Abstract
Background
Published work suggests that some types of endothelial cells undergo apoptosis in response to ligation of the receptor Fas (CD95, APO1) but other types are resistant. Because heterogeneity among endothelial cells from different tissues, has been demonstrated, the purpose of this study was to determine, if Fas ligation and/or activation by human Fas ligand induces apoptosis and caspase activities, in cultured human coronary artery endothelial cells, and the differences between TNF-a and FAS induced apoptosis in these cells.
Results
Cultured human coronary artery endothelial cells (HCAEC) were exposed to the monoclonal Fas-activating antibody CH-11, to purified recombinant human Fas ligand, to the Fas-neutralizing antibody ZB4, or to purified recombinant human TNF-α. Apoptosis was detected by assessment of chromatin condensation and nuclear fragmentation and by assay of the enzymatic activities of Caspase 1 and Caspase 3 with membrane-permeable substrates applied to intact cells. Fas protein was detected by immunoblotting of HCAEC lysates. Apoptosis was induced in HCAEC by purified Fas ligand or by the monoclonal activating antibody CH-11 at concentrations of 25 or 200 ng/ml, but not by nonspecific isotype-matched immunoglobulins. The apoptotic index elicited by either Fas activator was equal to that induced by TNF-a (3.0-3.6-fold versus control, p < 0.01). The Fas-neutralizing antibody ZB4 abrogated HCAEC apoptosis induced by CH-11, but had no inhibitory effect on apoptosis in response to TNF-a. Fas ligation significantly increased the activities of both Caspase 1 and Caspase 3 at 20 hours of stimulation (1.7- and 2.0-fold versus control, both p < 0.05); in contrast, purified TNF-a increased the activity of Caspase 3 but not Caspase 1 (2.1-fold, p < 0.05). Western blotting of HCAEC lysates with antibody CH-11 identified a single immunoreactive protein of 90 kDa.
Conclusions
Cultured human coronary artery endothelial cells express functional Fas capable of inducing apoptosis in response to either purified Fas ligand or receptor-activating monoclonal antibodies, at levels equal to those inducible by purified TNF-α. Immunologic studies and differential kinetics of caspase activation suggest that Fas and TNF-α induce apoptosis in HCAEC by signaling pathways that are distinct but equal in potency.
Keywords: FAS, apoptosis, atherosclerosis, heart failure, caspase, TNF-alpha
Background
The vascular endothelium regulates vascular function and homeostasis [1,2]. Injury to the human coronary artery endothelium may increase vascular permeability, blood coagulation, deposition of lipids, smooth muscle cells and monocytes and can facilitate atherosclerotic plaque development [3]. Apoptosis of endothelial cells has been observed as a prominent feature of advanced atherosclerosis, and has been implicated in the pathophysiology of acute coronary syndromes [4-6]. This concept is supported by the findings of increased expression of Caspase 1/interleukin-1β converting enzyme (ICE) and Caspase 3/ CPP-32 in atherosclerotic tissues [4-7]. Recently, it was shown [8] that foam cells within coronary arteries of patients with chronic ischemic heart disease express Fas (Apo1, CD95), a member of the tumor necrosis factor/nerve growth factor receptor family that induces apoptosis independent of TNF-α [9].
Previous work in endothelial cells have led to discordant reports of sensitivity or resistance to Fas induced apoptosis [10-14]. However, heterogeneity among endothelial cells from different tissues has been demonstrated and the effect of Fas on human coronary endothelial cells has not been extensively examined [15-17]. Moreover, in vitro observations suggest that the regulation of apoptosis in a vessel may be dependent not only on the cell type but on the local environment [12,18].
On this basis, we hypothesized that endothelial cells from different organs may respond differently to regulators of apoptosis as a result of cell-specific expression of receptors or downstream signaling molecules. The aim of the present study was to determine if cultured human coronary artery endothelial cells might undergo apoptosis in response to Fas activation, in contrast to other endothelial cell lines [10]. We report herein evidence that the activation of Fas in cultured human coronary artery endothelial cells induces apoptosis through signaling mechanisms distinct from those induced by TNF-α.
Results
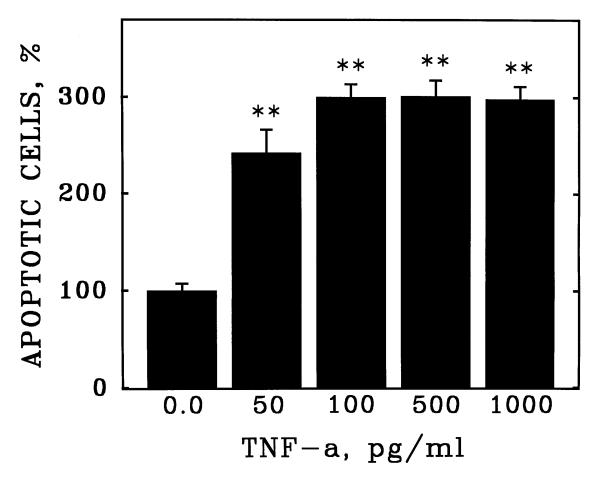
Apoptosis of human coronary artery endothelial cells (HCAEC) was quantitated by fluorescence detection of chromatin condensation and nuclear fragmentation in ethanol-fixed cells stained with propidium iodide (Figure 1). The reliability of this assay was confirmed by demonstration of the induction of apoptosis of HCAEC by purified recombinant human TNF-α (Figure 2), which stimulated apoptosis in a concentration-dependent manner with a maximal induction at 100 pg/ml.
Figure 1.
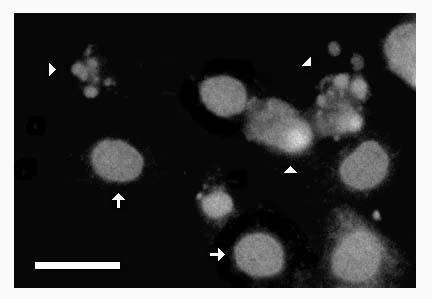
Fluorescence detection of apoptosis in cultured human coronary artery endothelial cells. Human coronary artery endothelial cells (HCAEC) were incubated with purified TNF-α (1 ng/ml) in serum-free medium. After 20 hours, the culture vessel was centrifuged to retain detached cells and was labeled with propidium iodide (see Materials and Methods). Greyscale image depicts red fluorescence (>590 nm) due to chromatin-bound propidium iodide in ethanol-fixed HCAEC treated with DNAse-free RNAse. Note condensed chromatin in discrete nuclear fragments in apoptotic cells (arrowheads). Arrows depict normal nuclei. Bar = 10 microns.
Figure 2.
Induction of apoptosis in cultured human coronary artery endothelial cells by purified human TNF-α. Human coronary artery endothelial cells (HCAEC) were incubated with purified recombinant human TNF-α at the indicated concentrations (ng/ml) in serum-free medium. After 20 hours, apoptosis was detected as described in Figure 1. Bars are the mean + S.E.M. of at least three determinations; ** = p < 0.01 versus 0.0 dose by ANOVA and Student-Newman-Keul's test.
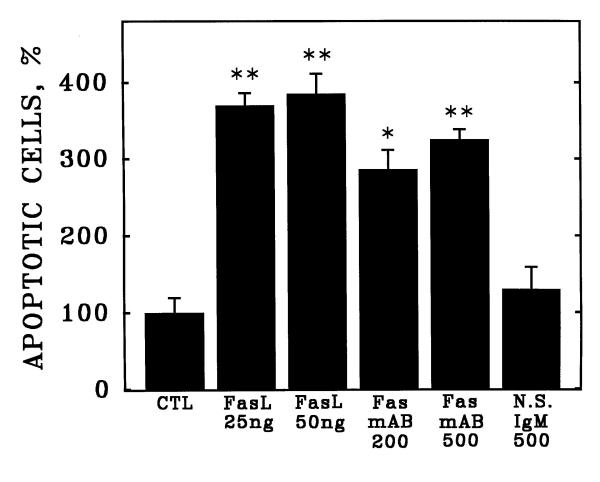
Apoptosis of HCAEC also was induced by either purified recombinant human Fas ligand (Fas L, Figure 3) or by a monoclonal antibody (Fas mAB, clone CH-11) known to activate the receptor in other cell types [15,17]. In contrast, a nonspecific isotype-matched control antibody (N.S.IgM) did not induce apoptosis at the same concentration of 500 pg/ml (Figure 3). By western blotting of HCAEC lysates, the anti-Fas monclonal antibody CH-11 recognized a single major immunoreactive protein with molecular mass approximately 90 kDa (Figure 4).
Figure 3.
Induction of apoptosis in cultured human coronary artery endothelial cells by activation of Fas. Human coronary artery endothelial cells (HCAEC) were incubated with purified recombinant human Fas ligand (FasL), Fas-activating monoclonal antibody CH-11 (Fas mAB), or nonspecific isotype-matched immunoglobulins (N.S.IgM) at the indicated concentrations (ng/ml) in serum-free medium. After 20 hours, apoptosis was detected as described in Figure 1. Bars are the mean + S.E.M. of at least three determinations; ** = p < 0.01 versus control (CTL) and * = p < 0.05 versus CTL by ANOVA and Student-Newman-Keul's test.
Figure 4.

Identification of Fas antigen in human coronary artery endothelial cells. Cell lysates were prepared from cultured HCAEC and were analyzed by western blotting with the same anti-Fas monoclonal antibody used for receptor activation in Figure 3. Note single major immunoreactive band (arrowhead). STD = molecular mass standards in kDa.
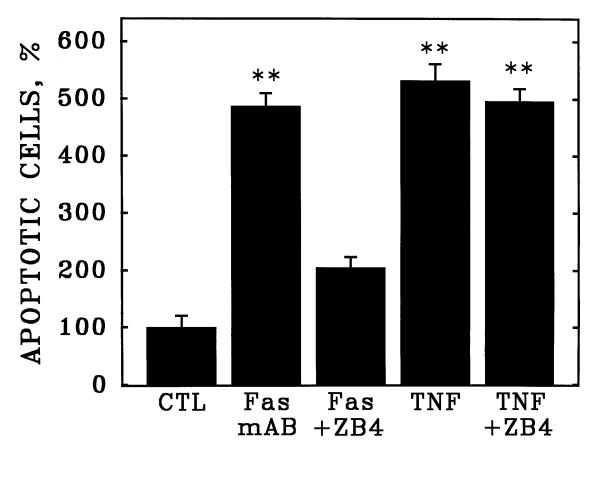
The induction of apoptosis by the CH-11 activating antibody (Fas mAB) could be abrogated by the Fas-neutralizing antibody ZB4 (Figure 5), which blocked about 80% of the induction of apoptosis by CH-11. However, the ZB4 neutralizing antibody did not inhibit HCAEC apoptosis in response to purified TNF-α, consistent with the notion that the induction of apoptosis by the CH-11 Fas-activating antibody does not involve the TNF receptor in HCAEC.
Figure 5.
Fas-neutralizing antibodies abrogate apoptosis of cultured human coronary artery endothelial cells in response to Fas but not TNF-α. Cultured HCAEC were incubated with Fas-activating monoclonal antibody CH-11 (Fas) or purified human TNF-α (TNF) in the presence or absence of the Fas-neutralizing monoclonal antibody, clone ZB4. Bars are the mean + S.E.M. of at least three determinations; ** = p < 0.01 versus control (CTL) by ANOVA and Student-Newman-Keul's test.
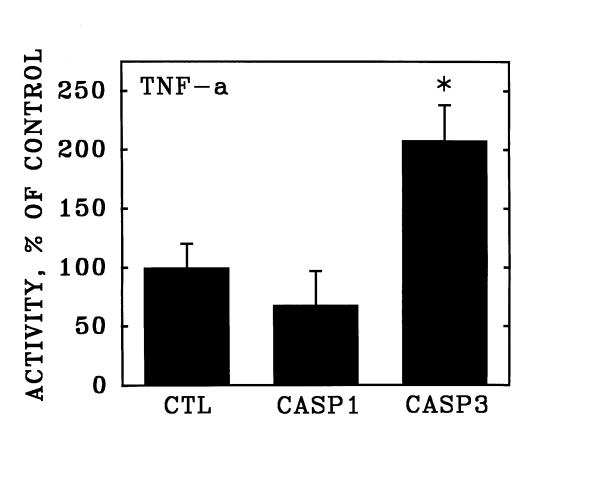
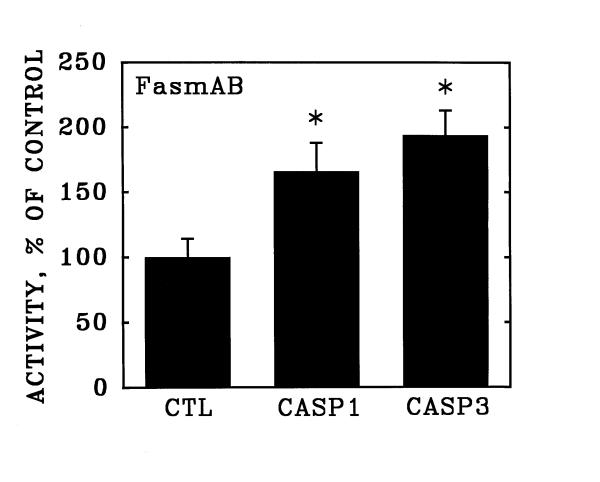
The activation of apoptosis with purified TNF-α was accompanied by a significant increase in the activity of Caspase 3 (Figure 6), but not in the activity of Caspase 1 measured after 20 hours exposure to the inducer (see Methods). In contrast, activation of Fas with the CH-11 monoclonal antibody induced significant increases in both Caspase 3 and Caspase 1 enzymatic activities under the same assay conditions (Figure 7).
Figure 6.
Stimulation of Caspase 3 activity in human coronary artery endothelial cells by purified TNF-α. Cultured human coronary artery endothelial cells were incubated for 20 hours in the presence and absence of purified recombinant TNF-α and were then detached from the culture surfaces. Over the subsequent hour, the cells were incubated in suspension culture with membrane-permeable fluorescent substrates specific for Caspase 1 (CASP1) and Caspase 3 (CASP3). Data are expressed as substrate cleaved per 106 cells, relative to unstimulated control cultures (CTL). Bars are the mean + S.E.M. of at least three determinations; * = p < 0.05 versus unstimulated control (CTL) by ANOVA and Student-Newman-Keul's test.
Figure 7.
Stimulation of Caspase 1 and Caspase 3 activities in human coronary artery endothelial cells by activation of Fas. Cultured human coronary artery endothelial cells were incubated for 20 hours in the presence and absence of anti-Fas clone CH-11 activating antibody, and were then detached from the culture surfaces. Over the subsequent hour, the activities of Caspase 1 (CASP1) and Caspase 3 (CASP3) were determined as described in Figure 6. Data are expressed as substrate cleaved per 106 cells, relative to unstimulated control cultures (CTL). Bars are the mean + S.E.M. of at least three determinations; * = p < 0.05 versus unstimulated control (CTL) by ANOVA and Student-Newman-Keul's test.
Discussion
Atherosclerosis is an inflammatory disorder, in which damage to the endothelium is an early causative event [1-3]. It has been suggested that endothelial cell apoptosis is an important feature in atherogenesis [4-8]. In support of this, recent evidence has shown that pro-atherosclerotic agents like angiotensin II and oxidized LDL induce endothelial cell apoptosis and that anti atherosclerotic agents are anti apoptotic [11]. Moreover, increased level of apoptosis and increased expression of pro apoptotic factors is observed in endothelial cells found in atherosclerotic lesions [23]. However, the apoptotic pathways that are altered in atherosclerosis have not been clarified.
Fas is a member of the TNF superfamily and is implicated in the initiation of apoptotic signaling in many tissues. It has been supported that endothelial cells are resistant to Fas mediated apoptosis, although other parameters like the oxidized LDL can sensitize endothelial cell to Fas apoptosis [10,11]. However, heterogeneity among endothelial cells from different tissues has been demonstrated and the effect of Fas on human coronary endothelial cells has not been extensively examined [15-17]. Heterogeneity among endothelial cells in various tissues has been implicated by both morphologic and functional studies. For example, endothelial cells isolated from large versus small vessels were found to vary in the number and nature of tight junctions and Weibel-Palade bodies [15]. Moreover, functional studies have shown that plasma from patients with idiopathic thrombotic thrombocytic purpura and hemolytic uremic syndrome induced the expression of Fas and apoptosis in dermal, renal and cerebral microvascular endothelial cells, but not in cells of pulmonary origin or in endothelial cells from large vessels [16,17]. Given these findings, it seems reasonable to suspect that responsiveness to activators of Fas might also vary with the origin and local environment from which endothelial cells were isolated.
In the present study, we observed that a monoclonal antibody capable of activating human Fas (clone CH-11) and purified human Fas ligand can induce apoptosis of cultured HCAEC to a degree similar to that induced by purified human TNF-α. Moreover, the failure of the Fas-neutralizing antibody ZB4 to abrogate TNF-induced apoptosis of HCAEC (Figure 5) supports the notion that Fas induces apoptosis in HCAEC by a mechanism independent of the TNF receptor. The finding of a different profile of activation of caspases 1 and 3 by Fas versus TNF-α (Figures 6 and 7) is also consistent with this interpretation.
In any case, the results of the present study are apparently contradictory to data published by others [10,12], who reported that Fas ligation does not induce apoptosis in endothelial cells. We hypothesize that our data, not only reflect anatomical and physiological differences in the regulation of apoptosis among different endothelial cells subpopulations, but also technical limitations in some of the studies. The lack of apoptosis in response to Fas ligation shown in some studies [10] may have been due to the use of antibody preparations which have been shown in at least one other cell type [19] to be incapable of activating Fas. Regardless, other authors have reported that Fas induces apoptosis in endothelial cells [13,23]. Fas protein expression increases in human coronary endothelial cells under conditions that promote apoptosis, such as anoxia-reoxygenation [24,25]. Moreover, Dang et. al [23] found concurrent apoptosis and Fas expression in the coronary arteries of transplanted patients with allograft arteriopathy; the Fas-positive cells were primarily endothelial cells. Other authors have observed strong Fas protein expression in the smooth muscle cells and endothelial cells of aortic allografts, and virtually all the apoptotic cells were located within the area of Fas expression [26].
Apoptosis also has been implicated in cardiomyopathies and after myocardial infarction, and has also been observed as a prominent feature in coronary artery disease associated with atherosclerosis and transplant arteriopathy [4,5,27]. TUNEL-positive endothelial cells also have been observed in tissues from patients with transplant coronary arteriopathy and endothelial dysfunction is a characteristic of chronic heart failure [6]. Together, these results and our findings support the concept that Fas expression by endothelial cells may play an important role in the initiation and progression of pathologies involving human coronary arteries.
Conclusion
In summary, the present study demonstrates that human coronary artery endothelial cells express functional Fas that induces apoptosis to a degree similar to that induced by TNF-α. Fas in HCAEC is activated by either purified human Fas ligand or by an activating monoclonal anti-Fas antibody (clone CH-11), but these activators act in a manner independent of the TNF receptor. These findings suggest functional differences in the responsiveness to inducers of apoptosis by endothelial cells of different origins.
Methods
Endothelial Cell Culture and Induction of Apoptosis
Primary human coronary artery endothelial cells (HCAEC, Clonetics Corp, San Diego, CA) were cultured in cell type-specific culture media optimized for this purpose (EBM, Clonetics). Cells were seeded on 24-well tissue culture-treated plates at a density of 5,000 cells per cm2, and were used at subconfluent densities of 80–90%. Depending on experimental design, apoptosis was induced with purified recombinant human tumor necrosis factor-alpha (TNF-α, Pharmingen, San Diego, CA), with activating antibodies specific for human Fas (FasmAB clone CH-11, Upstate Biotechnology, Saranac Lake, NY) or with purified human recombinant Fas ligand (FasL, Kamiya Biomedical, Seattle, WA). All test agents were applied in serum-free Ham's F12 medium and were applied for 20 hours at 37C in a 5% CO2 incubator.
Fluorescence Detection of Apoptosis
Apoptotic cells were quantitated by detection of chromatin condensation and nuclear fragmentation as described earlier [19-22]. Briefly, at the end of 20 hours incubation 100% ethanol was added directly to the cell culture vessels to a final concentration of 70% to fix the cells [19]. After at least 30 minutes, the culture plates were centrifuged to retain detached cells, the ethanol was carefully removed, and a solution of DNAse-free RNAse containing 5 ug/ml propidium iodide (PI) in PBS was applied and incubated 20 minutes at 37C° to digest RNA. Nuclear morphology was assessed by the fluorescence of chromatin-bound PI on a Ziess/Jena Sedival inverted epifluorescence microscope through a >570 nm long pass filter [15,16].
Caspase Assay
The enzymatic activities of Caspase 1/interleukin-1b converting enzyme (ICE) and Caspase 3/CPP32/YAMA were determined as described earlier for alveolar epithelial cells [19,20]. Briefly, the enzymes were measured in intact HCAEC suspension cultures that had been previously incubated in an identical fashion to those used for detection of apoptosis. After 18 hours incubation with activator, the cells were trypsinized from the culture plates and resuspended in serum-free culture medium containing activator (anti-Fas or TNF-α) at the same concentration used for morphologic assay for apoptosis. After one hour, fluorogenic peptides specific to each enzyme were added to cuvettes containing the cell suspensions for determination of total activity in the intact cells. For measurement of Caspase 1/ICE activity, the peptide substrate N-Acetyl-Tyr-Val-Ala-Asp-7-amino-4-methylcoumarin (Ac-YVAD-AMC, Pharmingen, San Diego, CA) was used at final concentration of 50 mM. For Caspase 3/CPP32/YAMA, the peptide substrate N-Acetyl-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin (Ac-DEVD-AMC, Upstate Biotechnology, Saranac Lake, NY) was added at 200 mM final concentration. Detection of fluorescent product over time was monitored in a spectroflourometer at 380 nm excitation and 450 nm emission. Data were normalized to equivalent cell numbers per treatment group, and are expressed as the intial reaction rates for a given activator relative to the unactivated control group.
Protein Electrophoresis
Cell lysates were prepared from unactivated HCAEC with lysis buffer containing the detergent NP40 and a panel pf protease inhibitors. Protein gel electrophoresis was performed on 10 ug of HCAEC protein on 7.5% polyacrylamide Ready-Gels (BioRad, Hercules, CA) run under reducing conditions. Resolved proteins were electrotransfered to PVDF membranes and were subjected to western blotting with the same monoclonal anti-Fas antibody used for Fas activation (clone CH-11). Bound antibody was detected with anti-mouse IgG-biotin secondary antibody, streptavidin-alkaline phosphatase and Nitro Blue Tetrazolium (NBT) chromogen.
Statistics
Statistical significance between treatment groups was analyzed by Analysis of Variance (ANOVA) followed by Student Newman Keul's post hoc analysis, through the software package Instat (Graphpad Software, San Diego, CA). Differences were considered significant at p < 0.05.
Acknowledgments
Acknowledgements
This work was supported by PHS HL-45136 to B.D.U, by the Womens' Board Endowment to the Cardiovascular Institute, Michael Reese Hospital, and by the Research and Education Foundation, Michael Reese Hospital.
Contributor Information
Gerasimos Filippatos, Email: geros@compulink.gr.
Edmund Ang, Email: bdu2@aol.com.
Claudia Gidea, Email: cgidea@tigger.uic.edu.
Erhan Dincer, Email: erhan_dincer@yahoo.com.
Rongqi Wang, Email: wrongqi@yahoo.com.
Bruce D Uhal, Email: Uhal@msu.edu.
References
- Abrams J. Role of endothelial dysfunction in coronary artery disease. Am J Cardiology. 1997;79:2–9. doi: 10.1016/S0002-9149(97)00379-2. [DOI] [PubMed] [Google Scholar]
- Rubanyi GM. The role of endothelium in cardiovascular homeostasis and diseases. J Cardiovasc Pharmacol. 1993;22:S1–S14. doi: 10.1097/00005344-199322004-00002. [DOI] [PubMed] [Google Scholar]
- Ruschitza F, Noll G, Luscher T. The endothelium in coronary artery disease. Cardiology. 1997;88:3–19. doi: 10.1159/000177500. [DOI] [PubMed] [Google Scholar]
- Geng YJ, Libby P. Evidence for apoptosis in advanced human atheroma: colocalization with interleukin-1b-converting enzyme. Am J Pathol. 1995;147:251–266. [PMC free article] [PubMed] [Google Scholar]
- Bochaton-Piallat M, Gabbiani F, Redard M, Desmouliere A, Gabbiani G. Apoptosis participates in cellular regulation during rat aortic intimal thickening. Am J Pathol. 1995;146:1059–1064. [PMC free article] [PubMed] [Google Scholar]
- Best E. Apoptosis: basic concepts and implications in coronary artery disease. Arterioscler Thromb Vasc Biol. 1999;19:14–22. doi: 10.1161/01.atv.19.1.14. [DOI] [PubMed] [Google Scholar]
- Mallat Z, Ohan J, Leseche G, Tedgui A. Colocalization of CPP-32 with apoptotic cells in human atherosclerotic plaques. Circulation. 1997;96:424–428. doi: 10.1161/01.cir.96.2.424. [DOI] [PubMed] [Google Scholar]
- Cai W, Devaux B, Schaper W, Schaper J. The role of Fas/Apo1 and apoptosis in the development of human atherosclerotic lesions. Atherosclerosis. 1997;131:177–186. doi: 10.1016/S0021-9150(97)06099-1. [DOI] [PubMed] [Google Scholar]
- Suda T, Nagata S. Purification and characterization of the Fas-ligand that induces apoptosis. J Exp Med. 1994;179:873–879. doi: 10.1084/jem.179.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson B, Lalwani N, Johnson K, Marks R. Fas ligation triggers apoptosis in macrophages but not endothelial cells. Eur J Immunol. 1994;24:2640–2645. doi: 10.1002/eji.1830241111. [DOI] [PubMed] [Google Scholar]
- Sata M, Walsh K. TNFa regulation of Fas ligand expression on endothelium modulates leukocyte infiltration of the blood vessel wall. Nat Med. 1998;4:415–420. doi: 10.1038/nm0498-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoudjit F, Vuori K. Matrix attachment regulates Fas-induced apoptosis in endothelial cells: a role for c-flip and implications for anoikis. J Cell Biol. 2001;152:633–643. doi: 10.1083/jcb.152.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardier JE, Schulte T, Kammer H, Kwak J, Cardier M. Fas (CD-95, APO-1) antigen expression and function in murine liver endothelial cells: implications for the regulation of apoptosis in liver endothelial cells. FASEB J. 1999;13:1950–1960. doi: 10.1096/fasebj.13.14.1950. [DOI] [PubMed] [Google Scholar]
- Janin A, Deschaumes C, Daneshpouy M, Estaquier J, Micic-Polianski J, Rajagopalan-Levasseur P, et al. CD 95 engagement induces disseminated endothelial cell apoptosis in vivo: immunopathologic implications. Blood. 2002;99:2940–2947. doi: 10.1182/blood.V99.8.2940. [DOI] [PubMed] [Google Scholar]
- Craig L, Spelman J, Strandberg J, Zink MC. Endothelial cells from diverse tissues exhibit differences in growth and morphology. Microvascular Research. 1998;55:65–76. doi: 10.1006/mvre.1997.2045. [DOI] [PubMed] [Google Scholar]
- Dang C, Magid M, Weksler B, Chadburn A, Laurence J. Enhanced endothelial cell apoptosis in splenic tissues of patients with thrombotic thrombocytopenic purpura. Blood. 1999;93:1264–1270. [PubMed] [Google Scholar]
- Laurence J, Mitra D, Steiner M, Staiano-Coico L, Jaffe E. Plasma from patients with idiopathic and human immunodefficiency virus-associated thrombotic thrombocytic purpura induces apoptosis in microvascular endothelial cells. Blood. 1996;87:3245–3254. [PubMed] [Google Scholar]
- Pollman M, Naumovski L, Gibbons G. Vascular cell apoptosis. Cell type-specific modulation by transforming growth factor-b1 in endothelial cells versus smooth muscle cells. Circulation. 1999;99:2019–2026. doi: 10.1161/01.cir.99.15.2019. [DOI] [PubMed] [Google Scholar]
- Uhal B, Gidea C, Bargout R, Bifero A, Ibarra-Sungam O, Papp M, Flynn K, Filippatos G. Captopril inhibits apoptosis in human lung epithelial cells: a potential antifibrotic mechanism. Am J Physiol. 1998;275:L1013–1017. doi: 10.1152/ajplung.1998.275.5.L1013. [DOI] [PubMed] [Google Scholar]
- Wang R, Zagariya A, Ibarra-Sunga O, Gidea G, Ang E, Deshmukh S, Chaudhary G, Baraboutis J, Filippatos G, Uhal B. Angiotensin II induces apoptosis in human and rat alveolar epithelial cells. Am J Physiol. 1999;276:L885–L889. doi: 10.1152/ajplung.1999.276.5.L885. [DOI] [PubMed] [Google Scholar]
- Wang R, Zagariya A, Ang E, Ibarra-Sunga O, Uhal B. Fas-induced apoptosis of alveolar epithelial cells requires angiotensin II generation and receptor interaction. Am J Physiol. 1999;277:L1245–L1250. doi: 10.1152/ajplung.1999.277.6.L1245. [DOI] [PubMed] [Google Scholar]
- Filippatos G, Leche J, Sunga R, Tsoukas A, Joshi I, Anthopoulos P, Bifero A, Pick R, Uhal B. Expression of Fas by surviving myocardial cells adjacent to fibrotic foci in the failing human heart is not associated with increased apoptosis. Am J Physiol. 1999;277:H445–51. doi: 10.1152/ajpheart.1999.277.2.H445. [DOI] [PubMed] [Google Scholar]
- Dong C, Wilson J, Winters G, McManus B. Human transplant coronary artery disease: Pathological evidence for Fas-mediated apoptotic cytotoxicity in allograft arteriopathy. Lab Invest. 1996;74:921–931. [PubMed] [Google Scholar]
- Li D, Tomson K, Baichun Y, Mehta P, Croker CP, Mehta JL. Modulation of constitutive nitric oxide synthase, bcl-2 and Fas expression in cultured human coronary endothelial cells exposed to anoxia-reoxygenation and angiotensin II: role of AT1 receptor activation. Cardiovasc Res. 1999;41:109–115. doi: 10.1016/S0008-6363(98)00196-5. [DOI] [PubMed] [Google Scholar]
- Li D, Weng S, Yang B, Mehta J. Angiotensin II augments anoxia-reoxygenation-mediated apoptosis of cultured human coronary endothelial cells: Modulation by AT1 receptor antagonist losartan. Circulation. 1997;96:411–418. [Google Scholar]
- Akyurek L, Johnsson C, Lange D, Georgii-Hemming P, Larsson E, Fellstrom B. Tolerance induction ameliorates allograft vasculopathy in rat aortic transplants. Influence of Fas-mediated apoptosis. J Clin Invest. 1998;101:2889–2899. doi: 10.1172/JCI1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D, Haudenschild C, Hong M, Tinkle B, Leon M, Liau G. Evidence for apoptosis in human atherogenesis and in rat vascular injury model. Am J Pathol. 1995;147:267–277. [PMC free article] [PubMed] [Google Scholar]