Abstract
Kidney stone is a common clinical problem faced by clinicians. The prevalence of the disease is increasing worldwide. As the affected population is getting younger and recurrence rates are high, dietary modifications, lifestyle changes, and medical management are essential. Patients with recurrent stone disease need careful evaluation for underlying metabolic disorder. Medical management should be used judiciously in all patients with kidney stones, with appropriate individualization. This chapter focuses on medical management of kidney stones.
Keywords: Calcium oxalate, kidney stones, renal stone
INTRODUCTION
Urinary tract stones (urolithiasis) are known to the mankind since antiquity.[1] Kidney stone is not a true diagnosis; rather it suggests a broad variety of underlying diseases. Kidney stones are mainly composed of calcium salts, uric acid, cysteine, and struvite. Calcium oxalate and calcium phosphate are the most common types accounting for >80% of stones, followed by uric acid (8–10%) and cysteine, struvite in remainders.
The incidence of urolithiasis is increasing globally, with geographic, racial, and gender variation in its occurrence. Epidemiological study (1979) in the western population reveals the incidence of urolithiasis to be 124 per 100,000 in males and 36 per 100,000 in females.[2] The lifetime risk of having urolithiasis is higher in the Middle East (20–25%) and western countries (10–15%) and less common in Africans and Asian population. Stone disease carries high risk of recurrence after the initial episode, of around 50% at 5 years and 70% at 9 years.[3,4]
Positive family history of stone disease, young age at onset, recurrent urinary tract infections (UTIs), and underlying diseases like renal tubular acidosis (RTA) and hyperparathyroidism are the major risk factors for recurrence. High incidence and recurrence rate add enormous cost and loss of work days.[1]
Though the pathogenesis of stone disease is not fully understood, systematic metabolic evaluation, medical treatment of underlying conditions, and patient-specific modification in diet and lifestyle are effective in reducing the incidence and recurrence of stone disease.[5–8]
TYPES OF STONE DISEASES AND RISK FACTORS
Kidney stones are made of organic and inorganic crystals amalgamated with proteins. Calcium stones are the most frequent type, accounting for up to 80% [Table 1]. Of these, calcium oxalate, calcium phosphate, struvite, and cystine are radio-opaque stones, while uric acid, xanthine, and hypoxanthine stones are radiolucent. The risk factors for stone disease are as shown in Table 2.
Table 1.
Types of stones
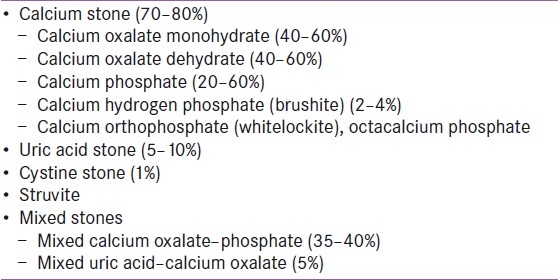
Table 2.
Risk factors for stone disease

MEDICAL MANAGEMENT OF STONE DISEASE
Management of stone disease needs individualization. Clinical presentation, proper history, and laboratory tests help to identify whether one needs urgent surgical or medical treatment.
Medical management is indicated for clinically stable patients with non-obstructive urinary stones, recurrent stone formers, and the patients with underlying systemic diseases. Detailed history of patient illness including family history, drug history, and history of previous similar illness and previous interventions needs to be recorded. Assessment of risk factors for stone disease [Table 2] should be carried out. Medical management of stone disease includes laboratory evaluation and treatment. As the laboratory evaluation of renal calculi has been discussed by Ranabir, Baruah and Ritu Devi in this issue,[8] we will focus on medical treatment.
Medical treatment of kidney stones includes dietary management, disease-specific therapies, and medical expulsion therapy (MET) of stones.
Dietary management
Fluid intake and dietary changes are important measures in preventing recurrence of kidney stones. Many trials have shown that increasing urine volume to at least 2 L/day OR 2 lit/day can reduce the recurrence of stone disease by up to 40–50%.[9] Fluid intake mainly should include water. As tea and coffee contain oxalate, milk (which binds free oxalate) should be added to them. However, increasing the urine volume has a disadvantage of reducing urinary citrate.
A small reduction in urinary oxalate has been found to be associated with significant reduction in the formation of calcium oxalate stones; hence, oxalate-rich foods like cucumber, green peppers, beetroot, spinach, soya bean, chocolate, rhubarb, popcorn, and sweet potato should be avoided. Many studies have found calcium restriction to increase the risk of stone disease; therefore, dietary calcium restriction is not recommended.
Hypocitraturia is a proven risk factor for stone formation and is found in about 16–63% of calcium stone formers.[10,11] Oral potassium citrate (Kcit) has been shown to be useful in increasing urinary citrate and reducing the stone recurrence. Dietary replacement with high citrate as a substitute to Kcit has been studied. Lemon juice when delivered as a lemonade therapy was found to increase urinary citrate.[12] Odvina found orange juice to increase urine pH and urinary citrate.[13] Kang et al. compared 11 patients taking Kcit with 11 matched patients on lemonade therapy and found both the therapies to increase urinary citrate. However, the effect with Kcit was significantly better than the effect with lemonade.[14] Yilmaz et al. studied tomato, orange, lemon, and mandarin juice for nutritional content.[15] Surprisingly, fresh tomato juice was found to have the highest citrate and low oxalate content. Reduction of animal protein intake is suggested in both calcium oxalate and uric acid stone formers.
Stone-specific therapies
Calcium oxalate stones
In patients with idiopathic hypercalciuria, thiazide diuretics have shown to reduce the recurrence rates by up to 70%.[9] It is the only medical therapy directed at reducing urinary calcium.[16] Citrate supplements as detailed earlier are useful. Pyridoxine sometimes can be useful in patients with primary hyperoxaluria, but not in idiopathic hyperoxaluria.[17] Oxalobacter formigenes is an oxalate degrading bacterium found in human gastrointestinal tract. It is thought that increased colonization of the gut might lead to decreased absorption of dietary oxalate and decrease in urinary oxalate excretion. Colonization with O. formigenes showed benefit in uncontrolled studies;[18,19] however, a prospective, randomized, placebo control, double-blind trial refuted such benefits.[20]
Uric acid stones
The aim of treatment in uric acid stones is to increase the solubility of uric acid in urine. It is achieved by increasing the urine volume and by alkali therapy. Allopurinol is a useful adjunct to the therapy.
Struvite stones
Struvite stones form in alkaline urine from infection with urea-splitting microorganisms. Antibiotics are the mainstay of the therapy with occasional use of acetohydroxamic acid.[17]
Cystine stones
This is a rare stone type. The aim of treatment is to reduce the concentration of free cystine and increase its solubility in urine. A high fluid intake up to 4-5 L/day OR 4-5 lit/day and alkalinization of urine with target urine pH >7 is desirable. Chelating agents like D-penicillamine or tiopronin are indicated when 24-hour urine cystine concentration exceeds 2000 μmol/l.[17]
General stone-expulsive therapies
MET is treatment with combination of drugs which facilitates the spontaneous passage of ureteric calculi. Ureteric colic is an emergency and management depends upon the severity of obstruction and degree of renal function deterioration. Approximately 90% of stones <5 mm and 15% of stones between 5 and 8 mm pass spontaneously within 4 weeks, while 95% of those larger than 8 mm require urological intervention.[21] The American Urology Association Nephrolithiasis Clinical Guidelines panel in its recent guidelines have found MET to facilitate and accelerate the spontaneous passage of ureteric stones and the stone fragments generated by shock wave lithotripsy.[22]
Smooth muscles of lower ureter innervated by alpha adrenoreceptors and abundance of calcium channels, which on stimulation causes peristalsis of ureter, maintain basal tone. Calculus in distal ureter causes ureteric spasm and increases contraction by activating these receptors. Mechanical effects include submucosal edema and associated inflammation. Combination therapy used includes alpha adrenoreceptor blockers, calcium channel blockers, corticosteroid, analgesics, and hydration.
In a recent prospective, randomized study which compared three drugs as MET for distal ureteral calculi, patients with symptomatic distal ureteral stones >4 mm were randomly assigned to three treatment groups : p0 hloroglucinol and corticosteroid, tamsulosin and corticosteroid, and nifedipine and corticosteroid.[23] Tamsulosin and corticosteroid was the most efficacious combination – stones passed more quickly and the need for analgesics was reduced.[23] A randomized, controlled, prospective study has also shown tamsulosin to be a useful addition to shock wave lithotripsy.[1,23]
Alpha adrenergic receptors are densely located in the smooth muscles of ureter. Alpha-1a-receptors predominate in bladder outlet, prostate, and proximal urethra, whereas alpha-1d-receptors are seen in lower ureter and detrussor muscle of bladder.[24] Drugs which block these receptors cause smooth muscle relaxation and inhibits peristalsis and relieves spasm.
Tamsulosin is effective in expulsion therapy as it increases the passage rate and reduces the passage time for stones up to 10 mm.[25,26] In a study of tamsulosin in renal colic, 100% expulsion rate was seen in the treatment group and 70% in the control group. The mean expulsion time in the treatment group was 65.7 h (range 2–288 h) compared with 111.1 h for the control group (range 12–240 h).[25] In this study, the mean stone size was 6.7 (SD 2.1) mm in the treatment group, compared with 5.8 mm in control subjects (P=0.047). A recent study using sustained release alfuzosin 10 mg once daily was found to be effective in enhancing the ureteral stone spontaneous passage rate, particularly for upper ureteral stones. The patients treated with alfuzosin required fewer analgesics and less surgical interventions like ureteroscopic lithotripsy and/or extracorporeal shock wave lithotripsy.[27]
Calcium channel blockers (CCBs) cause inhibition of calcium channels in distal ureter and decrease the contraction and spasm caused by distal ureter calculus.[28] A study of combination therapy with nifedepine and deflazacort in distal ureter stone resulted in stone expulsion in 79% of the treatment group and in only 35% of control subjects. The average stone size was 5.8 mm (range 3.5–10 mm) for the treatment group and 5.5 mm (range 3–10 mm) for the control group. CCBs can facilitate spontaneous passage for stones up to 10 mm, though no correlation was found between stone size and expulsion time in the treatment group.[29]
A comparative study between nifedepine and tamsulosin reveals a better stone expulsion rate of 97.1% in tamsulosin versus 77.1% in nifedepine group. Average expulsion times were 72 h and 120 h (P<0.001) in the two groups, respectively, despite the presence of larger stones in the tamsulosin group (tamsulosin 7.2 mm vs. nifedepine 6.2 mm; P=0.002).[21,29,30]
Steroids are also found to be useful as medical expulsive agents in distal ureteric stones. Calculus in distal ureter causes inflammation and submucosal edema which further aggravates the obstruction due to the stone per se. Being anti-inflammatory agents, steroids reduce the inflammation and neutrophil-induced damage. This class of drug, in combination with other agents described earlier, improves stone passage and reduces stone expulsion time.[30,31]
Patients receiving MET, who do not pass their stones within 4 weeks, should be reffered to a urologist since delay in definitive management may increase the rate of complications, including renal dysfunction, urosepsis, and intractable pain.[32] Side effect profile should be considered before initiation, and during therapy.
CONCLUSIONS
Kidney stones present as an important and challenging clinical problem. Medical therapy, when used judiciously in conjunction with dietary measures, can help in preventing recurrence and in expulsion of small size (<10 mm) stones. Awareness of the advantages and limitations of different modalities of medical therapy is necessary in order to provide the correct treatment to pateints presenting with this common complaint.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Miller NL, Lingeman JE. Management of kidney stones. BMJ. 2007;334:468–72. doi: 10.1136/bmj.39113.480185.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson CM, Wilson DM, O’Fallon WM, Malek RS, Kurland LT. Renal stone epidemiology : A0 25-year study in Rochester, Minnesota. Kidney Int. 1979;16:624–31. doi: 10.1038/ki.1979.173. [DOI] [PubMed] [Google Scholar]
- 3.Williams RE. Long term survey of 538 patients with upper urinary tract stone. Br J Urol. 1963;35:416–37. [PubMed] [Google Scholar]
- 4.Prezioso D, Di Martino M, Galasso R, Iapicca G. Laboratory assessment. Urol Int. 2007;79(Suppl 1):20–5. doi: 10.1159/000104437. [DOI] [PubMed] [Google Scholar]
- 5.Lotan Y, Cadeddu JA, Pearle MS. International comparison of cost effectiveness of medical management strategies for nephrolithiasis. Urol Res. 2005;33:223–30. doi: 10.1007/s00240-005-0463-9. [DOI] [PubMed] [Google Scholar]
- 6.Heilberg IP, Schor N. Renal stone disease : C0 auses, evaluation and medical treatment. Arq Bras Endocrinol Metabol. 2006;50:823–31. doi: 10.1590/s0004-27302006000400027. [DOI] [PubMed] [Google Scholar]
- 7.Levy FL, Adams-Huet B, Pak CY. Ambulatory evaluation of nephrolithiasis : A0 n update of a 1980 protocol. Am J Med. 1995;98:50–9. doi: 10.1016/S0002-9343(99)80080-1. [DOI] [PubMed] [Google Scholar]
- 8.Ranabir S, Baruah M, Ritu Devi K. Nephrolitiasis: Endocrine evaluation. Indian J Endocrinol Metab. 2012 doi: 10.4103/2230-8210.93740. [In Press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borghi L, Meschi T, Amato F, Briganti A, Novarini A, Giannini A. Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis : A0 5-year randomized prospective study. J Urol. 1996;155:839–43. [PubMed] [Google Scholar]
- 10.Hamm LL, Hering-Smith KS. Pathophysiology of hypocitraturic nephrolithiasis. (8).Endocrinol Metab Clin North Am. 2002;31:885–93. doi: 10.1016/s0889-8529(02)00031-2. [DOI] [PubMed] [Google Scholar]
- 11.Pak CY. Medical management of urinary stone disease. Nephron Clin Pract. 2004;98:c49–53. doi: 10.1159/000080252. [DOI] [PubMed] [Google Scholar]
- 12.Seltzer MA, Low RK, McDonald M, Shami GS, Stoller ML. Dietary manipulation with lemonade to treat hypocitraturic calcium nephrolithiasis. J Urol. 1996;156:907–9. [PubMed] [Google Scholar]
- 13.Odvina CV. Comparative value of orange juice versus lemonade in reducing stone-forming risk. Clin J Am Soc Nephrol. 2006;1:1269–74. doi: 10.2215/CJN.00800306. [DOI] [PubMed] [Google Scholar]
- 14.Kang DE, Sur RL, Haleblian GE, Fitzsimons NJ, Borawski KM, Preminger GM. Long-term lemonade based dietary manipulation in patients with hypocitraturic nephrolithiasis. J Urol. 2007;177:1358–62. doi: 10.1016/j.juro.2006.11.058. discussion 1362; quiz 1591. [DOI] [PubMed] [Google Scholar]
- 15.Yilmaz E, Batislam E, Basar M, Tuglu D, Erguder I. Citrate levels in fresh tomato juice: A possible dietary alternative to traditional citrate supplementation in stone-forming patients. Urology. 2008;71:379–83. doi: 10.1016/j.urology.2007.08.065. discussion 383-4. [DOI] [PubMed] [Google Scholar]
- 16.Moe OW, Pearle MS, Sakhaee K. Pharmacotherapy of urolithiasis : E0 vidence from clinical trials. Kidney Int. 2011;79:385–92. doi: 10.1038/ki.2010.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johri N, Cooper B, Robertson W, Choong S, Rickards D, Unwin R. An update and practical guide to renal stone management. Nephron Clin Pract. 2010;116:c159–71. doi: 10.1159/000317196. [DOI] [PubMed] [Google Scholar]
- 18.Kaufman DW, Kelly JP, Curhan GC, Anderson TE, Dretler SP, Preminger GM, et al. Oxalobacter formigenes may reduce the risk of calcium oxalate kidney stones. J Am Soc Nephrol. 2008;19:1197–203. doi: 10.1681/ASN.2007101058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campieri C, Campieri M, Bertuzzi V, Swennen E, Matteuzzi D, Stefoni S, et al. Reduction of oxaluria after an oral course of lactic acid bacteria at high concentration. Kidney Int. 2001;60:1097–105. doi: 10.1046/j.1523-1755.2001.0600031097.x. [DOI] [PubMed] [Google Scholar]
- 20.Goldfarb DS, Modersitzki F, Asplin JR. A randomized, controlled trial of lactic acid bacteria for idiopathic hyperoxaluria. Clin J Am Soc Nephrol. 2007;2:745–9. doi: 10.2215/CJN.00600207. [DOI] [PubMed] [Google Scholar]
- 21.Liu M, Henderson SO. Myth: Nephrolithiasis and medical expulsive therapy. Can J Emerg Med. 2007;9:463–5. doi: 10.1017/s1481803500015529. [DOI] [PubMed] [Google Scholar]
- 22.Preminger GM, Tiselius HG, Assimos DG, Alken P, Buck C, Gallucci M, et al. 2007 guideline for the management of ureteral calculi. J Urol. 2007;178:2418–34. doi: 10.1016/j.juro.2007.09.107. [DOI] [PubMed] [Google Scholar]
- 23.Gravina GL, Costa AM, Ronchi P, Galatioto GP, Angelucci A, Castellani D, et al. Tamsulosin treatment increases clinical success rate of single extracorporeal shock wave lithotripsy of renal stones. Urology. 2005;66:24–8. doi: 10.1016/j.urology.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Cervenakov I, Fillo J, Mardiak J, Kopecny M, Smirala J, Lepies P. Speedy elimination of ureterolithiasis in lower part of ureters with the alpha 1-blocker–Tamsulosin. Int Urol Nephrol. 2002;34:25–9. doi: 10.1023/a:1021368325512. [DOI] [PubMed] [Google Scholar]
- 25.Dellabella M, Milanese G, Muzzonigro G. Efficacy of tamsulosin in the medical management of juxtavesical ureteral stones. J Urol. 2003;170:2202–5. doi: 10.1097/01.ju.0000096050.22281.a7. [DOI] [PubMed] [Google Scholar]
- 26.De Sio M, Autorino R, Di Lorenzo G, Damiano R, Giordano D, Cosentino L, et al. Medical expulsive treatment of distal-ureteral stones using tamsulosin: A single-center experience. J Endourol. 2006;20:12–6. doi: 10.1089/end.2006.20.12. [DOI] [PubMed] [Google Scholar]
- 27.Chau LH, Tai DC, Fung BT, Li JC, Fan CW, Li MK. Medical expulsive therapy using alfuzosin for patient presenting with ureteral stone less than 10 mm: A prospective randomized controlled trial. Int J Urol. 2011;18:510–4. doi: 10.1111/j.1442-2042.2011.02780.x. [DOI] [PubMed] [Google Scholar]
- 28.Borghi L, Meschi T, Amato F, Novarini A, Giannini A, Quarantelli C, et al. Nifedipine and methylprednisolone in facilitating ureteral stone passage : A0 randomized, double-blind, placebo-controlled study. J Urol. 1994;152:1095–8. doi: 10.1016/s0022-5347(17)32511-9. [DOI] [PubMed] [Google Scholar]
- 29.Porpiglia F, Destefanis P, Fiori C, Fontana D. Effectiveness of nifedipine and deflazacort in the management of distal ureter stones. Urology. 2000;56:579–82. doi: 10.1016/s0090-4295(00)00732-9. [DOI] [PubMed] [Google Scholar]
- 30.Dellabella M, Milanese G, Muzzonigro G. Randomized trial of the efficacy of tamsulosin, nifedipine and phloroglucinol in medical expulsive therapy for distal ureteral calculi. J Urol. 2005;174:167–72. doi: 10.1097/01.ju.0000161600.54732.86. [DOI] [PubMed] [Google Scholar]
- 31.Cooper JT, Stack GM, Cooper TP. Intensive medical management of ureteral calculi. Urology. 2000;56:575–8. doi: 10.1016/s0090-4295(00)00658-0. [DOI] [PubMed] [Google Scholar]
- 32.Hubner WA, Irby P, Stoller ML. Natural history and current concepts for the treatment of small ureteral calculi. Eur Urol. 1993;24:172–6. doi: 10.1159/000474289. [DOI] [PubMed] [Google Scholar]


