Abstract
Primary hyperparathyroidism (PHPT) is associated with nephrolithiasis and nephrocalcinosis. Hypercalciuria is one of the multiple factors that is implicated in the complex pathophysiology of stone formation. The presence of a renal stone (symptomatic or asymptomatic) categorizes PHPT as symptomatic and is an indication for parathyroid adenomectomy. Progression of nephrocalcinosis is largely reversible after successful surgery, but the residual risk persists. PHPT is also associated with declining renal function. In case of asymptomatic mild PHPT, annual renal functional assessment is advised. Guidelines suggest that an estimated glomerular filtration rate (eGFR) < 60 ml / minute / 1.73 m2 is an indication for parathyroid adenomectomy. This article discusses how to monitor and manage renal stones and other related renal parameters in case of PHPT.
Keywords: Nephrolithiasis, primary hyperparathyroidism, renal cyst, renal stones
INTRODUCTION
The term primary hyperparathyroidism (PHPT) refers to the inappropriate or unregulated overproduction of the parathyroid hormone (PTH) leading to abnormal calcium homeostasis. High levels of PTH lead to increased renal resorption of calcium, phosphaturia, increased synthesis of 1,25(OH)2D (which increases intestinal calcium absorption), and increased resorption of the bone. The classical clinical manifestation of PHPT is the ‘stone and bone’ disease. Renal manifestations of PHPT include hypercalciuria, nephrolithiasis, nephrocalcinosis, chronic renal insufficiency, and renal tubular dysfunction. In this article, we discuss the monitoring and management of renal stones and other renal complications in patients with PHPT.
PREVALENCE OF RENAL DISEASE IN PRIMARY HYPERPARATHYROIDISM
Prevalence of renal disease in PHPT patients from western countries has changed significantly over the last few decades. In the 1970s and 1980s, renal complications were reported in as many as 40 to 60% of the patients.[1] PHPT has become an asymptomatic disease in the western world, with the introduction of routine calcium screening. Hence, a lower prevalence of renal stone disease in PHPT has been reported in most studies performed within the last two decades. A recent study has reported that only 19 (7.0%) of the 271 patients with mild PHPT had renal stones, as detected by the renal sonogram.[2]
In a cross-sectional survey of 294 PHPT patients, an estimated glomerular filtration rate below 60 ml / minute was found in 17% of the patients, indicating that renal impairment was a common finding in PHPT.[3] Development of renal insufficiency in PHPT was related to the degree and duration of hypercalcemia. During the period of medical observation (median follow-up of 2.9 years) for the mild asymptomatic PHPT cohort, the relative risks of developing renal stones and renal failure were 4.6 and 19.3%, respectively, compared to the healthy controls.[4] Nevertheless, others had observed that an absolute risk for renal function deterioration (over a two-to-three year duration) in the mild PHPT cohort was small or nil.[5]
The presence of a renal stone (symptomatic or asymptomatic) categorizes PHPT as symptomatic, and is an indication for parathyroid adenomectomy. Nephrocalcinosis progression is largely reversible after a successful surgery. Nevertheless, renal calcium excretion and rate of stone recurrence (30% over five years) after surgical cure, for PHPT, is comparable to idiopathic stone formers.[6]
A systematic review on PHPT, in India, collated the data reported by various tertiary care institutes (344 PHPT cases) across the country and reported 36% prevalence of renal disease.[7] Gopal et al., reported renal calculi including nephrocalcinosis in 40.5% of the PHPT patients and as a presenting complaint in 15.1% of the patients.[8] Renal stone disease was present in 70% of the PHPT patients and a recurrent renal stone was the presenting complaint in 21% of the patients in another series by Bhansali et al.[9] Asymptomatic PHPT constituted only 5% of the Indian PHPT patients. No detailed data is available on the creatinine clearance in the Indian PHPT cohort.
The parathyroid hormone inhibits proximal tubular bicarbonate reabsorption, which tends to cause mild metabolic acidosis (proximal tubular acidosis). However, this effect is usually counterbalanced by the alkali liberated as a result of increased bone resorption and tubular reabsorption of the bicarbonate, caused by hypercalcemia.
On the contrary, Muthukrishnan et al., reported distal renal tubular acidosis (RTA) (normal anion gap metabolic acidosis with urine pH > 5.5, a positive urine anion gap, and a positive ammonium chloride loading test) in four of the 53 patients with symptomatic PHPT.[10] On the basis of reversibility of the distal RTA (after surgical treatment of PHPT), the authors hypothesized PHPT as the primary cause of distal RTA in these cases.
The hereditary hyperparathyroidism–jaw tumor syndrome is a rare autosomal dominant disorder characterized by the parathyroid gland neoplasm (± single / multiglandular disease, ± cystic, ± malignant), ossifying fibromas of the mandible and maxilla, and uterine tumors. The affected patients may also have evidence of a variety of renal lesions including, cysts (two-third of the cases), hamartomas, renal cell carcinoma, and Wilms tumor.[11]
Simple kidney cysts (≥ 3 mm detected by ultrasonogram) occurred with higher prevalence in both male and female patients, with 172 PHPT (normal renal function), in comparison to healthy controls (34.9 vs. 16.2%, P < 0.001).[12] Aging and disease severity were associated with an increased risk of renal cysts. The authors concluded that simple renal cysts might be considered as a benign kidney complication of PHPT and might be related to the action of chronic elevated PTH levels on tubular epithelial cells. Table 1 summarizes the renal manifestations of PHPT.
Table 1.
Renal manifestations of primary hyperparathyroidism

PATHOPHYSIOLOGY OF RENAL DISEASE IN PRIMARY HYPERPARATHYROIDISM
The filtered load of calcium in the glomerulus increases proportionately with the degree of serum hypercalcemia. Hypercalciuria is one of evident factors in the complex pathophysiology of renal stone formation. Most renal stones in patients with PHPT are composed of calcium oxalate, although slightly alkaline urine may favor the precipitation of calcium phosphate stones. Stone formers are more likely to be hypercalciuric, but less than one-third of the hypercalciuric patients with PHPT actually develop renal stones. At present, it is not possible to confidently predict which asymptomatic patients with PHPT would develop a new onset of renal stone disease, based on the biochemical measurements in the blood or urine (including hypercalciuria).[13,14]
Young age, male gender, and high 1,25(OH)2D have been associated with an increased risk of renal stones. It is hypothesized that at a younger age, there is a relatively more viable renal mass, and hence, lower serum phosphorus. Both these factors lead to relatively higher 1,25(OH)2D levels, leading to increased intestinal calcium absorption (absorptive hypercalciuria).[15,16]
PHPT (milder variants) have an increased BMI compared to the general background population. Obesity per se is associated with increased excretion of urinary oxalate, uric acid, sodium, and phosphate; hence, it may be contributing to renal stone formation. This, is another speculation.[17]
Hypothesis of calcium renal leak stems from the observation of sustained risk (above baseline) for renal stone formation, after successful PHPT surgery. The proposed mineral disorder, that is, a renal calcium leak, is the basic forerunner for PTH excess, and hence, low serum phosphorus. This pathophsiology persists after surgery and so is renal stone risk.[18]
Polymorphism in the CaSR gene is associated with renal stone risk in general. Scillitani et al., has reported that patients with AGQ haplotype had a 3.8-fold higher risk of developing renal stones in a group of 225 patients with PHPT.[19]
MANAGEMENT ISSUES IN PRIMARY HYPERPARATHYROIDISM WITH RENAL DISEASE
Management criteria of asymptomatic PHPT have been modified over the last two decade [Table 2].[20,22]
Table 2.
Comparison of National Institute of Health criteria for parathyroidectomy for asymptomatic cases
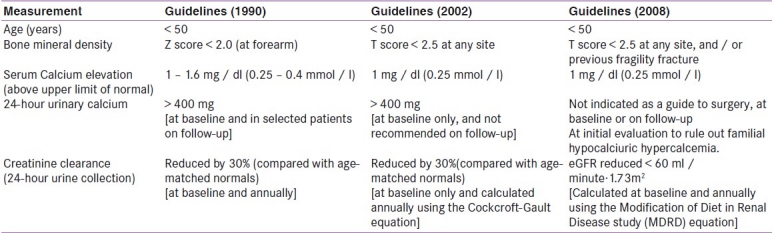
Hypercalciuria is not a predictor of renal stone disease in PHPT patients and is no more considered as an indication for surgery.[22] A 24-hour urinary calcium or spot calcium / creatinine ratio may be helpful at initial evaluation, to rule out familial hypocalciuric hypercalcemia.
The frequency of developing new kidney stones among those with history of nephrolithiasis declines after surgery.[23] Furthermore, over a 10-year follow-up period, all patients with a history of nephrolithiasis, who had chosen not to have parathyroidectomy had progression of disease.[24] Thus, presence of renal stone (symptomatic or asymptomatic) categorizes PHPT as symptomatic, and is an indication for parathyroid adenomectomy.
As per the recent guidelines [Table 3],[20–22] routine screening for renal stones (silent nephrolithiasis) at the time of the original evaluation, in asymptomatic PHPT, is not recommended and is restricted to those who have history suggestive of kidney stones. If renal stones are suspected, ultrasound is the recommended imaging modality of choice, followed by a computed tomography (CT) scan, if additional imaging is required. Low-dose non-contrast helical CT scan (radiation exposure 1.0 – 1.5 mSv) is one of the best radio-imaging techniques (sensitivity: 96% and specificity: 97%) for diagnosing renal calcification.[25] In a recent clinical review, authors have opined that all PHPT patients should initially be evaluated for renal calcifications by unenhanced helical computed tomography.[26]
Table 3.
Comparison of the National Institute of Health criteria for kidney stone screening in asymptomatic primary hyperparathyroidism cases

Renal calcium excretion and rate of stone recurrence (30% over five years) after surgical cure for PHPT, is comparable to idiopathic stone formers. Therefore, nephrolithiasis after successful parathyroid surgery should be monitored and managed similar to stone diseases caused by other etiologies.[26]
Guidelines suggest that an estimated glomerular filtration rate (eGFR) of < 60 ml / minute·1.73 m2 (stage 3 level of renal insufficiency) is an indication for parathyroid adenomectomy.[27] This cutoff is based on extrapolation from chronic kidney disease data where elevation in serum PTH is observed to be below GFR of 60 ml / minute·1.73 m2. However, there is no evidence that this threshold is actually associated with increasing level of PTH in PHPT patients, or whether correction of PHPT by successful surgery leads to improvement in GFR. Tassone et al., challenges the concept of PTH elevation in PHPT patients, below the threshold of 60 ml / minute of GFR. In their cohort of PHPT, PTH increased significantly below eGFR < 30 ml / minute·1.73 m2 (stage 4 level of renal insufficiency) only.[28]
For PHPT cases where surgery is not indicated, medical management needs to be optimized. It is advised to maintain adequate hydration (> 2 liters / day), to minimize the risk of nephrolithiasis. Physical activity should be encouraged to minimize bone resorption. As against idiopathic hypercalciuria, thiazide diuretic is contraindicated in PHPT, as serum calcium may rise with its use.[29] In symptomatic PHPT cases with severe vitamin D deficiency, replacing vitamin D may lead to a rise in calcium. However, in milder PHPT forms, routine vitamin D supplementation is not associated with a rise in serum or urinary calcium. In such cases it is recommended to maintain serum 25-OHD above 20 ng/ml.[27]
Another area of controversy is with regard to calcium supplementation. A low calcium diet may lead to further increases in PTH secretion and may aggravate bone disease.[30] Cooperberg et al., reported a protective effect of oral calcium supplementation on the rate of nephrolithiasis in PHPT men (19% vs. 46%, P = 0.027) and women (7% vs. 17%, P = 0.04) prior to surgery, for hyperparathyroidism.[31] Locker et al., have reported increase in urinary calcium with increasing oral calcium dosages and correlated it with higher plasma 1,25(OH)2D levels.[32] It is prudent to maintain a moderate calcium intake (up to 1000 mg / day), and consider monitoring of urinary calcium and serum calcitriol in selected cases.
SUMMARY
In India we still see the symptomatic variant of PHPT (renal stone being one of them), where the role of parathyroid surgery is unquestionable. Nephrolithiasis progression is largely reversible after successful surgery, but residual risk persists. Such cases should be managed similar to stone diseases caused by other etiologies. With the wide spread use of routine health check-ups (serum calcium, bone density), milder PHPT cases are more commonly diagnosed. Such cases need decision-making between conservative management and surgery. In such cases monitoring of the renal parameters is important. In addition to taking decisions about the definite treatment plan, the treating physicians should also optimize the interim medical therapy.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Pak CY, Nicar MJ, Peterson R, Zerwekh JE, Snyder W. A lack of unique pathophysiologic background for nephrolithiasis of primary hyperparathyroidism. J Clin Endocrinol Metab. 1981;53:536–42. doi: 10.1210/jcem-53-3-536. [DOI] [PubMed] [Google Scholar]
- 2.Suh JM, Cronan JJ, Monchik JM. Primary hyperparathyroidism: Is there an increased prevalence of renal stone disease? AJR Am J Roentgenol. 2008;191:908–11. doi: 10.2214/AJR.07.3160. [DOI] [PubMed] [Google Scholar]
- 3.Tassone F, Gianotti L, Emmolo I, Ghio M, Borretta G. Glomerular filtration rate and parathyroid hormone secretion in primary hyperparathyroidism. J Clin Endocrinol Metab. 2009;94:4458–61. doi: 10.1210/jc.2009-0587. [DOI] [PubMed] [Google Scholar]
- 4.Yu N, Donnan PT, Leese GP. Arecord linkage study of outcomes in patients with mild primary hyperparathyroidism: The Parathyroid Epidemiology and Audit Research Study (PEARS) Clin Endocrinol (Oxf) 2011;75:169–76. doi: 10.1111/j.1365-2265.2010.03958.x. [DOI] [PubMed] [Google Scholar]
- 5.Ambrogini E, Cetani F, Cianferotti L, Vignali E, Banti C, Viccica G, et al. Surgery or surveillance for mild asymptomatic primary hyperparathyroidism: A prospective, randomized clinical trial. J Clin Endocrinol Metab. 2007;92:3114–21. doi: 10.1210/jc.2007-0219. [DOI] [PubMed] [Google Scholar]
- 6.Mollerup CL, Lindewald H. Renal stones and primary hyperparathyroidism: Natural history of renal stone disease after successful parathyroidectomy. World J Surg. 1999;23:173–6. doi: 10.1007/pl00013175. [DOI] [PubMed] [Google Scholar]
- 7.Pradeep PV, Jayashree B, Mishra A, Mishra SK. Systematic review of primary hyperparathyroidism in India: The past, present, and the future trends. Int J Endocrinol. 2011;2011:921814. doi: 10.1155/2011/921814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gopal RA, Acharya SV, Bandgar T, Menon PS, Dalvi AN, Shah NS. Clinical profile of primary hyperparathyroidism from western India: A single center experience. J Postgrad Med. 2010;56:79–84. doi: 10.4103/0022-3859.65279. [DOI] [PubMed] [Google Scholar]
- 9.Bhansali A, Masoodi SR, Reddy KS, Behera A, das Radotra B, Mittal BR, et al. Primary hyperparathyroidism in north India: A description of 52 cases. Ann Saudi Med. 2005;25:29–35. doi: 10.5144/0256-4947.2005.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muthukrishnan J, Hari Kumar KV, Jha R, Jha S, Modi KD. Distal renal tubular acidosis due to primary hyperparathyroidism. Endocr Pract. 2008;14:1133–6. doi: 10.4158/EP.14.9.1133. [DOI] [PubMed] [Google Scholar]
- 11.Stålberg P, Carling T. Familial parathyroid tumors: diagnosis and management. World J Surg. 2009;33:2234–43. doi: 10.1007/s00268-009-9924-6. [DOI] [PubMed] [Google Scholar]
- 12.Corbetta S, Eller-Vainicher C, Vicentini L, Carnicelli S, Sardanelli F, Beck-Peccoz P, et al. High prevalence of simple kidney cysts in patients with primary hyperparathyroidism. J Endocrinol Invest. 2009;32:690–4. doi: 10.1007/BF03345742. [DOI] [PubMed] [Google Scholar]
- 13.Frøkjaer VG, Mollerup CL. Primary hyperparathyroidism: Renal calcium excretion in patients with and without renal stone disease before and after parathyroidectomy. World J Surg. 2002;26:532–5. doi: 10.1007/s00268-001-0262-6. [DOI] [PubMed] [Google Scholar]
- 14.Corbetta S, Baccarelli A, Aroldi A, Vicentini L, Fogazzi GB, Eller-Vainicher C, et al. Risk factors associated to kidney stones in primary hyperparathyroidism. J Endocrinol Invest. 2005;28:122–8. doi: 10.1007/BF03345354. [DOI] [PubMed] [Google Scholar]
- 15.Odvina CV, Sakhaee K, Heller HJ, Peterson RD, Poindexter JR, Padalino PK, et al. Biochemical characterization of primary hyperparathyroidism with and without kidney stones. Urol Res. 2007;35:123–8. doi: 10.1007/s00240-007-0096-2. [DOI] [PubMed] [Google Scholar]
- 16.Patron P, Gardin JP, Paillard M. Renal mass and reserve of vitamin D: Determinants in primary hyperparathyroidism. Kidney Int. 1987;31:1174–80. doi: 10.1038/ki.1987.125. [DOI] [PubMed] [Google Scholar]
- 17.Bolland MJ, Grey AB, Gamble GD, Reid IR. Association between primary hyperparathyroidism and increased body weight: A meta-analysis. J Clin Endocrinol Metab. 2005;90:1525–30. doi: 10.1210/jc.2004-1891. [DOI] [PubMed] [Google Scholar]
- 18.Parks JH, Coe FL, Evan AP, Worcester EM. Clinical and laboratory characteristics of calcium stone-formers with and withoutprimary hyperparathyroidism. BJU Int. 2009;103:670–8. doi: 10.1111/j.1464-410X.2008.08064.x. [DOI] [PubMed] [Google Scholar]
- 19.Vezzoli G, Terranegra A, Arcidiacono T, Gambaro G, Milanesi L, Mosca E, et al. Calcium kidney stones are associated with a haplotype of the calcium-sensing receptor gene regulatory region. Nephrol Dial Transplant. 2010;25:2245–52. doi: 10.1093/ndt/gfp760. [DOI] [PubMed] [Google Scholar]
- 20.Bilezikian JP, Silverberg SJ. Clinical practice.Asymptomatic primary hyperparathyroidism. N Engl J Med. 2004;350:1746–51. doi: 10.1056/NEJMcp032200. [DOI] [PubMed] [Google Scholar]
- 21.Eastell R, Arnold A, Brandi ML, Brown EM, D’Amour P, Hanley DA, et al. Diagnosis of asymptomatic primary hyperparathyroidism: Proceedings of the third international workshop. J Clin Endocrinol Metab. 2009;94:340–50. doi: 10.1210/jc.2008-1758. [DOI] [PubMed] [Google Scholar]
- 22.Silverberg SJ, Lewiecki EM, Mosekilde L, Peacock M, Rubin MR. Presentation of asymptomatic primary hyperparathyroidism: Proceedings of the third international workshop. J Clin Endocrinol Metab. 2009;94:351–65. doi: 10.1210/jc.2008-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mollerup CL, Vestergaard P, Frøkjaer VG, Mosekilde L, Christiansen P, Blichert-Toft MR. Risk of renal stone events in primary hyperparathyroidism before and after parathyroid surgery: Controlled retrospective follow up study. BMJ. 2002;325:807–11. doi: 10.1136/bmj.325.7368.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silverberg SJ, Shane E, Jacobs TP, Siris E, Bilezikian JP. A 10-year prospective study of primary hyperparathyroidism with or without parathyroid surgery. N Engl J Med. 1999;341:1249–55. doi: 10.1056/NEJM199910213411701. [DOI] [PubMed] [Google Scholar]
- 25.Hamm M, Knopfle E, Wartenberg S, Wawroschek F, Weckermann D, Harzmann R. Low dose unenhanced helical computerized tomography for the evaluation of acute flank pain. J Urol. 2002;167:1687–91. [PubMed] [Google Scholar]
- 26.Rejnmark L, Vestergaard P, Mosekilde L. Nephrolithiasis and renal calcifications in primary hyperparathyroidism. J Clin Endocrinol Metab. 2011;96:2377–85. doi: 10.1210/jc.2011-0569. [DOI] [PubMed] [Google Scholar]
- 27.Bilezikian JP, Khan A, Arnold A, Brandi ML, Brown E, Bouillon R, et al. Guidelines for the management of asymptomatic primary hyperparathyroidism: Summary statement from the third international workshop. J Clin Endocrinol Metab. 2009;94:335–9. doi: 10.1210/jc.2008-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tassone F, Gianotti L, Emmolo I, Ghio M, Borretta G. Glomerular filtration rate and parathyroid hormone secretion in primary hyperparathyroidism. J Clin Endocrinol Metab. 2009;94:4458–61. doi: 10.1210/jc.2009-0587. [DOI] [PubMed] [Google Scholar]
- 29.Strong P, Jewell S, Rinker J, Hoch D, Crapo L. Thiazide therapy and severe hypercalcemia in a patient with hyperparathyroidism. West J Med. 1991;154:338–40. [PMC free article] [PubMed] [Google Scholar]
- 30.Insogna KL, Mitnick ME, Stewart AF, Burtis WJ, Mallette LE, Broadus AE. Sensitivity of the parathyroid hormone-1,25-dihydroxyvitamin D axis to variations in calcium intake in patients with primary hyperparathyroidism. N Engl J Med. 1985;313:1126–30. doi: 10.1056/NEJM198510313131805. [DOI] [PubMed] [Google Scholar]
- 31.Cooperberg MR, Duh QY, Stackhouse GB, Stoller ML. Oral calcium supplementation associated with decreased likelihood of nephrolithiasis prior to surgery for hyperparathyroidism. Int J Urol. 2007;14:1113–5. doi: 10.1111/j.1442-2042.2007.01904.x. [DOI] [PubMed] [Google Scholar]
- 32.Locker FG, Silverberg SJ, Bilezikian JP. Optimal dietary calcium intake in primary hyperparathyroidism. Am J Med. 1997;102:543–50. doi: 10.1016/s0002-9343(97)00053-3. [DOI] [PubMed] [Google Scholar]


