Abstract
Objective:
Vitamin D deficiency is an unrecognized epidemic and a common health problem worldwide. This study was conducted to evaluate the vitamin D status in children living in Jeddah, Saudi Arabia and to study its relation to various variables.
Materials and Methods:
A cross-sectional study was conducted in the pediatric clinic in Jeddah Clinic Hospital-Kandarah, Jeddah, KSA, from October through December 2010, in which 510 healthy children aged 4–15 years were enrolled. Serum calcium, phosphorus, alkaline phosphatase and 25-hydroxyvitamin D [25(OH)D] were measured. Dietary vitamin D intake and duration of daily sunlight exposure were determined. 25(OH)D levels <20 ng/mL and <7 ng/mL were defined as relative and severe vitamin D deficiency, respectively.
Results:
The mean concentration of 25(OH)D was 13.07 ± 7.81 ng/mL. Seventy subjects (13.72%) had normal 25(OH)D level ranging 20–70 ng/mL. Three hundred (58.82%) had relative 25(OH)D deficiency and 140 (27.45%) had severe deficiency (P=0.000). 220 (43.14%) subjects were males and 290 (56.86%) were females having a statistically significant higher incidence of 25(OH)D deficiency (P=0.019). 54.9% were Saudis, 27.45% were Yemenis and 11.76% were Egyptians. Saudis and Yemenis were more subjected to 25(OH)D deficiency in comparison to Egyptians and other nationalities (P=0.01). There were significant inverse correlations between 25(OH)D levels and bony aches (P=0.000). 56.25% of asymptomatic children had vitamin D deficiency (P=0.000). Duration of sunlight exposure and daily intake of vitamin D had significant effects on serum level of vitamin D (P=0.000).
Conclusions:
A high prevalence of vitamin D deficiency in children living in Jeddah was observed in this study. Vitamin D supplementation of food products can prevent vitamin D deficiency in these children.
Keywords: Bony aches, children, fracture, vitamin D
INTRODUCTION
Vitamin D is a prohormone that is essential for normal absorption of calcium from the gut, and deficiency of vitamin D is associated with rickets in growing children and osteomalacia in adults.[1]
Various studies have identified potential associations between vitamin D deficiency and a variety of diseases, including diabetes mellitus, metabolic syndrome, cancer, cardiovascular disease, multiple sclerosis, and neuromuscular malfunction.[2]
Vitamin D is primarily made in the skin after exposure to ultraviolet radiation (UVR) and <10% is derived from dietary sources.[3]
However, there are few dietary sources of vitamin D available to meet the daily recommended requirement.[4] In addition, many individual and environmental factors interfere with the ability to have sufficient sun exposure to produce vitamin D endogenously.[5]
In November 2008, the American Academy of Pediatrics (AAP) doubled the recommended daily intake of vitamin D for infants and children from 200 IU/day (2003 recommendation) to 400 IU/day.[6]
As calcium and vitamin D are primarily important for bone growth and development, ensuring their adequate intake is an important nutritional goal for children.[7] Providing infants younger than 1 year with a total intake of 400 IU vitamin D/day and older children with 600 IU/day is advised by the 2011 Institute of Medicine IOM report to meet the needs of nearly (98%) all children older than 1 year.[8]
There is controversy, however, about what is the healthy level of 25(OH)D for children and even what level of 25(OH)D should be used to define vitamin D deficiency.[9]
The 2011 IOM committee,[7] in agreement with the Pediatric Endocrine Society,[1] targeted a serum value for 25(OH)D of at least 50 nmol/L (20 ng/ml) as meeting the needs of nearly all children (and adults).[10]
Currently, individuals with serum levels of <11 ng/mL4 are classified as being vitamin D deficient. However, there is evidence that biochemical and skeletal sequelae of vitamin D deficiency may actually manifest at a higher cutoff levels of 30–35 ng/mL.[11]
Vitamin D deficiency is an unrecognized epidemic and a common health problem worldwide. It has been reported from many countries, including those with a lot of sunshine.[12] The prevalence of vitamin D deficiency has been reported to range from 15 to 80%.[4]
This study was conducted to evaluate vitamin D status in children living in Jeddah, Saudi Arabia, and to study its correlation with various variables including, most importantly, vitamin D intake, dietary and non-dietary sources and possible clinical clues as bony aches.
MATERIALS AND METHODS
A cross-sectional study was conducted in the pediatric clinic in Jeddah Clinic Hospital-Kandarah (JCH-K), Jeddah, KSA, from October through December 2010 after obtaining approval from the research ethics committee. Five hundred and ten healthy children, aged 4 to 15 years, were enrolled. Serum calcium, phosphorus, alkaline phosphatase (ALP) and 25-hydroxyvitamin D [25(OH)D] were measured. Dietary vitamin D intake,[13,14] duration of daily sunlight exposure and the percentage of body surface area (BSA) exposed to sunlight were determined. Each parent filled a questionnaire including a 3-day food frequency and the children's daily sunlight exposure (minutes/day). The percentage of BSA exposed to sunlight was estimated by asking about the commonly worn daytime clothes. Face and neck 3%, plantar surface of hands 2.5%, half of the arms besides forearm 4%, feet 2.5% and calves 6% were estimated and BSA was calculated using the Mosteller formula:[15] BSA (m2) = {[height (cm) × weight (kg)]/3600}½. By multiplying the measured percentage of body surface exposed by the result of the formula, the exposed BSA was measured in m2 and then converted to cm2.
Levels <20 ng/mL and <7 ng/mL were defined as relative and severe vitamin D deficiency, respectively.[10]
The specimen collected consisted of 4mL blood in a plain tube and 2 mL serum at 2°C–8°C. 25(OH)D was assessed using Diasorin's chemiluminescent immunoassay liaison. Serum calcium, phosphorus and ALP were measured on Olympus AU 400 chemistry autoanalyzer, serum calcium by photometric color (Arsenazo method) test, serum inorganic phosphorus by photometric UV test (phosphomolybdate formation method) and serum ALP by kinetic color test (p-nitrophenyl phosphate substrate in amino methyl propanol buffer or AMP).
Statistical analysis
Data were tabulated and subjected to analysis using Microsoft Excel version 5.0 and the Statistical Package for Social Science (SPSS) version 11.0. The following methods were employed:
Frequency distributions and percentage distributions.
Mean, standard deviation and range of numerical data.
Comparison of means using the Student's t-test; testing differences between means for statistical significance.
Non-numerical data were compared using the chi-square test.
In general, P values less than 0.05 were considered significant, less than 0.01 as highly significant and those below 0.001 as very highly significant.
RESULTS
A cross-sectional study was performed on 510 children presenting to pediatric clinic in JCH-K for different reasons from September 2010 through December 2010 [Table 1]. Their ages ranged between 4 and 15 years, with a mean age of 8.36 ± 2.85 years. Of them, 220 (43.14%) were males and 290 (56.86%) were females who had statistically significant higher incidence of 25(OH)D deficiency than males (P=0.019). 54.9% were Saudis, 27.45% were Yemenis and 11.76% were Egyptians. Saudis and Yemenis were more subjected to 25(OH)D deficiency in comparison to Egyptians and other nationalities (P=0.01). Their mean body weight (BW) was 27.68 ± 13.68 kg, mean height was 123.98 ± 16.6 cm, and the mean body mass index (BMI) was 17.12 ± 4.9. The average daily sun exposure was for 7.64 ± 7.49 min and the mean BSA exposed to the sun was 920 ± 1367 cm2. The average daily vitamin D intake was 133 ± 77.18 IU/day with 82.74% of cases receiving less than 200 IU/day. Three hundred and fifty (68.6%) had bony aches with 5.9% having history of fracture. The mean serum calcium was 9.95 ± 0.62 mg/dL, with 6.9% having hypocalcemia (<9 mg/dL). The mean serum phosphorus was 4.2 ± 0.8 mg/dL. The mean serum ALP was 528.25 ± 210.53 U/L, with 15.7% having serum ALP>640 U/L. The mean concentration of 25(OH)D was 13.07 ± 7.81 ng/mL. Seventy subjects (13.73%) had normal 25(OH)D level ranging 20–70 ng/mL. Three hundred (58.82%) had relative 25(OH)D deficiency and 140 (27.45%) had severe deficiency (P=0.000). There were significant inverse correlations between 25(OH)D levels and both BW and height (P=0.000). There was a significant positive correlation between 25(OH)D deficiency and bony aches, fractures, decreasing serum calcium level and increasing serum phosphorus and serum ALP levels (P=0.000). There was a significant inverse correlation between 25(OH)D level and BMI (P<0.05), as well as significant direct correlation between 25(OH)D level and duration of sunlight exposure, BSA exposed to sun and daily vitamin D intake (P=0.000).
Table 1.
Characteristics and 25(OH) vitamin D status in 510 children in Jeddah Clinic Hospital between September 2010 through December 2010

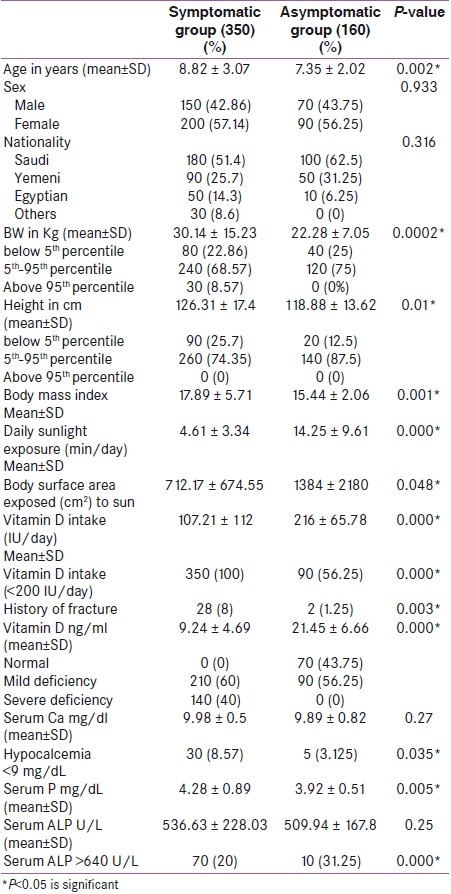
Table 2 presents a comparison between symptomatic cases having bony aches and asymptomatic cases. It is evident that symptomatic children showed statistically significant difference from asymptomatic group. They are older in age, have more BMI, less duration of daily sunlight exposure, lower BSA exposed to the sun, less daily vitamin D intake, higher incidence of fractures, lower serum level of vitamin D, higher serum phosphorus level, higher incidence of hypocalcemia and higher ALP levels (P<0.05).
Table 2.
Comparison between Symptomatic cases having bony aches and Asymptomatic cases
DISCUSSION
The prevalence of vitamin D deficiency in our cases was 72.55%, which is similar to that recorded by Khor et al.[16] in Malaysia and higher than that reported in other series.[4,17] It is lower than the 87% prevalence rate reported by McGillivray et al.[18] in East African immigrants living in Melbourne. Severe vitamin D deficiency (<7 ng/mL) was recorded in 27.45% of cases, which is a higher incidence than that found by Gordon et al.[19] and Das et al.[20]
The interesting issue in this study is the unexpected large proportion of healthy children showing vitamin D deficiency (72.55%) although KSA is a sunny country. There are increasing studies worldwide reporting on children with poor vitamin D status, including those in tropical countries.[21–24] The prevalence of vitamin D deficiency has been reported to range from 15 to 80%.[4] Increased prevalence among school-aged children and adolescents has been reported, reflecting modern-day lifestyle changes.[25]
Poor vitamin D status in children is likely to result from low dietary intake and inadequate exposure to sunshine. Unless fortified, most foods are poor sources of vitamin D, the exceptions being fish oils, egg yolk, and certain types of fish and sea food. Thus, it is not likely that children in KSA will obtain sufficient vitamin D from dietary sources alone.[16,26]
In the present study, the mean vitamin D was 13.07 ± 7.81 ng/mL, which is the lowest among other series.[26–28] Vitamin D deficiency is defined as a level <20 ng/mL as recommended by the 13th Workshop Consensus for Vitamin D Nutritional Guidelines held in British Columbia, Canada (April 2007).[29]
The age of our cases ranged between 4 and 15 years, with a mean of 8.36 ± 2.85 years. We found a significant increase in risk of vitamin D deficiency with older age (P=0.000) in accordance with the literature reports demonstrating that intake of vitamin D decreases with increasing age.[4] This is in contrast to the report of McGillivray[18] who found more vitamin D deficiency in younger age group. However, he studied only children up to 5 years of age.
Most children had normal growth and none was malnourished, according to the national reference of KSA,[30] as 70.6% of cases were between 5th and 95th percentiles and most of the children (96.5%) had normal height for age. This signifies that vitamin D deficiency is absolute rather than a part of general malnutrition and that nutrition education is important to avoid its deficiency.
In fact, we found significant inverse correlations between 25(OH)D levels and the BW, height and BMI (P=0.000).[2,4,19] This could be explained by the fact that the bioavailability of vitamin D, a fat-soluble vitamin, is decreased in obese individuals, which may result in the increased vitamin D deficiencies observed in such individuals.[31] This mechanism also could account for the inverse relationship between fat intake and vitamin D status. In contrast, other studies did not find any associations of BMI and/or fat mass with 25(OH)D levels in the pediatric population.[32,33]
Saudis and Yemenis were more subjected to 25(OH) D deficiency in comparison to Egyptians and other nationalities (P=0.01). Other studies found significant difference in the vitamin D levels between different ethnic groups,[19,25,26] giving special consideration to genetic variation and skin color.
There was a significant direct correlation between 25(OH) D levels and duration of sunlight exposure,[16,18,20] BSA exposed to sun and daily intake of vitamin D.[16,27,34] However, another study found that serum 25(OH)D concentration was not related to the estimated intake of vitamin D.[2,20]
The mean duration of sunlight exposure in our study was too short (7.64 ± 7.49 min/day) reflecting the absence of an important source of vitamin D as most of the children tend to spend more time indoors than outdoors. Moreover, indoor activity is sedentary in nature, e.g. doing homework, playing computer games and watching television. The same finding was found with Malaysians who generally avoid being outdoor during the day as the weather can be very hot and humid.[16] Increased urbanization and increased time spent indoors at work may lead to decreased time spent outdoors, and therefore decreased vitamin D synthesis, even in light-skinned populations. Shade reduces the amount of solar radiation by 60% and windowpane glass blocks UVR.[1]
Two hundred and twenty children (43.14%) were males and 290 (56.86%) were females having a statistically significant higher incidence of 25(OH)D deficiency (P=0.019).[9,16,18] Other series found no correlation of Vitamin D levels with sex.[9,32] This finding is more obvious in Arab Moslem countries as most of the girls follow a dress code of covered head, arms, and legs, similar to that found in Lebanese girls,[27] Kuwaiti women[1] and Saudi Arabian adolescents.[20]
There was a significant inverse correlation between 25(OH)D deficiency and BSA exposed to the sun,[20] which had an average of 920 ± 1367 cm2. This average was lower than that reported in other studies.[12,20] As at least 20% of the body's surface should be exposed to UV-B for blood vitamin D concentrations to increase, women and children in KSA, who wear traditional outfits, are at great risk for vitamin D deficiency. The nature of clothing is important, for example, black wool is twice as effective in absorbing and thus preventing transmission of incident UV-B radiation to the skin as white cotton.[1]
KSA is a tropical country, it has very hot weather in summer leading to restricted outdoor activity. So, we conducted our study in winter time, whereas sun and better climate allowing more outdoor activity and sun exposure; as known the majority (80%) of the vitamin D requirement comes from exposure to sunlight.[17] However, the results of vitamin D in our study were still very low. The major causes of low vitamin D levels seem to come from insufficient vitamin D supplementation, long-sleeved clothing, and limited outdoor lifestyle,[26] in addition to the use of sunscreen which we did not evaluate in this study as it is not usually used in our region due to the use of face veil and the usual avoidance of outdoor activity due to great humidity and hot weather.[1]
Both daily intake of vitamin D and sun exposure were significant predictors of vitamin D status, which is in agreement with other studies[12] and the fact that the main source of vitamin D is exposure to sunlight. The mean duration of sun exposure in our subjects was lower than that reported in other studies in Tehran.[20,27,35]
Unfortunately, most natural (unfortified) sources of vitamin D are not commonly consumed by children and there is possible inadequacy of the current level of vitamin D fortification of food products.[36] Therefore, fortifying food with vitamin D becomes important if there is inadequate sun exposure as in our study.[1]
However, we found that 82.74% of cases are receiving less than 200 IU vitamin D per day.[1,16] These results highlight the need to provide children with supplements and a diet rich in vitamin D.[12] In USA, where the national prevalence of vitamin D deficiency was 14%, approximately 50–60% of children receive the recommended USDA intake of 200 IU of vitamin D daily via diet and/or supplementation.[4] There is evidence that the recommended daily intake would need to be doubled to achieve a minimum serum 25(OH)D level of 20 ng/mL.[37]
There have been no studies of children, suggesting a level of sun exposure that would negate the need to comply with dietary vitamin D recommendations. Given the high prevalence of hypovitaminosis D, it seems clear that renewed attention must be paid to evaluate the adequacy of dietary and supplemental vitamin D intake and how much, if any, unprotected sun exposure is beneficial given the associated risks.[38]
In our study, we found a significant positive correlation between vitamin D deficiency and increasing level of ALP.[39] Elevated ALP was noted in 15.7% of our cases, which is higher than that reported in other studies[34] and lower than other reports.[18,40]
6.9% of cases had hypocalcemia, which is a lower incidence than that reported by Marwaha et al.[34] and higher than that reported by others.[18] There was significant direct correlation between the level of vitamin D and total serum calcium.[41]
In our study, there was a significant positive correlation between vitamin D deficiency and the incidence of bony aches as adequate vitamin D intake is of paramount importance to protect against bone metabolic diseases and prevent the occurrence of complications.[36,40]
The possibility of inadequate vitamin D intake should be considered in the differential diagnosis of chronic musculoskeletal pain. The process that links vitamin D to musculoskeletal pain is presumed to begin with a lack of circulating calcium due to inadequate vitamin D. This calcium deficiency, even if mild, stimulates increased parathormone secretion and sets in motion a cascade of biochemical reactions negatively affecting bone metabolism, as increased parathormone secretion can lead to softening of bone surfaces which generates pain in periosteal tissues covering the skeleton, as well as myopathy. In many cases involving pain and myopathy, defects of bone metabolism and osteomalacia may not be clinically detectable, but are nonetheless present or subclinical.[42]
Fracture prevalence in our study was 5.9%. Another study found a higher prevalence rate 15.4–18.6%.[27] There was significant positive correlation between vitamin D deficiency and the incidence of fractures.[36,43] 59% of African American children with fractures were vitamin D insufficient. This prevalence is higher than the baseline levels of vitamin D insufficiency reported in comparable populations. The effect of these insufficient levels on fracture risk in otherwise healthy children merits further careful evaluation.[44]
Bowden et al. did not find direct correlation between low vitamin D and fracture rate in their study, but they reported that their ability to detect a relationship may have been limited by the fact that all of the children in this referral group had previous fractures.[39] Schilling et al., in their study, found evidence of vitamin D deficiency, defined as a serum 25(OH)D level of <20 ng/mL, in >8% of the children with fractures.[45]
The limitations in our study include non-assessment of parathormone level and sun block use, and absence of a direct measure of fat mass besides BMI.
The point of strength in our study is that according to a recent search of database, this is the first study to evaluate vitamin D status in a wide range of the pediatric population and provides the prevalence rate of vitamin D deficiency in pediatric age group in Jeddah, KSA.
CONCLUSION
High prevalence of vitamin D deficiency in apparently healthy children living in Jeddah was observed in this study. Preschool routine screening for vitamin D status is advised to be done for all children. Parents and teachers should be provided with information on the importance of vitamin D in the growth and development of children. In addition, it is necessary to help parents make the correct dietary choices to improve vitamin D status of their children. Public health messages and interventions must educate the population about safe sun exposure. Vitamin D supplementation of food products, especially those directed to children, need to be enforced to prevent vitamin D deficiency. Further studies including larger number of subjects are recommended for proper evaluation; a broader approach would help in management of this unrecognized problem.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Misra M, Pacaud D, Petryk A, Collett-Solberg P, Kappy M. Vitamin D deficiency in children and its management: Review of current knowledge and recommendations. Pediatrics. 2008;122:398–417. doi: 10.1542/peds.2007-1894. [DOI] [PubMed] [Google Scholar]
- 2.Cole CR, Grant FK, Tangpricha V, Swaby-Ellis ED, Smith JL, Jacques A, et al. 25-Hydroxyvitamin D status of healthy, low-income, minority children in Atlanta, Georgia. Pediatrics. 2010;125:633–9. doi: 10.1542/peds.2009-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norris JM. Can the sunshine vitamin shed light on type 1 diabetes? Lancet. 2001;358:1476–8. doi: 10.1016/S0140-6736(01)06570-9. [DOI] [PubMed] [Google Scholar]
- 4.Saintonge S, Bang H, Gerber LM. Implications of a new definition of vitamin D deficiency in a multiracial US adolescent population: The national health and nutrition examination survey III. Pediatrics. 2009;123:797–803. doi: 10.1542/peds.2008-1195. [DOI] [PubMed] [Google Scholar]
- 5.Webb AR. Who, what, where and when: Influences on cutaneous vitamin D synthesis. Prog Biophys Mol Biol. 2006;92:17–25. doi: 10.1016/j.pbiomolbio.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Perrine CG, Sharma AJ, Jefferds ME, Serdula MK, Scanlon KS. Adherence to vitamin D recommendations among US infants. Pediatrics. 2010;125:627–32. doi: 10.1542/peds.2009-2571. [DOI] [PubMed] [Google Scholar]
- 7.National Academies Press. Washington, DC: Institute of Medicine; 2011. Dietary Reference Intakes for Vitamin D and Calcium. [Google Scholar]
- 8.Abrams SA. Dietary guidelines for calcium and vitamin D: A new era. Pediatrics. 2011;127:566–8. doi: 10.1542/peds.2010-3576. [DOI] [PubMed] [Google Scholar]
- 9.Mansbach JM, Ginde AA, Camargo CA., Jr Serum 25-Hydroxyvitamin D levels among US children aged 1 to 11 years: Do children need more vitamin D? Pediatrics. 2009;124:1404–10. doi: 10.1542/peds.2008-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagner CL, Greer FR. American Academy of Pediatrics Section on Breastfeeding; American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142–52. doi: 10.1542/peds.2008-1862. [DOI] [PubMed] [Google Scholar]
- 11.Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: Implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr. 2005;135:317–22. doi: 10.1093/jn/135.2.317. [DOI] [PubMed] [Google Scholar]
- 12.Ardestani PM, Salek M, Keshteli AH, Nejadnik H, Amini M, Hosseini SM, et al. Vitamin D status of 6- to 7-year-old children living in Isfahan, Iran. Endokrynol Pol. 2010;61:377–82. [PubMed] [Google Scholar]
- 13.Gebhardt SE, Lemar LE, Pehrsson PR, Exler J, Haytowitz DB, Patterson KK, et al. USDA National Nutrient Database for Standard Reference, Release 22. USDA National Nutrient Database for Standard Reference. 2009. Available: http://www.ars.usda.gov/nutrientdata .
- 14.Byrdwell WC. Comparison of analysis of vitamin D3 in Foods using ultraviolet and mass spectrometric detection. J Agric Food Chem. 2009;57:2135–46. doi: 10.1021/jf803398u. [DOI] [PubMed] [Google Scholar]
- 15.Mosteller RD. Simplified calculation of body surface area. N Engl J Med. 1987;317:1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 16.Khor GL, Chee WS, Shariff ZM, Poh BK, Arumugam M, Rahman AJ, et al. High prevalence of vitamin D insufficiency and its association with BMI-for-age among primary school children in Kuala Lumpur, Malaysia. BMC Public Health. 2011;11:95. doi: 10.1186/1471-2458-11-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holick MF. High prevalence of vitamin D inadequacy and implication for health. Mayo Clinic Proc. 2006;81:353–73. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 18.McGillivray G, Skull SA, Davie G, Kofoed SE, Frydenberg A, Rice J, et al. High prevalence of asymptomatic vitamin D and iron deficiency in East African immigrant children and adolescents living in a temperate climate. Arch Dis Child. 2007;92:1088–93. doi: 10.1136/adc.2006.112813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med. 2004;158:531–7. doi: 10.1001/archpedi.158.6.531. [DOI] [PubMed] [Google Scholar]
- 20.Das G, Crocombe S, McGrath M, Berry JL, Mughal MZ. Hypovitaminosis D among healthy adolescent girls attending an inner city school. Arch Dis Child. 2006;91:569–72. doi: 10.1136/adc.2005.077974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lips P. Vitamin D status and nutrition in Europe and Asia. J Steroid Biochem Mol Biol. 2007;103:620–5. doi: 10.1016/j.jsbmb.2006.12.076. [DOI] [PubMed] [Google Scholar]
- 22.Bener A, Al Ali M, Hoffmann GF. High prevalence of vitamin D deficiency in young children in a highly sunny humid country: A global health problem. Minerva Pediatr. 2009;61:15–22. [PubMed] [Google Scholar]
- 23.Peters BS, dos Santos LC, Fisberg M, Wood RJ, Martini LA. Prevalence of vitamin D insufficiency in Brazilian adolescents. Ann Nutr Metab. 2009;54:15–21. doi: 10.1159/000199454. [DOI] [PubMed] [Google Scholar]
- 24.Papandreou D, Malindretos P, Karabouta Z, Rousso I. Possible health implications and low vitamin D status during childhood and adolescence: An updated mini review. Int J Endocrinol. 2010;2010:472173. doi: 10.1155/2010/472173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagner CL, Greer FR. The Section on Breastfeeding and Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142–52. doi: 10.1542/peds.2008-1862. [DOI] [PubMed] [Google Scholar]
- 26.Oren Y, Shapira Y, Agmon-Levin N, Kivity S, Zafrir Y, Altman A, et al. Vitamin D insufficiency in a sunny environment: A demographic and seasonal analysis. Isr Med Assoc J. 2010;12:751–6. [PubMed] [Google Scholar]
- 27.Fuleihan GE, Nabulsi M, Choucair M, Salamoun M, Shahine CH, Kizirian A, et al. Hypovitaminosis D in healthy schoolchildren. Pediatrics. 2001;107:e53. doi: 10.1542/peds.107.4.e53. [DOI] [PubMed] [Google Scholar]
- 28.Reis JP, Mühlen DV, Miller ER, 3rd, Michos ED, Appel LJ. Vitamin D status and cardiometabolic risk factors in the United States adolescent population. Osteoporos Int. 2006;17:1133–40. doi: 10.1542/peds.2009-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norman AW, Bouillon R, Whiting SJ, Vieth R, Lips P. 13th workshop consensus for vitamin D nutritional guidelines. J Steroid Biochem Mol Biol. 2007;103:204–5. doi: 10.1016/j.jsbmb.2006.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al Herbish AS, Al Salloum AA, Al Omar AA, Al Qureshi MM, El Mouzan MI, Foster PJ, et al. Body mass index in Saudi Arabian children and adolescents: A national reference and comparison with international standards. Ann Saudi Med. 2009;29:342–7. doi: 10.4103/0256-4947.55162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong Y, Pollock N, Stallmann-Jorgensen IS, Gutin B, Lan L, Chen TC, et al. Low 25-hydroxyvitamin D levels in adolescents: Race, season, adiposity, physical activity, and fitness. Pediatrics. 2010;125:1104–11. doi: 10.1542/peds.2009-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parry J, Sullivan E, Scott AC. Vitamin D sufficiency screening in preoperative pediatric orthopaedic patients. J Pediatr Orthop. 2011;31:331–3. doi: 10.1097/BPO.0b013e3182104a94. [DOI] [PubMed] [Google Scholar]
- 33.Weng FL, Shults J, Leonard MB, Stallings VA, Zemel BS. Risk factors for low serum 25- hydroxyvitamin D concentrations in otherwise healthy children and adolescents. Am J Clin Nutr. 2007;86:150–8. doi: 10.1093/ajcn/86.1.150. [DOI] [PubMed] [Google Scholar]
- 34.Marwaha RK, Tandon N, Agarwal N, Puri S, Agarwal R, Singh S, et al. Impact of two regimens of vitamin D supplementation on calcium - vitamin D - PTH axis of schoolgirls of Delhi. Indian Pediatr. 2010;47:761–9. doi: 10.1007/s13312-010-0116-0. [DOI] [PubMed] [Google Scholar]
- 35.Dahifar H, Faraji A, Ghorbani A, Yassobi S. Impact of dietary and lifestyle on vitamin D in healthy student girls aged 11-15 years. J Med Invest. 2006;53:204–8. doi: 10.2152/jmi.53.204. [DOI] [PubMed] [Google Scholar]
- 36.Elsammak MY, Al-Wosaibi AA, Al-Howeish A, Alsaeed J. Vitamin D deficiency in Saudi Arabs. Horm Metab Res. 2010;42:364–8. doi: 10.1055/s-0030-1248296. [DOI] [PubMed] [Google Scholar]
- 37.Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25 hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204–10. doi: 10.1093/ajcn/77.1.204. [DOI] [PubMed] [Google Scholar]
- 38.Council on Environmental Health and Section on Dermatology. Ultraviolet radiation: A hazard to children and adolescents. Pediatrics. 2011;127:588–97. doi: 10.1542/peds.2010-3501. [DOI] [PubMed] [Google Scholar]
- 39.Bowden SA, Robinson RF, Carr R, Mahan JD. Prevalence of vitamin D deficiency and insufficiency in children with osteopenia or osteoporosis referred to a pediatric metabolic bone clinic. Pediatrics. 2008;121:e1585–90. doi: 10.1542/peds.2007-2111. [DOI] [PubMed] [Google Scholar]
- 40.Crocombe S, Mughal MZ, Berry JL. Symptomatic vitamin D deficiency among non-Caucasian adolescents living in the United Kingdom. Arch Dis Child. 2004;89:197–9. doi: 10.1136/adc.2003.026799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar J, Muntner P, Kaskel FJ, Hailpern SM, Melamed ML. Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001-2004. Pediatrics. 2009;124:e362–70. doi: 10.1542/peds.2009-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leavitt SB. Vitamin D- A neglected Analgesic for chronic musculoskeletal pain. Pain Treatment Topics. Practitionner briefing. 2008. Jun, [Last accessed on 2011 Aug 25]. Available from: http://www.Pain-Topics.org/VitaminD .
- 43.Davies JH, Shaw NJ. Preventable but no strategy: Vitamin D deficiency in the UK. Arch Dis Child. 2011;96:614–5. doi: 10.1136/adc.2010.191627. [DOI] [PubMed] [Google Scholar]
- 44.Ryan LM, Brandoli C, Freishtat RJ, Wright JL, Tosi L, Chamberlain JM. Prevalence of vitamin D insufficiency in African American children with forearm fractures: A preliminary study. J Pediatr Orthop. 2010;30:106–9. doi: 10.1097/BPO.0b013e3181d076a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paterson CR. Vitamin D deficiency and fractures in childhood. Pediatrics. 2011;127:973–4. doi: 10.1542/peds.2011-0086. [DOI] [PubMed] [Google Scholar]


