Abstract
Sugar chain abnormalities in glycolipids and glycoproteins are associated with various diseases. Here, we report an adult onset cardiac dilatation in a transgenic mouse line with Galβ1,3GalNAc α2,3-sialyltransferase II (ST3Gal-II) transgenes. The transgenic hearts at the end-stage, at around 7 months old, were enlarged, with enlarged cavities and thin, low-tensile walls, typical of dilated cardiomyopathy. Although no apparent change was found in heart gangliosides, glycosylation of heart proteins was altered. Interestingly, sugar moieties not directly related to the ST3Gal-II catalytic reaction were also changed. Significant increases in calreticulin and calnexin were observed in hearts of the transgenic mice. These results suggest that expression of ST3Gal-II transgenes induces abnormal protein glycosylation, which disorganizes the endoplasmic/sarcoplasmic reticulum quality control system and elevates the calreticulin/calnexin level, resulting in suppression of cardiac function. The transgenic mice showed 100% incidence of adult onset cardiac dilatation, suggesting great potential as a new model for dilated cardiomyopathy.
Keywords: ST3Gal-II, cardiac dilatation, calreticulin, calnexin, cardiomyopathy, ER stress
Introduction
Cardiomyopathy is a serious heart disease, and a heart transplant is the only therapeutic choice in extreme cases. Cardiomyopathies are defined as diseases of the myocardium associated with cardiac dysfunction.1) They are classified into five types according to symptomatic features: dilated cardiomyopathy (DCM), hypertrophic cardiomyopathy (HCM), restrictive cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy, and unclassified. Of the five types, DCM and HCM are the most common. In addition, specific cardiomyopathies are also defined as cardiomyopathies with systemic symptoms and/or those secondary to well-defined etiologies, e.g., alcoholism and hypertension. Thus, the etiology of cardiomyopathy is broad and complicated and the development of therapeutic strategies is therefore quite challenging.
Sugar chains in glycolipids and glycoproteins play important roles in many biological processes. Six forms (types I–VI) of β-galactoside α2,3-sialyltransferases have been described so far.2–6) Among them, types I and II are Galβ1,3GalNAc α2,3-sialyltransferases, which mediate the transfer of sialic acid via an α2,3-linkage to the galactose residue of terminal Galβ1,3GalNAc structures on O-linked oligosaccharides of glycoproteins or glycolipids. The type II enzyme (ST3Gal-II) acts on glycolipids and glycoproteins in vitro, but is highly expressed in the brain and has an acceptor substrate preference for glycolipids.3) This result suggests that the enzyme primarily operates in the biosynthesis of gangliosides, such as GM1b, GD1a, and GT1b.4,7) The mouse ST3Gal-II gene is developmentally expressed in the heart; it can be detected at 3 days old, but there is only a trace at 21 days and no expression by 49 days.4) The mouse type I enzyme (ST3Gal-I), which has an acceptor substrate preference for glycoproteins, is slightly expressed in the heart.8) In humans, ST3Gal-II mRNA is highly expressed in the heart and skeletal muscle, unlike in mice.9) The physiological significance of strict temporal control and species differences in ST3Gal-II expression remains unknown.
Protein glycosylation is critical for proper protein synthesis. Correct folding of secretory or membrane proteins is achieved in the endoplasmic/sarcoplasmic reticulum (ER/SR) with the aid of various molecular chaperones and oxidoreductases, which are called ER quality control (ERQC) mechanisms.10) In the system, two lectin-like chaperones, calreticulin and calnexin, bind to monoglycosylated forms of asparagine (N)-linked oligosaccharides. Calreticulin is a soluble Ca2+-binding chaperone, located in the ER lumen.11) Calnexin is a Ca2+-binding chaperone bound to the ER membrane.12) Thus, perturbation of protein glycosylation can be estimated by ERQC activity, and the activity can be monitored by measuring the expression levels of calreticulin and calnexin.
During the course of our research on sialic acid metabolism in transgenic mice, we found homozygous-specific, adult onset cardiac dilatation with 100% incidence in mice of a transgenic line harboring ST3Gal-II transgenes. The mice showed altered glycosylation of heart proteins and higher expression of calreticulin in hearts compared with wild-type mice, suggesting that the perturbation in protein glycosylation activated the ERQC machinery, particularly calreticulin expression, and that the high level of calreticulin impairs cardiac function. The mice would serve as a new model for diseases with cardiac dilatation, such as DCM.
Materials and methods
Mice.
All mice were housed under specific pathogen-free conditions with food (CMF, Oriental Yeast Co., Ltd., Tokyo, Japan) and water ad libitum. C57BL/6CrSlc and Slc:ICR mice were purchased from Japan SLC Inc. (Hamamatsu, Japan). All animal experiments were conducted in accordance with the guidelines for animal experiments of the National Institute of Infectious Diseases, Tokyo, Japan and the National Institute of Biomedical Innovation, Osaka, Japan.
Generation of transgenic mice.
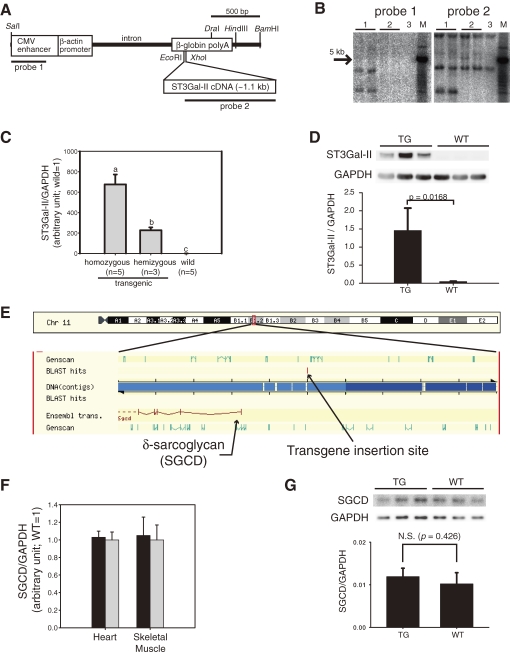
The mouse ST3Gal-II cDNA sequence (GenBank: X76989) was cloned in our previous study.3) The overexpression construct was generated by inserting the cDNA sequence into the multicloning site of a pCAGGS-based plasmid.13) The SalI-BamHI fragments of the plasmid (Fig. 1A) were injected into the pronuclei of C57BL/6CrSlc zygotes. The manipulated embryos were transferred into the oviducts of pseudopregnant Slc:ICR mice. Tail DNA of transgenic founder mice was first screened by PCR. Integration of the transgene into founder candidates was assessed by Southern blot analysis with EcoRI-digested genomic DNA (3 µg/lane) from 4-week-old mice (probe 1: SalI-SmaBI fragment of the CAGGS plasmid, 357 bp; probe 2: KpnI-XhoI fragment of ST3Gal-II cDNA, 930 bp; Fig. 1A). In our previous study,14) the insertion site of the transgene was mapped by Blast search for the flanking sequence of the transgene in the Ensembl database (http://www.ensembl.org/, Fig. 1E).
Figure 1.
Production of ST3Gal-II transgenic mice. (A) SalI-BamHI fragments of a pCAGGS-based plasmid containing mouse ST3Gal-II cDNA were injected into mouse zygotes. (B) Integration of the transgene was confirmed by Southern blot analysis. Lane 1: transgenic mice, Lane 2: non-transgenic (WT) mice, Lane 3: C57BL/6Cr. M: DNA size markers. (C) Expression of the transgene was confirmed by TaqMan based quantitative RT-PCR analysis of hearts from homozygous (n = 5), hemizygous (n = 3), and wild (n = 5) mice at 3 months of age. ST3Gal-II expression was normalized with GAPDH expression and displayed as mean ± S.D. with the average of wild values as 1. Values with different labels (a, b, and c) are significantly different (p < 0.05). (D) Western blot analysis indicated significantly higher expression (*, p < 0.05) of ST3Gal-II protein in homozygous TG hearts than WT hearts at 10 weeks of age (n = 3). (E) Our previous study14) indicated that the transgene was inserted at ∼200 kb upstream of the SGCD gene. (F) No significant difference in the SGCD expressions in hearts or skeletal muscles (rectus femoris) between TG (n = 4, black bars) and WT (n = 5, white bars) was found by TaqMan based quantitative RT-PCR analysis of 3-month-old mice. SGCD expression was normalized with GAPDH expression and displayed as mean ± S.D. with the average of wild values as 1. (G) Western blot analysis indicated no significant difference in expression of δ-sarcoglycan protein between TG and WT hearts at 10 weeks of age (n = 3).
Quantitative analysis of mRNA expressions.
The amounts of ST3Gal-II and δ-sarcoglycan (SGCD) mRNA in tissues of 3-month-old mice were examined by quantitative RT-PCR analyses with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA as an internal control. Total RNA was extracted from each tissue and cDNA was transcribed from the total RNA with reverse transcriptase (Superscript II, Invitrogen Corp., Carlsbad, CA, U.S.A.). Amounts of transcripts were measured by real-time PCR using 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, U.S.A.) with QuantiTect Probe PCR Kits (QIAGEN, Hilden, Germany) and TaqMan probes (Applied Biosystems) for three genes.
Protein extraction.
The hearts of TG and WT mice were collected and snap-frozen in liquid nitrogen. Heart proteins were extracted by unfractionated and fractionated methods. Unfractionated heart proteins were collected with the ReadyPrep Protein Extraction kit (Total protein, Bio-Rad, Hercules, CA, U.S.A.) for quantitative Western blot analysis according to the manufacturer’s protocol. Briefly, each heart was homogenized in 1 mL of extraction buffer (7 M urea, 2 M thiourea, 1% ASB-14, 40 mM Tris, 0.001% bromophenol blue) using a bead mill (TissueLyzer, QIAGEN). The homogenate was centrifuged (15,000 × g, 5 min), and the supernatant (TP) was collected for further analysis. Fractionated heart proteins were extracted sequentially for lectin blot analysis with the ReadyPrep kit (Bio-Rad), according to the manufacturer’s protocol. Briefly, each heart was homogenized in 1 mL of Buffer 1 (40 mM Tris, pH 7.4) supplemented with Complete Protease Inhibitor Mixture (Roche Diagnostics GmbH, Mannheim, Germany) using a bead mill. The homogenate was centrifuged (15,000 × g, 5 min), the supernatant (fraction 1) was collected, and the pellet was re-suspended in 500 µL of Buffer 2 (8 M urea, 4% CHAPS, 40 mM Tris, 2 mM tributylphosphine). After centrifugation, the supernatant (fraction 2) was collected. The protein concentration of each sample was measured with the EZQ Protein Quantitation kit (Invitrogen).
Quantitative Western blot analysis.
The amounts of ST3Gal-II, δ-sarcoglycan, calreticulin, and calnexin in unfractionated heart proteins were examined by quantitative Western blot analyses with GAPDH as an internal control. Heart proteins were separated by 4–12% Bis-Tris gel electrophoresis with MES SDS running buffer (Invitrogen), and transferred onto PVDF membranes (Pall Corp., Port Washington, NY, U.S.A.). Immunoblots were performed using SNAP i.d. (Millipore Corp., Billerica, MA, U.S.A.). The membranes were incubated with primary antibodies against ST3Gal-II (rabbit; Lifespan BioSciences, Inc., Seattle, WA, U.S.A.), δ-sarcoglycan (rabbit; Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.), calreticulin (goat; Santa Cruz Biotechnology) or calnexin (rabbit; Cell Signaling Technology, Beverly, MA, U.S.A.) and then the blots were visualized by chemiluminescence (ECL-plus, GE Healthcare U.K. Ltd., Buckinghamshire, U.K.) with horseradish peroxidase (HRP)-conjugated secondary antibodies against rabbit (Vector Laboratories Inc., Burlingame, CA, U.S.A.), or goat IgG (Jackson ImmunoResearch laboratories Inc., West Grove, PA, U.S.A.). Blot images were captured by a CCD camera (LAS-3000, Fujifilm, Tokyo, Japan). Then, all blots were treated with WB stripping solution (Nacalai Tesque, Kyoto, Japan), incubated with mouse anti-GAPDH antibodies (Millipore), and visualized by chemiluminescence with HRP-conjugated secondary antibodies against mouse IgG (Jackson ImmunoResearch). Blot images were captured with a CCD camera. The band intensities in each image were measured with imaging software (Multigauge, Fujifilm). The amounts of each protein were calculated as protein/GAPDH ratios.
Clinical observations.
The health status of both TG and WT mice was monitored every week for 1 year. The body weights of three male and three female WT and homozygous TG mice were also measured every week from age 40 to 300 days. The TG homozygous mice with dyspnea were used for pathological examinations. WT mice of the same age were used as controls. At necropsy, plasma samples were collected for plasma biochemical analyses using the DRI-CHEM 3000V system (Fujifilm Medical Co. Ltd., Tokyo, Japan).
Pathological examination.
Mice were deeply anesthetized with pentobarbital-sodium (Nembutal, Abbott, Chicago, IL, U.S.A.). Then, a thoracotomy was performed. After resecting the thymus, macroscopic photographs were taken to measure cardiac sizes against the thorax width. With these width parameters, we calculated cardiac dilatation indices similar to cardiac thoracic ratios, which are often used at diagnosis with chest radiographs. The thoracic and abdominal cavities were also examined. The vena cava was then resected, and the left ventricle was perfused with PBS and then reperfused with a 7:3 mixture of 10% neutral-buffered formalin and Carnoy fluid. Hearts were removed and fixed in neutral buffered formalin to present four-chamber views. The hearts were embedded in paraffin using conventional methods, cut into 3-µm slices, and specimens were prepared with hematoxylin and eosin staining. Microscopic images were captured under a light microscope (BX50, Olympus Corp., Tokyo, Japan) with a CCD camera (DP25, Olympus).
Ganglioside analysis.
Ganglioside fractions were obtained from heart, cerebrum and liver by the Folch extraction and partition of total lipids.15) The pooled upper phase was saponified with 0.5 mL of 0.2 N KOH/methanol and desalted with a Sep-Pak C18 reverse-phase cartridges (Waters Corp., Milford, MA, U.S.A.).16) Individual gangliosides were separated by thin-layer chromatography on a plate coated with super-fine silica gel 60 (Merck KGaA, Darmstadt, Germany) with chloroform:methanol:0.2% CaCl2 (60:35:8, by volume) and visualized with resorcinol reagent.17)
Lectin blot analysis of heart proteins.
Two protein fractions from 10-week-old TG and WT mice (∼2 µg/lane) were separated by 4–12% Bis-Tris gel electrophoresis with MES SDS running buffer (Invitrogen), transferred onto PVDF membranes (Pall). The protein transfer onto PVDF membranes was confirmed using a SYPRO Ruby blot stain kit (Invitrogen) before the lectin blots and the images were captured by a laser scanner (FX-Pro, Bio-Rad). Lectin binding assays were performed using SNAP i.d. (Millipore). For MAA assays, digoxygenin (DIG)-conjugated lectins (MAA) included in the DIG glycan differentiation kit (Roche Diagnostics) were used with HRP-conjugated anti-DIG antibody (Jackson ImmunoResearch). For ConA and PNA blots, fluorescein-conjugated ConA and PNA (Vector Laboratories) were used with HRP-conjugated anti-fluorescein antibody (Vector Laboratories). All blots were visualized with chemiluminescence (ECL-plus) and their images were captured with a CCD camera.
Lectin staining of heart and skeletal muscle tissues.
The sugar-chain status in frozen sections (6 µm) of hearts and rectus femoris muscles at 10 weeks of age was analyzed by binding of biotinylated PNA lectins (Vector Laboratories); the sections were treated with or without α2,3-sialidase (5 U/mL, Takara Bio Inc., Otsu, Japan). The lectins were visualized by 0.1 mg/mL FITC-avidin D (Vector Laboratories) under a fluorescence microscope (OPTIPHOTO, Nikon Corp., Tokyo, Japan) with a digital image capture unit (DXC-S500/OL, Olympus).
Statistical analyses.
Expressions of ST3Gal-II mRNA (Fig. 1C) were analyzed statistically among three genotypes using analysis of variance, followed by pairwise comparisons by the Tukey–Kramer method. Expressions of SGCD mRNA (Fig. 1F) and proteins (Figs. 1D, 1G, 6C, and 6D), the heart and body weight parameters (Fig. 2E), and biochemical analyses (Fig. 2F) were analyzed statistically between TG and WT mice using Student’s t-test. Differences with a p < 0.05 were considered statistically significant. All statistical analyses were conducted using StatView Version 5 (SAS Institute Inc., Cary, NC, U.S.A.).
Figure 6.
Lectin staining of two protein fractions extracted from the hearts of transgenic (TG) and wild-type (WT) mice (n = 3) at 10 weeks of age (A: fraction 1; B: fraction 2). After the confirmation of the presence of proteins on blot membranes by SyproRuby blot stain (one of three blots is shown for each fraction), the blots were stained with three lectins (ConA, MAA, PNA). Lectin binding was visualized with chemiluminescence. No evident difference was found on fraction 2 lectin blots containing proteins with lower solubility (e.g., membrane proteins). However, some differentially stained bands (arrows) between TG and WT hearts were found in fraction 1 (soluble proteins): ∼100 kDa by ConA, and ∼250 kDa by MAA and PNA. ConA staining indicated that ∼100 kDa proteins from TG hearts lost their high-mannose-type glycosylation, which was present in those of WT hearts. PNA staining, which recognizes Gal-GalNAc sugars, indicated that the sugar portion of the 250-kDa band in WT mice was highly sialylated, and less sialylated in TG mice. MAA staining, which recognizes α2,3-sialylation of sugars, such as Gal-GalNAc, confirmed higher sialylation of proteins of the same size in WT mice than TG mice. Thus, heart proteins in the TG mice were less sialylated than those of WT mice even though ST3Gal-II, one of the sialyltransferases, was overexpressed in the TG mice. M: Molecular weight markers. Western blots indicate that heart expression of calreticulin (C) and calnexin (D) were significantly higher in the TG hearts than that in WT hearts (p < 0.05).
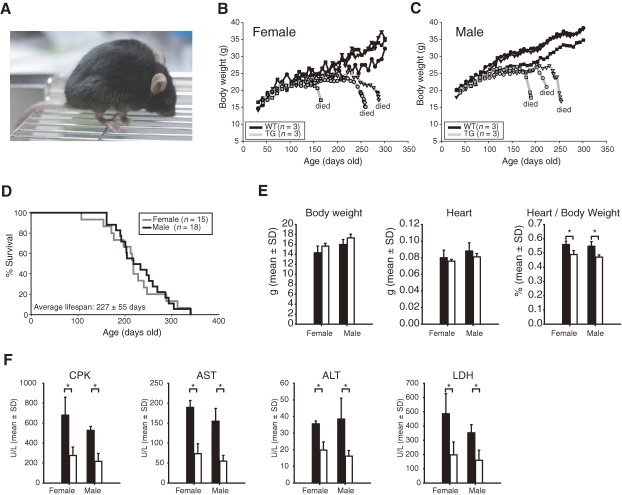
Figure 2.
(A) Appearance of 6-month-old transgenic (TG) mice homozygous for the ST3Gal-II transgene. Daily body weights gains (B, C) in TG and WT mice. (D) Kaplan–Meier survival plot for male and female TG mice. All hemizygous transgenic and WT mice survived until at least 1 year old (not plotted). (E) Body weights, heart weights, and heart:body weight ratios of TG (black bars) and WT (white bars) mice at 4 weeks of age (mean + SD; n = 3). (F) Biochemical analysis of mouse plasma enzymes (mean + SD; n = 5). Four enzyme activities were compared in plasma samples from TG (black bars) and WT (white bars) mice at 4 weeks of age. All plasma enzymes tested showed significantly higher activities in TG mice than in WT mice, even at a young age, long before the onset of symptoms. Asterisks in E and F indicate significant difference between WT and TG mice (n = 3; p < 0.05).
Results
Generation of transgenic mice.
Of two established transgenic lines (4C30 and 4C59), mice only from 4C30 line exhibited cardiac dilatation and was used for further studies. The presence of the transgenes (Fig. 1A) was confirmed by Southern blot analysis (Fig. 1B), and their expression was confirmed by quantitative RT-PCR (Fig. 1C) and Western blot analyses (Fig. 1D). Extremely higher levels of ST3Gal-II expression were found in hearts of transgenic mice than in those of wild mice. The mRNA level of ST3Gal-II in the heart was approximately three times higher in homozygous transgenic mice than in hemizygous transgenic mice. Our previous study14) showed that the transgene was inserted at upstream of the δ-sarcoglycan (SGCD) gene (Fig. 1E). However, neither mRNA nor protein expression of SGCD gene in hearts was influenced by genotype (Figs. 1F and 1G).
Clinical observations.
All mice of the homozygous transgenic (TG) line developed severe cardiac dilatation with dyspnea, deformation of the thorax, and rapid weight loss at the end-stage (Fig. 2A). Both wild-type (non-transgenic; WT) and hemizygous transgenic animals survived without abnormal symptoms for at least 1 year. The TG mice were born healthy and grew normally until approximately 150 days (Fig. 2B, 2C). At that point, they exhibited rapid weight loss and died about a week after the onset of symptoms. The average life span of the TG mice was 227 ± 55 days (mean ± SD, Fig. 2D). Approximately one-third of hemizygous transgenic mice older than 18-months also showed similar, but milder, cardiac symptoms compared with the homozygous transgenic mice (data not shown). Significantly increased heart:body weight ratios, indicating heart enlargement, were already evident in the TG mice at 4 weeks of age in comparison with WT mice even though the TG mice showed no clinical symptoms (Fig. 2E). Biochemical analyses indicated that the plasma CPK and LDH values were significantly higher in TG mice than in WT mice at 4 weeks of age, long before the onset of symptoms (Fig. 2F).
Pathological examination.
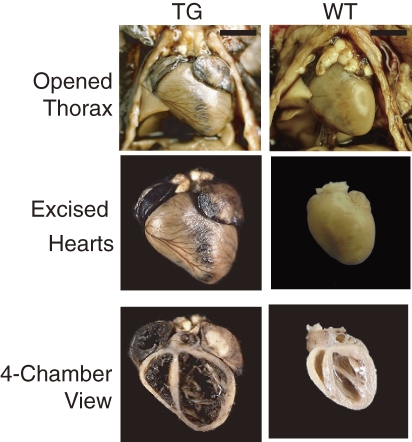
At necropsy of the TG mice, the hearts were enlarged and occupied a larger area inside the thorax, with all four chambers dilated and thin, with low tensile-strength walls (Fig. 3). Both atria and ventricles of TG hearts were larger compared with those of the WT hearts. From the width measurement of hearts and thoraxes, we calculated cardiac dilatation indices. Mice at 5–6 months of age in the TG group showed significantly larger indices (0.66 ± 0.44; mean ± SD, n = 4, range, 0.628–0.723) than those in the WT group (0.451 ± 0.039, n = 3, range, 0.408–0.485). Observation of the four chambered view of the heart presented extremely thin atrial walls in the TG group, with a high degree of dilatation and blood filling into the inner space. The left and right ventricles also exhibited thin free walls and septal walls with a high degree of dilatation. The papillary muscles in the left ventricles had also become thin. Except for pulmonary and hepatic congestion, no abnormality was found in the thoracic or abdominal cavities.
Figure 3.
The hearts of 6-month-old transgenic (TG) mice homozygous for the ST3Gal-II transgene were much larger than those of wild-type (WT) mice. TG heart shows severe dilatation of all four chambers with thin, low tensile-strength walls. Bars = 5 mm.
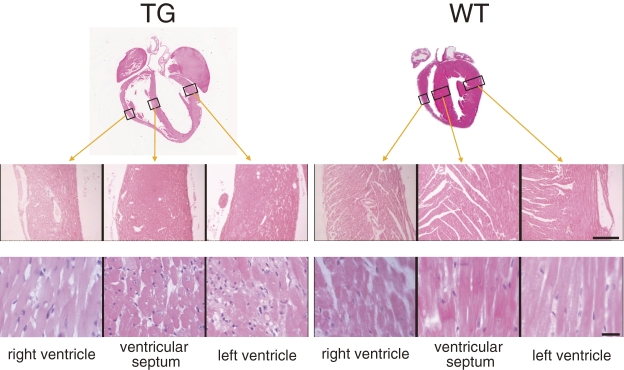
Our histological observations revealed that the width of myocardial fibers changed in both left and right atria as well as ventricular myocardial layers in TG hearts (Fig. 4). Particularly in ventricular muscles, thin and thick fibers were mixed, presenting a degenerated and irregular orientation. In the myocardium layer, connective tissues with slight fibrosis were sporadically observed, but there was no cell infiltration. No coronary artery in the atrium, ventricle, or myocardium presented with luminal narrowing or obstruction. No apparent abnormality was found in the myocardium of WT hearts. A survey of 45 additional organs in TG mice by hematoxylin and eosin staining revealed severe blood congestion in the lungs, liver, and spleen, and a slight, mild degeneration in skeletal muscles but no remarkable alteration in other organs (data not shown).
Figure 4.
Histopathological observations of hearts in 6-month-old transgenic (TG) and wild-type (WT) mice. Hematoxylin and eosin-stained tissue sections from three cavity walls, the positions of which are indicated by rectangles in the upper images and are shown in low (middle images, bar = 100 µm) and high (lower images, bar = 10 µm) magnifications. Slight fibrosis was observed sporadically in TG hearts, but no apparent cell infiltration was found in either TG or WT hearts.
Ganglioside analysis.
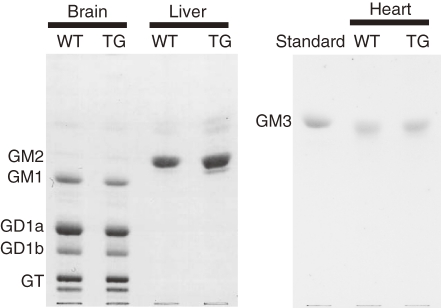
High-performance thin-layer chromatography analysis showed no difference in the ganglioside composition in the heart, brain, or liver between the TG and WT mice (Fig. 5).
Figure 5.
Ganglioside analysis in the brain, liver, and heart. No difference in ganglioside composition was found in the brain, liver, or heart between 6-month-old transgenic (TG) and wild-type (WT) mice, as analyzed by thin-layer chromatography and resorcinol staining. The amounts of gangliosides per lane were equivalent to 1.5 mg, 5 mg, and 5 mg dry weight of the brain, liver and heart, respectively.
Lectin blot analysis of heart proteins.
Alterations in the sugar moieties of heart glycoproteins were examined by lectin blot staining in two fractions of heart proteins from 10-week-old mice (Fig. 6A, 6B). Three lectins were used: Maackia amurensis seed lectins (MAA) recognize α2,3-sialylation of sugars, such as Gal-GalNAc; peanut lectins (PNA) recognize non-sialylated Gal-GalNAc sugars; concanavalin A (ConA) recognizes high-mannose-type sugars. Staining with MAA and PNA lectins indicated that heart proteins of TG mice were less sialylated than those of WT mice, even though ST3Gal-II was overexpressed in the TG mice. One differentially stained ConA band was found in fraction 1 (soluble proteins containing cytosolic proteins), indicating that some cytosolic heart proteins in TG mice lost their high-mannose-type glycosylation, which was present in those of WT mice.
Calreticulin and calnexin expression in hearts.
Quantitative Western blots with TG and WT heart proteins at 10 weeks of age revealed a significant increase (p < 0.05) in the amounts of calreticulin and calnexin in the TG heart (Fig. 6C, 6D).
Lectin staining of heart and skeletal muscle tissues.
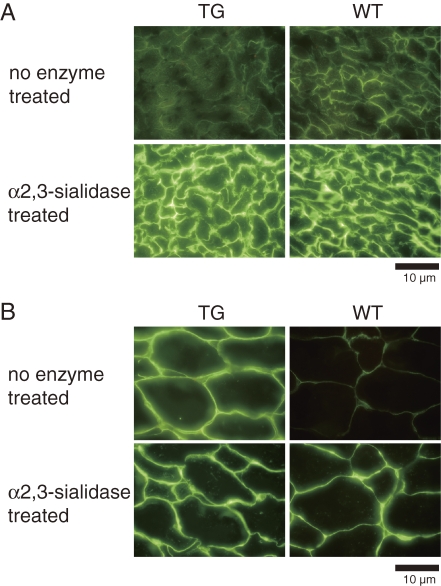
Sialylation in heart and skeletal muscle tissues with transgene-derived ST3Gal-II was examined by histochemical analysis using PNA lectin in combination with α2,3-sialidase treatment (Fig. 7). The ST3Gal-II enzyme converts sugars (Galβ1,3GalNAc) that are bound by PNA to sugars (SialGalβ1,3GalNAc) that are not bound by PNA by α2,3-sialylation of terminal galactose residues. The α2,3-sialidase enzyme reverses the reaction. In the heart, the staining was essentially the same between TG and WT mice with and without α2,3-sialidase treatment. In contrast, PNA staining in the TG skeletal muscle tissue was more intense than that in the WT tissue even without α2,3-sialidase treatment. Staining in skeletal muscle tissue was essentially the same between TG and WT mice after the α2,3-sialidase treatment. These results indicated that most of the terminal Galβ1,3GalNAc structures in pericellular regions (cell membranes and ECM) were sialylated via the α2,3 linkage of galactose residues in the heart. The sialylation decreased in skeletal muscle of TG mice compared with that in WT mice. After α2,3-sialidase treatment, the TG and WT tissues showed similar staining patterns, indicating that the differential staining by PNA without α2,3-sialidase treatment was attributable to differential α2,3-sialylation of Gal-GalNAc structures and not different amounts of Gal-GalNAc structures.
Figure 7.
Frozen sections of heart (A) and rectus femoris muscle (B) treated with or without α2,3-sialidase and stained with biotinylated PNA lectin, followed by FITC-avidin D. Bars = 10 µm. PNA binding was localized to pericellular regions without sialidase treatment, whereas the reactivity was essentially the same between TG and WG hearts. The reactivity in TG muscle was higher than in WT muscle. After sialidase treatment, PNA binding in both became evident equally in TG and WT heart and muscle.
Discussion
The mice that were homozygous for the ST3Gal-II transgene developed non-inflammatory cardiac dilatation with relatively late onset and 100% lethality. Our findings suggest a new concept that abnormal glycosylation of heart proteins can cause cardiac dilatation via perturbation of ER/SR environments. Thus, the mice may be a new model for examining the mechanism and developing therapeutics of non-inflammatory cardiac dilatation related to abnormal glycosylation.
The severity and onset of cardiac symptoms in 4C30 mice depended on a level of transgene expression. As a 1-year observation revealed that cardiac dilatation in 4C30 mice was homozygous-specific, we first considered two issues with regard to the possible mechanism of the cardiac dilatation: the effect of genome disruption by transgene insertion and the effect of transgene dosage. However, after longer observation and chromosomal mapping of the transgene, we concluded that the effect of the transgene itself was the primary cause for two reasons. First, some hemizygous transgenic mice older than 18 months, about three times older than the homozygous transgenic mice, also showed mild cardiac symptoms, suggesting that a level of transgene expression, about one third in the hemizygous compared to that in the homozygous (Fig. 1C), may be involved in the severity and onset of symptoms. Second, our previous report on chromosomal mapping of the transgene in 4C30 mice by genomic walking14) indicated that the transgenes were inserted into the B1.2 region of chromosome 11, approximately 200 kb upstream of the δ-sarcoglycan gene (Fig. 1E), an area in which no gene has been reported to date. Decreased δ-sarcoglycan expression was expected in homozygous mice because δ-sarcoglycan is a key component of the sarcomere complex in muscle tissues and its deficiency can cause degenerative heart diseases, including dilated cardiomyopathy.18) However, δ-sarcoglycan was equally expressed in TG and WT mice (Figs. 1F and 1G), suggesting that the transgene insertion had no adverse effect on genes near the insertion site in TG mice.
The histological analysis indicated that TG mice have cardiac dilatation with a non-inflammatory mechanism. The causes of cardiac dilatation in humans, such as dilated cardiomyopathies, are not fully understood. Some are known, such as inflammation of the myocardium in viral carditis and loss of or abnormalities in genes encoding structural proteins, particularly in hereditary cardiomyopathies.19) However, other idiopathic cardiomyopathies occur, for which the causes remain unknown. Our TG mice had no apparent disruption of genes encoding cardiomuscular proteins and no inflammatory response in heart tissues. These features suggest that our TG mice may be useful for examining the mechanism of some idiopathic cardiomyopathies.
Ganglioside abnormalities are unlikely to cause heart symptoms in the TG mice because no apparent change in ganglioside composition was detected in TG hearts, contrary to our expectation that the overexpressed ST3Gal-II would increase the content of sialylated glycolipids, such as GD1a. The absence of ganglioside changes suggests that the substrate preference predicted by in vitro experiments4) does not always coincide with the in vivo situation. The absence of changes may be because there is little GM1, a substrate for ST3Gal-II, in mouse hearts.20) GM3 is a major ganglioside in both mouse and human hearts.20,21) However, in human hearts, other gangliosides (GM1, GD1a, GD1b, and GT1b) are also detected by more sensitive assays with a GM1-specific choleragenoid.21) Additionally, GM1 can act as a cardioprotectant against hypoxic damage in neonatal rat myocardial cells.22) Thus, further analysis with more sensitive assays is needed to clarify the possible involvement of ganglioside abnormalities in cardiomyopathies.
In contrast to gangliosides, the glycosylation of heart glycoproteins was perturbed in TG mice, demonstrated by the protein and histochemical analyses with lectins. The effects of exogenous ST3Gal-II enzymes induced by transgenesis altered sugar portions of glycoproteins beyond the reaction catalyzed directly by the ST3Gal-II enzyme. However, sialylation changes in an abnormal state might be difficult to interpret because both skeletal and heart muscle tissues contain cytosolic sialidases with high activity at neutral pH.23) Elevated ST3Gal-II activity induced by transgenesis in TG mice might cause tissue damage in hearts and skeletal muscles by higher sialylation. In contrast, if the cytosol is exuded from damaged (necrotic/degenerated) tissue into interstitial spaces of the hearts and muscles, various cytosolic enzymes, including sialidases, may act on glycoproteins on the surface of adjacent muscle fibers, resulting in removal of terminal sialic acids from glycoproteins, particularly membrane-bound proteins. This tendency was evident in skeletal muscles (Fig. 7B). Complicated effects of exogenous ST3Gal-II are also suggested by transfection experiments of human ST3Gal-II in cell cultures.24) In the experiments, the level of ST3Gal-II mRNA are not necessary consistent with those of monosialosyl globopentaosylceramide (MSGb5), a product catalyzed by ST3Gal-II.24) Production of MSGb5 is highly dependent on cell types used for transfection, which may be due to cell-type-dependent sialidase activities.24) In addition, altered protein glycosylation might be attributed to mislocalization of ST3Gal-II itself and/or the other glycosyltransferases in the Golgi apparatus by overloading of exogenous ST3Gal-II because expression-dependent mislocalizations were reported for glycosylation-related enzymes like α2,6-sialyltransferase,25) GA2/GM2/GD2 synthase,26) and human galactosyltransferase.27) Thus, the perturbation in the glycoprotein sugar portions might have been caused by a complex imbalance of glycosylation–deglycosylation reactions in TG mice.
Elevated calreticulin and calnexin levels in TG hearts suggest that cardiac dilatation in TG mice is a response to ER-stress via elevated ERQC, caused by disorganized protein glycosylation in the ER/SR. Calreticulin and calnexin participate in a molecular chaperone system that integrates the processes of N-glycosylation and ERQC.10) Calreticulin is a multifunctional protein involved in many functions, such as regulation of calcium homeostasis,28) ERQC,29) and interactions with various nuclear hormone receptors.30) In the heart, calreticulin is highly expressed in the developing heart, but is strongly downregulated after birth.31,32) The low level of calreticulin expression in adult heart is important for normal heart function because overexpression of calreticulin in the mouse heart leads to severe cardiac pathology, such as cardiac chamber dilatation,33) resulting in complete heart block and sudden death.34) These harmful effects of calreticulin overexpression may be due to various forms of ER stress.35) Myocardiac cells overexpressing calreticulin are highly susceptible to apoptosis under oxidative stress.36) The presence of underglycosylated proteins generates a signal leading to increased GRP78 gene expression,37) which is an ER stress-associated protein.38) Thus, ST3Gal-II overexpression in our TG mice might have perturbed protein glycosylation, which evoked ER stress and stimulated calreticulin expression, and the elevated calreticulin may have caused cardiac dilatation. However, the mechanism is not so simple, because calreticulin is not always elevated in dilated hearts; the calreticulin level is not influenced in some cases of human dilated cardiomyopathy.39) Further investigations are needed to evaluate the involvement of calreticulin in TG hearts.
In conclusion, our transgenic mice (4C30 line) may represent a new form of cardiac dilatation with abnormal glycosylation. Although we do not yet have evidence for a direct connection between abnormal ST3Gal II expression and cardiac dilatation, our findings suggest that the glycosylation status of heart proteins should be evaluated carefully as a possible cause of DCM, especially noninflammatory cases, in humans. Our TG mice have potential as a new animal model for cardiac dilatation diseases, such as dilated cardiomyopathy, because the mice can be maintained as a homozygous line with 100% incidence of heart symptoms and a clear zygosity check system for the transgene allele is available.14) The 4C30 strain is available from JCRB Laboratory Animal Resource Bank at the National Institute of Biomedical Innovation (http://animal.nibio.go.jp/).
Acknowledgments
We thank Dr. J. Miyazaki of Osaka University for the gift of the pCAGGS plasmid and K. Takano for technical assistance. This work was supported by a grant from the Ministry of Health, Labour and Welfare, Japan.
Abbreviations
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- ConA
ConcanavalinA
- CPK
Creatine phosphokinase
- DCM
Dilated cardiomyopathy
- ER
Endoplasmic reticulum
- ERQC
Endoplasmic reticulum quality control
- FITC
Fluorescein isothiocyanate
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- GRP78
78-kDa Glucose-regulated protein
- HCM
Hypertrophic cardiomyopathy
- LDH
Lactate dehydrogenase
- MAA
Maackia amurensis seed lectin
- MSGb5
monosialosyl globopentaosylceramide
- PNA
Peanut lectin
- SGCD
δ-sarcoglycan
- SR
Sarcoplasmic reticulum
- ST3Gal-I
Galβ1,3GalNAc α2,3-sialyltransferase I (St3gal1)
- ST3Gal-II
Galβ1,3GalNAc α2,3-sialyltransferase II (St3gal2)
- TG
homozygous transgenic
- WT
wild (non-transgenic)
References
- 1).Richardson P., McKenna W., Bristow M., Maisch B., Mautner B., O’Connell J., Olsen E., Thiene G., Goodwin J., Gyarfas I., Martin I., Nordet P. (1996) Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation 93, 841–842 [DOI] [PubMed] [Google Scholar]
- 2).Lee Y.C., Kurosawa N., Hamamoto T., Nakaoka T., Tsuji S. (1993) Molecular cloning and expression of Galβ1,3GalNAc α2,3-sialyltransferase from mouse brain. Eur. J. Biochem. 216, 377–385 [DOI] [PubMed] [Google Scholar]
- 3).Lee Y.C., Kojima N., Wada E., Kurosawa N., Nakaoka T., Hamamoto T., Tsuji S. (1994) Cloning and expression of cDNA for a new type of Galβ1,3GalNAc α2,3-sialyltransferase. J. Biol. Chem. 269, 10028–10033 [PubMed] [Google Scholar]
- 4).Kono M., Ohyama Y., Lee Y.C., Hamamoto T., Kojima N., Tsuji S. (1997) Mouse β-galactoside α2,3-sialyltransferases: comparison of in vitro substrate specificities and tissue specific expression. Glycobiology 7, 469–479 [DOI] [PubMed] [Google Scholar]
- 5).Fukumoto S., Miyazaki H., Goto G., Urano T., Furukawa K. (1999) Expression cloning of mouse cDNA of CMP-NeuAc:Lactosylceramide α2,3-sialyltransferase, an enzyme that initiates the synthesis of gangliosides. J. Biol. Chem. 274, 9271–9276 [DOI] [PubMed] [Google Scholar]
- 6).Okajima T., Fukumoto S., Miyazaki H., Ishida H., Kiso M., Furukawa K., Urano T. (1999) Molecular cloning of a novel α2,3-sialyltransferase (ST3Gal VI) that sialylates type II lactosamine structures on glycoproteins and glycolipids. J. Biol. Chem. 274, 11479–11486 [DOI] [PubMed] [Google Scholar]
- 7).Kojima N., Lee Y.C., Hamamoto T., Kurosawa N., Tsuji S. (1994) Kinetic properties and acceptor substrate preferences of two kinds of Galβ1,3-GalNAc α2,3-sialyltransferase from mouse brain. Biochemistry 33, 5772–5776 [DOI] [PubMed] [Google Scholar]
- 8).Ellies L.G., Sperandio M., Underhill G.H., Yousif J., Smith M., Priatel J.J., Kansas G.S., Ley K., Marth J.D. (2002) Sialyltransferase specificity in selectin ligand formation. Blood 100, 3618–3625 [DOI] [PubMed] [Google Scholar]
- 9).Kim Y.J., Kim K.S., Kim S.H., Kim C.H., Ko J.H., Choe I.S., Tsuji S., Lee Y.C. (1996) Molecular cloning and expression of human Galβ1,3GalNAc α2,3-sialyltransferase (hST3Gal II). Biochem. Biophys. Res. Commun. 228, 324–327 [DOI] [PubMed] [Google Scholar]
- 10).Kopito R.R. (1997) ER quality control: the cytoplasmic connection. Cell 88, 427–430 [DOI] [PubMed] [Google Scholar]
- 11).Michalak M., Corbett E.F., Mesaeli N., Nakamura K., Opas M. (1999) Calreticulin: one protein, one gene, many functions. Biochem. J. 344 (Pt 2), 281–292 [PMC free article] [PubMed] [Google Scholar]
- 12).Wada I., Rindress D., Cameron P.H., Ou W.J., Doherty J.J., Louvard D., Bell A.W., Dignard D., Thomas D.Y., Bergeron J.J. (1991) SSRα and associated calnexin are major calcium binding proteins of the endoplasmic reticulum membrane. J. Biol. Chem. 266, 19599–19610 [PubMed] [Google Scholar]
- 13).Niwa H., Yamamura K., Miyazaki J. (1991) Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108, 193–199 [DOI] [PubMed] [Google Scholar]
- 14).Noguchi A., Takekawa N., Einarsdottir T., Koura M., Noguchi Y., Takano K., Yamamoto Y., Matsuda J., Suzuki O. (2004) Chromosomal mapping and zygosity check of transgenes based on flanking genome sequences determined by genomic walking. Exp. Anim. 53, 103–111 [DOI] [PubMed] [Google Scholar]
- 15).Folch J., Lees M., Stanley G.H.S. (1957) A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226, 497–509 [PubMed] [Google Scholar]
- 16).Williams M.A., McCluer R.H. (1980) The use of Sep-Pak C18 cartridges during the isolation of gangliosides. J. Neurochem. 35, 266–269 [DOI] [PubMed] [Google Scholar]
- 17).Svennerholm L. (1957) Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim. Biophys. Acta 24, 604–611 [DOI] [PubMed] [Google Scholar]
- 18).Sakamoto A., Ono K., Abe M., Jasmin G., Eki T., Murakami Y., Masaki T., Toyo-oka T., Hanaoka F. (1997) Both hypertrophic and dilated cardiomyopathies are caused by mutation of the same gene, δ-sarcoglycan, in hamster: an animal model of disrupted dystrophin-associated glycoprotein complex. Proc. Natl. Acad. Sci. U. S. A. 94, 13873–13878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Towbin J.A., Bowles N.E. (2002) The failing heart. Nature 415, 227–233 [DOI] [PubMed] [Google Scholar]
- 20).Leskawa K.C., Short C.S. (1988) Glycolipids of cardiac muscle: an interspecies comparison. Glycoconj. J. 5, 302 [Google Scholar]
- 21).Müthing J., Čačić M. (1997) Glycosphingolipid expression in human skeletal and heart muscle assessed by immunostaining thin-layer chromatography. Glycoconj. J. 14, 19–28 [DOI] [PubMed] [Google Scholar]
- 22).Jin Z.Q., Zhou H.Z., Zhu P., Honbo N., Mochly-Rosen D., Messing R.O., Goetzl E.J., Karliner J.S., Gray M.O. (2002) Cardioprotection mediated by sphingosine-1-phosphate and ganglioside GM-1 in wild-type and PKCε knockout mouse hearts. Am. J. Physiol. Heart Circ. Physiol. 282, H1970–H1977 [DOI] [PubMed] [Google Scholar]
- 23).Dairaku K., Miyagi T., Wakui A., Tsuiki S. (1986) Cytosolic sialidases of rat tissues with special reference to skeletal muscle enzyme. Biochem. Int. 13, 741–748 [PubMed] [Google Scholar]
- 24).Saito S., Aoki H., Ito A., Ueno S., Wada T., Mitsuzuka K., Satoh M., Arai Y., Miyagi T. (2003) Human α2,3-sialyltransferase (ST3Gal II) is a stage-specific embryonic antigen-4 synthase. J. Biol. Chem. 278, 26474–26479 [DOI] [PubMed] [Google Scholar]
- 25).Rabouille C., Hui N., Hunte F., Kieckbusch R., Berger E.G., Warren G., Nilsson T. (1995) Mapping the distribution of Golgi enzymes involved in the construction of complex oligosaccharides. J. Cell Sci. 108, 1617–1627 [DOI] [PubMed] [Google Scholar]
- 26).Giraudo C.G., Rosales Fritz V.M., Maccioni H.J. (1999) GA2/GM2/GD2 synthase localizes to the trans-Golgi network of CHO-K1 cells. Biochem. J. 342, 633–640 [PMC free article] [PubMed] [Google Scholar]
- 27).Teasdale R.D., Matheson F., Gleeson P.A. (1994) Post-translational modifications distinguish cell surface from Golgi-retained β1,4 galactosyltransferase molecules. Golgi localization involves active retention. Glycobiology 4, 917–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Michalak M., Robert Parker J.M., Opas M. (2002) Ca2+ signaling and calcium binding chaperones of the endoplasmic reticulum. Cell Calcium 32, 269–278 [DOI] [PubMed] [Google Scholar]
- 29).Zapun A., Darby N.J., Tessier D.C., Michalak M., Bergeron J.J., Thomas D.Y. (1998) Enhanced catalysis of ribonuclease B folding by the interaction of calnexin or calreticulin with ERp57. J. Biol. Chem. 273, 6009–6012 [DOI] [PubMed] [Google Scholar]
- 30).Dedhar S., Rennie P.S., Shago M., Hagesteijn C.Y., Yang H., Filmus J., Hawley R.G., Bruchovsky N., Cheng H., Matusik R.J., Giguère V. (1994) Inhibition of nuclear hormone receptor activity by calreticulin. Nature 367, 480–483 [DOI] [PubMed] [Google Scholar]
- 31).Mesaeli N., Nakamura K., Zvaritch E., Dickie P., Dziak E., Krause K.H., Opas M., MacLennan D.H., Michalak M. (1999) Calreticulin is essential for cardiac development. J. Cell Biol. 144, 857–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Papp S., Zhang X., Szabo E., Michalak M., Opas M. (2008) Expression of endoplasmic reticulum chaperones in cardiac development. Open Cardiovasc. Med. J. 2, 31–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Hattori K., Nakamura K., Hisatomi Y., Matsumoto S., Suzuki M., Harvey R.P., Kurihara H., Hattori S., Yamamoto T., Michalak M., Endo F. (2007) Arrhythmia induced by spatiotemporal overexpression of calreticulin in the heart. Mol. Genet. Metab. 91, 285–293 [DOI] [PubMed] [Google Scholar]
- 34).Nakamura K., Robertson M., Liu G., Dickie P., Guo J.Q., Duff H.J., Opas M., Kavanagh K., Michalak M. (2001) Complete heart block and sudden death in mice overexpressing calreticulin. J. Clin. Invest. 107, 1245–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Kageyama K., Ihara Y., Goto S., Urata Y., Toda G., Yano K., Kondo T. (2002) Overexpression of calreticulin modulates protein kinase B/Akt signaling to promote apoptosis during cardiac differentiation of cardiomyoblast H9c2 cells. J. Biol. Chem. 277, 19255–19264 [DOI] [PubMed] [Google Scholar]
- 36).Ihara Y., Urata Y., Goto S., Kondo T. (2006) Role of calreticulin in the sensitivity of myocardiac H9c2 cells to oxidative stress caused by hydrogen peroxide. Am. J. Physiol. Cell Physiol. 290, C208–C221 [DOI] [PubMed] [Google Scholar]
- 37).Chang S.C., Wooden S.K., Nakaki T., Kim Y.K., Lin A.Y., Kung L., Attenello J.W., Lee A.S. (1987) Rat gene encoding the 78-kDa glucose-regulated protein GRP78: its regulatory sequences and the effect of protein glycosylation on its expression. Proc. Natl. Acad. Sci. U. S. A. 84, 680–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Shintani-Ishida K., Nakajima M., Uemura K., Yoshida K. (2006) Ischemic preconditioning protects cardiomyocytes against ischemic injury by inducing GRP78. Biochem. Biophys. Res. Commun. 345, 1600–1605 [DOI] [PubMed] [Google Scholar]
- 39).Meyer M., Schillinger W., Pieske B., Holubarsch C., Heilmann C., Posival H., Kuwajima G., Mikoshiba K., Just H., Hasenfuss G. (1995) Alterations of sarcoplasmic reticulum proteins in failing human dilated cardiomyopathy. Circulation 92, 778–784 [DOI] [PubMed] [Google Scholar]