To the Editor
Cross-reactive carbohydrate determinants are widely occurring IgE epitopes. Glycan-related IgE reactivity has been demonstrated in most allergen sources, especially in the plant kingdom.1 The clinical effect of these cross-reactive carbohydrate determinants is debated.
We were recently able to show that IgE Abs to the cat IgA, present in cat-sensitized patients, are mainly directed to a glycan moiety localized on the α-chain.2 In addition, we have reported that these carbohydrates are present on IgM Abs from cat, as well as on IgM from many different mammalian species, but not human immunoglobulins.3 Interestingly, IgE antibodies to cat IgM and cat IgA show a complete cross-reactivity, whereas cat IgG does not, suggesting an identical oligosaccharide on the 2 former immunoglobulin classes. Because this is the first mammalian carbohydrate IgE epitope found, it is of major interest to identify the carbohydrate structure responsible for the broad cross-reactivity.
Chung et al4 have recently investigated subjects with anaphylactic reactions after treatment with the drug cetuximab, a chimeric mouse–human IgG1 mAb against the epidermal growth factor receptor, which is approved for use in colorectal cancer and squamous-cell carcinoma of the head and neck. The authors found that a carbohydrate epitope on the mouse Fab portion, galactose-α-1,3-galactose, a part of the Galα1,3Galβ1,4GlcNAc-R (α-gal) epitope, was responsible for the IgE binding. Furthermore, in most subjects, the IgE antibodies against cetuximab were present in serum before therapy.
The α-gal epitope is expressed on many different glycoproteins in mammals, except for old world monkeys, apes, and human beings. Species lacking the α-gal residues produce large quantities of IgG antibodies to this epitope.5 Studies have demonstrated that approximately 1% of antibodies in all healthy subjects are directed to α-gal.6 These antibodies also react with closely related carbohydrate structures in the ABO blood group and are one of the major obstacles in xenotransplantation.
Here we investigated whether α-gal is present on cat IgA and whether it is a major epitope responsible for IgE binding to cat IgA.
Cat IgA was purified from cat serum,3 and α-gal–human serum albumin was obtained from Dextra Laboratories, Reading, United Kingdom. To investigate the presence of α-gal on cat IgA, a monoclonal anti-Gal antibody was used in ELISA. Plates were coated with 5 μg/mL α-gal, cat IgA, or recombinant Fel d 1,7 which was included as negative control. Incubation with monoclonal anti-Gal antibodies (Alexis Biochemicals, Lausen, Switzerland), diluted 1:25, was followed by antimouse–IgG-alkaline phosphatase (Dako, Glostrup, Denmark) and substrate solution (Sigma, Steinheim, Germany). We found that the anti-Gal reactivity to α-gal and cat IgA was almost identical, whereas no reactivity was detected to recombinant Fel d 1 (Table I).
TABLE I.
Comparison of monoclonal antigalactose reactivity to solid phase bound α-gal, cat IgA, and recombinant Fel d 1 (rFel d 1) by ELISA
| Antigen | OD (450 nm) |
|---|---|
| α-gal | 0.69 |
| Cat IgA | 0.67 |
| rFel d 1 | 0.05 |
Twenty sera from the United States, 9 from patients who were found to have IgE antibodies to the α-gal epitope on cetuximab by using the streptavidin CAP technique,8 (range, 0.79 to >100 kilo Units per Liter [kUA/L]; median, 61.1 kUA/L) and 11 negative controls were examined. Eight of the 9 patients showed positive IgE responses to cat dander (range, 0.61–61.8 kUA/L; median, 17.8 kUA/L). The US sera were tested blind. Sera from 6 Swedish patients with cat allergy with IgE responses to cat IgA (range, 0.47–13.0 kUA/L; median, 1.5 kUA/L)2 were included.
We investigated by ELISA the correlation between IgE responses to cat IgA and α-gal, whether the cetuximab-positive sera from the United States had IgE antibodies to cat IgA, and whether this response could be blocked by α-gal. Likewise, we elucidated whether our Swedish cat IgA-positive sera had IgE antibodies to α-gal and whether the IgE reactivity to cat IgA could be inhibited by α-gal.
Direct ELISA with solid-phase bound α-gal and cat IgA was performed as described.2 Nine US sera showed IgE reactivity to cat IgA (OD, range, 0.302–2.712; median, 1.548) as well as to α-gal (OD, range, 0.364–2.575; median, 1.291) and were later confirmed to be cetuximab-positive sera. The 11 negative controls exhibited low values for IgA (OD, range, 0.066–0.196; median, 0.129) and α-gal (range, 0.043–0.207; median, 0.107). All but 1 of the cat IgA-positive Swedish sera displayed IgE responses to α-gal (OD, range, 0.043–0.989; median, 0.412). The reason for the single negative result is presumably the presence of epitopes other than α-gal on cat IgA. Our previous study revealed the presence of epitopes other than carbohydrates, and 1 serum displayed even higher IgE reactivity to cat IgA after de-glycosylation.2 The results in direct ELISA demonstrated a high correlation between IgE reactivity to α-gal and cat IgA (r = 0.98; Fig 1).
FIG 1.
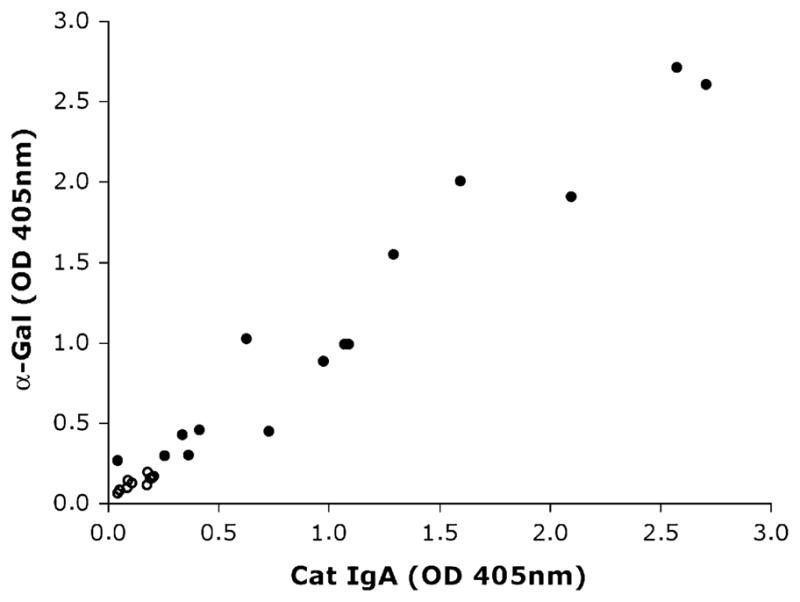
Reaction of IgE antibodies in sera from 6 Swedish patients with cat allergy, 9 US cetuximab-reactive patients (closed circles), and 11 US controls (open circles), tested by ELISA on wells coated with α-gal (y-axis) and cat IgA (x-axis).
In the next step, inhibition ELISA was performed with 1 Swedish and 1 US sera by using solid-phase bound cat IgA (5 μg/mL) and α-gal as competing antigen (3-fold dilutions from 300 μg/mL to 0.14 μg/mL). The responses were dose-dependent and similar for both sera (Fig 2). Complete inhibition was achieved at the highest antigen concentration (300 μg/mL), whereas no inhibition was detected with the lowest antigen concentration.
FIG 2.
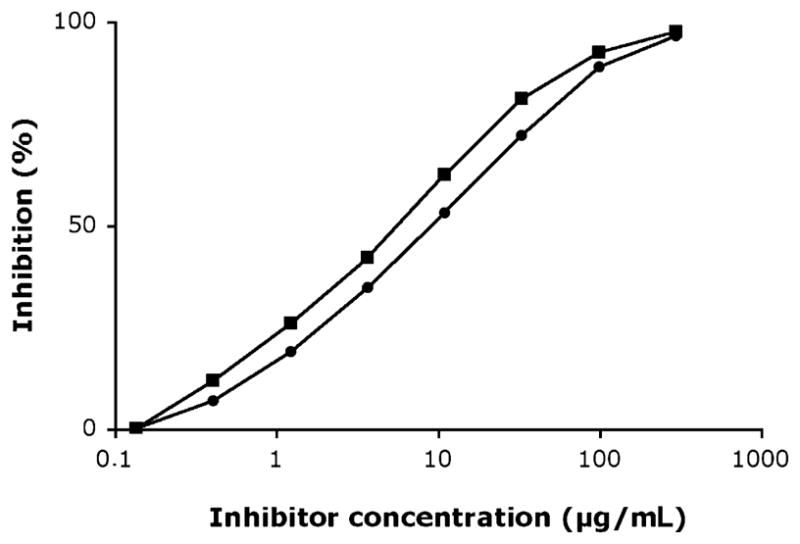
IgE competition ELISA using solid-phase bound cat IgA and α-gal as inhibitor performed on 1 serum with IgE antibodies to cat IgA from Sweden (filled boxes) and 1 serum with IgE antibodies to cetuximab from the United States (filled circles).
In conclusion, we have shown that the previously described dominant IgE epitope on cat IgA2 is the carbohydrate α-gal. This epitope exists on a large number of other mammalian proteins. Although we are exposed to mammalian proteins carrying the α-gal epitope both in the air (eg, cat, dog, or horse immunoglobulins) and by ingestion of foods such as beef, pork, or cow’s milk,9 current evidence suggests that IgE responses to α-gal may be induced by infections or parasitic agents. This epitope has, in addition, been demonstrated to be responsible for potentially life-threatening anaphylactic reactions to cetuximab in patients who have specific IgE antibodies to α-gal. The results suggest that screening for IgE antibodies to cat IgA or the α-gal epitope itself might help to identify patients who are at risk for anaphylaxis when treated with cetuximab.
Acknowledgments
Supported by grants from the Swedish Asthma and Allergy Association’s Research Foundation, the Swedish Cancer and Allergy Foundation, Konsul Th Bergs Foundation, the Swedish Research Council, the Swedish Heart-Lung Foundation, the Center for Allergy Research, the King Gustaf V 80th Birthday Foundation, the Hesselman Foundation, Karolinska Institutet, and the Stockholm County Council.
Footnotes
Disclosure of potential conflict of interest: H. Grönlund and M. van Hage have received research support from the Swedish Cancer and Allergy Foundation and the Asthma and Allergy Association’s Research Foundation. M. van Hage has also received research support from the Swedish Heart-Lung Foundation. S. P. Commins has received a fellowship grant from GlaxoSmithKline and the American Academy of Allergy, Asthma & Immunology. T. A. E. Platts-Mills has served on the scientific advisory board of Indoor Biotechnologies and has received research support from Phadia and ImClone, Inc. The other author has declared that he has no conflict of interest.
References
- 1.Altmann F. The role of protein glycosylation in allergy. Int Arch Allergy Immunol. 2006;142:99–115. doi: 10.1159/000096114. [DOI] [PubMed] [Google Scholar]
- 2.Adedoyin J, Grönlund H, Oman H, Johansson SG, van Hage M. Cat IgA, representative of new carbohydrate cross-reactive allergens. J Allergy Clin Immunol. 2007;119:640–5. doi: 10.1016/j.jaci.2006.11.637. [DOI] [PubMed] [Google Scholar]
- 3.Adedoyin J, Johansson SG, Grönlund H, van Hage M. Interference in immunoassays by human IgM with specificity for the carbohydrate moiety of animal proteins. J Immunol Methods. 2006;310:117–25. doi: 10.1016/j.jim.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358:1109–17. doi: 10.1056/NEJMoa074943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macher BA, Galili U. The Galalpha1,3Galbeta1,4GlcNAc-R (alpha-Gal) epitope: a carbohydrate of unique evolution and clinical relevance. Biochim Biophys Acta. 2008;1780:75–88. doi: 10.1016/j.bbagen.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galili U, Rachmilewitz EA, Peleg A, Flechner I. A unique natural human IgG antibody with anti-alpha-galactosyl specificity. J Exp Med. 1984;160:1519–31. doi: 10.1084/jem.160.5.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grönlund H, Bergman T, Sandstrom K, Alvelius G, Reininger R, Verdino P, et al. Formation of disulfide bonds and homodimers of the major cat allergen Fel d 1 equivalent to the natural allergen by expression in Escherichia coli. J Biol Chem. 2003;278:40144–51. doi: 10.1074/jbc.M301416200. [DOI] [PubMed] [Google Scholar]
- 8.Erwin EA, Custis NJ, Satinover SM, Perzanowski MS, Woodfolk JA, Crane J, et al. Quantitative measurement of IgE antibodies to purified allergens using streptavidin linked to a high-capacity solid phase. J Allergy Clin Immunol. 2005;115:1029–35. doi: 10.1016/j.jaci.2004.12.1131. [DOI] [PubMed] [Google Scholar]
- 9.Commins SP, Satinover SM, Hosen J, Mozena J, Borish L, Lewis BD, et al. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2009;123:426–33. doi: 10.1016/j.jaci.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]


