Abstract
The amygdala has been implicated in affective and social processing for more than a century. Animals with damage to the amygdala have altered affective and social behavior patterns, though the precise nature of these behavioral changes depends on a number of factors including lesion technique, age of the subject at the time of lesion, rearing, and housing environments. Interpretations of amygdala lesion studies are further complicated by the potentially confounded nature of affective and social stimuli (e.g., social interactions with a conspecific partner that is consistently aggressive). In the present study, we evaluated affective responding to socially and emotionally evocative video stimuli in a group of rhesus macaques that received bilateral amygdala lesions as neonates. The stimuli were produced in order to vary independently in terms of their affective and social content. The responses of the amygdala-lesioned animals were compared to a group of age-matched controls and a group of animals that had sustained bilateral hippocampus damage as neonates. As compared to control animals, amygdala-lesioned animals had blunted responding to both positive and negative stimuli, regardless of social content, but did differentiate between categories of social content. Taken together, these findings suggest that early amygdala damage permanently compromises affective processing while leaving intact the ability to distinguish between socially meaningful contexts.
Keywords: amygdala, hippocampus, affect, emotion, nonhuman primate, Macaca mulatta, rhesus
It has long been known that regions in the temporal lobe, and in particular the amygdala, are important for normal affective and social processing (Brown & Schäfer, 1888; Rosvold, Mirsky & Pribram, 1954). A large human functional neuroimaging literature has demonstrated that the amygdala is active while subjects perceive affective and social stimuli such as facial depictions of emotion (e.g., Whalen et al., 1998; for a meta-analytic review see Fusar-Poli et al, 2009) and while subjects engage in social interactions (e.g., Eisenberger, Gable, & Lieberman, 2007; Rilling et al., 2007). Much of what is known about the critical functions of the amygdala (those for which the amygdala is necessary) come from studies of animals, and in particular nonhuman primates, that have sustained experimental amygdala damage. These studies have demonstrated that adult macaques with amygdala damage respond inappropriately to discrete affective stimuli (e.g., snakes, spiders, novel objects; e.g., Aggleton & Passingham, 1981; Chudasama, Izquierdo & Murray, 2009; Izquierdo, Suda & Murray, 2005; Machado, Kazama & Bachevalier, 2009; Mason, Capitanio, Machado, Mendoza & Amaral, 2006; Meunier, Bachevalier, Murray, Málková & Mishkin, 1999; Stefanaci, Clark & Zola, 2003; Zola-Morgan, Squire, Alverez-Royo & Clower, 1991) and during social interactions (e.g., Rosvold et al., 1954; Emery et al., 2001; Machado et al., 2008). It is difficult to determine, however, if the amygdala-lesioned animals have deficits in processing the affective content, the social stimuli or a combination of the two because the discrete affective stimuli used to test amygdala function are often social (e.g., pictures of faces depicting “fear”) and social interactions are rarely neutral (e.g., social rejection is negative). To disentangle these stimulus properties, the present experiment used video stimuli in which the affective and social content could be easily manipulated. Such stimuli are more “life-like” than still objects commonly used in previous studies, potentially providing for a more robust and diverse behavioral repertoire to be generated in the experimental subjects. Not only are videos are more life-like than objects, but their content can be easily controlled and consistently presented to all subjects, unlike the behavior of partner animals who typically serve as “stimuli” in semi-naturalistic social interaction tasks. To illustrate the critical importance of disentangling the affective from social responding in the study of amygdala function, we presently review evidence implicating the nonhuman primate amygdala in affective and social processing.
The Nonhuman Primate Amygdala and Affective Processing
The amygdala has been historically studied in the context of understanding negative affective states (e.g., fear, anxiety, etc). For example, the amygdala is critical for learning the threatening nature of stimuli via associative learning (what is often called “fear” learning). Adult macaques with damage to the amygdala fail to learn the association between neutral stimuli and noxious stimuli presented concurrently (Antoniadis, Winslow, Davis, & Amaral, 2007; 2009), suggesting that they are incapable of learning what is threatening. Similarly, adult rhesus macaques with amygdala damage readily interact with potentially dangerous objects known to generate robust negative affective responses in neurologically intact animals (e.g., Aggleton & Passingham, 1981; Chudasama, Izquierdo & Murray, 2009; Izquierdo, Suda & Murray, 2005; Machado et al., 2009; Mason et al., 2006; Meunier et al., 1999; Stefanaci, Clark & Zola, 2003; Zola-Morgan et al., 1991). Neonatal damage restricted predominately to the amygdala (as opposed to complete medial temporal lobe damage) produces a nearly identical response to objects (Bliss-Moreau, Toscano, Bauman, Mason & Amaral, 2010, 2011; Prather et al., 2001) indicating that the brain does not compensate for the amygdala’s affective function despite the substantial neural development that occurs after damage was sustained.
Accumulating evidence suggests that the amygdala may process positive stimuli as well. For example, the amygdala is active not only during learning about negative value (as discussed above), but also when stimuli predict the presence of reward (Belova, Paton, Morrison & Salzman, 2007; Paton, Belova, Morrison & Salzman, 2006; Hirai, Hosokawa, Inoue, Miyachi & Mikami, 2009). Similarly, macaques with selective amygdala damage are unable to modify their behavior based on changing reward values when those changes occur as a result of the animals’ own altered physiological state (i.e., when a food item is no longer rewarding because the animal has eaten it to satiety; Izquierdo & Murray, 2007; Málková, Gaffan & Murray, 1997). The importance of the amygdala in both negative and positive affective processing may help to explain the altered patterns of social behavior observed after damage to the amygdala. In this view, disruption of the amygdala may be responsible for an inability to accurately assess the affective properties of social stimuli or affective properties of the context in which social interactions occur, thus leading to inappropriate or abnormal social behavior.
The Nonhuman Primate Amygdala and Social Behavior
In the earliest report of social behavior following amygdala lesions, Rosvold and colleagues (1954) reported that two of three adult amygdala-lesioned animals fell in the dominance hierarchy as indicated by a decreased competition for food dropped from a small feeder (that could only be accessed by one animal at a time). One possible explanation for this behavior is that the food reward no longer was valued by amygdala-lesioned animals and therefore not “worth” the competitive effort required to obtain the food. Lesioned animals were rated to be more aggressive when housed individually suggesting that they were still capable of enacting the aggressive behavior that might have been necessary to maintain their dominance at the food dispenser (Rosvold et al., 1954). Contemporary studies also document perturbations of social behavior following amygdala damage. Following neurotoxic lesions to the amygdala (which spare the fibers of passage through the amygdala) adult macaques were more socially engaged in dyadic (Emery et al., 2001) and tetradic (Machado et al., 2008) interactions, indicated by greater numbers of prosocial behaviors (physical contact and grooming) and more time spent in proximity to their interaction partners. One interpretation of these data is that lesioned animals readily engage new social interaction partners because, in the absence of the amygdala, they fail to appreciate the possibility of social threat. This interpretation is consistent with data that suggest that pharmacologically increasing the activity of the amygdala activity leads to a reduction of social interaction (Málková, et al., 2003).
Early damage to the amygdala also perturbs social behavior, although the pattern of effects appears to be related to early rearing contexts and lesion technique. Young, nursery-reared macaques with aspiration amygdala lesions initiate fewer social behaviors when paired with conspecifics at two and six months of age (Bachevalier, 1994). Research from our laboratory has also documented changes in social behavior resulting from early damage to the amygdala (Bauman, Lavenex, Mason, Capitanio & Amaral, 2004a, 2004b). Two week-old rhesus macaques received ibotenic acid lesions (sparing the fibers of passage) to the amygdala or the hippocampus, or sham operations, and then were reared with their mothers with access to larger social “play groups” five days per week. These amygdala-lesioned monkeys did not differ from controls in the amount of time spent socially interacting (e.g., contacting, playing, grooming) with age-matched conspecifics during the first year of life. At this early timepoint, they were, however, more affectively expressive than controls and hippocampus-lesioned animals. Amygdala-lesioned animals generated more frequently behaviors related to “fear” (e.g., grimacing, screaming, crooktail, etc.) in familiar social contexts. They also generated more behaviors related to positive affect (or “prosocial behavior”) such as cooing, grunting, lipsmacking, etc. when interacting with novel age matched peers (Bauman et al., 2004a). In contrast to the heightened affectivity described above, the amygdala-lesioned monkeys did not produce distress calls when separated from their mothers (Bauman et al., 2004a) and produced fewer behaviors associated with aggression during interactions with novel partners (Bauman et al., 2004b) and during food competition (Bauman, Toscano, Mason, Lavenex & Amaral, 2006). Taken together, these studies illustrate the complexity of social and affective behavioral changes following neonatal amygdala damage.
The Present Experiment
In the present study, we investigated the impact of neonatal damage to the amygdala on adult macaques global (both positive and negative) affective responding to salient video stimuli. Given the impact of amygdala lesions on affective processing and normal social behavior, we were interested in exploring affective responding to stimuli that varied both in terms of their affective content and their social content. To that end, monkeys viewed 30-second video clips that were positive, negative or neutral in affective content. We varied social content in three categories—videos in which no other monkeys were present (“nonsocial”), videos in which other monkeys could be seen alone or interacting with other monkeys (“social non-engaging”), and videos in which monkeys were directing their activity towards the camera and thus appeared to be engaging the research subject (“social engaging”). This design allowed us to test whether behavioral deficits observed as a result of early amygdala damage were related to processing difficulties of affective content, social content, or both. To our knowledge, these properties have never been disentangled in the study of the amygdala’s role in nonhuman primates. We hypothesized that amygdala-lesioned animals would show blunted affective responding to evocative videos (i.e., positive or negative videos). It is important to note that videos of this sort readily capture adult macaques’ attention (Machado, Bliss-Moreau, Platt, & Amaral, 2011; Capitanio, 2002) and have previously been paired with a food reward, in an experimental design similar to the present experiment, in order to index their value (Rudebeck et al., 2006). It was our hope that the use of dynamic attention capturing videos, rather than static stimuli, would create a context in which animals would engage their robust and diverse behavioral repertories.
In addition to testing our cohort of adult animals that received bilateral neurotoxic lesions to the amygdala at 2-weeks of age and their neurologically intact control counterparts, we also tested age and rearing history matched animals that received bilateral hippocampus-lesions at 2-weeks of age. In addition to serving as an operated control group, these animals have allowed us to document the minimal impact of early hippocampus damage in affective responding across their development (e.g., Bliss-Moreau et al., 2010; 2011).
Methods
All experimental procedures were developed in consultation with the veterinary staff at the California National Primate Research Center (CNPRC). All protocols were approved by the University of California Davis Institutional Animal Care and Use Committee.
Animals and Living Conditions
Subject selection and rearing history has been fully described in previous publications (Bauman, 2004a, 2004b; Bliss-Moreau et al., 2010; 2011). Subjects for the present experiment were 23 adult rhesus macaque monkeys that received bilateral ibotenic acid lesions to either the amygdala (four females, three males) or hippocampus (five females, three males), or sham control operations (four females, four males). A male amygdala-lesioned animal died at approximately 1 year of age due to congenital heart defect and was subsequently replaced with an alternative amygdala-lesioned male who underwent surgery at the same time as the original cohort and was raised with his mother only for his first year of life. He became a subject in the present study at 1 year and 3 months of age. A female amygdala-lesioned animal died at approximately 3 years of age; she was not replaced as a subject. The cause of both animals’ deaths was unrelated to their lesion condition.
Surgical Procedures
Lesion surgeries were performed at 12-16 days after birth. The surgical procedures summarized below are described in greater detail in previous publications (Bauman et al., 2004a, 2004b). On the day of surgery, each animal was anesthetized with ketamine hydrochloride (15 mg/kg i.m.) and medatomidine (30μg/kg), and then placed in an MRI-compatible stereotaxic apparatus (Crist Instruments Co., Inc., Damascus, MD). The animal’s brain was imaged using a General Electric 1.5 T Gyroscan MRI, T1-weighted Inversion Recovery Pulse sequence with a slice thickness of 1 mm ( (TR) = 21, (TE) =7.9, NEX 3, FOV = 8cm, Matrix, 256 × 256). Ibotenic acid injection coordinates for the amygdala or hippocampus were calculated from these images. A stable level of anesthesia was maintained using a combination of isoflurane (1.0% - varied as needed to maintain an adequate level of anesthesia) and intravenous infusion of fentanyl (7-10 μg/kg/hour). A midline incision was made on the scalp, the skin was laterally displaced to expose the skull, two craniotomies were made over the amygdala or the hippocampus, depending on the pre-determined lesion condition, and the dura was reflected to expose the surface of the brain. Ibotenic acid (IBO, Biosearch Technologies Inc., Novato, CA, 10 mg/ml in 0.1 M phosphate buffered saline) was injected simultaneously bilaterally into the amygdala or hippocampus using 10 μl Hamilton syringes (26 gauge beveled needles) at a rate of 0.2 μl/min. Sham-operated controls underwent the same pre-surgical preparations and received a midline incision in order to expose the skull. The control animals were maintained under anesthesia for the average duration of the lesion surgeries and the fascia and skin were sutured in two separate layers. Following the surgical procedure, all infants were monitored by a veterinarian and returned to their mothers once they were fully alert.
Lesion Analysis
The animals are continuing behavioral testing and therefore have not been euthanized. T2-weighted MR images acquired ten days after surgery were used to examine the extent of the edema associated with the lesion. Images were collected using a General Electric 1.5 T Gyroscan magnet; 1.5 mm thick sections were taken using a T2 weighted Inversion Recovery Pulse sequence (TR = 4000, TE = 102, NEX 3, FOV = 8 cm, Matrix, 256 × 256). The hyper-intense T2-weighted signal for each of the sixteen lesion animals (eight amygdala lesion, eight hippocampus lesion) was evaluated to confirm the general target and extent of the lesions (i.e., amygdala lesion sparing the hippocampus or hippocampus lesion sparing the amygdala). T2-weighted images of coronal sections through the mid portion of the amygdala are illustrated in previous publications (Bauman et al., 2004a, 2004b), providing substantial reassurance that the ibotenic acid was injected and was focused in the amygdaloid complex or hippocampal formation. Additionally, lesion extent was further characterized in T1-weight MRI images when animals were four years of age (Machado, Snyder, Cherry, et al., 2008). On average, amygdala tissue was reduced in volume by 72.00% (SD=4.34%) in animals with amygdala lesions while of hippocampal volume was reduced by 76.65% (SD=7.46%) in animals with hippocampus lesions. The extent of the targeted lesion was confirmed using histological evaluation in the one amygdala-lesioned animal that died due to an unrelated illness (see Bauman et al., 2004a, 2004b; Bliss-Moreau et al., 2010, 2011).
Animal Housing
All animals were returned to their mothers following surgery and housed in standard home cages (61 cm W × 66 cm D × 81 cm H). While housed indoors, animals were maintained on a 12-hour light/12-hour dark schedule. Throughout the course of infancy, all subjects and their mothers participated in socialization groups for three hours, five times per week. Each playgroup included six subjects (two from each lesion condition) and an adult male. An adult female was added to the group when the subjects were weaned and separated from their mothers at six months of age. Beginning at one year of age, all animals were housed 24 hours per day in their socialization groups in large indoor chain-link enclosures (2.1m W × 3.3m D × 2.4m H). At 3.5 years of age, female subjects were moved into large outdoor enclosures (4.9 m W × 4.3 m D × 2.4 m H) in groups. Each social group consisted of one female from each lesion group and one adult male with which the females had no prior experience. Males were relocated into smaller (2.5 m W × 4.8 m D × 2.1 m H) outdoor enclosures and maintained in same-sex pairs. At approximately 6.5 years of age, animals were moved indoors and maintained in male-female pairs. At the time of testing, all but 2 animals (1 control male and 1 hippocampus-lesioned male) were housed in stable male-female pairs. Each pair was allowed complete access to each for a minimum of 8 hours per day, 5 days a week. Testing in the current study occurred when animals were on average 7.73 years old (SD=.11 year).
Behavioral Testing
Experimental Overview
To evaluate the impact of early amygdala damage on responsiveness to affective and social stimuli, all animals completed a Video Responsiveness Task during which they viewed a series of videos that varied in terms of both affective and social content. The structure of each trial in the Video Responsiveness Task was similar to that of tasks these animals had previously completed (Bliss-Moreau et al., 2010; 2011) and to tasks typically used to assess macaques’ responsivity to salient stimuli (e.g., Aggleton & Passingham, 1981; Mason et al., 2006; Machado et al., 2009). Specifically, during each 30-second video presentation, animals were concurrently presented with a desired food reward placed near the video screen. The length of time required for each animal to retrieve each food reward is thought to be an index of how interesting/engaging/salient the concurrently presented stimulus is. In this view, food retrieval latencies are long when the stimuli are very salient, because animals either spend a long period of time inspecting the stimulus or avoid it (and the food reward) altogether. Given the importance of food retrieval, we first determined which food items animals preferred during Food Preference Testing, and then ensured that the animals would retrieve the food items in the testing cage in the presence of a control video during the Test Cage Acclimation. Specific details of these phases are detailed below.
Food Preference Testing
Prior to the start of video testing, animals’ preference for three different food rewards was assessed in their home-cages. Each animal completed five trials on each of four test days. On each trial, a grape, a banana chip and a mini-marshmallow were placed in individual wells of an ice-cube tray. The ice-cube tray was held in front of an opening on the cage through which the animals could easily reach the food item (presentation time= 30 seconds maximum). Placement of the food items (left to right) in the ice-cube tray was counterbalanced across trials and days. The order that animals retrieved food items was recorded. The food item that animals selected most frequently was used during the responsiveness testing phase. Data are available upon request.
Testing Apparatus
All behavioral testing occurred in a large, modified laboratory care cage in which the animals had previously undergone testing (Bliss-Moreau et al., 2010; 2011). The cage had a clear plastic front with two small openings through which the animal could reach. In front of the clear plastic front was an opaque guillotine door that could be raised and lowered between trials to occlude the animal’s view of the room. A small table sat directly in front of the clear window and held a 17” flat screen TV incased in a clear Plexiglas box. Food rewards were placed in a small depression in the table, directly in front of the TV. Two experimenters sat diagonally approximately 2 m in front of the cage.
Test Cage Acclimation
Animals were acclimated to the test cage prior to behavioral testing. During each acclimation day, animals were placed individually in the test cage. There were 10 30-second trials during which a “baseline” video (PC screen saver) was played on the TV and a desired food reward was presented on the platform. Each 30-second trial began when the opaque guillotine door was lifted and ended when the opaque door was closed. Animals were considered acclimated and allowed to move to the task phase when they retrieved the food item within 30 seconds during eight out of ten trials on two consecutive days.
Video Responsiveness Task
On each of 8 testing days, animals completed a series of 7 trials during which a video was shown on the TV and a food reward was placed in the food well (first trial was considered a “warm-up” and data from it were discarded). Each test day began with a trial during which the “baseline” video was shown (same PC screen saver that was used during test cage acclimation) and the same video was subsequently shown before each experimental video.
Video stimuli were created such that they varied on three levels of social content and three categories of affective content. “Nonsocial” videos included footage of nature scenes (e.g., leaves blowing in the wind, clouds) and included no images of other monkeys. “Social non-engaging” videos included footage of monkeys interacting with each other (e.g., two monkeys grooming), or alone (e.g., playing in gravel), but in no cases were the pictured monkeys directly looking at the camera or engaging the person recording the video. “Social engaging” videos included footage where a monkey was directly engaging the camera (e.g., threatening, lipsmacking). The content for positive videos included objects believed to be pleasant (i.e., the non-social videos; e.g., granola pouring into a bin), affiliative interactions (i.e., the social non-engaging videos; e.g., monkeys grooming and mounting), or a monkey directing an affiliative signal to the observer (i.e., the social engaging videos; e.g., lipsmacking). The content for negative videos included objects believed to be unpleasant (i.e., the non-social videos; e.g., monkey catcher net waving), aggressive/antagonistic interactions (i.e., the social non-engaging videos; e.g., monkeys fighting), or a monkey directing a clear aggressive/unpleasant signal to the observer (i.e., the social engaging videos; e.g., threatening). Finally, the content for neutral videos included objects believed to have no affective content—scenes from nature (i.e., the nonsocial videos), monkeys not engaged in interactions (i.e., the social non-engaging videos; e.g., monkeys playing in gravel), and finally monkeys looking directly at the camera but not making any sort of facial expressions (i.e., the social engaging videos; e.g., monkey staring at camera). Each category had at least two videos (see Table 1). A trained observer recorded a number of behaviors using an established ethogram or catalogue of behaviors (see Table 2) during each 30-second trial using The Observer (Noldus Information Technology; Leesburg, VA).
Table 1.
Video Library
| Social Content | Affective Content | Video Description |
|---|---|---|
| Nonsocial | Positive | Popcorn popping from popcorn maker into bin Granola pouring into pan |
| Negative | Capture net waving in air Room cleaning squeegee moving along floor |
|
| Neutral | Leaves blowing in the wind Dandelion blowing in the wind Feather in grass Cage gravel Sky with clouds |
|
| Social Non-Engaging | Positive | Male and female engaging in consortship behavior Young animals playing |
| Negative | Monkeys engaging in aggression Monkeys engaging in subordination |
|
| Neutral | Young animal orally exploring cage Female sleeping Young animal chewing on plastic Male orally exploring cage |
|
| Social Engaging | Positive | Male “lipsmacking” Female “presenting rump” with species typical sexual swelling |
| Negative | Male “threatening” Female “threatening” |
|
| Neutral | Male looking at camera Female looking at camera |
Table 2.
Behavioral Ethogram
| Behavior | Description |
|---|---|
| Affective Expressions | |
| Bark* | Low pitched, sharp, guttural sound. |
| Crooktail | Stiff-legged strut and tail held in stiff “?” shape |
| Grimace | Exaggerated grin with teeth. |
| Repetitive Grimace | Multiple instance of a grimace occurring within 3 seconds after the first instance. |
| Grunt | Deep, muffled, low-intensity vocalization. |
| Lipsmack | Rapid lip movements with pursed or puckered lips. |
| Repetitive Lipsmack | Multiple instance of a lipsmack occurring within 3 seconds after the first instance. |
| Present mount | Rigid posture with rump and tail elevated and oriented toward another (may or may not occur in the context of sex). |
| Scream* | High-pitched vocalization with extreme high intensity; usually sounds like “eeeeeee”. |
| Threat | Scored when one or more of these components are present: open mouth stare, head bobbing, ear flaps, bark vocalizations, or lunges. |
| Repetitive Threat | Multiple instance of a threat occurring within 3 seconds after the first instance. |
| Tooth Grind | Repetitive, audible rubbing of upper and lower teeth. |
| Exploratory Behavior | |
| Cage Pick* | Animal picks at or fiddles with cage. |
| Manual Exploration | Animal physically touches TV. |
| Take Food | Animal takes food reward. |
| Stereotypies | |
| Backflip | Animal flips backwards at least 2 times in a row. |
| Bounce * | Repetitive hopping or bouncing in the same place (must occur more than 3 times in a row to score). |
| Extended Pace | The animal makes at least another 2 complete revolutions around the experimental cage once the initial pace event is scored |
| Feces Smear* | Wiping feces on or around cage walls or floor. |
| Head-twist | Animal twists neck in a dramatic display; often seen when turning at corners and/or in conjunction with pacing. |
| Other Stereotypy | Repetitive motor pattern not described by any of the above definitions. |
| Pace | Repetitive undirected pacing with the same path repeated (must last longer than 3 seconds to score). |
| Rock* | Rhythmic movement back and forth. |
| Salute | Animal covers hand over eye or holds hand over eye. |
| Self-bite | Biting oneself. |
| Self-clasp* | Unusual holding of body part or limb with another body part. |
| Spin* | Repetitive twirling or spinning for at least 2 rotations |
| Swinging | Repetitive swinging in the same place (must last at least 3 seconds to score). |
| Miscellaneous | |
| Cage Shake | Vigorous shaking of cage bars, or animals slams body against cage. |
| Freeze | Scored when animal maintains rigidly fixed body position for at least 3 seconds. |
| Scratch | Animal scratches own body. |
| Self-groom | Grooming oneself. |
| Self-sex | Anogenital exploration of self. |
| Self-shake | Vigorous shaking of own body. |
| Yawn | Open mouth, exposing teeth. |
|
| |
| Position in Cage and Looking Direction | |
| Back, Facing Away from TV |
The animal’s head is positioned in the rear of the cage with its face directed away from the front of the cage for at least 3 seconds. |
| Back, Facing Towards TV | The animal’s head is positioned in the rear of the cage with its face directed towards the front of the cage for at least 3 seconds. |
| Front, Facing Away from TV |
The animal’s head is positioned in the front of the cage with its face directed away from the front of the cage for at least 3 seconds. |
| Front, Facing Towards TV | The animal’s head is positioned in the front of the cage with its face directed towards the front of the cage for at least 3 seconds. |
| Nonspecific Activity | Animal does not remain in the front or rear of the cage. Default state in which each sample is started. |
Note: = Behavior was not scored for any monkey during entire study.
Data processing
Behavior frequency was summed across each behavioral class (Table 2) for each individual video. Means were then computed for each video category. Duration and latency data were also averaged across each video category. Data were log transformed in cases where they were not normally distributed (as indicated below); in these cases, raw data are presented in tables for ease of interpretation (transformed data are available upon request). Data were subjected to analysis of variance (ANOVA) with lesion condition (i.e., 3 groups: amygdala, hippocampus or control) as the between subjects factor and social (i.e., 3 levels: nonsocial, social non-engaging, social engaging) and affective content (i.e., 3 levels: positive, negative, neutral) as the two repeated measures. Mauchly’s test of sphericity was used to assess whether the data violated the assumption of sphericity. In those cases, degrees of freedom were Greenhouse-Geisser corrected. Following omnibus tests, the overall effect of lesion group on each trial type (e.g., positive nonsocial videos, negative social engaging videos, etc.) was computed using univariate ANOVAs with lesion group as the between subjects factor. When the p-value associated with the omnibus F-test was significant (p<.05) or trend (p<.08), p-values from significant LSD post-hoc comparisons are presented in the figures (and were verified using t-tests). We elected to report post-hoc comparisons following marginally significant omnibus tests because the effect sizes were moderate. Marginal means from those trial specific ANOVAs were assessed, the data were visually inspected and in cases where the marginal means suggested a difference between two groups (e.g., amygdala v. control), t-tests were preformed comparing just those groups. Results from those t-tests are reported in the figure captions. In cases where the effect of lesion was assessed across multiple trial types, specific lesion group differences were computed using either paired or independent samples t-tests. Levene’s test for equality of variances was used to evaluate the variance structure for t-tests; corrected degrees of freedom are reported in cases where there were unequal group variances. For the sake of brevity, only significant behavioral results are discussed. Additional analyses are available upon request.
Results
Test cage acclimation
On average, animals passed acclimation after 5.73 days (SD=2.00). One control male was removed from the sample because he did not pass acclimation after 17 days. Once this male was removed from the sample, experimental groups did not differ in the number of acclimation days required to reach criterion, F(2, 21)= 1.41, p<.267, np2=.130 (amygdala-lesioned animals: M=5.0, SD=0; hippocampus-lesioned animals: M=5.5, SD=1.4; control animals: M=6.7, SD=3.1).
Video Responsiveness Task
Retrieval of Food Reward
All animals retrieved food on all baseline trials. There were no effects of social or affective video content, or lesion condition on the frequency to retrieve the food item during all non-baseline video trials (log transformed). Animals retrieved the food item on nearly every trial.1
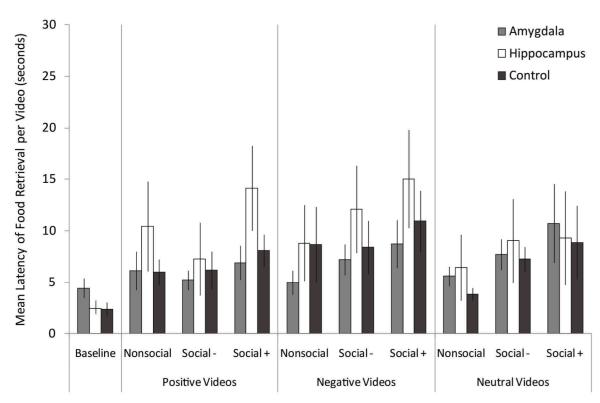
There was no difference in animals’ speed of food retrieval on baseline trials, F(2, 19)= 1.85, p<.185, np2=.163. Lesion groups did not differ in terms of how quickly they retrieved foods during the non-baseline videos, F(2, 19)= .01, p<.992, np2=.001. Food retrieval did differ significantly based on social content, F(2, 4)= 8.92, p<.001, np2=.319, but not affective content, F(1.52, 28.97)= 1.38, p<.263, np2=.068 such that all animals retrieved food fastest on trials with non-social content and slowest on trials with videos with social engaging content regardless of affective content (log transformed). Finally, there were no significant interactions: affective content X lesion, F(4, 38)= 1.12, p<.360, np2=.106; social content X lesion, F(2, 38)= .17, p<.954, np2=.017; affective content X social content, F(4, 76)= 1.81, p<.136, np2=.087; affective content X social content X lesion F(8, 76)= .49, p<.856, np2=.050. See Figure 1.
Figure 1.
Food Retrieval Latency
Data columns depict the average time to retrieve food per video type; error bars represent standard errors of the means. Note that the figure uses raw data for the ease of interpretation, although log transformed data were analyzed. Amygdala: amygdala-lesioned animals; Hippocampus: hippocampus-lesioned animals; Control: control animals. Baseline: baseline trials with screen saver video. Affective Content Categories—Positive: videos with positive affective content; Negative: videos with negative affective content. Neutral: videos with neutral affective content. Social Content Categories—Nonsocial: Nonsocial videos. Social−: Social non-engaging videos. Social+: Social engaging videos.
There were no significant, or marginally significant, lesion group differences in the latency to retrieve food within each specific stimulus type positive nonsocial videos, negative social engaging videos, etc.).
Frequency of “Expressions”
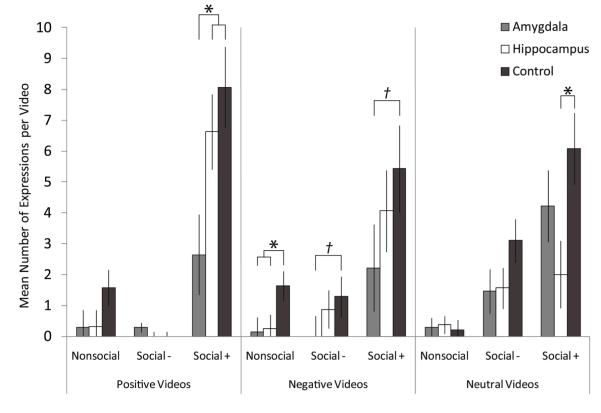
The “expressions” category includes all indexed behaviors which are thought to signal general affective or specific emotional states (e.g., grimacing, lipsmacking, grunting) as well as communicative social behaviors (e.g., presenting “rump”). Across all videos, amygdala-lesioned animals were the least expressive and control animals were the most expressive, F(2, 19)= 3.68, p<.044, np2=.279. See Figure 2. Amygdala-lesioned animals produced significantly fewer expressions than control animals, t(12)=3.01, p<.011, but did not differ significantly from hippocampus-lesioned animals, t(13)=.54, p<.597. Controls tended to be more expressive than hippocampus-lesioned animals, t(13)=1.79, p<.096.
Figure 2.
Expressions Directed Towards Video
Data columns depict the mean number of expressions per video type; error bars represent standard errors of the estimated marginal means. Amygdala: amygdala-lesioned animals; Hippocampus: hippocampus-lesioned animals; Control: control animals. Baseline: baseline trials with screen saver video. Affective Content Categories—Positive: videos with positive affective content; Negative: videos with negative affective content. Neutral: videos with neutral affective content. Social Content Categories—Nonsocial: Nonsocial videos. Social−: Social non-engaging videos. Social+: Social engaging videos. * indicates significant between group differences based on post-hoc LSD comparisons or t-tests. † indicates marginally significant (trend) between group differences based on post-hoc LSD comparisons or t-tests.
Amygdala-lesioned animals were significantly less responsive to positive social engaging videos than hippocampus-lesioned and control animals, F(2, 22)= 4.68, p<.023, np2=.330 (amygdala-lesioned < hippocampus-lesioned, control, p<.05). Amygdala- and hippocampus-lesioned were marginally less responsive than controls to negative nonsocial videos, F(2, 22)= 3.01, p<.07, np2=.245 (amygdala-lesioned, hippocampus-lesioned < control, p<.05). Post-hoc tests following a marginally significant effect of lesion condition on responsivity neutral social engaging videos F(2, 22)= 3.279, p<.06, np2=.257, revealed that hippocampus-lesioned animals were significantly less responsive than controls (p<.05). Amygdala-lesions animals were marginally less responsive than controls to negative social nonengaging videos, t(6)=2.12, p<.08, and negative social engaging videos t(12)=2.12, p<.06.
Overall, animals were most expressive towards the social-engaging videos compared to social non-engaging and non-social videos, F(1.16, 22.02)= 40.82, p<.0001, np2=.682. The effect of social content (main effect) did not differ across lesion conditions, F(2.32, 22.02)= 1.52, p<.239, np2=.138. On average, animals were equally expressive in the presence of positive, negative and neutral videos, F(1.61, 30.65)= 1.23, p<.299, np2=.061. Lesion condition did not impact the influence of affective content on expressiveness, F(3.23, 30.65)= 2.02, p<.117, np2=.138.
Social and affective content together influenced expressivity, F(2.74, 52.10)= 7.90, p<.0001, np2=.294. When content was positive or negative, animals were most responsive to the social engaging videos and equally responsive to the nonsocial and social non-engaging videos. In contrast, when the video content was neutral, animals were differentially expressive across social content categories—specifically, animals were most responsive to the social engaging videos and least responses to non-social videos with an intermediate level of responsivity to social non-engaging videos. The interaction between social and affective content varied for the different lesion groups, F(5.48, 52.10)= 3.23, p<.011, np2=.254. Compared to control animals, amygdala-lesioned animals were less expressive during both positive and negative social-engaging videos. Control and amygdala-lesioned animals displayed comparable levels of expressivity when the social-engaging video content was neutral; hippocampus-lesioned animals were less expressive than both control and amygdala-lesioned animals during neutral social-engaging videos.
Given that all animals were most responsive to the social-engaging videos, we evaluated responsivity to those videos only by lesion condition. Amygdala-lesioned animals were equally expressive when content was positive, negative or neutral, F(2, 12)= 2.31, p<.142, np2=.278. In contrast, hippocampus-lesioned and control animals were not consistently responsive across affective categories. Control animals were most responsive to videos with positive content and least responsive to videos with negative content, although the omnibus test did not reach conventional levels of significance, F(1.13, 6.81)= 4.25, p<.077, np2=.414. Responsivity to positive videos was significantly greater than to neutral videos, t(6)=2.863, p<.029, and tended to be greater than to negative videos, t(6)=2.042, p<.087. Responsivity to neutral and negative videos did not differ, t(6)=.891, p<.407. Hippocampus-lesioned animals were most responsive to videos with positive content and least responsive to videos with neutral content (with negative content as the intermediate), F(2, 14)= 4.891, p<.025, np2=.411. They were significantly more responsive to videos with positive content as compared to videos with neutral content, t(7)=2.871, p<.024.
Discussion
Neurologically intact control animals responded robustly to videos with affective and social content by generating many communicative signals while watching the video clips. As such, they appear to be engaging the video stimuli on the television as if they are “real” stimuli. Neonatal damage to the amygdala resulted in remarkably reduced affective responding to all stimuli. Compared to control monkeys, monkeys that received bilateral neurotoxic lesions of the amygdala at two weeks of age generated significantly fewer communicative signals (i.e., “affective expressions”) while watching videos with social and nonsocial affective content. Despite the overall reduction in amygdala lesioned animals’ affective responding, amygdala lesioned animals did respond most robustly to social engaging stimuli and least robustly to nonsocial stimuli. In other words, amygdala lesioned animals resembled control monkeys in their ability to differentially respond to stimuli based on social content, but differed from control monkeys in their overall level of affective responding. Taken together, these findings suggest that early damage to the amygdala causes a global blunting of affective responding in adulthood, while leaving intact the ability to assess the intensity of social stimuli and to respond appropriately to them.
It is important to note that in the present study, we elected to equate “affective responding” with the generation of both affiliative and aggressive signals, as well as body postures that convey social meaning (e.g., presenting “rump”). We elected this analysis strategy to account for individual differences in affective signaling between monkeys and the expectation that we could not a priori determine what “appropriate” responding was for each category. For example, when faced with a large male monkey generating a threat facial signal, it might be appropriate to threaten the animal back if the perceiving animal is also a large, dominant, male monkey. In contrast, the advantageous response for a small, submissive female monkey might be to generate submissive expressions such as lipsmacking or present rump. This approach to the data seemed to be the most appropriate given that the experimental animals are heterogeneous in terms of size, sex and rank. In fact, there was a good deal of variation in terms of specific signals generated within a given video category. Unpacking specific deficits in positive or negative affective responding related to amygdala-damage is a potentially fruitful avenue for future research.
The effect of early amygdala damage on affective responding to discrete nonsocial stimuli has been remarkably consistent over time. When the animals in the present study were tested at approximately 9, 18 and 36 months of age in the presence of novel and threat-provoking objects, amygdala-lesioned animals did not generate species typical responses to the objects (e.g., avoidance) and rather interacted with them readily (Bliss-Moreau, et al., 2010, 2011). One interpretation of these findings is that interaction with the objects was made possible by a lack of species typical fear, anxiety or neophobia—in other words, a lack of, or blunted, negative affect. The present findings build upon that previous work by demonstrating that the amygdala-lesioned animals’ blunted affect is not restricted to the processing of negative, threatening or fear provoking stimuli, but instead encompasses processing of both positive and negative stimuli. An avenue for future analysis will be to map these animals’ deficits in responding to discrete affective stimuli to positive and negative social responsivity across their lives.
Amygdala-lesioned animals’ responses, like control animals’ responses, did vary based on social content. All animals were most responsive to the social engaging videos and least responsive to the nonsocial videos. This overall effect was influenced by affective content as well, such that for positive and negative affective content, animals were most responsive to positive and negative social engaging stimuli and equally lowly responsive to social non-engaging and nonsocial stimuli. In contrast, responsivity to neutral information was calibrated with sociality such that animals were most responsive to social engaging stimuli, least responsive to nonsocial stimuli with social non-engaging stimuli as an intermediate. This pattern held for the amygdala-lesioned and control animals, but not for the hippocampus-lesioned animals. These findings highlight two important points—first, that hippocampus-lesioned animals’ affective responses differed from control animals’ responses in some contexts and second, that stimuli with “neutral” content (on the basis of human assessment) were not necessarily perceived to be neutral by the rhesus monkey subjects.
As discussed above, the present findings also suggest that early hippocampus damage does influence affective processing, although not to the extent that early amygdala damage does. Hippocampus-lesioned animals’ number of expressions were typically intermediate between amygdala-lesioned and control animals (even if the differences were not significant), and the magnitude of their responses decreased monotonically across affective categories (from positive to negative to neutral). The one notable exception to that pattern is their response to neutral, social-engaging stimuli—hippocampus animals were significantly less responsive to those stimuli as compared to amygdala-lesioned and control animals. Over the course of these animals’ lives, we have observed inconsistent patterns of affective deficits related to hippocampus damage. For example, at 9 months of age, hippocampus-lesioned animals behaved just like control animals in the presence of novel and threat-related stimuli insofar as they did not touch the objects (Experiment 1; Bliss-Moreau, et al., 2010). Yet at 18 months of age, they physically explored threat-related objects that were similar to those with which they had experience (e.g., a toy snake) much like amygdala-lesioned animals (Experiment 2; Bliss-Moreau et al., 2010). Finally, at approximately 36 months of age, they once again behaved like controls in the presence of threat-related objects (Bliss-Moreau et al., 2011). The inconsistency within the hippocampus-lesioned group is mirrored in the adult lesion literature in which some groups have found affective responding deficits resulting from hippocampus damage (e.g., Chudasama, et al., 2009; Chudasama, Wright & Murray, 2007) while other groups do not (e.g., Zola-Morgan et al., 1991). Additional research is needed to further characterize the affective deficits of the current hippocampus-lesioned experimental group. Further, histological examination of these animals’ brains will allow us to characterize the neural reorganization that may underlie their affective deficits.
A second interesting finding from the present study is that control and amygdala-lesioned animals generated robust responses to “neutral” social engaging stimuli. The stimuli were selected because the human experimenters detected no inherent affective signal generated from the monkeys that were filmed (i.e., no facial expressions or communicative signals). One interpretation of these findings is that even though the stimuli appeared to be neutral (to the human experimenters), they actually did have subtle affective value. This is consistent with the idea that a stimulus’ affective meaning (or affective “value”) can be indexed by the response that it generates in the perceiver (Barrett & Bliss-Moreau, 2009). In this view, clear and directed social stimuli (e.g., conspecifics looking directly at the subject) are inherently valued even if the conspecifics in those stimuli are not generating an affective response themselves. Future research is needed to investigate what specific properties of social stimuli are necessary to generate an affective response in the observer.
The present findings have important implications for understanding the etiology of disorders characterized by a lack of affective responding. One possible source of such blunted affect may be related to a poorly functioning amygdala from birth. This conclusion is consistent with both the structural and functional neuroimaging literatures in psychiatric neuroscience which point to altered amygdala volumes and abnormal amygdala functioning in a host of psychiatric disorders ranging from bipolar disorder to autism (for reviews: Price & Drevets, 2010; Schumann, Bauman, & Amaral, 2011). Further investigation is warranted to determine whether the affective deficits documented in the present study relate to variation in naturalistic social interactions, the ability to build or maintain social relationships, or variation daily affective experience.
Acknowledgments
This research was supported by a grant from the National Institute of Mental Health (R37MH57502) and by the base grant of the California National Primate Research Center (RR00169). This work was also supported through the Early Experience and Brain Development Network of the MacArthur Foundation. Portions of this data set were presented at the Society for Neuroscience Meeting in San Diego, California, November 2011. We thank the veterinary and husbandry staff of the California National Primate Research Center for excellent care of the animal subjects. We also thank Dr. Pierre Lavenex, Jeffrey Bennett and Pamela Tennant for assistance with surgical procedures and Jessica Toscano for help with data collection.
Footnotes
Lesion conditions did not differ across videos, F(2, 19)= 1.859, p<.183, np2=.164. Overall, food retrieval frequency did not vary based on videos’ social content, F(1.48, 28.18)= 1.97, p<.167, np2=.094, nor affective content, F(2, 38)= 1.35, p<.272, np2=.066. Similarly, no interactions were significant: affective content X lesion, F(4, 38)= 2.085, p<.102, np2=.180; social content x lesion, F(4, 38)= 1.85, p<.140, np2=.094 ; affective content X social content, F(4, 76)= .774, p<.545, np2=.039; affective content X social content X lesion, F(8, 76)= .891, p<.529, np2=.086. Means available upon request.
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne.
References
- Aggleton JP, Passingham RE. Syndrome produced by lesions of the amygdala in monkeys (Macaca mulatta) Journal of Comparative and Physiological Psychology. 1981;95:961–977. doi: 10.1037/h0077848. [DOI] [PubMed] [Google Scholar]
- Antoniadis EA, Winslow JT, Davis M, Amaral DG. Role of the primate amygdala in fear-potentiated startle: effects of chronic lesions in the rhesus monkey. Journal of Neuroscience. 2007;27:7386–7396. doi: 10.1523/JNEUROSCI.5643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniadis EA, Winslow JT, Davis M, Amaral DG. The nonhuman primate amygdala is necessary for the acquisition but not the retention of fear-potentiated startle. Biological Psychiatry. 2009;65:241–248. doi: 10.1016/j.biopsych.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachevalier J. Medial temporal lobe structures and autism: a review of clinical and experimental findings. Neuropsychologia. 1994;32:627–648. doi: 10.1016/0028-3932(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Barrett LF, Bliss-Moreau E. Affect as a psychological primitive. Advances in Experimental Social Psychology. 2009;41:167–218. doi: 10.1016/S0065-2601(08)00404-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman MD, Lavenex P, Mason WA, Capitanio JP, Amaral DG. The development of mother-infant interactions after neonatal amygdala lesions in rhesus monkeys. Journal of Neuroscience. 2004a;24:711–721. doi: 10.1523/JNEUROSCI.3263-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman MD, Lavenex P, Mason WA, Capitanio JP, Amaral DG. The development of social behavior following neonatal amygdala lesions in rhesus monkeys. Journal of Cognitive Neuroscience. 2004b;16:1388–1411. doi: 10.1162/0898929042304741. [DOI] [PubMed] [Google Scholar]
- Bauman MD, Toscano JE, Mason WA, Lavenex P, Amaral DG. The expression of social dominance following neonatal lesions of the amygdala or hippocampus in Rhesus Monkeys (Macaca mulatta) Behavioral Neuroscience. 2006;120:749–760. doi: 10.1037/0735-7044.120.4.749. [DOI] [PubMed] [Google Scholar]
- Belova MA, Paton JJ, Morrison SE, Salzman CD. Expectation modulates neuronal response to pleasant and aversive stimuli in primate amygdala. Neuron. 2007;55:970–84. doi: 10.1016/j.neuron.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss-Moreau E, Toscano JE, Bauman MD, Mason WA, Amaral DG. Neonatal amygdala or hippocampus lesions influence responsiveness to objects. Developmental Psychobiology. 2010;52:487–503. doi: 10.1002/dev.20451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss-Moreau E, Toscano JE, Bauman MD, Mason WA, Amaral DG. Neonatal amygdala lesions alter responsiveness to objects in juvenile rhesus macaques. Neuroscience. 2011;178:132–132. doi: 10.1016/j.neuroscience.2010.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S, Schäfer EA. An investigation into the functions of the occipital and temporal lobes of the monkey’s brain. Philosophical Transactions of the Royal Society B. 1888;179:303–327. [Google Scholar]
- Capitanio JP. Sociability and responses to video playback in adult male rhesus monkeys (Macaca mulatta) Primates. 2002;43:169–177. doi: 10.1007/BF02629645. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Izquierdo A, Murray EA. Distinct contributions of the amygdala and hippocampus to fear expression. European Journal of Neuroscience. 2009;30:2327–2337. doi: 10.1111/j.1460-9568.2009.07012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Wright KS, Murray EA. Hippocampal lesions in rhesus monkeys disrupt emotional responses but not reinforcer devaluation effects. Biological Psychiatry. 2007;63:1084–1091. doi: 10.1016/j.biopsych.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Gable SL, Lieberman MD. Functional magnetic resonance imaging responses relate to differences in real-world social experience. Emotion. 2007;7:745–754. doi: 10.1037/1528-3542.7.4.745. [DOI] [PubMed] [Google Scholar]
- Emery NJ, Capitanio JP, Mason WA, Machado CJ, Mendoza SP, Amaral DG. The effects of bilateral lesions of the amygdala on dyadic social interactions in rhesus monkeys (Macaca mulatta) Behavioral Neuroscience. 2001;115:515–544. [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, et al. Functional atlas of emotional faces processing: a voxel-based meta analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry Neuroscience. 2009;34:418–432. [PMC free article] [PubMed] [Google Scholar]
- Hirai D, Hosokawa T, Inoue M, Miyachi S, Mikami A. Context-dependent representation of reinforcement in monkey amygdala. Neuroreport. 2009;20:558–562. doi: 10.1097/WNR.0b013e3283294a2f. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Murray EA. Selective bilateral amygdala lesions in rhesus monkeys fail to disrupt object reversal learning. Journal of Neuroscience. 2007;27:1054–1062. doi: 10.1523/JNEUROSCI.3616-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Suda RK, Murray EA. Comparison of the effects of bilateral orbital prefrontal cortex lesions and amygdala lesions on emotional responses in rhesus monkeys. The Journal of Neuroscience. 2005;25:8534–8542. doi: 10.1523/JNEUROSCI.1232-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, Bliss-Moreau E, Platt M, Amaral DG. Visual attention to social and nonsocial stimuli. (under review) [DOI] [PMC free article] [PubMed]
- Machado CJ, Emery NJ, Capitanio JP, Mason WA, Mendoza SP, Amaral DG. Bilateral neurotoxic amygdala lesions in Rhesus monkeys (Macaca mulatta): Consistent pattern of behavior across different social contexts. Behavioral Neuroscience. 2008;22:251–266. doi: 10.1037/0735-7044.122.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, Kazama AM, Bachevalier J. Impact of amygdala, orbitofrontal, or hippocampal lesions on threat avoidance and emotional reactivity in nonhuman primates. Emotion. 2009;9:147–163. doi: 10.1037/a0014539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, Snyder AZ, Cherry SR, Lavenex P, Amaral DG. Effects of neonatal amygdala or hippocampus lesions on resting brain metabolism in the macaque monkey: a microPET imagining study. Neuroimage. 2008;15:832–846. doi: 10.1016/j.neuroimage.2007.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Málková L, Gaffan D, Murray EA. Excitotoxic lesions of the amygdala fail to produce impairment in visual learning for auditory secondary reinforcement but interfere with reinforcer devaluation effects in rhesus monkeys. Journal of Neuroscience. 1997;17:6011–6020. doi: 10.1523/JNEUROSCI.17-15-06011.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Málková L, Barrow KW, Lower LL, Gale K. Decreased social interactions in monkeys after unilateral blockade of GABAA receptors in the basolateral amygdala. Annals of the New York Academy of Sciences. 2003;985:540–541. [Google Scholar]
- Mason WA, Capitanio JP, Machado CJ, Mendoza SP, Amaral DG. Amygdalectomy and responsiveness to novelty in Rhesus monkeys (Macaca mulatta): Generality and individual consistency of effects. Emotion. 2006;6:73–81. doi: 10.1037/1528-3542.6.1.73. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Murray EA, Málková L, Mishkin M. Effects of aspiration versus neurotoxic lesions of the amygdala on emotional responses in monkeys. European Journal of Neuroscience. 1999;11:4403–4418. doi: 10.1046/j.1460-9568.1999.00854.x. [DOI] [PubMed] [Google Scholar]
- Paton JJ, Belova MA, Morrison SE, Salzman CD. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439:865–870. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather MD, Lavenex P, Mauldin-Jourdain ML, Mason WA, Capitanio JP, Mendoza SP, Amaral DG. Increased social fear and decreased fear of objects in monkeys with neonatal amygdala lesions. Neuroscience. 2001;106:653–658. doi: 10.1016/s0306-4522(01)00445-6. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, Glenn AL, Jairam MR, Pagnoni G, Goldsmith DR, Elfenbein HA, Lilienfeld SO. Neural correlates of social cooperation and non-cooperation as a function of psychopathy. Biological Psychiatry. 2007;61:1260–1271. doi: 10.1016/j.biopsych.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Rosvold HE, Mirsky AF, Pribram KH. Influence of amygdalectomy on social behavior in monkeys. Journal of Comparative and Physiological Psychology. 1954;47:173–178. doi: 10.1037/h0058870. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Buckley MJ, Walton ME, Rushworth MFS. A role for macaque anterior cingulate gyrus in social valuation. Science. 2006;313:1310–1312. doi: 10.1126/science.1128197. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Bauman MD, Amaral DG. Abnormal structure or function of the amygdala is a common component of neurodevelopmental disorders. Neuropsychologia. 2011;49:745–759. doi: 10.1016/j.neuropsychologia.2010.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanacci L, Clark RE, Zola SM. Selective neurotoxic amygdala lesions in monkeys disrupt reactivity to food and object stimuli and have limited effects on memory. Behavioral Neuroscience. 2003;117:1029–1043. doi: 10.1037/0735-7044.117.5.1029. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rouch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Alverez-Royo P, Clower RP. Independence of memory functions and emotional behavior: Separate contributions of the hippocampal formation and the amygdala. Hippocampus. 1991;1:207–220. doi: 10.1002/hipo.450010208. [DOI] [PubMed] [Google Scholar]