Colorectal cancer (CRC) was the second leading cause of cancer death among men and women nationally and in Maryland in 2000.1,2 African Americans in Maryland had an age-adjusted CRC mortality rate that was 1.4 times that of white people (31.1 per 100,000 population vs. 22.1 per 100,000 population for black and white people, respectively).2 American Cancer Society (ACS) recommendations for CRC screening published in 1997 provided a menu of screening options.3 Of these, fecal occult blood test (FOBT) and sigmoidoscopy screening have been covered under Medicare Part B since 1998. In 2001, Medicare Part B initiated coverage for colonoscopy screening.4 In 1999, only 29.1% of Marylanders aged 50–64 years reported having an FOBT in the last year, and only 41.2% had a sigmoidoscopy or colonoscopy within the previous five years.5 Screening disparities existed with lower screening rates in individuals with lower income, without health insurance, and of minority race/ethnicity.6
Although CRC screening has been shown to reduce mortality,7,8 prior to 2000, only New York State had initiated a public health screening program that used FOBT for screening its low-income uninsured population.9 In 2002, the U.S. Preventive Services Task Force (USPSTF) recommended CRC screening for those aged 50 years and older.10 Tests and intervals had been further defined by the ACS, and screening test options for those at average risk included (1) annual FOBT, (2) flexible sigmoidoscopy every five years, (3) annual FOBT with flexible sigmoidoscopy every five years, (4) double-contrast barium enema (DCBE) every five years, or (5) colonoscopy every 10 years. While FOBT may detect bleeding from some larger, advanced adenomas and CRCs, non-bleeding lesions can only be found and removed during sigmoidoscopy and colonoscopy. For those at increased risk of CRC or with symptoms, colonoscopy is recommended.
Following the multistate Master Tobacco Settlement Agreement, Maryland established the Cigarette Restitution Fund (CRF) in 2000.11 The goal of the CRF is to decrease cancer mortality and reduce racial/ethnic disparities in cancer mortality. The Maryland Department of Health and Mental Hygiene (MDHMH) initially developed and now supervises a statewide cancer-control program administered by its local health departments (LHDs). Under the CRF law, each of Maryland's 24 LHDs formed a community health coalition that helped determine the cancers targeted for screening. In addition, Maryland funded local minority outreach and technical assistance (MOTA) programs to ensure the participation of racial/ethnic minority groups in the CRF cancer and tobacco programs in the 17 jurisdictions with the highest percentage of minority residents. Because screening for CRC was recommended but underutilized,6,12 23 jurisdictions initially targeted CRC; Baltimore City chose other cancers for its screening program.
We report on the first eight years of this nearly statewide program and evaluate the feasibility, outcomes, and lessons learned from CRC screening targeting low-income, uninsured, medically underserved, and minority adults.
METHODS
Program development
The MDHMH convened a CRC Medical Advisory Committee, comprising a medical oncologist, three gastroenterologists, a pathologist, a radiation oncologist, and two primary care providers, to formulate medical guidelines for the program. The guidelines were based on national guidelines3,13 and stated that local programs should offer CRC screening either by colonoscopy or by FOBT with flexible sigmoidoscopy to clients who met income and health insurance eligibility requirements. Some programs also offered FOBT screening without endoscopy to clients who did not meet income or insurance eligibility requirements for endoscopy; however, programs instructed these clients to see their doctor and complete their screening with either sigmoidoscopy or colonoscopy. DCBE was an alternative but was not chosen by providers as a first-line screening test. Each LHD decided on the screening method(s), income eligibility requirements (a maximum of 250% of the federal poverty level [FPL]), and whether to accept clients with gastrointestinal (GI) symptoms or a history of neoplasia.
The MDHMH awarded CRF funding to each LHD;11 managed local grants; provided clinical, technical, and administrative guidance; developed a clinical and management database; and distributed CRC fact sheets, consent forms, and templates of medical provider contracts. To provide outreach and education about screening opportunities to the public and providers, LHDs hired staff or contracted with other organizations and partnered with local MOTA programs. Emphasis was placed on outreach to minority and medically underserved adults.
LHDs hired clinical case-management staff and signed contracts with private doctors, endoscopy facilities, laboratories, and hospitals for physical exams, sigmoidoscopy, colonoscopy, DCBE, pathology, and other clinical services. Contracts required providers to follow the CRC state guidelines and accept Medicare rates for reimbursement of screening procedures (including colonoscopy and biopsy). For half of the jurisdictions that had sufficient funds to pay for diagnosis (beyond colonoscopy and biopsy) or treatment when cancer was detected, diagnosis and treatment services were paid at Medicaid rates. Programs without sufficient funds for treatment linked clients to treatment by helping with Medicaid applications or by helping arrange hospital charity care, uncompensated care, and payment plans.
Client enrollment, consent, and case management
Local programs recruited eligible clients through outreach to existing LHD clients (e.g., federally funded breast and cervical cancer screening clients and their male partners) at LHD clinics (e.g., influenza vaccination clinics); through referral from primary care providers, colonoscopists, or MOTA programs; at community sites or events (e.g., churches and health fairs); and through the media (e.g., television, radio, print media, and flyers).
Each client screened with only FOBT signed a consent form that acknowledged the limitations of the FOBT and the need for additional visualization of the colon to complete the screening. Each client who was screened with FOBT, sigmoidoscopy, colonoscopy, or DCBE signed a consent form acknowledging that the screening data would be shared with MDHMH and its data contractor to be used for quality assurance and program evaluation. In those LHDs not covering diagnosis and treatment costs, consent acknowledged that the program would link to care but would not cover these costs if further tests or treatments were needed. Medical providers ordered bowel preparation and obtained clinical consent for the procedures performed. Either providers or program staff explained bowel preparation to clients.
The MDHMH established case-management guidelines for appropriate follow-up and referral of clients based on the test(s) and results. Local case managers navigated the client through screening with reminders about appointments and bowel preparation, follow-up calls, and transportation; collected and entered data from patients and providers; notified clients of results; and recalled clients for their next CRC test. MDHMH oversaw the quality of clinical and case-management services of medical providers and the local program staff through data reviews and site visits.
Data collection and analysis
MDHMH established standard data elements and forms. Data included self-reported demographic information, personal and family CRC risk factors, symptoms, and previous history of CRC screening. A client was considered at increased risk for CRC if he or she had a personal history of CRC, adenoma(s), or a polyp of unknown type, and/or a family history of CRC, adenoma(s), or a polyp of unknown type.14 We included a history of polyp of unknown type in our definition of increased risk so we would not miss those who were at increased risk when the clients' pathology results were not known. A client was considered at average risk if he or she had no increased risk for CRC.
Screening procedure results, pathology results (if any), unplanned events or complications of the procedure(s), and treatment and staging of diagnosed cancers were recorded in the database. Programs accepted the providers' customary reports; providers were not required to complete additional standardized forms. Adverse events that occurred during the procedure and required termination of the procedure, or occurred within 30 days following endoscopy and required medical evaluation, were considered endoscopic complications.
Data were entered into the secure, confidential, intranet-based client database (CDB). For quality assurance, on clients with cancer or adenoma(s), MDHMH compared coded data in the CDB with the text of colonoscopy and pathology reports to assure the accuracy of data entry for lesion size and histology.
We analyzed data from the initial screening cycle for each client during the first eight years of the program. We categorized each screening into one of four categories based on the most advanced procedure the client had in the cycle using the following hierarchy (from most advanced to least advanced): colonoscopy (with or without FOBT or other procedures in the cycle), sigmoidoscopy (with or without FOBT and without colonoscopy), DCBE (with or without FOBT and without colonoscopy or sigmoidoscopy), or FOBT only.
Based on gross and histologic findings of all procedures in a cycle, a final diagnosis for the screening cycle was assigned as the most advanced finding of the following: colorectal adenocarcinoma, high-risk adenoma, low-risk adenoma, hyperplastic polyp, other finding, or “normal” colonoscopy (i.e., a colonoscopy without these findings). A high-risk adenoma was one with high-grade dysplasia, villous or tubulovillous histology, ≥10 millimeters (mm) in diameter, or noted as large on the colonoscopy report. A low-risk adenoma was one with tubular histology without a villous component and <10 mm in diameter. Other findings included hemorrhoids, diverticula, other non-neoplastic diagnoses (e.g., inflammatory bowel disease and lymphoid aggregates), and polyps that were noted on the colonoscopy report but either had no pathology results (i.e., those that were ablated or lost during retrieval or processing) or were noted as normal mucosa on pathology. In clients with more than one lesion, the final diagnosis and adenoma classification were based on the most advanced lesion. Anal and rectal squamous cell carcinomas, lymphoma, and carcinoid tumors of the colon or rectum were categorized separately for this analysis.
We conducted the analysis using Microsoft® Excel and SAS® version 9.2.15 We examined differences in client characteristics by screening test using Chi-square statistics.
RESULTS
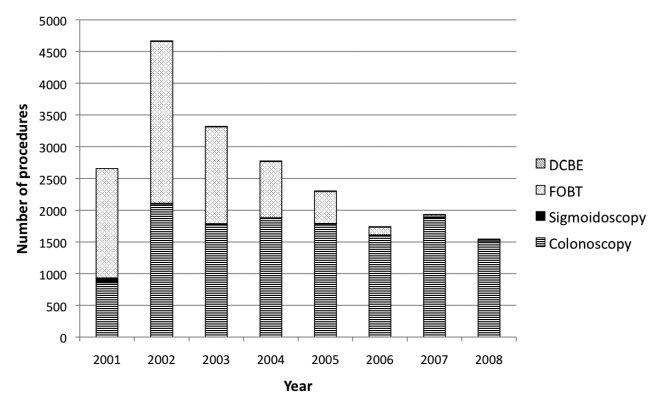
In 2000, colonoscopy was selected as the primary CRC screening method for those at average risk in four programs, FOBT with flexible sigmoidoscopy was selected as the primary CRC screening method in 18 programs, and FOBT alone was selected as the primary screening method in one program. Positive FOBTs were followed by colonoscopy in the program for those who were eligible; those who were not eligible were referred to their own providers. Figure 1 shows the distribution of CRC tests by year. By 2004, the primary CRC screening method became colonoscopy in 22 programs, and one program stopped CRC screening. Ten of the 22 programs additionally offered FOBT to those who were not eligible for colonoscopy in their program or to those who were eligible for but not yet willing to accept colonoscopy.
Figure 1.
Number of colorectal cancer testing procedures, by year: Maryland Cigarette Restitution Fund program, 2000–2008
DCBE = double-contrast barium enema
FOBT = fecal occult blood test
Programs contracted with 189 endoscopists statewide to perform colonoscopy, including individual gastroenterologists or gastroenterology practices (n=161) and surgeons or surgical practices (n=28). All 23 programs chose to enroll clients regardless of pre-enrollment CRC screening and results; 22 programs enrolled clients who reported GI symptoms, including GI bleeding.
From July 1, 2000, to December 31, 2008, the LHDs enrolled 17,065 clients. They recorded 20,951 screening cycles (17,065 first screening cycles and 3,886 subsequent cycles) and performed 13,588 colonoscopies, 155 sigmoidoscopies, 227 DCBEs, and 8,316 FOBT procedures.
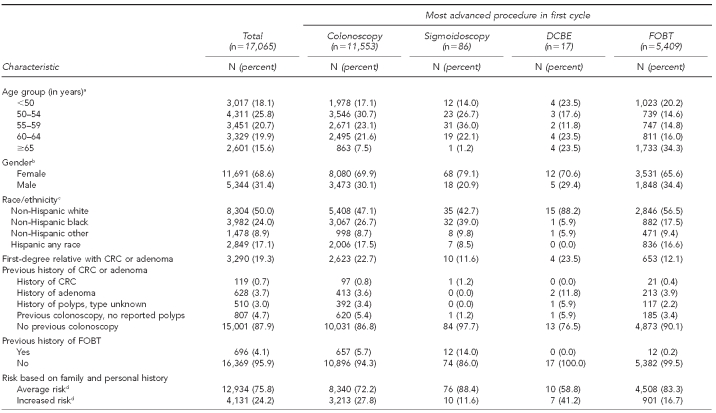
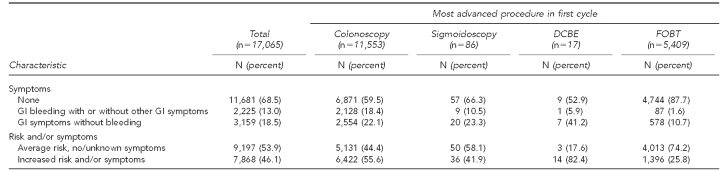
The Table shows the demographics, risk history, and symptoms of the 17,065 clients categorized by the most advanced procedure that the individual had in his or her first cycle. Overall, 81.9% of the clients were aged 50 years or older, 68.6% were female, and 50.0% were of a racial/ethnic minority group. Those considered at increased risk for CRC accounted for 24.2% of the total, based on having a first-degree relative with CRC or adenoma (19.3%) and/or a personal history of CRC (0.7%), adenoma (3.7%), or unspecified type of polyp (3.0%). At enrollment, 68.5% reported no GI symptoms, 13.0% noted one or more episodes of rectal bleeding or blood in the stool (with or without other GI symptoms), and 18.5% reported GI symptoms without bleeding (i.e., lower abdominal pain, change in bowel habits, unexplained weight loss, or other symptoms). Of all clients screened, 53.9% were of average CRC risk without GI symptoms on enrollment to the first cycle. Colonoscopy was the most advanced procedure in 11,553 (67.7%) cycles, sigmoidoscopy in 86 (0.5%) cycles, DCBE in 17 (0.1%) cycles, and FOBT in 5,409 (31.7%) cycles.
Table.
Demographic and colorectal cancer risk characteristics for 17,065 clients, by most advanced screening procedure in the first cycle: Maryland Cigarette Restitution Fund program, 2000–2008
aAge was missing for 356 clients with FOBT.
bGender was missing for 30 clients with FOBT.
cRace/ethnicity was missing for 74 clients with colonoscopy, four clients with sigmoidoscopy, and 374 clients with FOBT.
dAverage risk was defined as all clients without increased risk of CRC. Increased risk was defined as a client with a personal history of CRC, adenoma(s), polyp of unknown type, and/or a family history of CRC, adenoma, or polyp of unknown type.
DCBE = double-contrast barium enema
FOBT = fecal occult blood test
CRC = colorectal cancer
GI = gastrointestinal
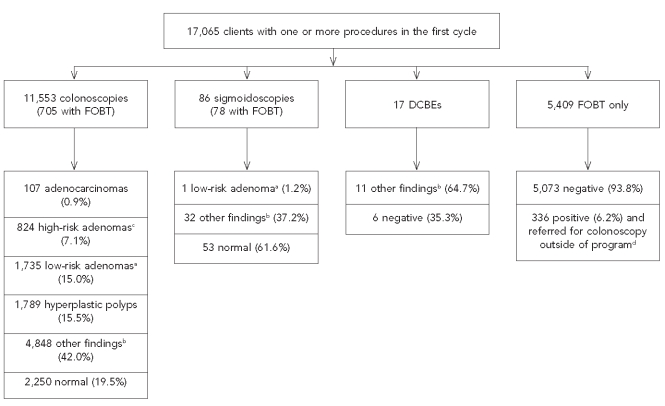
Of the initial colonoscopies in the first cycle, 7.6% were considered inadequate by the endoscopist. Figure 2 shows the final diagnosis of the first cycle of screening by the most advanced procedure paid for by the program. In addition to the 107 adenocarcinomas (Figure 2), seven carcinoid tumors, one colonic lymphoma, two rectal squamous cell carcinomas, and three anal squamous cell carcinomas were identified in the first cycle (data not shown).
Figure 2.
Flow chart of clients by the most advanced testing procedure in the first colorectal cancer testing cycle and final diagnosis or test result: Maryland Cigarette Restitution Fund program, 2000–2008
aLow-risk adenoma is a tubular histology without a villous component but <1 cm in size.
bOther findings included hemorrhoids, diverticula, inflammatory bowel disease, other non-neoplastic diagnoses, or polyps that were noted on the colonoscopy report but either had no pathology results (i.e., those that were ablated or lost during retrieval or processing) or were noted as normal mucosa on pathology.
cHigh-risk adenoma is an adenoma with high-grade dysplasia, villous component, or ≥1 cm in size.
dResults included cancer (n=6), adenomas (n=7), other findings (n=112), and referred but follow-up information not obtained (n=211).
FOBT = fecal occult blood test
DCBE = double-contrast barium enema
cm = centimeter
Of the 107 clients diagnosed with adenocarcinoma, two died of advanced disease without treatment; 55 had all or part of their treatment funded by the CRF program; and 50 were linked to treatment services paid for by Medicaid, hospital charity care, or private funds. Ninety percent of the treated clients began their treatment within 60 days of diagnosis of cancer (diagnosis made at the time of colonoscopy or at the time of a subsequent procedure) (data not shown).
Staging was reported for 87 (81.3%) of the adenocarcinomas: 16 (15.0%) were Stage 1, 23 (21.5%) were Stage 2, 27 (25.2%) were Stage 3, and 21 (19.6%) were Stage 4. The LHD did not obtain the final stage of cancer following diagnosis for 20 (18.7%) cancer cases (data not shown).
Adverse events were reported in 91 of the 13,588 colonoscopies (0.7%); 23 (0.2%) were considered major. There were six bowel perforations, one of which was associated with biopsy of a lesion, one that was associated with fulguration of a polyp, two that were associated with diverticulosis, and two that were associated with difficulty advancing the colonoscope. One client had a stroke within 30 days of the colonoscopy. There were 16 major bleeding episodes, each occurring after biopsy or polypectomy, 11 requiring hospitalization or surgery, and five requiring an emergency room visit. Minor complications included 68 events such as bleeding that did not require an emergency room visit, arrhythmia, hypo- or hypertension, intra- or post-procedure abdominal pain, nausea, vomiting, diarrhea, inability to sedate for the colonoscopy, or combativeness (data not shown).
DISCUSSION
We report the development and results of a complex, ongoing public health CRC testing program that targets low-income, uninsured, and racial/ethnic minority adults. Funding from the CRF made it possible for Maryland to implement a program that addressed many aspects foreseen in the National Colorectal Cancer Roundtable Strategic Plan.16 More than 46% of our clients were at increased risk and/or reported some degree of GI symptoms, including those with family risk, a personal history of CRC, adenomas or polyps of unknown type, and GI symptoms that were not suggestive of CRC. While most of our clients received screening colonoscopies, some were surveillance colonoscopies, and a few were diagnostic colonoscopies. We have therefore referred to this program as a testing program.
During the first eight years, the program tested more than 17,000 clients. The program successfully reached people in racial/ethnic minority groups: the percentage of clients enrolled who were aged 50 years and older and of a racial/ethnic minority group was more than twice the percentage of those residing in the counties with the CRC screening program (51% vs. 24%). The program was very effective in reaching this underserved population in Maryland: of the 13,692 clients aged 50 years and older at enrollment, 14% reported being previously tested with a colonoscopy or sigmoidoscopy compared with 63% of adults aged 50 years and older in the Maryland population in 2004.17 Based on 2004 estimates, there were 73,618 Marylanders aged 50–64 years who were uninsured,18 approximately 12% of whom received a colonoscopy in our program during 2000–2008.
If roughly 40% of high-risk adenomas progress to cancer,19,20 we estimate this program may have prevented at least 300 cases of CRC through polypectomy. The rate of major complications in our program falls within the rates cited for the systematic review of the USPSTF, released in 2008.21
Maryland's 23 local programs received guidance, administrative oversight, and funding from the MDHMH,22 but each was designed and administered locally. Local providers, hospitals, community groups, and clients in communities with and without racial/ethnic minority groups embraced this successful public-private partnership as a benefit to their communities.
Rather than excluding participants because of increased risk, prior screening, or symptoms, the local programs enrolled clients representing a cross-section of risk, symptoms, and prior medical and CRC screening histories. Despite broadening our eligibility and serving more clients in need, our cancer and adenoma detection rates are comparable with U.S. studies cited in a meta-analysis of screening colonoscopies in asymptomatic people.23 We compared the stages of CRC diagnosed in the program with stages of CRC reported in Maryland from 2001 to 2008. The proportion of cancers with known stage in the CRF program that were at the local stage (Stage 1) was 18.4% compared with 40.5% for the state; 57.5% in the CRF program were at the regional stage (25.2% Stage 2 and 19.6% Stage 3) vs. 39.5% for the state; and 24.1% in the CRF program were at the distant stage (Stage 4) vs. 20.1% for the state.24 Detecting later stages may reflect that we reached the underserved who otherwise might not have been reached for screening (especially with colonoscopy).
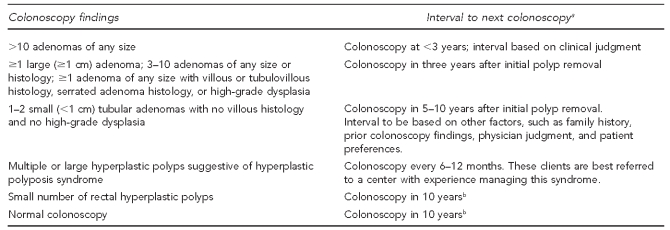
MDHMH staff assured standardization and quality by developing and monitoring standardized clinical guidelines including recall intervals based on findings (Figure 3) and standardized data elements in colonoscopy reports. The MDHMH oversaw the local programs through frequent communication; routine quality review of results and recall intervals in the client database; and annual on-site program reviews, including individual chart reviews and review of colonoscopy and pathology reports. These reviews provided feedback on whether the providers' management and recall intervals agreed with program guidelines, and whether the local programs had accurately entered data in the CDB and managed cases appropriately.
Figure 3.
Guidelines for the interval to next colonoscopy based on findings of colonoscopya per colorectal cancer minimal elements: Maryland Department of Health and Mental Hygiene, March 2009
aInterval is based on colonoscopy being complete (i.e., the bowel preparation was adequate and cecum was reached) and neoplastic lesions being completely removed. If not, repeat colonoscopies should be performed at very short intervals (0–6 months).
bInterval should take family history into account. Patients with family history of colorectal cancer or adenomatous polyps in a first-degree relative (FDR) aged <60 years or ≥2 FDRs at any age should consider repeat colonoscopy within 5–10 years.
cm = centimeter
The CRC testing methods changed over time: by 2006, 22 of 23 programs selected colonoscopy as the primary screening method (Figure 1). Factors that influenced this change included the time, expense, and difficulty of operating an annual FOBT-based screening program; the ease of case-managing clients who had no findings on colonoscopy and a 10-year recall; the inability of finding providers to perform sigmoidoscopy; the national shift toward colonoscopy as the most recommended CRC screening test;25,26 and the ability of colonoscopy to both diagnose and treat some cancers as well as prevent cancer through polypectomy.
Programs additionally found it difficult to limit FOBT distribution to asymptomatic people of average risk. Hoping to reach the largest number of people with a low-cost screening test, several programs offered FOBT kits at health fairs and community events in the program's early years. Of those who took kits, more than 20% of those screened only with an FOBT in their first cycle were younger than 50 years of age (and not eligible for average risk screening) and more than 25% reported some GI symptom or risk and should have received a colonoscopy rather than an FOBT, according to program guidelines. Also, some jurisdictions initially found that starting CRC screening with an FOBT was more acceptable to clients who were not yet ready for endoscopy. Those with positive FOBTs who were ineligible for colonoscopy in the program due to income or insurance were notified of results and referred for colonoscopy; however, follow-up information on these clients was often difficult to obtain.
The income limit for eligibility increased over time in many jurisdictions. Programs found it difficult to recruit clients who had incomes ≤100% FPL. Clients with income eligibility matching that of the federal breast and cervical cancer program in Maryland (250% FPL) were easier to locate and recruit.
Recruitment and outreach efforts included linkages with medical clinics serving low-income/uninsured patients, physician offices, outreach to low-income workers at their worksites, hair salon/barber shop outreach, flyers in Laundromats and restrooms, and door-to-door outreach in low-income and minority neighborhoods. Despite these efforts, it was more difficult to recruit men than women. The percentage of men in the screening program has increased to only 36% in the last two years of the program despite outreach efforts (data not shown).
LHD staff remained integrally involved in their clients' care after the diagnosis of cancer was made, whether or not treatment was funded by the CRF program. Anecdotally, counties that linked their clients to other payment methods for treatment invested a significant amount of time helping clients complete and submit applications for Medicaid, hospital charity care, and other sources of funding in a timely manner.
Since its inception, the Maryland program has consulted with numerous other state programs and with the Centers for Disease Control and Prevention CRC Screening Demonstration Program,27,28 which funded Baltimore City as a CRC screening demonstration site in 2005.
CONCLUSIONS
The Maryland CRF program has successfully shown that low-income and underserved members of the community will take advantage of CRC screening. We have also shown that a program based predominantly on colonoscopy is challenging but feasible. It is acceptable to clients and providers, and it is associated with good outcomes for primary and secondary prevention of CRC. If health-care reform is enacted, many underserved clients will gain access to CRC screening and other preventive services, but the public health roles of outreach, education, enrollment, and overcoming barriers through navigation will still be necessary to improve screening rates.
Acknowledgments
The authors acknowledge the Maryland Colorectal Cancer Medical Advisory Committee Members for their clinical guidance of the screening program, including Stanley Watkins, MD (Chairman) (Anne Arundel Medical Specialists, The Johns Hopkins University School of Medicine); Marshall Bedine, MD (The Johns Hopkins University School of Medicine); Anthony Calabrese, MD (Anne Arundel Gastroenterology Associates); Michael Choti, MD, MBA (The Johns Hopkins University School of Medicine); Cinthia Drachenberg, MD (University of Maryland [UM] Medical Center); Francis Giardiello, MD, MBA (The Johns Hopkins University School of Medicine); Bruce Greenwald, MD (UM School of Medicine); and Harris Yfantis, MD (Veterans Administration Maryland Health Care System).
The authors acknowledge the assistance of Carlessia Hussein, RN, DrPH, for directing the Cigarette Restitution Fund (CRF) program at the Maryland Department of Health and Mental Hygiene (MDHMH); the staff of the Center for Cancer Surveillance and Control (CCSC) at MDHMH, including Alyse Weinstein Cooper, MS, Ahmed Elmi, MPH, Donna Gugel, MHS, Catherine Musk, RN, MS, Sarah Kanchuger, RN, MPH, Maya King, MPH, Lorraine Underwood, and William Wiseman, MAHE; and former staff of the UM Department of Epidemiology and Preventive Medicine—Ebenezer Israel, MD, MPH, and Raza Hasan, MS—for their assistance in program implementation.
The Institutional Review Board (IRB) of the MDHMH exempted this program evaluation project, and the UM IRB approved the project.
REFERENCES
- 1.American Cancer Society. Cancer facts and figures, 2000. Atlanta: ACS; 2000. [Google Scholar]
- 2.Maryland Department of Health and Mental Hygiene. Annual cancer report. Baltimore: Cigarette Restitution Fund Program. 2003. [cited 2011 Oct 4]. Also available from: URL: http://fha.maryland.gov/pdf/cancer/CRF_Annual_Cancer_Report_2003.pdf.
- 3.Byers T, Levin B, Rothenberger D, Dodd DG, Smith RA. American Cancer Society guidelines for screening and surveillance for early detection of colorectal polyps and cancer: update 1997. American Cancer Society Detection and Treatment Advisory Group on Colorectal Cancer. CA Cancer J Clin. 1997;47:154–60. doi: 10.3322/canjclin.47.3.154. [DOI] [PubMed] [Google Scholar]
- 4. Medicare, Medicaid, and SCHIP Benefits Improvement and Protection Act of 2000. H.R. 5661. Pub. L. 106-554 (2000)
- 5.Trends in screening for colorectal cancer—United States, 1997 and 1999. MMWR Morb Mortal Wkly Rep. 2001;50(9):162–6. [PubMed] [Google Scholar]
- 6.Screening for colorectal cancer—United States, 1997. MMWR Morb Mortal Wkly Rep. 1999;48(6):116–21. [PubMed] [Google Scholar]
- 7.Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328:1365–71. doi: 10.1056/NEJM199305133281901. [DOI] [PubMed] [Google Scholar]
- 8.Winawer SJ, Flehinger BJ, Schottenfeld D, Miller DG. Screening for colorectal cancer with fecal occult blood testing and sigmoidoscopy. J Natl Cancer Inst. 1993;85:1311–8. doi: 10.1093/jnci/85.16.1311. [DOI] [PubMed] [Google Scholar]
- 9.Promising Practices. New York State Colorectal Cancer Screening Program. [cited 2011 Oct 4]. Available from: URL: http://promisingpractices.fightchronicdisease.org/programs/detail/new_york_state_colorectal_cancer_screening_program.
- 10.U S. Preventive Services Task Force. Screening for colorectal cancer: recommendation and rationale. Ann Intern Med. 2002;137:129–31. doi: 10.7326/0003-4819-137-2-200207160-00014. [DOI] [PubMed] [Google Scholar]
- 11. Annotated Code of Maryland, Ch. 6, Health-General §§13-1101 to 13-1119.
- 12.Guide to clinical preventive services. 2nd ed. Washington: U.S. Government Printing Office; 1996. U.S. Preventive Services Task Force. [Google Scholar]
- 13.Rex DK, Johnson DA, Lieberman DA, Burt RW, Sonnenberg A. Colorectal cancer prevention 2000: screening recommendations of the American College of Gastroenterology. Am J Gastroenterol. 2000;95:868–77. doi: 10.1111/j.1572-0241.2000.02059.x. [DOI] [PubMed] [Google Scholar]
- 14.Winawer S, Fletcher R, Rex D, Bond J, Burt R, Ferrucci J, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale—update based on new evidence. Gastroenterology. 2003;124:544–60. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]
- 15.SAS Institute, Inc. SAS®: Version 9.2. Cary (NC): SAS Institute, Inc.; 2008. [Google Scholar]
- 16.Levin B, Smith RA, Feldman GE, Colditz GA, Fletcher RH, Nadel M, et al. Promoting early detection tests for colorectal carcinoma and adenomatous polyps: a framework for action: the strategic plan of the National Colorectal Cancer Roundtable. Cancer. 2002;95:1618–28. doi: 10.1002/cncr.10890. [DOI] [PubMed] [Google Scholar]
- 17.Maryland Department of Health and Mental Hygiene. Maryland DHMH cancer-related surveillance data and reports. [cited 2011 Oct 4]. Available from: URL: http://fha.maryland.gov/cancer/surv_data-reports.cfm.
- 18.Maryland Department of Health and Mental Hygiene. Behavioral Risk Factor Surveillance System (BRFSS) (MD Health Study) [cited 2011 Oct 4]. Available from: URL: http://fha.maryland.gov/ohpp/brfss.cfm.
- 19.Winawer SJ, Zauber AG. The advanced adenomas as the primary target of screening. Gastrointest Endosc Clin N Am. 2002;12:1–9. doi: 10.1016/s1052-5157(03)00053-9. [DOI] [PubMed] [Google Scholar]
- 20.Brenner H, Hoffmeister M, Stegmaier C, Brenner G, Altenhofen L, Haug U. Risk of progression of advanced adenomas to colorectal cancer by age and sex: estimates based on 840,149 screening colonoscopies. Gut. 2007;56:1585–9. doi: 10.1136/gut.2007.122739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitlock EP, Lin JS, Liles E, Beil TL, Fu R. Screening for colorectal cancer: a targeted, updated systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:638–58. doi: 10.7326/0003-4819-149-9-200811040-00245. [DOI] [PubMed] [Google Scholar]
- 22.Maryland Department of Health and Mental Hygiene. Cigarette Restitution Fund—cancer prevention, education, screening, and treatment program, colorectal cancer screening. [cited 2011 Oct 4]. Available from: URL: http://fha.maryland.gov/cancer/crc_screening.cfm.
- 23.Niv Y, Hazazi R, Levi Z, Fraser G. Screening colonoscopy for colorectal cancer in asymptomatic people: a meta-analysis. Dig Dis Sci. 2008;53:3049–54. doi: 10.1007/s10620-008-0286-y. [DOI] [PubMed] [Google Scholar]
- 24.Maryland Department of Health and Mental Hygiene. Center for Cancer Surveillance and Control. [cited 2011 Oct 4]. Available from: URL: http://fha.maryland.gov/cancer.
- 25.Klabunde CN, Frame PS, Meadow A, Jones E, Nadel M, Vernon SW. A national survey of primary care physicians' colorectal cancer screening recommendations and practices. Prev Med. 2003;36:352–62. doi: 10.1016/s0091-7435(02)00066-x. [DOI] [PubMed] [Google Scholar]
- 26.Klabunde CN, Lanier D, Nadel MR, McLeod C, Yuan G, Vernon SW. Colorectal cancer screening by primary care physicians: recommendations and practices, 2006-2007. Am J Prev Med. 2009;37:8–16. doi: 10.1016/j.amepre.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeGroff A, Holden D, Goode Green S, Boehm J, Seeff LC, Tangka F. Start-up of the colorectal cancer screening demonstration program. Prev Chronic Dis. 2008;5:A38. [PMC free article] [PubMed] [Google Scholar]
- 28.Seeff LC, DeGroff A, Tangka F, Wanliss E, Major A, Nadel M, et al. Development of a federally funded demonstration colorectal cancer screening program. Prev Chronic Dis. 2008;5:A64. [PMC free article] [PubMed] [Google Scholar]