Abstract
The free-living eukaryotic protozoan Tetrahymena is a potentially useful model for the thermoadaptive membrane regulation because of easy growth in the axenic culture, systematic isolation of subcellular organelles, and quick response to temperature stress. Exposure of Tetrahymena cells to the cold temperature induces marked alterations in the lipid composition and the physical properties (fluidity) of various membranes. The increase in fatty acid unsaturation of membrane phospholipids is required to preserve the proper fluidity. In this homeoviscous adaptive response, acyl-CoA desaturase plays a pivotal role and its activity is regulated by induction of the enzyme via transcriptional activation.
Keywords: Tetrahymena, homeoviscous adaptation, membrane fluidity, membrane lipid composition, fatty acid desaturase
Introduction
There is substantial evidence that poikilothermic organisms possess the potential ability to modify the fatty acyl composition of their membrane phospholipids in response to changes in environmental temperature. The general trend is an increase in unsaturated fatty acids at lower growth temperatures and an increase in saturated fatty acids at higher temperatures. Therefore, when exposed to a down-shift in the growth temperature, cells are required to increase the level of unsaturated fatty acids. Such compositional adaptation of membrane lipids, which was called a homeoviscous adaptation process, serves to maintain the correct membrane fluidity at the new growth temperature. This homeoviscous adaptation occurs in diverse micro-organisms and in plants.1)
The unicellular eukaryotic protozoan Tetrahymena exerts marked changes in membrane lipid composition when exposed to altered growth temperatures. Thus this cell has proved to be a potentially suitable model system for studying the molecular mechanisms of the thermoadaptive control of membrane lipids and physical properties (fluidity). From data obtained by ultrastructural and physico-chemical techniques, it was indicated that, in Tetrahymena cells, the temporal adjustment of membrane fluidity coincided with the proportional changes in the levels of unsaturated and saturated fatty acids; i.e. increases in palmitoleic (C16:1Δ9), linoleic (C18:2Δ9,12) and γ-linolenic (C18:3Δ6,9,12) acids and a decrease in palmitic acid (C16:0). This adaptive regulation of fatty acid composition can be achieved by modifying the activity of Δ9-desaturase, which catalyses the introduction of the first double bond into palmitic acid or stearic acid (C18:0). Upon exposure of Tetrahymena cells to low-temperature, the activities of palmitoyl-CoA and stearoyl-CoA desaturases were increased. The increased activity of the desaturase enzyme was principally due to enhanced expression of the enzymes, probably via induced transcription of its mRNA. Membrane fluidity-mediated activation of the desaturase, possibly by a conformational change was suggested to be involved in the early adaptive response.
General description of Tetrahymena cell
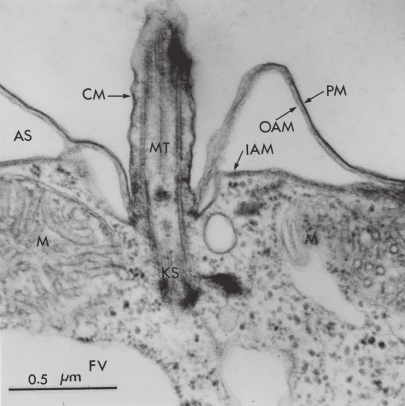
The ciliate protozoan Tetrahymena, which is approximately 60 µm in length and 20 µm in width, grows well axenically in lipid-free media and it can be therefore considered a closed system with respect to the economy of its membrane components. All lipids and proteins are synthesized de novo within the cell, primarily in one compartment, and are then disseminated to various structurally and functionally different membranes for utilization. A wealth of structural and biochemical details reveals Tetrahymena to be a typical cell.2) Tetrahymena cell contains the subcellular organelles typically found in eukaryotic cells, such as mitochondria, lysosomes, endoplasmic reticulum, peroxisomes, but no typical Golgi apparatus. The highly specialized surface structures are less typical (Fig. 1). Underlying the plasma membrane is an extensive anastomosing system of alveolar sacs of unknown function. The outer alveolar membrane is closely apposed to the plasma membrane and actually joined to it by cross bridges. This entire system of cortical membranes is referred to as the pellicle. Another membrane specialization of the cell surface is the oral apparatus, which is a permanently fixed structure designed for the endocytosis. Being a free-living unicellular organism, it furnishes the key advantages of bacteria with respect to rapid growth and easy manipulation. Thus, Tetrahymena has been chosen as an appropriate model for the use in studying the structure and function of biological membranes, particularly membrane biogenesis and membrane adaptation (lipids and fluidy) to environmental stresses.3–6)
Figure 1.
General feature of ultrathin-sectioned T. pyriformis. PM, plasma membrane; OAM, outer alveolar membrane; IAM, inner alveolar membrane; CM, ciliary membrane; M, mitochondria; KS, kinetosome; MT, microtubule; AS, alveolar space; FV, food vacuole. From Nozawa (1975).3)
The lipid composition of the functionally different membranes has been studied extensively. In order to examine the metabolic interrelationships among the different membrane types, it is highly desirable to recover in purified form as many organelles as possible. Accordingly, we have developed the procedure,7) which separates cilia, pellicles, mitochondria, and microsomes as well as postmicrosomal supernatant. Table 1 shows the remarkable differences in phospholipid composition among several organelles. The major phospholipids are phosphatidylcholine (PC), phosphatidylethanolamine (PE), and the rather unusual 2-aminoethylphosphonolipid (AEPL).8) Thirty percent of the phosphoglycerides contain an ether-linked hydrocarbon side chain at the C-1 position in place of the more typical fatty acyl group. The ether side chains are localized mainly in AEPL and PC. The resistance of the phosphonolipid with ether linkage to attack by endogenous lipolytic enzymes has been proposed as the factor responsible for its accumulation in the metabolically isolated surface membranes. The assortment of lipids present in a membrane is important in determining the membrane’s physical properties (fluidity). The principal neutral lipid is the pentacyclic triterpenoid tetrahymanol,9) which serves as a fluidity modulator similar to cholesterol in mammalian cells. There is a 9-fold higher molar ratio of tetrahymanol to phospholipids in ciliary membranes as compared with microsomal membranes. The stabilizing effect of tetrahymanol is reflected in the fluidity properties of these membranes.
Table 1.
Major phospholipids of various membrane fractions from T. pyriformis WH-14 cells
| Membrane fraction | Total phospholipids (mole%) | Tetrahymanol (moles/mole lipid phosphorus) | Glyceryl ethers (moles/100 moles lipid phosphorus) | |||||
|---|---|---|---|---|---|---|---|---|
| Lyso-PC | PC | Lyso-AEPL/ PE | PE | AEPL | CL | |||
| Whole cells | 2 | 33 | 0 | 37 | 23 | 5 | 0.057 | 29.7 |
| Cilia | 1 | 28 | 9 | 11 | 47 | 1 | 0.30 | 52.6 |
| Ciliary supernatant | 8 | 19 | 13 | 16 | 35 | 1 | 0.16 | 23.1 |
| Pellicles | 5 | 25 | 3 | 34 | 30 | 2 | 0.084 | 32.8 |
| Mitochondria | 2 | 35 | 0 | 35 | 18 | 10 | 0.048 | 24.7 |
| Nuclear membranes | 6 | 31 | 6 | 26 | 23 | 3 | 0.036 | — |
| Microsomes | 1 | 35 | 3 | 34 | 23 | 1 | 0.041 | 18.3 |
| Postmicrosomal supernatant | 5 | 34 | 4 | 30 | 22 | 2 | 0.016 | 27.4 |
Many analyses of Tetrahymena fatty acids have been reported.3,6,10) As in the case of phospholipid polar head groups, each membrane maintains a characteristic fatty acid pattern. Tetrahymena synthesizes large amounts of polyunsaturates, with γ-linolenic acid (C18:3Δ6,9,12) being the predominant species present. As has been observed in many other tissues, each phospholipid class in Tetrahymena has its own unique fatty acid distribution.
Of particular advantage is abundant information regarding the physical properities (fluidity) of Tetrahymena membranes. The most detailed comparative analysis of Tetrahymena intracellular membranes has involved freeze-fracture electron microscopy. When cells were exposed to temperature shift-down, the alveolar membrane displayed an obvious lateral movement of protein particles into dense aggregates, leaving behind large particle-free areas of lipids in the crystalline phase. Other membranes, such as endoplasmic reticulum, plasma membrane and nuclear envelope, also showed particle-free domains, but pronounced lateral movement of particles was not distinct. On the other hand, the ciliary membrane underwent no particle rearrangement. Kitajima and Thompson11) have developed a method for quantitative comparison of particle rearrangement in several membranes. The temperature at which each particular membrane first showed particle-free regions varied with the growth temperature of the cells, but the relative orders remained unchanged.
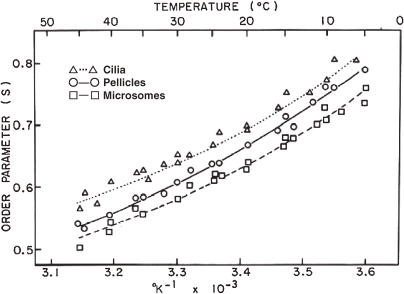
Fluidity-related parameters of isolated membrane fractions have been examined by several physico-chemical techniques, such as electron spin resonance (ESR), X-ray diffraction, fluorescence depolarization and 31PNMR. The fact that general agreement is found using these basically different physical-chemical tools inspires a high degree of confidence that each membrane type within a eukaryotic cell possesses very specific physical properties determined largely by its lipid composition. ESR study with the use of the probe 5-nitrostearate demonstrated that the membranes are more fluid in the order, microsomes > pellicles > cilia10) (Fig. 2). The localization of certain lipids is striking in particular Tetrahymena membrane fractions, which perhaps reflects a diversity in the physical state of the membranes. Taking into consideration that the content of tetrahymanol increases in the order, cilia > pellicles > microsomes, one would expect that this specific sterol-like lipid might be involved in stabilizing membrane fluidity, as observed in cholesterol of mammalian cells.
Figure 2.
The order parameter of 5-nitroxide stearate label in various membranes of T. pyriformis as a function of the reciprocal of the absolute temperature. Data from Nozawa et al. (1974).10)
Another useful technique for probing the physical state of Tetrahymena membranes is fluorescence labeling. The fluidity or microviscosity was measured for isolated membranes by using the fluorescent probe (DPH).12) There are pronounced differences in the microviscosity of various membrane fractions, which are compatible with data from ESR.10) The microviscosity of the ciliary membranes is markedly greater than the microsomal and pellicular membranes. Such diversity in the microviscosity reflects the different lipid compositions of the three membrane fractions. The ciliary membranes contain a considerably higher level of tetrahymanol and 2-aminoethylphosphonolipid (AEPL), whereas the more fluid microsomes have the lowest content of these two lipids together with the greatest degree of unsaturation in fatty acyl chains (Table 1).
Effects of growth temperature on membrane lipid composition and fluidity
Experimentation in many laboratories including our own has established a firm base of information concerning the membrane biochemistry of T. pyriformis. Some strains of this ciliate are capable of adapting to rather high growth temperatures while others are more temperature sensitive. A moderately adaptable strain, WH-14, was used for the earlier studies as above described, because of the capability to alter its membrane lipid composition and fluidity. However, we have discovered that another strain of T. pyriformis, which was isolated from a natural hot spring in New Mexico by Phelps, A. (1961) and provisionally designated this strain NT-1,13) can adapt to higher temperatures and alter its membrane lipids in a much more pronounced fashion than can strain WH-14. Because of its rapid and extensive temperature response, we have chosen this thermotolerant strain for futher studies of the molecular mechanisms underlying temperature adaptation.
Tetrahymena NT-1 cells were grown isothermally at 15, 24, and 39 ℃, and the generation time at these temperatures was 16, 4, and 3 h, respectively. The profound changes were observed in the fatty acid composition of phospholipids from several subcellular fractions.13) The principal effects of decreasing temperature are an increase in linoleic and linolenic acid and a decrease in palmitic acid thus producing a generally higher degree of unsaturation (Table 2). For example, the ratios of total unsaturated to saturated fatty acids of microsomal phospholipids from cells grown at 15, 24, and 39 ℃ are 4.51, 3.97, and 2.66, respectively.
Table 2.
Lipid composition of various membranes from T. pyriformis NT-1 cells grown at different temperatures
| Cilia | Pellicles | Microsomes | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 15 ℃ | 24 ℃ | 39 ℃ | 15 ℃ | 24 ℃ | 39 ℃ | 15 ℃ | 24 ℃ | 39 ℃ | |
| Phospholipid composition | |||||||||
| 2-Aminoethylphosphonolipids (AEPL) | 40.0 | 37.0 | 36.4 | 30.8 | 32.9 | 19.1 | 22.9 | 20.8 | 14.7 |
| Phosphatidylethanolamines (PE) | 20.8 | 21.4 | 20.0 | 33.2 | 36.2 | 49.2 | 34.1 | 35.5 | 43.9 |
| Phosphatidylcholines (PC) | 15.8 | 18.1 | 30.0 | 18.9 | 21.4 | 23.5 | 30.0 | 27.6 | 32.4 |
| Fatty acid composition of phospholipids | |||||||||
| C14:0 | 6.0 | 3.9 | 6.7 | 8.4 | 9.6 | 8.9 | 7.2 | 7.4 | 7.3 |
| C16:0 | 16.8 | 19.4 | 16.8 | 12.7 | 15.8 | 16.4 | 8.9 | 10.4 | 13.2 |
| C16:1Δ9 | 14.4 | 7.7 | 8.3 | 9.9 | 7.4 | 7.1 | 8.8 | 9.8 | 8.7 |
| C18:0 | 2.5 | 3.8 | 6.1 | 0.7 | 2.0 | 3.0 | 0.5 | 1.4 | 2.2 |
| C18:1Δ9 | 6.9 | 8.0 | 11.7 | 7.4 | 8.5 | 12.6 | 8.1 | 7.1 | 13.9 |
| C18:2Δ9,12 | 9.0 | 9.7 | 8.1 | 16.2 | 13.8 | 10.5 | 19.5 | 17.7 | 13.0 |
| C18:3Δ6,9,12 | 27.2 | 28.2 | 20.7 | 28.0 | 27.4 | 18.5 | 31.8 | 31.2 | 21.4 |
Data from Fukushima et al. (1976).13)
It was also rather surprising to observe that there were marked differences in the phospholipids head group composition of cells grown at the three different temperatures. There was a marked increase in phosphatidylethanolamines (PE) as the growth temperature increased, and the relative concentration of phosphatidylcholines (PC) remained constant in pellicles and microsomes. In contrast, the level of AEPL was increased at the low temperature.14) The positional distribution of fatty acids shows a characteristic profile for each phospholipid. When compared with 39 ℃-grown cells, the C-1 position of PC from 15 ℃-grown cells undergoes a large increase in palmitoleic (C16:1Δ9) and γ-linolenic (C18:3Δ6,9,12) acids with a corresponding decrease of myristic (C14:0) and palmitic (C16:0) acids. At C-2 position of 15 ℃-grown cells, linoleic (C18:2Δ9,12) and γ-linolenic acids in PC, and linoleic acid in AEPL increase with a large decrease of palmitoleic acid in both phospholipids and of γ-linolenic acid in AEPL.
It can therefore be speculated that such pronounced alterations in the membrane lipid composition play an important role in adapting the membrane fluidity to changed temperatures. Wunderlich et al.15) showed that the physical properties of membranes of T. pyriformis GL cells and the fatty acid composition of the cellular phospholipids were dependent upon the growth temperature of the cells, with apparent fluidity visualized by freeze-fracture electron microscopy and fatty acid unsaturation both being greater in cells grown at lower temperatures. We observed a similar but more distinct pattern of changing fatty acid composition using the thermotolerant T. pyriformis NT-1 cell and were able to infer by ESR analysis that the fluidity of membranes from low temperature-grown cells was greater than that of high temperature cells measured at the same temperature.12) The fluidities of major membrane fractions isolated from 15 ℃- and 39 ℃-grown cells were measured by ESR as a function of temperature. The membranes of cilia, pellicles,16) and microsomes from cells grown at 15 ℃ were more fluid than those from cells grown at 39 ℃.16) The effect of growth temperature on the membrane fluidity was also examined by wide-angle X-ray diffraction.17) The solid-to-fluid phase transition temperatures of phospholipids from mitochondria, microsomes and pellicles were 21, 19 and 26 ℃ for cells grown at 39 ℃, and −8, −3 and 6 ℃ for cells grown at 15 ℃, respectively. All phospholipids were found in a completely fluid state at these growth temperatures.
Adaptive membrane alterations induced during low-temperature acclimation
As observed above, fatty acids of Tetrahymena phospholipids were considerably more unsaturated when the cells were grown at low temperatures. The logical advantage to a cell of increasing fatty acid unsaturation with decreasing temperature is that its membrane fluidity can thereby be maintained at an optimal level for metabolic function (homeoviscous adaptation).
In order to gain more insight mechanisms of the thermal membrane adaptation, we have done temperature shift-down (chilling) experiments. When Tetrahymena NT-1 cells were exposed to a rapid temperature drop, cell division was quickly arrested, and the cells remained motile but relatively inactive for a period of several hours before eventually resuming growth at a slower rate characteristic of their new environmental temperature. Tetrahymena cells were grown initially at 39 ℃ and then shifted to 15 ℃ over a period of 30 min. This cooling treatment caused a growth cessation for a period of 10 h. During this lag period with little apparent metabolic activity, the critically important alterations should occur in membrane lipid dynamics, e.g. membrane lipid composition and membrane fluidity.
Alterations in membrane lipid composition.
Although, as described above, the large differences in polar head groups were found to exist between 39 and 15 ℃-grown cells,13) rather surprisingly, these changes did not take place during the initial low-temperature acclimation period of 10 h following chilling, when the most significant fatty acid changes occur and the cells regain their capacity to divide.18) Instead, there was a slow transition from the characteristic 39 ℃ pattern to the 15 ℃ pattern during the first few generations of cell growth at 15 ℃, with AEPL increasing drastically at the expense of PE. It is thus assumed that the polar head group changes constitute a secondary response to the lowered temperature, thereby readjusting structural associations resulting from the initial fatty acid changes.
On the other hand, a rapid response to the temperature shift-down was observed in the fatty acid composition of membrane lipids. There was typically a rapid rise in the percentage of palmitoleic acid (16:1Δ9) concurrent with a decrease in palmitic acid (16:0), followed by a slower decline of that fatty acid as other species gradually increase. The compositional changes accompanying low-temperature acclimation do not take place concurrently in various membranes19) (Table 3). The immediate modifications of fatty acids occur considerably more quickly in microsomal membranes than in the peripheral membranes, such as pellicles. The much slower modification of the cell surface membrane fatty acid pattern was completed at roughly the same time (10 h) that low-temperature acclimation was achieved, as judged by the resumption of cell division. The microsomal fatty acid composition was rapidly modified to the pattern observed in microsomes from 15 ℃-grown cells, as compared to the peripheral cellular membranes.
Table 3.
Temporal changes in fatty acid composition of various membrane fractions from T. pyriformis NT-1 cells during temperature acclimation
| Fatty acids | Pellicles | Mitochondria | Microsomes | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 h | 2 h | 4 h | 6 h | 10 h | 0 h | 2 h | 4 h | 6 h | 10 h | 0 h | 2 h | 4 h | 6 h | 10 h | |
| C14:0 | 8.1 | 8.1 | 8.6 | 6.8 | 7.5 | 5.4 | 5.3 | 5.8 | 4.8 | 4.2 | 7.0 | 7.8 | 7.9 | 6.7 | 6.7 |
| C16:0 | 17.6 | 15.4 | 13.4 | 10.4 | 10.9 | 12.4 | 10.9 | 10.1 | 7.7 | 6.6 | 15.5 | 12.7 | 10.9 | 8.1 | 7.6 |
| C16:1Δ9 | 9.6 | 12.3 | 13.4 | 13.0 | 11.7 | 10.0 | 11.8 | 11.5 | 11.5 | 10.5 | 10.9 | 14.5 | 14.5 | 14.6 | 13.6 |
| C18:1Δ9 | 11.9 | 8.5 | 7.4 | 7.4 | 7.8 | 9.4 | 7.4 | 6.8 | 7.0 | 6.0 | 12.4 | 6.5 | 6.6 | 6.1 | 6.0 |
| C18:2Δ9,12 | 15.4 | 16.2 | 16.3 | 17.9 | 17.9 | 19.0 | 19.4 | 17.9 | 20.3 | 20.3 | 16.0 | 16.4 | 16.8 | 17.4 | 18.4 |
| C18:3Δ6,9,12 | 19.7 | 20.9 | 20.8 | 25.3 | 25.1 | 27.2 | 28.9 | 29.5 | 32.7 | 36.2 | 20.5 | 21.8 | 23.4 | 26.8 | 27.9 |
| U/S | 1.60 | 1.76 | 1.82 | 2.43 | 2.24 | 2.62 | 2.92 | 3.04 | 3.79 | 4.38 | 1.94 | 2.00 | 2.22 | 2.71 | 2.97 |
U/S, A ratio of unsaturated to saturated fatty acids. Data from Yamauchi et al. (1981).19)
The fatty acid composition was also examined for each phospholipid class of whole cells at various times after the temperature shift.20) The ratios of unsaturated to saturated fatty acid content following chilling (0, 2, 4, 10 h) were 1.1, 2.8, 2.4, 3.0 for PC; 1.8, 3.9, 4.6, 5.7 for PE; 2.7, 4.7, 7.9, 14.0 for AEPL, respectively, indicating that all three phospholipids have the maximum level of unsaturated fatty acid content at 10 h. It is generally accepted that the physical state of a phospholipid or mixture of phospholipids is sensitive not only to the nature of their fatty acid constituents, but also to the positioning of fatty acids within the phospholipid molecules. In general, naturally occurring phospholipids contain saturated fatty acids (in some cases, ether side chains) at C-1 position and unsaturated fatty acids at C-2 position. Tetrahymena phospholipids show this distributional pattern, particularly when grown at moderate to high temperatures. However, a dramatic change in fatty acid positional specificity occurs upon the temperature shift-down. This change is reflected in the appearance of unusually high levels of polyunsaturated fatty acids at C-1 position of certain phospholipids.21) Upon temperature shift from 39 to 15 ℃, palmitic acid at C-1 position began to be replaced by γ-linolenic acid in all 1,2-diacyl-types of PE, PC, and AEPL from microsomes and mitochondria, while pellicles did not exhibit such rapid and pronounced incorporation of this most unsaturated fatty acid into the C-1 position.22) This rather mild modification in pellicles might reflect the slow transfer from microsomes of phospholipids with modified fatty acid compositions. On the other hand, as for alkyl-acyl types of AEPL and PC which are unique in containing a high level of γ-linolenic acid, there were no marked differences between pellicles, mitochondria and microsomes in temperature-induced rearrangement of the fatty acid composition at C-2 position. Of great interest was the tremendous increase of γ-linolenic acids in 1-O-hexadecyl-2-acyl-PC at C-2 position, which was compensated mainly by the reduction of oleic and palmitoleic acids. In contrast, there were only small or no significant changes in 1-O-hexadecyl-2-γ-linolenoyl AEPL. Thus, the renewal of two principal molecular species, 1-γ-linolenoyl-2-linoleoyl-PE and 1-O-hexadecyl-2-γ-linolenoyl-PC, plays a crucial role for adjusting rapidly the membrane fluidity to the newly exposed low temperature.
Alterations in membrane fluidity.
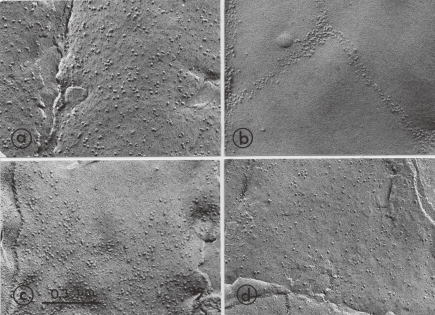
Lowering the temperature of Tetrahymena cells induced the rearrangement of membrane protein particles, as detected by freeze-fracture electron microscopy.5,18,23) When cells grown at 39 ℃ were cooled to 15 ℃ over a 30-min period, the alveolar membrane displayed a marked lateral movement of particles, thereby producing densely aggregated areas and particle-free domains (Fig. 3). Other membranes, such as endoplasmic reticulum, plasma membrane, and nuclear envelope, also showed the perturbed distribution of membrane particles, but the lateral movement of particles was not distinct.11) Therefore, the alveolar membrane may serve as an effective thermosensor to detect the environmental temperature changes and to trigger the rapid modification of membrane lipid composition. During the low-temperature acclimation, the altered distribution of alveolar membrane particles was remodeled.18) The membrane particles induced the lateral movement within the plane of membrane, finally resulting in a random, homogeneous arrangement at 10 h after the temperature shift-down. Of particular note was that the cells regained their ability to grow and divide at about this time, when the particle distribution was restored to the initial random distribution. Such a restoration of the distribution of membrane protein particles coincided with the modification of phospholipids fatty acid composition.
Figure 3.
Changes in distribution of intercalated-membrane particles of the outer alveolar membrane during low-temperature acclimation. The 39 ℃-grown culture (thermotolerant T. pyrifomis NT-1) was shifted down to 15 ℃ over a 30-min period and continued to grow at this low temperature. Samples were taken at indicated time intervals for freeze-fracture electron microscopy. a) 39 ℃ (before shift to 15 ℃); b) 0 h; c) 6 h; d) 11 h after shift. Data from Nozawa and Kasai (1978).18)
The temporal changes in membrane fluidity during cold acclimation were also examined by ESR spectroscopy.19) The increase in fluidity in microsomal and pellicular lipids during the temperature shift from 39 to 15 ℃ corresponded with the increase in the ratio of total unsaturated to saturated fatty acid content. However, unexpectedly, despite the increase in the unsaturated to saturated fatty acid ratio, the fluidity of mitochondrial lipids was found to be rather constant within 10 h after the temperature shift. A distinct difference in lipid composition between mitochondria and the other two membranes (pellicles and microsomes) was the much higher content of cardiolipin in mitochondria. Accordingly, it can be expected that this lipid may play a role in maintaining a consistent fluidity. The correlation between the fluidity of three phospholipids (AEPL, PE, PC) and their fatty acid composition was examined at various intervals after the temperature shift.20) The fluidity of AEPL increased within the first 10 h of the cold acclimation, with the content of γ-linolenic acid in this phospholipid being highest. In contrast, the fluidities of PE and PC showed gradual decreases up to 24 h after the shift, although their γ-linolenic acid contents were highest after 10 h of 15 ℃ incubation. Thus, the fluidity changes of these two phospholipids could be due to the altered content of other fatty acids as well as to γ-linolenic acid.
Fluorescence polarization measurements showed that upon the temperature shift from 39 to 15 ℃ over 30 min, rapid and pronounced alterations in the DPH polarization occurred in the microsomal lipids, while distinct changes in polarization of pellicle lipids were observed after 1 h of a lag time.24) The decrease in polarization of microsomes was much more rapid than that of pellicular membranes, but it did not reach the level of the 15 ℃-grown cells. The polarization of pellicles became almost identical with the value of the 15 ℃-grown cells at 4 h after the temperature shift. The fluidity changes in the microsomal lipids correlated well with the level of fatty acid unsaturation. On the other hand, fluidity of mitochondrial lipids was kept constant up to 10 h, which was compatible with the result obtained by ESR.19,25)
When examined by X-ray wide-angle diffraction, the phase transition temperature was lowered gradually in microsomal and pellicular phospholipids, whereas the transition temperature of mitochondrial phospholipids was unchanged for 10 h after chilling.26) Transition temperatures of microsomal, pellicular, and mitochondrial phospholipids reached the growth temperature (15 ℃) at 6, 10, and 24 h after the temperature shift, respectively. In the case of the membrane phospholipids extracted from cells grown at 39 ℃, lowering the temperature increased the solid phase in all three membrane fractions. However, in the case of the phospholipids from cells acclimated to 15 ℃ for 10 h, the increase in the solid phase was significantly smaller in mitochondrial phospholipids than in the phospholipids of two other membrane fractions.
Molecular mechanisms for adaptive regulation of membrane phospholipid fatty acyl unsaturation
As described above, a critical cellular response to the low-temperature acclimation is increased proportion of unsaturated fatty acids in membrane phospholipids and this is thought to compensate for the direct ordering effects of the cold exposure. The fluidity of membrane phospholipids is largely a property of their unsaturation level and it is generally accepted that the fluidity of membrane lipids are related to the number of unsaturation bonds. However, there is also strong evidence to suggest that the introduction of the first unsaturation bond has the greatest effect on membrane physical state and successive bonds have progressively smaller effects. This initial reaction can be achieved by modifying the activity of the enzyme, Δ9-desaturase, which incorporates the first double bond into palmitic or stearic acid at the (C9–C10) position, resulting in production of plamitoleic or oleic acid. The characterization of this regulatory mechanism is therefore of great importance for better understanding the low-temperature acclimation in Tetrahymena cells.
Fatty acyl-CoA desaturase system in Tetrahymena cell.
Numerous studies of various microorganisms have provided substantial evidence that both molecular oxygen and NADH or NADPH are essential for the microsomal desaturase system, which catalyzes unsaturation of long-chain fatty acids. In addition, an obligatory requirement has been established for flavoproteins (NADH-cytochrome b5 reductase and NADPH-cytochrome c reductase) and cytochrome b5 in the oxidative desaturation of fatty acids in microsomal membranes. The activity of the terminal component of the desaturase system changes in parallel with the total fatty acid desaturase, while the flavoproteins and cytochrome b5 of this system are not affected in mammalian cells,27) suggesting that the terminal component is a rate-limiting enzyme involved in regulation of the overall fatty acid desaturation system. The electrons provided by NADH (or NADPH) are mediated by NADH-cytochrome b5 reductase (or NADPH-cytochrome c reductase) and cytochrome b5 as sequential electron carriers and they are finally transported to the terminal component. Regarding the topology of the microsomal enzyme system, it was shown that three integral membrane components of the microsomal fatty acid desaturase system, including NADH-cytochrome b5 reductase, cytochrome b5, and the terminal component, are localized on the cytoplasmic surface of the endoplasmic reticulum.
On the other hand, it has been shown in Tetrahymena that, in parallel with large changes in the fatty acyl (palmitoyl, stearoyl)-CoA desaturase activity of microsomes in the acute stage (2 h) of low-temperature acclimation, a concurrent, pronounced alteration in the activity of the terminal component of the desaturase system occurred.28,29) However, smaller change was observed in the activity of microsomal NADH-cytochrome c reductase. We have purified a b-type hemoprotein, cytochrome b560ms, which was functionally similar to but not identical to mammalian cytochrome b5, from Tetrahymena microsomes.30,31) Cytochrome b560ms was reduced by NADH in the presence of an NADH-cytochrome b5 reductase purified from rat liver microsomes, suggesting that the pathway of electrons to the cytochrome b560ms-linked fatty acyl-CoA desaturase in Tetrahymena microsomes is mediated via microsomal NADH-cytochrome b560ms reductase.
Regulatory mechanisms of acyl-CoA desaturase activity.
The three independent regulatory mechanisms, which were thought most likely to operate for adaptively increased desatuse activity, have been proposed and evaluated experimentally; 1) the control of fatty acid desaturation by level of dissolved O2 as a co-substrate, 2) induced synthesis of fatty acid desaturase enzyme, and 3) membrane fluidity-mediated activation of pre-existing desaturase enzyme.
It was proposed that the much greater solubility of O2 in aqueous environments at low temperature would automatically raise the concentration of the O2 co-substrate for the desaturation reaction. In fact, the good correlation was observed in some plants between O2 level and desaturation activity. However, when the O2 levels in cultures of Tetrahymena were experimentally changed over the range of values that the cells might encounter in nature at high and low temperature, there was no significant effect on the degree of phospholipid fatty acid desaturation.32) Also, at the constant O2 tension, the temperature shifts did induce the expected changes in fatty acid composition. Thus, the O2 tension may not be implicated in the increased desaturase activity by cold exposure.
The classical studies of desaturase regulation during cold exposure in some bacteria have provided evidence for increased expression of the desaturase enzyme, as an acclimatory response. Induction of the microsomal desaturase enzyme in Tetrahymena has been suggested to be caused by environmental temperature shift-down. When cells were pulse-labeled with 14C-palmitic acid (C16:0) at intervals after the temperature shift-down (39 to 15 ℃), the 14C-pamitoleic acid (C16:1Δ9) level immediately rose after the shift, reaching its maximal level 2 h thereafter.18) The maximum of the conversion rate of 14C-palmitic to 14C-palmitoleic acid was comparable to the palmitoyl-CoA desaturase activiy. After the temperature shift to 15 ℃, there were rapid increases in the activities of palmitoyl- and stearoyl-CoA desaturases in microsomes (Table 4).28) Unlike the situation in mammalian cells, the temperature shift-down also produced small but significant increases in NADH-cytochrome c reductase activity and cytochrome b560ms content. The pre-treatment with cycloheximide prevented the increases in the desaturase and reductase activities after the temperature shift, suggesting that the increase in overall desaturase activities during the cold acclimation may be due to the induction of of NADH-cytochrome b560ms reductase and cytochrome b560ms protein, as well as the terminal component of the desaturase system. Similar findings have also been obtained in the starved Tetrahymena cells which were deprived of the pre-existing desaturase enzyme.33) Although the starved cells have little or no desaturase activity, they showed a lowered temperature-induced rise in the desaturase activity to the level of control-chilled cells. This provides evidence to support the concept that the increased level of total desaturase activity results from induction of the desaturase enzyme protein.
Table 4.
Palmitoyl-CoA and stearoyl-CoA desaturase activities in T. pyriformis NT-1 microsomes from control and cycloheximide-treated cells after temperature shift-down
| Growth condition | nmol/min/mg protein | |||
|---|---|---|---|---|
| Palmitoyl-CoA desaturase | Stearoyl-CoA desaturase | |||
| Control | Cycloheximide | Control | Cycloheximide | |
| 39 ℃-cells | 0.90 | 0.65 | 6.31 | 4.80 |
| After shift (39 ℃ → 15 ℃) | ||||
| 0 h | 2.29 | 0.74 | 12.25 | 4.65 |
| 1 h | 3.40 | 0.39 | 15.50 | 6.20 |
| 2 h | 3.90 | 0.24 | 17.84 | 4.10 |
| 4 h | 2.07 | 0.15 | 4.90 | 2.65 |
| 6 h | 0.54 | 0.08 | 2.70 | 1.30 |
| 10 h | 0.32 | 0.05 | 1.50 | 0.70 |
| 24 h | 0.77 | — | 5.85 | — |
| 15 ℃-cells | 0.59 | 5.00 | ||
Data from Umeki et al. (1982).28)
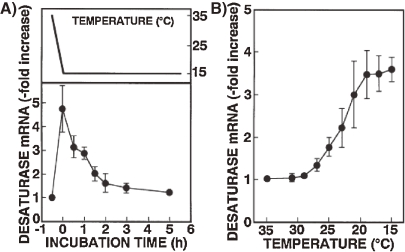
In order to gain further insight into induction of desaturase enzyme, we have isolated a gene that encodes Δ9-fatty acid desaturase from T. thermophila and examined its expression during cold acclimation.34) The nucleotide sequence indicates that the 1.4 kbp gene encodes a polypeptide of 292 amino acid residues which shows marked sequence similarity to Δ9 acyl-CoA desaturase from other sources, e.g. rat, mouse, and Saccharomyces. This desaturase protein has three histidine-cluster motifs (one HXXXXH and two HXXHH), and two hydrophobic regions which are conserved among Δ9 acyl-CoA desatuases. The level of the desaturase mRNA was sensitive to decreasing the temperature of the culture media, and was close to maximum immediately after the temperature was shifted down from 35 to 15 ℃ (0.8 ℃/min) (Fig. 4). Thereafter, the amount of mRNA gradually decreased with time, but remained above the control level for at least 5 h. Furthermore, during the cooling process to 15 ℃, the increased expression of the desaturase mRNA became evident at 27 ℃. Nuclear run-on analysis and actinomycin D chase experiments revealed that the elevation of the mRNA level was due to increases in both transcription and mRNA stability. These results suggest that the enhanced desaturase activity during the cold acclimation is controlled, at least in part, at the transcriptional level. However, the molecular mechanisms for signal transduction mediated by the cold stress leading to increased desaturase gene expression remain to be disclosed.
Figure 4.
Changes in the level of dasaturase mRNA after (A) or during (B) temperature shift-down (39 ℃ to 15 ℃). T. thermophila grown at 35 ℃ were cooled to 15 ℃ over 25 min. After (A) or during (B) the shift-down to 15 ℃, total RNA was extracted at the indicated time intervals. Data from Nakashima et al. (1996).34)
On the other hand, much attention has been paid to the direct influence of the membrane lipid fluidity on desaturase activity in endoplasmic reticulum. Decreased membrane fluidity has been correlated, along with enhanced desaturase activity, with the increased incorporation of unsaturated fatty acids into membrane phospholipids and the consequent adaptive increase in fluidity. Thompson and coworkers35,36) suggested that a chilling-induced compositional modification of fatty acids in Tetrahymena was due to the increased activity of pre-existing desaturase enzymes by the decreased fluidity of the microsomal membrane. There is experimental evidence to indicate that the fluidity of the Tetrahymena microsomal membrane lipids is an important factor in regulating the activity of fatty acid desaturases. Supplementation of polyunsaturated fatty acids (C18:2, C18:3) leading to increased fluidity offset the increased desaturase activity in the control chilled cells.23,37) Furthermore, exposure to chimyl alcohol (1-O-hexadecylglycerol), the precursor to alkyl-ether-containing phospholipids, induced an enhancement of unsaturated fatty acids and a large increase in fluidity in membrane phospholipids.38) Subsequent cooling of the cells induced little or no change in desaturase activity. These results indicate that when the chimyl alcohol-treated cells, with more-fluid membranes, were chilled to a low temperature, enhancement of desaturase activity to the extent observed in untreated control cells was not required. Accordingly it is reasonable to consider that decreasing fluidity would have a stimulatory effect on fatty acid desaturases activity, possibly by inducing conformational change of the desaturase enzyme. Moreover, when membrane fluidity was modified isothermally by several procedures, the microsomal fatty acid desaturase activity was altered in a fluidity-dependent fashion. The fluidizing compounds, such as methoxy fatty acids39) and anesthetics,40,41) reduced fatty acid desaturation in a way which should restore membrane fluidity back into the optimal level. Decreased fluidity was almost invariably accompanied by increased desaturase activity and vice versa. It was thus suggested that fluidity-mediated regulation would be involved in the early adaptive response.
Deacylation and reacylation.
As described above, the temperature shift-down exerted a profound modification of the membrane phospholipid molecular species. For this adaptive process, the deacylation–reacylation plays an important role, which can be assayed by changes in radioactivity whose fatty acyl chain and glycerol-backbone are labeled with different radioisotopes. When 39 ℃-grown Tetrahymena cells prelabeled with both [32P] Pi and [14C] palmitic acid were shifted to 15 ℃ and then linolenic and γ-linolenic acids, which are known to increase during cold acclimation, were added to the culture medium, the (14C/32P) ratios of major phospholipids were progressively decreased until 5 h after the temperature shift. This suggests that preexisting fatty acids of membrane phospholipids are deacylated and then, after the adequate modification by desaturation and/or elongation, are reacylated into lysophospholipids.42) This concept is supported by the finding that the active replacement of the phospholipid fatty acyl chains occurs at the C-1 position; the principal saturated fatty acid, palmitic acid is replaced by polyunsaturated fatty acids, mainly γ-linolenic acid.22) In this context, it is to be noted that phospholipase A1(PLA1) rather than PLA2 was rich in T. pyriformis NT-1 cells.43) This PLA1 activity is Ca2+-dependent and cleaves fatty acyl chains from the C-1 position of phospholipids, giving rise to free fatty acids and 2-acylysophospholipids. Usually, the reacylation of lysophospholipids occurs at the 2-position and plays a crucial role in determining molecular species of phospholipids in mammalian cells. However, in Tetrahymena cells, a marked alteration of fatty acid composition is observed at the C-1 position of phospholipids during temperature acclimation. The reacylation process is mediated by acyltransferases. We have shown that there are reacylation activities not only for acyl-GPC (glycerol-3-phosphorylcholine) and 1-acyl-GPE (glycerol-3-phosphorylethanolamine), but also for 2-acyl-GPC and 2-acyl-GPE, using various acyl-CoAs as donors.44) The specificities for acyl-CoAs were very similar between 2-acyl-GPC acyltranserase and 2-acyl-GPE acyltransferase, which could transfer not only saturated fatty acyl-CoAs but also unsaturated ones, despite enrichment of saturated fatty acids at the C-1 position of PC and PE. Since it was observed that the specificity of acyltransferases for various acyl-CoAs was unchanged during temperature shift-down, the contribution of acyltranserase to the thermal adaptive modification of membrane phospholipid fatty acid composition, may be small.
In the light of these observations, we may conclude that the fatty acyl-CoA desaturase plays a pivotal role in the cold acclimation of membrane phospholipid acyl chain composition; its enhanced activity supplies sufficient amounts of unsaturated fatty acids and subsequently they are utilized to produce the newly adapted molecular species of membrane phospholipids.
Acknowledgments
I would like to express sincere thanks to all colleagues, especially Prof. Guy A. Thompson (University of Texas at Austin) who were involved in this Tetrahymena membrane adaptation project, and also to Prof. Tamio Yamakawa, m.j.a., for encouraging me to contribute this review article.
Profile
Yoshinori Nozawa received MD (1962) and PhD (1966, Biochemistry) from Gifu Prefectural Medical University. He then joined the Department of Biochemistry (Professor Yuki Ito) as a research associate and started his research on the biochemistry of the surface structures of the pathogenic fungal cells. He was appointed to Lecturer (1968) and an Associate Professor (1969). He spent two years (1969–l971) at the University of Texas at Austin as a research associate where he worked with Professor Guy A. Thompson on the membrane biogenesis and the membrane adaptation mechanisms during thermal acclimation in the ciliate protozoan Tetrahymena cell. After return, he has extended these studies to investigate the membrane dynamic structure (fluidity) by using electron spin resonance, wide-angle X-ray diffraction etc., mainly in this suitable model Tetrahymena cell and also some mammalian cells. In 1978 he was promoted to Professor and served as Dean of Gifu University School of Medicine (1996–1999). After retirement (1999) he became a Professor Emeritus of Gifu University, and moved to Gifu International Institute of Biotechnology as Vice-President and then appointed to the President in 2002 (to present), where besides the membrane lipid signaling, he started the new research project studying the molecular mechanisms of phytochemicals including polyphenols as the cell signaling modulator. In 2007, he was appointed to a Vice-Director of the Research & Development Foundation of Gifu prefecture. In 2009, he was appointed to a Professor, Department of Food and Health Sciences, Tokai Gakuin University. He has received the Hutner Award (American Society of Protozoology, 1982), the Doctor Honoris Causa from Semmelweis University (Hungary, 1990), the Japanese Society of Medical Mycology Award (1990) and Tokai Yomiuri Award in Medicine (1999).
References
- 1).Cossins, A.R. (1994) Temperature Adaptation of Biological Membranes. Portland Press, London. [Google Scholar]
- 2).Hill, D.L. (1972) The Biochemistry and Physiology of Tetrahymena Academic Press, New York. [Google Scholar]
- 3).Nozawa, Y. (1975) Isolation of subcellular membrane components from Tetrahymena. In Methods in Cell Biology, Vol. 10 (ed. Prescott, D.). Academic Press, New York, pp. 105–133. [PubMed] [Google Scholar]
- 4).Nozawa, Y. and Thompson, G.A. (1979) Lipids and membrane organization in Tetrahymena. In Biochemistry and Physiology of Protozoan (eds. Levandowsky, M. and Hutner, S.H.). Academic Press, New York, pp. 276–338. [Google Scholar]
- 5).Thompson, G.A. and Nozawa, Y. (1984) The regulation of membrane fluidity in Tetrahymena. In Biomembranes, Vol. 12 (Membrane Fluidity) (eds. Kates, M. and Manson, L.A.). Plenum Publishing, New York, pp. 397–432. [Google Scholar]
- 6).Thompson G.A., Nozawa Y. (1977) Tetrahymena—A system for studying dynamic membrane alterations within the eukaryotic cell. Biochim. Biophys. Acta (Biomembrane, Review)472, 55–92 [DOI] [PubMed] [Google Scholar]
- 7).Nozawa Y., Thompson G.A. (1971) Studies of membrane formation in Tetrahymena pyriformis II. Isolation and lipid analysis of cell fractions. J. Cell Biol. 49, 712–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Thompson G.A., Nozawa Y. (1972) Lipids of protozoa—Phospholipids and neutral lipids. Annu. Rev. Microbiol. 26, 249–278 [DOI] [PubMed] [Google Scholar]
- 9).Thompson G.A., Bambery R.J., Nozawa Y. (1971) Further studies of the lipid composition and biochemical properties of Tetrahymena pyriformis membrane systems. Biochemistry 10, 4441–4447 [DOI] [PubMed] [Google Scholar]
- 10).Nozawa Y., Iida H., Fukushima H., Ohki K., Ohnishi S. (1974) Studies on Tetrahymena membranes—Temperature-induced alterations in fatty acid composition of various membrane fractions in Tetrahymena pyriformis and its effect on membrane fluidity as inferred by spin-label study. Biochim. Biophys. Acta 367, 134–147 [DOI] [PubMed] [Google Scholar]
- 11).Kitajima Y., Thompson G.A. (1977) Tetrahymena strives to maintain the fluidity interrelationship of all its membrane constant—Electron microscopic evidence. J. Cell Biol. 72, 744–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Shimonaka H., Fukushima H., Kawai K., Nagao S., Okano Y., Nozawa Y. (1978) Altered microviscosity of in vivo lipid-manipulated membranes in Tetrahymena pyriformis—A fluorescence study. Experientia 34, 586–587 [DOI] [PubMed] [Google Scholar]
- 13).Fukushima H., Martin C.E., Iida H., Kitajima Y., Thompson G.A., Nozawa Y. (1976) Changes in membrane lipid composition during temperature adaptation by a thermotolerant stain of Tetrahymena pyriformis. Biochim. Biophys. Acta 431, 165–179 [DOI] [PubMed] [Google Scholar]
- 14).Watanabe T., Fukushima H., Nozawa Y. (1980) Studies on thermal adaptation in Tetrahymena membrane lipids—Positional distribution of fatty acid in diacyl- and alkyl-acyl-phosphatidylcholines and -(2-aminoethyl) phospholipids from cells grown at different temperatures. Biochim. Biophys. Acta 620, 133–141 [DOI] [PubMed] [Google Scholar]
- 15).Wunderlich F., Speth V., Kleinig H. (1973) The effect of temperature on membrane core structures and fatty acid composition of Tetrahymena. Biochim. Biophys. Acta 298, 39–49 [DOI] [PubMed] [Google Scholar]
- 16).Iida H., Maeda T., Ohki K., Nozawa Y., Ohnishi S. (1978) Transfer of phosphatidylcholine between different membranes in Tetrahymena as studied by spin labeling. Biochim. Biophys. Acta 508, 55–64 [DOI] [PubMed] [Google Scholar]
- 17).Nakayama H., Goto M., Ohki K., Mitsui T., Nozawa Y. (1983) An X-ray diffraction study on phase transition temperatures of various membranes isolated from Tetrahymena pyriformis cells grown at different temperatures. Biochim. Biophys. Acta 730, 17–24 [DOI] [PubMed] [Google Scholar]
- 18).Nozawa Y., Kasai R. (1978) Mechanism of thermal adaptation of membrane lipids in Tetrahymena pyriformis NT-I—Possible evidence for temperature-mediated induction of palmitoyl-CoA desaturase. Biochim. Biophys. Acta 529, 54–66 [PubMed] [Google Scholar]
- 19).Yamauchi T., Ohki K., Maruyama H., Nozawa Y. (1981) Thermal adaptation of Tetrahymena membranes with special reference to mitochondria—Role of cardiolipin in fluidity of mitochondrial membranes. Biochim. Biophys. Acta 649, 385–392 [DOI] [PubMed] [Google Scholar]
- 20).Ohki K., Kasai R., Nozawa Y. (1979) Correlation between fluidity and fatty acid composition of phospholipid species in Tetrahymena pyriformis during temperature acclimation. Biochim. Biophys. Acta 558, 273–281 [DOI] [PubMed] [Google Scholar]
- 21).Watanabe T., Fukushima H., Kasai R., Nozawa Y. (1981) Studies on thermal adaptation in Tetrahymena membrane lipids—Changes in positional distribution of fatty acids in diacylphospholipids and alkyl-acyl-phospholipids during temperature acclimation. Biochim. Biophys. Acta 665, 66–73 [DOI] [PubMed] [Google Scholar]
- 22).Maruyama H., Banno Y., Watanabe T., Nozawa Y. (1982) Studies on thermal adaptation in Tetrahymena membrane lipids—Modification of positional distribution of phospholipid acyl chains in plasma membranes, mitochondria and microsomes. Biochim. Biophys. Acta 711, 229–244 [PubMed] [Google Scholar]
- 23).Martin C.E., Hiramitsu K., Kitajima Y., Nozawa Y., Skriver L., Thompson G.A. (1976) Molecular control of membrane properties during temperature acclimation—Fatty acid desaturase regulation of membrane fluidity in acclimating Tetrahymena cells. Biochemistry 15, 5218–5227 [DOI] [PubMed] [Google Scholar]
- 24).Martin C.E., Thompson G.A. (1978) Use of fluorescence polarization to monitor intracellular membrane changes during temperature acclimation. Correlation with lipid compositional and ultrastructural changes. Biochemistry 17, 3581–3587 [DOI] [PubMed] [Google Scholar]
- 25).Ohki K., Goto M., Nozawa Y. (1984) Thermal adaptation of Tetrahymena membranes with special reference to mitochondria II. Preferential interaction of cardiolipin with specific molecular species of phospholipids. Biochim. Biophys. Acta 769, 563–570 [DOI] [PubMed] [Google Scholar]
- 26).Nakayama H., Ohki K., Mitsui T., Nozawa Y. (1984) Changes in thermal phase transition of various membranes during temperature acclimation in Tetrahymena. Biochim. Biophys. Acta 769, 311–316 [DOI] [PubMed] [Google Scholar]
- 27).Oshino N., Sato R. (1972) The dietary control of the micorsomal stearyl CoA desaturation enzyme system in rat liver. Arch. Biochem. Biophys. 149, 369–377 [DOI] [PubMed] [Google Scholar]
- 28).Umeki S., Fukushima H., Watanabe T., Nozawa Y. (1982) Temperature acclimation mechanisms in Tetrahymena pyriformis—Effects of decreased temperature on microsomal electron transport. Biochem. Int. 4, 101–107 [Google Scholar]
- 29).Umeki S., Fukushima H., Nozawa Y. (1983) Variations in the activity of fatty acyl-CoA desaturases and electrontransport components in Tetrahymena microsomes with temperature and age of culture. J. Therm. Biol. 8, 353–360 [Google Scholar]
- 30).Fukushima H., Takeda T., Sasaki N., Watanabe T., Nozawa Y. (1983) Purification and characterization of microsomal cytochrome b560ms from a unicellular eukaryote Tetrahymena pyriformis. J. Biol. Chem. 258, 11991–11996 [PubMed] [Google Scholar]
- 31).Fukushima H., Takeda T., Sasaki N., Watanabe T., Nozawa Y. (1984) Isolation of detergent-solubilized NADPH-cytochrome c reductase from Tetrahymena microsomes, with regard to thermoadaptive regulation of fatty acyl-CoA desaturase activity. Comp. Biochem. Physiol., B 78, 855–858 [Google Scholar]
- 32).Skriver L., Thompson G.A. (1976) Environmental effects on Tetrahymena membranes: Temperature induced changes in membrane fatty acid unsaturation are independent of molecular oxygen concentration. Biochim. Biophys. Acta 431, 180–188 [DOI] [PubMed] [Google Scholar]
- 33).Kasai R., Nozawa Y. (1980) Regulatory mechanism of palmitoyl-CoA desaturase activity in thermal adaptation—Induction in non-growing Tetrahymena cells deprived of pre-existing desaturase. Biochim. Biophys. Acta 617, 161–166 [DOI] [PubMed] [Google Scholar]
- 34).Nakashima S., Zhao Y., Nozawa Y. (1996) Molecular cloning of Δ9 fatty acid desaturase from the protozoan Tetrahymena thermophila and its mRNA expression during thermal membrane adaptation. Biochem. J. 317, 29–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Skriver L., Thompson G.A. (1979) Temperature-induced changes in fatty acid unsaturation of Tetrahymena membranes do not require induced fatty acid desaturase synthesis. Biochim. Biophys. Acta 572, 376–381 [DOI] [PubMed] [Google Scholar]
- 36).Thompson, G.A. (1980) Regulation of membrane fluidity during temperature acclimation of Tetrahymena pyriformis. In Membrane Fluidity-Biophysical Techiniques and Cellular Regulation (eds. Kates, M. and Kuksis, A.). The Humana Press, New York, pp. 381–398. [Google Scholar]
- 37).Kasai R., Kitajima Y., Martin C.E., Nozawa Y., Skriver L., Thompson G.A. (1976) Molecular control of membrane properties during temperature acclimation—Membrane fluidity regulation of fatty acid desaturase action? Biochemistry 15, 5228–5233 [DOI] [PubMed] [Google Scholar]
- 38).Umeki S., Nozawa Y. (1984) Regulation of microsomal desaturase and electron-transport enzyme activities in lipid-manipulated Tetrahymena cells—Extent of unsaturated fatty acid production is dependent on membrane fluidity before temperature down-shift. Biochim. Biophys. Acta 793, 123–128 [PubMed] [Google Scholar]
- 39).Kitajima Y., Thompson G.A. (1977) Self regulation of membrane fluidity—The effect of saturated normal and methoxy fatty acid supplementation on Tetrahymena membrane physical properties and lipid composition. Biochim. Biophys. Acta 468, 73–80 [DOI] [PubMed] [Google Scholar]
- 40).Nandini-Kishore S.G., Kitajima Y., Thompson G.A. (1977) Membrane fluidizing effects of the general anesthetic methoxy-fulurene elicit an acclimation response in Tetrahymena. Biochim. Biophys. Acta 471, 157–161 [DOI] [PubMed] [Google Scholar]
- 41).Umeki U., Nozawa Y. (1986) Effects of local anesthetics on stearoyl-CoA desaturase of Tetrahymena microsomes. Biol. Chem. Hoppe-Seyler 367, 61–65 [DOI] [PubMed] [Google Scholar]
- 42).Kameyama Y., Yoshioka S., Nozawa Y. (1984) Mechanism for adaptive modification during cold acclimation of phospholipid acyl chain composition in Tetrahymena. Biochim. Biophys. Acta 793, 28–33 [PubMed] [Google Scholar]
- 43).Arai H., Inoue K., Natori Y., Banno Y., Nozawa Y., Nojima S. (1985) Intracellular phospholipase activities of Tetrahymena pyriformis. J. Biochem. 97, 1525–1532 [DOI] [PubMed] [Google Scholar]
- 44).Yoshioka S., Kameyama Y., Nozawa Y. (1984) Mechanism for adaptive modification during cold acclimation of phospholipid acyl chain composition in Tetrahymena. II. Activities of 2-acyl-glycerol-3-phosphorylcholine and 2-acyl-glycerol-3-phosphorylethanolamine acyltransferases involving the reacylation. Biochim. Biophys. Acta 793, 34–41 [PubMed] [Google Scholar]