Abstract
Background
Mechanosensing and its downstream responses are speculated to involve sensory complexes containing Ca2+-permeable mechanosensitive channels. On recognizing osmotic signals, plant cells initiate activation of a widespread signal transduction network that induces second messengers and triggers inducible defense responses. Characteristic early signaling events include Ca2+ influx, protein phosphorylation and generation of reactive oxygen species (ROS). Pharmacological analyses show Ca2+ influx mediated by mechanosensitive Ca2+ channels to influence induction of osmotic signals, including ROS generation. However, molecular bases and regulatory mechanisms for early osmotic signaling events remain poorly elucidated.
Results
We here identified and investigated OsMCA1, the sole rice homolog of putative Ca2+-permeable mechanosensitive channels in Arabidopsis (MCAs). OsMCA1 was specifically localized at the plasma membrane. A promoter-reporter assay suggested that OsMCA1 mRNA is widely expressed in seed embryos, proximal and apical regions of shoots, and mesophyll cells of leaves and roots in rice. Ca2+ uptake was enhanced in OsMCA1-overexpressing suspension-cultured cells, suggesting that OsMCA1 is involved in Ca2+ influx across the plasma membrane. Hypo-osmotic shock-induced ROS generation mediated by NADPH oxidases was also enhanced in OsMCA1-overexpressing cells. We also generated and characterized OsMCA1-RNAi transgenic plants and cultured cells; OsMCA1-suppressed plants showed retarded growth and shortened rachises, while OsMCA1-suppressed cells carrying Ca2+-sensitive photoprotein aequorin showed partially impaired changes in cytosolic free Ca2+ concentration ([Ca2+]cyt) induced by hypo-osmotic shock and trinitrophenol, an activator of mechanosensitive channels.
Conclusions
We have identified a sole MCA ortholog in the rice genome and developed both overexpression and suppression lines. Analyses of cultured cells with altered levels of this putative Ca2+-permeable mechanosensitive channel indicate that OsMCA1 is involved in regulation of plasma membrane Ca2+ influx and ROS generation induced by hypo-osmotic stress in cultured rice cells. These findings shed light on our understanding of mechanical sensing pathways.
Background
Plants need to sense and respond to mechanical stresses, such as wind, touch, and changes in osmotic pressure [1-3]. Elevation of cytosolic free Ca2+ concentration ([Ca2+]cyt) is induced in response to various stimuli, such as chemical, physical, and mechanical stimuli [2,4-7]. During this process, [Ca2+]cyt levels rise through the opening of Ca2+ channels located on the plasma membrane and endomembranes. Electrophysiological and bioinformatic studies have revealed the existence of plasma membrane Ca2+-permeable channels activated by mechanical stimuli, although the structural entity involved and their physiological functions remain largely unknown [8-12].
Molecular and electrophysiological studies have shown that Arabidopsis thaliana MSL9 and MSL10, homologs of the bacterial mechanosensitive channel MscS, are required for mechanosensitive channel activity in the plasma membrane of root cells, and are more permeable to Cl- than Ca2+ [13,14]. We have recently identified two plasma membrane proteins as putative Ca2+-permeable mechanosensitive channels, MCA1 (At4g35920) and MCA2 (At2g17780), from Arabidopsis [15,16], and showed that ectopic overexpression of MCA1 increases Ca2+ uptake in roots, and also enhances [Ca2+]cyt elevation upon hypo-osmotic shock. However, the direct effects of MCA proteins on osmotic-induced Ca2+ influx through the plasma membrane and the osmotic signaling pathways are little understood.
Upon recognition of osmotic signals, plant cells initiate activation of a widespread signal transduction network that induces second messengers and triggers inducible defense responses. Characteristic early signaling events include Ca2+ influx, protein phosphorylation and generation of reactive oxygen species (ROS) [17-20]. These downstream events are often prevented when Ca2+ influx is compromised by either Ca2+ chelators, such as ethylene glycol-bis-(2-aminoethylether)-N, N, N', N'-tetra acetic acid (EGTA), or Ca2+-channel blockers, such as La3+ [21]. In tobacco cells, hypo-osmotic shock-induced ROS generation reportedly requires activation of mechanosensitive Ca2+ channels [22]. These results suggest that Ca2+ influx mediated by mechanosensitive Ca2+ channels is involved in the induction of osmotic signals including ROS generation. However, in osmotic responses, molecular bases and regulation mechanisms remain poorly elucidated.
In the present study, we have identified a sole MCA ortholog in the rice genome and developed both overexpression and suppression lines. Studies of these lines with altered levels of this putative mechanosensitive Ca2+ channel indicated that OsMCA1 is involved in regulation of plasma membrane Ca2+ influx and ROS generation induced by hypo-osmotic stress in cultured rice cells.
Results
Identification of OsMCA1 and its expression patterns
Full-length cDNA of OsMCA1 was obtained by a rapid amplification of cDNA ends (RACE)-PCR method. It encodes a polypeptide of 418 amino acid residues with a calculated molecular mass of 47,417 (GenBank Accession No. AB601973). The predicted protein showed 66.7% and 57.6% amino acid sequence identity compared with Arabidopsis MCA1 and MCA2, respectively; the TopPred program http://www.sbc.su.se/~erikw/toppred2/ suggests that OsMCA1 has two potential transmembrane segments (S1 and S2) (Additional file 1), while other transmembrane segment prediction programs suggest different numbers of putative transmembrane segments (data not shown). The PLAC8 motif was found by TMpred prediction http://www.ch.embnet.org/software/TMPRED_form.html in the C-terminal region (Additional file 1). A database search of the whole genome (Rice BLAST; http://riceblast.dna.affrc.go.jp/) indicated that rice has no other homolog of OsMCA1.
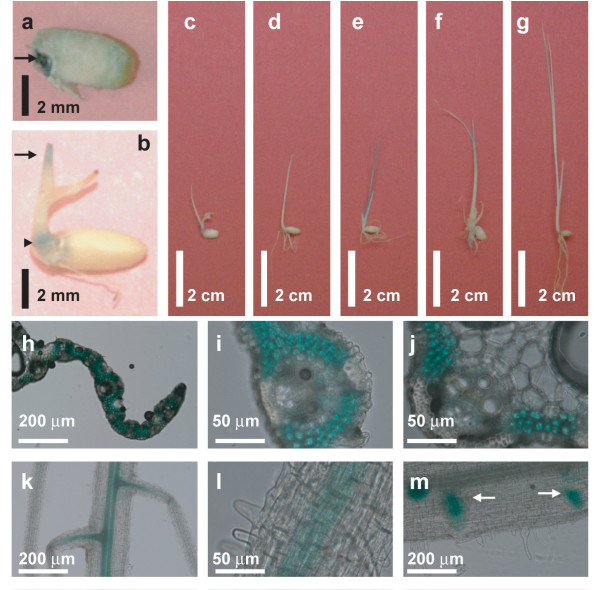
Quantitative reverse transcriptase (RT)-PCR analysis showed OsMCA1 mRNA to be expressed in mature leaves, shoots, roots and suspension-cultured cells, suggesting that OsMCA1 mRNA is expressed throughout the plants in seedlings as well as in cultured cells (Additional file 2). We also consulted the microarray expression database (Rice XPro; http://ricexpro.dna.affrc.go.jp/GGEP/index.html, OsMCA1 locus ID; Os03g0157300), showing expression of OsMCA1 mRNA throughout the developmental stages, including root, leaf blade, panicle, anther, pistil, and ovary as well as embryo (data not shown). The spatial pattern of OsMCA1 expression was examined using an OsMCA1 promoter::β-glucuronidase (GUS) fusion reporter gene construct (OsMCA1p::GUS). Figure 1a shows OsMCA1p::GUS is expressed in the seed, with relatively high levels in embryo. In the seedling stage, OsMCA1p::GUS is highly expressed in proximal and apical regions of shoots (Figure 1b-g). Cross sections of the leaves indicate that OsMCA1p::GUS is highly expressed in mesophyll cells, but expressed in vascular tissues at relatively very low levels (Figure 1h-j). OsMCA1p::GUS was also expressed in the root, with relatively high levels in the center of primary root as well as the lateral root primordia (Figure 1k-m). These results suggest that OsMCA1 transcription may be regulated throughout developmental stages. The expression pattern of OsMCA1 was similar to those of the Arabidopsis MCA2.
Figure 1.
Spatial patterns of OsMCA1 transcription as revealed by GUS staining. Transgenic rice plants harboring OsMCA1p::GUS were stained in X-Gluc solution and cleared in methanol. (a) Half of a seed. An arrow indicates the seed embryo. (b-g) Whole seedlings grown for 7, 7, 7, 8, 13 and 11 days, respectively. Shoot apices and proximal regions are indicated with arrows and arrowheads, respectively. (h-j) Cross sections of leaves from plants grown for 14 days. Representative staining images for three transgenic plants are shown. (k-m) Roots from plants grown for 7, 14 and 14 days, respectively. Arrows indicate lateral root primordial. Sections are 50 or 200 μm thick.
Intracellular localization of the OsMCA1 protein
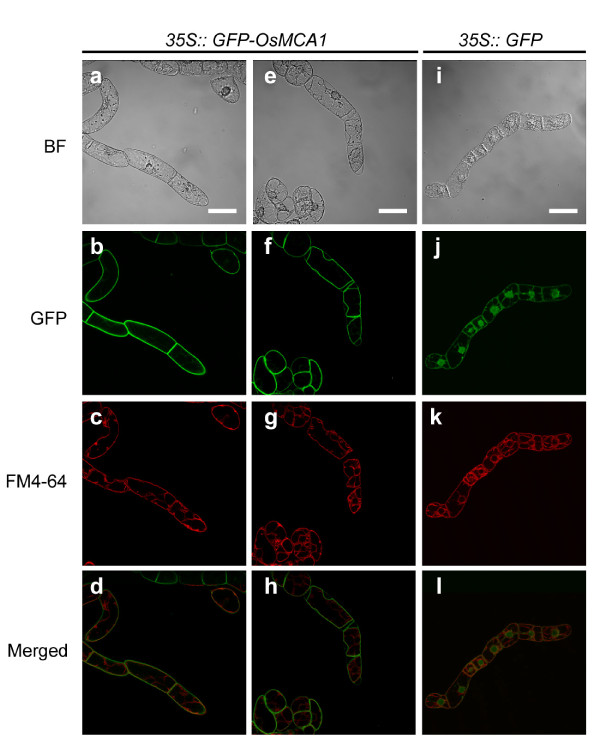
To investigate intracellular localization of the OsMCA1 protein, we introduced a green fluorescent protein (GFP) construct fused to the coding sequence of the N-terminus of OsMCA1 into tobacco BY-2 cells and examined its intracellular localization using confocal laser scanning microscopy. When GFP alone was expressed, it localized to the nucleus and the cytoplasm (Figure 2i, j). In contrast, GFP-OsMCA1 fusion protein localized specifically to the plasma membrane (Figure 2a, b). This pattern was reinforced by treatment with a high osmotic solution, 1 M mannitol, which induced plasmolysis (Figure 2e, f). In addition, fluorescent images and behavior of GFP-OsMCA1 before and after plasmolysis were different from those of the intracellular staining marker FM4-64 (Figure 2c, g, k and 2d, h, l), indicating that OsMCA1 is localized to the plasma membrane.
Figure 2.
Intracellular localization of the OsMCA1 protein. Confocal fluorescence images (b-d, f-h, j-l) and differential interference contrast (DIC) images (a, e, i) of tobacco BY-2 cells expressing GFP-OsMCA1 (a-h) or GFP (i-l) stained with FM4-64 (4.25 μM) for 3 h. Fluorescence of GFP (b, f, j) and FM4-64 (c, g, k). (e-h) Plasmolyzed cells. Scale bar: 20 μm.
Effects of OsMCA1 overexpression on Ca2+ uptake in cultured rice cells
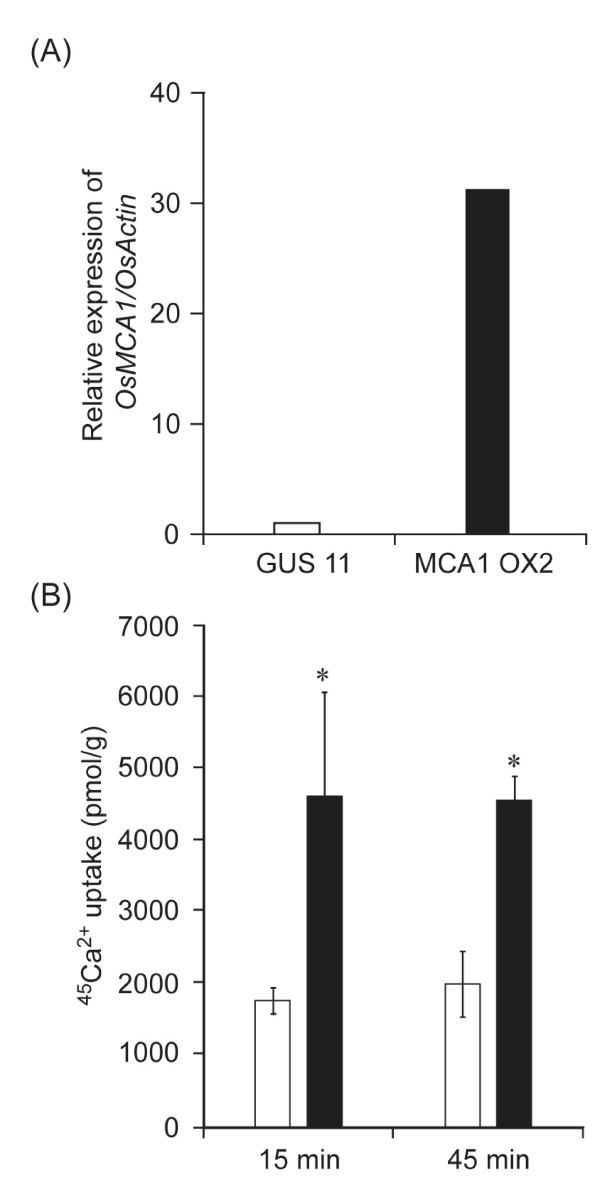
To test if OsMCA1 plays a role in Ca2+ transport, we generated cultured cells overexpressing OsMCA1 and analyzed whether expression levels of OsMCA1 affected Ca2+ uptake activity. As shown in Figure 3A, B, Ca2+ uptake activity was higher in OsMCA1-overexpressing cells than in GUS-expressing control cells, suggesting that OsMCA1 is involved in Ca2+ uptake across the plasma membrane in rice. We also generated OsMCA1-overexpressing plants, which showed no significant visible phenotypes (data not shown).
Figure 3.
Effect of OsMCA1-overexpression on Ca2+ uptake in cultured rice cells. Open and closed bars represent GUS-expressing control line (GUS No.11) and OsMCA1-overexpressing line (OX No. 2), respectively. (A) Quantification of OsMCA1 mRNA by real-time quantitative PCR. The amount of OsMCA1 mRNA was calculated from the threshold point in the log-linear range of the RT-PCR. The relative level of OsMCA1 mRNAs in the GUS-expressing control line was standardized as 1. (B) 45Ca2+ uptake into cultured rice cells. Data are means ± SD, n = 3 independent samples. *P < 0.05; significantly different compared with the control.
Effect of OsMCA1 suppression on growth and development in planta
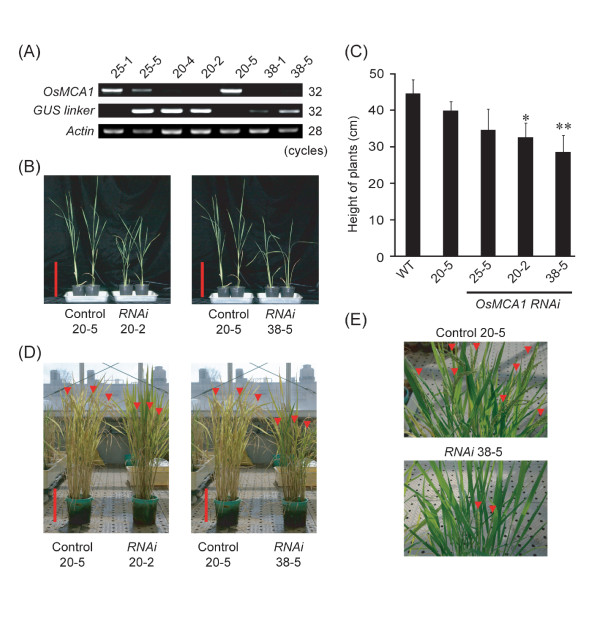
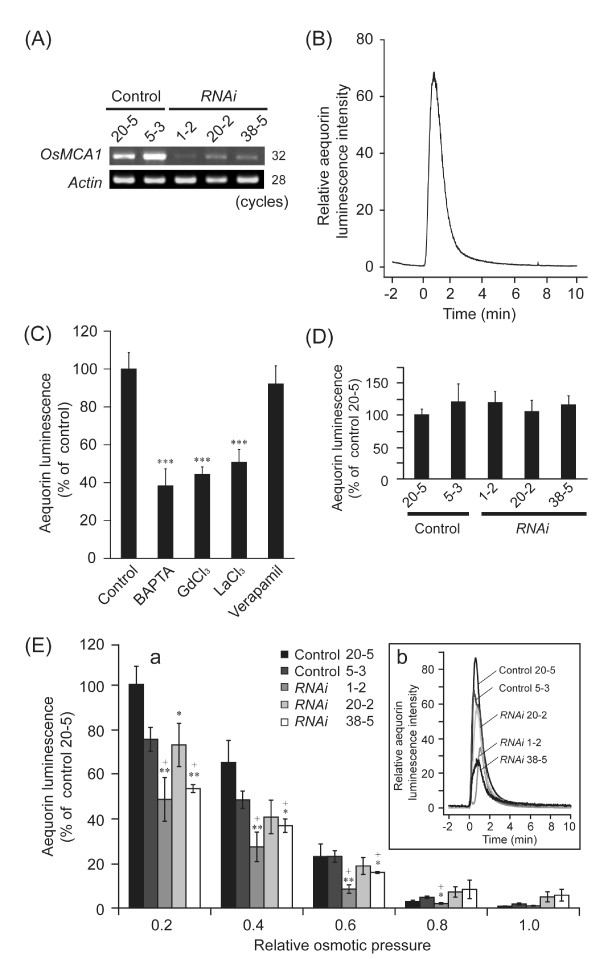
To elucidate the physiological roles of OsMCA1, transgenic plants were generated in which OsMCA1 expression was suppressed by RNA interference (RNAi) using gene-specific sequences (a 400-bp region of OsMCA1). Five independent transgenic plants were generated using Agrobacterium-mediated transformation. Non-transgenic plants were investigated simultaneously as controls, whose transduced genes were removed by heterozygous segregation. RT-PCR analyses revealed significant reductions in OsMCA1 mRNA levels compared with controls (Figure 4A).
Figure 4.
Effects of OsMCA1 suppression on growth and development in planta. (A) RT-PCR analysis of OsMCA1 in five independent RNAi lines. Expression of GUS linker indicates RT-PCR products of GUS linker region, and expression of trigger dsRNA. Actin cDNA was used as internal control; PCR products were analyzed by agarose gel electrophoresis. (B) Phenotype of OsMCA1-suppressed lines of 3-week-old plants. Bars indicate 10 cm. (C) Heights of plants shown in (B) were quantified. Data are means ± SD, n = 6-13 independent plants. *P < 0.05; **P < 0.01; significantly different compared with two control lines (WT and Control 20-5). (D, E) Phenotype of OsMCA1-suppressed lines grown for 4 months (D) and 100 days (E) in a greenhouse. Arrowheads indicate ears; bars indicate 10 cm.
The OsMCA1-suppressed lines showed slower growth in adult plants (Figure 4B, C). Though germination rates (data not shown) and seedling growth of suppression lines were comparable to controls in the Murashige and Skoog medium (MS medium) (Additional file 3), growth of suppression lines was remarkably retarded after transplantation into soil in a greenhouse, suggesting that OsMCA1 suppression leads to hypersensitivity to environmental stresses. This phenotype was exhibited in all 5 independent T2 transgenic RNAi lines; severity of the phenotypes correlated well with expression levels of OsMCA1 transcripts (Figure 4A, C). Furthermore, unlike Arabidopsis mca mutants, rachises of the OsMCA1-suppressed lines were significantly shorter than those of controls (Figure 4D, E), suggesting that OsMCA1 plays a different role from Arabidopsis MCAs in some developmental stages.
Effects of OsMCA1-suppression on cell growth and Ca2+ sensitivity in cultured rice cells
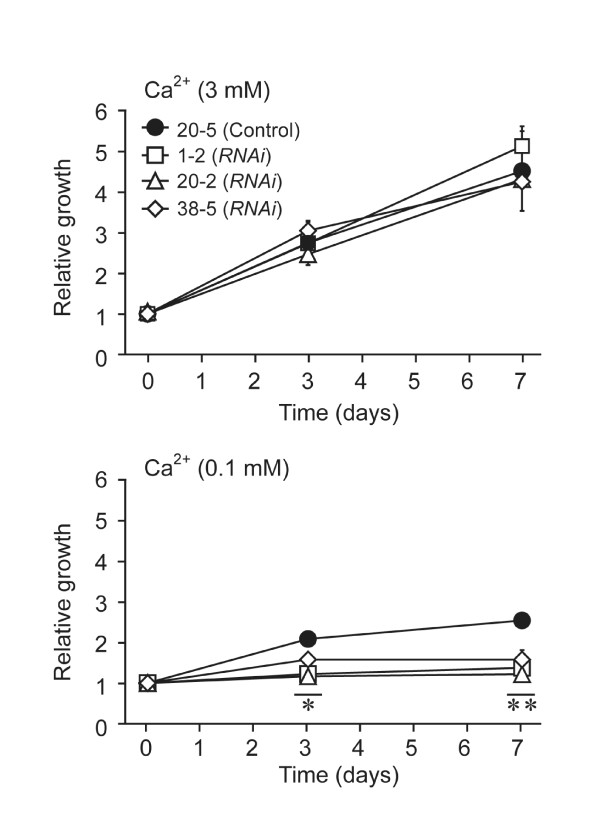
We tested whether OsMCA1 suppression affects Ca2+ sensitivity to growth in rice cells. In regular medium containing 3 mM of Ca2+, growth rates of OsMCA1-suppressed lines were comparable to controls (Figure 5). In contrast, when Ca2+ concentration of the medium was decreased to 0.1 mM (Figure 5), growth of OsMCA1-suppressed lines was significantly restricted compared with controls, suggesting possible involvement of OsMCA1 in acquisition of Ca2+ for cell growth under Ca2+ limitation.
Figure 5.
Effects of OsMCA1 suppression on cell growth and Ca2+ sensitivity in cultured rice cells. Seven day-old-cultured cells were assayed. Cells (fresh weight 0.5 g) were transferred to L medium with standard or low Ca2+ concentration. After culturing for 0, 3, or 7 days, the fresh weight of cells was measured. Data are the mean ± SE for three independent experiments. * P < 0.05; ** P < 0.01; significantly different compared with control.
Involvement of OsMCA1 in mechanical stress-induced [Ca2+]cyt changes
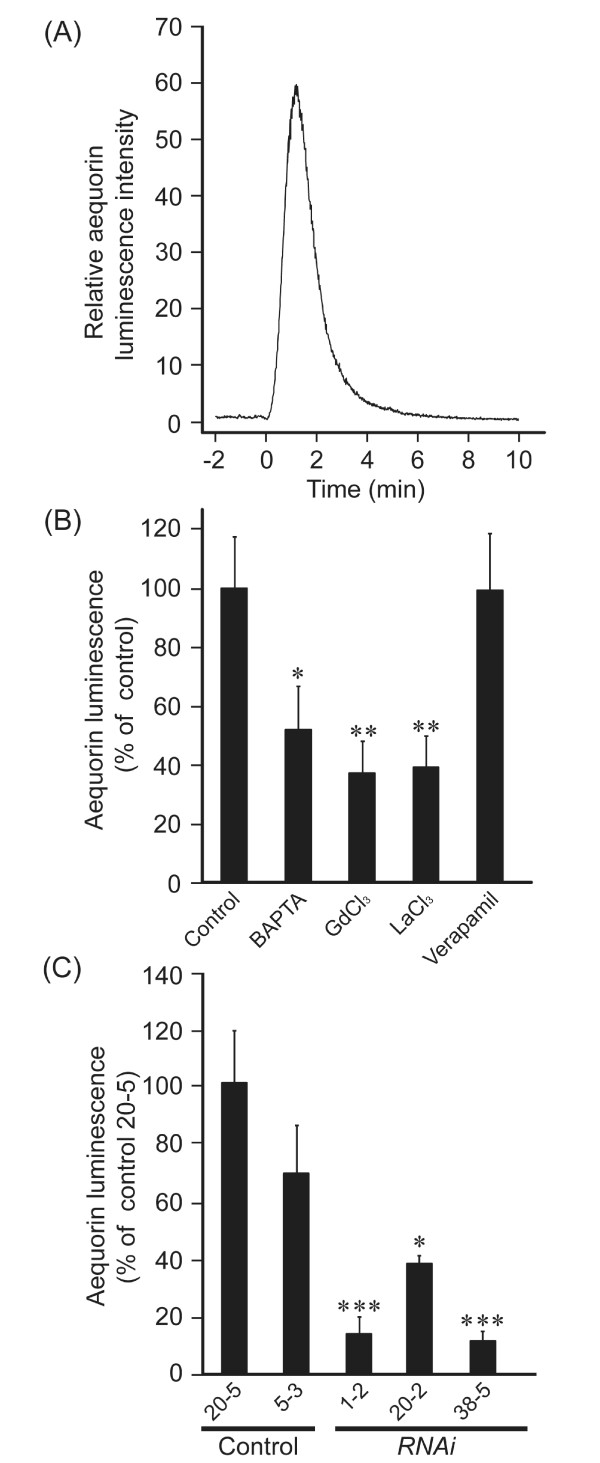
To test for possible involvement of OsMCA1 in regulation of Ca2+ influx induced by various stimuli, we generated OsMCA1-suppressed lines harboring the Ca2+-sensitive bioluminescent protein aequorin (Figure 6A). Hypo-osmotic shock-induced transient [Ca2+]cyt change in cultured rice cells (Figure 6B) was inhibited by a Ca2+ chelator, 1,2-bis-(2-aminophenoxy)ethane-N, N, N', N'-tetra-acetic acid (BAPTA), and Ca2+ channel blockers (GdCl3 and LaCl3) but not by verapamil, an inhibitor for voltage-dependent Ca2+ channels, (Figure 6C), suggesting that plasma membrane Ca2+ influx mediated by Gd3+-sensitive mechanosensitive Ca2+-permeable channel(s) is induced by hypo-osmotic shock. The hypo-osmotic shock-induced [Ca2+]cyt change was partially impaired in the OsMCA1-suppressed cells (Figure 6E), and was proportional to levels of OsMCA1 expression in various OsMCA1-suppressed cells. On the other hand, increased [Ca2+]cyt triggered by Nacetylchito-oligosaccharides, a major microbe-associated molecular pattern (MAMP) recognized by plasma membrane receptors in rice [23,24], was not affected by OsMCA1 expression levels (Figure 6D). These results suggest that OsMCA1 participates in the plasma membrane Ca2+ influx triggered by hypo-osmotic shock but not by MAMPs.
Figure 6.
Involvement of OsMCA1 in the regulation of hypo-osmotic shock-induced [Ca2+]cyt changes. (A) RT-PCR analysis of OsMCA1 in three independent RNAi lines expressing apoaequorin mRNA. (B) Hypo-osmotic shock-induced [Ca2+]cyt changes in cultured rice cells. Cells were diluted four-fold with water at 0 min. A representative result of five experiments is shown. (C) Pharmacological analyses of hypo-osmotic shock-induced [Ca2+]cyt changes. Cells were diluted four-fold with water at 0 min. BAPTA (5 mM), GdCl3 (5 mM), LaCl3 (5 mM) and verapamil (1 mM) were added to the cell suspension 15 min before hypo-osmotic shock treatment. No treatment prior to hypo-osmotic shock in the "control." Peak intensities of aequorin chemiluminescence were compared; relative luminescence level in the control was standardized as 100% (C and D). Data are mean ± SE for five independent experiments. ***P < 0.005; significantly different compared with control. (D) Effects of OsMCA1 suppression on N-acetylchito-oligosaccharides-induced [Ca2+]cyt changes. Cells were treated with N-acetylchitoheptaose (1 μM). Error bars indicate SE of the mean of five experiments. (E-a) 1 mL of water, medium, or diluted medium was added to OsMCA1-suppressed cells harboring apoaequorin at 0 min to generate wide-ranging changes in extracellular osmotic pressure. The relative amount of luminescence accumulated for 10 min after hypo-osmotic shock was plotted versus relative extracellular osmotic pressure. Data are means ± SE, n = 3-7. +*P < 0.05; +**P < 0.01; significantly different compared with two control lines (No. 20-5 and 5-3). *P < 0.05; significantly different compared with the control line (No. 20-5). (E-b) Hypo-osmotic shock-induced Ca2+ signature in OsMCA1-suppressed lines. Cells were diluted four-fold with water at 0 min. A representative result of several experiments is shown.
Trinitrophenol (TNP) is a potent compound to generate membrane distortion to activate mechanosensitive channels and mimic mechanical stimuli in plants [15] and animal cells. We investigated the effects of TNP on [Ca2+]cyt and possible involvement of OsMCA1 in its regulation in cultured rice cells. TNP induced transient [Ca2+]cyt change, which was inhibited by BAPTA, GdCl3, and LaCl3 but not by verapamil (Figure 7A, B). The pharmacology of [Ca2+]cyt transients triggered by hypo-osmotic shock and TNP was basically similar (Figure 6C, 7B). TNP-induced [Ca2+]cyt change was also impaired in OsMCA1-suppressed lines (Figure 7C), suggesting the possible involvement of OsMCA1 as a putative mechanosensitive Ca2+-permeable channel in the regulation of mechanical stress-triggered plasma membrane Ca2+ influx.
Figure 7.
Involvement of OsMCA1 in the regulation of Trinitrophenol (TNP)-induced [Ca2+]cyt changes. (A) TNP-induced [Ca2+]cyt changes in cultured rice cells. Cells were treated with TNP (1 mM) at 0 min. A representative result of five experiments is shown. (B) Pharmacological analyses of TNP-induced [Ca2+]cyt changes. BAPTA (5 mM), GdCl3 (5 mM), LaCl3 (5 mM) and verapamil (1 mM) were added to the cell suspension 15 min prior to the TNP treatment; DMSO is used as the control. The peak intensities of aequorin chemiluminescence were compared; the relative luminescence level in the control was standardized as 100%. Data are the mean ± SE for three independent experiments. *P < 0.05; **P < 0.01; significantly different compared with control. (C) Effects of OsMCA1-suppression on TNP-induced [Ca2+]cyt changes. Cells were treated with TNP (1 μM). The peak intensities of aequorin chemiluminescence were compared; the relative luminescence level in the control line (20-5) was standardized as 100%. Data are the mean ± SE for three independent experiments. *P < 0.05; ***P < 0.005; significantly different compared with two control lines (20-5 and 5-3).
We also tried to examine the effect of overexpression of OsMCA1 on mechanical stress-triggered Ca2+ influx. However, we observed a strong reduction in the total aequorin luminescence in all transgenic cell lines overexpressing OsMCA1 (data not shown). Thus it was impossible to measure [Ca2+]cyt using OsMCA1-overexpressing lines. Real-time RT-PCR analysis revealed that the expression level of aequorin gene in the OsMCA1-overexpressing lines was comparable to the control (data not shown). Thus constitutive overexpression of OsMCA1 does not affect the expression but may affect the stability of aequorin protein or inhibit the aequorin chemiluminescence.
Effects of OsMCA1-overexpression on sensitivity to hypo-osmotic shock and generation of reactive oxygen species
Hypo-osmotic shock has been shown to trigger ROS generation following [Ca2+]cyt increase in cultured tobacco cells [18,22]. As OsMCA1 has been suggested to affect regulation of hypo-osmotic shock-induced Ca2+ influx (Figure 6E), we investigated possible OsMCA1 involvement in hypo-osmotic shock-induced ROS generation in cultured rice cells, using two distinctive methods sensitive for superoxide anion radical (•O2-) and hydrogen peroxide (H2O2).
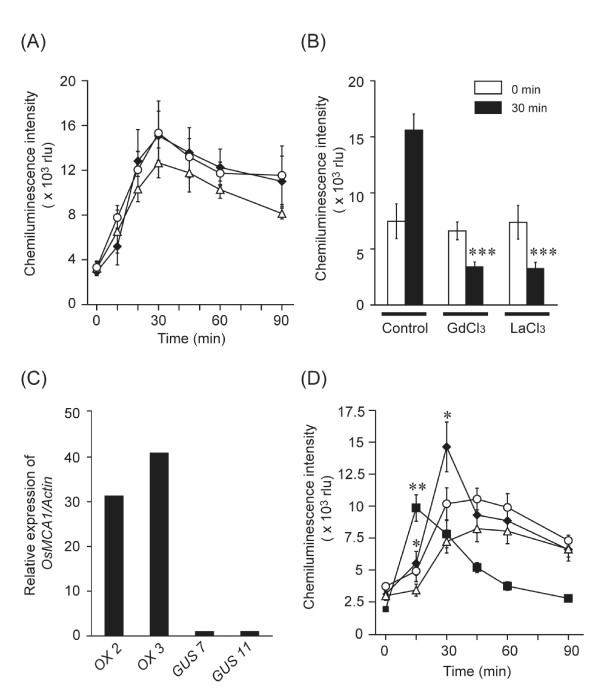
Hypo-osmotic shock triggered ROS generation within 5 min (Figure 8A), which was markedly inhibited by Ca2+ channel blockers (GdCl3 and LaCl3), suggesting an important role f Ca2+ influx in hypo-osmotic shock-induced ROS generation (Figure 8B). Diphenylene iodonium (DPI; 10 μM), an NADPH oxidase inhibitor, significantly suppressed ROS generation (Additional file 4). Peroxidase-catalyzed reactions have been also proposed for osmotic shock-induced ROS generation in cultured tobacco and Arabidopsis cells [19]. However, a peroxidase inhibitor, salicylhydroxamic acid (SHAM, 3 mM), scarcely affected hypo-osmotic shock-induced ROS generation (Additional file 5), suggesting that a major part of hypo-osmotic shock-induced ROS generation is attributable to ROS-producing NADPH oxidases in cultured rice cells. Hypo-osmotic shock-induced generation of both •O2- (Figure 8C, D) and H2O2 (Additional file 6) was either significantly enhanced or more rapid in OsMCA1-overexpressing lines than in the control. Generation of ROS in OsMCA1-suppressed lines was comparable to the control (Figure 8A).
Figure 8.
Effects of the expression level of OsMCA1 on hypo-osmotic shock-induced ROS generation. (A) Effects of OsMCA1-suppression on hypo-osmotic shock-induced ROS generation measured by MCLA chemiluminescence. Data are the mean ± SE for five independent experiments for the control line (closed diamond for control 20-5) and two independent RNAi lines (open circle for RNAi 20-2; open triangle for RNAi 38-5) are shown. As a hypo-osmotic shock, growth medium was replaced by four-fold diluted medium at 0 min (A and B). (B) The effect of several Ca2+ channel blockers on hypo-osmotic shock-induced ROS generation in the wild type. GdCl3 (1 mM) and LaCl3 (1 mM) were added to the cells 60 min prior to hypo-osmotic shock treatment. Data are the mean ± SE for three or four independent experiments. ***P < 0.005; significantly different compared with the control. (C) Quantitative expression levels of OsMCA1 mRNAs in the OsMCA1 overexpressor lines by real-time quantitative PCR. The relative level of the OsMCA1 mRNAs in the control cells (GUS No. 7) was standardized as 1. (D) Effects of OsMCA1 overexpression on hypo-osmotic shock-induced ROS generation. As a hypo-osmotic shock, growth medium was replaced by three-fold diluted medium at 0 min. Data are the mean ± SE for five independent experiments for two control lines (open circle for GUS No. 11; open triangle for GUS No. 7) and two overexpressor lines (closed diamond for OX No. 2; closed square for OX No. 3) are shown. *P < 0.05; **P < 0.01; significantly different compared with the control line (GUS No. 7).
Discussion
It has been suggested that Ca2+ plays a crucial role in mechanical sensing [7,25]. However, little is known of the molecular mechanisms responsible for Ca2+ mobilization. Functional characterization of the OsMCA1-RNAi lines as well as overexpressors suggests that OsMCA1 is involved in hypo-osmotic shock-induced Ca2+ influx and ROS generation.
Possible functions of OsMCA1 in the regulation of growth and development of rice
OsMCA1-suppressed plants displayed stunted growth and shortened rachises (Figure 4D, E). These phenotypes are frequently observed under drought-stress conditions [26]. The suppression of OsMCA1 might have affected adaptation to drought. Drought stress is known to lead to osmotic stress at a cellular level. Since hypo-osmotic shock-induced [Ca2+]cyt changes were impaired in the OsMCA1-suppressed lines, these lines may have defects in osmotic sensing/responses and ability to adapt to drought stress. Future studies to characterize physiological reactions to drought and mechanical signaling in OsMCA1-suppressed plants would further elucidate the in vivo roles of OsMCA1 in intact plants.
In Arabidopsis, the mca1 mca2 double mutant shows a growth defect in soil, and reduced accumulation of Ca2+ as well as enhanced sensitivity to Mg2+ [16]. The balance of Ca2+ and Mg2+ in soil is an important factor for normal plant growth [27]. Since the growth of the OsMCA1-suppressed lines was significantly restricted compared with the control under Ca2+-limitation (Figure 5), growth retardation in the OsMCA1-suppressed plants may be attributed to reduced Ca2+ uptake, resulting in a low Ca2+-Mg2+ ratio.
Possible involvement of OsMCA1 in osmotic signaling in cultured rice cells
The GFP-OsMCA1 fusion protein localized specifically to the plasma membrane (Figure 2), suggesting that OsMCA1 is a plasma membrane protein. In cultured rice cells, both hypo-osmotic shock- and TNP-induced [Ca2+]cyt transients, which were inhibited by La3+ and Gd3+, were impaired in OsMCA1-suppressed lines (Figures 6 and 7). The temporal pattern of the MAMP-induced [Ca2+]cyt transient was similar (data not shown), but was unaffected by OsMCA1 suppression (Figure 6D). These results suggest that OsMCA1 affects regulation of Ca2+ influx across the plasma membrane in response to mechanical stimulation in cultured rice cells.
Hypo-osmotic shock triggers ROS generation following a [Ca2+]cyt increase [18,20,22]. Extracellular Ca2+ is required for both Ca2+ influx and NADPH oxidase-mediated ROS generation induced by hypo-osmotic shock (Figures 6C and 8B, Additional file 4), suggesting that ROS generation requires Ca2+ influx across the plasma membrane. Overexpression of OsMCA1 enhances ROS generation (Figure 8D, Additional file 6). Binding Ca2+ to the EF-hand regions of cytosolic regulatory domains of plant NADPH oxidases directly activates them [28-30]. A functional NADPH oxidase AtrbohC/RHD2 reportedly affects mechanical stress-induced ROS generation in a Ca2+-dependent manner [31]. Overproduction of the plasma membrane Ca2+-permeable channels may induce the mobilization of excess Ca2+ in response to mechanical stimuli, which may cause enhanced activation of NADPH oxidases.
In OsMCA1-suppressed lines challenged with hypo-osmotic shock, Ca2+ influx was partially impaired (Figure 6E), but no significant influence of the impairment on subsequent ROS generation was detected under our assay conditions (Figure 8A). Similar effects of overexpression and a loss-of-function mutation were also observed with another putative Ca2+-permeable channel, OsTPC1 [32]. A certain level of Ca2+ increment may be sufficient for NADPH oxidase-mediated ROS generation. Alternatively, other Ca2+-permeable channels activated by hypo-osmotic shock may redundantly play a role in bypassing OsMCA1. It has been suggested that Arabidopsis MSL9 and MSL10, homologs of the bacterial mechanosensitive channel MscS, are required for mechanosensitive channel activity in root cell plasma membranes, and are able to translocate cations including Ca2+ [13,14]. Rice MSL homologs may therefore be candidates for such Ca2+-permeable channels.
Conclusions
The present study indicates that OsMCA1 is involved in regulation of plasma membrane Ca2+ influx and NADPH oxidase-mediated ROS generation induced by hypo-osmotic stress in cultured rice cells. These findings shed light on our understanding of mechanical sensing pathways.
Methods
Plant materials and cell culture
Surface-sterilized seeds of rice, Oryza sativa L. cv. Nipponbare, were germinated on MS medium [33] containing 0.8% agar and grown for 10 days in a growth chamber under long day conditions (16 h light/8 h darkness, 28°C). Seedlings were transplanted into soil and grown in a greenhouse (16 h light/8 h darkness, 28°C and 60% humidity). Calli were suspension-cultured at 25°C in a liquid L medium [34] containing 2,4-D (0.5 mg L-1) in the dark and subcultured in fresh medium every week. Cells were filtered through a 20-mesh screen every 2 weeks to make fine aggregates. Cells at 5 days after subculture were used for experiments with osmotic stress and defense responses. N-acetylchito-oligosaccharides were kindly provided by Prof. Naoto Shibuya (Meiji University).
Isolation of OsMCA1 cDNA
The estimated coding region of OsMCA1 was amplified by PCR using two primers: OsMCA1 forward, 5'-GAAGAAGAAGAAGAAGAAGAAGCCGAGTAG-3'; OsMCA1 reverse, 5'-TATTTATGCTTACCCTGCATTGTTTGTGTT-3'. Total RNA was isolated from rice leaves using Trizol (Invitrogen, Carlsbad, CA, USA) in accordance with manufacturer's protocol and quantified spectrophotometrically. First-strand cDNA was synthesized from 3 μg of total RNA with the oligo-dT primer and reverse transcriptase (Invitrogen). To obtain full-length cDNAs for OsMCA1 and to define the open reading frame, 3'-RACE PCR and 5'-RACE PCR were performed with a 3'-full RACE core kit (Takara, Ohtsu, Japan) and a 5'-RACE system (Invitrogen) in accordance with manufacturers' protocols.
RNA isolation and RT-PCR analyses
Total RNA was isolated using Trizol reagent in accordance with manufacturer's protocol and quantified using a spectrophotometer. First-strand cDNA was synthesized from 3 μg total RNA with an oligo-dT primer and reverse transcriptase. PCR amplification was performed with an initial denaturation at 95°C for 3 min followed by indicated numbers of cycles of incubations at 94°C for 30 s, 55°C for 90 s, and 72°C for 1 min by using specific primers for OsMCA1. Actin was used as a quantitative control [35]. Aliquots of individual PCR products were resolved by agarose gel electrophoresis and visualized using ethidium bromide staining and exposure to UV light.
Real-time RT-PCR quantification
Real-time RT-PCR assays were performed as described by Kurusu et al. (2010) [36]. First-strand cDNA was synthesized from 3 μg of total RNA using an oligo-dT primer and reverse transcriptase. Real-time PCR was performed using an ABI PRISM 7300 sequence detection system (Applied Biosystems, Foster City, CA, USA) with SYBR Green real-time PCR Master Mix (Toyobo, Osaka, Japan) and OsMCA1 specific primers OsMCA1-RealF, 5'-TGGTCTCAAGCAGAGGATCATACA-3'; OsMCA1-RealR, 5'-CTCTGAACAGCAACCAAGCAAA-3'. Relative mRNA levels were calculated using the standard curve method and normalized to the corresponding OsActin1 gene levels. Standard samples of known template amounts were used to quantify PCR products.
Spatial pattern of OsMCA1 expression using OsMCA1p::GUS-expressing plants
A DNA fragment of the OsMCA1 promoter region was prepared using PCR by synthesizing the 5'-non-coding region spanning -1.5 to 0 kb from the OsMCA1 initiation codon, using rice (Nipponbare) genomic DNA as a template and the following primers: OsMCA1pF, 5'-CACCAACAACCCCTAACATGCCTAA-3'; OsMCA1pR, 5'-TGCCGTCGTCTACTCGGCTTCTTCT-3' (the CACC sequence used with the Gateway system), subcloned into a pENTR/D-TOPO cloning vector, and then cloned into a Ti-based promoterless GUS expression vector, pHGWFS7 [37] using the LR clonase reaction; Agrobacterium-mediated transformation of rice calli was performed. Transformed calli were screened by hygromycin selection (50 μg mL-1); transgenic plants were then regenerated.
The T2 transgenic plants were grown at 28°C under a 16 h light/8 h dark cycle for experiments. Histochemical localization of GUS activity in situ was performed as follows. Samples were fixed for 1 h with 90% acetone in Eppendorf tubes placed on ice and washed four times with 100 mM sodium phosphate buffer, pH 7.0. Samples were then incubated for 24 h at 37°C in X-Gluc buffer (0.5 mg/mL 5-bromo-4-chloro-3-indolyl glucuronidase (Nacalai Tesque, Osaka, Japan), 50 mM sodium phosphate buffer, pH 7.0, 5% methanol), and then cleaned and fixed by rinsing for 1 h each with 50%, 70%, 90%, and 100% (v/v) ethanol successively. Fixed samples were stored in 100% ethanol before being photographed.
Generation of OsMCA1-overexpressing and suppressed lines
To generate RNA-silencing-triggered inverted repeat constructs, a region corresponding to 400 bp of the 3'-UTR of OsMCA1 was amplified using RNAiFW, 5'-CACC CTCTTATCCAAACTTGCCAT-3' and RNAiRV, 5'- AATGTTCCACAGGGGAAAAAGAATGTTCTC-3' as specific primers, subcloned into a pENTR/D-TOPO cloning vector, and cloned into a Ti-based RNAi vector, pANDA [38] using the LR clonase reaction. The construct was introduced into rice calli using Agrobacterium-mediated transformation, according to the method of Tanaka et al. 2001 [39]. Transformed calli were screened by hygromycin selection (50 μg mL-1); transgenic plants were then regenerated. Transgenic cell lines derived from T2 plants were used for various analyses.
To overexpress OsMCA1 and GUS cDNAs, sequences were cloned into a Ti-based vector pPZP2H-lac [40] downstream of the maize Ubiquitin promoter, and Agrobacterium-mediated transformation of rice calli was performed. Transformed calli were screened by hygromycin selection (50 μg mL-1), followed by the regeneration of transgenic plants.
To express cytoplasm-targeted apoaequorin cDNA [41] in OsMCA1-suppressed plants, sequences were cloned into a Ti-based vector pSMAB704 [42] downstream of a CaMV 35S promoter, and Agrobacterium-mediated transformation of rice calli was performed. Transformed calli were screened using bialaphos (Meiji Seika, Tokyo, Japan) selection (5 μg mL-1), followed by the regeneration of transgenic plants.
Subcellular localization of OsMCA1 in tobacco BY-2 cells
To generate transgenic BY-2 cells expressing GFP-OsMCA1, the coding region was amplified using OsMCA1(GFP)F, 5'-CACCATGGCGTCGTGGGAGAACCT-3' and OsMCA1(GFP)R, 5'-TTAGTGTTCCATGTACTGAA-3' as specific primers, subcloned into a pENTR/D-TOPO cloning vector, and then cloned into a pH7WGF2 vector encoding a N-terminal EGFP fusion [37] using the LR clonase reaction.
Transformation of BY-2 cells was carried out in accordance with An (1985) [43] with minor modifications as follows: 4 mL of 3-day-old exponentially growing culture was transferred to 90-mm Petri dishes and incubated at 28°C with 100 μL of fresh overnight-culture of Agrobacterium tumefaciens pGV2260 containing the binary vector pH7WGF2. After a 48-h co-cultivation, the tobacco cells were washed and plated on to LS agar medium containing hygromycin (50 μg mL-1) and carbenicillin (250 μg mL-1). Every 3-4 weeks, transformants were selected and transferred onto fresh medium for continued selection.
The fluorescent styryl membrane probe FM4-64 (Molecular Probes, Carlsbad, CA, USA) was kept as a 17 mM stock solution in sterile water, and used at a final concentration of 4.25 μM to label the vacuolar membrane (tonoplast). Five-day-old BY-2 cells were treated with FM4-64 for 3 h and washed twice with culture medium.
Measurement of cytosolic Ca2+ concentration
Measurements of Ca2+ mobilization were made in accordance with the method described by Kurusu et al. (2011) [44]. Briefly, apoaequorin-expressing rice cells (5 day after subculture) were incubated with 1 μM coelenterazine for at least 12 h at 25°C. Cell suspension (250 μL) was transferred to 1.1-cm-diameter culture tubes, and set in a luminometer (Lumicounter 2500, Microtech Nition, Chiba, Japan). In the luminometer, culture tubes rotated 17 revolutions every 3 s clockwise and counterclockwise in turn, agitating the cells. After a 15-min incubation to stabilize the cells, Ca2+-dependent aequorin luminescence was measured and expressed as relative luminescence units (rlu).
Ca2+ uptake in cultured cells
Rice cells 5 days after subculture were used to measure Ca2+ uptake. The rice cells were incubated in Ca2+-free medium for at least 3 h at 25°C. The cell suspension (80 mg mL-1) was transferred to medium containing 0.1 mM CaCl2 and incubated for 1 h. Ca2+ uptake was initiated by adding 45CaCl2 solution to a final concentration of 33 kBq/g. Cells were then agitated at 25°C; 1 mL of cells was collected at 0, 15 and 45 min after the addition of 45CaCl2. Cells were filtered using Whatman filters (GF/C) presoaked with 5 mM CaCl2 and washed 5 times with an ice-cold solution of 5 mM CaCl2, and 2 mM LaCl3 to remove 45Ca2+ from cell walls. Radioactivity retained on each filter was counted as described previously [45].
Measurement of ROS
Rice cells (cv. Nipponbare) 5 days after subculture were used for measurement of •O2- and H2O2 in the extracellular medium. •O2--dependent chemiluminescence was monitored in growth medium supplemented with 20 μM methoxylated cypridina luciferin analog (MCLA (2-methyl-6-[p-methoxyphenyl]-3,7-dihydroimidazo [1,2-α]pyrazin-3-one); Invitrogen) using a luminometer (Lumicounter 2500) under the same conditions as for measuring [Ca2+]cyt [46].
To monitor H2O2 produced in extracellular medium, cells (80 mg mL-1) were washed and resuspended in 5 mM MES buffer (pH 7.0) containing 0.5 mM CaCl2, 0.5 mM K2SO4 and with or without 175 mM mannitol (Kurusu et al. 2005). A 25-μL aliquot of medium was mixed in a 96-well microtiter plate with 150 μl 50 mM Tris-HCl (pH 8.0) and 25 μL 0.462 mM luminol in 50 mM Tris-HCl, pH 8.0. Potassium ferricyanide (25 μL, 11.76 mM) was added, and H2O2-dependent chemiluminescence was recorded for 15 s using a luminometer (MicroLumat Plus LB96V, Berthold Technologies, Bad Wildbad, Germany).
Statistical analysis
Statistical significance was determined using an unpaired Student's t test; P < 0.05 indicated significance.
Abbreviations
BAPTA: 1,2-bis-(2-aminophenoxy)ethane-N, N, N', N'-tetra acetic acid; [Ca2+]cyt: cytosolic free Ca2+ concentration; DIC: differential interference contrast; DPI: diphenylene iodonium; EGTA: ethylene glycol-bis-(2-aminoethylether)-N, N, N', N'-tetra acetic acid; GFP: green fluorescent protein; GUS: β-glucuronidase; H2O2: hydrogen peroxide; MAMP: microbe-associated molecular pattern; MCLA: 2-methyl-6-[p-methoxyphenyl]-3,7-dihydroimidazo [1,2-α]pyrazin-3-one; •O2-: superoxide anion radical; MS medium: Murashige and Skoog medium; RACE: rapid amplification of cDNA ends; rlu: relative luminescence units; RNAi: RNA interference; RT: reverse transcriptase; ROS: reactive oxygen species; SHAM: salicylhydroxamic acid; TNP: trinitrophenol.
Authors' contributions
TK, DN, and YY carried out most of the experiments and data analyses. TK and KK designed the study and wrote the manuscript. DN, YY and MG participated in confocal imaging analyses. MN, TY and KI carried out Ca2+ uptake experiments in yeast. HH and HS participated in constructing transgenic lines and PCR analyses. YN, KS, HI participated in the design of the study and critically revised the manuscript. All authors read and approved the final manuscript.
Authors' information
YN Present address: Laboratory of Cell Biology, Institute for Molecular and Cellular Regulation, Gunma University, Maebashi, Gunma 371-8510, Japan.
Supplementary Material
Multiple amino acid sequence alignment of rice OsMCA1 and Arabidopsis MCA1 and MCA2 using Clustal W. Asterisks indicate identical or conserved residues in the whole sequence in the alignment. Colons indicate conserved substitutions. Dots indicate semi-conserved substitutions. Light and dark gray box indicate the PLAC8 motif and coiled-coil motif, respectively. Two putative transmembrane segments (S1 and S2) are underlined.
Expression of the OsMCA1 gene in rice tissues. The expression of OsMCA1 in rice plants was determined by quantitative RT-PCR analysis. Total RNA was extracted from various tissues of rice plants as well as cultured cells. The amount of OsMCA1 mRNA was calculated from the threshold point in the log-linear range of the RT-PCR. The relative OsMCA1 mRNA level in cultured cells was standardized as 1. Data are means ± SD; n = 2-3 independent samples.
Growth phenotype of OsMCA1-suppressed seedlings in MS medium. Length of roots and shoots of the control line (20-5) and the OsMCA1-suppressed lines (20-2 and 38-5) of 10-days-old seedlings grown on MS medium plate in a growth chamber under long-day conditions (16 h light/8 h darkness, 28°C). Data are means ± SD; n = 7-10 independent seedlings.
Effect of NADPH oxidase inhibitor on hypo-osmotic shock-induced ROS generation. H2O2 concentration in extracellular medium was determined by ferricyanide-catalyzed oxidation of luminol. Diphenylene iodonium (DPI; 10 μM) was added to the rice cells 30 min before hypo-osmotic shock treatment. Data are the mean ± SE for five independent experiments for the wild type. ***P < 0.005; significantly different compared with the control.
Effect of salicylhydroxamic acid, a peroxidase inhibitor, on hypo-osmotic shock-induced ROS generation. The concentration of •O2- in extracellular medium was measured by MCLA chemiluminescence. Salicylhydroxamic acid (SHAM; 3 mM) was added to the rice cells 30 min before hypo-osmotic shock treatment. Average values and SE of three independent experiments for the wild type.
Effect of OsMCA1-overexpression on hypo-osmotic shock-induced ROS generation. H2O2 concentration in the extracellular medium was determined by ferricyanide-catalyzed oxidation of luminol. As a hypo-osmotic shock, growth medium was replaced by three-fold diluted medium at 0 min. Data are the mean ± SE for four independent experiments for two control lines (open circle for GUS No. 11; open triangle for GUS No. 7) and two overexpressor lines (closed diamond for OX No. 2; closed square for OX No. 3) are shown. *P < 0.05, significantly different compared with two control lines (GUS No. 7 and 11).
Contributor Information
Takamitsu Kurusu, Email: kurusu@rs.noda.tus.ac.jp.
Daisuke Nishikawa, Email: dnishikaw@gmail.com.
Yukari Yamazaki, Email: cuctus7216@hotmail.co.jp.
Mariko Gotoh, Email: marimekko0711@yahoo.co.jp.
Masataka Nakano, Email: masa_nak@u-gakugei.ac.jp.
Haruyasu Hamada, Email: j6409705@ed.noda.tus.ac.jp.
Takuya Yamanaka, Email: tyam88@hotmail.com.
Kazuko Iida, Email: iida-kz@igakuken.or.jp.
Yuko Nakagawa, Email: nakayuko@showa.gunma-u.ac.jp.
Hikaru Saji, Email: hsaji@nies.go.jp.
Kazuo Shinozaki, Email: sinozaki@rtc.riken.jp.
Hidetoshi Iida, Email: iida@u-gakugei.ac.jp.
Kazuyuki Kuchitsu, Email: kuchitsu@rs.noda.tus.ac.jp.
Acknowledgements
We would like to thank Mr. Yasuhiro Sakurai for helpful technical assistance, Drs. Hiroaki Shimada and Tadamasa Sasaki for helpful technical suggestions, Drs. Daisuke Miki and Ko Shimamoto for the RNAi plasmid (pANDA vector), and Dr. Naoto Shibuya for the gift of N-acethylchitoheptaose.
This work was supported in part by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science & Technology for Scientific Research on Innovative Areas (21200067) to TK, for Exploratory Research (21658118) to KK, for Young Scientists (B) (21780041) to TK, for Scientific Research on Priority Area (21026009) to HI, for Scientific Research B (19370023) to KK and (21370017) to HI, and by grants from Japan Science and Technology Agency, for Adaptable and Seamless Technology Transfer Program through target-driven R&D (AS221Z03504E) to TK and for CREST to HI and KK.
References
- Reddy ASN. Calcium: Silver bullet in signaling. Plant Sci. 2001;160:381–404. doi: 10.1016/S0168-9452(00)00386-1. [DOI] [PubMed] [Google Scholar]
- Sanders D, Pelloux J, Brownlee C, Harper JF. Calcium at the crossroads of signaling. Plant Cell. 2002;14:S401–S417. doi: 10.1105/tpc.002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Poovaiah BW. Calmodulin-mediated signal network in plants. Trends Plant Sci. 2003;8:505–512. doi: 10.1016/j.tplants.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Fasano JM, Massa GD, Gilroy S. Ionic signaling in plant responses to gravity and touch. J Plant Growth Regul. 2002;21:71–88. doi: 10.1007/s003440010049. [DOI] [PubMed] [Google Scholar]
- Braam J. Genome-wide identification of touch- and darkness-regulated Arabidopsis genes: a focus on calmodulin-like and XTH genes. New Phytol. 2005;165:373–389. doi: 10.1111/j.1469-8137.2004.01238.x. [DOI] [PubMed] [Google Scholar]
- Toyoda M, Furuichi T, Tatsumi H, Sokabe M. Cytoplasmic calcium increases in response to change in the gravity vector in hypocotyls and petioles of Arabidopsis seedling. Plant Physiol. 2008;146:505–514. doi: 10.1104/pp.107.106450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd AN, Kudla J, Sanders D. The language of calcium signaling. Annu Rev Plant Biol. 2010;2:593–620. doi: 10.1146/annurev-arplant-070109-104628. [DOI] [PubMed] [Google Scholar]
- Véry AA, Sentenac H. Cation channels in the Arabidopsis plasma membrane. Trends Plant Sci. 2002;7:168–175. doi: 10.1016/S1360-1385(02)02262-8. [DOI] [PubMed] [Google Scholar]
- White PJ, Bowen HC, Demidchik V, Nichols C, Davies JM. Genes for calcium-permeable channels in the plasma membrane of plant root cells. Biochim Biophys Acta. 2002;1564:299–309. doi: 10.1016/S0005-2736(02)00509-6. [DOI] [PubMed] [Google Scholar]
- Dutta R, Robinson KR. Identification and characterization of stretch-activated ion channels in pollen protoplasts. Plant Physiol. 2004;135:1398–1406. doi: 10.1104/pp.104.041483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Z, Kishigami A, Nakagawa Y, Iida H, Sokabe M. A mechanosensitive anion channel in Arabidopsis thaliana mesophyll cells. Plant Cell Physiol. 2004;45:1704–1708. doi: 10.1093/pcp/pch194. [DOI] [PubMed] [Google Scholar]
- Telewski FW. A unified hypothesis of mechanoperception in plants. Am J Bot. 2006;93:1466–1476. doi: 10.3732/ajb.93.10.1466. [DOI] [PubMed] [Google Scholar]
- Haswell ES, Peyronnet R, Barbier-Brygoo H, Meyerowitz EM, Frachisse JM. Two MscS homologs prvide mechanosensitive channel activities in the Arabidopsis root. Curr Biol. 2008;18:730–734. doi: 10.1016/j.cub.2008.04.039. [DOI] [PubMed] [Google Scholar]
- Peyronnet R, Haswell ES, Barbier-Brygoo H, Frachisse JM. AtMSL9 and AtMSL10: Sensors of plasma membrane tension in Arabidopsis roots. Plant Signal Behav. 2008;3:726–729. doi: 10.4161/psb.3.9.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y, Katagiri T, Shinozaki K, Qi Z, Tatsumi H, Furuichi T, Kishigami A, Sokabe M, Kojima I, Sato S, Kato T, Tabata S, Iida K, Terashima A, Nakano M, Ikeda M, Yamanaka T, Iida H. Arabidopsis plasma membrane protein crucial for Ca2+ influx and touch sensing in roots. Proc Natl Acad Sci USA. 2007;104:3639–3644. doi: 10.1073/pnas.0607703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka T, Nakagawa Y, Mori K, Nakano M, Imamura T, Kataoka H, Terashima A, Iida K, Kojima I, Katagiri T, Shinozaki K, Iida H. MCA1 and MCA2 that mediate Ca2+ uptake have distinct and overlapping roles in Arabidopsis. Plant Physiol. 2010;152:1284–1296. doi: 10.1104/pp.109.147371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingarelli L, Marré MT, Massardi F, Lado P. Effects of hyperosmotic stress on K+ fluxes, H+ extrusion, transmembrane electric potential difference and comparison with the effects of fusicoccin. Physiol Plant. 1999;106:287–295. doi: 10.1034/j.1399-3054.1999.106305.x. [DOI] [Google Scholar]
- Beffagna N, Buffoli B, Busi C. Modulation of reactive oxygen species production during osmotic stress in Arabidopsis thaliana cultured cells: involvement of the plasma membrane Ca2+-ATPase and H+-ATPase. Plant Cell Physiol. 2005;46:1326–1339. doi: 10.1093/pcp/pci142. [DOI] [PubMed] [Google Scholar]
- Rouet MA, Mathieu Y, Barbier-Brygoo H, Laurière C. Characterization of active oxygen-producing proteins in response to hypo-osmolarity in tobacco and Arabidopsis cell suspensions: identification of a cell wall peroxidase. J Exp Bot. 2006;57:1323–1332. doi: 10.1093/jxb/erj107. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Harada A, Sakai T, Takagi S. Ca2+ transient induced by extracellular changes in osmotic pressure in Arabidopsis leaves: differential involvement of cell wall-plasma membrane adhesion. Plant Cell Environ. 2006;29:661–672. doi: 10.1111/j.1365-3040.2005.01447.x. [DOI] [PubMed] [Google Scholar]
- Moran N. Osmoregulation of leaf motor cells. FEBS Lett. 2007;581:2337–2347. doi: 10.1016/j.febslet.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Cazalé AC, Rouet-Mayer MA, Barbier-Brygoo H, Mathieu Y, Laurière C. Oxidative burst and hypoosmotic stress in tobacco cell suspensions. Plant Physiol. 1998;116:659–669. doi: 10.1104/pp.116.2.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaku H, Nishizawa Y, Ishii-Minami N, Akimoto-Tomiyama C, Dohmae N, Takio K, Minami E, Shibuya N. Plant cells recognize chitin fragments for defense signaling through a plasma membrane receptor. Proc Natl Acad Sci USA. 2006;103:11086–11091. doi: 10.1073/pnas.0508882103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miya A, Albert P, Shinya T, Desaki Y, Ichimura K, Shirasu K, Narusaka Y, Kawakami N, Kaku H, Shibuya N. CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. Proc Natl Acad Sci USA. 2007;104:19613–19618. doi: 10.1073/pnas.0705147104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewavas A, Knight M. Mechanical signalling, calcium and plant form. Plant Mol Biol. 1994;26:1329–1341. doi: 10.1007/BF00016478. [DOI] [PubMed] [Google Scholar]
- Ji XM, Raveendran M, Oane R, Ismail A, Lafitte R, Bruskiewich R, Cheng SH, Bennett J. Tissue-specific expression and drought responsiveness of cell-wall invertase genes of rice at flowering. Plant Mol Biol. 2005;59:945–964. doi: 10.1007/s11103-005-2415-8. [DOI] [PubMed] [Google Scholar]
- Brady KU, Kruckeberg AR, Bradshaw HD Jr. Evolutionary ecology of plant adaptation to serpentine soils. Annu Rev Ecol Evol Syst. 2005;36:243–266. doi: 10.1146/annurev.ecolsys.35.021103.105730. [DOI] [Google Scholar]
- Ogasawara Y, Kaya H, Hiraoka G, Yumoto F, Kimura S, Kadota Y, Hishinuma H, Senzaki E, Yamagoe S, Nagata K, Nara M, Suzuki K, Tanokura M, Kuchitsu K. Synergistic activation of the Arabidopsis NADPH oxidase AtrbohD by Ca2+ and phosphorylation. J Biol Chem. 2008;283:8885–8892. doi: 10.1074/jbc.M708106200. [DOI] [PubMed] [Google Scholar]
- Takeda S, Gapper C, Kaya H, Bell E, Kuchitsu K, Dolan L. Local positive feedback regulation determines cell shape in root hair cells. Science. 2008;319:1241–1244. doi: 10.1126/science.1152505. [DOI] [PubMed] [Google Scholar]
- Kimura S, Kaya H, Kawarazaki T, Hiraoka G, Senzaki E, Michikawa M, Kuchitsu K. Protein phosphorylation is a prerequisite for the Ca2+-dependent activation of Arabidopsis NADPH oxidases and may function as a trigger for the positive feedback regulation of Ca2+ and reactive oxygen species. Biochim Biophys Acta. 2011;1823:398–405. doi: 10.1016/j.bbamcr.2011.09.011. [DOI] [PubMed] [Google Scholar]
- Monshausen GB, Bibikova TN, Weisenseel MH, Gilroy S. Ca2+ regulates reactive oxygen species production and pH during mechanosensing in Arabidopsis roots. Plant Cell. 2009;21:2341–2356. doi: 10.1105/tpc.109.068395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurusu T, Yagala T, Miyao A, Hirochika H, Kuchitsu K. Identification of a putative voltage-gated Ca2+ channel as a key regulator of elicitor-induced hypersensitive cell death and mitogen-activated protein kinase activation in rice. Plant J. 2005;42:798–809. doi: 10.1111/j.1365-313X.2005.02415.x. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Kuchitsu K, Kikuyama M, Shibuya N. N-acetylchitooligosaccharides, biotic elicitors for phytoalexin production, induce transient membrane depolarization in suspension-cultured rice cells. Protoplasma. 1993;174:79–81. doi: 10.1007/BF01404046. [DOI] [Google Scholar]
- Kojima S, Takahashi Y, Kobayashi Y, Monna L, Sasaki T, Araki T, Yano M. Hd3a, a rice ortholog of the Arabidopsis FT gene, promotes transcription to flowering downstream of Hd1 under short-day conditions. Plant Cell Physiol. 2002;43:1096–1105. doi: 10.1093/pcp/pcf156. [DOI] [PubMed] [Google Scholar]
- Kurusu T, Hamada J, Nokajima H, Kitagawa Y, Kiyoduka M, Takahashi A, Hanamata S, Ohno R, Hayashi T, Okada K, Koga J, Hirochika H, Yamane H, Kuchitsu K. Regulation of microbe-associated molecular pattern-induced hypersensitive cell death, phytoalexin production and defense gene expression by calcineurin B-like protein-interacting protein kinases, OsCIPK14/15, in rice cultured cells. Plant Physiol. 2010;153:678–692. doi: 10.1104/pp.109.151852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi M, Inzé D, Depicker A. GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci. 2002;7:193–195. doi: 10.1016/S1360-1385(02)02251-3. [DOI] [PubMed] [Google Scholar]
- Miki D, Shimamoto K. Simple RNAi vectors for stable and transient suppression of gene function in rice. Plant Cell Physiol. 2004;45:490–495. doi: 10.1093/pcp/pch048. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Kayano T, Ugaki M, Shiobara F, Onodera H, Ono K, Tagiri A, Nishizawa Y, Shibuya N. Ultra-fast transformation technique for monocotyledons. International Patent Application. 2001. No. WO 01/06844 A1.
- Fuse T, Sasaki T, Yano M. Ti-plasmid vectors useful for functional analysis of rice genes. Plant Biotechnol. 2001;18:219–222. doi: 10.5511/plantbiotechnology.18.219. [DOI] [Google Scholar]
- Knight MR, Campbell AK, Smith SM, Trewavas AJ. Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature. 1991;352:524–526. doi: 10.1038/352524a0. [DOI] [PubMed] [Google Scholar]
- Igasaki T, Ishida Y, Mohri T, Ichikawa H, Shinohara K. Transformation of Populus alba and direct selection of transformants with the herbicide bialaphos. Bulletin of FFPRI. 2002;1:235–240. [Google Scholar]
- An G. High efficiency transformation of cultured tobacco cells. Plant Physiol. 1985;79:568–570. doi: 10.1104/pp.79.2.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurusu T, Hamada H, Sugiyama Y, Yagala T, Kadota Y, Furuichi T, Hayashi T, Umemura K, Komatsu S, MIyao A, Hirochika H, Kuchitsu K. Negative feedback regulation of microbe-associated molecular pattern-induced cytosolic Ca2+ transients by protein phosphorylation. J Plant Res. 2011;124:415–424. doi: 10.1007/s10265-010-0388-4. [DOI] [PubMed] [Google Scholar]
- Iida H, Yagawa Y, Anraku Y. Essential role for induced Ca2+ influx followed by [Ca2+]i rise in maintaining viability of yeast cells late in the mating pheromone response pathway. A study of [Ca2+]i in single Saccharomyces cerevisiae cells with imaging of fura-2. J Biol Chem. 1990;265:13391–13399. [PubMed] [Google Scholar]
- Kurusu T, Hamada H, Hanamata S, Kuchitsu K. Roles of calcineurin B-like protein-interacting protein kinases in plant innate immunity in rice. Plant Signal Behav. 2010;5:1045–1047. doi: 10.4161/psb.5.8.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multiple amino acid sequence alignment of rice OsMCA1 and Arabidopsis MCA1 and MCA2 using Clustal W. Asterisks indicate identical or conserved residues in the whole sequence in the alignment. Colons indicate conserved substitutions. Dots indicate semi-conserved substitutions. Light and dark gray box indicate the PLAC8 motif and coiled-coil motif, respectively. Two putative transmembrane segments (S1 and S2) are underlined.
Expression of the OsMCA1 gene in rice tissues. The expression of OsMCA1 in rice plants was determined by quantitative RT-PCR analysis. Total RNA was extracted from various tissues of rice plants as well as cultured cells. The amount of OsMCA1 mRNA was calculated from the threshold point in the log-linear range of the RT-PCR. The relative OsMCA1 mRNA level in cultured cells was standardized as 1. Data are means ± SD; n = 2-3 independent samples.
Growth phenotype of OsMCA1-suppressed seedlings in MS medium. Length of roots and shoots of the control line (20-5) and the OsMCA1-suppressed lines (20-2 and 38-5) of 10-days-old seedlings grown on MS medium plate in a growth chamber under long-day conditions (16 h light/8 h darkness, 28°C). Data are means ± SD; n = 7-10 independent seedlings.
Effect of NADPH oxidase inhibitor on hypo-osmotic shock-induced ROS generation. H2O2 concentration in extracellular medium was determined by ferricyanide-catalyzed oxidation of luminol. Diphenylene iodonium (DPI; 10 μM) was added to the rice cells 30 min before hypo-osmotic shock treatment. Data are the mean ± SE for five independent experiments for the wild type. ***P < 0.005; significantly different compared with the control.
Effect of salicylhydroxamic acid, a peroxidase inhibitor, on hypo-osmotic shock-induced ROS generation. The concentration of •O2- in extracellular medium was measured by MCLA chemiluminescence. Salicylhydroxamic acid (SHAM; 3 mM) was added to the rice cells 30 min before hypo-osmotic shock treatment. Average values and SE of three independent experiments for the wild type.
Effect of OsMCA1-overexpression on hypo-osmotic shock-induced ROS generation. H2O2 concentration in the extracellular medium was determined by ferricyanide-catalyzed oxidation of luminol. As a hypo-osmotic shock, growth medium was replaced by three-fold diluted medium at 0 min. Data are the mean ± SE for four independent experiments for two control lines (open circle for GUS No. 11; open triangle for GUS No. 7) and two overexpressor lines (closed diamond for OX No. 2; closed square for OX No. 3) are shown. *P < 0.05, significantly different compared with two control lines (GUS No. 7 and 11).