Abstract
Objective
An increasing proportion of gonorrhea in the United States is diagnosed in the private sector, posing a challenge to existing national surveillance systems. We described gonorrhea epidemiology outside sexually transmitted disease (STD) clinic settings.
Methods
Through the STD Surveillance Network (SSuN), health departments in the San Francisco, Seattle, Denver, Minneapolis, and Richmond, Virginia, metropolitan areas interviewed systematic samples of men and women reported with gonorrhea by non-STD clinic providers from 2006 through 2008.
Results
Of 2,138 interviews, 10.0% were from San Francisco, 26.4% were from Seattle, 25.2% were from Denver, 22.9% were from Minneapolis, and 15.5% were from Richmond. A total of 1,165 women were interviewed; 70.1% (815/1,163) were ≤24 years of age, 51.3% (598/1,165) were non-Hispanic black, and 19.0% (213/1,121) reported recent incarceration of self or sex partner. Among 610 men who have sex with only women, 50.9% were ≤24 years of age, 65.1% were non-Hispanic black, 14.1% reported incarceration of self or sex partner, and 16.7% reported anonymous sex. Among 363 men who have sex with men (MSM), 20.9% were ≤24 years of age, 61.6% were non-Hispanic white, 39.8% reported anonymous sex, 35.7% reported using the Internet to meet sex partners, and 12.1% reported methamphetamine use.
Conclusions
These data identified two concurrent gonorrhea epidemics in minority populations: a young, black, heterosexual epidemic with frequently reported recent incarceration, and an older, mostly white MSM epidemic with more frequently reported anonymous sex, Internet use to meet sex partners, and methamphetamine use.
Untreated Neisseria gonorrhoeae (N. gonorrhoeae) infection may lead to pelvic inflammatory disease, ectopic pregnancy, infertility, and chronic pelvic pain.1 In addition, infection with N. gonorrhoeae increases the risk of human immunodeficiency virus (HIV) transmission and acquisition.2 Gonorrhea, a reportable disease in all 50 U.S. states, is the second most common notifiable disease.3 In 2008, approximately 350,000 gonorrhea cases were reported to the Centers for Disease Control and Prevention (CDC), with a national rate of 111.6 cases per 100,000 population.4
Despite the large number of individuals affected and the severity of the sequelae of gonorrhea, national surveillance data contain only basic information on the gender, age, race/ethnicity, and county of residence of individuals with gonorrhea. In 2008, 77% of all reported cases of gonorrhea came from providers outside the sexually transmitted disease (STD) clinic and 54% were among women. National case-report data show that gonorrhea rates vary widely by race/ethnicity and residence. In 2009, rates were 20 times higher among non-Hispanic black people than among non-Hispanic white people, with estimates suggesting three cases of gonorrhea for every 100 black 15- to 19-year-old women. In 2008, 22.3% of 3,141 counties in the U.S. had gonorrhea rates that were higher than 100 cases per 100,000 population, with the majority of these cases concentrated in the South.4 In addition to limited data on infected individuals, current gonorrhea surveillance is limited by incomplete reporting. Many infections are asymptomatic and, thus, go undiagnosed; diagnosed infections may be unreported; and information on reported cases is often incomplete.5 In 2008, for example, race or ethnicity was missing on 20.3% of case reports.4
In addition to case-report data, another source of national data on patients with gonorrhea has been through the Gonococcal Isolate Surveillance Project (GISP), a sentinel surveillance project that collects gonococcal specimens and demographic, behavioral, and clinical data on men diagnosed with gonorrhea in approximately 30 STD clinics. GISP has been an important source for understanding trends in gonococcal susceptibility and characteristics of men who seek care for gonorrhea at publicly funded STD clinics. However, GISP does not provide information on women with gonorrhea, nor on the approximately 70% of men who receive care for gonorrhea from providers other than STD clinics.4
To better support the prevention and control of gonorrhea, additional as well as more complete information is needed on a broad cross-section of affected individuals on a routine basis. In response to such surveillance needs, CDC established in 2005 the STD Surveillance Network (SSuN),6 a dynamic STD surveillance network comprising a number of local health departments following common protocols to collect, analyze, and interpret disease data. The purpose of the SSuN was to fill critical gaps in national surveillance and improve the capacity of state and local STD programs to act rapidly.
In this descriptive analysis, enhanced gonorrhea surveillance data from SSuN Cycle 1 (2005–2008) were used to describe the epidemiology of gonorrhea among men and women diagnosed outside the STD clinic setting in five geographically diverse metropolitan areas in the U.S. We discuss implications of the findings for gonorrhea-control programs and future gonorrhea surveillance activities.
METHODS
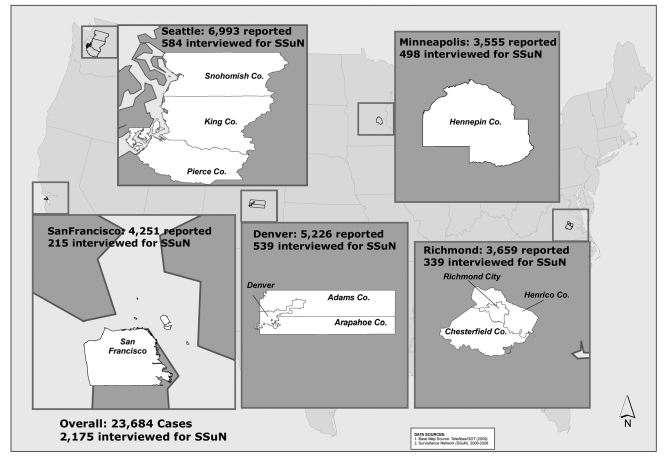
Participating SSuN Cycle 1 sites identified one or more counties in which to conduct enhanced gonorrhea surveillance activities. For this analysis, the term “metropolitan area” was used to informally describe the clusters of counties in which surveillance activities were conducted around the cities of San Francisco, California; Seattle, Washington; Denver, Colorado; Minneapolis, Minnesota; and Richmond, Virginia (Figure). The data-collection time period varied by metropolitan area: San Francisco (August 1, 2006, to August 5, 2008), Seattle (April 1, 2006, to December 31, 2008), Denver (March 1, 2006, to December 31, 2008), Minneapolis (July 1, 2006, to August 31, 2008), and Richmond (January 1, 2007, to December 31, 2008).
Figure.
Number of gonorrhea cases reported in five metropolitan areas and number of gonorrhea cases interviewed through the SSuN, 2006–2008
SSuN = Sexually Transmitted Disease Surveillance Network
Each site collected a common set of demographic, clinical, and behavioral data on a sample of patients reported with gonorrhea. In Denver, Minneapolis, Richmond, and Seattle, the first patients reported by providers or laboratories to the health department in participating counties each calendar month were selected for interview until 10 male and 10 female patients were interviewed. In San Francisco, a variable proportion of patients was sampled from all cases reported by providers or laboratories on a weekly basis, with adjustments made for nonresponse, for a target interviewed sample size of 100 men and 100 women annually. All sites excluded patients reported by STD clinics from SSuN county sampling, as the SSuN had a separate system of data collection for STD clinic patients.
To integrate with existing disease-control activities, interviews of SSuN patients were conducted by health department staff using locally developed data-collection instruments. However, data-collection instruments from all sites used a common set of demographic, clinical, and behavioral variables. Prior to initiation, this activity was determined to be a non-research surveillance activity by the CDC National Center for HIV, Viral Hepatitis, STD, and Tuberculosis Prevention.
We examined SSuN interview data from March 1, 2006, through December 31, 2008. For this evaluation, we defined men who have sex with men (MSM) as men who either reported sex with a man in the three months before being tested for gonorrhea (asked in all areas) or did not report the sex of their partner and reported that they were gay/homosexual or bisexual (asked only in Denver and San Francisco). We defined men who have sex only with women (MSW) as men who reported sex only with women in the three months before being tested for gonorrhea or did not report being gay/homosexual or bisexual. Race and ethnicity were coded separately in data-collection instruments at all sites but were combined for this analysis, such that any patient who reported being of Hispanic ethnicity was classified as Hispanic irrespective of reported race.
All analyses were stratified by gender and sexual behavior or sexual orientation. Because only nine MSW in San Francisco and 17 MSM in Richmond were interviewed, data on these patients are not presented in any analysis stratified by sexual behavior or orientation to avoid over-interpretation of data from samples that were too small to be reliable. We assessed representativeness using Chi-square statistics comparing the proportions of SSuN-interviewed patients #24 years of age and of non-Hispanic black race/ethnicity with those of non-STD clinic non-interviewed patients. Chi-square statistics were also used to assess the significance of differences in characteristics among women, MSW, and MSM. We conducted all analyses using SAS® version 9.1.7
RESULTS
The five metropolitan areas received case reports of 23,684 individuals with gonorrhea during the surveillance period (range by site: 3,555 in Minneapolis to 6,993 in Seattle) (Figure). Of these gonorrhea case reports, 6,623 were reported by the primary STD clinics and excluded. Of the remaining 17,061 cases, 10,006 were selected for SSuN interviews, of whom 2,175 were successfully interviewed. This figure corresponded to an interview success rate of 21.7% overall, with slight variation by site: Seattle = 19.0%, Richmond = 19.2%, Denver = 20.0%, San Francisco = 27.1%, and Minneapolis = 29.6% (data not shown).
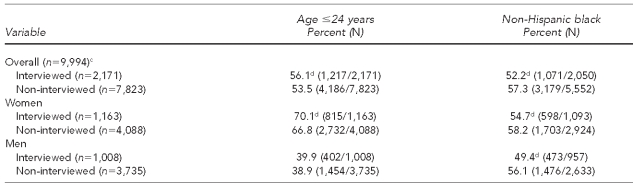
To better understand how a low response rate might be affecting the SSuN sample, we compared SSuN-interviewed patients with patients who were selected for interview but could not be interviewed for any reason (e.g., four interviewed patients were removed from analysis: two due to lack of information on age and two due to gender reported as transgender). Interviewed and non-interviewed men were of similar age (39.9% of interviewed men and 38.9% of non-interviewed men were #24 years of age); however, interviewed men were significantly less likely to be non-Hispanic black than were non-interviewed men (49.4% vs. 56.1%, respectively). Compared with non-interviewed women, interviewed women were significantly more likely to be #24 years of age (70.1% vs. 66.8%, respectively) and less likely to be non-Hispanic black (54.7% vs. 58.2%, respectively) (Table 1).
Table 1.
Comparison of age and race/ethnicity between interviewed patients and patients who were selected but not intervieweda through the STD Surveillance Network,b 2006–2008
aDue to missing race/ethnicity, 121 patients were excluded.
bThe STD Surveillance Network comprised health departments in the San Francisco, Seattle, Denver, Minneapolis, and Richmond, Virginia, metropolitan areas.
cTwo interviewed patients were excluded due to missing age, and two interviewed patients were excluded due to gender reported as transgender.
dStatistically significant at p<0.05 between interviewed and non-interviewed patients
STD = sexually transmitted disease
We excluded 37 males (3.8%) from the analysis who had missing gender of sex partners and unknown sexual orientation. Of 2,138 interviews, 10.0% were from San Francisco, 26.4% were from Seattle, 25.2% were from Denver, 22.9% were from Minneapolis, and 15.5% were from Richmond. Consistent with the SSuN sampling methodology, which was stratified by gender, 1,165 of 2,138 SSuN interviews (54.5%) were women, 610 were MSW (28.5%), and 363 were MSM (17.0%). Of 973 men reporting either gender of sex partners, sexual orientation, or both, the proportion of men with gonorrhea who were MSM varied widely by site: San Francisco (92.0%), Seattle (43.8%), Denver (29.6%), Minneapolis (25.6%), and Richmond (12.5%) (data not shown).
Women
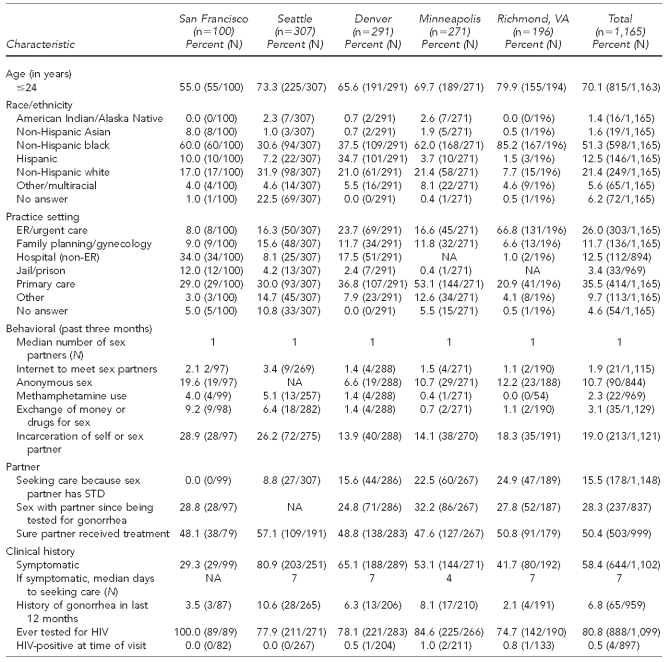
A total of 1,165 women with gonorrhea were interviewed through the SSuN (range by site: 100 in San Francisco to 307 in Seattle); 70.1% were #24 years of age (range by site: 55.0% in San Francisco to 79.9% in Richmond). Overall, the majority of interviewed women were non-Hispanic black (51.3%), although this percentage varied widely from 30.6% in Seattle to 85.2% in Richmond. Overall, 21.4% of interviewed women were non-Hispanic white; this proportion ranged from 7.7% in Richmond to 31.9% in Seattle. Denver was the only area where a sizable proportion of women reported Hispanic ethnicity (34.7%) (Table 2).
Table 2.
Reported characteristics of women with gonorrhea interviewed in the STD Surveillance Network, 2006–2008
STD = sexually transmitted disease
ER = emergency room
NA = not available
HIV = human immunodeficiency virus
Overall, the most common practice setting reporting women with gonorrhea was primary care (35.5%). However, a significant proportion of women in Richmond were diagnosed in emergency room (ER)/urgent care settings (66.8%); in San Francisco, 34.0% of women were diagnosed in non-ER hospital settings and 12.0% were diagnosed in jails/prisons. Overall, 80.8% of women reported ever being tested for HIV (range by site: 74.7% in Richmond to 100.0% in San Francisco) and 0.5% reported being HIV-positive. A total of 6.8% of women had a history of gonorrhea in the past 12 months (Table 2).
Symptoms of gonorrhea were reported by 58.4% of women, though this percentage varied widely by site (29.3% in San Francisco to 80.9% in Seattle). Women reporting symptoms said they waited a median of seven days (individual range: 0–365) before seeking care. A total of 15.5% of women reported seeking care because a sex partner had an STD, though this figure also varied widely by site (range: 0.0% in San Francisco to 24.9% in Richmond). Among women, 28.3% reported having sex with their partner since being diagnosed with gonorrhea. Only 50.4% of women reported being sure that their partner had been treated, with minimal variation by site for both issues (Table 2).
Incarceration of self or sex partner in the past three months was reported by 19.0% of women with gonorrhea interviewed through the SSuN (range by site: 13.9% in Minneapolis to 28.9% in San Francisco). All other measured risk factors were relatively uncommon among women with gonorrhea. Although uncommon in all sites, West Coast sites (San Francisco and Seattle) reported slightly higher Internet use to meet sex partners, anonymous sex, exchange of money or drugs for sex, and methamphetamine use (Table 2).
MSW
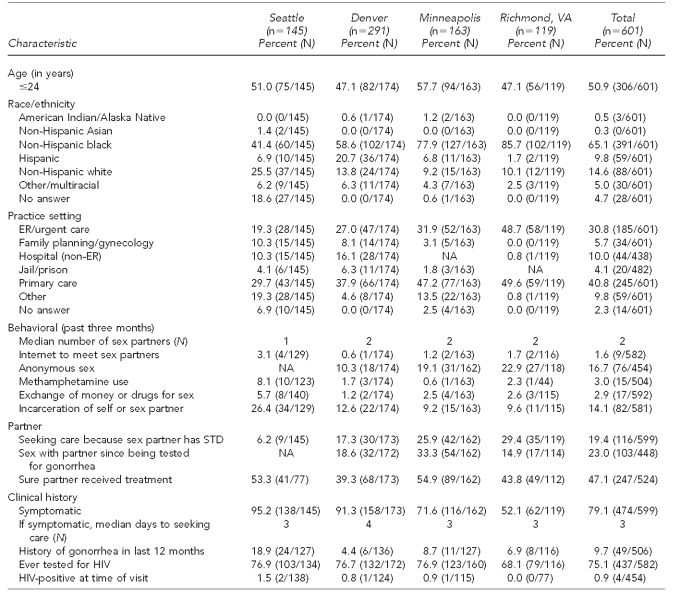
A total of 601 MSW reported with gonorrhea were interviewed through the SSuN (range by site: 119 in Richmond to 163 in Minneapolis; total excludes nine MSW from San Francisco). Of these 601 MSW, 50.9% were #24 years of age. The majority of MSW with gonorrhea in all SSuN sites were non-Hispanic black (65.1%), though this figure ranged from 41.4% in Seattle to 85.7% in Richmond. Seattle was the only site with a sizable proportion (25.5%) of non-Hispanic white MSW. In Denver, 20.7% of MSW were Hispanic (Table 3).
Table 3.
Reported characteristics of MSW with gonorrhea interviewed in the STD Surveillance Network, 2006–2008a
aData for nine MSW in San Francisco were not included due to small numbers.
MSW = men who have sex with only women
STD = sexually transmitted disease
ER = emergency room
NA = not available
HIV = human immunodeficiency virus
MSW with gonorrhea presented most frequently to primary care providers (40.8%; range by site: 29.7% in Seattle to 49.6% in Richmond). However, all SSuN sites also reported a large proportion of MSW from ERs/urgent care centers (30.8%; range by site: 19.3% in Seattle to 48.7% in Richmond). By self-report, 75.1% of all MSW had ever been tested for HIV, but only 0.9% were HIV-positive at the time of current visit. Though 9.7% of MSW reported a history of gonorrhea in the last 12 months, this figure ranged from 4.4% in Denver to 18.9% in Seattle. At the time of diagnosis, 79.1% of all MSW reported symptoms of gonorrhea (range by site: 52.1% in Richmond to 95.2% in Seattle); the median number of days before seeking care for symptomatic men was three (individual range: 0–120) (Table 3).
Overall, 19.4% of MSW sought care because a sex partner had an STD. Among MSW, 23.0% reported having had sex since being tested for gonorrhea. Only 47.1% of MSW were sure that their partner had received treatment for gonorrhea (Table 3).
Risk behaviors reported by MSW varied across SSuN sites. Overall, the most common risk behaviors reported were anonymous sex (16.7%) and incarceration of self or sex partner (14.1%). The median reported number of sex partners for MSW was two in the past three months. In general, methamphetamine use, exchange of sex for drugs or money, and use of the Internet to meet sex partners were uncommonly reported by MSW (Table 3).
MSM
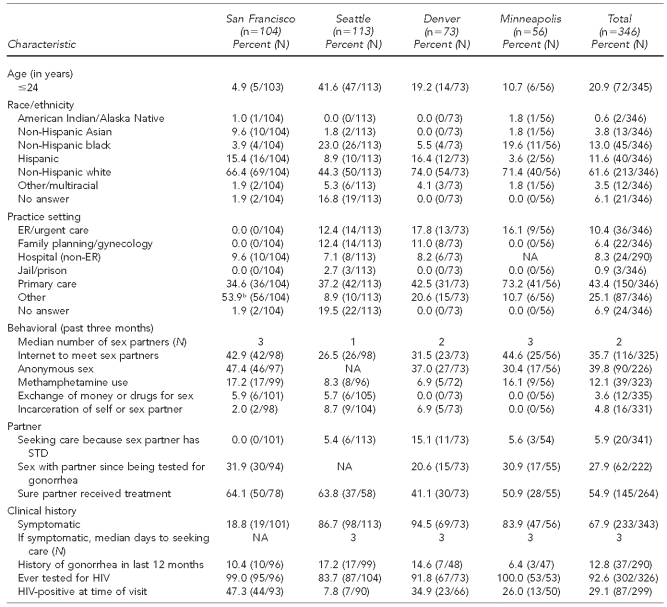
A total of 346 MSM reported with gonorrhea were interviewed through the SSuN (range by site: 56 in Minneapolis to 113 in Seattle; total excludes 17 MSM from Richmond); 20.9% were #24 years of age. The majority of MSM reported with gonorrhea in all SSuN sites were non-Hispanic white (61.6%; range by site: 44.3% in Seattle to 74.0% in Denver). Only Seattle and Minneapolis reported any significant proportions of non-Hispanic black MSM (23.0% and 19.6%, respectively) (Table 4).
Table 4.
Reported characteristics of MSM with gonorrhea interviewed in the STD Surveillance Network, 2006–2008a
aData for 17 MSM in Richmond, Virginia, were not included due to small numbers.
b“Other” in San Francisco included a gay men’s health center.
NA = not available
MSM = men who have sex with men
STD = sexually transmitted disease
ER = emergency room
NA = not available
HIV = human immunodeficiency virus
Although overall the most common practice setting reporting MSM with gonorrhea was primary care (43.4%; range by site: 34.6% in San Francisco to 73.2% in Minneapolis), 53.9% of MSM with gonorrhea in San Francisco were reported by other providers, principally a gay men’s health center. Overall, 92.6% of MSM had ever been tested for HIV. Self-reported HIV positivity at the time of visit was 29.1% overall, ranging from 7.8% in Seattle to 47.3% in San Francisco. A history of gonorrhea in the past 12 months was reported by 12.8% of MSM with gonorrhea.
Overall, 67.9% of MSM reported symptoms of gonorrhea; however, in San Francisco, only 18.8% of MSM were symptomatic compared with Minneapolis, Seattle, and Denver, where 83.9%, 86.7%, and 94.5% of MSM, respectively, were symptomatic. For symptomatic MSM, the median number of days before seeking care was three (individual range: 0–210) (Table 4). Rectal infection was reported among 37.5% of MSM in San Francisco, 12.5% of MSM in Minneapolis, 5.5% of MSM in Denver, and 5.3% of MSM in Seattle. Pharyngeal infection was reported among 40.4% of MSM in San Francisco, 3.5% of MSM in Seattle, 2.7% of MSM in Denver, and 1.8% of MSM in Minneapolis. It was not possible to assess the underlying differences in screening practices among areas in this SSuN database (data not shown).
Overall, only 5.9% of MSM sought care because a sex partner had an STD. A total of 27.9% of MSM had had sex since being tested for gonorrhea, yet only 54.9% were sure their partner had received treatment (Table 4).
Overall, both anonymous sex and use of the Internet to meet sex partners were more likely to be reported at all sites (39.8% and 35.7%, respectively) compared with women and MSW. Reported methamphetamine use was also high in San Francisco and Minneapolis (17.2% and 16.1%, respectively). Overall, incarceration of self or sex partner (4.8%) and exchange of money and drugs for sex (3.6%) were rarely reported.
DISCUSSION
The SSuN project provides critical epidemiologic data to supplement limited data that are routinely collected from passive case-based national surveillance. Data presented in this study suggest that in the metropolitan areas that participate in the SSuN, two overlapping epidemics of gonorrhea are occurring simultaneously in minority populations: one among young, black heterosexuals and another among older, mostly white MSM. Identifying these concurrent epidemics from nationally reportable data would not have been possible, as gender of sex partners is not routinely collected or reported to CDC.
In SSuN interviews, heterosexual gonorrhea patients were significantly more likely than MSM to report recent incarceration of themselves or their partners (p<0.0001, data not shown), whereas MSM reported much higher frequencies of methamphetamine use (p<0.0001), use of the Internet to meet sex partners (p<0.0001), and anonymous sex (p<0.0001) than did heterosexual gonorrhea patients. HIV co-infections were rare among heterosexual SSuN patients; however, more than one in four MSM with gonorrhea reported being co-infected with HIV (p<0.0001). MSM were significantly more likely to report having been tested for HIV (p<0.0001). ER providers diagnosed a greater proportion of women and heterosexual men than MSM (p<0.0001), and symptomatic women waited longer than symptomatic men before seeking care (p<0.0001).
Such findings suggest that a one-size-fits-all approach to gonorrhea prevention and control efforts is likely to be ineffective. A health department responsible for a population in which patients with gonorrhea are primarily women and heterosexual men may want to ensure that prevention, screening, and treatment services are available and acceptable to populations that are young and black. If services are either not available or acceptable to these populations, then health departments may need to identify means of reaching these populations. For example, efforts to increase screening and treatment in incarceration facilities may have a greater impact on a young minority heterosexual epidemic than an older MSM epidemic. Given that more than one-quarter of heterosexuals with gonorrhea were diagnosed in ER and urgent care settings, targeted screening of adolescents seeking care in these settings should be considered.8
In contrast, health departments with a significant population of MSM with gonorrhea should use different strategies for the prevention, screening, and treatment of MSM. For example, to reach MSM, health departments could consider screening at known MSM meeting venues, using the Internet to target prevention messages to MSM who are meeting partners on the Internet, or working with substance abuse programs to provide prevention counseling messages or gonorrhea screening for their clients. Ensuring that extragenital testing is widely available and STD screening is integrated into routine HIV care may be useful activities for local gonorrhea-control programs.
Health departments may have a combination of heterosexual and MSM epidemics. In an ideal world with unlimited resources, gonorrhea-control efforts could be rolled out widely for both populations. However, given that gonorrhea is rarely a health priority, most health departments have resources for only a limited number of interventions. To target control measures to reduce health disparities, it is necessary to have information on which populations to target. SSuN data suggest that gender of sex partner is a fundamentally important category of information for understanding a gonorrhea epidemic; all health departments are encouraged to more routinely collect data on gender of sex partners through case-based surveillance.
Currently, national case-report data for gonorrhea do not routinely include gender of sex partner. In 2007, gender of sex partner was unknown for 91.9% of gonorrhea morbidity reports received at CDC (Unpublished data, CDC, Division of STD Prevention, 2010). It is possible that health departments have this information available locally but are not transmitting it nationally. However, it is more likely that gender of sex partner is not recorded routinely on reporting forms in many states either because the form does not allow for the collection of such data or the data are not provided by the reporting clinician or laboratory. Many health departments rely heavily on laboratory reports, and unlike traditional demographic information (e.g., age, gender, and race/ethnicity), gender of sex partner may not be collected routinely for administrative purposes. For syphilis reporting, many health departments overcome this limitation by interviewing reported patients. However, given the significantly larger case burden for gonorrhea, few health departments have the resources to routinely interview patients with gonorrhea. Through supplemental surveillance platforms such as the SSuN, additional epidemiologic information can be collected outside of the traditional, passive case-based surveillance system to better address health disparities. Several innovative approaches to enhance gonorrhea surveillance, such as the use of geocoding, spatial analysis, and tablet personal computers for interviewing, have been explored and described in a variety of settings.9,10
Limitations
This study was subject to several limitations. As similar projects have found, successfully locating and interviewing patients with gonorrhea can be challenging.11,12 SSuN sites were able to successfully interview only approximately one out of every five patients selected for interview, despite determined efforts to improve the response rate. All SSuN sites attempted to contact patients at least three and sometimes up to 10 times through a variety of methods, including different times of day and different days of the week. In the overwhelming majority of cases, the reason for non-interview was that the patient could not or refused to be located by phone (77.5%). Of note, of those patients who were successfully contacted by SSuN staff, only 3.7% of patients declined to be interviewed, 1.4% quit before the interview was completed, and 1.0% had a language barrier to being interviewed. Although interviews were generally conducted in English, interviewers in at least three sites were able to conduct interviews in Spanish, as necessary.
Informal discussions with SSuN interviewers suggest that poor contact information was a frequent barrier, especially among case reports originating from laboratories. Poor contact information could be the result of incomplete collection of data by health personnel; intentional provision of invalid contact information on the part of a patient (to avoid association with the stigma of having an STD); or unintentionally provided invalid contact information related to young age, poverty, or other factors associated with the economic or social instability of many patients with gonorrhea.13–15
The low response rate encountered by the SSuN also introduced a potential response bias to the analysis. This response bias was examined through comparison of age and race for SSuN-interviewed patients with patients who were selected but could not be interviewed for any reason. Compared with nonrespondents, SSuN participants were more likely to be white, and female participants were more likely to be young. In addition, there were likely other differences between the two groups that could not be assessed. Given the paucity of population-based epidemiologic data on gonorrhea in the U.S., however, these data are still informative and useful for guiding public health interventions to control gonorrhea to an extent not possible through routine case-report data.
An additional limitation to SSuN methodology and resultant data was that the gonorrhea surveillance activity in SSuN Cycle 1 described in this article was limited to five metropolitan areas that were not randomly selected. Therefore, SSuN patient data should not be construed as representing all non-STD clinic patients with gonorrhea in the U.S. SSuN Cycle 2 is currently underway in 12 geographically diverse metropolitan areas that account for more than 20% of all U.S. gonorrhea morbidity and is, therefore, likely to improve how well SSuN data reflect the gonorrhea epidemic across the U.S.
SSuN collaborators developed a common protocol used by all sites for the collection of supplemental epidemiologic data. However, SSuN surveillance activities were integrated into existing systems, not implemented as a formal, independent research protocol. As a result, each SSuN site had its own local protocols, and SSuN activities were implemented in the context of existing diagnosis, treatment, and prevention interventions. For example, screening of the pharynx and rectum of patients reporting exposure at these sites is widely practiced in San Francisco, thus providing one possible explanation for why a greater proportion of MSM from San Francisco had reported pharyngeal and rectal gonorrhea.16 Another example of the possible effect of using data from a surveillance rather than a research activity can be seen in the widely varying proportion of women presenting with symptoms (29.3% in San Francisco to 80.9% in Richmond), as the proportion of symptomatic women will be strongly affected by how much gonorrhea screening of asymptomatic women is conducted in the clinic.
To ensure a common definition across all SSuN sites for the sexual orientation of patients, a three-month time frame for elicitation of the sex of sexual partners was used. However, male patients who had sex with a man more than three months prior to the interview or who did not report being gay or bisexual would, therefore, be classified for this analysis as MSW, thus potentially introducing a classification bias.
CONCLUSIONS
Despite these limitations, the data obtained from the SSuN provide information that cannot be obtained through routine surveillance for targeting of gonorrhea-control activities in five metropolitan areas. The analysis shows the differences and similarities among the gonorrhea epidemic in five different areas. In addition, the SSuN experience is useful for providing a better understanding of how to collect data on patients reported with gonorrhea. The data tell a tale of two different epidemics among minority populations: one of a young, black, heterosexual epidemic with frequently reported incarceration, and another of an older, white, MSM epidemic with high levels of HIV coinfection and high-risk sexual behaviors. Such findings suggest that a one-size-fits-all approach to gonorrhea may not be effective in reducing health disparities. Rather, health departments should improve data collection on gender of sex partners and other key risk behaviors to allow for targeting of gonorrhea-control interventions to the most affected populations.
Footnotes
The STD Surveillance Network (SSuN) was established through funds by the Centers for Disease Control and Prevention (CDC) grant #AA-055. These activities were also supported in part by SSuN-participating state and city health departments: San Francisco Department of Public Health, Colorado Department of Public Health and Environment, Minnesota Department of Health, Washington State Department of Health, and Virginia Department of Health.
This article was written on behalf of the many SSuN collaborators who contributed to the data collection, management, and implementation of this project. The authors thank Darlene Davis for managing the national SSuN database, Brian Lewis for his assistance in mapping, and Robert Nelson for his programming support.
No protocol approval was needed for this study because data were obtained from existing sources. The SSuN was determined by CDC to be a non-research public health activity to control disease and conduct surveillance. The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of CDC.
REFERENCES
- 1.Hook EW, III, Handsfield HH. Holmes KK, Sparling PF, Stamm WE, Piot P, Wasserheit JN, Corey L, et al. Sexually transmitted diseases. 4th ed. New York: McGraw-Hill; 2008. Gonococcal infections in the adult; pp. 627–45. [Google Scholar]
- 2.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75:3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall-Baker PA, Nieves E, Jr, Jajosky RA, Adams DA, Sharp P, Anderson WJ, et al. Summary of notifiable diseases—United States, 2008. MMWR Morb Mortal Wkly Rep. 2010;57(54):1–94. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (US), Division of STD Prevention. Atlanta: CDC; 2009. Sexually transmitted disease surveillance, 2008. November. [Google Scholar]
- 5.Turner CF, Rogers SM, Miller HG, Miller WC, Gribble JN, Chromy JR, et al. Untreated gonococcal and chlamydial infection in a probability sample of adults. JAMA. 2002;287:726–33. doi: 10.1001/jama.287.6.726. [DOI] [PubMed] [Google Scholar]
- 6.Rietmeijer CA, Donnelly J, Bernstein KT, Bissette JM, Martins S, Pathela P, et al. Here comes the SSuN: early experiences with the STD Surveillance Network. Public Health Rep. 2009;124(Suppl 2):72–7. doi: 10.1177/00333549091240S211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.SAS Institute Inc. SAS®: Version 9.1 for Windows. Cary (NC): SAS Institute, Inc.; 2003. [Google Scholar]
- 8.Newman LM, Donnelly J, Marcus J, Martins S, Stenger M, Stover J, et al. Use of emergency medical facilities for the treatment of Neisseria gonorrhoeae infection in the United States. Presented at the 18th International Society for Sexually Transmitted Diseases Research; 2009 Jun 28-Jul 1; London.
- 9.Gaffga NH, Samuel MC, Stenger MR, Stover JA, Newman LM. The OASIS Project: novel approaches to using STD surveillance data. Public Health Rep. 2009;124(Suppl 2):1–4. doi: 10.1177/00333549091240S203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stover JA. Use of tablet PCs in enhanced gonorrhea surveillance. [cited 2011 Dec 21]. Presented at the 2006 National STD Prevention Conference; 2006 May 8-11; Jacksonville, Florida. Also available from: URL: http://cdc.confex.com/cdc/std2006/techprogram/P10773.htm.
- 11.Malotte CK, Ledsky R, Hogben M, Larro M, Middlestadt S, St Lawrence JS, et al. Comparison of methods to increase repeat testing in persons treated for gonorrhea and/or Chlamydia at public sexually transmitted disease clinics. Sex Transm Dis. 2004;31:637–42. doi: 10.1097/01.olq.0000143083.38684.9d. [DOI] [PubMed] [Google Scholar]
- 12.Bernstein KT, Zenilman J, Olthoff G, Marsiglia VC, Erbelding EJ. Gonorrhea reinfection among sexually transmitted disease clinic attendees in Baltimore, Maryland. Sex Transm Dis. 2006;33:80–6. doi: 10.1097/01.olq.0000187233.53622.8a. [DOI] [PubMed] [Google Scholar]
- 13.Sionéan C, DiClemente RJ, Wingood GM, Crosby R, Cobb BK, Harrington K, et al. Socioeconomic status and self-reported gonorrhea among African American female adolescents. Sex Transm Dis. 2001;28:236–9. doi: 10.1097/00007435-200104000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Rice RJ, Roberts PL, Handsfield HH, Holmes KK. Sociodemographic distribution of gonorrhea incidence: implications for prevention and behavioral research. Am J Public Health. 1991;81:1252–8. doi: 10.2105/ajph.81.10.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dolan C, Delcher C. Monitoring health inequities and planning in Virginia: poverty, human immunodeficiency virus, and sexually transmitted infections. Sex Transm Dis. 2008;35:981–4. doi: 10.1097/OLQ.0b013e318182a571. [DOI] [PubMed] [Google Scholar]
- 16.San Francisco Department of Public Health, STD Prevention and Control Services. STD screening and diagnostic testing guidelines in community settings. April 2009. [cited 2011 Nov 22]. Available from: URL: http://www.sfcityclinic.org/providers/SFDPH_STDScreeningRecs2009v2.pdf.