The autonomic and the immune systems play major roles in the pathogenesis of cardiovascular disease and hypertension. To date, those two systems have been studied extensively but independently by cardiovascular biologists and by immunologists. The notion that the autonomic system can modulate the immune system and thereby influence the pathogenesis of cardiovascular disease and hypertension and their clinical outcome is novel and critical.
In this brief review we focus on that interaction and an integrated understanding of the neuro-immune axis. We also highlight recent progress and future research directions.
The main theme is that dysregulation of the autonomic system enhances the inflammatory response of the innate and adaptive immune systems leading to the initiation or acceleration of pathological processes and worsening of cardiovascular risks. The therapeutic potential of restoring an optimal autonomic control of the immune system is very promising.
Both components of the neuro-immune axis may be involved in its disruption. One is the autonomic nervous system which may be dysregulated or imbalanced with increased sympathetic and decreased parasympathetic activation. The other is the immune system itself which may be abnormally sensitive to the modulatory influence of the autonomic system. These two components are briefly described below.
-
The autonomic dysregulation in chronic hypertension. The complexity of cardiovascular mechanisms that contribute to hypertension has been challenging. The roles of vascular and renal abnormalities are undeniable. Changes in vasomotor tone, sodium retention, and renal renin release are all well established. For decades the contribution of the sympathetic nervous system to chronic hypertension was not fully appreciated. Its importance is now well recognized [1–5]. In a 1982 review we described the neural sites and mechanisms involved in exaggerated sympathetic nerve activity in several animal models as well as in human hypertension [1]. Moreover, there has been a surge of data highlighting the damaging cardiovascular effects and the increased mortality and morbidity associated with exaggerated sympathetic nerve activity (SNA) in a variety of cardiovascular diseases [6–10]. In the Cannon Lecture of 2009 entitled, “In Search of Autonomic Balance: the Good, the Bad, and the Ugly” the parasympathetic nerve activity (PSNA) was portrayed as good, the sympathetic activity as bad, and the generation of reactive oxygen species (ROS) by the renin-angiotensin-aldosterone system (RAAS) as the ugly [11]. Although an essential role of the kidney has been established in angiotensin II hypertension [12] the RAAS can initiate and maintain a state of sympathetic overactivity through its action on central neurons in the subfornical organ of the forebrain [3,11,13].
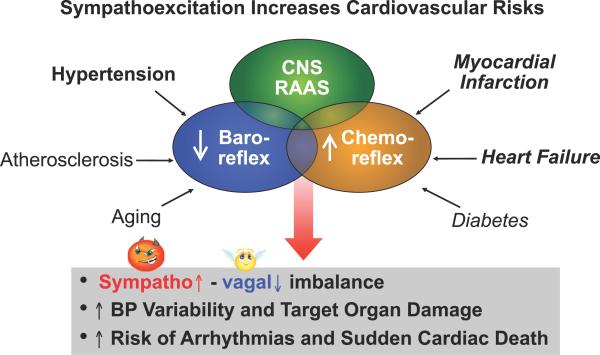
Additionally, the decrease in baroreceptor reflex activity [1,2,3,11] and increase in chemoreceptor activity [3,5,14,15], often seen in hypertension and heart failure, enhance the excessive SNA and suppress the PSNA. This combination increases cardiovascular risks not only in hypertension, but also in several other cardiovascular disease states [8, 9, 16] (Figure 1).
Sensitivity of the immune system to autonomic modulation. A hallmark study demonstrated that vagal nerve stimulation protected mice from LPS induced sepsis, and was associated with a decrease in the secretion of TNF-alpha [17]. In later studies this effect was shown to be mediated by the alpha7-nicotinic acetylcholine (ACh) receptor [18]. The suppression of the immune responses has been coined the “Cholinergic Inflammatory Reflex”, drawing an analogy to the vago-vagal neural reflex [19]. These studies set the stage for further exploration of the autonomic regulation of the immune system in cardiovascular disease. The enhanced inflammatory response in hypertension or other cardiovascular diseases may thus be due to the loss of the inhibitory parasympathetic influence or an increased sympathetic influence. It could also be due to abnormal sensitivities of excitatory or inhibitory receptors on the immune cells.
Figure 1.
Mechanisms involved in excessive sympathetic activity and reduced parasympathetic activity in cardiovascular disease. This dysautonomia is associated with increased morbidity and mortality [11].
Interactions of the Neuro-Immune Circuit in Cardiovascular Disease
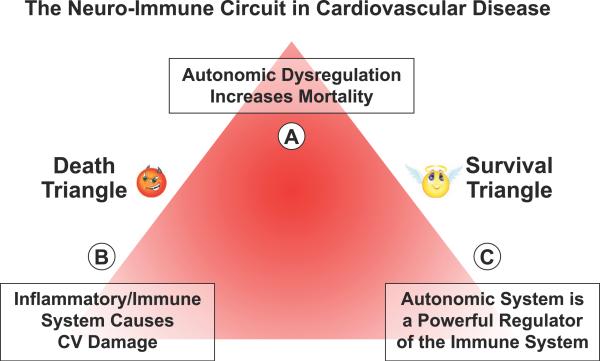
The interactions of the nervous system, immune system and cardiovascular disease can be visualized as a triangle (Figure 2). One angle (A) represents autonomic dysregulation which may increase mortality as a result of excessive sympathetic activity; another angle (B) represents the inflammatory/immune system which causes significant cardiovascular damage; and the third angle (C) represents the powerful neuro-immune interaction. In a state of homeostasis, there is a balance between the parasympathetic and the sympathetic nerve activities. However, when the sympathetic nervous system prevails, the triangle becomes a “Death Triangle” with overactivation of the inflammatory response, whereas when the parasympathetic nervous system prevails, the triangle becomes a “Survival Triangle” as a result of suppression of the inflammatory response (Figure 2).
Figure 2.
The triangle reflects the convergence of the neural and immunological mechanisms in cardiovascular disease. It can be either a “Death Triangle” with excessive sympathetic and RAAS activities that enhance the inflammatory immune response and increase mortality, or a “Survival Triangle” with enhanced parasympathetic activity which has been shown to suppress the inflammatory immune response and prolong survival.
(A) Autonomic Dysregulation Increases Cardiovascular Mortality
A number of studies reported a significant association between high sympathetic and low parasympathetic activity and mortality following acute myocardial infarction, heart failure, diabetes and hypertension. As early as 1984 Cohn reported that survival of patients in congestive heart failure was less than 10% in 2 years when their plasma norepinephrine (NE) levels were >800 pg/ml and more than 50% at 4 years if their NE levels were <400 pg/ml [6]. An increase in high frequency heart rate variability measured by spectral analysis reflects the salutory prevalence of increased PSNA whereas an increased low frequency component may reflect an enhanced SNA and its associated morbid consequences [8–10, 16].
The most compelling data indicating a profound beneficial effect of sympathoinhibition and vagal activation on survival came from recent work in animal models of heart failure.
Stimulation of carotid sinus nerves and vagal nerves in animal models
In a canine model of rapid pacing-induced heart failure, electrical carotid sinus nerve stimulation resulted in an increased survival benefit by suppressing the sympathetic and enhancing the parasympathetic arms of the autonomic nervous system [20]. Similarly, stimulation of the right vagus nerve in a rat model of ischemic cardiomyopathy resulted in a 90% survival 4 months post coronary artery ligation, compared to only a 50% survival in rats without vagal stimulation [21]. Taken together, these studies indicate a beneficial effect of autonomic balance that not only spans different animal models and etiologies of heart failure, but also shows that the effects are long-lasting. In the rat model the survival extended beyond the vagal stimulation time-period. The mechanism of protection transcends the direct hemodynamic effect.
Uemura et al. demonstrated in an ischemia-reperfusion model of myocardial injury that vagal stimulation enhances the activity of tissue inhibitors of metalloproteinase -1 and suppresses the active matrix metalloproteinase-9 [22]. Conversely, beta adrenergic stimulation induces apoptosis of ventricular myocytes, since propranolol, but not prazosin abrogates the norepinephrine induced apoptosis [23]. The sustained lowering of blood pressure in dogs with chronic renal hypertension during carotid sinus nerve stimulation is a compelling demonstration of a renin-independent antihypertensive effect of prolonged reflex suppression of the sympathetic nervous system, and activation of the parasympathetic system [24].
Prehypertensive increase in sympathetic nerve activity
An important question in evaluating the role of SNA in hypertension is whether increased SNA contributes to the initiation of hypertension. In other words, does the impaired baroreceptor reflex and enhanced chemoreceptor reflex induce the development of hypertension or do they simply sustain a hypertensive state. In the Spontaneously Hypertensive Rat (SHR), a genetic model of hypertension, there is evidence of an increase in SNA to several vascular beds prior to the onset of hypertension [25]. We have shown that in the prehypertensive state, when the SHR is less than 5 weeks of age, there is a significant increase in gene expression of the acid sensing ion channels (ASIC 1 and 3) and the two pore domain acid sensing K+ (TASK) channels in glomus cells [15], which would increase chemoreceptor sensitivity. We also found a reduced expression of ASIC2 and Ca2+-activated potassium channels (BK) in aortic baroreceptor neurons, which may explain their reduced mechanosensitivity and decreased excitability in SHR prior to the onset of hypertension [26]. The abnormal gene expression of baro- and chemosensing channels may contribute to the early prehypertensive increase in SNA and the development of hypertension.
Clinical trials of carotid sinus and vagus nerve stimulation
The translational significance of the animal studies is now being tested in patients. The unexpected but significant beneficial influence of nerve stimulations on cardiovascular mortality and morbidity led to the implementation of major clinical trials involving the direct electrical stimulation of the carotid sinus nerves in patients with drug resistant hypertension, and recently in patients with heart failure [27–29]. Also, results of vagus nerve stimulation in experimental models are leading to clinical trials in patients with advanced heart failure [30–32].
(B) The Inflammatory/Immune System Causes Cardiovascular Damage
In parallel with the recognition of the powerful influence of the autonomic nervous system on cardiovascular risks is an equally powerful recognition of the functional and structural damages that ensue from inflammation of the cardiovascular system [33]. This is evident in a variety of cardiovascular diseases. In atherosclerosis, plaques contain immune cells (lipid-laden macrophages, T cells, and dendritic cells). In heart failure, cytokine “storms” and the enhanced innate immune response with TLR activation in macrophages-dendritic cells cause cardiac dysfunction.
In hypertension such associations include early reports in 1976 that thymectomy in mice prevents the development of DOCA/salt hypertension [34]. The neonatal thymic transplants from WKY normotensive controls into SHR delays the onset of hypertension in the SHR to over 32 weeks [35]. The immune dysfunction in the SHR involves a mononuclear immune cell subpopulation that induced dysfunction of T-lymphocytes [36]. Moreover, the administration of the immunosuppressive agents, cyclophosphamide and mycophenolate, was shown to stop the progression of hypertension in SHR [37].
Although, Ang II is known to induce hypertension in experimental models, it also induces upregulation of IL1-β, TNF-α, MCP-1 and ICAM-1 [38]. Elevated levels of ICAM-1 and IL-6 have also been documented in apparently healthy men [39]. These findings are significant because IL-6 causes myocardial hypertrophy, fibrosis, apoptosis in experimental models of hypertension, and appears to be involved in the pathogenesis of hypertension [40]. Moreover, IL-6 KO mice are refractory to Ang II induced hypertension [41].
Another model that confirms the dependence of Ang II induced hypertension on the immune system is the immune-deficient RAG−/− mouse. In this model the pressor response to Ang II infusion was blunted [42]. It was restored to the high levels seen in the C57BL/6 control mouse by the adoptive transfer of T lymphocytes. The transfer of B lymphocytes did not restore the pressor response [42].
The possibility that a latent activation of the innate immune system may exist in the prehypertensive state and initiate the development of hypertension is suggested by two observations. One is our early result in the young prehypertensive SHR that indicates a significant enhancement of cytokine release from splenocytes in response to activation of Toll-Like Receptors (TLR) which induce the transcription of cytokines [43]. This prehypertensive enhancement of cytokine release may explain the increase in regional sympathetic activity reported in the prehypertensive phase of SHR by Cabassi et al. [25].
The other observation by David Harrison's group proposes that in the prehypertensive state an initial stress which may be of neural origin, causes some vascular damage and the release of endogenous “neoantigens” [44]. These activate the innate immune system and the release of cytokines which in turn activate the T lymphocytes and the adaptive immune system resulting in inflammatory consequences and hypertension.
C) Autonomic System is a Powerful Regulator of the Immune System
The neurogenic origin of hemodynamic changes in hypertension is well accepted [2, 11, 45]. However the concept that the autonomic nervous system exerts long-term effects on the cardiovascular system in pathological states through the regulation of the immune system is novel. It represents a major shift in our thinking about the mechanisms of cardiovascular actions of the autonomic nervous system. The shift is from accepting the primacy of the direct hemodynamic effects to the recognition that the more chronic functional and structural pathological processes are mediated through the interaction of the autonomic and the immune systems. There is strong evidence to support the direct sympathetic innervation of immune organs [46]. Immunomodulation by cholinergic and adrenergic agonists has also been shown, strengthening the functional significance of this anatomical association [47].
Three sets of experimental observations have been selected to illustrate the influence of autonomic regulation on the immune system.
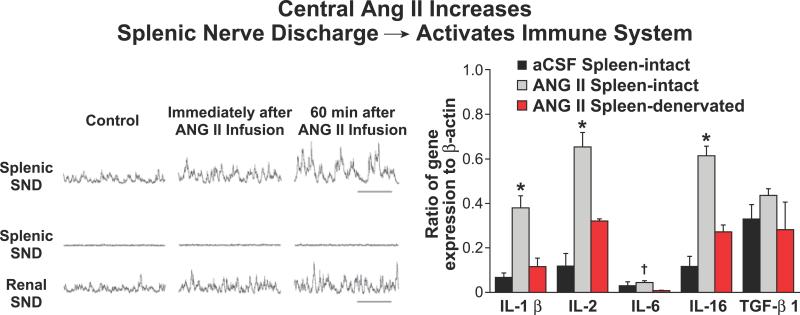
First is the evidence that an increase in central sympathetic nerve activation by intracerebral ventricular (ICV) infusion of Ang II enhances the immune response [48]. Although Ang II is responsible for a variety of actions that contribute to the development of hypertension, its central actions activate the peripheral sympathetic nervous system and affect the immune responses. A direct in vivo link between central angiotensin II, the increase in splenic sympathetic nerve activity, and the enhanced splenic cytokine (IL-1β, IL-2, IL-5, IL-16 and TGFβ1) gene expression was convincingly demonstrated, as shown in Figure 3. Selective splenic denervation caused a significant suppression of the enhanced centrally-driven sympathetically-mediated immune response to ICV infusion of Ang II while the increase in renal sympathetic discharge was preserved [48].
Figure 3.
Centrally-driven increase in splenic sympathetic nerve discharge (SND) by Ang II infusions (ICV) induces a marked increase in splenic gene expression of several cytokines. Selective splenic denervation abrogates the responses while the increase in renal SND is preserved. Adapted from Ganta et al. [48].
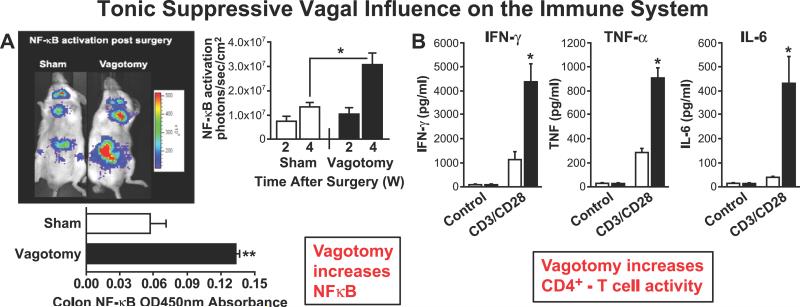
Second is the evidence that vagus nerve activity restrains the inflammatory system. A compelling indication of a suppressive influence of resting vagal tone is the demonstration of a marked increase in NFκB-activation in the colon by photon emission tomography 4 weeks after sub-diaphragmatic vagotomy (Figure 4A) [49]. The colon is densely populated with lymphoid tissue. A significant increase in NFκB, most likely in the innate immune cells, is the ultimate product of cellular inflammation and a major determinant of gene expression of cytokines [49]. In a parallel experiment, vagotomy increased significantly the cytokine production including IFNυ, TNF and IL-6 in CD3/CD28(CD4+) T-lymphocytes from murine spleen (Figure 4B) [50]. Additionally, activation of nicotinic cholinergic receptors has also been shown to play a role in B-lymphocyte maturation [51]. Recently, studies have shown that T-lymphocytes can synthesize and release acetylcholine, thus serving as effectors of the vagal nerve circuit [52].
Figure 4.
A: Subdiaphragmatic vagotomy induces pronounced NFκB-activation in the colon in vivo over a period of 4 weeks (w) post-vagotomy. Adapted from Omahony et al. [49]. B: Vagotomy results in a significant increase in gene expression of IFNγ, TNF, and IL-6 in isolated splenic murine CD4+T lymphocytes. Adapted from Karimi et al. [50].
Third is the evidence that vagal nerve stimulation exerts a protective anti-inflammatory effect and abrogates the detrimental responses in heart failure and endotoxic shock. In a canine model of pacing-induced heart failure, vagus nerve stimulation improved left ventricular ejection fraction, decreased cardiac size, and increased baroreflex sensitivity, when compared to the sham unstimulated animals [53]. This functional improvement was associated with a significant reduction of the clinically used biomarker of inflammation, C reactive protein, at 4 weeks and its nearly total suppression at 8 weeks [53].
Vagus nerve stimulation was also protective in a murine model of sepsis [16]. It attenuated the hypotension or shock induced in mice by endotoxin. It also suppressed serum as well as hepatic TNF-alpha [17]. These anti-inflammatory effects were later determined to be mediated by the activation of alpha7 nicotinic ACh receptors on macrophages [18].
Recent Progress and Future Directions
Some of the most recent work and ideas for future directions in the area of inflammation/immunity relevant to hypertension cover the communications between the nervous system and the immune system. These occur both at a central and peripheral levels [54].
Central Interaction
At the level of the CNS, circulating cytokines, or cytokines released from astrocytes and microglia may activate specific nuclei in the hypothalamus or brain stem and induce an increase in SNA and hypertension. Alternatively, the migration of innate immune cells such as macrophages and monocytes or T regulatory or cytotoxic lymphocytes from the circulation to the CNS may induce an inflammatory response and a similar neuronal activation.
An intriguing hypothesis, proposed by Julian Paton, is that hypoperfusion of the brain stem may evoke hypertension [45]. The hypothesis is based on the analogy that hypertension in the giraffe is an essential adjustment to maintain the cerebral circulation. The link between brain hypoperfusion and hypertension may be an inflammation of the brain microvasculature triggered by increased expression of adhesion molecules on the endothelium, which attract leukocytes and lymphocytes [45, 55]. Inflammation of the brain microvasculature causes localized ischemia. The transduction mechanisms that evoke sympathoexcitation and hypertension might include vascular-glial-neuronal signaling pathways that require further exploration [56]. The proposition is that long term control of blood pressure may need to be viewed in the context of maintenance of cerebral blood flow by a process that involves central inflammatory/immune mechanisms.
Peripheral Interaction
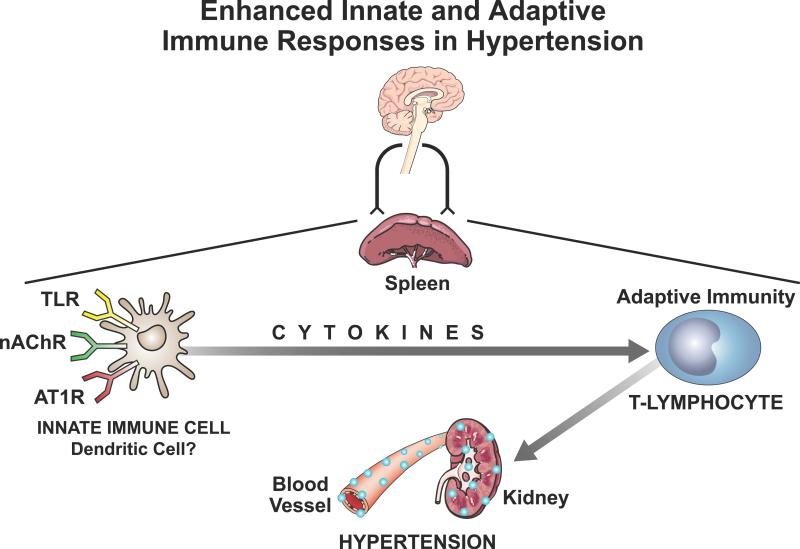
A second level of communication occurs between the autonomic nervous system and the peripheral immune system. The spleen and the gut are the organs with the most dense reservoirs of immune cells. They represent the innate and the adaptive immune systems. The innate immune cells which recognize pathogenic antigens and release cytokines have adrenergic, cholinergic and angiotensin receptors. When activated, these “autonomic receptors” may certainly alter the immunological response.
The work of Kevin Tracey and colleagues has focused on the vagus nerve dependent circuits that control innate immunity [18, 19]. He proposed the “Inflammatory Reflex” as a hypothesis to explain a negative feedback regulation of cytokine release. The vagus nerve is the mediator of both the afferent and efferent signals of the reflex. Vagal afferents, which are widely distributed in most visceral organs, including the heart and gut, may be activated by cytokines released as a result of tissue injury or inflammation. The reflex is then centrally mediated through the nucleus tractus solitarius (NTS) and the dorsal motor nucleus of the vagus and activates the vagal efferents. These, in turn, suppress cytokine release from macrophages in the spleen or the gut. This feedback requires ACh signaling through α7 nicotinic receptors (Figure 5) [18]. The release of ACh in the spleen during vagal stimulation is puzzling since the splenic nerve fibers lack the enzymatic processes for ACh synthesis. The recent discovery that a memory phenotype T-cell population in the spleen produces ACh and is integral to the “inflammatory reflex” resolves this question [52]. Thus, this T-lymphocyte is a component of the peripheral neural control of innate immunity and a possible therapeutic target in inflammatory states and autoimmune diseases.
Figure 5.
The autonomic, neurohumoral sympathetic and vagal signals regulate both the innate and adaptive immune cells. The activation of cholinergic (nAChR), adrenergic (α/β ADR) or angiotensin receptors (AT1R) on innate cells may dramatically alter the TLR-mediated cytokine release in pathologic states such as hypertension [43]. This, in turn, activates the adaptive cytotoxic T-lymphocytes, which cause end-organ damage in blood vessels and kidney. [42, 44, 60].
Dysregulation of Neuro-Immune Interactions
In pursuit of the relevance of this nicotinic modulation of innate immune cells in an animal model of genetic hypertension we studied the SHR [43]. We tested the hypothesis that there is a heightened innate immune response and cytokine release from splenocytes of prehypertensive SHR, compared to their WKY controls.
The transcriptional regulation of cytokine expression occurs through activation of Toll-like Receptors (TLR) on innate immune cells. TLRs, are the primary innate immune receptors that detect “pathogen associated molecular patterns” or PAMPs from bacteria, fungi or viruses (e.g. LPS, flagellin, CpG-containing DNA) [57]. They also recognize products of cellular damage from the host as “damage associated molecular patterns” or DAMPs (e.g. HMGB1, Heat Shock proteins, IL-Los, Annexins) [58].
We demonstrated a significant enhancement of the IL-6 response to TLR activation in splenocytes from SHR when nicotinic and AT1 receptors are stimulated [43]. This enhanced immune response in SHR may contribute to the early prehypertensive activation of the sympathetic nervous system through a central action of circulating cytokines. In contrast, we found an inhibitory influence of nicotine in WKY. This suggested that the inhibitory α7 nicotinic receptors may be lacking in SHR and that other subunits of nicotinic receptors may mediate instead an excitatory response. In a recent report, nicotine was shown to enhance NF-κB in aortic vascular muscle in an interaction that involves the expression of osteopontin [59]. The pronounced pro-inflammatory response in SHR innate immune cells, which are the antigen presenting cells to T lymphocyte, should also evoke profound activation of the adaptive immune system (Figure 5).
T-lymphocytes are Essential in Hypertension
The assertion that T lymphocytes are essential determinants of hypertension was made by David Harrison and colleagues who were first to identify the dependence of angiotensin-hypertension on the presence of T lymphocytes and to proclaim that hypertension is indeed an immunologic disease (Figure 5) [42]. Different types of T lymphocytes define the nature of the immune response as either protective or cytotoxic. For example T regulatory cells which release IL-10 and adenosine suppress cytotoxic T cells (CD8+ cells) and prevent auto-immune disease. In contrast, the recently identified Th17 cells which release IL-17 are cytotoxic against endogenous antigens causing severe auto-immune disease. The same TH17 lymphocytes release IL-22 which is effective against exogenous microbial antigens.
The migration of T lymphocytes to renal or vascular tissues would contribute to the inflammatory and pathological process in hypertension. This positive feedback interaction between the activation of the innate and adaptive immune systems supports the notion that hypertension is in part an immunologic disease profoundly regulated by the autonomic system. Harrison reinforced the role of IL-17 specifically as the cytotoxic cytokine in renal and vascular tissues [44]. CD8+ cells are the responsible lymphocytes that release IL17 in this model and CD4+ cells may be protective. The mechanisms by which the T lymphocytes migrate to the end organs were explored [60]. An initial neural stimulus to the target organ, possibly an increase in sympathetic nerve activity, may provoke an initial tissue damage. A mild “borderline” elevation of arterial pressure may initiate a vascular tension that may induce the expression of matrix metalloproteinase-12. This then may function as a neoantigen that activates an innate immune response and creates the chemoattracting environment that results in migration of the cytotoxic T lymphocytes.
Conclusions and Implications
The important concept that emerges from this brief review is the realization that autonomic balance is not only an essential determinant of acute circulatory adjustments to stresses. It is a powerful regulator of pathological processes that define cardiovascular mortality and morbidity. We are now able to measure non-invasively this autonomic balance and to intervene therapeutically with drugs and direct nerve stimulations in human and animal models and confirm the strong association between autonomic balance, cardiovascular risks and cardiovascular pathology.
A second important concept is the realization that the immune system, which is a major determinant of most cardiovascular pathological processes, can be modulated extensively by the autonomic system. This means that our notion of autonomic control of the circulation needs to be expanded beyond its hemodynamic influence and extended into its influence on cellular and structural pathological processes. These are accentuated by the pro-inflammatory activation of the innate and adaptive immune systems.
Our biggest challenge resides in the complexity of the immune system, its cellular components, and the multiplicity of cytokines they generate. We need to define their origin and sites of action, the processes that determine their damaging influences and more importantly the interventions that block or reverse those influences. On the other hand a better understanding of the autonomic control of cardiovascular immune processes is extremely promising. We have tools to quantify that autonomic influence and tools to modulate it.
Our take home message from this review is: The pronounced influence of the autonomic imbalance on cardiovascular morbidity and mortality is not simply a function of hemodynamic effects. Recent studies have revealed that a major determinant of the pathological process results from autonomic dysregulation of the inflammatory/immune system.
Acknowledgments
This brief review was prepared following the presentation of a symposium entitled “Neural Regulation of the Immune System—Implications for Hypertension and Cardiovascular Risks,” at the Meeting of the Council on High Blood Pressure Research of the American Heart Association (September 20–24, 2011). We thank Shawn Roach for the preparation of the illustrations of this manuscript.
Sources of Funding The work of the authors referred to in this review was supported by research and training grants from the National Institutes of Health (Grants HL 14388 and HL 007121) and the U.S. Department of Veterans Affairs.
Footnotes
Disclosures None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abboud FM. The sympathetic system in hypertension. State-of-the-art review. Hypertension. 1982;4:208–225. [PubMed] [Google Scholar]
- 2.Esler M. The sympathetic nervous system through the ages: from Thomas Willis to resistant hypertension. Exp Physiol. 2011;96:611–622. doi: 10.1113/expphysiol.2010.052332. [DOI] [PubMed] [Google Scholar]
- 3.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 4.Mancia G, Grassi G, Giannattasio C, Seravalle G. Sympathetic activation in the pathogenesis of hypertension and progression of organ damage. Hypertension. 1999;34:724–728. doi: 10.1161/01.hyp.34.4.724. [DOI] [PubMed] [Google Scholar]
- 5.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, Rector T. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984;311:819–823. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- 7.Korner P, Bobik A, Oddie C, Friberg P. Sympathoadrenal system is critical for structural changes in genetic hypertension. Hypertension. 1993;22:243–252. doi: 10.1161/01.hyp.22.2.243. [DOI] [PubMed] [Google Scholar]
- 8.La Rovere MT, Bigger JT, Jr., Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351:478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 9.Mortara A, La Rovere MT, Pinna GD, Prpa A, Maestri R, Febo O, Pozzoli M, Opasich C, Tavazzi L. Arterial baroreflex modulation of heart rate in chronic heart failure: clinical and hemodynamic correlates and prognostic implications. Circulation. 1997;96:3450–3458. doi: 10.1161/01.cir.96.10.3450. [DOI] [PubMed] [Google Scholar]
- 10.Robinson TG, Dawson SL, Eames PJ, Panerai RB, Potter JF. Cardiac baroreceptor sensitivity predicts long-term outcome after acute ischemic stroke. Stroke. 2003;34:705–712. doi: 10.1161/01.STR.0000058493.94875.9F. [DOI] [PubMed] [Google Scholar]
- 11.Abboud FM. The Walter B. Cannon Memorial Award Lecture, 2009. Physiology in perspective: The wisdom of the body. In search of autonomic balance: the good, the bad, and the ugly. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1449–1467. doi: 10.1152/ajpregu.00130.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci U S A. 2006;103:17985–17990. doi: 10.1073/pnas.0605545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res. 2004;95:210–216. doi: 10.1161/01.RES.0000135483.12297.e4. [DOI] [PubMed] [Google Scholar]
- 14.Fukuda Y, Sato A, Trzebski A. Carotid chemoreceptor discharge responses to hypoxia and hypercapnia in normotensive and spontaneously hypertensive rats. J Auton Nerv Syst. 1987;19:1–11. doi: 10.1016/0165-1838(87)90139-1. [DOI] [PubMed] [Google Scholar]
- 15.Tan ZY, Lu Y, Whiteis CA, Simms AE, Paton JF, Chapleau MW, Abboud FM. Chemoreceptor hypersensitivity, sympathetic excitation, and overexpression of ASIC and TASK channels before the onset of hypertension in SHR. Circ Res. 2010;106:536–545. doi: 10.1161/CIRCRESAHA.109.206946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawrence IG, Weston PJ, Bennett MA, McNally PG, Burden AC, Thurston H. Is impaired baroreflex sensitivity a predictor or cause of sudden death in insulin-dependent diabetes mellitus? Diabet Med. 1997;14:82–85. doi: 10.1002/(SICI)1096-9136(199701)14:1<82::AID-DIA290>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 17.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 18.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 19.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 20.Zucker IH, Hackley JF, Cornish KG, Hiser BA, Anderson NR, Kieval R, Irwin ED, Serdar DJ, Peuler JD, Rossing MA. Chronic baroreceptor activation enhances survival in dogs with pacing-induced heart failure. Hypertension. 2007;50:904–910. doi: 10.1161/HYPERTENSIONAHA.107.095216. [DOI] [PubMed] [Google Scholar]
- 21.Li M, Zheng C, Sato T, Kawada T, Sugimachi M, Sunagawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation. 2004;109:120–124. doi: 10.1161/01.CIR.0000105721.71640.DA. [DOI] [PubMed] [Google Scholar]
- 22.Uemura K, Li M, Tsutsumi T, Yamazaki T, Kawada T, Kamiya A, Inagaki M, Sunagawa K, Sugimachi M. Efferent vagal nerve stimulation induces tissue inhibitor of metalloproteinase-1 in myocardial ischemia-reperfusion injury in rabbit. Am J Physiol Heart Circ Physiol. 2007;293:H2254–2261. doi: 10.1152/ajpheart.00490.2007. [DOI] [PubMed] [Google Scholar]
- 23.Communal C, Singh K, Pimentel DR, Colucci WS. Norepinephrine stimulates apoptosis in adult rat ventricular myocytes by activation of the beta-adrenergic pathway. Circulation. 1998;98:1329–1334. doi: 10.1161/01.cir.98.13.1329. [DOI] [PubMed] [Google Scholar]
- 24.Lohmeier T, EIliescu R. Chronic lowering of blood pressure by carotid baroreflex activation: mechanisms and potential for hypertension therapy. Hypertension. 2011;57:880–886. doi: 10.1161/HYPERTENSIONAHA.108.119859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cabassi A, Vinci S, Calzolari M, Bruschi G, Borghetti A. Regional sympathetic activity in pre-hypertensive phase of spontaneously hypertensive rats. Life Sci. 1998;62:1111–1118. doi: 10.1016/s0024-3205(98)00034-4. [DOI] [PubMed] [Google Scholar]
- 26.Lu Y, Whiteis CA, Chapleau MW, Abboud FM. Deceased mRNA expression of ASIC2a in nodose sensory ganglia is associated with development of hypertension in SHR (Abstract) FASEB J. 2007;21:A1405. [Google Scholar]
- 27.Doumas M, Guo D, Papademetriou V. Carotid baroreceptor stimulation as a therapeutic target in hypertension and other cardiovascular conditions. Expert Opin Ther Targets. 2009;13:413–425. doi: 10.1517/14728220902780185. [DOI] [PubMed] [Google Scholar]
- 28.Heusser K, Tank J, Engeli S, Diedrich A, Menne J, Eckert S, Peters T, Sweep FC, Haller H, Pichlmaier AM, Luft FC, Jordan J. Carotid baroreceptor stimulation, sympathetic activity, baroreflex function, and blood pressure in hypertensive patients. Hypertension. 2010;55:619–626. doi: 10.1161/HYPERTENSIONAHA.109.140665. [DOI] [PubMed] [Google Scholar]
- 29.Wustmann K, Kucera JP, Scheffers I, Mohaupt M, Kroon AA, de Leeuw PW, Schmidli J, Allemann Y, Delacretaz E. Effects of chronic baroreceptor stimulation on the autonomic cardiovascular regulation in patients with drug-resistant arterial hypertension. Hypertension. 2009;54:530–536. doi: 10.1161/HYPERTENSIONAHA.109.134023. [DOI] [PubMed] [Google Scholar]
- 30.Hauptman PJ, Mann DL. The vagus nerve and autonomic imbalance in heart failure: past, present, and future. Heart Fail Rev. 2011;16:97–99. doi: 10.1007/s10741-010-9222-2. [DOI] [PubMed] [Google Scholar]
- 31.Sabbah HN. Electrical vagus nerve stimulation for the treatment of chronic heart failure. Cleve Clin J Med. 2011;78(Suppl 1):S24–29. doi: 10.3949/ccjm.78.s1.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sabbah HN, Ilsar I, Zaretsky A, Rastogi S, Wang M, Gupta RC. Vagus nerve stimulation in experimental heart failure. Heart Fail Rev. 2011;16:171–178. doi: 10.1007/s10741-010-9209-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roifman I, Beck PL, Anderson TJ, Eisenberg MJ, Genest J. Chronic inflammatory diseases and cardiovascular risk: a systematic review. Can J Cardiol. 2011;27:174–182. doi: 10.1016/j.cjca.2010.12.040. [DOI] [PubMed] [Google Scholar]
- 34.Svendsen UG. Evidence for an initial, thymus independent and a chronic, thymus dependent phase of DOCA and salt hypertension in mice. Acta Pathol Microbiol Scand A. 1976;84:523–528. doi: 10.1111/j.1699-0463.1976.tb00150.x. [DOI] [PubMed] [Google Scholar]
- 35.Ba D, Takeichi N, Kodama T, Kobayashi H. Restoration of T cell depression and suppression of blood pressure in spontaneously hypertensive rats (SHR) by thymus grafts or thymus extracts. J Immunol. 1982;128:1211–1216. [PubMed] [Google Scholar]
- 36.Pascual VH, Oparil S, Eldridge JH, Jin H, Bost KL, Pascual DW. Spontaneously hypertensive rat: lymphoid depression is age dependent and mediated via a mononuclear cell subpopulation. Am J Physiol. 1992;262:R1–7. doi: 10.1152/ajpregu.1992.262.1.R1. [DOI] [PubMed] [Google Scholar]
- 37.Khraibi AA. Association between disturbances in the immune system and hypertension. Am J Hypertens. 1991;4:635–641. doi: 10.1093/ajh/4.7.635. [DOI] [PubMed] [Google Scholar]
- 38.Huang XR, Chung AC, Yang F, Yue W, Deng C, Lau CP, Tse HF, Lan HY. Smad3 mediates cardiac inflammation and fibrosis in angiotensin II-induced hypertensive cardiac remodeling. Hypertension. 2010;55:1165–1171. doi: 10.1161/HYPERTENSIONAHA.109.147611. [DOI] [PubMed] [Google Scholar]
- 39.Chae CU, Lee RT, Rifai N, Ridker PM. Blood pressure and inflammation in apparently healthy men. Hypertension. 2001;38:399–403. doi: 10.1161/01.hyp.38.3.399. [DOI] [PubMed] [Google Scholar]
- 40.Melendez GC, McLarty JL, Levick SP, Du Y, Janicki JS, Brower GL. Interleukin 6 mediates myocardial fibrosis, concentric hypertrophy, and diastolic dysfunction in rats. Hypertension. 2010;56:225–231. doi: 10.1161/HYPERTENSIONAHA.109.148635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brands MW, Banes-Berceli AK, Inscho EW, Al-Azawi H, Allen AJ, Labazi H. Interleukin 6 knockout prevents angiotensin II hypertension: role of renal vasoconstriction and janus kinase 2/signal transducer and activator of transcription 3 activation. Hypertension. 2010;56:879–884. doi: 10.1161/HYPERTENSIONAHA.110.158071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harwani SC, Chapleau MW, Legge K, Ballas Z, Abboud FM. Autonomic dysregulation of innate immunity in genetic hypertension (Abstract) Hypertension. 2011;58:e50. [Google Scholar]
- 44.Harrison DG, Vinh A, Lob H, Madhur MS. Role of the adaptive immune system in hypertension. Curr Opin Pharmacol. 2010;10:203–207. doi: 10.1016/j.coph.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zubcevic J, Waki H, Raizada MK, Paton JF. Autonomic-immune-vascular interaction: an emerging concept for neurogenic hypertension. Hypertension. 2011;57:1026–1033. doi: 10.1161/HYPERTENSIONAHA.111.169748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987–2007) Brain Behav Immun. 2007;21:736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schauenstein K, Felsner P, Rinner I, Liebmann PM, Stevenson JR, Westermann J, Haas HS, Cohen RL, Chambers DA. In vivo immunomodulation by peripheral adrenergic and cholinergic agonists/antagonists in rat and mouse models. Ann N Y Acad Sci. 2000;917:618–627. doi: 10.1111/j.1749-6632.2000.tb05427.x. [DOI] [PubMed] [Google Scholar]
- 48.Ganta CK, Lu N, Helwig BG, Blecha F, Ganta RR, Zheng L, Ross CR, Musch TI, Fels RJ, Kenney MJ. Central angiotensin II-enhanced splenic cytokine gene expression is mediated by the sympathetic nervous system. Am J Physiol Heart Circ Physiol. 2005;289:H1683–1691. doi: 10.1152/ajpheart.00125.2005. [DOI] [PubMed] [Google Scholar]
- 49.O'Mahony C, van der Kleij H, Bienenstock J, Shanahan F, O'Mahony L. Loss of vagal anti-inflammatory effect: in vivo visualization and adoptive transfer. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1118–1126. doi: 10.1152/ajpregu.90904.2008. [DOI] [PubMed] [Google Scholar]
- 50.Karimi K, Bienenstock J, Wang L, Forsythe P. The vagus nerve modulates CD4+ T cell activity. Brain Behav Immun. 2010;24:316–323. doi: 10.1016/j.bbi.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 51.Skok M, Grailhe R, Changeux JP. Nicotinic receptors regulate B lymphocyte activation and immune response. Eur J Pharmacol. 2005;517:246–251. doi: 10.1016/j.ejphar.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 52.Rosas-Ballina M, Olofsson PS, Ochani M, Valdes-Ferrer SI, Levine YA, Reardon C, Tusche MW, Pavlov VA, Andersson U, Chavan S, Mak TW, Tracey KJ. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y, Popovic ZB, Bibevski S, Fakhry I, Sica DA, Van Wagoner DR, Mazgalev TN. Chronic vagus nerve stimulation improves autonomic control and attenuates systemic inflammation and heart failure progression in a canine high-rate pacing model. Circ Heart Fail. 2009;2:692–699. doi: 10.1161/CIRCHEARTFAILURE.109.873968. [DOI] [PubMed] [Google Scholar]
- 54.Trakhtenberg EF, Goldberg JL. Immunology - Neuroimmune communication. Science. 2011;334:47–48. doi: 10.1126/science.1213099. [DOI] [PubMed] [Google Scholar]
- 55.Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, Sriramula S, Francis J, Sumners C, Raizada MK. Brain microglial cytokines in neurogenic hypertension. Hypertension. 2010;56:297–303. doi: 10.1161/HYPERTENSIONAHA.110.150409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Waki H, Liu B, Miyake M, Katahira K, Murphy D, Kasparov S, Paton JF. Junctional adhesion molecule-1 is upregulated in spontaneously hypertensive rats: evidence for a prohypertensive role within the brain stem. Hypertension. 2007;49:1321–1327. doi: 10.1161/HYPERTENSIONAHA.106.085589. [DOI] [PubMed] [Google Scholar]
- 57.Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30:16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 58.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y, Zhang F, Yang W, Xue S. Nicotine induces pro-inflammatory response in aortic vascular smooth muscle cells through a NFkappaB/osteopontin amplification loop-dependent pathway. Inflammation. 2011 doi: 10.1007/s10753-011-9324-6. In Press. [DOI] [PubMed] [Google Scholar]
- 60.Thabet S, Wu J, Chen W, Marvar P, Gongora M, Madhur M, Binder Y, Weyand H, Harrison DG. The role of CD8+ T Cells, IP-10, and MMP12 in hypertension (Abstract) Hypertension. 2011;58:e49. [Google Scholar]