Abstract
Background
Sin Nombre virus (SNV) establishes a persistent infection in the deer mouse, Peromyscus maniculatus. A strong antibody response occurs in response to SNV infection, but the role of the innate immune response is unclear. To address this issue, we have initiated an effort to identify and characterize deer mouse cytokine and chemokine genes. Such cytokines and chemokines are involved in various aspects of immunity, including the transition from innate to adaptive responses, type I and type II responses, recruitment of leukocytes to sites of infection, and production of mature cells from bone marrow progenitors.
Results
We established a colony of SNV antibody-negative deer mice and cloned 11 cytokine and chemokine partial cDNA sequences using directed PCR. Most of the deer mouse sequences were highly conserved with orthologous sequences from other rodent species and functional domains were identified in each putative polypeptide.
Conclusions
The availability of these sequences will allow the examination of the role of these cytokines in deer mouse responses to infection with Sin Nombre virus.
Background
Deer mice (Peromyscus maniculatus) are the principal natural host of Sin Nombre virus (SNV), the etiologic agent of most hantavirus pulmonary syndrome (HPS) cases in humans in North America [1-3]. Deer mice are found throughout most of North America, except for the eastern seaboard and southeast United States [4].
SNV predominantly infects capillary endothelial cells without discernible pathology [5]. A prominent feature of HPS is capillary leakage and subsequent hypotension that causes death by cardiac failure. In addition to resident alveolar macrophages, lymphocytes infiltrate the lungs of patients and contribute to the pathology by secreting proinflammatory cytokines, including interferon-γ (IFNγ), interleukin-2 (IL-2), tumor necrosis factor (TNF), and lymphotoxin (LT) [6]. These characteristics suggest HPS may be a cytokine-mediated immunopathology.
As is usual with most long-term reservoir hosts, deer mice exhibit little pathology during acute infection with SNV [7]. The virus infects capillary endothelial cells of deer mouse lungs, but lymphocyte infiltration or inflammation are not observed. After acute infection, the virus establishes persistence in many tissues [8] and most, if not all, deer mice remain infected for the remainders of their lives.
The role of the immune response in infected deer mice is unclear because few reagents of defined specificity have been developed to characterize their immune function. A strong IgG antibody response occurs [7,9], suggesting that B cells and helper T cells participate in containment of the virus, but the role of cytokines has not been characterized. As part of our continuing efforts to develop the deer mouse as a model for SNV research [10,11]; (Green et al., in press; Richens et al., submitted), we established a colony of deer mice captured in a region where SNV is prevalent [12,13]. Splenocytes obtained from deer mice from this colony were used to clone several cytokine and chemokine cDNA sequences. These sequences represent several components of the immune response, including the transition from innate to adaptive responses (IL-12a, IL-21, IL-23a), type I (IL-2) and type II responses (IL-6, IL-13), a colony stimulating factor (GM-CSF), and chemokines (CCL2, CCL3, CCL4, CXCL2). The availability of such sequences will permit experimental examination of the roles of these cytokines in deer mice infected with SNV.
Results
Establishment of a deer mouse colony
We identified a location that contained SNV-infected deer mice and trapped 10 deer mice. Nine (4 males and 5 females) had no IgG antibody to SNV at capture, as well as after 36 days of quarantine [14], and were used to establish the colony. Breeders were kept on a 16:8 light:dark cycle and were provided food and water ad libitum. The colony has been reproductively vigorous and has produced nearly 300 offspring in 3 years.
Interleukins
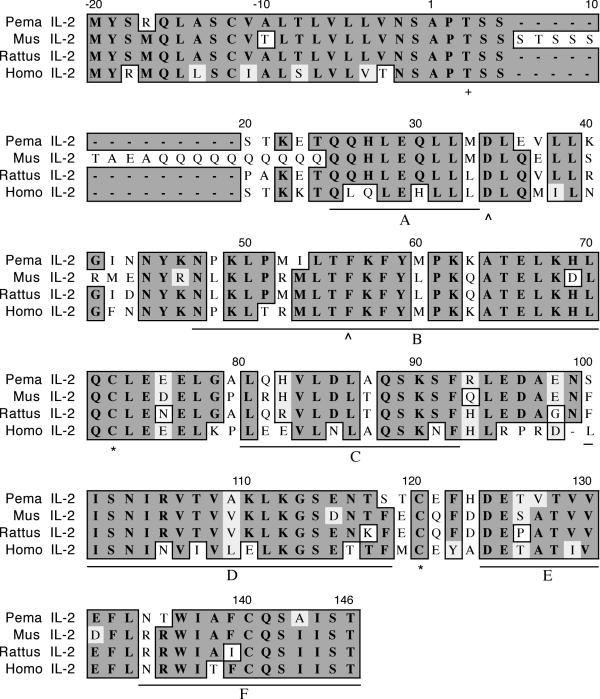
The fragment encoding deer mouse IL-2 was 456 nucleotides (nt) in length and represents all but the final three amino acids (Figure 1) based upon the known sequence of house mouse IL-2. House mouse IL-2 exhibits an unusual N-terminal structure that is not present in IL-2 of the other species. Discounting this region, deer mouse IL-2 shares 76% identity and 82% similarity to house mouse IL-2. As with the other species, deer mouse IL-2 possesses a 20 residue signal sequence. All three cysteines are conserved in IL-2 orthologs and C72 and C120 of the deer mouse polypeptide are predicted to form an intrachain disulfide bond [15]. The threonine at position 3 of the mature polypeptide is conserved and serves as a potential glycosylation site [16]. The predicted α-helices (A-F) are present, although helix F is incomplete [15].
Figure 1.
Amino acid alignment of deer mouse (Pema), house mouse (Mus), rat (Rattus) and human (Homo) IL-2. The house mouse, rat and human sequences were truncated to align with the cloned deer mouse fragment. The sequence accession numbers are listed in Table 3 and were aligned using the clustalw multiple alignment algorithm in MacVector DNA analysis software. Identical amino acids are boxed with dark background, similar amino acids are boxed with light background, and nonidentities have white background. The deer mouse polypeptide represents all but the final 3 amino acids. An unusual feature of house mouse IL-2 is the presence of a serine- and glutamine-rich region near the N-terminus. A conserved threonine (+) that is a potential glycosylation site is located at position 3. All three cysteine residues are conserved in deer mouse IL-2 and it is predicted that a disulfide bond is formed between C72 and C120 (*). Conserved residues involved in receptor binding are located at positions 20 and 42 (^). The predicted alpha helices are denoted by lettered (A-F) underlines.
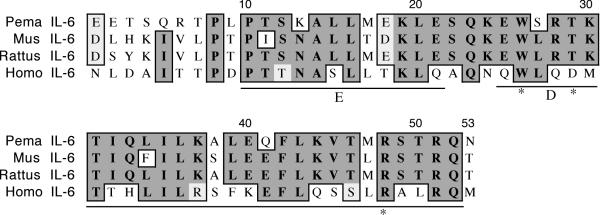
We cloned a 225 nt fragment of deer mouse IL-6 that encodes the 53 C-terminal residues, which represents 25% of the coding region (Figure 2). The polypeptide shares 66% identity and 70% similarity with house mouse IL-6. The polypeptide possesses the predicted D (E25-R51) and E (P10-E21) helices [17]. The tryptophan at 157 that is involved in receptor interaction [18] is conserved in deer mouse IL-6, as is the arginine at position 48. Mutations in this residue have been shown to reduce IL-6 activity by 100-fold [19].
Figure 2.
Amino acid alignment of deer mouse (Pema), house mouse (Mus), rat (Rattus) and human (Homo) IL-6. Sequences were aligned as described in Figure 1. The fragment represents approximately 25% of the polypeptide. The E and D helices (underlined) and residues implicated in receptor binding are denoted (*).
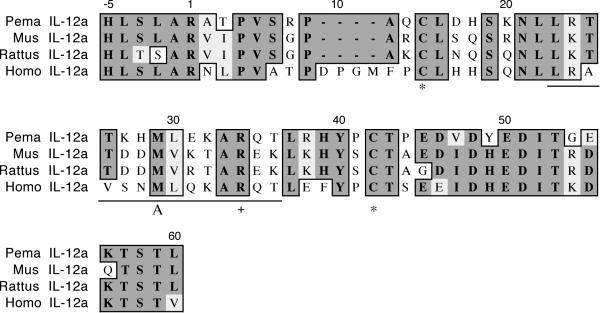
The cloned IL-12a fragment is 185 nt and represents 28% of the polypeptide (IL-12 p35, Figure 3). It shares 62% identity and 74% similarity with house mouse p35 and includes the first 60 residues of the mature polypeptide. The human p35 polypeptide has four consecutive residues not found in the rodent p35 polypeptides (D9-M12). Conserved cysteines are found at positions 15 and 42, the latter of which is involved in intrachain disulfide bond formation [20]. The conserved arginine at position 34 plays a role in the interchain interface with the IL-12 p40 subunit. Helix A, which provides a large interlocking topology for the p40 subunit, is present in the deer mouse polypeptide.
Figure 3.
Amino acid alignment of deer mouse (Pema), house mouse (Mus), rat (Rattus) and human (Homo) IL-12a. Sequences were aligned as described in Figure 1. The deer mouse fragment represents 28% of the polypeptide. The polypeptide includes helix A (underlined) and conserved cysteines noted with an asterisk (*). The conserved arginine (+) is involved with dimerization to the p40 subunit.
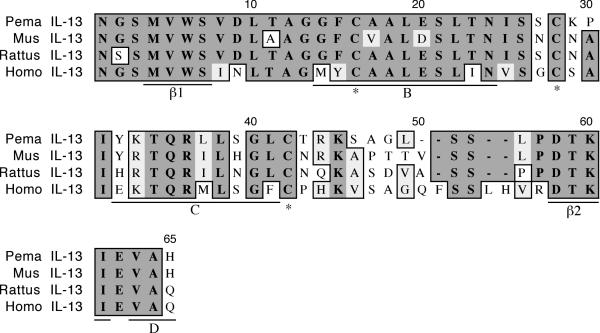
The cloned deer mouse IL-13 fragment is 184 nt in length and represents 47% of the coding region (Figure 4). It possesses 76% identity and 84% similarity to house mouse IL-13. The deer mouse polypeptide includes helices B (G14-T23) and C (Y32-G40), and the first three residues of helix D (V63-H65) [21]. It also includes both the β1 (M4-S6) and β2 (D58-I61) strands and the amino acids are completely conserved between the rodent and human sequences. A hydrogen bond between V5 and I61 of these interloop strands is predicted to structurally stabilize IL-13. Three of the four expected cysteines that form intrachain disulfide bonds are present. The deer mouse IL-13 fragment is four amino acids shorter than the human fragment, while the house mouse and rat fragments are three residues shorter than the human fragment. These differences are between helix C and the β2 strand.
Figure 4.
Amino acid alignment of deer mouse (Pema), house mouse (Mus), rat (Rattus) and human (Homo) IL-13. Sequences were aligned as described in Figure 1. The deer mouse fragment represents 47% of the polypeptide. The fragment includes helices B and C and part of helix D, and both β-strands (underlined). Three of the four expected cysteines (*) involved in intrachain disulfide bonds are also present.
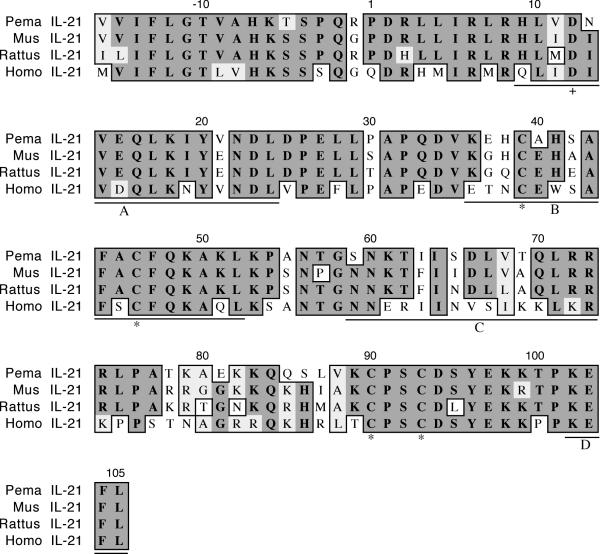
The cloned IL-21 fragment is 371 nt in length and represents 84% of the coding region (Figure 5). It shares 81% identity and 86% similarity with house mouse IL-21. The polypeptide includes the first three and part of the fourth helices (A 9–24; B 36–52; C 59–73; D 102–105) typical of the four-helix-bundle family of cytokines [22]. Residue D12, believed to be involved in receptor interaction, is conserved in all four species [23].
Figure 5.
Amino acid alignment of deer mouse (Pema), house mouse (Mus), rat (Rattus) and human (Homo) IL-21. Sequences were aligned as described in Figure 1. The deer mouse fragment represents 84% of the polypeptide and includes the first three and part of the fourth helices (A-D, underlined). Conserved cysteines (*) and an aspartic acid (+) involved in receptor binding are also present in deer mouse IL-21.
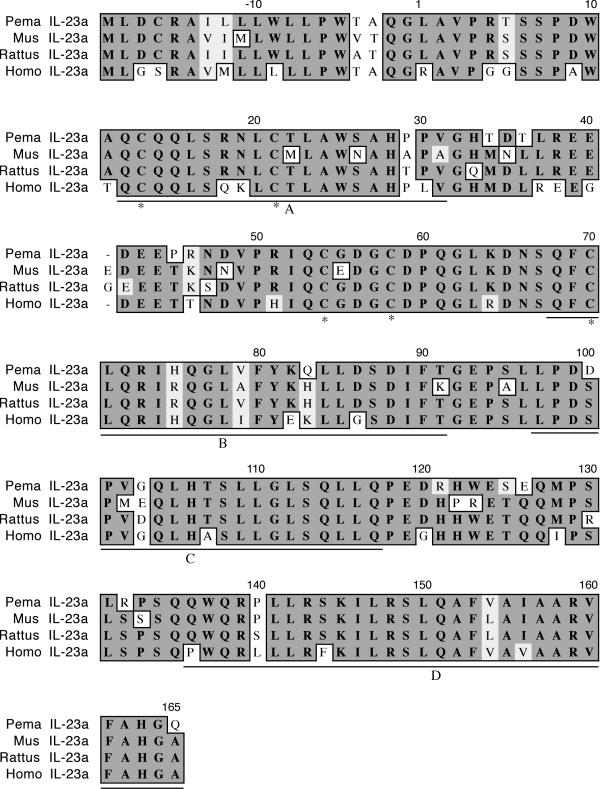
The cloned IL-23a cDNA is 579 nt and represents 94% of the coding region, including the complete N-terminus (Figure 6). The polypeptide is 81% identical and 86% similar to house mouse IL-23a. The fragment contains all five cysteine residues and all four helices are present (A Q12-V31; B Q68-T91; C L97-Q117; D Q136-Q165) [24].
Figure 6.
Amino acid alignment of deer mouse (Pema), house mouse (Mus), rat (Rattus) and human (Homo) IL-23a. Sequences were aligned as described in Figure 1. The deer mouse fragment represents 94% of the polypeptide. All five cysteines (*) are present as well as the four α-helices (A-D, underlined).
GM-CSF
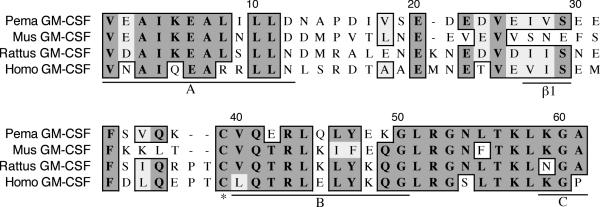
The granulocyte macrophage-colony stimulating factor (GM-CSF) fragment is 175 nt long and represents 39% of the coding region (Figure 7). It shares 65% identity and 73% similarity to house mouse GM-CSF. All of helix B (V40-G50) and parts of helices A (V1-D12) and C (K59-A61) are present in the fragment [25]. The first β-strand is located at positions I27-S29. The cysteine at position 39 participates in one of two intrachain disulfide bonds. The A helix is highly conserved between rodents and plays an important role in binding to the GM-CSF receptor [26].
Figure 7.
Amino acid alignment of deer mouse (Pema), house mouse (Mus), rat (Rattus) and human (Homo) GM-CSF. Sequences were aligned as described in Figure 1. The deer mouse fragment represents 39% of the polypeptide. It includes partial A and C helices and the entire B helix and the β1-strand (underlined). One conserved cysteine is also present in deer mouse GM-CSF (*).
Chemokines
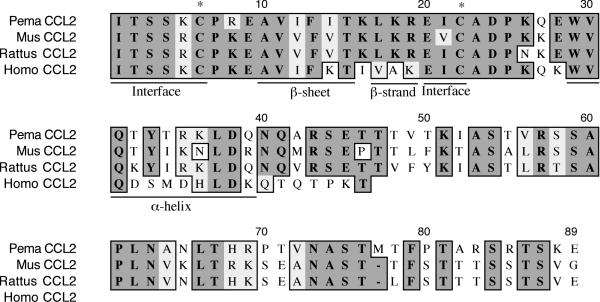
The CCL2 fragment is 297 nt and encodes 60% of the polypeptide (Figure 8). It is 61% identical and 75% similar to house mouse CCL2. The rodent CCL2 polypeptides are 43 residues longer than human CCL2. The third and fourth conserved cysteines (C6, C22) are also present in the deer mouse polypeptide. Residues 1–6 of the polypeptide form a loop that is important in forming the homodimer interface of CCL2 [27]. A β-sheet is preserved from A10-T15 as well as a β-strand from L17-R19. A helix is encoded from W29-Q39.
Figure 8.
Amino acid alignment of deer mouse (Pema), house mouse (Mus), rat (Rattus) and human (Homo) CCL2. Sequences were aligned as described in Figure 1. The fragment represents 60% of the polypeptide. Conserved secondary structures (underlined) and cysteines are present in deer mouse CCL2. Two highly conserved regions that form the homodimer interface are also conserved in deer mouse CCL2. Rodent CCL2 polypeptides are substantially longer than human CCL2.
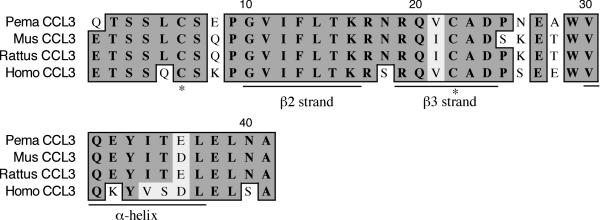
The CCL3 fragment is 260 nt and encodes the last 41 residues (Figure 9). It represents 45% of the polypeptide and exhibits 83% identity and 90% similarity to house mouse CCL3. The deer mouse polypeptide possesses the β2 (G10-K16) and β3 (N18-D24) strands as well as the C-terminal α-helix (V30-L37) [28]. The third (C6) and fourth (C22) cysteines involved with intrachain disulfide bonds were also present.
Figure 9.
Amino acid alignment of deer mouse (Pema), house mouse (Mus), rat (Rattus) and human (Homo) CCL3. Sequences were aligned as described in Figure 1. The deer mouse fragment represents 45% of the polypeptide and includes the second and third β-strands, and the α-helix (underlined). The third and fourth conserved cysteines (*) are also present.
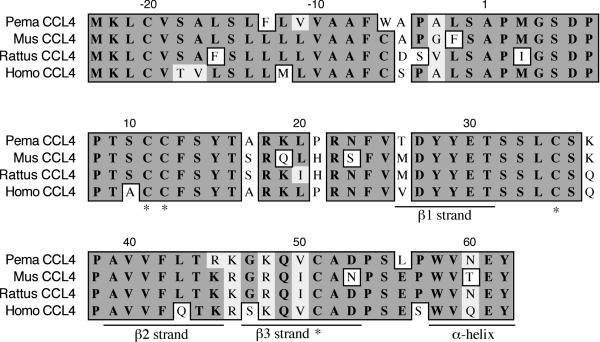
The CCL4 fragment is 257 nt fragment and represents all but the last 7 expected amino acids (Figure 10). It shares 80% identity and 87% similarity with house mouse CCL4. The polypeptide includes all four conserved cysteines that form intrachain disulfide bonds (C11-C35, C12-C51) [28], including the adjacent cysteines that are the hallmark of CC chemokines. All three β-strands are present (β1 T26-T31; β2 A39-R45; β3 G47-D53) as is the α-helix (W58-Y62).
Figure 10.
Amino acid alignment of deer mouse (Pema), house mouse (Mus), rat (Rattus) and human (Homo) CCL4. Sequences were aligned as described in Figure 1. The deer mouse fragment represents 92% of the polypeptide. All four conserved cysteines (*) and all three β-strands are present, as is part of the α-helix.
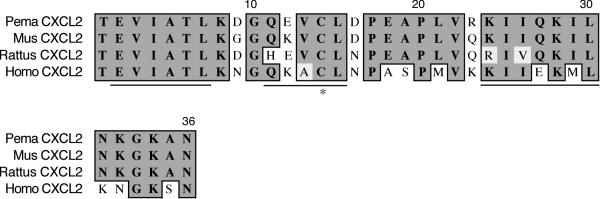
The CXCL2 fragment is 198 nt long, encodes the last 36 amino acids, and represents about half of the mature polypeptide (Figure 11). It shares 92% identity and similarity to house mouse CXCL2. The fourth conserved cysteine involved in intrachain disulfide bond formation is residue 14 [29]. The β2 (E2-L7) and β3 (Q11-L15) strands that are involved in homodimer formation are also present, as is the C-terminal α-helix (R23-L30).
Figure 11.
Amino acid alignment of deer mouse (Pema), house mouse (Mus), rat (Rattus) and human (Homo) CXCL2. Sequences were aligned as described in Figure 1. The deer mouse fragment represents 49% of the polypeptide and possesses the last two β-strands, the α-helix, and the fourth conserved cysteine (*)
Discussion
Pathogenic hantaviruses induce acute inflammatory responses in humans that contribute to HPS disease progression [5,6]. Much of the pathology occurs because of the production of several inflammatory cytokines in the lungs, including IL-2, IFNγ, tumor necrosis factor (TNF) and lymphotoxin (LT). In addition, pulmonary T cell infiltrates are observed in patients that die from HPS. It is evident that immunopathology contributes to HPS progression, given that SNV does not cause discernible direct cytopathology.
We targeted several deer mouse cytokines important in different aspects of immune responses. Our approach was to identify highly conserved regions of orthologous sequences from rodents and humans and to design primers from them. In many instances no consensus was found, so degenerate primer sets were used. In our analysis we determined the approximate relative sizes of the fragments based upon the known length of M. musculus orthologs; it is likely that some deer mouse genes will be longer or shorter when full-length sequences become available.
We also used cells from ostensibly SNV-susceptible deer mice. It is plausible that, in response to coadaptation with the virus, some selective pressure has occurred over time on deer mouse cytokine genes.
IL-2 is the principal T cell growth factor and is secreted by T helper 1 (Th1) cells during immune responses [30,31]. It is also produced by pulmonary infiltrates in HPS patients [6] and probably plays an indirect role in inflammation by augmenting TNF expression [32]. IL-21 is an IL-2 family member that is also secreted by Th1 cells. It was initially described as an activator of NK cells and an augmenter of B cell and T cell proliferation [22]. More recently, it has been shown to play a role in the transition from innate to adaptive responses by limiting NK cell responses and activating cytotoxic T lymphocyte responses [33]. It also skews the immune response towards a type I response by augmenting expression of the T-bet transcription factor, IFNγ and receptors for IL-2, IL-12 and IL-18 [34].
Although GM-CSF is secreted by Th1 cells [31], it is commonly used to generate bone marrow-derived dendritic cells (DC). The receptor-binding region of deer mouse GM-CSF is highly conserved with respect to house mouse and rat GM-CSF. This suggested to us that recombinant house mouse GM-CSF (rGM-CSF), which is commercially available, might bind to the deer mouse GM-CSF receptor, and we have obtained preliminary evidence suggesting that it does (data not shown). Human DC have recently been shown to support the replication of Hantaan virus [35], which is related to SNV. It should be possible to generate deer mouse DC with rGM-CSF to determine whether they can be infected by SNV and to determine whether there is a functional consequence of infection.
Both IL-12a and IL-23a polypeptides form heterodimers with the IL-12b (p40) subunit to form IL-12 and IL-23, respectively [24,36]. IL-12 facilitates the transition from the innate response to a type I adaptive immune response, while IL-23 appears to sustain proliferation of Th1 cells during the course of an immune response [24,37]. Both are produced by macrophages and dendritic cells and influence T cell maturation towards a Th1 phenotype.
In addition to Th2 cells, IL-6 is expressed by many other cell types and has broad biological activity, including a major role in augmenting antibody production in activated B cells [38]. IL-13 is closely linked to the IL-4 gene cluster in humans and house mice and has a role in class switching and controlling inflammatory responses [39,40].
Chemokine production in humans infected with SNV has not been examined in detail. TNF is produced in the lungs of patients that die from HPS [6] and mononuclear infiltrates are present in pulmonary tissues [5]. Since chemokines are potent recruiters of blood leukocytes into infected tissues, there must be a prominent role for these proteins in HPS. Deer mice exhibit no conspicuous recruitment of leukocytes into the lungs [7], thus comparison of chemokine responses in humans and deer mice infected with SNV may provide clues as to how their respective immune systems respond to the virus and how the virus evades the immune response in deer mice to establish persistence.
We did not find any conspicuous differences in the amino acid sequences of deer mouse cytokines or chemokines compared to rat or house mouse orthologs that would suggest a functional difference in the mechanism by which these molecules exert their effects in vivo. More likely, immunological decisions are made during acute infection in deer mice that lead to a qualitative difference in cytokine profiles. These sequences and others that we previously reported [11] will be useful in characterizing cytokine and chemokine responses in deer mice. By comparing the profiles of these responses in deer mice and humans, it is possible that therapeutic targets can be identified in humans infected with hantaviruses. For HPS, treatment strategies must function quickly because of the rapidity of patient decline; death can occur within hours after medical treatment is sought [5,41]. Although antiviral drugs may be developed against the virus, it is likely that an immunomodulatory approach that prevents or reverses pulmonary inflammation would be useful in treating patients.
We believe that the primer sets developed in this work can be used to amplify orthologous sequences from a variety of rodent species. Deer mice are New World rodents, while house mice and rats (Rattus sp.) are Old World rodents. Deer mice are about 50 million years divergent (myd) from house mice, while house mice and the laboratory rat (R. rattus) are only 15–25 myd [42]. We attempted to perform phylogenetic analyses; however, the short fragments representing most of the genes possessed insufficient data for meaningful interpretation. We are currently pursuing additional sequence data to address this deficiency (Palmer et al., manuscript in preparation).
Conclusions
We have cloned a number of deer mouse cytokine and chemokine cDNA sequences. These represent several components of immune responses, including transition from innate to adaptive immunity, type I and type II responses, chemotaxis, and a cytokine useful for producing mature monocytic cells from bone marrow. The availability of these sequences will allow the characterization of a portion of the cytokine and chemokine responses in deer mice acutely or persistently infected with SNV.
Methods
Establishment of deer mouse colony
Deer mice were trapped under license from the Colorado Division of Wildlife. Sherman live traps were set at a site where deer mice are known to occur (N 38° 59' 18.9", W 108° 17' 13.3") [12] and baited as described elsewhere [13]. Ten captured deer mice (P. maniculatus nebrascensis) were trapped and bled from the retro-orbital capillary beds and tested for IgG antibody by a standard enzyme-linked immunosorbent assay (ELISA) [43]. Each deer mouse was quarantined outdoors in the shade in buried (10 cm) 20 L plastic buckets with ventilated sealed lids, bedding, lab mouse chow, and apple slices for water and inspected daily. None of the animals died during the quarantine period. One deer mouse was seropositive at capture and was discarded. At day 30 of quarantine, the deer mice were again bled and tested for IgG antibody to SNV by ELISA and still were seronegative. On day 36 of the quarantine, the 9 uninfected deer mice were transported to Mesa State College where they were used to establish a colony with approval of the Institutional Animal Care and Use Committee.
Cloning and sequencing of deer mouse cytokines and chemokines by directed RT-PCR
The procedure for cloning deer mouse genes has been described elsewhere [11]. Briefly, total RNA was extracted from splenocyte cultures stimulated with concanavalin A or lipopolysaccharide and reverse transcribed with oligo-dT primers (Superscript II, Invitrogen, Carlsbad, CA). Degenerate primer sets were designed from highly conserved orthologous sequences from other rodent species and humans (Table 2). First attempts for PCR amplification (PCR Core Kit, Qiagen, Valencia, CA) were done with 58°C annealing temperature. If products were not amplified, PCR was repeated with 5 cycles of 58°C annealing, 5 cycles of 54°C annealing, then 27 cycles with 58°C annealing. Amplicons were cloned into pGEM-T Easy (Promega, Madison, WI) and transformed into NovaBlue competent cells (Novagen, Madison, WI). In some instances it was necessary to gel-purify PCR products (NucleoTrap, CLONTech, Palo Alto, CA). Colonies were screened by PCR and those with inserts were grown in LB/amp broth and the plasmids sequenced (Big Dye Terminator, Applied Biosystems Inc., Foster City, CA) using an ABI 310 DNA Analyzer. Sequences from three independent clones for each gene were assembled using Sequencher 4 software and identified by BLAST against house mouse entries in Genbank. House mouse, rat and human sequences (Table 3) were downloaded from the NCBI and used for comparative analysis with deer mouse sequences using the clustal algorithm [44] in MacVector software (Accelerys, San Diego, CA) and its default translation table. Amino acid gaps were considered nonidentities.
Table 2.
Primer sets used to clone deer mouse cDNA sequences.
| Gene | Forward Primer1 | Reverse Primer |
| IL-2 | ATG TAC AGC AKG CAG CTC GC | TGT TGA GAT GRY RCT TTG AC |
| IL-6 | GAG RGR AGA CTT CAC AGA GG | CAG GAT ATR TTT TCT GAC CAC AG |
| IL-12a | RAC CAC CTC AST TYG GCC AG | TGG TAC ATC TTC AAG TCY TC |
| IL-13 | CAA YRG CAG CAT GGT ATG GAG | STG GGC YAC YTC GAT TTT GG |
| IL-21 | GTA GTC ATC TTC TTG GGG AC | CTT TCT AGG AAT TCT TTG GG |
| IL-23a | AGC CAG ATC TGA GAA GCA GG | CTG CTC CRT GGG CAA AGA CC |
| GM-CSF | GTA GAK GCC ATC AAA GAA GC | AGG CRC CMT TGA GTT TGG TG |
| CCL2 | ATC ACC AGC AGC ARG TGT CC | RRT CAC ACT AGT TCW CTG TC |
| CCL3 | SAG ACC AGC AGC CTT TGC TC | RRT GTG GCT ACT TGG CAG C |
| CCL4 | ACC ATG AAG CTC TGC CTG TC | RTA CTC ATT GAC CCA GGG C |
| CXCL2 | GAC RGA AGT CAT AGC CAC TC | TCA GGW ACG ATC CAG GCT TC |
1Sequences are listed 5' to 3'. IUPAC nomenclature: K = G or T; R = A or G; Y = C or T; S = C or G; M = A or C; W = A or T.
Table 3.
Accession numbers of polypeptide sequences used for alignments in this work.
| Gene | Homo sapiens | Rattus rattus | Mus musculus | Peromyscus maniculatus |
| IL-2 | 1009281A | NP_446288 | X01772 | AY247760 |
| IL-6 | P05231 | NP_036721 | NM_031168 | AY256518 |
| IL-12a | NP_000873 | NP_445842 | NM_008351 | AY247763 |
| IL-13 | P35225 | NP_446280 | NM_008355 | AY311480 |
| IL-21 | NP_068575 | XP_227047 | NM_021782 | AY247761 |
| IL-23a | NP_057668 | NP_569094 | NM_031252 | AY259629 |
| GM-CSF | NP_000749 | P48750 | X02333 | AY247762 |
| CCL2 | NP_002973 | NP_113718 | NM_011333 | AY271904 |
| CCL3 | P10147 | AAA80608 | NM_011337 | AY247759 |
| CCL4 | P13236 | P50230 | NM_013652 | AY247758 |
| CXCL2 | NP_002080 | NP_446099 | NM_009140 | AY271905 |
Table 1.
Deer mouse cytokine and chemokine cDNA sequences compared to house mouse, rat, and human orthologs.
| Similarity (%) to:2 | |||||
| Gene | Size (bp) | Est. coding region (%)1 | House mouse | Rat | Human |
| IL-2 | 456 | 98 | 82 | 89 | 80 |
| IL-6 | 225 | 25 | 70 | 83 | 40 |
| IL-12a | 185 | 28 | 74 | 75 | 69 |
| IL-13 | 184 | 47 | 84 | 80 | 71 |
| IL-21 | 371 | 84 | 86 | 84 | 70 |
| IL-23a | 579 | 94 | 86 | 91 | 83 |
| GM-CSF | 175 | 39 | 73 | 69 | 62 |
| CCL2 | 297 | 60 | 75 | 82 | 70 |
| CCL3 | 260 | 45 | 90 | 88 | 81 |
| CCL4 | 257 | 92 | 87 | 88 | 86 |
| CXCL2 | 198 | 49 | 92 | 92 | 69 |
1Estimated coding region is based upon length of house mouse polypeptide. 2Similarity is based upon amino acid comparisons, where gaps are treated as nonidentities, except as noted in the text for IL-2 (house mouse) and CCL2 (human).
List of Abbreviations
IL, interleukin; CC, CC chemokine; CXC, CXC chemokine; GM-CSF, granulocyte macrophage-colony stimulating factor; SNV, Sin Nombre virus; TNF, tumor necrosis factor; IFNγ, interferon-γ ; LT, lymphotoxin
Authors' Contributions
TS provided conceptual efforts on establishment of the colony and the overall cloning strategy and cloned the IL-13, IL-23a, CCL2 and CXCL2 genes. RG cloned and sequenced the IL-6 gene. BD cloned and sequenced the GM-CSF gene. AB cloned the IL-2 and IL-21 genes. TH cloned and sequenced the IL-12a, CCL3 and CCL4 genes. JJR supervised the trapping and bled the deer mice. FD cared for the deer mice during the quarantine period. CHC and BJB performed diagnostic testing of the deer mouse serum samples for SNV infection.
Acknowledgments
Acknowledgments
We are grateful to R. Mackie for assistance during the trapping of the deer mice and A. D~N. Palmer for preliminary phylogenetic analyses. This work was supported by funds from the βββ Biological Honor Society (BD, RG, AB, TR), and the Mesa State College School of Natural Sciences and Mathematics (TS). Additional funding was provided by NIH grant AI25489.
Contributor Information
Tony Schountz, Email: tschount@mesastate.edu.
Renata Green, Email: rmgrenata@hotmail.com.
Bennett Davenport, Email: bennettdavenport@hotmail.com.
Amie Buniger, Email: aburns@mesastate.edu.
Tiffany Richens, Email: trichens@colostate.edu.
J Jeffrey Root, Email: jroot@colostate.edu.
Forbes Davidson, Email: davidson@mesastate.edu.
Charles H Calisher, Email: calisher@cybersafe.net.
Barry J Beaty, Email: bbeaty@colostate.edu.
References
- Hughes JM, Peters CJ, Cohen ML, Mahy BW. Hantavirus pulmonary syndrome: an emerging infectious disease. Science. 1993;262:850–851. doi: 10.1126/science.8235607. [DOI] [PubMed] [Google Scholar]
- Elliott LH, Ksiazek TG, Rollin PE, Spiropoulou CF, Morzunov S, Monroe M, Goldsmith CS, Humphrey CD, Zaki SR, Krebs JW, et al. Isolation of the causative agent of hantavirus pulmonary syndrome. Am J Trop Med Hyg. 1994;51:102–108. doi: 10.4269/ajtmh.1994.51.102. [DOI] [PubMed] [Google Scholar]
- Nichol ST, Spiropoulou CF, Morzunov S, Rollin PE, Ksiazek TG, Feldmann H, Sanchez A, Childs J, Zaki S, Peters CJ. Genetic identification of a hantavirus associated with an outbreak of acute respiratory illness. Science. 1993;262:914–917. doi: 10.1126/science.8235615. [DOI] [PubMed] [Google Scholar]
- Mills JN, Childs JE. Ecologic studies of rodent reservoirs: their relevance for human health. Emerg Infect Dis. 1998;4:529–537. doi: 10.3201/eid0404.980403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki SR, Greer PW, Coffield LM, Goldsmith CS, Nolte KB, Foucar K, Feddersen RM, Zumwalt RE, Miller GL, Khan AS, et al. Hantavirus pulmonary syndrome. Pathogenesis of an emerging infectious disease. Am J Pathol. 1995;146:552–579. [PMC free article] [PubMed] [Google Scholar]
- Mori M, Rothman AL, Kurane I, Montoya JM, Nolte KB, Norman JE, Waite DC, Koster FT, Ennis FA. High levels of cytokine-producing cells in the lung tissues of patients with fatal hantavirus pulmonary syndrome. J Infect Dis. 1999;179:295–302. doi: 10.1086/314597. [DOI] [PubMed] [Google Scholar]
- Botten J, Mirowsky K, Kusewitt D, Bharadwaj M, Yee J, Ricci R, Feddersen RM, Hjelle B. Experimental infection model for Sin Nombre hantavirus in the deer mouse (Peromyscus maniculatus) Proc Natl Acad Sci U S A. 2000;97:10578–10583. doi: 10.1073/pnas.180197197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botten J, Mirowsky K, Kusewitt D, Ye C, Gottlieb K, Prescott J, Hjelle B. Persistent Sin Nombre Virus Infection in the deer mouse (Peromyscus maniculatus) Model: Sites of Replication and strand-specific expression. J Virol. 2003;77:1540–1550. doi: 10.1128/JVI.77.2.1540-1550.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Hjelle B, Lanzi R, Morris C, Anderson B, Jenison S. Antibody responses to Four Corners hantavirus infections in the deer mouse (Peromyscus maniculatus): identification of an immunodominant region of the viral nucleocapsid protein. J Virol. 1995;69:1939–1943. doi: 10.1128/jvi.69.3.1939-1943.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn J, Schountz T. Discrimination of Peromyscus maniculatus leukocytes by flow cytometry. BIOS. 2003;74:79–86. [Google Scholar]
- Herbst MM, Prescott J, Palmer AD, Schountz T. Sequence and expression analysis of deer mouse interferon-gamma, interleukin-10, tumor necrosis factor, and lymphotoxin alpha. Cytokine. 2002;17:203–213. doi: 10.1006/cyto.2001.0998. [DOI] [PubMed] [Google Scholar]
- Root JJ, Black IV WC, Calisher CH, Wilson KR, Mackie RS, Schountz T, Mills JN, Beaty BJ. Analyses of gene flow among populations of deer mice (Peromyscus maniculatus) at sites near hantavirus pulmonary syndrome case-patient residences. J Wildlife Dis. 2003;39:287–298. doi: 10.7589/0090-3558-39.2.287. [DOI] [PubMed] [Google Scholar]
- Calisher CH, Sweeney W, Mills JN, Beaty BJ. Natural history of Sin Nombre virus in western Colorado. Emerg Infect Dis. 1999;5:126–134. doi: 10.3201/eid0501.990115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botten J, Ricci R, Hjelle B. Establishment of a deer mouse (Peromyscus maniculatus rufinus) breeding colony from wild-caught founders: comparison of reproductive performance of wild-caught and laboratory-reared pairs. Comp Med. 2001;51:314–318. [PubMed] [Google Scholar]
- Brandhuber BJ, Boone T, Kenney WC, McKay DB. Three-dimensional structure of interleukin-2. Science. 1987;238:1707–1709. doi: 10.1126/science.3500515. [DOI] [PubMed] [Google Scholar]
- Robb RJ, Greene WC. Internalization of interleukin 2 is mediated by high-affinity interleukin 2 receptor. J Exp Med. 1987;165:1201–1206. doi: 10.1084/jem.165.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers W, Stahl M, Seehra JS. 1.9 A crystal structure of interleukin 6: receptor dimerization and signaling. Embo J. 1997;16:989–997. doi: 10.1093/emboj/16.5.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paonessa G, Graziani R, De Serio A, Savino R, Ciapponi L, Lahm A, Salvati AL, Toniatti C, Ciliberto G. Two distinct and independent sites on IL-6 trigger gp 130 dimer formation and signalling. Embo J. 1995;14:1942–1951. doi: 10.1002/j.1460-2075.1995.tb07186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savino R, Lahm A, Giorgio M, Cabibbo A, Tramontano A, Ciliberto G. Saturation mutagenesis of the human interleukin-6 receptor binding site: implications for its three-dimensional structure. Proc Natl Acad Sci U S A. 1993;90:4067. doi: 10.1073/pnas.90.9.4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon C, Johnston SC, Tang J, Stahl M, Tobin JF, Somers WS. Charged residues dominate a unique interlocking topography in the heterodimeric cytokine interleukin-12. Embo J. 2000;19:3530–3541. doi: 10.1093/emboj/19.14.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JP, Debinski W. Mutants of interleukin 13 with altered reactivity toward interleukin 13 receptors. J Biol Chem. 1999;274:29944–29950. doi: 10.1074/jbc.274.42.29944. [DOI] [PubMed] [Google Scholar]
- Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, Johnston J, Madden K, Xu W, West J, Schrader S, Burkhead S, Heipel M, Brandt C, Kuijper JL, Kramer J, Conklin D, Presnell SR, Berry J, Shiota F, Bort S, Hambly K, Mudri S, Clegg C, Moore M, Grant FJ, Lofton-Day C, Gilbert T, Rayond F, Ching A, Yao L, Smith D, Webster P, Whitmore T, Maurer M, Kaushansky K, Holly RD, Foster D. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- Parrish-Novak J, Foster DC, Holly RD, Clegg CH. Interleukin-21 and the IL-21 receptor: novel effectors of NK and T cell responses. J Leukoc Biol. 2002;72:856–863. [PubMed] [Google Scholar]
- Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams JS, Moore KW, Rennick D, de Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/S1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- Walter MR, Cook WJ, Ealick SE, Nagabhushan TL, Trotta PP, Bugg CE. Three-dimensional structure of recombinant human colony-stimulating factor. J Mol Biol. 1992;224:1075–1085. doi: 10.1016/0022-2836(92)90470-5. [DOI] [PubMed] [Google Scholar]
- Diederichs K, Boone T, Karplus PA. Novel fold and putative receptor binding site of colony-stimulating factor. Science. 1991;254:1779–1782. doi: 10.1126/science.1837174. [DOI] [PubMed] [Google Scholar]
- Handel TM, Domaille PJ. Heteronuclear (1H, 13C, 15N) NMR assignments and solution structure of the monocyte chemoattractant protein-1 (MCP-1) dimer. Biochemistry. 1996;35:6569–6584. doi: 10.1021/bi9602270. [DOI] [PubMed] [Google Scholar]
- Czaplewski LG, McKeating J, Craven CJ, Higgins LD, Appay V, Brown A, Dudgeon T, Howard LA, Meyers T, Owen J, Palan SR, Tan P, Wilson G, Woods NR, Heyworth CM, Lord BI, Brotherton D, Christison R, Craig S, Cribbes S, Edwards RM, Evans SJ, Gilbert R, Morgan P, Hunter MG, et al. Identification of amino acid residues critical for aggregation of human CC chemokines macrophage inflammatory protein (MIP)-1alpha, MIP-1beta, and RANTES. Characterization of active disaggregated chemokine variants. J Biol Chem. 1999;274:16077–16084. doi: 10.1074/jbc.274.23.16077. [DOI] [PubMed] [Google Scholar]
- Shao W, Jerva LF, West J, Lolis E, Schweitzer BI. Solution structure of murine macrophage inflammatory protein-2. Biochemistry. 1998;37:8303–8313. doi: 10.1021/bi980112r. [DOI] [PubMed] [Google Scholar]
- Yokota T, Arai N, Lee F, Rennick D, Mosmann T, Arai K. Use of a cDNA expression vector for isolation of mouse interleukin 2 cDNA clones: expression of T-cell growth-factor activity after transfection of monkey cells. Proc Natl Acad Sci U S A. 1985;82:68–72. doi: 10.1073/pnas.82.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- Nedwin GE, Svedersky LP, Bringman TS, Palladino M. A., Jr., Goeddel DV. Effect of interleukin 2, interferon-gamma, and mitogens on the production of tumor necrosis factors alpha and beta. J Immunol. 1985;135:2492–2497. [PubMed] [Google Scholar]
- Kasaian MT, Whitters MJ, Carter LL, Lowe LD, Jussif JM, Deng B, Johnson KA, Witek JS, Senices M, Konz RF, Wurster AL, Donaldson DD, Collins M, Young DA, Grusby MJ. IL-21 limits NK cell responses and promotes antigen-specific T cell activation: a mediator of the transition from innate to adaptive immunity. Immunity. 2002;16:559–569. doi: 10.1016/S1074-7613(02)00295-9. [DOI] [PubMed] [Google Scholar]
- Strengell M, Sareneva T, Foster D, Julkunen I, Matikainen S. IL-21 up-regulates the expression of genes associated with innate immunity and Th1 response. J Immunol. 2002;169:3600–3605. doi: 10.4049/jimmunol.169.7.3600. [DOI] [PubMed] [Google Scholar]
- Raftery MJ, Kraus AA, Ulrich R, Kruger DH, Schonrich G. Hantavirus infection of dendritic cells. J Virol. 2002;76:10724–10733. doi: 10.1128/JVI.76.21.10724-10733.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gately MK, Desai BB, Wolitzky AG, Quinn PM, Dwyer CM, Podlaski FJ, Familletti PC, Sinigaglia F, Chizonnite R, Gubler U, et al. Regulation of human lymphocyte proliferation by a heterodimeric cytokine, IL-12 (cytotoxic lymphocyte maturation factor) J Immunol. 1991;147:874–882. [PubMed] [Google Scholar]
- Murphy KM, Ouyang W, Farrar JD, Yang J, Ranganath S, Asnagli H, Afkarian M, Murphy TL. Signaling and transcription in T helper development. Annu Rev Immunol. 2000;18:451–494. doi: 10.1146/annurev.immunol.18.1.451. [DOI] [PubMed] [Google Scholar]
- Hirano T, Yasukawa K, Harada H, Taga T, Watanabe Y, Matsuda T, Kashiwamura S, Nakajima K, Koyama K, Iwamatsu A, et al. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986;324:73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- McKenzie AN, Culpepper JA, de Waal Malefyt R, Briere F, Punnonen J, Aversa G, Sato A, Dang W, Cocks BG, Menon S, et al. Interleukin 13, a T-cell-derived cytokine that regulates human monocyte and B-cell function. Proc Natl Acad Sci U S A. 1993;90:3735–3739. doi: 10.1073/pnas.90.8.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punnonen J, Aversa G, Cocks BG, McKenzie AN, Menon S, Zurawski G, de Waal Malefyt R, de Vries JE. Interleukin 13 induces interleukin 4-independent IgG4 and IgE synthesis and CD23 expression by human B cells. Proc Natl Acad Sci U S A. 1993;90:3730–3734. doi: 10.1073/pnas.90.8.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters CJ, Simpson GL, Levy H. Spectrum of hantavirus infection: hemorrhagic fever with renal syndrome and hantavirus pulmonary syndrome. Annu Rev Med. 1999;50:531–545. doi: 10.1146/annurev.med.50.1.531. [DOI] [PubMed] [Google Scholar]
- Crew MD, Bates LM, Douglass CA, York JL. Expressed Peromyscus maniculatus (Pema) MHC class I genes: evolutionary implications and the identification of a gene encoding a Qa1-like antigen. Immunogenetics. 1996;44:177–185. doi: 10.1007/s002510050109. [DOI] [PubMed] [Google Scholar]
- Feldmann H, Sanchez A, Morzunov S, Spiropoulou CF, Rollin PE, Ksiazek TG, Peters CJ, Nichol ST. Utilization of autopsy RNA for the synthesis of the nucleocapsid antigen of a newly recognized virus associated with hantavirus pulmonary syndrome. Virus Res. 1993;30:351–367. doi: 10.1016/0168-1702(93)90101-R. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1006/jmbi.1990.9999. [DOI] [PubMed] [Google Scholar]