Summary
Cases of aneurysm associated with the occlusion of both common carotid arteries are very rare. We present a case of ruptured aneurysms of the basilar bifurcation and posterior cerebral artery coexisting with bilateral common carotid artery occlusion, successfully treated by endovascular coil embolization with a double-balloon remodeling technique. Finally, we review the literature. A 62-year-old woman presented with severe headache; a computed tomography scan demonstrated subarachnoid hemorrhage. Angiography revealed that the bilateral common carotid arteries were occluded.
The muscle branches of the vertebral arteries had anastomosed to the bilateral external carotid arteries. Bilateral posterior communicating arteries had developed and supplied the bilateral internal carotid arteries. Two aneurysms (a saccular aneurysm of the P1 portion of the left posterior cerebral artery and a wide-necked aneurysm of the basilar bifurcation) were also observed. Endovascular embolization of the aneurysms was successfully performed using a double-balloon remodeling technique.
The patient made a full recovery after treatment, and the aneurysms remained obliterated 12 months after embolization. We believe that this is the first report of ruptured aneurysms associated with bilateral common carotid artery occlusion successfully treated by endovascular coiling. The double-balloon remodeling technique was useful for treatment of wide-necked basilar bifurcation aneurysm.
Key words: bilateral common carotid artery occlusion, cerebral aneurysm, endovascular coiling, balloon neck remodeling
Introduction
It is been well known that the most important etiological factor for intracranial saccular aneurysm is hemodynamic stress. In particular, occlusion of one or more feeding vessels may enhance the possibility of aneurysm formation at large arterial forks subjected to increased hemodynamic stress associated with collateral flow1,2. Cases of aneurysms coexistent with the occlusion of one or both internal carotid arteries have been already reported3-6, but cases of aneurysm coexistent with the occlusion of both common carotid arteries are rare7-10. We present the first case of ruptured aneurysms of the basilar bifurcation and posterior cerebral artery coexisting with the occlusion of both common carotid arteries successfully treated by endovascular coil embolization with the balloon remodeling technique.
Case Report
A 62-year-old Japanese woman suffered sudden onset of severe headache, nausea and vomiting followed by transient loss of consciousness, and was immediately admitted to our hospital. She had been diagnosed as having hypertension twenty years previously, but did not receive any medical treatment. On admission, she complained of severe headache and nausea, and had no neurological abnormalities. The pulsation of the left radial artery was weak, but the blood pressure was 160-100 mmHg in the right upper extremity. Laboratory data revealed no definitive abnormality, including C-reactive protein, indicating that there was no active inflammation. An initial computed tomography (CT) scan disclosed diffuse subarachnoid hemorrhage (SAH). Angiography revealed occlusion of the bilateral common carotid arteries and dilatation of the bilateral vertebral arteries (Figure 1). The left subclavian artery was occluded (Figure 1). The muscle branches of vertebral arteries had anastomosed to the bilateral external carotid arteries. Bilateral posterior communicating arteries had developed and supplied the bilateral internal carotid arteries. Right vertebral angiography revealed a basilar bifurcation aneurysm (Figure 2). CT angiography demonstrated two aneurysms at the top of the basilar artery (a wide-necked aneurysm of the basilar bifurcation and a small saccular aneurysm of the P1 portion of the left posterior cerebral artery) (Figure 3). Because of the high-lying position of the basilar terminus above the posterior clinoid processes, we scheduled endovascular treatment. While the patient was waiting for treatment of the aneurysms on the day after her admission, her blood pressure suddenly became unstable and she lapsed into a coma. CT revealed thick SAH, especially in the prepontine cistern. We performed endovascular treatment of the aneurysms on day 2 because of the patient’s general condition. Under general anesthesia and systemic heparinization to maintain an activated coagulation time between 200 and 300 seconds, a 5-French guiding sheath (Shuttle, Cook, Inc., Bloomington, IN) was placed in the left vertebral artery via the right femoral artery. The initial attempt to treat the aneurysm of the P1 portion of the left posterior cerebral artery (PCA) by a conventional endovascular approach was unsuccessful because the microcatheter and coil protruded into the parent artery. A balloon-assisted technique was then employed. An Excelsior SL-10 microcatheter with two tip markers (Boston Scientific/Target Therapeutics, Fremont, CA) and a HyperForm balloon microcatheter (Micro Therapeutics, Irvine, CA) primed with a Xpedion guidewire (Micro Therapeutics) were introduced into the left PCA within the guiding sheath. With the tip of the microcatheter within the aneurysm, the balloon inflated for support of the microcatheter and coils, the aneurysm was successfully embolized with Guglielmi detachable coils (GDCs) (Boston Scientific/Target Therapeutics) (Figure 4A).
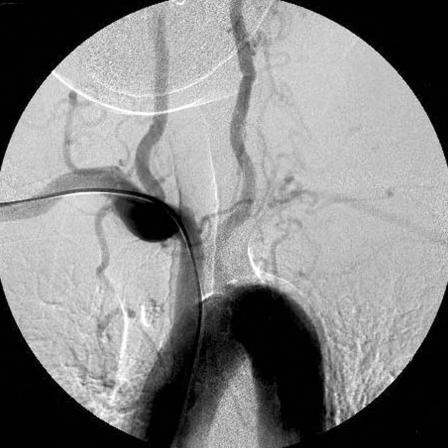
Figure 1.
An aortogram revealed occlusion of the bilateral common carotid arteries and dilatation of the bilateral vertebral arteries. The left subclavian artery was occluded.
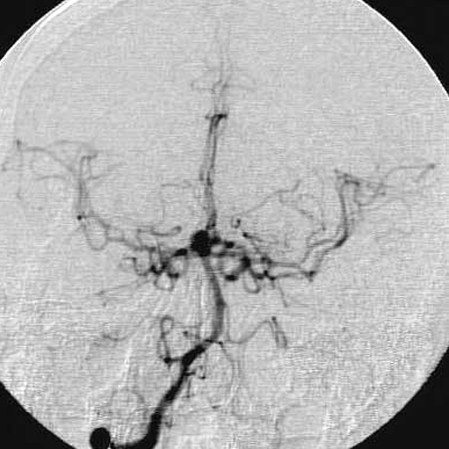
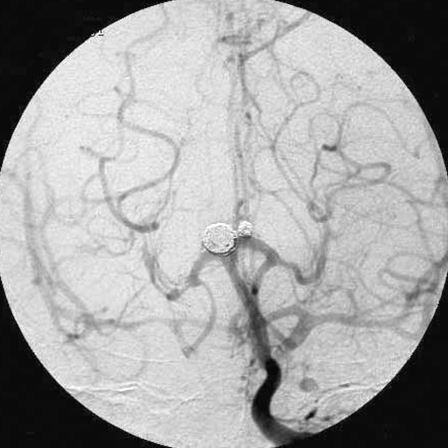
Figure 2.
Right vertebral angiograms, anterioposterior (A) and lateral views (B), showed normal filling of the posterior circulation, excellent collateral flow to the territories of the both internal carotid arteries via both posterior communicating arteries, and a basilar bifurcation aneurysm.
A.
B.
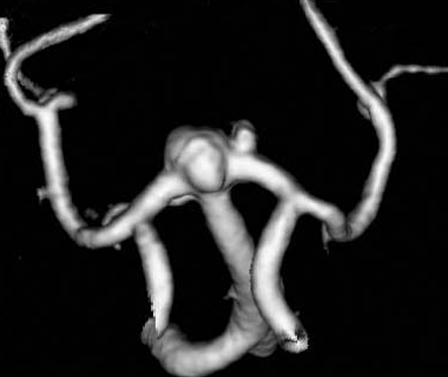
Figure 3.
Three-dimensional CT angiography demonstrated two aneurysms in the region of the top of basilar artery (a wide-necked aneurysm of the basilar bifurcation and a small saccular aneurysm of the P1 portion of the left posterior cerebral artery).
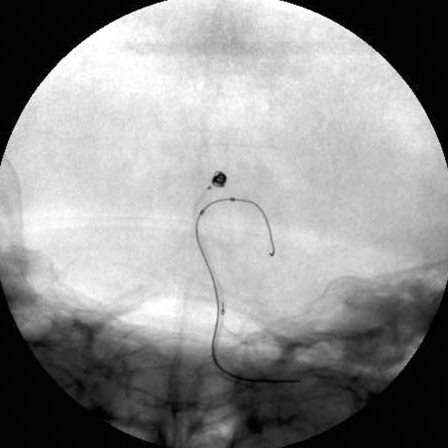
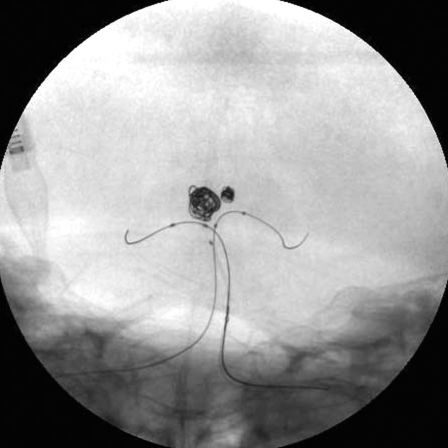
Figure 4.
Skull radiogram during balloon remodeling coil embolization. A) The aneurysm of the P1 portion of the left posterior cerebral artery was treated by single-balloon remodeling technique. B) The basilar bifurcation aneurysm was treated by double-balloon remodeling technique. Note two balloon catheters with microguidewires were positioned in both basilar-P1 junctions.
A.
B.
Next, we attempted to treat the wide-necked aneurysm of the basilar bifurcation with the same single balloon-assisted technique. But treatment was unsuccessful because the neck-to-fundus ratio of the aneurysm was greater than 1.0 and the blood flow from the basilar artery into the aneurysmal sac was too strong as almost the entire cerebral blood flow came through only the basilar artery. Then a second guiding catheter, 6-French Envoy guiding catheter (Cordis, Miami Lakes, FL., USA) was advanced into the right vertebral artery through the left femoral artery. A Sentry balloon catheter (Boston Scientific/Target Therapeutics) with Transend 010 guidewire (Boston Scientific/Target Therapeutics) was positioned into the basilar-right PCA junction through the Shuttle sheath. A HyperForm balloon microcatheter with an Xpedion guidewire was positioned into the basilar-left PCA junction through the Envoy guiding catheter. During the insertion of GDCs, both balloons were inflated to occlude a distal segment of the basilar artery and bilateral PCAs. Double protective balloons from distal basilar artery to the bilateral PCAs were inflated for the prevention of coil protrusion during the insertion of GDCs (Figure 4B). After each coil was positioned in the aneurysmal sac, but before detachment, both balloons were deflated to confirm the stability of the coil in the aneurysmal sac. Since the coil did not move, the coil was detached. Seven GDCs with a total length of 86 cm were introduced and the embolization resulted in satisfactory elimination of the aneurysm. Immediately after the embolization, the patient complained of mild left hemiparesis, but the patient’s hemiparesis resolved completely two hours later. Continuous lumbar drainage was undertaken for one week. The patient was discharged after the fifth week with no neurological deficits. Six months later, follow-up angiography revealed no coil compaction (Figure 5). One year later, magnetic resonance imaging and angiography disclosed no cerebral infarction or relapse of aneurysms.
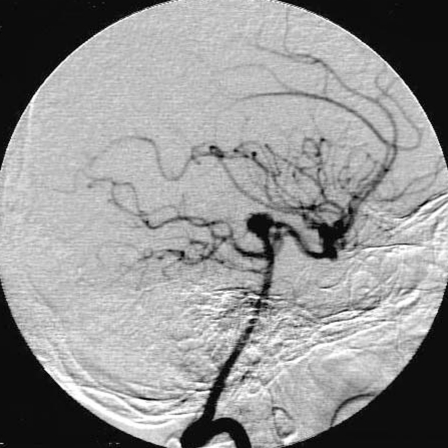
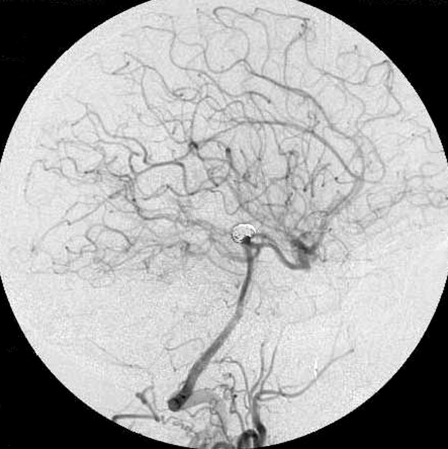
Figure 5.
Left vertebral angiograms, anterioposterior (A) and lateral (B) views acquired at the end of the procedure showed that the aneurysms were occluded completely.
A.
B.
Discussion
Common carotid artery occlusion is rare. The incidence of symptomatic common carotid artery occlusion in patients of ischemic stroke is estimated to be from 0.24 to 5%7,11-14. So, bilateral common carotid artery occlusion is still rarer. The lesions are generally caused by an advanced atherosclerotic process at the carotid bifurcation or at the origin of the common carotid artery. Reported causes of common carotid artery occlusion include atherothrombosis, arteritis/aortitis, fibromuscular dysplasia, thrombocytosis, aortic arch dissection, aortic arch aneurysm, aortic arch angiography, mediastinal tumors, cardiac source embolism, post-irradiation arteriopathy, blunt or open craniocervical trauma, and idiopathic occlusion15. The present case had no previous illness except systemic hypertension, and we diagnosed the patient’s bilateral common carotid artery occlusion as atherosclerotic change.
Only six cases of ruptured intracranial aneurysm associated with bilateral common carotid artery occlusion have been reported, including the present case (Table). All cases were women and the aneurysms predominately arose in the vertebrobasilar systems. The aneurysms probably develop due to hemodynamic stress caused by changes in blood circulation. There are several clinical or experimental studies reporting development of de novo saccular aneurysms associated with unilateral or bilateral internal carotid artery occlusion1,3-5. Araki et Al. reported that bilateral common carotid artery occlusion caused increased blood flow and hemodynamic stress in the vertebrobasilar system, and development of de novo aneurysms mainly in the posterior circulation7. Ages of patients ranged from 41 to 83 years, with an average age of 62.7 years. The etiology of the common carotid artery occlusion included both aortitis syndrome and arteriosclerosis, but the average age of the cases caused by aortitis syndrome (50 years) was younger than that caused by arteriosclerosis (75.3 years) because the peak age for the manifestation of aortitis syndrome is un the twenties and the aneurysm developed about 30 years after that10. All four patients who were treated conservatively or by surgical wrapping for ruptured aneurysms have died, while one case treated by surgical clipping and the present case treated by endovascular coil embolization had a good clinical course. It seems that a poor outcome is caused by technical difficulties of surgical treatment for ruptured aneurysms in the posterior circulation or serious brain damage because of SAH and the latent ischemic brain condition associated with bilateral common carotid artery occlusion.
Table 1.
Summary of previously reported cases of bilateral common carotid artery occlusion with ruptured cerebral aneurysms.
| Authors (year) | Age/ | Etiology | Location | Treatment | Outcome |
|---|---|---|---|---|---|
| Sex | of aneurysm | ||||
|
| |||||
| Kumagai et Al, (1981) | 51/F | aortitis | Rt. & Lt. BA-SCA | wrapping | Dead |
|
| |||||
| 41/F | aortitis | BA top, Lt.PCA-PcomA | clipping | GR | |
|
| |||||
| Matsuzawa et Al, (1982) | 54/F | aortitis | BA top | - | Dead |
|
| |||||
| Kataoka and Taneda, (1982) | 81/F | arteriosclerosis | BA trunk | Dead | |
|
| |||||
| Araki et Al, (2002) | 83/F | arteriosclerosis | Rt.PCA(P2), Rt.PCA-PcomA | - | Dead |
|
| |||||
| Present case | 62/F | arteriosclerosis | BA top, Lt.PCA(P1) | coiling | GR |
|
| |||||
| BA, basilar artery; SCA, superior cerebellar artery; PCA, posterior cerebral artery; PcomA, posterior communicating artery; | |||||
| GR, good recovery; Lt., left; Rt., right. | |||||
Patients with ruptured basilar tip aneurysms who are not surgical clipping candidates should be offered endovascular coil embolization as a treatment option16. Recent advances in endovascular techniques have greatly expanded the scope of intracranial aneurysms amenable to endovascular elimination. One of the techniques for the treatment of difficult wide-necked intracranial aneurysms is the balloon-assisted, so-called remodeling, technique. The technique consists of remodeling the arterial wall by temporarily inflating a non-detachable balloon in front of the neck of the aneurysm during each coil placement. But several complications of the balloon remodeling technique have been reported, such as thromboembolic events, rupture of the aneurysmal sac, damage to the arterial wall (mechanical rupture, dissection, vasospasm, endothelial cell damage), brain ischemia from prolonged balloon inflation and so on17,18. One modification of this technique is the double-balloon remodeling technique19,20. The advantage of double-balloon remodeling is that it permits the treatment of wider-necked aneurysms, for which the single-balloon technique cannot enable the side branch and neck to seal together. However, the potential risk factors such as thromboembolic events during coiling increase with this method10. In the present case, the bilateral carotid arteries were occluded and most cerebral blood flow was maintained by the vertebrobasilar system alone. Temporal occlusion of the parent artery of the aneurysm by double balloons, therefore, could induce global brain ischemia, and we had to repeat balloon inflation and deflation within a short time during coil embolization.
Although the use of the balloon remodeling technique helps obtain better immediate results in the treatment of intracranial aneurysms, recurrence of the aneurysm is still a major problem. In the present case especially, most cerebral blood flows through the terminal portion of the basilar artery and the coil-embolized aneurysms are prone to recanalization secondary to the hemodynamics and the resulting high-pressure pulsatile flow directly at the coil masses. The present case, therefore, needs long-term clinical follow-up.
Recently, the development of intracranial stents has increased the options for the treatment of a wide-necked or a large/giant aneurysm. The stent serves as a mechanical scaffold for the placement of aneurysm coils, prevents coil protrusion into the parent vessels, and may allow safer packing of the aneurysm with a denser coil mesh. In addition, intracranial stents may help prevent aneurysm recanalization by redirecting flow and facilitating endothelialization. For wide-necked basilar bifurcation aneurysms, successful coiling is most commonly achieved by use of a Y-configuration double stent-assisted technique in which stents are delivered and deployed in series from the parent vessel into each limb of the bifurcation21. Stent placement is associated with problems such as thromboembolic events, stent migration and so on19. Either technique, balloon remodeling or stent-assisted coil embolization, requires both understanding of the advantages and mastery of the potential pitfalls for successful treatment of wide-necked aneurysms.
References
- 1.Hashimoto N, Handa H, et al. Experimentally induced cerebral aneurysms in rats: Part V. Relation of hemodynamics in the circle of Willis to formation of aneurysms. Surg Neurol. 1980;13:41–45. [PubMed] [Google Scholar]
- 2.Stehbens WE. Etiology of intracranial berry aneurysms. . J Neurosurg. 1989;70:823–831. doi: 10.3171/jns.1989.70.6.0823. [DOI] [PubMed] [Google Scholar]
- 3.Batjer H, Mickey B, Samson D. Enlargement and rupture of distal basilar artery aneurysm after iatrogenic carotid occlusion. Neurosurgery. 1987;20:624–628. doi: 10.1227/00006123-198704000-00021. [DOI] [PubMed] [Google Scholar]
- 4.Wolf RL, Imbesi SG, et al. Development of a posterior cerebral artery aneurysm subsequent to occlusion of the contralateral internal carotid artery for giant cavernous aneurysm. Neuroradiology. 2002;44:443–446. doi: 10.1007/s00234-001-0723-5. [DOI] [PubMed] [Google Scholar]
- 5.Yamanaka C, Hirohata T, et al. Basilar bifurcation aneurysm associated with bilateral internal carotid occlusion. Neuroradiology. 1987;29:84–88. doi: 10.1007/BF00341047. [DOI] [PubMed] [Google Scholar]
- 6.Yousaf I, Gray WJ, et al. Development of posterior circulation aneurysm in association with bilateral internal carotid artery occlusion. British J Neurosurg. 2003;17:471–472. doi: 10.1080/02688690310001613871. [DOI] [PubMed] [Google Scholar]
- 7.Araki T, Fujiwara H, et al. A case of aneurysmal subarachnoid hemorrhage associated with bilateral common carotid artery occlusion. No Shinkei Geka. 2002;30:853–358. [PubMed] [Google Scholar]
- 8.Kataoka K, Taneda M. Cerebral aneurysm with bilateral carotid occlusion. Neurol Med Chir (Tokyo) 1982;22:744–750. doi: 10.2176/nmc.22.744. [DOI] [PubMed] [Google Scholar]
- 9.Kumagai Y, Sugiyama H, et al. Two cases of pulseless disease with cerebral aneurysm. No Shinkei Geka. 1981;9:611–615. [PubMed] [Google Scholar]
- 10.Masuzawa T, Shimabukuro H, et al. The development of intracranial aneurysms associated with pulseless disease. Surg Neurol. 1982;17:132–136. doi: 10.1016/s0090-3019(82)80041-4. [DOI] [PubMed] [Google Scholar]
- 11.Collice M, D'Angelo V, Arena O. Surgical treatment of common carotid artery occlusion. Neurosurgery. 1983;12:515–524. doi: 10.1227/00006123-198305000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Hass WK, Fields WS, et al. Joint study of extracranial arterial occlusion. II. Arteriography, techniques, sites, and complications. JAMA. 1968;11:961–968. [PubMed] [Google Scholar]
- 13.Keller HM, Valavanis A, et al. Patency of external and internal carotid artery in the presence of an occluded common carotid artery: noninvasive evaluation with combined cerebrovascular Doppler examination and sequential computer tomography. Stroke. 1984;15:149–156. doi: 10.1161/01.str.15.1.149. [DOI] [PubMed] [Google Scholar]
- 14.Podore PC, Rob CG, et al. Chronic common carotid occlusion. Stroke. 1981;12:98–100. doi: 10.1161/01.str.12.1.98. [DOI] [PubMed] [Google Scholar]
- 15.Levine SR, Welch KMA, et al. Common carotid artery occlusion. Neurology. 1989;39:178–186. doi: 10.1212/wnl.39.2.178. [DOI] [PubMed] [Google Scholar]
- 16.Eskridge JM, Song JK. Endovascular embolization of 150 basilar tip aneurysms with Guglielmi detachable coils: results of the Food and Drug Administration multicenter clinical trial. J Neurosurg. 1998;89:81–86. doi: 10.3171/jns.1998.89.1.0081. [DOI] [PubMed] [Google Scholar]
- 17.Mericle RA, Wakhloo AK, et al. Temporary balloon protection as an adjunct to endosaccular coiling of wide-necked cerebral aneurysms. Technical note. Neurosurgery. 1997;41:975–978. doi: 10.1097/00006123-199710000-00045. [DOI] [PubMed] [Google Scholar]
- 18.Moret J, Cognard C, et al. The “remodeling technique” in the treatment of wide neck intracranial aneurysms. Angiographic results and clinical follow-up in 56 cases. . Interventional Neuroradiology. 1997;3:21–35. doi: 10.1177/159101999700300103. [DOI] [PubMed] [Google Scholar]
- 19.Arat A, Cil B. Double-balloon remodeling of wide-necked aneurysms distal to the circle of Willis. Am J Neuroradiol. 2005;26:1768–1771. [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi A. Neck plastic intra-aneurysmal GDC embolization with double protective balloons. Method of multiple guiding catheter introduction. Interventional Neuroradiology. 1998;4:177–179. doi: 10.1177/159101999800400211. [DOI] [PubMed] [Google Scholar]
- 21.Biondi A, Janardhan V, et al. Neuroform stent-assisted coil embolization of wide-neck intracranial aneurysms. Strategies in stent development and midterm follow-up. Neurosurgery. 2007;61:460–469. doi: 10.1227/01.NEU.0000290890.62201.A9. [DOI] [PubMed] [Google Scholar]