Summary
As endovascular surgery (EVS) of symptomatic unruptured aneurysms can result in symptom exacerbation due to intra-aneurysmal thrombosis or lump formation by coils, this treatment remains controversial.
We present five women ranging in age from 58 to 76 years (mean 65.6 years) who suffered post-EVS symptom exacerbation attributable to local inflammation.
The aneurysms measured from 8 to 25 mm (mean 19 mm) and were located at the cavernous portion in four patients and at the origin of the ophthalmic artery in one. All underwent endosaccular embolization under local anesthesia. Immediately after embolization, 24 h anti-coagulation therapy was started via the continuous injection of heparin; they also received anti-platelet therapy.
At one to three days post-EVS, all five patients manifested worsening of their cranial nerve symptoms.
In three other patients the symptoms were improved after EVS. We posit that inflammation induced by coil embolization may worsen cranial nerve symptoms transiently.
Our findings suggest that post-EVS follow-up is necessary and that patients exhibiting an inflammatory reaction be treated with anti-inflammatory drugs.
Key words: aneurysms, inflammation, coiling, complications
Introduction
Endovascular surgery (EVS) of symptomatic unruptured aneurysms exerting mass effect on the cranial nerves remains controversial1-3. EVS can result in symptom exacerbation due to intra-aneurysmal thrombosis or mass effect by the coils. Elsewhere we reported the efficacy of EVS for symptomatic unruptured aneurysms4. To avoid symptom exacerbation, we insert coils tightly into the aneurysmal neck and less tightly on the dome side. We use anti-coagulation therapy to avoid thrombosis.
The immediate treatment outcome in some of our patients subjected to EVS was not favorable. Closer examination showed that their complications were related to post-EVS inflammation. To our knowledge, the relationship between EVS for intracranial aneurysms and local inflammation due to intra-aneurysmal thrombosis has not been documented previously. Here we report the occurrence of inflammation post-EVS for symptomatic intracranial aneurysms. We assessed the inflammatory reaction by counting the number of white blood cells (WBC) and by comparing the level of hypersensitive C-reactive protein (hs-CRP) before and one and seven days after the operation. We used these data to evaluate the relationship between postoperative symptom exacerbation and inflammatory reaction.
Material and Methods
Between April 1992 and April 2007 we treated 26 patients with unruptured internal carotid artery (ICA) aneurysms with cranial nerve palsy. Here we retrospectively evaluated five patients whose symptoms worsened after EVS. All were women aged from 58 to 76 years (mean 65.6 years). The aneurysms were located at the cavernous portion of the ICA in four patients and at the origin of the ophthalmic artery in one. The lesions measured from 8 to 25 mm (mean 19 mm). Details on the five patients are summarized in the table.
Table.
Summary of five patients with post-EVS symptom exacerbation.
| Case | Age/ | Location | Size of An | EVS | Result | Final | Timing of CNS |
|---|---|---|---|---|---|---|---|
| Sex | of An | (mm) | of EVS | CNS | worse (day) | ||
|
| |||||||
| 1 | 58F | RT IC cavernius | 17 | ESE | Neck remnant | Improvement | post EVS 3 |
|
| |||||||
| 2 | 60F | RT IC-Opthalmic | 25 | ESE | Neck remnant | No change | 2 |
|
| |||||||
| 3 | 74F | LT IC cavernius | 8 | ESE | Neck remnant | Improvement | 2 |
|
| |||||||
| 4 | 76F | RT IC cavernius | 25 | PAO | Complete | No change | 2 |
|
| |||||||
| 5 | 60F | RT IC cavernius | 20 | ESE | Complete | Improvement | 1 |
|
| |||||||
| EVS: endovascular surgery, ESE: endosaccular embolization, PAO: parent artery occlusion, CNS: cranial nerve symptoms | |||||||
All embolization procedures were performed under local anesthesia. All patients received a systemic infusion of heparin with a bolus of 2000 U, followed by the continuous injection of 200 U/min. The activated coagulation time (ACT) was maintained at more than twice the control value. A microcatheter was carefully inserted into the aneurysm over a guide wire and the aneurysms were embolized with detachable coils. To avoid post-EVS symptom exacerbation, the coils were inserted tightly into the aneurysmal neck and more loosely into the dome. Appropriate embolization was confirmed angiographically and the procedure was finished. Post-embolization we delivered anti-coagulation therapy via the 24 h continuous infusion of heparin; anti-platelet therapy consisted of the oral administration of aspirin and ticlopidine.
We evaluated post-EVS cranial nerve symptoms and signs. All patients were followed by magnetic resonance (MR) angiography and thin-slice MR imaging. To assess the inflammatory reaction we counted the number of WBC and compared the hs-CRP levels before and one and seven days after the operation. To evaluate the relationship between post-EVS symptom changes and inflammatory reaction we compared data obtained on five patients with and three patients without post-EVS symptom exacerbation.
Results
We performed endosaccular embolization in four patients, the other patient underwent parent artery occlusion. Complete occlusion was achieved in two patients; in three there was a neck remnant. All five patients suffered post-treatment worsening of their cranial nerve symptoms; two patients each reported exacerbated optic nerve, oculomotor nerve, and abducens palsy. Worsening of the symptoms occurred at one to three days after the EVS procedure. Symptom improvement was achieved in three patients.
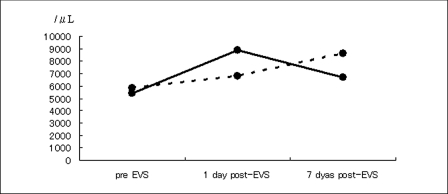
Before EVS, the mean number of WBC and the mean hs-CRP level was 5,400/ µL (range 4,600-7,000/µL) and 140 µg/dL (range 46-364 µg/dL), respectively. One and seven days post-EVS these values were 8,860/µL (range 7,100-10,600/µL) and 6,660/µL (range 520-7,600/µL), and 1,678 µg/dL (range 220-4,600 µg/dL) and 430 µg/dL (range 30-1,443 µg/dL), respectively. These findings indicate marked post-EVS inflammatory reaction. In three other patients without post-EVS symptom exacerbation the mean number of WBC before and one and seven days after EVS was 5,833/µL (range 5,200-6,200/µL), 6,766/µL (range 6,100-6,766/µL), and 8,633/µL (range 6,200-10,400/µL); the mean level of hs-CRP was 58 µg/dL (range 36-100 µg/dL), 113 µg/dL (range 53-221 µg/dL), and 67 µg/dL (range 30-139 µg/dL).
As shown in figure 1A,B, post-EVS, the five patients with symptom exacerbation manifested a greater degree of inflammatory reaction than the patients without. After EVS, the body temperature of the five patients rose by range 0.8-3.0oC. One patient manifested an increase in the number of eosinocytes.
Figure 1.
Post-EVS inflammatory reaction as determined by the number of WBC (A) and the hs-CRP level (B) in five patients with and three patients without symptom exacerbation. Solid line: patients without exacerbation; dotted line: patients with exacerbation.
A.
B.
Case Presentation
Case 1
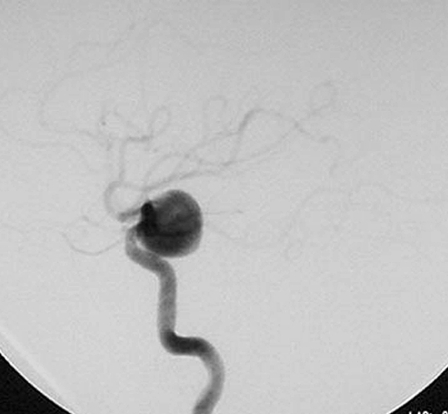
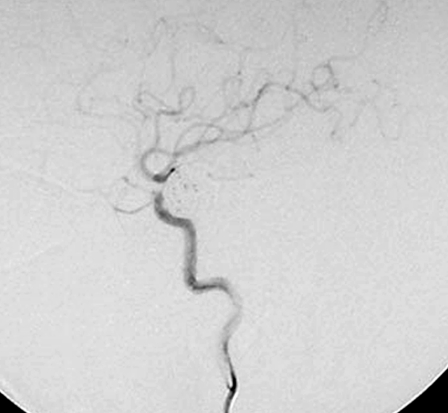
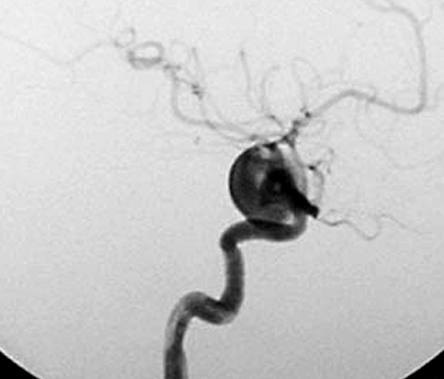
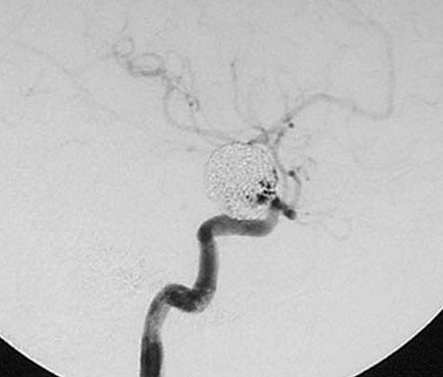
This 58-year-old woman had a four month history of progressive visual impairment. At admission she manifested pollinosis. MRI showed an aneurysm in the cavernous portion of the ICA. Angiography demonstrated that it was 17 mm in diameter (figure 2A). She was started on anti-platelet drugs and underwent EVS under local anesthesia. At the start of the procedure she received a heparin bolus of 2,000 U; during the procedure we infused heparin systemically (200 U/min). Her activated coagulation time (ACT) was maintained at more than twice the control value. EVS was via the right femoral artery using an ENVOY guiding catheter (6-Fr; Cordis/ Johnson & Johnson, Miami, FL, USA). An Excelsior 1018 microcatheter (Boston Scientific, Natick, MA, USA) was introduced into the aneurysm over the guide wire and the aneurysm was embolized using 18 Guglielmi detachable coils (GDC; Boston Scientific). A post-embolization angiogram showed inflow into the aneurysm via a neck remnant (figure 2B). Systemic heparin infusion was continued during the first 24h after EVS.
Figure 2.
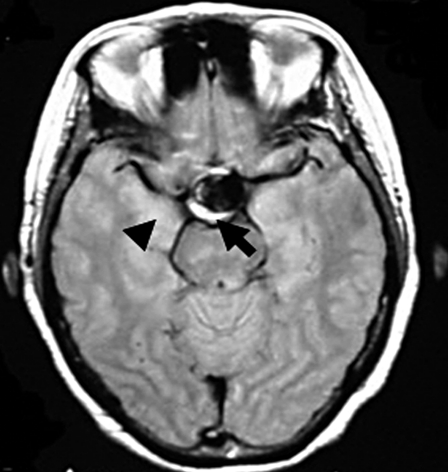
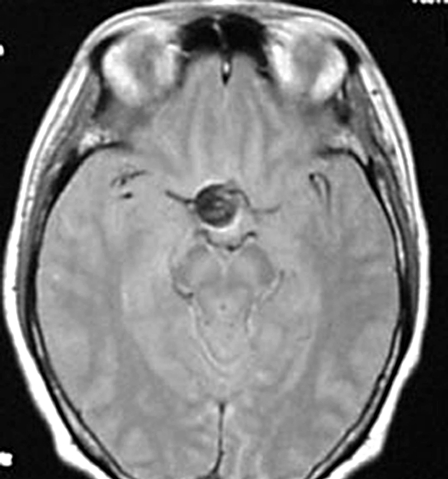
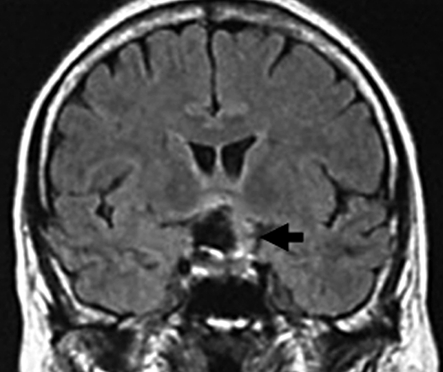
A) Pre-embolization angiograph showing a large intracranial aneurysm at the left internal carotid artery. B) Post-embolization angiograph showing inflow into the aneurysm via a neck remnant. C) MR images obtained 7 days post-EVS showing intra-aneurysmal thrombosis (arrow) and brain edema around the aneurysm (arrowhead). D) MR images obtained two years post-EVS showing disappearance of intra-aneurysmal thrombosis.
A.
B.
C.
D.
One day post-EVS her left visual acuity used by Landolt ring decreased from 0.07 to 0.06, her temperature was 38°C, her WBC rose from 5,300 to 10,600/µL, and her hs-CRP level from 98 to 220 µg/dL. Steroid administration led to fever alleviation and her WBC and hs-CRP fell to 7,600/µL and 31 µg/dL, respectively, by the seventh post-EVS day. At that time her left eyesight was 0.04. Post-EVS MRI disclosed intra-aneurysmal thrombosis (figure 2C) and brain edema around the aneurysmal site (figure 2C). On MRI scans obtained years years later there was no evidence of intra-aneurysmal thrombosis (figure 2D) and her left visual acuity was 0.1.
Case 2
At a clinical survey, this 60-year-old woman was found to have a decrease in her bilateral eyesight and a homonymous quadrantic hemianopia. MRI performed at admission showed an aneurysm at the ophthalmic portion of the ICA. There was no history of allergic disease. Angiography demonstrated an aneurysm measuring 25 mm in diameter at the ophthalmic portion of the ICA (figure 3A). She was started on anti-platelet drugs and underwent EVS under local anesthesia. After the delivery of a 2,000 U bolus, she received a continuous infusion of 200 U/min heparin. Her ACT was maintained at more than twice the control value. EVS was via the right femoral artery using an ENVOY guiding catheter (6-Fr; Cordis/ Johnson & Johnson). An Excelsior 1018 microcatheter (Boston Scientific, Natick, Massachusetts, USA) was introduced into the aneurysm over the guide wire and the aneurysm was embolized using 22 GDC (Boston Scientific) and TRUFILL detachable coils (Cordis/ Johnson & Johnson). A post-embolization angiograph showed inflow into the aneurysm via a neck remnant (figure 3B). We continued the systemic infusion of heparin during the first 24 h after EVS.
Figure 3.
A) Pre embolization angiograph showing an internal carotid artery-ophthalmic artery aneurysm. B) Post-EVS angiograph showing inflow into the aneurysm via the neck remnant. C) MR images obtained 10 days post-EVS showing intra-aneurysmal thrombosis (arrow).
A.
B.
C.
Two days after EVS her right and left visual acuity used by Landolt ring decreased from 1.0 to 0.7 and from 0.8 to 0.5, respectively and the homonymous quadrantic hemianopia increased to 1/2. Her temperature was 38°C, her WBC rose from 7,000 to 7,900/ µL and her hs-CRP from 35 to 645 µg/dL. The administration of heparin and steroids resulted in fever alleviation and a decrease in the WBC and hs-CRP level to 7,400/µL and 37 μg/dL, respectively, at ten days post-EVS. Her bilateral eyesight was 0.3 at that time. Post-EVS MRI showed intra-aneurysmal thrombosis (figure 3C). At three months post-EVS her left and right visual acuity was 0.9 and 0.01, respectively. Her visual field defect did not improve.
Discussion
The cranial nerve symptoms in patients with unruptured intracranial aneurysms are due to mass effect or water hammer injury by pulsatile motion. The purpose of treatment is to remove the mechanical pressure the aneurysm exerts on the cranial nerves and to stop its pulsation. Although aneurysmal clipping serves this purpose, it may be complicated by morphological or anatomical factors. Therefore, EVS is now an accepted modality to treat intracranial aneurysms.
Although EVS effectively addresses symptomatic unruptured intracranial aneurysms, this treatment remains controversial because it may result in symptom exacerbation due to intra-aneurysmal thrombosis or mass effect attributable to the inserted coils. On the other hand, unlike clipping surgery, EVS avoids direct iatrogenic injury to the brain. Elsewhere we reported the efficacy of EVS for symptomatic unruptured aneurysms4: cranial nerve symptoms were completely resolved in 46% of our patients and 33% manifested some improvement.
Stiver et Al5 reported intra-aneurysmal changes after endosaccular embolization. Initially, inflammatory cells migrate into the aneurysm wall and later the center of the aneurysm contains organized fibrous tissue with newly formed capillaries. They encountered no whole-body inflammation attributable to uncoated coils. Guglielmi et Al6 and Suzuki et Al7 examined the efficacy of electrical thrombosis using uncoated coils. In experimental aneurysm models, Dawson et Al8 and Murayama et Al9 used collagen-coated platinum- and Matrix coils to elicit a local inflammatory reaction to increase the aneurysmal occlusion rate. However, the results were not satisfactory. The reaction elicited by uncoated platinum and tungsten coils was found to be different10.
The five patients presented here suffered post-EVS symptom exacerbation due to acute intra-aneurysmal thrombosis and manifested increased WBC and hs-CRP levels one day post-embolization. As expected, the embolization elicited an inflammatory reaction in the aneurysm and in tissue around the aneurysm. Allergic reactions to the inserted coils and intra-aneurysmal thrombosis due to local blood coagulation may be causative factors in patients exhibiting strong inflammatory reactions post-EVS. According to Takahashi et Al11 the lymphocyte transformation test is more useful than the patch test to assess allergies to nickel, cobalt, palladium, gold, chromium, and mercury and although platinum coils are thought not to elicit strong allergic reactions, this issue needs further study.
Kang et Al12 studied hs-CRP as a marker of inflammation and reported a relationship between hs-CRP levels and the degree of vascular atherosclerosis. Based on our findings we suggest that patients with increased hs-CRP levels post-EVS require the administration of anti-inflammatory drugs, e.g. steroids, and anti-allergic drugs in addition to anti-coagulation drugs.
Conclusions
As the inflammatory reaction elicited by aneurysmal coil embolization may result in the exacerbation of cranial nerve symptoms due to aneurysmal mass effect, patients treated by EVS require the administration of anti-inflammatory drugs in addition to anti-coagulants.
References
- 1.Halbach VV, Higashida RT, et al. The efficacy of endosaccular aneurysm occlusion in alleviating neurological deficits produced by mass effect. J Neurosurg. 1994;80:659–666. doi: 10.3171/jns.1994.80.4.0659. [DOI] [PubMed] [Google Scholar]
- 2.Tsuura M, Terada T, et al. Magnetic resonance signal intensity and volume changes after endovascular treatment of intracranial aneurysms causing mass effect. Neuroradiology. 1998;40:184–188. doi: 10.1007/s002340050565. [DOI] [PubMed] [Google Scholar]
- 3.Kim DJ, Kim DI, et al. Unruptured aneurysms with cranial nerve symptoms: Efficacy of endosaccular Guglielmi detachable coil treatment. Korean J Radiol. 2003;4(3):141–145. doi: 10.3348/kjr.2003.4.3.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suzuki S, Kurata A, et al. Efficacy of endovascular surgery for unruptured internal carotid artery aneurysms presenting with cranial nerve symptoms. Interventional Neuroradiology. 2007;13(Sup 1):163–169. doi: 10.1177/15910199070130S125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stiver SI, Porter PJ, et al. Acute human histopathology of an intracranial aneurysm treated using Guglielmi detachable coils: Case report and review of literature. Neurosurg. 1998;43:1203–1208. doi: 10.1097/00006123-199811000-00106. [DOI] [PubMed] [Google Scholar]
- 6.Guglielmi G, Viñuela F, et al. Electrothrombosis of saccular aneurysms via endovascular approach. 1: Electrochemical basis, technique, and experimental results. J Neurosurg. 1991;75:1–7. doi: 10.3171/jns.1991.75.1.0001. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki S, Kurata A, et al. Experimental determination of minimal stimulation current and period for electrical thrombosis in dogs. Interventional Neuroradiology. 2004;10:225–230. doi: 10.1177/159101990401000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dawson RC, Kirisht AF, et al. Treatment of experimental aneurysms using collagen-coated microcoils. Neurosurgery. 1995;36:133–140. doi: 10.1227/00006123-199501000-00017. [DOI] [PubMed] [Google Scholar]
- 9.Murayama Y, Viñuela F, et al. Cellular responses of bioabsorbable polymeric material and Guglielmi detachable coil in experimental aneurysms. Stroke. 2002;33:1120–1128. doi: 10.1161/01.str.0000014423.20476.ee. [DOI] [PubMed] [Google Scholar]
- 10.Byrne JV, Hope KA, et al. The nature of thrombosis induced by platinum and tungsten coils in saccular aneurysms. Am J Neuroradiol. 1997;18:29–33. [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi S, Kawashima J, et al. Lymphocyte transformation test in comparison with patch test using nickel, cobalt, palladium, gold, chromium and mercury. Environ Dermatol. 2003;10:64–69. [Google Scholar]
- 12.Kang WQ, Song DL, Guo XG. Relationship between serum vasoactive factor and plaque morphology in patients with acute coronary syndrome. Zhonghua Xin Xue Guan Bing Za Zhi. 2007;35:1020–1023. [PubMed] [Google Scholar]