola 1996). The multisensory episodic memories that are produced through relational processing rely on the integration of sensory information from a variety of cortical regions. The current paper is one in a series of studies (Amaral 1987; Insausti, Amaral et al. 1987a; Insausti 1987b; Suzuki and Amaral 1994; Suzuki and Amaral 1994; Kobayashi and Amaral 2003; Kobayashi and Amaral 2007) that were designed to define the raw material on which these memories are built. Given that the entorhinal cortex is the main interface between sensory neocortex and the other fields of the hippocampal formation (Van Hoesen, Pandya et al. 1975; Van Hoesen and Pandya 1975a; Van Hoesen and Pandya 1975b) this program has been designed first to determine which sensory areas project to the entorhinal cortex.
In previous studies on the inputs to the macaque monkey entorhinal cortex, we have highlighted some of the quantitative aspects of this input (Insausti, Amaral et al. 1987a). The perirhinal and parahippocampal cortices, for example, were estimated to give rise to approximately 60% of the input to the entorhinal cortex, the retrosplenial cortex provided some 20% of the input and all other cortical inputs were substantially smaller in magnitude. Given the laminar and regional differences of the cortical inputs demonstrated in the current paper, it is perhaps appropriate to briefly discuss the potential influences of these projections on the production and storage of new memories.
Other than the olfactory system that projects directly to layer I of EO, most of the other sensory information originates in polysensory cortical areas. It remains difficult to provide a simple descriptor of the type of sensory information that is provided to the hippocampal formation from its cortical sources. Both the perirhinal and parahippocampal cortices, for example, are heavily visual (Suzuki and Amaral 1994) although both also receive inputs from cortices associated with auditory and somatic sensation (Jones and Powell 1970) and their neurons demonstrate polysensory responsivity (Desimone and Gross 1979). The perirhinal cortex is increasingly portrayed as the culmination of the object processing system and is involved in sophisticated cognitive processes (Murray and Richmond 2001). Taylor et al. (2006) have suggested, for example, that the perirhinal cortex may play an important role in forming a meaningful multimodal representation of an object (Taylor, Moss et al. 2006). This complex percept, then, would be directed to the entorhinal cortex and on to the other fields of the hippocampal formation for subsequent processing, leading ultimately to an episodic memory.
Both the parahippocampal cortex and the retrosplenial cortex are typically portrayed as providing visuospatial information to the hippocampal formation (Suzuki and Amaral 1994; Epstein, Parker et al. 2007). Epstein et al. (2007) used fMRI in adult subjects to provide evidence that the parahippocampal cortex supports spatial perception in real time whereas the retrosplenial cortex may support memory retrieval mechanisms that allow a scene to be localized within the broader spatial environment. Whether the parahippocampal cortex is preferentially involved in spatial information processing is not yet clear. Aminoff et al. (Aminoff, Gronau et al. 2007), for example, have demonstrated that the parahippocampal cortex plays a role in processing contextual associations in general, and that these associations are not restricted to spatial information. The entorhinal cortex also receives input from the dorsal bank of the superior temporal sulcus, the so-called superior temporal gyrus polysensory area (Bruce, Desimone et al. 1981). This area has been implicated in the perception of complex visual objects and of biological motion (Perrett, Harries et al. 1989; Vaina and Gross 2004; Lange and Lappe 2006). Thus, virtually all of the major projections to the superficial layers of the entorhinal cortex provide high level, multimodal sensory information that could be further integrated to form a polysensory representation of an episode of one's life. As indicated above and in Figure 23, this sensory information has a somewhat segregated entry to the hippocampal formation, entering either rostral or caudal levels of the entorhinal cortex. Of course, there is ample opportunity for further integration of this information within the networks of the dentate gyrus and hippocampus.
But, what about the projections from orbitofrontal and infralimbic (or medial frontal) cortices. There are several reasons to believe that these inputs have a distinctly different role in the synaptic economy of the entorhinal cortex. First, these projections terminate mainly in layers V and VI of the entorhinal cortex (Fig. 23). These are the layers that receive the return projections from CA1 of the hippocampus and the subiculum and these layers give rise to the major efferent projections of the hippocampal formation. Second, the orbital and medial frontal projections tend to innervate the entire rostrocaudal extent of the entorhinal cortex as if having a more global influence on its function. The orbitofrontal cortex is a complex region that is currently the subject of intense research (Zald and Rauch 2006). This cortical area is itself a polysensory region that participates in a number of local and distributed cortical networks (Ongur and Price 2000). There appears to be an emerging consensus that the orbitofrontal cortex is not so much critical for perception of the sensory features of stimuli but of their saliency or their reinforcing value. Thus, while the orbitofrontal cortex receives gustatory information, taste responsive neurons are modulated by the satiation of the animal to a specific food (Rolls, Sienkiewicz et al. 1989). In fact, human functional magnetic resonance imaging studies demonstrate that the orbitofrontal cortex codes the reward value of stimuli across all modalities (O'Doherty and Dolan 2006; Wallis 2007). Thus, one possibility is that the input from the orbitofrontal cortex to the entorhinal cortex can gate the output of the hippocampal formation to presumed memory storage sites in the neocortex based on the reward or punishment outcome of the encoded episode. Thus, the encoding of episodes that lead either to a species-typical reward or punishment could be facilitated through the orbitofrontal input. One implication of this relationship is that lesions of the orbitofrontal cortex would not be expected to have a profound impact on making new episodic memories (Fujii, Suzuki et al. 2005) although the differential encoding a salient versus nonsalient information may be impaired. Interestingly, Ranganath et al. (Ranganath, Heller et al. 2005) have demonstrated that there is increased functional connectivity between the hippocampal formation and the orbitofrontal cortex (but not the dorsolateral prefrontal cortex) during successful memory formation. We have emphasized that the cortical inputs to the entorhinal cortex are likely influencing both the input neurons to the hippocampal formation and the output neurons to the cerebral cortex. Clearly, detailed electrophysiological studies are needed to define how these inputs condition the response properties of the entorhinal cortex. These interactions of cortical inputs and outputs may favor a controlled and flexible processing of internal representations that presumably leads to the formation and consolidation of declarative memory.
DEDICATION
We would like to dedicate this paper to the memory of W. Maxwell (Max) Cowan who was a mentor and friend to both of the authors. Max initiated this series of studies and carefully shepherded the initial projects through design, implementation and publication. We learned much from Max and think of him often as we continue our quest to understand the organization of the hippocampal formation.
Acknowledgments
Grant support: This research was supported in part by NIH grant NS16980 to D.G.A., BFI 2000-0418 from the Ministry of Science and Technology (Spain), BFI2003-09581, BFU2006-12964 and GC-02-022 from the Department of Science and Technology, Autonomous Government of Castilla-La Mancha (Spain) to R.I. These studies were conducted, in part, at the California National Primate Research Center (RR00169).
ABBREVIATIONS
- A13l
Area 13 of Brodmann, lateral field
- A13m
Area 13 of Brodmann, medial field
- A14
Area 14 of Brodmann
- A24
Area 24 of Brodmann
- A25
Area 25 of Brodmann
- A29m
Area 29 of Brodmann, medial field
- A29l
Area 29 of Brodmann, lateral field
- A30
Area 30 of Brodmann
- A35
Area 35 of Brodmann
- A36
Area 36 of Brodmann
- A36r
Area 36 of Brodmann, rostral field
- A36c
Area 36 of Brodmann, caudal field
- A36rv
Area 36 of Brodmann, rostroventral temporopolar field
- A
amygdaloid complex
- Acc
Nucleus accumbens
- ac
anterior commissure
- amts
anterior middle temporal sulcus
- C
Caudal
- cas
calcarine sulcus
- cs
cingulate sulcus
- E
Entorhinal cortex
- EC
Entorhinal cortex, caudal subfield
- EI
Entorhinal cortex, intermediate subfield
- ECL
Entorhinal cortex, caudal limiting subfield
- ELr
Entorhinal cortex, lateral rostral subfield
- ELc
Entorhinal cortex, lateral caudal field
- EO
Entorhinal cortex, olfactory subfield
- ER
Entorhinal cortex, rostral subfield
- H
Hippocampus
- I
Layer I of the Entorhinal cortex
- II
Layer II of the Entorhinal cortex
- III
Layer III of the Entorhinal cortex
- IV
Layer IV of the Entorhinal cortex
- Ia
Agranular insular cortex
- Id
Disgranular insular cortex
- I
Insular cortex
- ils
inferior limiting sulcus
- ips
intraparietal sulcus
- L
Lateral
- las
lateral sulcus
- LD
Lamina dissecans
- M
Medial
- MF
Medial frontal cortex
- OF
Orbitofrontal cortex
- ots
occipitotemporal sulcus
- ot
optic tract
- Pi
Parainsular cortex
- PC
Posterior cingulate cortex
- PH
Parahippocampal cortex
- PR
Perirhinal cortex
- Pul
Pulvinar
- R
Rostral
- rs
rhinal sulcus
- STG
Superior temporal gyrus
- sts
superior temporal sulcus
- TE
Area TE of von Bonin and Bailey
- TF
Area TF of von Bonin and Bailey
- TH
Area TH of von Bonin and Bailey
- V
Layer V of the Entorhinal cortex
- v
ventricle
- VI
Layer VI of the Entorhinal cortex
LITERATURE CITED
- Amaral DG, Insausti R. Evidence for a direct projection from the superior temporal gyrus to the entorhinal cortex in the monkey. Brain Res. 1983;275:263–277. doi: 10.1016/0006-8993(83)90987-3. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Insausti R, Cowan WM. The entorhinal cortex of the monkey. I. Cytoarchitectonic organization. J. Comp. Neurol. 1987;264:326–355. doi: 10.1002/cne.902640305. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL. An air pressure system for the injection of tracer substances into the brain. J Neurosci Methods. 1983;9(1):35–43. doi: 10.1016/0165-0270(83)90107-3. [DOI] [PubMed] [Google Scholar]
- Aminoff E, Gronau N. The parahippocampal cortex mediates spatial and nonspatial associations. Cereb Cortex. 2007;17(7):1493–503. doi: 10.1093/cercor/bhl078. [DOI] [PubMed] [Google Scholar]
- Barbas H, Ghashghaei H. Medial prefrontal cortices are unified by common connections with superior temporal cortices and distinguished by input from memory-related areas in the rhesus monkey. J Comp Neurol. 1999;410(3):343–67. doi: 10.1002/(sici)1096-9861(19990802)410:3<343::aid-cne1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Bruce C, Desimone R. Visual properties of neurons in a polysensory area in superior temporal sulcus of the macaque. J Neurophysiol. 1981;46(2):369–84. doi: 10.1152/jn.1981.46.2.369. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG. Perirhinal and postrhinal cortices of the rat: interconnectivity and connections with the entorhinal cortex. The Journal of Comparative Neurology. 1998;391:293–321. doi: 10.1002/(sici)1096-9861(19980216)391:3<293::aid-cne2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefontal cortex in macaque monkeys. J. Comp. Neurol. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Cavada C, Company T. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb Cortex. 2000;10(3):220–42. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. J Comp Neurol. 1989;287(4):393–421. doi: 10.1002/cne.902870402. [DOI] [PubMed] [Google Scholar]
- Chrobak JJ, Amaral DG. Entorhinal cortex of the monkey: VII. intrinsic connections. J Comp Neurol. 2007;500(4):612–33. doi: 10.1002/cne.21200. [DOI] [PubMed] [Google Scholar]
- Cowan WM, Gottlieb DI. The autoradiographic demonstration of axonal connections in the central nervous system. Brain Res. 1972;37(1):21–51. doi: 10.1016/0006-8993(72)90344-7. [DOI] [PubMed] [Google Scholar]
- Desimone R, Gross CG. Visual areas in the temporal cortex of the macaque. Brain Res. 1979;178(2–3):363–80. doi: 10.1016/0006-8993(79)90699-1. [DOI] [PubMed] [Google Scholar]
- Ding SL, Van Hoesen G. Inferior parietal lobule projections to the presubiculum and neighboring ventromedial temporal cortical areas. J Comp Neurol. 2000;425(4):510–30. doi: 10.1002/1096-9861(20001002)425:4<510::aid-cne4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Epstein RA, Parker WE. Where am I now? Distinct roles for parahippocampal and retrosplenial cortices in place recognition. J Neurosci. 2007;27(23):6141–9. doi: 10.1523/JNEUROSCI.0799-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T, Suzuki M, et al. Normal memory and no confabulation after extensive damage to the orbitofrontal cortex. J Neurol Neurosurg Psychiatry. 2005;76(9):1309–10. doi: 10.1136/jnnp.2004.061846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Selemon LD, et al. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience. 1984;12(3):719–43. doi: 10.1016/0306-4522(84)90166-0. [DOI] [PubMed] [Google Scholar]
- Insausti R, Amaral DG, et al. The entorhinal cortex of the monkey. II. Cortical afferents. J. Comp. Neurol. 1987a;264:356–395. doi: 10.1002/cne.902640306. [DOI] [PubMed] [Google Scholar]
- Insausti R, Amaral DG, Cowan WM. The entorhinal cortex of the monkey. III. Subcortical afferents. J. Comp. Neurol. 1987b;264:396–408. doi: 10.1002/cne.902640307. [DOI] [PubMed] [Google Scholar]
- Jones BF, Witter MP. Cingulate cortex projections to the parahippocampal region and hippocampal formation in the rat. Hippocampus. 2007;17(10):957–76. doi: 10.1002/hipo.20330. [DOI] [PubMed] [Google Scholar]
- Jones EG, Burton H. Areal differences in the laminar distribution of thalamic afferents in cortical fields of the insular, parietal and temporal regions of primates. J Comp Neurol. 1976;168(2):197–247. doi: 10.1002/cne.901680203. [DOI] [PubMed] [Google Scholar]
- Jones EG, Powell TP. An anatomical study of converging sensory pathways within the cerebral cortex of the monkey. Brain. 1970;93(4):793–820. doi: 10.1093/brain/93.4.793. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: II. Cortical afferents. J Comp Neurol. 2003;466(1):48–79. doi: 10.1002/cne.10883. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: III. Cortical efferents. J Comp Neurol. 2007;502(5):810–33. doi: 10.1002/cne.21346. [DOI] [PubMed] [Google Scholar]
- Kohler C. Morphological details of the projection from the presubiculum to the entorhinal area as shown with the novel PHA-L immunohistochemical tracing method in the rat. Neuroscience Letters. 1984;45:285–290. doi: 10.1016/0304-3940(84)90240-4. [DOI] [PubMed] [Google Scholar]
- Kosel KC, Van Hoesen GW. Nonhippocampal cortical projections from the entorhinal cortex in the rat and rhesus monkey. Brain Res. 1982;244:201–213. doi: 10.1016/0006-8993(82)90079-8. [DOI] [PubMed] [Google Scholar]
- Lange J, Lappe M. A model of biological motion perception from configural form cues. J Neurosci. 2006;26(11):2894–2906. doi: 10.1523/JNEUROSCI.4915-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenex P, Suzuki WA. Perirhinal and parahippocampal cortices of the macaque monkey: Projections to the neocortex. Journal of Comparative Neurology. 2002;447(4):394–420. doi: 10.1002/cne.10243. [DOI] [PubMed] [Google Scholar]
- Leichnetz GR, Astruc J. Efferent connections of the orbitofrontal cortex in the marmoset (Saguinus oedipus) Brain Res. 1975;84(2):169–80. doi: 10.1016/0006-8993(75)90973-7. [DOI] [PubMed] [Google Scholar]
- Leichnetz GR, Astruc J. Preliminary evidence for a direct projection of the prefrontal cortex to the hippocampus in the squirrel monkey. Brain Behav Evol. 1975;11(5–6):355–64. doi: 10.1159/000123645. [DOI] [PubMed] [Google Scholar]
- Leichnetz GR, Astruc J. The efferent projections of the medial prefrontal cortex in the squirrel monkey (Saimiri sciureus) Brain Res. 1976;109(3):455–72. doi: 10.1016/0006-8993(76)90027-5. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey. III: Efferent cortical output and comments on function. J Comp Neurol. 1982;212(1):38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Van Hoesen GW, et al. Limbic and sensory connections of the inferior parietal lobule (area PG) in the rhesus monkey: a study with a new method for horseradish peroxidase histochemistry. Brain Res. 1977;136(3):393–414. doi: 10.1016/0006-8993(77)90066-x. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Cipolloni PB. Cytoarchitecture and cortical connections of the posterior cingulate and adjacent somatosensory fields in the rhesus monkey. J Comp Neurol. 2004;469(1):37–69. doi: 10.1002/cne.10980. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Geula C, et al. Cytoarchitecture and neural afferents of orbitofrontal cortex in the brain of the monkey. J Comp Neurol. 1992;323(3):341–58. doi: 10.1002/cne.903230304. [DOI] [PubMed] [Google Scholar]
- Morris R, Petrides M, et al. Architecture and connections of retrosplenial area 30 in the rhesus monkey (Macaca mulatta) Eur J Neurosci. 1999;11(7):2506–18. doi: 10.1046/j.1460-9568.1999.00672.x. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Pandya DN. Some observations on the course and composition of the cingulum bundle in the rhesus monkey. J Comp Neurol. 1984;225(1):31–43. doi: 10.1002/cne.902250105. [DOI] [PubMed] [Google Scholar]
- Munoz M, Insausti R. Cortical efferents of the entorhinal cortex and the adjacent parahippocampal region in the monkey (Macaca fascicularis) Eur J Neurosci. 2005;22(6):1368–88. doi: 10.1111/j.1460-9568.2005.04299.x. [DOI] [PubMed] [Google Scholar]
- Murray EA, Richmond BJ. Role of perirhinal cortex in object perception, memory, and associations. Curr Opin Neurobiol. 2001;11(2):188–93. doi: 10.1016/s0959-4388(00)00195-1. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Dolan R. The Orbitofrontal Cortex. Oxfod University Press; Oxford: 2006. The role of human orbitofrontal cortex inreqest prediction and behavioral choice: insights from neuroimaging; pp. 265–283. D. Zald and S. Rauch. [Google Scholar]
- Ongur D, Price J. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Perrett DI, Harries MH, et al. Frameworks of analysis for the neural representation of animate objects and actions. J Exp Biol. 1989;146:87–113. doi: 10.1242/jeb.146.1.87. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur J Neurosci. 2002;16(2):291–310. doi: 10.1046/j.1460-9568.2001.02090.x. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Kelly JL, Amaral DG. Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the entorhinal cortex in the macaque monkey. Hippocampus. 2002;12:186–205. doi: 10.1002/hipo.1099. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Heller A. Functional connectivity with the hippocampus during successful memory formation. Hippocampus. 2005;15(8):997–1005. doi: 10.1002/hipo.20141. [DOI] [PubMed] [Google Scholar]
- Rempel-Clower NL, Barbas H. The laminar pattern of connections between prefrontal and anterior temporal cortices in the Rhesus monkey is related to cortical structure and function. Cereb Cortex. 2000;10(9):851–65. doi: 10.1093/cercor/10.9.851. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Sienkiewicz ZJ, et al. Hunger Modulates the Responses to Gustatory Stimuli of Single Neurons in the Caudolateral Orbitofrontal Cortex of the Macaque Monkey. Eur J Neurosci. 1989;1(1):53–60. doi: 10.1111/j.1460-9568.1989.tb00774.x. [DOI] [PubMed] [Google Scholar]
- Room P, Groenewegen HJ. Connections of the parahippocampal cortex. I. Cortical afferents. J Comp Neurol. 1986;251(4):415–50. doi: 10.1002/cne.902510402. [DOI] [PubMed] [Google Scholar]
- Rosene DL, Van Hoesen GW. Hippocampal efferent reach widespread areas of cerebral cortex and amygdala in the rhesus monkey. Science. 1977;198:315–317. doi: 10.1126/science.410102. [DOI] [PubMed] [Google Scholar]
- Saleem KS, Tanaka K. Divergent projections from the anterior inferotemporal area TE to the perirhinal and entorhinal cortices in the macaque monkey. J Neurosci. 1996;16(15):4757–75. doi: 10.1523/JNEUROSCI.16-15-04757.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders RC, Rosene DL. A comparison of the efferents of the amygdala and the hippocampal formation in the rhesus monkey: I. Convergence in the entorhinal, prorhinal, and perirhinal cortices. J Comp Neurol. 1988;271(2):153–84. doi: 10.1002/cne.902710202. [DOI] [PubMed] [Google Scholar]
- Squire L, Zola S. Structure and function of declarative and nondeclarative memory systems. PNAS. 1996;93(24):13515–13522. doi: 10.1073/pnas.93.24.13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Zola SM. Episodic memory, semantic memory, and amnesia. Hippocampus. 1998;8:205–11. doi: 10.1002/(SICI)1098-1063(1998)8:3<205::AID-HIPO3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Steward O, Scoville SA. Cells of origin of entorhinal cortical afferents to the hippocampus and fascia dentata of the rat. Journal of Comparative Neurology. 1976;169:347–370. doi: 10.1002/cne.901690306. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. J Comp Neurol. 1994;350:497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Topographic organization of the reciprocal connections between the monkey entorhinal cortex and the perirhinal and parahippocampal cortices. J. Neurosci. 1994;14:1856–1877. doi: 10.1523/JNEUROSCI.14-03-01856.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo J, Cowan WM. A stereotaxic atlas of the brain of the cynomolgus monkey (Macaca fascicularis) J Comp Neurol. 1984;222(2):265–300. doi: 10.1002/cne.902220208. [DOI] [PubMed] [Google Scholar]
- Taylor KI, Moss HE, et al. Binding crossmodal object features in perirhinal cortex. Proc Natl Acad Sci U S A. 2006;103(21):8239–44. doi: 10.1073/pnas.0509704103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaina LM, Gross CG. Perceptual deficits in patients with impaired recognition of biological motion after temporal lobe lesions. Proc Natl Acad Sci U S A. 2004;101(48):16947–51. doi: 10.1073/pnas.0407668101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoesen GW, Pandya DN. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. I. Temporal lobe afferents. Brain Res. 1975a;95:1–24. doi: 10.1016/0006-8993(75)90204-8. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Pandya DN. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. III. Efferents connections. Brain Res. 1975b;95:39–59. doi: 10.1016/0006-8993(75)90206-1. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Pandya DN, et al. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. II. Frontal lobe afferents. Brain Res. 1975;95:25–38. doi: 10.1016/0006-8993(75)90205-x. [DOI] [PubMed] [Google Scholar]
- Wallis JD. Orbitofrontal cortex and its contribution to decision-making. Annu Rev Neurosci. 2007;30:31–56. doi: 10.1146/annurev.neuro.30.051606.094334. [DOI] [PubMed] [Google Scholar]
- Wellman BJ, Rockland KS. Divergent cortical connections to entorhinal cortex from area TF in the macaque. J Comp Neurol. 1997;389(3):361–76. [PubMed] [Google Scholar]
- Whitlock DG, Nauta WJH. Subcortical projections from the temporal neocortex in macaca mulatta. Journal of Comparative Neurology. 1956;106:183–212. doi: 10.1002/cne.901060107. [DOI] [PubMed] [Google Scholar]
- Witter MP, Amaral DG. Entorhinal cortex of the monkey: V. Projections to the dentate gyrus, hippocampus, and subicular complex. J. Comp. Neurol. 1991;307:437–459. doi: 10.1002/cne.903070308. [DOI] [PubMed] [Google Scholar]
- Witter MP, Van Hoesen GW. Topograpical organization of the entorhinal projection to the dentate gyrus of the monkey. J. Neurosci. 1989;9:216–228. doi: 10.1523/JNEUROSCI.09-01-00216.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald D, Rauch S. The Orbitofrotnal Cortex. Oxford University Press; Oxford: 2006. [Google Scholar]