Abstract
Protein-energy wasting (PEW) is common in patients with chronic kidney disease (CKD). PEW is one of the strongest predictors of mortality in patients with CKD. The International Society of Renal Nutrition and Metabolism (ISRNM) expert panel has defined PEW as a, “state of decreased body stores of protein and energy fuels (body protein and fat masses)”. The ISRNM panel has also proposed diagnostic criteria of PEW with four categories. Cachexia is a severe form of PEW. The proposed causes of PEW are multi-factorial and include nutritional and non-nutritional mechanisms. The literature indicates that PEW can be mitigated or corrected with an appropriate diet and enteral nutritional support that targets dietary protein intake. Dietary requirements and enteral nutritional support must also be considered in patients with CKD and diabetes mellitus and in children with CKD, in addition to dialysis patients. Features of ideal dietary supplement have also been discussed. Dietary interventions such as enteral feeding with high-protein meals or supplements might improve the nutritional status and outcomes in dialysis patients.
Keywords: Chronic kidney disease, nutritional intervention, protein energy malnutrition, protein energy wasting
INTRODUCTION
Management of the nutritional aspects of chronic kidney disease (CKD) presents a number of challenges. In the general population, overnutrition is a major problem. Overnutrition is considered a serious risk factor for developing metabolic syndrome, cardiovascular disease, and CKD, with a subsequent increase in the risk of mortality. However, in patients with CKD, and especially in those undergoing maintenance dialysis, the so-called uremic malnutrition (also referred to as protein-energy wasting [PEW]) is by far the strongest risk factor for adverse outcomes and death.[1] Patients undergoing dialysis die of the short-term consequences of PEW and do not live long enough to die of risk factors associated with overnutrition. This ‘time discrepancy hypothesis’[2] suggests that, in a patient with CKD, whose risk of short-term mortality is high, interventions that improve nutritional status and prevent or correct wasting and sarcopenia have the potential to save lives, as compared to the conventional interventions, such as, treating hypercholesterolemia, hypertension or obesity. Furthermore, studies related to the nutritional status have shown that malnutrition / wasting is common in CKD, as approximately 18 – 75% of patients with CKD, undergoing maintenance dialysis therapy, show evidence of wasting.[3,4]
One recently published 10-year cohort study evaluated serum albumin, C-reactive protein, and carotid atherosclerosis as predictors of a 10-year mortality in hemodialysis patients. The results indicated that serum albumin concentration was far superior as a predictor of mortality than inflammatory markers (C-reactive protein) or the intima-media thickness of the common carotid artery.[5] PEW, therefore, seemed to be a strong predictor of mortality in patients with CKD, and improving nutritional status by dietary and non-dietary interventions could be an important step toward improving the outcomes in CKD.[6]
NOMENCLATURE OF MALNUTRITION / WASTING SYNDROMES IN CHRONIC KIDNEY DISEASE
Various different terms and definitions have been used by different authors for conditions associated with loss of muscle and fat tissue, malnutrition, and inflammation in patients with CKD. These include uremic malnutrition, uremic (renal) cachexia, protein-energy malnutrition, malnutrition-inflammation atherosclerosis syndrome or malnutrition-inflammation complex (or cachexia) syndrome. Use of non-uniform and ill-defined terminologies may lead to both conceptual errors and misinterpretation of data. Hence, to avoid confusion the International Society of Renal Nutrition and Metabolism (ISRNM)'s expert panel has recommended the term ‘protein-energy wasting’ (PEW).[7]
Protein-energy wasting
The ISRNM expert panel has defined PEW to describe a “state of decreased body stores of protein and energy fuels (body protein and fat masses)”. This abnormality is often associated with diminished functional capacity related to metabolic stresses.[7] The possible reasons for PEW in kidney disease have been listed in Table 1 (Adapted from[7])
Table 1.
Potential causes of protein-energy wasting syndrome in kidney disease
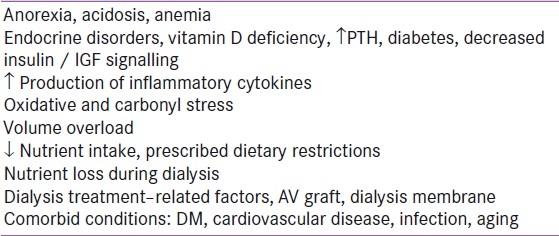
As protein wasting and energy wasting may occasionally occur separately from each other, the term ‘protein wasting’ or ‘energy wasting’ may be used to indicate the isolated occurrence of only one of these phenomena.
Cachexia
In recent times, the word ‘cachexia’ has been suggested as a term to denote PEW included in the setting of kidney disease.[8] The ISRNM expert panel has suggested the use of cachexia for a severe form of protein-energy wasting.[7] Cachexia refers to a very severe form of PEW, often associated with profound physiological, metabolic, psychological, and immunological disorders.[9] The difference in PEW compared to cachexia is that the latter encompasses only severe forms of metabolic depletion, whereas, PEW can refer to mild degrees of depleted protein and energy mass.
Kidney disease wasting
According to the ISRNM expert panel, kidney disease wasting (KDW) refers to the occurrence of protein-energy wasting in CKD or acute kidney injury (AKI), regardless of the cause. The clear majority of the ISRNM panel members prefer PEW to KDW for most circumstances. The panel believes that the term KDW is not a suitable substitute for PEW. The KDW simply implies that PEW is likely to occur in people with CKD.[7]
PROTEIN-ENERGY WASTING IN CHRONIC KIDNEY DISEASE: CLINICAL IMPLICATIONS
Several small and large scale cohort studies have revealed that protein-energy malnutrition is associated with increased morbidity, mortality, and impaired quality of life. The potential consequences of PEW are provided in Table 2 [Adapted from[7]].
Table 2.
Potential manifestations of the protein-energy wasting syndrome in kidney disease

The data from the United States Renal Data System (USRDS) database of 5058 patients concluded that patients who were considered malnourished by their physicians, had a 27% greater risk of cardiovascular death. In addition it was shown that for every one-unit decrease in body mass index (BMI) the risk for cardiovascular death rose by 6%.[10] Results from the large Dialysis Outcomes and Practice Patterns Study (DOPPS) cohort also confirmed that malnourished dialysis patients had an increased risk of mortality.[11]
Protein-energy wasting, mortality, and albumin levels
Evidence indicates that surrogates of PEW, such as, low serum levels of albumin or inadequate protein intake, correlate with mortality. Measuring the serum levels of albumin remains the simplest test that is readily available. Indeed, a low serum albumin concentration is by far the strongest predictor of poor outcomes and mortality, at least in patients on dialysis, when compared with any other risk factor, including the traditional risk factors (hypertension, hypercholesterolemia, diabetes mellitus, and obesity) and the nonconventional risk factors (measures of anemia, mineral and bone surrogates, and dialysis modality).[1] In the United States Renal Data System (USRDS) database results, every 1 g / dl fall in the serum albumin level is associated with a 39% increase in the risk of cardiovascular death.[10] The association between serum albumin levels and mortality is highly incremental and linear. There is an ongoing debate of whether low serum levels of albumin in patients with CKD are a surrogate of inadequate protein intake or other conditions related to PEW, such as inflammation and comorbidity.[12] However, there is less disagreement with regard to the consistent association of hypoalbuminemia with poor outcomes in dialysis patients and those with nondialysis-dependent CKD.[13] Reports indicate that patients attending dialysis clinics that provide superior care and have a good performance, exhibit higher serum albumin levels and better survival rates, than patients who attend clinics with an inferior performance.[14,15] Longevity has consistently been observed in those patients with CKD who have a better nutritional status, including larger muscle mass, fat mass, better appetite, and higher protein intake.[6]
DIAGNOSIS OF PROTEIN-ENERGY WASTING
The diagnostic criteria for PEW (proposed by ISRNM) fall into four distinct categories: (1) biochemical indicators, (2) low body weight, reduced body fat or weight loss, (3) decreased muscle mass, and (4) low protein or energy intake [Table 3].
Table 3.
Readily utilizable criteria for the clinical diagnosis of protein energy wasting in chronic kidney disease criteria

NUTRITIONAL REQUIREMENTS IN CHRONIC KIDNEY DISEASE
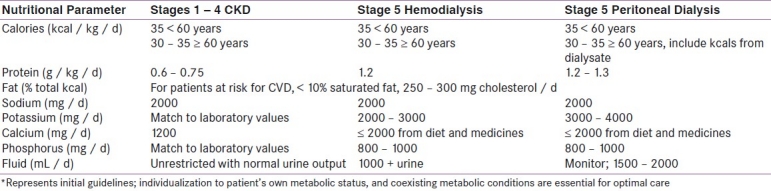
Regarding dietary protein recommendations in CKD, the current Kidney Dialysis Outcome Quality Initiative (K / DOQI) guidelines suggest a protein intake of 0.6 – 0.75 grams of protein per kilogram of body weight per day (g / kg / d) for patients in stages 1 – 4 of CKD. In stage 5, when the patients are receiving dialysis, increased protein intake is suggested (approx. 1.2 g / kg / d).[16] Table 4 has mentioned selected nutritional parameter requirements for varying levels of kidney disease based on the American Dietetic Association guidelines.[17]
Table 4.
Selected nutritional parameters for varying levels of kidney disease*
IMPROVING NUTRITIONAL STATUS IN CHRONIC KIDNEY DISEASE
Oral nutritional consideration: Intervention may begin with suggestions to enhance oral intake in malnourished CKD patients [Refer Table 5 – Adapted from[18] ]
Table 5.
Strategies to enhance oral intake

Dietary sodium intake is frequently restricted to 2000 – 4000 mg per day for patients with CKD, in an effort to aid in the control of hypertension, and to avoid excessive thirst and fluid consumption in those patients with oliguria or anuria. Salt substitutes frequently contain potassium chloride, and patients should be instructed to avoid salt substitutes that are not approved by their dietitian or physician.
Patients with CKD also frequently experience hyperphosphatemia. Hyperphosphatemia is associated with a number of deleterious consequences, such as secondary hyperparathyroidism, arterial calcification, and renal osteodystrophy. A dietary phosphorus restriction of 800 – 1000 mg per day should be implemented when serum phosphorus rises > 4.6 mg / dL.
Need for oral nutritional supplements
The recommended DEI for patients undergoing hemodialysis and peritoneal dialysis is 30 – 35 kcal / kg per day. The suggested mean dietary protein intake (DPI) is 1.2 g / kg per day in patients on hemodialysis, and 1.3 g / kg per day in patients on peritoneal dialysis.[16,17] Most patients on dialysis, however, have a lower DEI and DPI than the recommended intake. In 1901 adult patients on the HEMO Study, the mean DEI and DPI were 23.2 ± 9.5 kcal / kg per day and 0.96 ± 0.43 g / kg per day, respectively, on nondialysis days, and 22.2 ± 9.6 kcal / kg per day and 0.90 ± 0.41 g / kg per day, respectively, on dialysis days.[19] Nutritional support for patients on dialysis should make it possible to ensure that the nutritional intake is in accordance with the current guidelines. Oral supplementation can provide an additional 7 – 10 kcal / kg per day of energy and 0.3 – 0.4 g / kg per day of protein, which makes it possible to meet the recommended targets of both DEI and DPI. To reach such energy and protein levels, oral supplements should be given two-to-three times a day, preferably one hour after the main meals.
In a recent review by Kalantar-Zadeh et al.,[6] the authors have identified clinical trials with at least 10 participants, wherein the effects of enteral nutritional interventions were examined in malnourished patients on dialysis. They observed, “In most of these studies, enteral therapy was associated with improved nutritional status or other clinical outcomes.” Eight out of nine randomized trials that used serum albumin concentration as a surrogate outcome measure, reported statistically significant improvements in hypoalbuminemia after the nutritional support.[6]
Enteral feedings
Enteral feeding allows the provision of complete nutrition in a minimal fluid volume, which is associated with fewer complications of infection and is significantly less expensive than parenteral nutrition. The hospitalized patients may benefit from a period of nasogastric feeding until the acute illness resolves. A patient's willingness to accept the nasogastric tube (NGT) placement is frequently dependent on the method of presentation. If the feeding tube placement is discussed as an important and beneficial part of their overall care, a patient is much more likely to agree to NGT placement. Experience shows that ‘threatening’ a patient with NGT placement if they do not eat adequately, seldom changes the factors that limit intake, and is frequently perceived as a ‘punishment’ and only serves as a barrier to providing the needed nutritional support. Some patients may require small bowel positioning of the feeding tube tip if they have a history of gastroparesis or if they do not tolerate gastric feedings. Patients with active pancreatitis should have the tip of the feeding tube placed beyond the ligament of Treitz.[18]
Parenteral nutrition
Total parenteral nutrition is associated with increased infectious complications and is significantly more expensive than enteral feedings. Parenteral nutrition also requires a greater fluid volume to meet the calorie and protein needs than equivalent enteral nutrition. Parenteral nutrition should be reserved only for those patients who are unable to receive enteral nutrients.[18]
SPECIAL ISSUES IN MANAGEMENT
Nutrition in chronic kidney disease patients with diabetes
There are concerns regarding the glycemic burden of nutritional interventions in patients with diabetes mellitus. However, such considerations might have less relevance in malnourished patients on dialysis. Indeed, in approximately one-third of the patients on dialysis with diabetes mellitus, a state of ‘burnt-out diabetes’ is observed, in which frequent episodes of hypoglycemia necessitate a decrease or even total discontinuation of most or all diabetic medications, including insulin injections and oral hypoglycemic agents.[20,21] Many of these patients exhibit normal-to-low levels of hemoglobin A1c, even without medication for diabetes mellitus and when they originally suffered from diabetic nephropathy as the etiology of their CKD. In the opinion of the authors, in a recent review, a history of diabetes mellitus is not a contraindication for oral nutritional therapy or meals during hemodialysis, and should not be a reason to withhold nutritional interventions, especially among dialysis patients with hypoalbuminemia and normal-to-low levels of hemoglobin A1c.[6]
Enteral nutrition in children with chronic kidney disease
Protein-energy wasting often presents as growth retardation in children with CKD. One of the major goals in the treatment of infants and children with CKD is to achieve normal growth and development. Infants and children with CKD, who experience anuria and polyuria, have very different food and fluid requirements from those who do not have these conditions. Both these subsets of children require adequate nutrition to maximize growth. Children with polyuria may be maintained on a diluted formula and may undergo pre-emptive kidney transplantation, thus never requiring dialysis. However, it is important to monitor the amount of nutrients being delivered to infants with polyuria. Those who are oliguric or anuric often require frequent (sometimes daily) dialysis, to offset the large-volume feeds that occur with standard formulas. In the absence of pediatric renal feeding supplements, adult renal products that are available with normal and reduced protein content, and designed to be calorie dense and low in minerals and electrolytes, can be recommended for children who are aged > 4 years. Adult supplements have also been successfully used at diluted strength in children aged < 1 year.[22] A study shows that children with CKD and hyperkalemia have demonstrated improved growth rates while receiving adult renal formulas, which have been well-tolerated and effective in lowering potassium exposure.[23] As normal growth and development in children is important, monitored in-center enteral nutrition therapy for all infants and children who require maintenance dialysis treatment has been encouraged.[6]
Ideal nutritional supplement in chronic kidney disease
At present, various products (dietary food supplements) are available in our country, which are calorie dense and low in electrolyes. They can be broadly classified in two categories: (1) High protein content (per 100 gm) — for dialysis patients (2) Low protein content (per 100 gm) — for predialysis patients / renal supplement for nondialysis patients. Regarding the protein quality, guidelines suggest at least 50% of the protein should be of high biological value. Biological value refers to how well and how quickly the body can actually use the protein. Considering this fact, whey protein seems most suitable, as the whey protein concentrate has a high biological value (104). This will ensure better utilization of protein in the body.
As discussed in oral nutrition section, hyperphosphatemia is an important risk in dialysis patients. Large observational studies have shown a graded association between the levels of serum phosphate and all-cause mortality in patients undergoing dialysis.[24] Hence, a supplement that is free from free phosphorous would be ideal. Whey protein does not contain phosphorous. Hence, a supplement with 100% whey protein may be preferred, due to its advantage of high biological value and absence of free phosphorous.
Many of the available protein supplements for dialysis patients contain vitamin A and / or vitamin K. According to the EBPG (European best practice guidelines) — guidelines on nutrition; Vitamin A supplements are not recommended for the maintenance hemodialysis patients.[25] The serum plasma levels of vitamin A are elevated in patients with CKD. Furthermore, vitamin A is not removed during MHD (maintenance hemodialysis) and deficiencies are rare. As vitamin A toxicity includes hypercalcemia, anemia, and hypertriglyceridemia, supplementation is not recommended for hemodialysis patients. Moreover, the EBPG guidelines also state, “There is no need for vitamin K supplementation, except in patients receiving long-term antibiotic treatment or those with altered coagulant activity”.[25] High plasma vitamin K levels have been associated with soft tissue calcifications in MHD patients. Hence, a supplement that does not contain vitamin K may be suitable for dialysis patients.
The ideal supplement should have good protein (100% whey protein) and should preferably be free from phosphorous (P), vitamin A, and vitamin K (PAK - free).
CONCLUSIONS
Fifty years after the first dialysis treatment, nutrition is still a recurrent issue and many disorders are currently not well understood. However, there has been progress in understanding the nutritional targets in CKD patients. Before dialysis, there is good evidence that a longstanding nutritional care plan, with control of protein intake, is efficient for correcting many metabolic disorders, including proteinuria, and it is cost-effective. PEW is a distinct condition in CKD patients. PEW is common in CKD and is associated with adverse outcomes. Dietary interventions and nutritional support seem to be effective in mitigating or correcting PEW and improving the outcomes in patients with CKD. All patients with CKD should be assessed periodically (monthly or quarterly) for the presence of PEW and should be offered oral nutritional support whenever required. Providing meals or oral nutritional supplements and other nutritional interventions to patients with CKD is the most promising way to increase serum albumin concentration and improve longevity and quality of life in this patient population.
Footnotes
Source of Support: Nil
Conflict of Interest: Dr. Yashpal Jadeja is an employee of Cadila Healthcare Limited
REFERENCES
- 1.Kovesdy CP, Kalantar-Zadeh K. Why is protein-energy wasting associated with mortality in chronic kidney disease? Semin Nephrol. 2009;29:3–14. doi: 10.1016/j.semnephrol.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalantar-Zadeh K, Block G, Horwich T, Fonarow GC. Reverse epidemiology of conventional cardiovascular risk factors in patients with chronic heart failure. J Am Coll Cardiol. 2004;43:1439–44. doi: 10.1016/j.jacc.2003.11.039. [DOI] [PubMed] [Google Scholar]
- 3.Kopple JD. McCollum Award Lecture, 1996: Protein–energy malnutrition in maintenance hemodialysis patients. Am J Clin Nutr. 1996;65:1544–57. doi: 10.1093/ajcn/65.5.1544. [DOI] [PubMed] [Google Scholar]
- 4.Kalantar-Zadeh K, Ikizler TA, Block G, Avram MM, Kopple JD. Malnutrition–inflammation complex syndrome in dialysis patients: Causes and consequence. Am J Kidney Dis. 2003;42:864–81. doi: 10.1016/j.ajkd.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 5.Kato A, Takita T, Furuhashi M, Maruyama Y, Hishida A. Comparison of serum albumin, C-reactive protein and carotid atherosclerosis as predictors of 10-year mortality in hemodialysis patients. Hemodial Int. 2010;14:226–32. doi: 10.1111/j.1542-4758.2009.00432.x. [DOI] [PubMed] [Google Scholar]
- 6.Kalantar-Zadeh K, Cano NJ, Budde K, Chazot C, Kovesdy CP, Mak RH, et al. Diets and enteral supplements for improving outcomes in chronic kidney disease. Nat Rev Nephrol. 2011;7:369–84. doi: 10.1038/nrneph.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73:391–8. doi: 10.1038/sj.ki.5002585. [DOI] [PubMed] [Google Scholar]
- 8.Kalantar-Zadeh K. Recent advances in understanding the malnutrition–inflammation–cachexia syndrome in chronic kidney disease patients: What is next? Semin Dial. 2005;18:365–9. doi: 10.1111/j.1525-139X.2005.00074.x. [DOI] [PubMed] [Google Scholar]
- 9.Martignoni ME, Kunze P, Friess H. Cancer cachexia. Mol Cancer. 2003;2:36. doi: 10.1186/1476-4598-2-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fung F, Sherrard DJ, Gillen DL, Wong C, Kestenbaum B, Seliger S, et al. Increased risk for cardiovascular mortality among malnourished end-stage renal disease patients. Am J Kidney Dis. 2002;40:307–14. doi: 10.1053/ajkd.2002.34509. [DOI] [PubMed] [Google Scholar]
- 11.Pifer TB, McCullough KP, Port FK, Goodkin DA, Maroni BJ, Held PJ, et al. Mortality risk in hemodialysis patients and changes in nutritional indicators: DOPPS. Kidney Int. 2002;62:2238–45. doi: 10.1046/j.1523-1755.2002.00658.x. [DOI] [PubMed] [Google Scholar]
- 12.Friedman AN, Fadem SZ. Reassessment of albumin as a nutritional marker in kidney disease. J Am Soc Nephrol. 2010;21:223–30. doi: 10.1681/ASN.2009020213. [DOI] [PubMed] [Google Scholar]
- 13.Kovesdy CP, George SM, Anderson JE, Kalantar-Zadeh K. Outcome predictability of biomarkers of protein-energy wasting and inflammation in moderate and advanced chronic kidney disease. Am J Clin Nutr. 2009;90:407–14. doi: 10.3945/ajcn.2008.27390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lacson E, Jr, Wang W, Hakim RM, Teng M, Lazarus JM. Associates of mortality and hospitalization in hemodialysis: Potentially actionable laboratory variables and vascular access. Am J Kidney Dis. 2009;53:79–90. doi: 10.1053/j.ajkd.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 15.Lacson E, Jr, Wang W, Lazarus JM, Hakim RM. Hemodialysis facility-based quality-of-care indicators and facility-specific patient outcomes. Am J Kidney Dis. 2009;54:490–7. doi: 10.1053/j.ajkd.2009.01.260. [DOI] [PubMed] [Google Scholar]
- 16.National Kidney Foundation. Clinical practice guidelines for nutrition in chronic renal failure. [Last Accessed 2012 Feb 10]. Available from: http: / / www.kidney.org /professionals / kdoqi / guidelines_updates / doqi_nut.html .
- 17.Beto JA, Bansal VK. Medical nutrition therapy in chronic kidney failure: Integrating clinical practice guidelines. J Am Diet Assoc. 2004;104:404–9. doi: 10.1016/j.jada.2003.12.028. [DOI] [PubMed] [Google Scholar]
- 18.Parrish CR. Nutrition in renal failure: Myths and management. Pract Gastroenterol. 2004;20:40–59. [Google Scholar]
- 19.Burrowes JD, Larive B, Cockram DB, Dwyer J, Kusek JW, McLeroy S, et al. Effects of dietary intake, appetite, and eating habits on dialysis and non-dialysis treatment days in hemodialysis patients: Cross-sectional results from the HEMO study. J Ren Nutr. 2003;13:191–8. doi: 10.1016/s1051-2276(03)00069-4. [DOI] [PubMed] [Google Scholar]
- 20.Kalantar-Zadeh K, Derose SF, Nicholas S, Benner D, Sharma K, Kovesdy CP. Burnt-out diabetes: Impact of chronic kidney disease progression on the natural course of diabetes mellitus. J Ren Nutr. 2009;19:33–7. doi: 10.1053/j.jrn.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kovesdy CP, Park JC, Kalantar-Zadeh K. Glycemic control and burnt-out diabetes in ESRD. Semin Dial. 2010;23:148–56. doi: 10.1111/j.1525-139X.2010.00701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gast T, Bunchman T, Barletta GM. Nutritional management of infants with CKD / ESRD with use of “adult” renal-based formulas [abstract] Perit Dial Int. 2007;27(Suppl 1):S34. [Google Scholar]
- 23.Hobbs DJ, Gast TR, Ferguson KB, Bunchman TE, Barletta GM. Nutritional management of hyperkalemic infants with chronic kidney disease, using adult renal formulas. J Ren Nutr. 2010;20:121–6. doi: 10.1053/j.jrn.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Tonelli M, Pannu N, Manns B. Oral phosphate binders in patients with kidney failure. N Engl J Med. 2010;362:1312–24. doi: 10.1056/NEJMra0912522. [DOI] [PubMed] [Google Scholar]
- 25.Fouque D, Vennegoor M, ter Wee P, Wanner C, Basci A, Canaud B, et al. EBPG guideline on nutrition. Nephrol Dial Transplant. 2007;22(Suppl 2):ii45–87. doi: 10.1093/ndt/gfm020. [DOI] [PubMed] [Google Scholar]


