Summary
The study assessed the effectiveness and safety of endovascular covered stents in the management of intracranial pseudoaneurysms, fusiform aneurysms and direct carotid-cavernous fistulas.
Fourteen endovascular covered stents were used to repair three pseudoaneurysms, six fu-siform aneurysms and six direct carotid-cavernous fistulas. Aneurysms were in the carotid artery in seven cases, in the vertebral artery two cases. It was not possible to treat two additional cases transcutaneously for technical reasons 2/15.
Percutaneous closure of the lesions with an endovascular covered stent was successful in 13 of 15 cases. Initial follow-up showed good stent patency. No complications were observed after stent implantation. During follow-up, stent thromboses were detected in two of nine patients with follow-up digital subtracted angiography. One carotid-cavernous fistula of Barrow Type A transformed into Barrow Type D at nine month follow-up study was cured with a procudure of Onyx-18 injection.
Endovascular covered stents may be an option for percutaneous closure of intracranial pseudoaneurysms, fusiform aneurysms and direct carotid-cavernous fistulas. Endoluminal vascular repair with covered stents offers an alternative therapeutic approach to conventional modalities.
Key words: covered stent, endovascular treatment, aneurysm, carotid-cavernous fistula
Introduction
The covered stent has recently been proved to be an acceptable alternative to treatment of carotid, vertebral aneurysms and direct carotid-cavernous fistulas (DCCFs)1-8,10-22. Carotid artery pseudoaneurysm treated by covered stent graft has been performed by Ahuja et Al.1. Pero et Al reported that a middle cerebral artery aneurysm was cured by placing a covered stent17. Manda et Al. described placement of a covered stent as another option for DCCFs16. These early results with the covered stent are encouraging and supportive of the need to continue to pursue this route in applicable cases. The present manuscript can potentially add to the published literature on the management of these lesions.
Patients and Techniques
Fifteen patients with intracranial pseudoaneurysms, fusiform aneurysms and DCCFs (from June 2005 to December 2005) were included in this study. Thirteen consecutive male patients were treated percutaneously with implantation of a covered stent (JoStent; JoMed, Abbott) for repair of lesions. We considered this treatment in two additional patients. We elected to have the stent-coil technique performed in the two cases because of tortuous parent vessels.
All patients underwent cerebral angiographic evaluations before treatment. Of the seven internal carotid aneurysms, four were located at the ophthalmic segment, three at the cavernous segment. Two vertebral aneurysms were located at the subdural segment, where no indispensable cerebral perforating arteries originated. Among these aneurysms there were three pseudoaneurysms secondary to head trauma or surgical injury, and six fusiform aneurysms, all of which were difficult to obliterate with detachable coils. The six patients with DCCF were all initially treated with detachable balloons in other hospitals. The fistulae were recurrent because of deflation of the balloons in four patients. The balloon could not flow into the fistula in one patient because of a linear tear in the cavernous portion of the internal carotid artery. In another patient the fistula was partially occluded with a 2# detachable balloon. We initially treated only those patients in whom conventional modalities were considered impossible or particularly risky. All patients were explained the nature of their treatment and signed an informed consent statement. Intracranial stent placement into intracranial vessels is viewed as a standard therapy in our hospital. The patients mean age was 40 years (14 to 62 years). Digital subtraction angiography showed a carotid pseudoaneurysm in three patients, a carotid dissection in four patients, a vertebral dissection in two patients, a DCCF in six patients. Four aneurysms were =or >10 mm and the other five aneurysms were <10 mm.
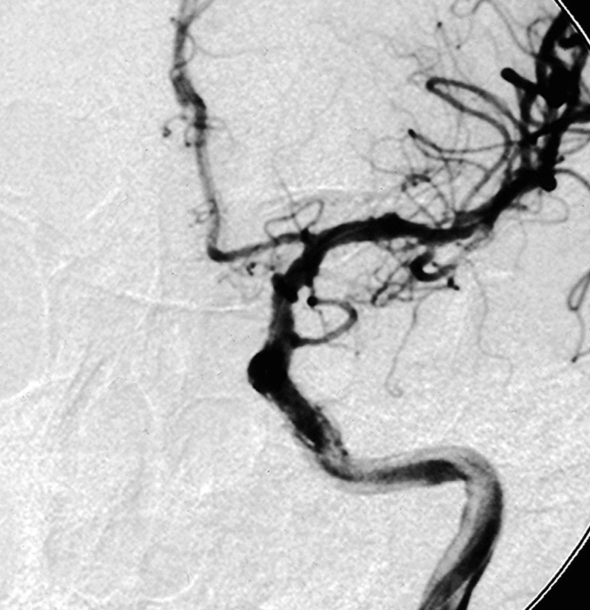
Figure 1.
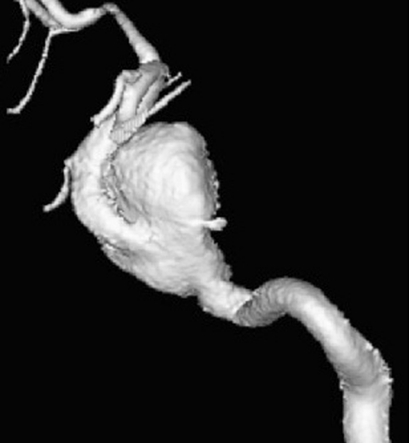
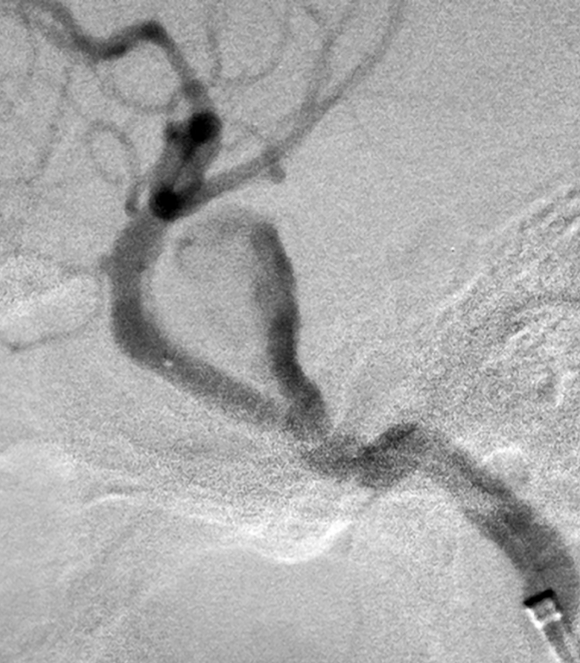
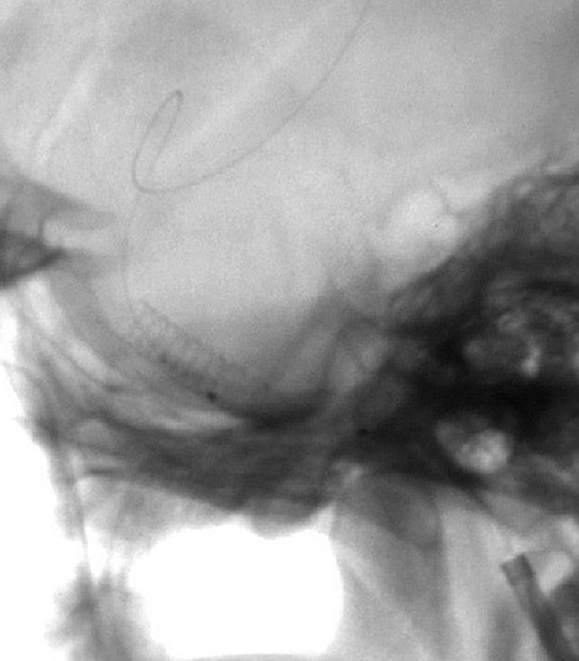
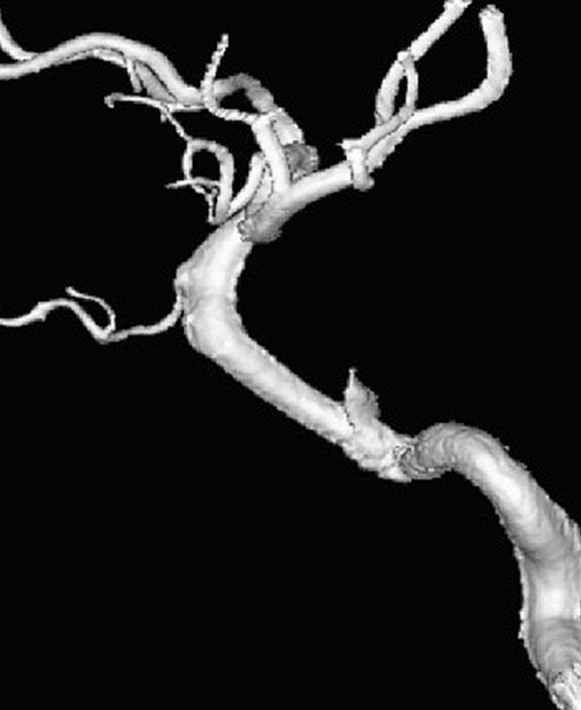
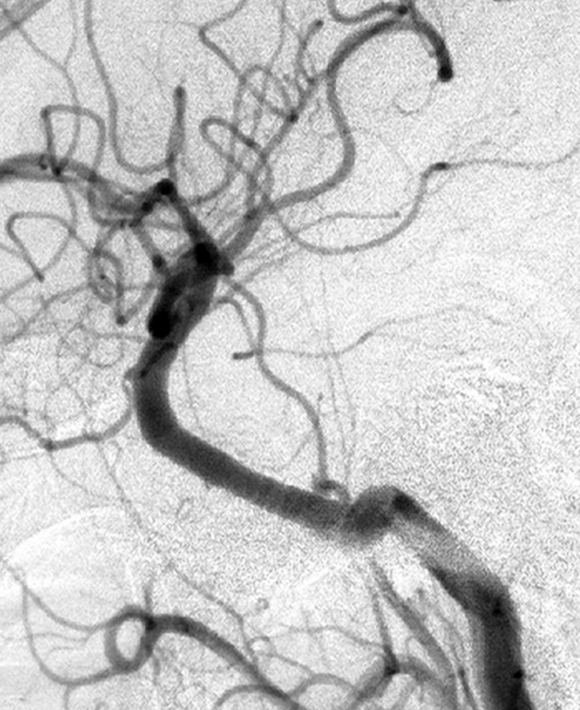
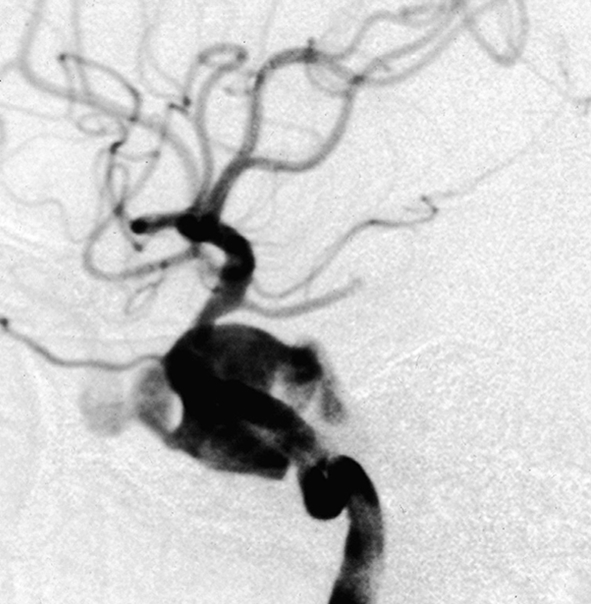
Patient 6. This 40-year-old man presented with a giant fusiform aneurysm involving the cavernous carotid artery. Left internal carotid angiogram, 3-D reconstruction (A) showing the aneurysm. The left internal carotid angiogram after deployment of the first covered stent (4.5 mm x 16 mm), lateral projection (B) demonstrated the aneurysm was still filled. C) Fluoroscopic image demonstrated another 4.5 mm x 19 mm covered stent was advanced over the exchange wire. Note the first covered stent in place. Left internal carotid angiogram 3-D reconstruction (D) after embolization showing the aneurysm was subtotally obliterated. Left common carotid angiogram lateral projection (E) after six months showing the aneurysm was completely occluded with patent carotid artery.
A.
B.
C.
D.
E.
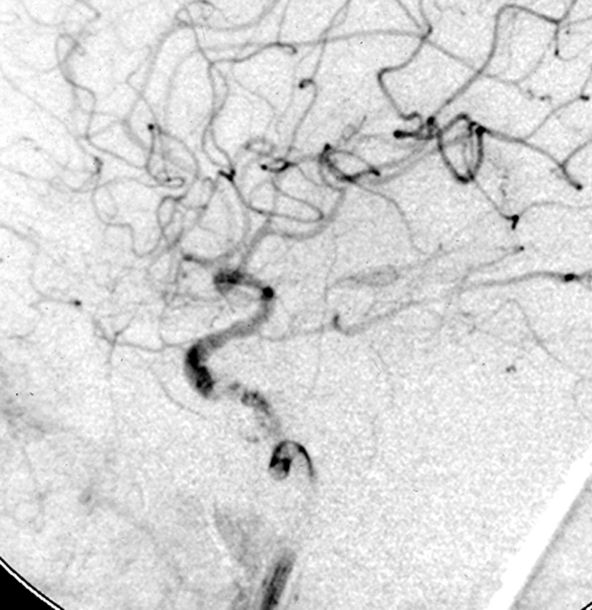
Figure 2.
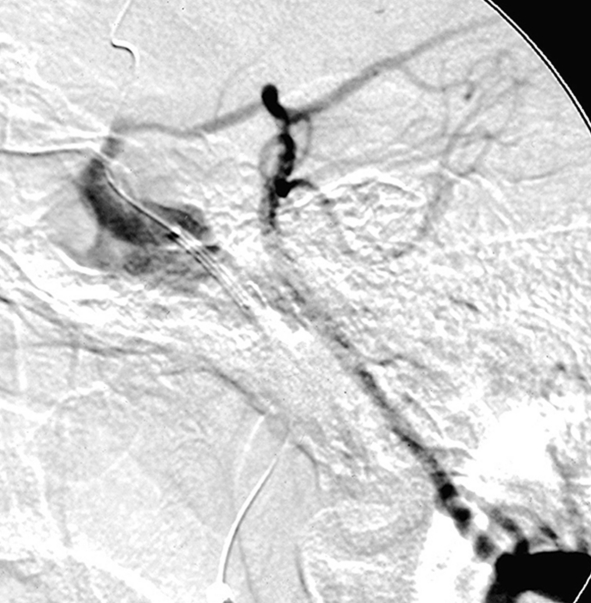
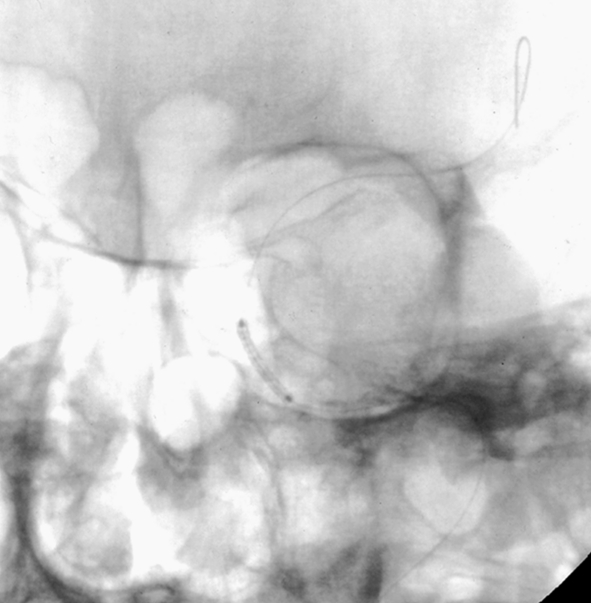
Patient 11. This 20-year-old man was involved in a high-speed motor vehicle accident and suffered a cranial injury. Two months later, chemosis and III, VI cranial palsy developed and the patient was diagnosed as CCF of Barrow Type A, which could not be closed by using a 2 detachable balloon because of a linear tear in the cavernous portion of the ICA. Left internal carotid angiogram lateral projection (A) showing a CCF fed by left internal carotid artery, mainly drained to the superior ophthalmic vein. The left vertebral angiogram, lateral projection (B) demonstrated the exchanging wire was crossed the fistula point and the tip was placed in the middle cerebral artery. Note the covered stent was advanced to the fistula point. C) Fluoroscopic image demonstrates the endovascular treatment using a 4 mm x 19 mm covered stent (JoStent; JoMed, Abbott). Left internal carotid angiogram, anteroposterior projection (D) after embolization showing the fistula was completely obliterated. Left internal carotid (E) angiogram after six months showing the CCF was completely occluded.
A.
B.
C.
D.
E.
Endovascular therapy
All patients were premedicated with clopidogrel, 7 mg/day, and aspirin, 300 mg/day, starting three days before the treatment and were treated under general anesthesia. Systemic heparinization was achieved during the procedures with heparin 5000U bolus followed by 1000U of heparin every hour. After placement of an 8F guiding catheter (Cordis, Miami Lakes, FL) in the parent artery via a transfemoral approach. The lesion was bypassed with a microcatheter distal to the aneurysm neck or the fistula. A 300-cm 0.014-inch exchanging wire (Floppy, Boston Scientific) was placed through the microcatheter and the microcatheter was withdrawn. A Jostent coronary stent graft, the size of which was determined according to the size of the lesion and the parent artery lumen (see Table 1), was navigated over the exchanging wire and placed across the aneurysmal neck or the fistula. Before deployment of the stent, cerebral angiograms must be evaluated carefully to confirm optimal position and to avoid compromising any important branches, such as the anterior choroidal artery and the posterior inferior cerebellar artery.
Table 1.
Clinical data of the fifteen patients with intracranial aneurysms and carotid-cavernous fistulas.
| Stent | Clinical | |||||||
|---|---|---|---|---|---|---|---|---|
| Patient | Age | Aneurysm | Size | Trau- | size | Follow-up | follow-up | |
| No. | yr/Sex | Location | (mm) | Symptom | ma | (mm) | Angiography | outcome |
|
| ||||||||
| 1 | 37 M | ICA-Oph | 16x11 | Epistaxis | + | 4x16 | 3-m, no recanalization | 12-m. |
| ICA occlusion | no symptoms | |||||||
|
| ||||||||
| 2 | 40 M | ICA-Oph | 4x6 | SAH | + | 3.5x12 | 6-m, no recanalization | 18-m, |
| ICA occlusion | no symptoms | |||||||
|
| ||||||||
| 3 | 55 M | ICA-Cav | 6x6 | 6n Palsy | + | 4x12 | no | 14-m, |
| no symptoms | ||||||||
|
| ||||||||
| 4 | 62 M | ICA-Cav | 18x20 | 3n, 6n Palsy | – | 4x19 | 6-m, no recanalization | 16-m, |
| patent ICA | no symptoms | |||||||
|
| ||||||||
| 5 | 14 M | ICA-Oph | 8x7 | Headache | – | 3.5x12 | no | 24-m, |
| no symptoms | ||||||||
|
| ||||||||
| 6 | 40 M | ICA-Cav | 21x27 | 3n, 6n Palsy | – | 4.5x16,4.5x19 | 6-m, no recanalization | 13-m, |
| patent ICA | no symptoms | |||||||
|
| ||||||||
| 7 | 40 M | VA-Sub | 8x15 | SAH | – | 4x19 | 6-m, no recanalization | 25-m, |
| patent ICA | no symptoms | |||||||
|
| ||||||||
| 8 | 55 F | ICA-Oph | 10x10 | Headache | – | 4x19 (failed) | no | 17-m, |
| no symptoms | ||||||||
|
| ||||||||
| 9 | 42 M | VA-Sub | 6x10 | SAH | – | 4x19 (failed) | no | 12-m, |
| no symptoms | ||||||||
|
| ||||||||
| M, male; F, female; ICA-Oph, ophthalmic segment of the internal carotid artery; | ||||||||
| ICA-Cav, cavernous segment of the internal carotid artery; VA-Sub, subdural segment of the vertebral artery; | ||||||||
| CCF, carotid-cavernous fistula; IIIcn, third cranial nerve; VI cn, sixth cranial nerve; SAH, subarachnoid hemorrhage; y, year; m, month | ||||||||
The origin of ophthalmic artery can be covered because the ophthalmic artery can be refilled via the branches of the external carotid artery. The posterior communicating artery can also be covered if it is not of fetal type. Control angiograms were obtained to confirm full coverage of the aneurysm neck or the fistula by the stent. The balloon was always inflated very slowly up to the nominal pressure of 12-14 atm so that the stent would be expanded to a diameter of 3.5-4.5 mm. If a contrast endoleak into the aneurysm or the fistula was noted upon deflation of the balloon, we often obtained another control angiogram after five to 15 minutes. If the endoleak persisted, the balloon was reinflated up to a pressure of 14-16 atm in order to appose the stent graft to the vessel wall. If necessary another covered stent would be deployed. Systemic heparinization was administered for 24 hours after procedure and subcutaneous injection of low molecular weight heparin was given for two days. Antiplatelet agents (clopidogrel 75 mg and aspirin 300 mg daily) were administered orally for six months after that. Follow-up angiography was performed in nine patients and all patients were contacted at two year follow-up period by telephone.
Results
Stent placement was technically possible and was successfully carried out in 13 of 15 patients. All the aneurysms (n=9) and DCCFs (n=6) were totally closed in 13 patients. In one patient, a second larger (4.5 mm) covered stent was used to appose the proximal end of the stent graft to the ICA wall, which was longer than the first stent graft, sealing the endoleak into the aneurysm. Fourteen stent grafts were successfully deployed 13 patients leading to total obliteration of the aneurysms and fistulae with patent parent arteries and favorable clinical outcomes. There were two technical failures secondary to proximal tortuosity, one with ophthalmic segment aneurysm and one with vertebral aneurysm, they were treated with stent-coil technique and parent artery occlusion with detachable coils respectively and the clinical outcomes were good.
Follow-up Results
Among nine patients with follow-up angiography, two had angiographic morbidity and developed asymptomatic occlusion of the ICA. Their immediate control angiogram demonstrated excellent flow with no obvious ICA dissection. The two patients interrupted the prescribed antiplatelet medications soon after discharge, which we believe precipitated thrombotic occlusion of the stent graft. The three month and six month follow-up angiograms demonstrated the occlusion the stented ICAs and preservation of the intracranial circulation through the contralateral flow. The other seven patients had angiographic follow-up at six months, all showing persistent exclusion of the aneurysm and fistula with patent stent graft and intracranial ICA and VA. Only one patient with CCF of Barrow Type A demonstrated a CCF of Barrow Type D, which was cured with Onyx-18 throughout his nine month follow-up. The other six patients had no evidence of intimal hyperplasia. The mean clinical follow-up period was 17.2 months (range 12 to 25 months), and all 15 patients were without neurological deficits.
Discussion
Covered stents have been successfully used for the treatment of fusiform aneurysm, iatrogenic injury of the cavernous carotid artery and cavernous-carotid fistula2,4,5,9,10,16-22. This technique may be a reasonable alternative for fusiform or pseudo aneurysms or DCCFs if neither coil embolization nor detachable balloons is possible1-22. We have presented the results of use of covered stents for intracranial aneurysms and DCCFs that could not be repaired with conventional modalities. Our study on 15 patients potentially adds to the published literature on the management of these lesions. Stent placement was possible and successful in 13 of 15 patients, and the pseudoaneurysms, fusiform aneurysms and DCCFs were totally closed. Two other patients were considered for this therapy, but we misjudged how difficult transversing tortuous carotid artery would be.
With regard to the use of stent grafts within intracranial vessels, an appropriate case selection is crucial for successful results. Closure of the perforating branches from the parent artery is the main concern in the use of covered stent. Therefore the use of stent grafts has to be confined to segments of the vasculature not giving rise to critical branches, which significantly restricts their application in cerebrovascular diseases. The use of stent grafts for the treatment of intracranial aneurysms or fistulasis limited to the lesions located in the internal carotid artery proximal to the anterior choroidal artery origin or in the vertebral artery away from the posterior inferior cerebellar artery origin. Another drawback associated with the placement of stent grafts in intracranial vessels is their inflexibility with poor navigation. The use of covered stents in the intracranial circulation is exploding and will continue to do so as covered stents are further refined. The concept of forming a new lumen across pathology is logical and exciting but these stents are not without problems. In addition to the complications with uncovered stents such as the need for antiplatelet therapy, covered stents have the added problems such as poor navigation and the increased possibility of a stroke. The Jostent is a balloon mounted covered stent which results in poorer navigation due to the fact that it requires both the balloon in addition to the stent to navigate the intracranial vessels. Our case series clearly demonstrated this as the stent could not be deployed in two out of 15 patients despite only treating pathology in the proximal intracranial circulation. Increased stroke risk is due to the concern that perforators and branches may also been excluded from the intracranial circulation in addition to the pathology and therefore limits the use of this stent to areas that perforators are not found and no branches that would be included in the treatment of the pathology. Complications that may result from this rigidity are perforation of the cerebral vessels, and if necessary, another covered stent will be needed7. Other potential complications include thrombosis and intimal hyperplasia. Regular use of anti-platelet agents may help to prevent these events. The PTFE layer may reduce the rate of neointimal hyperplasia and stent-related stenosis by inhibiting the migration of inflammatory cells and by attenuating the diffusion of cytokines2,9,13,19. Two of our patients had carotid occlusion, and six-month follow-up angiograms in seven cases showed no stenosis of the parent arteries. In our experience we encountered proximal or distal endoleak and endoleak due to porosity of the graft covering material. Differentiating between the two types of endoleak in practice could be somewhat difficult. Our first strategy was waiting five to 15 minutes. If the endoleak persisted, the balloon was reinflated up to a higher pressure to make the stent graft closely appose to the vessel wall. Further inflation of the balloon may also lead to displacement of the graft. In a patient with a wide-necked giant aneurysm we had to lay two stent grafts to obliterate the proximal or distal endoleak.
Conclusions
Implantation of endovascular covered stents is a feasible method for percutaneous closure of intracranial pseudoaneurysms, fusiform aneurysms and direct carotid-cavernous fistulas. Thus, endoluminal vascular repair with covered stents offers an alternative therapeutic approach to conventional modalities.
References
- 1.Ahuja V, Tefera G. Successful covered stent-graft exclusion of carotid artery pseudoaneurysm: two case reports and review of literature. Ann Vasc Surg. 2007;21(3):367–372. doi: 10.1016/j.avsg.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 2.Archondakis E, Pero G, et al. Angiographic follow-up of traumatic carotid cavernous fistulas treated with endovascular stent graft placement. Am J Neuroradiol. 2007;28(2):342–347. [PMC free article] [PubMed] [Google Scholar]
- 3.Auyeung KM, Liu WM, et al. Massive epistaxis related to petrous carotid artery pseudoaneurysm after radiation therapy: Emergency treatment with covered stent in two cases. . Am J Neuroradiol. 2003;24:1449–1452. [PMC free article] [PubMed] [Google Scholar]
- 4.Burbelko MA, Dzyak LA, et al. Stent-graft placement for wide-neck aneurysm of the vertebrobasilar junction. Am J Neuroradiol. 2004;25(4):608–610. [PMC free article] [PubMed] [Google Scholar]
- 5.Chiaradio JC, Guzman L, et al. Intravascular graft stent treatment of a ruptured fusiform dissecting aneurysm of the intracranial vertebral artery: technical case report. Neurosurgery. 2002;50(1):213–217. doi: 10.1097/00006123-200201000-00034. [DOI] [PubMed] [Google Scholar]
- 6.Cohen TE, Grigoriadis S, Gomori JM. Petrous carotid artery pseudoaneurysm in bilateral carotid fibromuscular dysplasia: treatment by means of self-expanding covered stent. Surg Neurol. 2007;68(2):216–220. doi: 10.1016/j.surneu.2006.08.082. [DOI] [PubMed] [Google Scholar]
- 7.Dieter RS, Ikram S, et al. Perforation complicating carotid artery stenting: the use of a covered stent. Catheterization & Cardiovascular Interventions. 2006;67(6):972–975. doi: 10.1002/ccd.20744. [DOI] [PubMed] [Google Scholar]
- 8.Fusonie GE, Edwards JD, Reed AB. Covered stent exclusion of blunt traumatic carotid artery pseudoaneurysm: case report and review of the literature. Ann Vasc Surg. 2004;18(3):376–379. doi: 10.1007/s10016-004-0037-2. [DOI] [PubMed] [Google Scholar]
- 9.Gomez F, Escobar W, et al. Treatment of carotid cavernous fistulas using covered stents: midterm results in seven patients. Am J Neuroradiol. 2007;28:1762–1768. doi: 10.3174/ajnr.A0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kocer N, Kizilkilic O, et al. Treatment of iatrogenic internal carotid artery laceration and carotid cavernous fistula with endovascular stent-graft placement. Am J Neuroradiol. 2002;23(3):442–446. [PMC free article] [PubMed] [Google Scholar]
- 11.Layton KF, Kim YW, Hise JH. Use of covered stent grafts in the extracranial carotid artery: report of three patients with follow-up between 8 and 42 months. Am J Neuroradiol. 2004;25(10):1760–1763. [PMC free article] [PubMed] [Google Scholar]
- 12.Li MH, Li YD, et al. A new covered stent designed for intracranial vasculature: application in the management of pseudoaneurysms of the cranial internal carotid artery. Am J Neuroradiol. 2007;28(8):1579–1585. doi: 10.3174/ajnr.A0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lupatteli T, Garaei FG, et al. Covered stent deployment and follow-up of a case of internal carotid artery pseudoaneurysm. Cerebrovasc Dis. 2003;16(1):98–101. doi: 10.1159/000070125. [DOI] [PubMed] [Google Scholar]
- 14.Lv XL, Li YX, et al. A complex cavernous sinus dural arteriovenous fistula secondary to covered stent placement for a traumatic carotid artery-cavernous sinus fistula. J Neurosurg. 2008;108(3):588–590. doi: 10.3171/JNS/2008/108/3/0588. [DOI] [PubMed] [Google Scholar]
- 15.Lylyk P, Cohen JE, et al. Endovascular reconstruction of intracranial arteries by stent placement and combined techniques. J Neurosurg. 2002;97(6):1306–1313. doi: 10.3171/jns.2002.97.6.1306. [DOI] [PubMed] [Google Scholar]
- 16.Madan A, Mujic A, et al. Traumatic carotid artery-cavernous sinus fistula treated with a covered stent. Report of two cases. J Neurosurg. 2006;104(6):969–973. doi: 10.3171/jns.2006.104.6.969. [DOI] [PubMed] [Google Scholar]
- 17.Pero G, Denegri F, et al. Treatment of a middle cerebral artery giant aneurysm using a covered stent. Case report. J Neurosurg. 2006;104(6):965–968. doi: 10.3171/jns.2006.104.6.965. [DOI] [PubMed] [Google Scholar]
- 18.Redekop G, Marotta T, Weill A. Treatment of traumatic aneurysms and arteriovenous fistulas of the skull base by using endovascular stents. J Neurosurg. 2001;95(3):412–419. doi: 10.3171/jns.2001.95.3.0412. [DOI] [PubMed] [Google Scholar]
- 19.Saatci HI, Cekirge MS, et al. Treatment of internal carotid artery aneurysms with a covered stent: experience in 24 patients with mid-term follow-up results. Am J Neuroradiol. 2004;25:1742–1749. [PMC free article] [PubMed] [Google Scholar]
- 20.Schonholz C, Krajcer Z, et al. Stent-graft treatment of pseudoaneurysms and arteriovenous fistulae in the carotid artery. Vascular. 2006;14(3):123–129. doi: 10.2310/6670.2006.00034. [DOI] [PubMed] [Google Scholar]
- 21.Tseng A, Ramaiah V, et al. Emergent endovascular treatment of a spontaneous internal carotid artery dissection with pseudoaneurysm. J Endovasc Ther. 2003;10(3):643–646. doi: 10.1177/152660280301000334. [DOI] [PubMed] [Google Scholar]
- 22.Vanninen RL, Manninen HI, Rinne J. Intrasellar latrogenic carotid pseudoaneurysm: endovascular treatment with a polytetrafluoethylene-covered stent. Cardiovasc Intervent Radiol. 2003;26(3):298–301. doi: 10.1007/s00270-003-2728-4. [DOI] [PubMed] [Google Scholar]