Summary
Ontogenetically, the ventricular venous system may develop in order to drain the gray matter (cells of the mantle layer of the neural tube) which migrates dorsally. On primitive brain vesicles of submammals especially fish, amphibian and reptile, the ventricular venous system is the major venous collector located on the mid-dorsal surface, in between the meningeal layers comparable to the subarachnoid space in mammals. The ventricular venous system functions as a major drainage system for the brain vesicles in these submammals but its role decreases when the other two venous systems develop.
Concerning the route of venous exit from the brain vesicles, we found that it resembles the spinal cord but could not be found all the way along the brain vesicles.
Key words: cerebral veins, anatomy, spinal cord, comparative anatomy
General Introduction
Thinking about the craniospinal venous system as a whole among vertebrates, it has ontogenetic and phylogenic similarities but little diversity depending on each species. We proposed a new cranial venous system classification and explained the evolution of the dorsal and lateral-ventral venous system in parts I and II of this article series. This study describes another system “the ventricular venous system” which can be found early in the brain of submammals.
Materials and Methods
Literature on the cranial venous anatomy in vertebrates was reviewed. Using the area of venous drainage, the veins involved and their functions, we classify the cranial venous system in vertebrates into four systems compared to the venous drainage of the five brain vesicles in man. We also add the extracranial venous system found in vertebrates. The vertebrates reviewed are fish (Myxine glutinosa, Eptatretus stouti, and Danio rerio), amphibians (Amblystoma tigerinum), reptiles (Testudo geometrica), birds (Larus argentatus and hen), rodents (inbred Sprague-Dawley strain of rats, guinea pigs), tree shrews (Tupaia glis), opossums (Didelphis virginiana), domestic animals (dogs, cats, rabbits, pigs, horses, oxen, sheep and goats), primates (Macaca mulatta, Cebus paella, Papio ursinus, Cercopithecus pygerithrus, Galago senegalensis) and hominids.
Results
Ventricular venous system
We propose the forerunner of median procencephalic vein territories in human embryo as another system. The name “ventricular system” comes from its major role of venous drainage from the ventricles in man. It is possible that it occurs as a major route of drainage because of the migration of gray matter dorsally in primitive brain vesicles of lower vertebrates onward.
Phylogenic evolution of the ventricular venous system
Submammals.
From the study of the hagfish1, the term “sagittal sinus” used to refer to the dorsal midline vein extending from the diencephalon to the rhombencephalon. At time of meningeal evolution of cyclostomes, they do not have a well-developed dura mater. Therefore, the venous sinuses seen in mammals could not exist in this species.
In tiger salamander2, the “choroid veins” of telencephalon and diencephalon empty into the “nodus vasculosus” over the paraphysis on the dorsal surface of the brain. The “sinus oblique” is the main channel for blood flow from the choroid plexus of the fourth ventricle. Blood from the cerebellum is drained through the “vena mesencephali” into the “endolymphatic rete”.
Schepers et Al3 reported that the “common choroidal vein”, which is on the medial surface of both hemispheres of the turtle, does not only receive the “choroidal veins” but also receives the “septal vein” which drains the septal area outside the ventricles. It empties dorsally into the “dorsal longitudinal vein”. They also described small choroid vessels of the fourth ventricle, which enter the “anastomotic vein” over the roof of this cavity. The cerebellar venous drainage, the “central cerebellar vein” and “lateral cerebellar vein”, can join both the “dorsal longitudinal vein” and “anastomotic vein”.
Mammals.
On the study of brain vessels in guinea pig4, the tributaries of the internal cerebral veins are the vein of septum pellucidum, thalamostriate vein, choroid vein. They drain the subcortical nuclei of the telencephalon, diencephalon, walls of the ventricles and choroid plexuses.
The ventricular system of domestic animals resembles that of man. The internal cerebral veins arise from the junction of choroidal and thalamostriate veins. They drain the deeper structures of the cerebrum, diencephalon and mesencephalon, and empty into the “great cerebral vein”. There is an anastomosis between the basal vein and the internal cerebral veins through the thalamic veins5,6.
Among monkeys, the system seems to have a similar pattern of tributaries and course of the internal cerebral and great cerebral veins as in man7,8.
On the roof of the diencephalon in human embryo, the evolution of the telencephalic choroid plexus is accompanied by simultaneous differentiation of a single, dorsal located vein coursing from the paraphysis to the marginal (interhemispheric) sinus. It drains blood from the choroid plexuses bilaterally. It was named the median prosencephalic vein of Markowski9 or the primitive internal cerebral vein of Padget10. Since the choroid plexus develops before the neural parenchyma is penetrated by vessels, this vein is therefore the first to drain the intracerebral structures9.
In the study of human embryo during the initial fetal stage11 when the cerebellum starts to develop, the primary fissure approximately establishes the limits of the superior venous drainage. The superior vermian veins principally drain the regions of the superior vermis and the posteriorsuperior portions of the cerebellar hemisphere (paleocerebellum).
Hypothesis of the comparative ventricular venous system anatomy.
Concerning the migration of the primitive cell in mantle zone (future gray matter) dorsally from the neural tube to be the structures in different brain vesicles of vertebrate embryos, this event may have a role of inducing the beginning circumferential venous plexus surrounding the neural tube to be in a dorsal position.
Venous drainage of the brain of adult fish, amphibian and reptile can be divided into two simple venous systems. One is the large vein located on the mid-dorsal surface of the brain vesicles (named “the ventricular venous system”). Another is the veins lying on the lateral surface (some of them lie in between the olfactory lobes, named “the lateral-ventral venous system”).
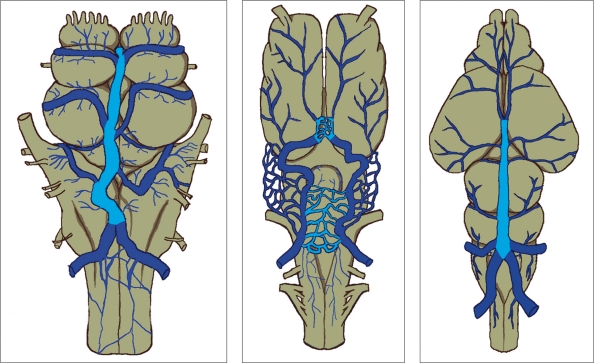
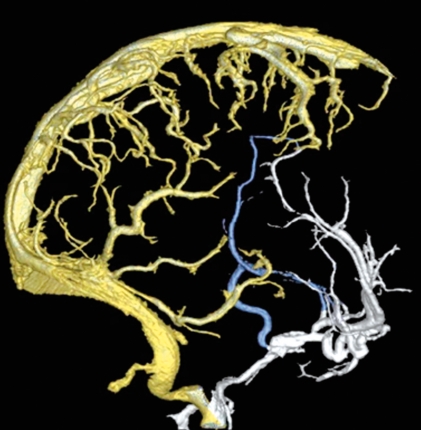
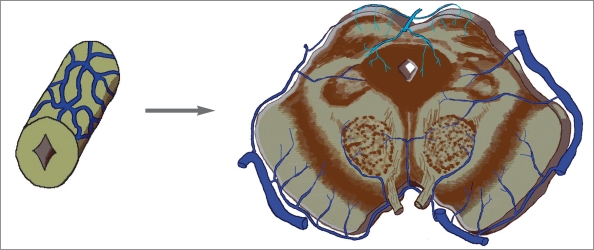
It is interesting that the large mid-dorsal longitudinal vein extending just in front of and over the habenula, diencephalon and mesencephalon as seen in hagfish and Testudo geometrica, and a part of the venous plexus over the paraphysis and mesencephalon of Amblystoma tigerinum, is the main collector receiving the veins from the brain vesicles in these animals. As confirmed on histological examination, all endocranial veins of Testudo geometrica lie in the “arachnoidal spaces”3. This vein is likely to be located in the comparable subarachnoid space in higher vertebrates. Due to the tremendous growth of telencephalon and rhombencephalon, and the decreased size of the tectum of mesencephalon with phylogenic ascent, this vein could be the forerunner of the median procencephalic vein in human embryo (figure 1).
Figure 1.
Three different submammalian brains with the mid-dorsal located vein(s) (in blue) lie(s) in the comparable subarachnoid space of mammals, which could be the forerunner of the median prosencephalic vein of Markowski of human embryo.
Figure 2.
Some variations of the ventral venous system in man. This venous system can connect to the dorsal venous system by different ways: A) through the ventral venous group (posterior pericallosal vein in blue) to the dorsal venous system (falcine sinus in blue); B) through the lateral venous group (inferior ventricular vein and tentorial sinus in blue) to the dorsal venous system. It also exits the brain using the lateral venous group to by-pass the dorsal venous system; C) subependymal veins connect the lateral venous group (in blue) and exit the cranium through the condyloid vein; D) the subependymal vein connects the lateral venous group (in blue) and exit the brain through the petrosal vein.
A.
B.
C.
D.
There are differences in the relative sizes and anatomic disposition of the choroid plexuses, ventricles and brain among species of vertebrates. Among cyclostomes, hagfish have greatly reduced ventricles and no choroid plexuses, whereas lampreys have large ventricles and a prominent choroid plexus that contains approximately two thirds of the total brain blood volume. In dipnoans, well-developed choroid plexuses are characteristic12. In birds and mammals, where the ventricular system is relatively smaller and located deep in the hemispheres, the complex folding of the choroid plexuses compensates for their large surface area. It seems that the venous drainage of choroid plexus of the telencephalon of all species of vertebrates empties into the forerunner of the great cerebral vein.
Like the phylogenic evolution of the cerebellum, petromyzonts and myxinoids have a primordial cerebellum comprising the structures homologous with the flocculi and nodulus in higher vertebrates. The homologue of the cerebellar vermis begins to develop in certain types of fish and reptiles, the so-called paleocerebellum. Having looked at the venous drainage of the cerebellum among vertebrates, in hagfish, tiger salamander and Testudo geometrica, the venous drainage of cerebellum can empty into both the forerunner of the Galenic vein or transverse sinus.
Concerning the archicerebellum, the related venous drainage can be compared with the petrosal (superior petrosal) vein in man. It often empties into the superior petrosal sinus. For that of the paleocerebellum, the superior vermian vein, it empties into the vein of Galen.
Comparative anatomy of the venous outlet of spinal cord and its homology with the five brain vesicles
Looking at the craniospinal venous system as a whole, another system, a homologous spinal radicular vein of the spinal cord, can be seen. This system would be called the "emissary bridging venous system" (table 1). We use this term to describe how venous blood exits each level of brain vesicles.
Table 1.
The emissary bridging venous system of each brain vesicle in man compared to the spinal cord.
| Level of the | Homologous |
|---|---|
| emissary bridging | venous |
| venous system | structures |
|
| |
| Spinal cord | Emissary (Radicular) veins |
|
| |
| Myelencephalon | Condyloid vein |
| (the bridging vein | |
| draining the vicinity | |
| of the medulla) | |
|
| |
| Metencephalon | Petrosal vein |
| (superior petrosal vein) | |
| or superior vermian vein | |
| or basal vein of Rosenthal | |
|
| |
| Mesencephalon | Basal vein |
|
|
of Rosenthal |
| Diencephalon | or vein of Galen |
|
| |
| Telencephalon | Deep middle cerebral vein |
| or sphenoparietal sinus | |
On this topic, we discuss how cephalization affects the venous drainage of five brain vesicles. Attention is drawn only to how blood flows from different parts of the neural tube.
Considering the venous anatomy of the vertebrates’ spinal cord, a similar location of the perineural venous plexus (in the subarachnoid space) and the exit routes from the cord can be found. Even though the evolution of the meninges moves onward from submammals to mammals, the layer of the meninges where the perineural venous plexus is located could be comparable.
The most primitive meninges evolution belongs to cyclostomes. The study of the lamprey meninges13 found that the so-called “meninx primitiva”14 was actually divided into four layers. The first (outermost), second (cellular) and forth (innermost) layers correspond to the dura, arachnoid and pia of the meninges of mammals, respectively. Due to the lack of communication between the ventricle and any space outside the brain in lampreys, the third (“gelatinous”) layer is assumed to be the forerunner of the subarachnoid space found in mammals. The perineural vascular plexus is located on the forth layer, whereas the epidural vascular plexus is located on the first layer.
The embryonic neural tube of vertebrates is divided on each side by the sulcus limitans extending longitudinally and separating the dorsal sensory part from the ventral motor part, except in cyclostomes. Each of the dorsal and ventral regions can be subdivided into somatic and visceral columns15. On a study of the neural tube vascularization in quail embryos and chick–quail chimeras16, the neural tube receives its first blood vessels by a combination of two different processes, dorsal immigration of isolated migrating angioblastic cells, and ventral sprouting of endothelial cells which derive from perfused vessels. The dorsal invasive angioblasts contribute to the developing intraneural vascular plexus after having traversed the neural tube.
Major tributaries of venous drainage of the cephalic area develop from the plexus of capillaries over the neural tube. On each side, these tributaries drain laterally into three superficial dural stems10. During the first six weeks of life, most of the neural tube seems to have a “lateral-ventral direction” of venous drainage17.
While the neural tube is closing, the walls become thickened and stratified. The cells and their process are arranged in three layers: ependymal, mantle and marginal layers. In the region of the forerunner of spinal cord, continued germinal cell proliferation in the ependymal layer produces thickenings in the mantle layer to form the anterior and posterior horns of the cord (gray matter). The marginal layer comprises processes from cells in the mantle and ependymal layers18.
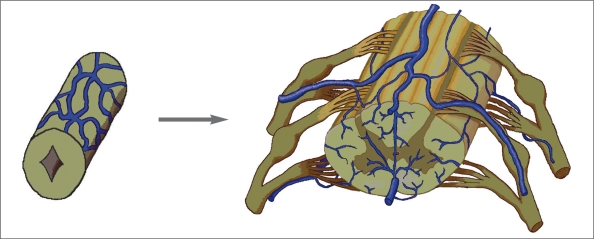
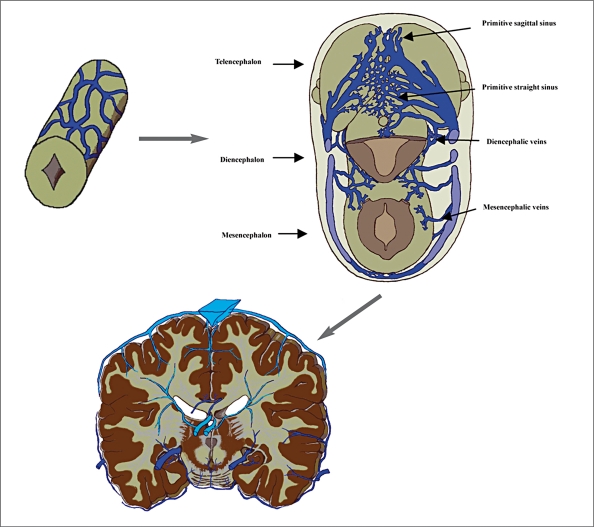
On the study of pig embryo19, veins of the spinal cord develop from the capillary plexus on the surface. The first appear on the ventrolateral surface from capillaries which are continuous laterally with those of the surrounding mesenchyme, and medially with lateral branches of the primitive arterial tract. The veins drain laterally into the anterior radicular veins. The posterior radical veins develop alongside the dorsal ganglion. At first they drain blood from the dorsal surface and then from all of the cord. In the adult spinal cord, there is no constant correspondence between arteries and veins. In the cervical and thoracic regions the radicular veins do not follow the nerve roots along their entire length as a single vessel. The veins on the ventral and dorsal surface of the cord are anastomosed by the venous network or “circumferential venous plexus” (figure 3). We propose the venous outlet of the spinal cord as the “emissary bridging veins”, whose homologues can be found along the ventral-lateral surface of cephalic brain vesicles.
Figure 3.
Evolution of the perineural venous plexus to the spinal cord vein in man (from Lasjaunias9 with modification).
When cephalization leads to the secondary migration of the neural tube cells in the five brain vesicle regions, the pattern of venous drainage of these brain vesicles also changed. It is possible that the migration of gray matter has a role of inducing changes in the pattern of venous drainage from the circumferential pattern in the spinal cord region to the dorsal position in the cephalic region found in lower vertebrates.
Myelencephalon
In the myelencephalic region, the walls of the neural tube are pushed laterally. The alar plate lies lateral to the basal plate. Cells of the basal plate migrate to and from the motor nuclei of the IX, X, XI, and XII cranial nerves. The sensory nuclei derive from the alar plate.
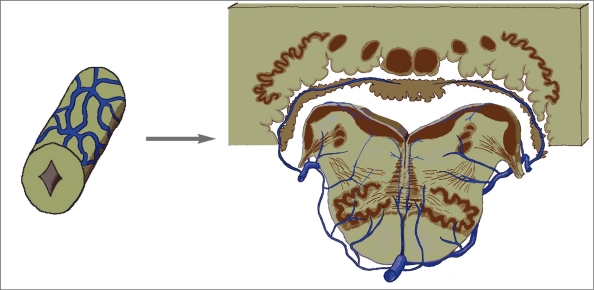
In lower vertebrates such as hagfish, Amblystoma tigerinum, and Testudo geometrica, the venous drainage of the myelencephalic region empties dorsally before exiting the cranial cavity into the veins which curve ventrally around the myelencephalon and connect to the jugular veins1-3. From the study of brain venous system of dog5, the pattern of venous drainage begins to be similar to that in man. The medullary veins frequently anastomose across the midline. Veins from the dorsal medulla and choroid plexus of the fourth ventricle converge and empty into the sigmoid sinus.
Considering the venous drainage in this region in man, the venous plexus surrounding the myelencephalon still continues from the spinal cord. There are bridging veins, which are comparable to the spinal cord radicular veins: the “condyloid veins” or “inferior petrosal veins”20 emptying either into the jugular bulb, marginal sinus or anterior condylar vein. Probably with the result of cephalization, another anatomical variation of the venous exit is found on the mid-dorsal surface of the medulla (just below the foramen of Magendie) connected with the occipital plexus21. The venous anastomosis occurs both on the dorsal and ventral surfaces as seen in the cord. The most cephalic dorsal venous anastomosis is the vein of lateral recess of the fourth ventricle (figure 4). The lateral-ventral venous anastomosis: anterior, lateral and transverse myelencephalic veins, connects the circumferential veins from the spinal cord to the metencephalic region. This anastomosis begins to be a new system, “the lateral-ventral venous system of the brain vesicles”, which is well-developed in mammals possibly due to the development of recent deep nuclei on the ventral surface of the vesicles.
Figure 4.
Evolution of the perineural venous plexus at the myelencephalic region in man (from Duvernoy22 with modification).
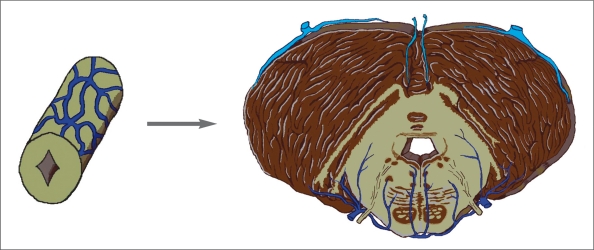
Metencephalon
The pons and cerebellum are from the anterior and posterior part of the metencephalic region, respectively. The ventral portion of the tegmentum of pons is a recent acquisition of phylogenic development. The posterolateral parts of the two alar plates in the region of the isthmus bend posteriorly and medially to and from the rhombencephalic lips. They enlarge to form the cerebellum. The oldest acquisitions of the cerebellum (archicerebellum) are nodulus and flocculi. Due to the development of the neocerebellum (cerebellar hemispheres) and paleocerebellum (most of the cerebellar vermis), the venous drainage of the metencephalic region has been changed when compared to the spinal cord and myelencephalon. Three systems are involved.
The venous drainage of the cerebellum had been changing over time with phylogenic ascent. The archicerebellum found in fish onward uses the vein which empties into the homologue of the transverse sinus as the major drainage. For the paleocerebellum, the forerunner of the internal cerebral veins plays this role. We put the vein of Galen in the category of bridging veins. It seems that after evolution of the dorsal venous system in birds onward, the vein of Galen acts as the connection between this system and the ventricular system.
After the evolution of the dura mater along with the development of the neocerebellum after reptiles, the dorsal venous system which related to most of the cerebral venous sinuses takes the action of venous drainage of this new acquisition of the brain (figure 5).
Figure 5.
Evolution of the perineural venous plexus at the metencephalic region in man (from Duvernoy22 with modification).
The venous drainage of the ventral metencephalic region in submammals such as hagfish, Amblystoma tigerinum, and Testudo geometrica1-3 uses the veins which curve dorsally going either into the homologue of the Galenic vein or transverse sinus, and anastomose with the veins of the ventral region of mesencephalon and myelencephalon. The pontine veins of dogs receive tributaries from the lateral and ventral aspects of the pons and proceed dorsally to anastomose either with the homologue of the superior petrosal sinus or basal cerebral vein 5.
In man, the continuous (lateral-ventral) venous system from the myelencephalic region plays an important role of venous drainage on the lateral and ventral part. It consists of the anterior, lateral and transverse pontine veins anastomosed together as the venous plexus. The bridging veins of this region go into the petrosal (superior petrosal) vein.
Mesencephalon
The mantle layer of the alar plate undergoes marked cellular proliferation in the mesencephalon. Many of the newly formed cells migrate into the marginal layer where they become arranged into the tectum. The tegmentum is developed from the basal plate. It contains the motor nulei of the third and fourth cranial nerves, recently acquired red nuclei, and fiber tracts. The peduncle consists of fiber tracts which develop in the marginal layer of the basal parts of this region, and the substantia nigra.
Among submammals1-3, the venous blood from the tectum of mesencephalon is drained in the same pattern into the homologue of the Galenic vein of human. The venous anastomosis on the lateral and ventral parts can be seen but is not as complex as in mammals.
The pattern of venous drainage in this region of dogs5 is similar to man. The vein of tectum enters the great cerebral vein and anastomoses to circumscribe each quadrigeminal plate and also with other cerebellar veins. The blood from the tegmentum is drained into the basal veins (a part of the lateral-ventral venous system) which are well-developed in higher mammals.
In man, the venous drainage of this region continues from the rhombencephalon on the lateral, ventral parts. It consists of the posterior communicating vein, peduncular vein, and medial, lateral, transverse mesencephalic veins. As we proposed the role of cephalization on the dorsally oriented venous drainage, the collicular(tectal) veins can empty dorsally into the Galenic vein or ventrally into the continued lateral-ventral venous system (basal vein of Rosenthal) (Figure 6).
Figure 6.
Evolution of the perineural venous plexus at the mesencephalic region in man (from Duvernoy22 with modification).
Diencephalon
All the diencephalic structures are derived from the enlarged alar plates. The part of the alar plate which is inferior to the hypothalamic sulcus differentiates into the hypothalamic nuclei. The part which lies superior to it differentiate into the thalami. The roof plate of the diencephalon consists of a layer of ependymal cells covered by vascular mesenchyme. The optic vesicle and stalk develop from this brain vesicle.
Considering the venous drainage of this region in submammals1-3, the veins lie lateral in the groove between the primitive telencephalon and the diencephalon or mesencephalon. They drain structures of this region from ventral and lateral surfaces and enter dorsally either into the forerunner of the great cerebral vein or transverse sinus in mammals. The veins of this region in domestic animals5 resemble those of man. The lateral-ventral venous system continues from the mesencephalic region. The system receives many small tributaries from the base of the brain which drain the optic tract, tuber cinereum, mammillary bodies and geniculate bodies. They can anastomose across the midline in the interpeduncular space. The thalamic veins connect the great cerebral vein and the basal veins.
In man, the lateral-ventral venous system still continues from the mesencephalon as the basal vein of Rosenthal and connects with the same of the telencephalic region. The venous outlets of this region are similar to those of the mesencephalon.
Telencephalon
The structures of the telencephalon (corpus striatum and pallia) develop from expanded alar plates and enclose the lateral ventricles. The corpus striatum is developed in the mantle layer of the ventrolateral walls of the telencephalon adjacent to the diencephalon. Cells in the germinal zone surrounding the lateral ventricle migrate outwards to become neuronal and glial cells upon maturation in pallia.
When comparing the corpus striatum among vertebrates, the primordial pallidum can be found in primitive vertebrates. The development of the neostriatum (the caudate nucleus and putamen) parallels the development of the thalamus and neopallium. It first appears in reptiles (primordial neostriatum or hypopallium). The neostriatum is large and well-developed in birds onward15,23. It is possible that due to the effect of corpus striatum evolution, the development of the lateral-ventral venous plexus is more complex and appeared in mammals.
We found that the each pallium likely has its own related venous drainage. The archipallium (hippocampus) in submammals1-3 has its own venous drainage located on the midline between the two hemispheres emptying into the homologous great cerebral vein. When the archipallium is pushed caudally and ventrally by the development of the corpus callosum and neopallium in mammals, its venous position is moved together and is connected by the continuous lateral-ventral venous plexus from the brain vesicles caudally.
The paleopallium has a complex of the position of the venous drainage. Its venous drainage first empties into the forerunner of the great cerebral vein in submammals. Due to the development of the neopallium between the first two pallia as phylogenic ascent, the paleopallium is moved laterally away from the archipallium with its veins from dorsal-lateral position in submammals to lateral-ventral in mammals, and to definite ventral position in man. Then, it is captured by the same plexus as the archipallium from the diencephalon and brain stem. Its venous emptying site has changed from the homologue of the internal cerebral veins in submammals to the transverse sinus in rodents and domestic animals, either to the superior petrosal sinus or transverse sinus in primates and fetal period of human. The medial direction of venous drainage of the paleopallium into the cavernous sinus is a recent acquisition.
With a tremendous growth of the neopallium and well-developed dura mater in birds onward, the newly developed venous system or the “dorsal venous system”(most of the dural venous sinuses) functions as a venous collector of the neopallium (figure 7).
Figure 7.
Evolution of the perineural venous plexus at the prosencephalic region (from Padget10 with modification).
As far as the middle cerebral veins are not captured with the cavernous sinus, the venous drainage of the telencephalon uses only the dorsal venous system and the lateral-ventral venous system (the basal vein of Rosenthal) as the major routes of exit. As the middle cerebral veins captured with the cavernous sinus occur specifically in man, the cavernous sinus plays a role of venous drainage outside this brain vesicle.
An aspect of clinical application of the emissary bridging venous system
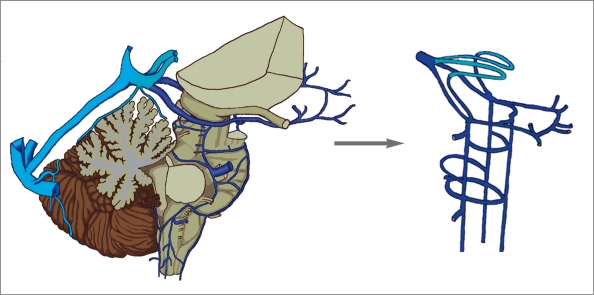
In clinical practice, we usually face the problem of how the venous blood of the brain drains if the dorsal venous system does not function. For example, patients with cerebral venous sinus thrombosis, vein of Galen aneurysmal malformation, dural arteriovenous fistula, etc. When we cut the brain vesicles off the venous systems, we can see the connections between the lateral-ventral venous system and the ventricular venous system clearly. The fabulous role of the emissary bridging venous system acts like pipes connecting the venous systems of the brain vesicles to the dural/epidural venous system (figure 8).
Figure 8.
Three venous systems of the brain vesicles in the lateral view (A). A diagram of the emissary bridging venous system (B) shows how venous blood leaves the brain or some venous variations can be expected.
Some comparative aspects on pathways of cranial venous outflow
Jugular veins
The external jugular vein is apparent in mammals. In lower vertebrates e.g. fish, amphibians, reptiles and birds, the internal jugular vein is more dominant.
The external jugular vein becomes larger than the internal jugular vein in rats, opossums, domestic animals and monkeys. It is not only because it drains the face, which is proportionally larger than the cranial cavity, but also because of the partial cerebral venous drainage through the postglenoid foramen8.
Vertebral venous plexus
The vertebral plexus appears to be the largest and most immediately available system for collateral circulation. The vertebral plexuses are continuous with the dorsal venous system in vertebrates.
The vertebral venous plexus becomes evident in all vertebrates. But its appearance seems to play an important role of cranial venous drainage in the upright vertebrates. Evidence is found that the vertebral venous plexus often plays a dominant role in cerebral venous drainage depending upon body posture24, especially in man25,26. Standing position leads to a collapse of the internal jugular vein, and opening of the vein with increasing central venous pressure.
On the other hand, blood flow in the vertebral veins is negligible in supine position, but surpasses the internal jugular flow at 45 degrees. At standing baseline the cerebral outflow pathway is predominantly through the vertebral venous plexus, but the flow is redirected through the internal jugular vein with increasing central venous pressure. Negative epidural pressure and non-collapsed epidural veins may facilitate venous outflow of the brain through the vertebral venous plexus in the upright position and compensate for a cessation of the jugular flow.
Extracranial venous systems
Diploic veins
The evidence of diploic veins can be found in only few literature reports. These veins play a role in connecting extracranial veins or plexuses with the intracranial dural venous sinus. The appearance of the diploic veins in domestic animals varies among species but the arrangement seems to be similar6. For a descriptive example of diploic veins found in dogs, they comprise the rostral or frontal, middle or parietal, and caudal or occipital groups.
The frontal group connects the angular vein of the eye and opens caudally into either the “dorsal sagittal sinus” or a “dorsal cerebral vein” (a counterpart of cerebral vein in man). The second group anastomoses with the occipital diploic vein and empties into the mid-dorsal sagittal sinus. The last group anastomoses with the parietal diploic vein and enters the transverse sinus. They form a collateral circulation between the sagittal sinus and the transverse sinus5.
Conclusions
Classifications and variations of the cerebral venous system are reported in the literature. Most of them depend on human anatomy rather than the comparative anatomy among vertebrates. The knowledge of how the venous systems develop from vertebrates series can widen our scope of education. The major defect of this article series is the scant descriptive data possibly due to a lack of interest in venous system evolution.
References
- 1.Cecon S, Minnich B, Lametschwandtner A. Vascularization of the Brains of the Atlantic and Pacific Hagfishes, Myxine glutinosa and Eptatretus stouti: A Scanning Electron Microscope Study of Vascular Corrosion Casts. J Morphol. 2002;253:51–63. doi: 10.1002/jmor.1112. [DOI] [PubMed] [Google Scholar]
- 2.Roofe PG. The Endocranial Blood Vessels of Amblystoma Tigerinum. J Comp Neurol. 1934;61(2):257–293. [Google Scholar]
- 3.Schepers GWH. The Blood Vascular System of the Brain of Testudo Geometrica. J Anat. 1939;73(pt3):451–495. [PMC free article] [PubMed] [Google Scholar]
- 4.Majewska-Michalska E. Vascularization of the brain in guinea pig. I: Gross anatomy of the arteries and veins. Folia Morphol (Warsz) 1994;53(4):249–268. [PubMed] [Google Scholar]
- 5.Armstrong LD, Horowitz A. The Brain Venous System of the Dog. Am J Anat. 1971;132(4):479–490. doi: 10.1002/aja.1001320406. [DOI] [PubMed] [Google Scholar]
- 6.Ghoshal NG, Koch T, Popesko P. The Venous Drainage of the Domestic Animals. W.B. Saunders Company; 1981. Veins of the head and neck and thoracic wall and thoracic cavity; pp. 39–93. [Google Scholar]
- 7.Lake AR, Van Niekerk I, et al. Angiology of the brain of the baboon Papio ursinus, the vervet monkey Cercopithecus pygerithrus, and the bushbaby Galago senegalensis. Am J Anat. 1990;187(3):277–286. doi: 10.1002/aja.1001870307. [DOI] [PubMed] [Google Scholar]
- 8.Madeira MC, Watanabe IS. Anatomical data on the intracranial venous drainage of the tufted capuchin, Cebus apella Linnaeus, 1758. Anat Rec. 1975;183(4):589–598. doi: 10.1002/ar.1091830409. [DOI] [PubMed] [Google Scholar]
- 9.Lasjaunias P, terBrugge KG, Berenstein A. Clinical Vascular anatomy and Variations. 2nd ed. Vol 1. Springer; 2001. Surgical Neuroangiography. [Google Scholar]
- 10.Padget DH. The development of the cranial venous system in man from the viewpoint of comparative anatomy. Contrib Embryol. 1957;36:81–151. [Google Scholar]
- 11.Miquel MA, Mateu JMD, et al. A Hakuba. Vol. 1996. Tokyo: Editor Springer; Embryogenesis of the Veins of the Posterior Fossa: An Overview, in Surgery of the Intracranial Venous System; pp. 14–25. [Google Scholar]
- 12. Cserr HF, Bundgaard M, et al. On the anatomic relation of the choroid plexus to brain: a comparative anatomy. Am J Physiol. 1980;238(1):R76–R81. doi: 10.1152/ajpregu.1980.238.1.R76. [DOI] [PubMed] [Google Scholar]
- 13.Nakao T. Electron Microscopic Studies on the Lamprey Meninges. J Comp Neurol. 1979;183:429–454. doi: 10.1002/cne.901830213. [DOI] [PubMed] [Google Scholar]
- 14.Kuhlenbeck H. Morphologic Pattern of the Vertebrate Neuraxis, in The central nervous system of vertebrates: a general survey of its comparative anatomy with an introduction to the pertinent fundamental biologic and logical concepts. New York: S. Karger Editor; 1897. pp. 668–728. [Google Scholar]
- 15.Sarna HB, et al. ed Evolution of the Nervous System. New York: Oxford University Press; 1974. [Google Scholar]
- 16.Kurz H, et al. First Blood Vessels in the Avian Neural Tube Are Formed by a Combination of Dorsal Angioblast Immigration and Ventral Sprouting of Endothelial Cells. Dev Biol. 1996;173:133–147. doi: 10.1006/dbio.1996.0012. [DOI] [PubMed] [Google Scholar]
- 17.Lasjaunias P, terBrugge KG, Berenstein A. Surgical Neuroangiography. Clinical and Interventional Aspects in Children. 2nd ed. Vol 3. Springer; 2006. [Google Scholar]
- 18.Truex RC, Carpenter MB. Human Neuroanatomy. 6th ed. Baltimore: The Williams & Wilkins Company; 1971. [Google Scholar]
- 19.Tureen LL. Circulation of the spinal cord and the effect of vascular occlusion. Res Publ Ass Res Nerv Ment Dis. 1938;18:394–437. [Google Scholar]
- 20.Rhoton AJ. The posterior fossa veins. Neurosurgery . 2000;47(3 Sup):S69–92. doi: 10.1097/00006123-200009001-00012. [DOI] [PubMed] [Google Scholar]
- 21.Wackenheim A, Braun JP. ed The Veins of the Posterior Fossa: Normal and Pathologic Findings. Berlin, Heidelberg, New York: Springer-Verlag; 1978. [Google Scholar]
- 22.Duvernoy HM. ed Human Brain Stem Vessels. 2nd ed . Berlin, Heidelberg: Springer; 1999. [Google Scholar]
- 23.Smeets WJ, Marín O, González A. Evolution of the basal ganglia: new perspectives through a comparative approach. J Anat. 2000;196:501–517. doi: 10.1046/j.1469-7580.2000.19640501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zippel KC, Lillywhite HB, Mladinich CR. New Vascular System in Reptiles: Anatomy and Postural Hemodynamics of the Vertebral Venous Plexus in Snakes. J Morphol. 2001;250:173–184. doi: 10.1002/jmor.1063. [DOI] [PubMed] [Google Scholar]
- 25.Valdueza JM, von Münster T, et al. Postural Dependency of the Cerebral Venous Outflow. Lancet. 2000;335(9199):200–201. doi: 10.1016/s0140-6736(99)04804-7. [DOI] [PubMed] [Google Scholar]
- 26.Gisolf J, van Lieshout JJ, et al. Human Cerebral Venous Outflow Pathway Depends on Posture and Central Venous Pressure. J Physiol. 2004;560(Pt 1):317–327. doi: 10.1113/jphysiol.2004.070409. [DOI] [PMC free article] [PubMed] [Google Scholar]