Abstract
Detection of light in the eye underlies image-forming-vision, but also regulates adaptive responses in physiology and behavior. Typically these adaptive responses do not involve image-forming-vision, but depend on a relatively absolute measure of brightness (non-image-forming irradiance detection). The goal of this study was to further understand how image-forming-vision and non-image-forming irradiance detection contribute to the effects of light on behavior. Three light dependent behaviors were assessed in wild-type, Rpe65−/− and rd1 mice. In Rpe65−/− mice, non-image-forming irradiance detection is severely attenuated, but rod based visual acuity is relatively preserved. In rd1 mice visual acuity is non-recordable, but non-image-forming responses are less severely attenuated than Rpe65−/−. Positive masking, a image-forming-vision dependent increase in wheel running, was absent in rd1 and restricted to higher irradiances in Rpe65−/−. Negative masking, a suppression of wheel running sensitivity with non-image-forming irradiance detection input, was increased in rd1, but reduced in Rpe65−/− mice. By contrast, light-aversion, an avoidance of brightly lit areas, was abolished in both Rpe65−/−and rd1. This shows that image-forming-vision is not sufficient for light-aversion, suggesting non-image-forming irradiance detection motivates this behavior. Further, the differing effects of disease suggest that negative masking and light-aversion are distinct responses with specialized non-image-forming irradiance detection pathways.
Keywords: light-aversion, melanopsin, negative masking, place-preference, predation
The eye of mammals serves two distinct types of light-detection task. Detection of relative contrast on the array of rod and cone photoreceptors in the retina supports image-forming-vision (Stryer, 1996). Separate from image-forming-vision, detection of the amount of light in relatively absolute terms, regulates adaptive changes in physiology and behavior (Foster, 2002). This non-image-forming function of the eye depends on melanopsin ganglion cell photoreceptors (melGCs) that respond proportionally to irradiance (Guler et al., 2008; Hattar et al., 2003). Despite an identified retinal cellular basis for these distinct functions, how image-forming-vision and non-image-forming irradiance detection contribute to the effects of light on behavior is not fully understood.
For a singly housed animal in a controlled laboratory environment, image-forming-vision could most obviously alter behavior by enhancing the ability to perform tasks. In complete darkness, despite absence of image-forming-vision, other spatial cues (tactile and olfactory) support remarkably high levels of wheel running. However, if placed in dim-light, there is a marked increment in wheel running. The most plausible explanation is that much as our own motility improves when we can see what we are doing, image-forming-vision aids wheel running (Thompson, Philp, & Stone, 2008). This effect of light is termed ‘positive masking’ because it masks the baseline amount of activity in a positive direction.
In the natural environment, ambient light levels also affect the quality of image-forming-vision of other animals. The implicit benefit of nocturnal activity in mice is to minimize visual search predation. In addition to this circadian segregation of activity, mice exhibit two acute light driven changes in behavior that would reduce risk of visual target acquisition by a predator: negative masking and light-aversion. Negative masking is a suppression of wheel running under bright light, and light-aversion is a preference for dimly illuminated areas (Lamprea, Cardenas, Setem, & Morato, 2008; Mrosovsky, 1999; Recober et al., 2009; Simon, Dupuis, & Costentin, 1994). As responses best served by an objective measure of visual conditions, we hypothesize that both would depend on non-image-forming irradiance detection. Consistent with this, negative masking depends on melGCs, and is independent of positive masking (Guler et al., 2008; Mrosovsky & Hattar, 2003; Mrosovsky & Thompson, 2008). However, light-aversion involves a spatial preference, so it is less clear whether the response depends on non-image-forming irradiance detection, image-forming-vision, or a combination of both.
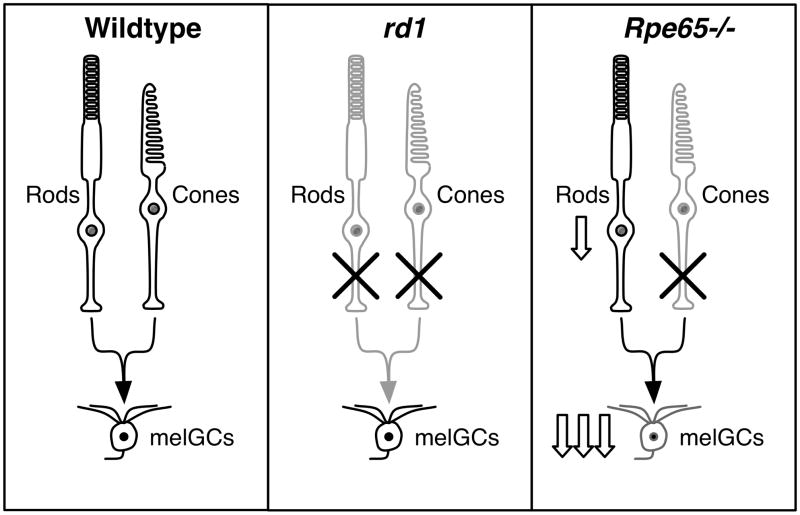
The retinal cellular specialization for image-forming-vision and non-image-forming irradiance detection means that retinal diseases differentially affect these functions of the eye (Thompson, Mullins, Philp, Stone, & Mrosovsky, 2008). Therefore, to further understand the basis of light-dependent behaviors in mice, we compared positive masking, negative masking, and light-aversion in three strains of mice with contrasting retinal function: wild-type, rd1, and Rpe65−/− mice (Figure 1). In Rpe65−/− mice, loss of RPE65 all-trans-retinoid isomerase activity results in chromophore deficits (Pang et al., 2005; Seeliger et al., 2001). Rpe65−/− responses originating with non-image-forming irradiance detection are severely attenuated, but rod based visual acuity is relatively preserved (Doyle, Castrucci, McCall, Provencio, & Menaker, 2006; Doyle, Yoshikawa, Hillson, & Menaker, 2008; Seeliger et al., 2001; Thompson, Mullins et al., 2008; Tu et al., 2006). In contrast, in rd1 mice loss of cGMP-phosphodiesterase-6b hydrolysis activity results in early degeneration of rods, with associated loss of cones (Carter-Dawson, LaVail, & Sidman, 1978). rd1 image-forming-vision is abolished well before the age tested, but non-image-forming irradiance detection is less severely attenuated than Rpe65−/− (Foster et al., 1991; Mrosovsky, Foster, & Salmon, 1999; Thompson, Mullins et al., 2008).
Figure 1. Effect of disease on the function of photoreceptors.
In wild-type mice, rod, cone and melanopsin ganglion cell photoreceptors (melGCs) all respond to light. In rd1 mice rods and cones have degenerated so responses to light arise only from melGCs. This abolishes image-forming-vision but non-image-forming irradiance detection responses are retained relative to Rpe65−/− mice. In Rpe65−/− mice, cones are degenerated, rod function is retained with reduced sensitivity, and melGCs appear to have severely attenuated function (reduction of sensitivity is implied by down arrows). This severely diminishes non-image-forming irradiance detection but image-forming-vision remains intact.
Methods
Animals and general methods
All experiments were performed in accordance with the American Psychological Association statement for the Use of Animals in Research and were approved by the University of Iowa Animal Care and Use Review. Mice were on a B6 (C57) backcrossed background from Jackson Laboratories (Bar Harbor, ME): B6(A)-Rpe65rd12/J (Rpe65−/−), B6.C3-Pde6brd1 Hps4le/J (rd1) and their common control, C57BL/6J (wild-type). Animals were raised and maintained under a 12-hour light cycle at ~17 (micro) μWcm−2s−1, with access to food and water ad libitum. The rd1 was selected because the severe rod-cone loss was known to abolish image-forming-vision, and to result in a characteristic hyper-sensitization of negative masking (Mrosovsky et al., 1999; Thompson, Mullins et al., 2008). The Rpe65−/−mouse was selected as a disease model with contrasting phenotypes in previous studies, having positive masking consistent with image-forming-vision, but severely desensitized non-image-forming irradiance detection (Doyle et al., 2006; Doyle et al., 2008; Thompson, Mullins et al., 2008; Tu et al., 2006).
Positive and negative masking responses
The effect of light on wheel running activity was measured in Rpe65−/− (n = 15), rd1 (n = 13) and wild-type (n = 18) mice using previously reported methods (Thompson, Philp et al., 2008). Male mice were individually housed in wheel cages (Thoren Caging Inc) mounted in environment control cabinets (University of Iowa Medical Instruments, Iowa City, IA), with food and water available ad libitum. Wheel running was continuously recorded using a customized ClockLab data acquisition system (Actimetrics, Inc. Evanston, IL). A 12-hour light: 12-hour dark cycle of fluorescent white light at ~19 μWcm−2s−1 was napplied throughout. Animals were allowed to acclimatize to wheel cages for 21 days prior to testing so that they were 76 to 85 days old at the first test. A 3-day repeating experimental cycle of pre-pulse baseline day, pulse day and maintenance day was applied. On pulse days, a remotely controlled 1-hour pulse was applied to mice in their home cage, starting one hour after daily dark onset. Light was from a ‘cool white’ fluorescent bulb F40CW/RS/EW/ALTO (Philips Lighting, Somerset, NJ), with Cinegel neutral density film used to adjust irradiance (Rosco, Stamford, CT). Nine light levels between 3.3×10−7 and 15.4 μWcm−2s−1 were applied in a sequence that distributed bright and dim pulses over the course of testing. Light measurements were taken in μWcm−2s−1 using a PM103 power meter (Macam Photometrics Ltd, Livingston, UK).
Changes in activity with applied light were calculated as % of baseline activity at the corresponding time on the preceding day for each animal. Variable slope sigmoid dose response curves were fitted to data in Prism (GraphPad Software Inc, La Jolla, CA) with a fixed constant for the minimum set at 0%. The irradiance producing a half maximal response (EC50) and hill-slope were calculated from fitted curves, and compared with an F-test of a two-fit comparison, also in Prism. Briefly, curves were fitted to the data sets for two genotypes independently and then to the combined data set for both genotypes, the effect of combining data sets on the quality of fit to a given parameter was then used to calculate if there was a difference. Bonferroni corrections were applied to P.
Light-aversion
Light-aversion was measured in Rpe65−/− (n = 6), rd1 (n = 11) and wild-type (n = 10) mice using a dark-chamber light-chamber paradigm as previously reported (Recober et al., 2009). Mice were previously untested, male and female, and between 80 and 120 days old. Prior to testing, animals were group housed at 2 to 5 mice per cage. Behavioral testing was performed during mid-light phase, between 3 and 9 hours after lights on, in a quiet room with constant temperature (23 oC). After transit to the testing facility, mice were allowed to acclimate to the testing room, in their home cages, for at least 1 hour with background pre-test room lighting at 80 μWcm−2s−1, ~240 lux.
Mice were individually tested in a custom made arena (60 L x 60 W x 45 H cm) with two equally sized chambers connected at the mid point of the dividing wall by a small portal (7 x 7 cm). An open topped chamber was painted white and illuminated at 330 μWcm−2s−1, 1000 lux using of a Fiber-lite 180 fiber optic light source with EEG2823 dual fibers (Dolan-Jenner Industries, Boxborough, MA). The dark chamber was painted black and covered, with seepage of light through the portal giving an illumination of 2.5 μWcm−2s−1, ~7 lux. Use of a fiber-optic avoided differences in temperature of the dark and illuminated chambers. Light measurements were taken in μWcm−2s−1 using a PM103 power meter, and in lux using a Light Meter (VWR International LLC, West Chester, PA).
Each mouse was gently held on the investigator’s palm with minimal tail restrain and placed in the center of the illuminated chamber of the box, facing away from the portal to the dark chamber. A video tracking system (View Point Life Sciences, Montreal, Canada) was used to monitor behavior and to quantify the total time that mice spent in the illuminated versus the dark chamber during a 10-minute trial. The chambers were thoroughly cleaned between animals. The proportion of time spent in the illuminated chamber as a measure of light-aversion was recorded by automated video tracking. Number of full body transitions between chambers, latency to first entry to the dark-chamber, and latency to first re-entry to the dark-chamber were measured to determine whether difficulty finding the portal was a factor in the observed behavior, and whether there were differences in anxiety-related behavior (transitions only). Portal transition timing and number were carefully calculated by watching the video. Differences between genotypes were identified using one-way ANOVA, then where P < 0.05, genotypes were compared using Tukey’s multiple comparison test. Bonferroni corrections were applied to P.
Results
Positive and negative masking
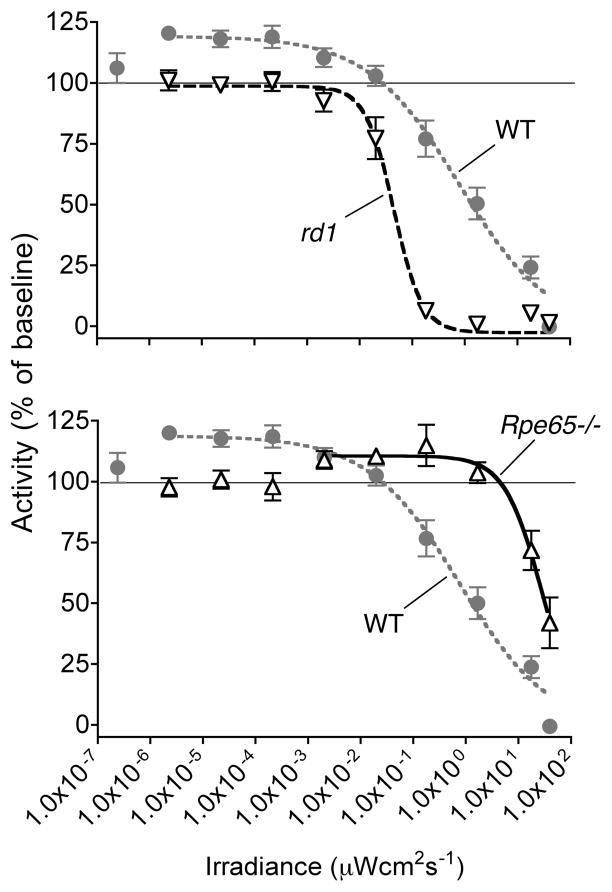
In wild-type, the effect of light on running wheel activity varied with irradiance (Figure 2). At the lowest irradiance, there was no effect. Above 1.0×10−6 μWcm−2s−1, positive masking, the effect of image-forming-vision on running wheel use, was apparent. Then above 0.1 μWcm−2s−1, a negative masking response was induced, with the degree of suppression of activity increasing with irradiance. For the negative masking effect of light, the irradiance producing a half-maximal response (EC50) in wild-type was 5.3 μWcm−2s−1.
Figure 2. Negative masking responses and visual guidance of locomotion.
The irradiance response relationship of positive and negative masking is shown for rd1 mice (top panel), and compared with Rpe65−/− mice (lower panel). Wild-type responses are shown in both panels. Mean and SEM activity at each irradiance is shown as a percentage of baseline, defined as activity in complete darkness (line at 100%). Positive masking (the effect of image-forming-vision on wheel running) is seen above 100%, and negative masking (suppression of activity by light) below 100%. Variable slope sigmoid dose response curves are fitted to data excluding points at lower irradiances where no response is induced.
The effect of retinal dysfunction was different for positive and negative masking, and between rd1 and Rpe65−/− mice. Rpe65−/− mice retained positive masking but only above 0.002 μWcm−2s−1, a much higher irradiance than that sufficient for image-forming-vision in wild-type mice. In contrast, positive masking was completely absent from rd1 mice, consistent with the absence of rod-cone image-forming-vision. For negative masking, irradiance sensitivity was affected in opposite directions in rd1 and Rpe65−/− mice. When compared to wild-type, the EC50 for the negative masking response was significantly increased in Rpe65−/− (EC50 73 μWcm−2s−1; P = 0.0075), but rd1 it was decreased (EC50 0.067 μWcm−2s−1; P < 0.0001)
Light-aversion
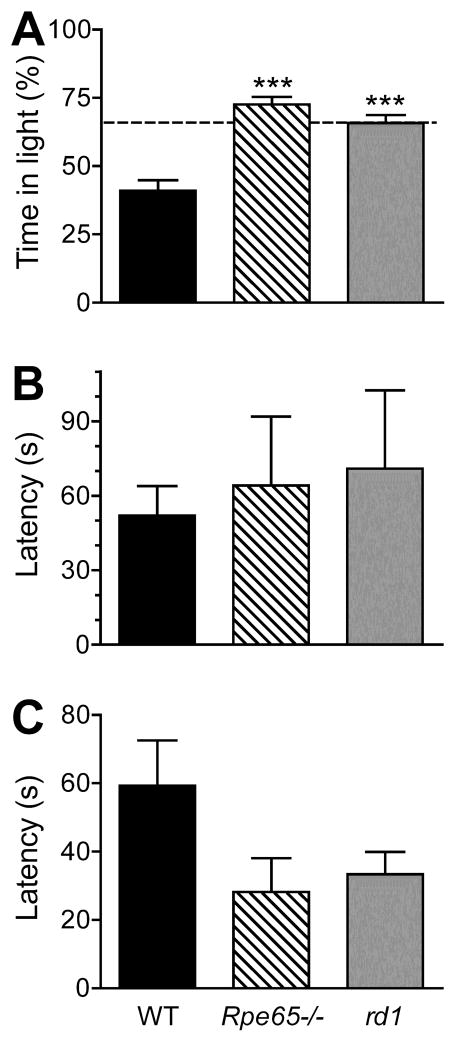
Wild-type mice spent less than half of the total test time in the ‘open’ brightly illuminated chamber (Figure 3). In comparison, both rd1 and Rpe65−/− spent significantly more time in the light-chamber (both P < 0.001 by Tukey’s multiple comparison test). However, there was no difference between genotypes in the latency to first transition into the dark chamber (Kruskal Wallis test P = 1.0), or in the number of transitions between chambers during the test (Mean and SD: wild-type 25.2 ± 4.8; rd1 18.9 ± 7.9; Rpe65−/− 18.9 ± 7.9; one-way ANOVA P = 0.124). Finally, there was a non-significant trend for increased latency to return to the light-chamber in wild-type (one-way ANOVA P = 0.40).
Figure 3. Light-aversion responses.
Mean ± SEM of responses under a two-choice chamber light-aversion assay are shown for wild-type, Rpe65−/− and rd1 mice. Lighting in the open chamber was 330 μWcm2s−1, and tests were over a 600 second trial. Statistical comparison was to wildtype, with *** indicating P < 0.0001, and no stars indicating P > 0.05. (A) Percent time spent in the brightly illuminated area. Dotted line at 65% shows the no-stimulus proportion of time spent in the open side in highly light-aversive mice. (B) Latency to first entry into the dark-chamber after test start. (C) Latency to first re-entry to the light-chamber.
Discussion
Light dependent behaviors were compared in mice with different types of photoreceptive dysfunction to further understand how light affects behavior. We find that: (1) image-forming-vision is supported in Rpe65−/− mice but having reduced sensitivity, only increases motility in brighter light; (2) negative masking has increased sensitivity in rd1 but decreased sensitivity in Rpe65−/− mice; (3) light-aversion is not supported by image-forming-vision alone, but originates with non-image-forming irradiance detection; and (4) non-image-forming irradiance detection input to light-aversion is distinct from that generating negative masking.
Positive masking
Consistent with previous studies, image-forming-vision (positive masking) was present in wild-type and absent in rd1 mice (Mrosovsky et al., 1999; Thompson, Philp et al., 2008). Positive masking was retained in Rpe65−/− mice as previously suggested, but by defining responses across a full irradiance range, positive masking was shown to be restricted to considerably higher light levels than in wild-type (Thompson, Mullins et al., 2008). This can be attributed to the retained rod photoreceptors having reduced sensitivity to light (Seeliger et al., 2001; Thompson, Mullins et al., 2008). This means that image-forming-vision only affects behavior in Rpe65−/− mice in relatively bright light.
Negative masking
Consistent with previous studies negative masking was hypersensitized in rd1 mice when compared to wild-type, resembling a threshold-dependent response rather than an incremental response with irradiance (Mrosovsky et al., 1999; Thompson, Philp et al., 2008). Negative masking was retained in Rpe65−/− mice, but with reduced sensitivity - the only retinal pathology with identified loss of sensitivity in negative masking. This suggests defects in the visual cycle impact melanopsin function, and consistent with this hypothesis, recent evidence has shown that 11-cis-retinal, absent in Rpe65−/− mice, is the optimal retinoid for melanopsin function (Doyle et al., 2006; Doyle et al., 2008; Thompson, Mullins et al., 2008; Tu et al., 2006; Walker, Brown, Cronin, & Robinson, 2008).
Light-aversion
As defined by time spent in the illuminated chamber, light-aversion was present in wild-type but absent in rd1 and Rpe65−/− mice. For rd1 and Rpe65−/− mice, the proportion of time in the light-chamber, approximated that seen in otherwise light-aversive mice with no difference in illumination between chambers (Bourin & Hascoet, 2003; Recober, Kaiser, Kuburas, & Russo, 2010). Mice are initially introduced to the open chamber, so a plausible explanation for this baseline bias is that, given the novelty of the test environment, initial introduction to the open chamber makes it more familiar and preferred during early exploration.
Importantly, our findings show that image-forming-vision does not account for differences in light-aversion. First, time to find the portal and numbers of transitions between chambers are not different between genotypes, so ocular function does not affect the ability to find the portal between chambers. The non-significant increased latency to return to the light-chamber in wild-type is most easily explained as a consequence of light-aversion, and is consistent with our previous studies (Recober et al., 2010). Second, Rpe65−/− mice show no light-aversion despite having image-forming-vision (positive masking) at the level of illumination used in this test. Third, although circumstantial, there is a disconnect between the sensitivity of light-aversion (only in bright light) and the dynamic range of useful image-forming-vision in mice – with positive masking at light levels more than five orders of magnitude dimmer than that generating light aversion in wild-type mice (Bourin & Hascoet, 2003). Therefore, the photic input motivating these behaviors almost certainly originates with non-image-forming irradiance detection.
This is consistent with the predicted requirement for a relatively absolute measure of irradiance given the likely ‘predation avoidance’ role of light-aversion behavior. These findings strongly suggest that light-aversion-based behavioral tests are inappropriate as a measure of image-forming-vision, and where bright light is used to induce anxiety, care should be taken in using strains with retinal degeneration (CBA, C3H, DBA) (Chang et al., 2002; Chang et al., 1999).
Different roles of non-image-forming irradiance detection
These findings also suggest that different non-image-forming irradiance detection pathways underlie light-aversion and negative masking. Light-aversion is absent in rd1 and Rpe65−/− at irradiances that would induce negative masking in both genotypes. Further, phenotype effects of retinal dysfunction in rd1 and Rpe65−/− are opposite for negative masking but the same for light-aversion. The different types of melGCs and diversity of their central projections can explain these differences (Hattar et al., 2006). The sub-paraventricular zone (sPVZ) of the hypothalamus receives input from type1 melGCs, and is thought to mediate the negative masking response. This implies that input from a different type of melGC and/or involvement of another area of the CNS, such as the posterior thalamus, generates light-aversion (Noseda et al., 2010).
As negative masking and light-aversion appear to have distinct behavioral drives, further investigation of differences in the photic input to these behaviors is suggested. Study of mice with ablated melGCs could positively identify melGCs as part of the input pathway. Further, mice that selectively lack melanopsin function could show whether normal function of rods and cones is sufficient to induce light-aversion in the absence of melanopsin activity. As in this study, such comparison can show that input to behaviors is different in the model of study. However, retinal remodeling is a common effect of disease, and it must be understood that such findings do not absolutely demonstrate input under normal conditions (Haverkamp et al., 2006). One approach would be more detailed examination of wild-type mice, which could include full dose-response curves for light-aversion that can then be compared to in vitro properties of the different types of melGC.
Sum of effects on behavior
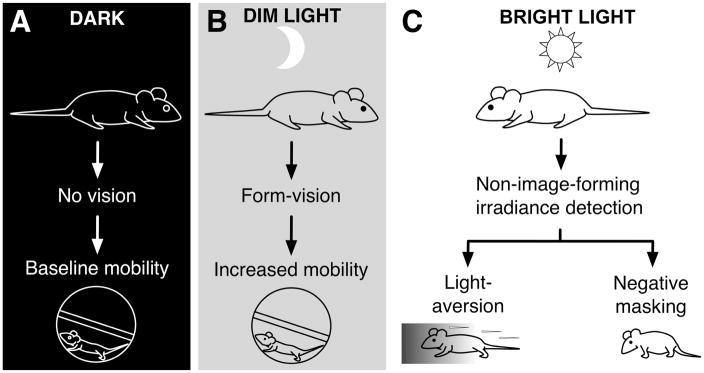
We therefore hypothesize that the interaction of image-forming-vision and two distinct non-image-forming irradiance detection mechanisms determine the acute effects of light on behavior in mice (Figure 4). In total darkness, guided by tactile and olfactory cues, mice still show baseline levels of exploratory or wheel running activity. In dim light conditions that do not induce any predation avoidance behaviors, light levels can still be sufficient to support image-forming-vision. This has a performance enhancing effect on motility, apparent as greater than baseline running wheel activity (positive masking). Finally, in bright light, two separate predation avoidance behaviors originate with non-image-forming irradiance detection: light-aversion, and suppression of non-essential movement (negative masking).
Figure 4. Coordination of light dependent predation avoidance behaviors.
Hypothesized basis for the effects of light on behavior in mice in (A) total darkness, (B) dim light, and (C) bright light.
Relevance to humans
Irradiance detection in people synchronizes the circadian clock, regulates behavioral states such as alertness, and physiological parameters such as the widely acting pineal hormone, melatonin (Foster, 2002). Abnormal irradiance detection can cause disorders of circadian rhythms, sleep, alertness, mood (Foster & Wulff, 2005; Lockley, Dijk, Kosti, Skene, & Arendt, 2008; Roecklein et al., 2008). More recent evidence even identifies abnormal irradiance detection as a risk factor in breast cancer and possibly type-2-diabetes (Flynn-Evans, Stevens, Tabandeh, Schernhammer, & Lockley, 2009; Stevens, 2009). However, being able ameliorate the effects of abnormal irradiance detection will depend on better understanding of how specific eye diseases and artificial light configurations affect irradiance responses. This study advances this goal by showing how two types of eye disease can affect a class of light-aversion responses that in people is most consistent with photophobia, an aversion to light in a number of conditions including migraine (Lockley et al., 2008; Recober et al., 2010; Recober et al., 2009; Thiels, Hoffman, & Gorin, 2008).
Acknowledgments
This study was funded by the Howard Hughes Medical Institute (EMS/VCS), the Foundation Fighting Blindness (EMS), and NIH R01 DE016511 (AFR).
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
References
- Bourin M, Hascoet M. The mouse light/dark box test. Eur J Pharmacol. 2003;463(1–3):55–65. doi: 10.1016/s0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- Carter-Dawson LD, LaVail MM, Sidman RL. Differential effect of the rd mutation on rods and cones in the mouse retina. Invest Ophthalmol Vis Sci. 1978;17(6):489–498. [PubMed] [Google Scholar]
- Chang B, Hawes NL, Hurd RE, Davisson MT, Nusinowitz S, Heckenlively JR. Retinal degeneration mutants in the mouse. Vision Res. 2002;42(4):517–525. doi: 10.1016/s0042-6989(01)00146-8. [DOI] [PubMed] [Google Scholar]
- Chang B, Smith RS, Hawes NL, Anderson MG, Zabaleta A, Savinova O, Roderick TH, Heckenlively JR, Davisson MT, John SW. Interacting loci cause severe iris atrophy and glaucoma in DBA/2J mice. Nat Genet. 1999;21(4):405–409. doi: 10.1038/7741. [DOI] [PubMed] [Google Scholar]
- Doyle SE, Castrucci AM, McCall M, Provencio I, Menaker M. Nonvisual light responses in the Rpe65 knockout mouse: rod loss restores sensitivity to the melanopsin system. Proc Natl Acad Sci U S A. 2006;103(27):10432–10437. doi: 10.1073/pnas.0600934103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle SE, Yoshikawa T, Hillson H, Menaker M. Retinal pathways influence temporal niche. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0801728105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn-Evans EE, Stevens RG, Tabandeh H, Schernhammer ES, Lockley SW. Total visual blindness is protective against breast cancer. Cancer Causes Control. 2009;20(9):1753–1756. doi: 10.1007/s10552-009-9405-0. [DOI] [PubMed] [Google Scholar]
- Foster RG. Keeping an eye on the time: the Cogan Lecture. Invest Ophthalmol Vis Sci. 2002;43(5):1286–1298. [PubMed] [Google Scholar]
- Foster RG, Provencio I, Hudson D, Fiske S, De Grip W, Menaker M. Circadian photoreception in the retinally degenerate mouse (rd/rd) J Comp Physiol [A] 1991;169(1):39–50. doi: 10.1007/BF00198171. [DOI] [PubMed] [Google Scholar]
- Foster RG, Wulff K. The rhythm of rest and excess. Nat Rev Neurosci. 2005;6(5):407–414. doi: 10.1038/nrn1670. [DOI] [PubMed] [Google Scholar]
- Guler AD, Ecker JL, Lall GS, Haq S, Altimus CM, Liao HW, Barnard AR, Cahill H, Badea TC, Zhao H, Hankins MW, Berson DM, Lucas RJ, Yau KW, Hattar S. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453(7191):102–105. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497(3):326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, Yau KW. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424(6944):75–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp S, Michalakis S, Claes E, Seeliger MW, Humphries P, Biel M, Feigenspan A. Synaptic plasticity in CNGA3(−/−) mice: cone bipolar cells react on the missing cone input and form ectopic synapses with rods. J Neurosci. 2006;26(19):5248–5255. doi: 10.1523/JNEUROSCI.4483-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprea MR, Cardenas FP, Setem J, Morato S. Thigmotactic responses in an open-field. Braz J Med Biol Res. 2008;41(2):135–140. doi: 10.1590/s0100-879x2008000200010. [DOI] [PubMed] [Google Scholar]
- Lockley SW, Dijk DJ, Kosti O, Skene DJ, Arendt J. Alertness, mood and performance rhythm disturbances associated with circadian sleep disorders in the blind. J Sleep Res. 2008;17(2):207–216. doi: 10.1111/j.1365-2869.2008.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrosovsky N. Masking: history, definitions, and measurement. Chronobiol Int. 1999;16(4):415–429. doi: 10.3109/07420529908998717. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N, Foster RG, Salmon PA. Thresholds for masking responses to light in three strains of retinally degenerate mice. J Comp Physiol [A] 1999;184(4):423–428. doi: 10.1007/s003590050341. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N, Hattar S. Impaired masking responses to light in melanopsin-knockout mice. Chronobiol Int. 2003;20(6):989–999. doi: 10.1081/cbi-120026043. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N, Thompson S. Negative and positive masking responses to light in retinal degenerate slow (rds/rds) mice during aging. Vision Res. 2008;48(10):1270–1273. doi: 10.1016/j.visres.2008.02.016. [DOI] [PubMed] [Google Scholar]
- Noseda R, Kainz V, Jakubowski M, Gooley JJ, Saper CB, Digre K, Burstein R. A neural mechanism for exacerbation of headache by light. Nat Neurosci. 2010;13(2):239–245. doi: 10.1038/nn.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Chang B, Hawes NL, Hurd RE, Davisson MT, Li J, Noorwez SM, Malhotra R, McDowell JH, Kaushal S, Hauswirth WW, Nusinowitz S, Thompson DA, Heckenlively JR. Retinal degeneration 12 (rd12): a new, spontaneously arising mouse model for human Leber congenital amaurosis (LCA) Mol Vis. 2005;11:152–162. [PubMed] [Google Scholar]
- Recober A, Kaiser EA, Kuburas A, Russo AF. Induction of multiple photophobic behaviors in a transgenic mouse sensitized to CGRP. Neuropharmacology. 2010;58(1):156–165. doi: 10.1016/j.neuropharm.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recober A, Kuburas A, Zhang Z, Wemmie JA, Anderson MG, Russo AF. Role of calcitonin gene-related peptide in light-aversive behavior: implications for migraine. J Neurosci. 2009;29(27):8798–8804. doi: 10.1523/JNEUROSCI.1727-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roecklein KA, Rohan KJ, Duncan WC, Rollag MD, Rosenthal NE, Lipsky RH, Provencio I. A missense variant (P10L) of the melanopsin (OPN4) gene in seasonal affective disorder. J Affect Disord. 2008 doi: 10.1016/j.jad.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeliger MW, Grimm C, Stahlberg F, Friedburg C, Jaissle G, Zrenner E, Guo H, Reme CE, Humphries P, Hofmann F, Biel M, Fariss RN, Redmond TM, Wenzel A. New views on RPE65 deficiency: the rod system is the source of vision in a mouse model of Leber congenital amaurosis. Nat Genet. 2001;29(1):70–74. doi: 10.1038/ng712. [DOI] [PubMed] [Google Scholar]
- Simon P, Dupuis R, Costentin J. Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav Brain Res. 1994;61(1):59–64. doi: 10.1016/0166-4328(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Stevens RG. Electric light causes cancer? Surely you're joking, Mr. Stevens. Mutat Res. 2009;682(1):1–6. doi: 10.1016/j.mrrev.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Stryer L. Vision: from photon to perception. Proc Natl Acad Sci U S A. 1996;93(2):557–559. doi: 10.1073/pnas.93.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiels E, Hoffman EK, Gorin MB. A reliable behavioral assay for the assessment of sustained photophobia in mice. Curr Eye Res. 2008;33(5):483–491. doi: 10.1080/02713680802130347. [DOI] [PubMed] [Google Scholar]
- Thompson S, Mullins RF, Philp AR, Stone EM, Mrosovsky N. Divergent phenotypes of vision and accessory visual function in mice with visual cycle dysfunction (Rpe65 rd12) or retinal degeneration (rd/rd) Invest Ophthalmol Vis Sci. 2008;49(6):2737–2742. doi: 10.1167/iovs.07-1546. [DOI] [PubMed] [Google Scholar]
- Thompson S, Philp AR, Stone EM. Visual function testing: a quantifiable visually guided behavior in mice. Vision Res. 2008;48(3):346–352. doi: 10.1016/j.visres.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Tu DC, Owens LA, Anderson L, Golczak M, Doyle SE, McCall M, Menaker M, Palczewski K, Van Gelder RN. Inner retinal photoreception independent of the visual retinoid cycle. Proc Natl Acad Sci U S A. 2006;103(27):10426–10431. doi: 10.1073/pnas.0600917103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MT, Brown RL, Cronin TW, Robinson PR. Photochemistry of retinal chromophore in mouse melanopsin. Proc Natl Acad Sci U S A. 2008;105(26):8861–8865. doi: 10.1073/pnas.0711397105. [DOI] [PMC free article] [PubMed] [Google Scholar]