Abstract
Classically, glia have been regarded as non-excitable cells that provide nourishment and physical scaffolding for neurons. However, it is now generally accepted that glia are active participants in brain function that can modulate neuronal communication via several mechanisms. Investigations of anatomical plasticity in the magnocellular neuroendocrine system of the hypothalamic paraventricular and supraoptic nuclei led the way in the development of much of our understanding of glial regulation of neuronal activity. In this review, we provide an overview of glial regulation of magnocellular neuron activity from a historical perspective of the development of our knowledge of the morphological changes in evident in the paraventricular and supraoptic nuclei and focus on recent data from the authors’ laboratories that were presented at the 9th World Congress on Neurohypophysial Hormones and that have contributed to our understanding of the multiple mechanisms by which glia modulate the activity of neurons, including: gliotransmitter modulation of synaptic transmission; trans-synaptic modulation by glial neurotransmitter transporter regulation of neurotransmitter spillover; and glial neurotransmitter transporter modulation of excitability by regulation of ambient neurotransmitter levels and their action on extrasynaptic receptors. The magnocellular neuroendocrine system secretes oxytocin and vasopressin from the posterior pituitary gland to control birth, lactation and body fluid balance and we finally speculate as to whether glial regulation of individual magnocellular neurons might co-ordinate population activity to respond appropriately to altered physiological circumstances.
Keywords: Paraventricular nucleus, supraoptic nucleus, oxytocin, vasopressin, dehydration, lactation
Introduction
Historically, glia have been regarded as non-excitable cells that primarily serve a support and structural role in central nervous system function. However, investigations over the last 10–15 years have, with increasing frequency, been changing this perception. Glial cells, and in particular astrocytes, have been identified as active and dynamic participants along with neurons in generating, coordinating and sustaining electrical activity in the brain. Indeed, the field of glial signalling and neuronal-glial interactions is one of the fastest growing areas of neuroscience today, in large part thanks to work done in the magnocellular neuroendocrine system.
The magnocellular neuroendocrine cells are part of the hypothalamic-neurohypophysial neurosecretory system, and are comprised of two largely distinct populations of neurosecretory cells, oxytocin- and vasopressin-producing cells. Both the oxytocin cells and the vasopressin cells are located in the paraventricular nucleus (PVN) and supraoptic nucleus (SON) of the hypothalamus and each cell sends a single axonal projection to the posterior lobe of the pituitary gland, the neurohypophysis, where they secrete oxytocin and vasopressin into the bloodstream. Oxytocin is secreted in a pulsatile fashion during parturition and in response to suckling to induce uterine contractions and milk ejection from the mammary glands (1). Vasopressin, the antidiuretic hormone, is secreted into the blood in a graded fashion in response to a decrease in blood osmolality or blood pressure, and acts on the kidneys to increase water reabsorption and on the arteries to increase vasoconstriction (2). Both the oxytocin and vasopressin neurons display characteristic bursting behaviours when activated, although their bursting patterns are very different from one another. During reflex milk ejection and parturition, oxytocin neurons generate high-frequency bursts of action potentials that are synchronized among all the oxytocin neurons of the PVN and SON; vasopressin neurons generate a lower-frequency, episodic (‘phasic’) bursting activity that is asynchronous among the vasopressin neurons (2). The respective mechanisms of burst generation between the two cell types and burst synchronization among the oxytocin neurons have been active areas of investigation over the years.
It is now generally well accepted that glia are active participants in brain function by their ability to modulate synaptic transmission, but it has not always been so. Early seminal studies on the morphological plasticity of the hypothalamic magnocellular neuroendocrine system paved the way for much of the breakthrough work on glial-neuronal interactions of the last several years. Recent investigations of the magnocellular neuroendocrine system, the focus of this review, have been able to build on these early studies to move the field forward, and have done so by exploiting the remarkable structural plasticity of the magnocellular system that these studies revealed. The influential work being done by these investigators in the area of neuronal-glial interactions would not be possible without the foundations laid down by pioneers in the field of structural plasticity in the magnocellular system.
The magnocellular neuroendocrine system’s remarkable capacity for structural plasticity was first discovered over 30 years ago by two groups, that of the late Glenn Hatton in the U.S. and the team of Dionysia Theodosis and Dominique Poulain in France. In 1971, Tweedle and Hatton (3) reported that dehydration causes a dramatic neuronal-glial reorganization in the SON and the nucleus circularis of the hypothalamus, characterized by decreased glial content between magnocellular neurons and increased direct magnocellular neuron-to-neuron membrane contact. Theodosis and Poulain then reported that a similar neuronal-glial structural plasticity resulting in glial retraction from between neighbouring magnocellular neurons occurs in the SON during lactation (Fig. 1), which they suggested provided a morphological substrate for inter-neuronal electrical interactions that could contribute to the synchronization of oxytocin neuron bursting activity responsible for the pulsatile release of oxytocin (4). These changes were found to occur immediately prior to parturition and last through the lactation period (5), and similar changes in the interactions between magnocellular axons and pituicytes were also found to occur in the neurohypophysis (6). It was later found that these morphological changes in the glial coverage of magnocellular neurons were specific to the oxytocin neurons, which was consistent with increased synchronization of oxytocin neuron bursting (7), and that these changes were underpinned by the high estrogen levels of pregnancy (8), which also induced morphological changes in the oxytocin neurons themselves (9). While a more recent study indicated that the observed glial retraction and magnocellular neuron-to-neuron juxtaposition may not be necessary for synchronized oxytocin neuron bursting and milk ejection (10), these early studies and subsequent pivotal studies by these investigators firmly established the magnocellular neuroendocrine system as a model system for the study of neuronal-glial plasticity and for the critical role of astrocytes in the regulation of synaptic function; in this review, we focus on recent ground-breaking studies from the authors’ laboratories that were presented at the 9th World Congress on Neurohypophysial Hormones, which have expanded our understanding of neuronal-glial interactions at the synaptic and cellular level in the neurohypophysial system, and of how these interactions impact whole animal physiology.
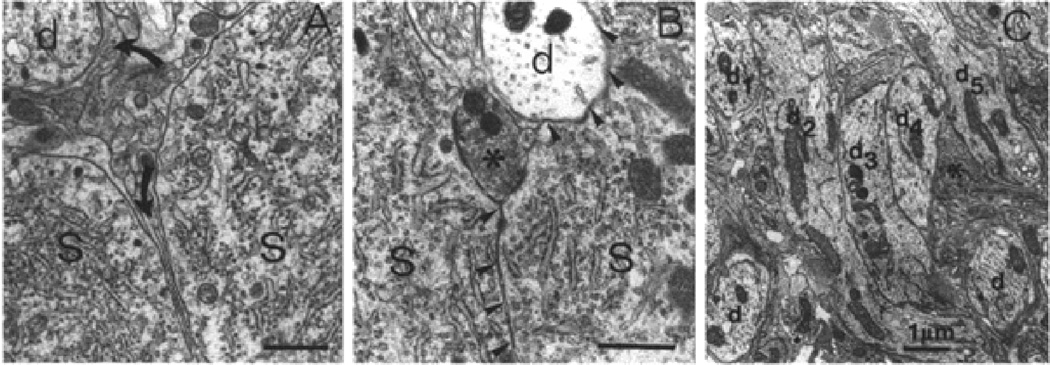
Figure 1.
Neuronal-glial plasticity in the supraoptic nucleus during lactation. A. In the non-lactating rat SON, the membranes of magnocellular neuronal somata (S) and dendrites (d) are separated by glial tissue (arrows). B. Under stimulated conditions such as lactation, glial processes retract from between magnocellular neuronal somata (S) and the plasma membranes of neighbouring magnocellular neurons come into direct contact with each other, without glial interposition. C. A similar glial retraction and direct neuronal membrane apposition occurs between dendrites, seen in a dendrite cluster in the SON nucleus. Taken from Montagnese et al., 1990 (8) with permission.
The tripartite synapse
Neurons and glia are now recognised as essential partners in the nervous system. In the peripheral nervous system, perisynaptic Schwann cells modulate dialogue between nerve and muscle at the neuromuscular junction (11–14). In the central nervous system, many types of glial cells play important roles in brain function but it is the astrocytes that are the third partner of the tripartite synapse (15–20). A single astrocyte can contact tens of thousands of synapses, yet individual astrocytes occupy distinct, non-overlapping domains within the brain (21, 22) suggesting that astrocytes do not respond to individual neurons, but more likely control information flow through their allocated volume domains. Pioneering work on the tripartite synapse established that astrocytes contribute to short-term plasticity by altering neurotransmitter release from nearby presynaptic elements (23–26) and by directly activating postsynaptic glutamate receptors (27). Astrocytes were subsequently shown to play an important role in clearing glutamate, thereby regulating cleft concentration (28) and limiting diffusion to neighbouring synapses (29). Interconnected astrocytes also play an important role in regulating neuronal and synaptic function, particularly over long distances (17, 30, 31). There is now compelling evidence that astrocytes release gliotransmitters, such as adenosine, ATP, D-serine, glutamate and TNF-α, to modulate synaptic efficacy (18, 19, 26, 32–38). More recent studies have shown that astrocytes are critical for the induction of classical, activity-dependent long-term potentiation (LTP) at excitatory synapses in the hypothalamus (34) and hippocampus (39) and can also affect heterosynaptic depression through the recruitment of a glial network (30).
As outlined above, astrocytes in the PVN and the SON exhibit remarkable anatomical plasticity. During dehydration (40–42) or lactation (7, 43, 44), astrocytes retract their fine processes, effectively unwrapping synaptic contacts in the PVN and SON. These dramatic morphological changes have allowed us, and others to take advantage of this system to study how astrocytes impact synaptic transmission and plasticity (28, 29, 33, 34, 37, 45, 46). This includes the finding that astrocytes control activity-dependent LTP and LTD by regulating the synaptic levels of D-serine (34).
Astrocytes express an abundance of G protein-coupled receptors for various neurotransmitters, but due to their pivotal roles in regulating magnocellular neuroendocrine system output, we focused on the recruitment of astrocytes by noradrenaline and glutamate. We first focussed on noradrenergic inputs to the PVN and showed that noradrenaline acts at α1-adrenoceptors on PVN astrocytes to stimulate ATP release (33). When released from astrocytes in response to noradrenaline, ATP binds to P2X7 receptors on the postsynaptic magnocellular neuron. Opening of these ionotropic, cation-permeable receptors (47–50) leads to an influx of calcium into the neuron. Combined with the activation of phosphotidyl inositol 3-kinase, this causes the insertion of AMPA receptors to increase sensitivity of the magnocellular neuron to synaptically-released glutamate (Fig. 2).
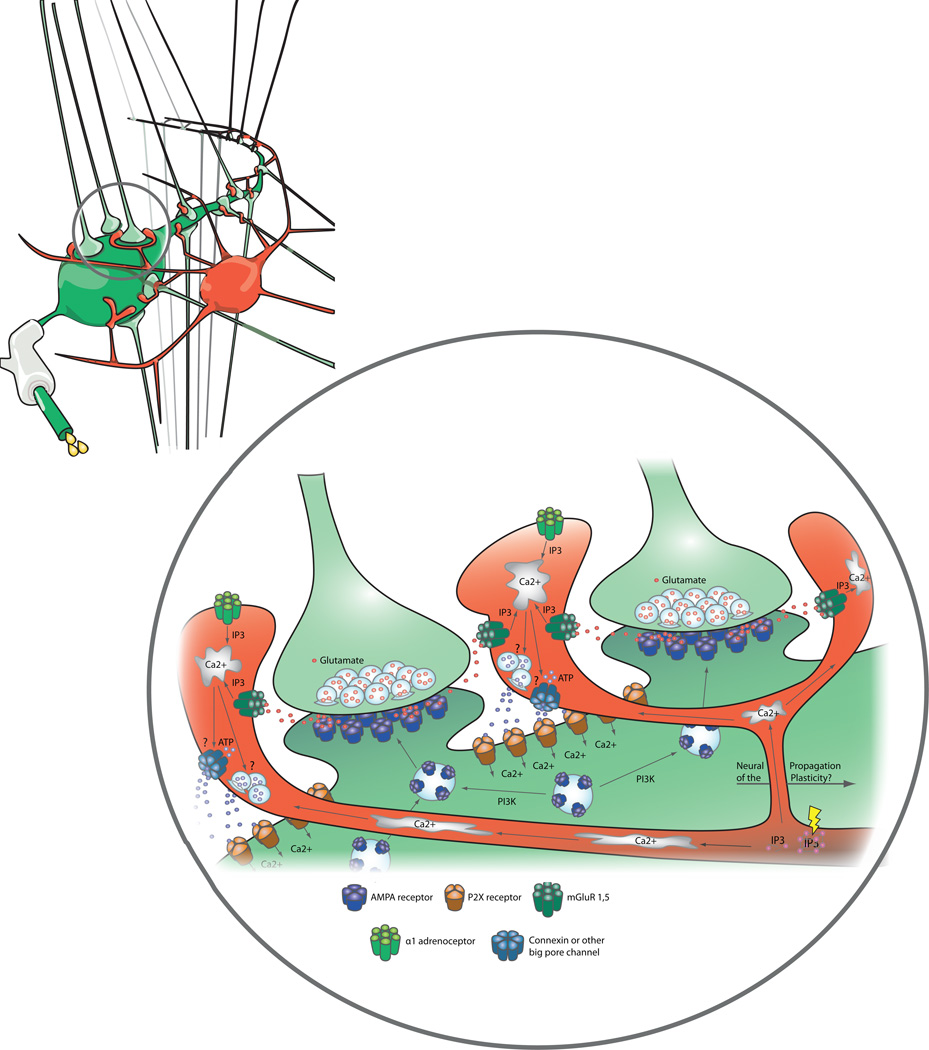
Figure 2.
Intracellular calcium is increased in astrocytes is response to activation of group 1 and 5 mGluRs by glutamate released from presynaptic terminals, or by the activation of α1-adrenoreceptors. The increase in astrocyte calcium triggers ATP release, to activate P2X receptors on the postsynaptic magnocellular neuron. Calcium influx through P2X receptors on the magnocellular neuron membrane activates phosphotidyl inositol 3-kinase to increase AMPA receptor insertion into the postsynaptic membrane, mediating a long-term potentiation in synaptic efficacy (synaptic scaling).
In a second study, we focussed on the ability of glutamatergic inputs to themselves liberate glial ATP. We used an approach where we combined patterned electrical stimulation of afferent, glutamatergic inputs with 2-photon imaging and whole-cell recordings from magnocellular neurons to demonstrate that ATP has similar consequences when released from astrocytes in response to synaptically released glutamate to those observed when ATP is released in response to noradrenaline. In this scenario, glutamate, released from presynaptic terminals in response to stimulation using patterns of activity similar to those described for forebrain regions innervating the PVN (51) acts at metabotropic glutamate receptors (mGluR1,5) on astrocytes, also stimulated ATP release (37, 52). What is particularly intriguing about this series of experiments is that the recruitment of astrocytes does not correlate with an increase in calcium in the soma of the astrocyte. Rather, there is an obligatory increase in the calcium signal in the fine processes of the astrocytes in the immediate vicinity of the neuronal process under investigation. This suggests that somatic astrocyte calcium levels are not reliable indicators of astrocyte recruitment and that changes in calcium levels in the fine processes can occur independent of any changes in somatic calcium and have important consequences for the physiology of the synapse. Importantly, in these experiments, we also demonstrated that this increase in postsynaptic sensitivity to glutamate was not limited to the activated synapse on the postsynaptic cell. Rather, all glutamate synapses on the recorded neuron were strengthened. We have coined the term ‘global scaling’ to describe this phenomenon. At this stage, we have a reasonable understanding of the sequence of biochemical events necessary for potentiation by the noradrenaline pathway, we know considerably less about how the glutamate pathway either potentiates active synapses or scales inactive synapses. This remains an on-going area of investigation in our laboratory.
We have combined electrophysiology and 2-photon imaging from slices with glial retraction to make two key discoveries. First, we demonstrated that, in response to noradrenaline, astrocytes release ATP, which acts on postsynaptic targets to potentiate glutamate synapses (33). We then showed that all three elements of the tripartite synapse were necessary for potentiating glutamate synapses following activation of astrocytes by synaptically-released glutamate. This potentiation was distributed throughout the postsynaptic neuron and also required ATP release from astrocytes (37). Both scenarios require the release of ATP from the astrocytes. The mechanism(s) through which this release is accomplished, however, remain unresolved. Once released, ATP acts on postsynaptic P2X receptors. In one scenario, P2X7 receptors appear to be essential, but it is not clear whether ATP released in response to glutamate activates P2X7 or another subtype of the P2X receptor. Finally, while both noradrenergic and glutamatergic inputs increase magnocellular neuron excitability (53, 54) to increase hormone release (55–57), it is not clear whether these signals acts independently or in concert to ensure appropriate hormone responses in basal conditions and in response to acute physiological challenge.
Reproductive modulation of neuronal-glial interactions
Over the last decade, in vitro electrophysiological recordings have permitted us to obtain new insights on the cellular consequences associated with the reduction of the astrocytic coverage of magnocellular neurons in the PVN and SON during lactation that were initially described by Theodosis and Poulain (4). This structural plasticity has proved to be a unique experimental model to investigate the contribution of glial cells to synaptic transmission and to activity-dependent synaptic plasticity (28, 33, 34, 58–60). One direct consequence of the modification of the glial environment of magnocellular neurons is a change in the diffusion properties in the extracellular space (ECS). Glial withdrawal that accompanies the anatomical remodelling of the SON in lactating animals causes a significant reduction of tortuosity, an index of restriction on diffusion in the tissue in comparison with an obstacle-free medium. We found this to have significant consequences on volume transmission and more specifically on the action of synaptically-released glutamate whose inhibitory action on neighbouring GABAergic inputs is facilitated in the SON of lactating animals (59). As glutamate diffuses away from its site of release, it causes the activation of metabotropic glutamate receptors (mGluRs) expressed by GABAergic terminals, thereby inhibiting GABA release. This phenomenon, known as heterosynaptic depression, is facilitated when glial coverage of SON neurons is reduced in lactating animals (59). This result indicates that the anatomical remodelling enhances the range of action of signalling molecules in the tissue. Whether the diffusion of other neurotransmitters and neuromodulators, such as noradrenaline, GABA, OT or VP, is also facilitated remains to be demonstrated.
The withdrawal of glial processes from the vicinity of synapses has a very strong impact on the concentration and diffusion of glutamate. In addition to acting as a physical barrier, glial processes also contribute very actively to glutamatergic homeostasis by ensuring glutamate clearance from the ECS via high affinity transporters. Under conditions where the glial coverage of neurons and synapses in the SON is reduced, this clearance is delayed, resulting in a build-up of the excitatory amino acid in the ECS. Such enhanced levels of glutamate have several consequences on glutamatergic synaptic transmission, including an enhanced activation of presynaptic mGluRs which inhibit transmitter release (28, 61) As a result of this augmented tonic presynaptic inhibition, synaptic efficacy is significantly reduced at glutamatergic inputs impinging onto magnocellular neurons.
A further consequence of glial retraction is a change in the mode of operation of presynaptic kainate receptors (KARs). Activation of presynaptic KARs can regulate the release of glutamate or GABA in a positive or negative fashion, thereby playing a key role in modulating the excitability of neuronal networks (62). Interestingly, bidirectional modulation of transmitter release by KAR activation has been described in the different structures such as the hippocampus (63, 64), the cerebellum (65), amygdala (66) and the spinal cord (67, 68). In these areas, an experimentally-induced increase in the concentration of KAR agonist resulted in a switch from facilitation to inhibition of transmitter release. The underlying mechanisms of such a switch, as well as its physiological and/or pathological relevance of this switch, are presently unknown. We have recently reported that a similar switch occurs in the SON during lactation (60), providing the first evidence of a physiological role for this switch. It appears that the enhanced glutamate levels in the ECS that result from glial withdrawal cause a dramatic switch in the mode of operation of the presynaptic KARs controlling GABA release at inhibitory terminals impinging upon oxytocin neurons. While KAR activation in virgin rats causes facilitation of GABAergic transmission, an inhibition is observed in lactating rats. Interestingly, the facilitation is associated with the canonical ionotropic mode of action of KARs, whereas the inhibition involved a metabotropic coupling to a second messenger pathway implicating protein kinase C (60).
Another process that is dramatically modified by the structural plasticity of the SON is gliotransmission, or more specifically, the impact of gliotransmission onto magnocellular neurons. As explained above, gliotransmission is the process by which glial cells release active signalling molecules that influence neighbouring neurons. The major gliotransmitters are glutamate, ATP, taurine and D-serine. D-serine is an amino acid which serves as an endogenous co-agonist of NMDA receptors (NMDARs). We found that when astrocytic coverage of magnocellular neurons is reduced during lactation NMDAR activity was impaired due to a deficiency in D-serine (34) (Fig. 3). As a consequence, NMDAR-dependent processes such as synaptic plasticity was affected astrocytes. Thus, it appears that the glial environment of neurons is of utmost importance for controlling NMDAR activity and synaptic plasticity.
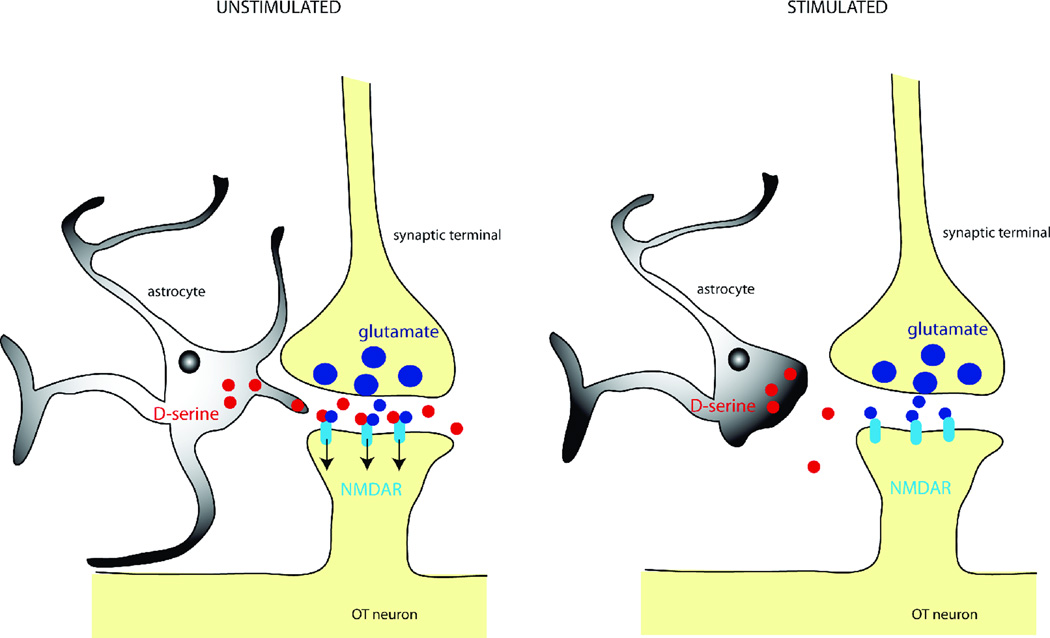
Figure 3.
Under unstimulated conditions (left panel), astrocytes provide D-serine to synaptic NMDA receptors (NMDARs) located on oxytocin neurons making them fully available whenever glutamate is released from presynaptic terminals. The withdrawal of astrocytic processes occurring under stimulated conditions (right panel), reduces the levels of D-serine present in the cleft, thereby impairing NMDAR activation.
Glial modulation of extrasynaptic receptor activation
A growing body of evidence indicates that in addition to acting on conventional postsynaptic receptors, the neurotransmitters glutamate and GABA can also activate receptors located extrasynaptically (69–71). Extrasynaptic receptors have been typically considered a non-functional pool of receptors, which could be recruited to active synapses on an ‘on-demand’ basis (72, 73). It is now recognized however, that extrasynaptic receptors play important roles in information processing and integration within central neuronal circuits. Interestingly, a growing body of evidence shows quit remarkable differences between synaptic- and extrasynaptic-mediated signalling modalities. For example, conventional glutamate synaptic transmission is mediated by high concentration, transient ‘bursts’ of neurotransmitters in the synaptic cleft, which bind to low-affinity and rapidly desensitizing receptors (74). These characteristics result in a spatially and temporally restricted transfer of information (i.e., an excitatory postsynaptic current, EPSC) between two neurons. Conversely, extrasynaptic transmission is mediated by low, sustained levels of neurotransmitter in the extracellular space, which bind to molecularly distinct receptors that show a much higher affinity and little desensitization to the binding transmitter (70). Upon activation, extrasynaptic receptors typically mediate a persistent, ‘tonic’ current, believed to more globally influence neuronal excitability, as well as the overall gain within a network of neurons. Thus, the notion of a compartmentalized signalling mechanism mediated by synaptic and extrasynaptic receptors is steadily growing, which can lead to distinct, and even opposing functional outcomes for glutamate, depending on the specific pool of receptors activated. A clear example of such glutamate-mediated compartmentalized signalling was recently demonstrated in hippocampal neurons, in which activation of synaptic NMDARs resulted in a neuroprotective effect, whereas activation of extrasynaptic NMDARs promoted apoptosis and neuronal death (75). Given this growing body of evidence, and the well-established importance of glutamate and NMDARs in the regulation of magnocellular neuron activity and hormone release (76–78), we recently investigated whether extrasynaptic glutamate-mediated signalling regulates the activity of magnocellular neurons of the SON (79). We provide here a comprehensive summary of this work, highlighting the main findings related to the general properties, functional relevance and state-dependent modulation of glutamate-mediated extrasynaptic signalling in these neurons.
To test for the presence of extrasynaptic-mediated tonic glutamate signalling, we obtained whole-cell patch clamp recordings from magnocellular neurons in an acute slice preparation. Bath application of the broad-spectrum glutamate receptor blocker kynurenic acid (1 mM) not only blocked EPSCs (i.e., fast synaptic transmission), but also induced a sustained outward shift in the holding current, of ~8 pA. This simple experiment indicates that under basal conditions, glutamate receptors are persistently activated by an endogenous glutamate tone, resulting in a sustained inward current. The use of more selective glutamate receptor antagonists revealed that ~95% of the sustained inward current was mediated by NMDARs, ~50% of which contained the NR2B receptor subunit. We termed this persistent current as tonic INMDA. Highlighting the importance of tonic INMDA, we found that despite its relative small magnitude, most of the glutamate charge transfer per unit of time was indeed carried by tonic INMDA, rather than EPSCs (~80% versus ~20%, respectively), likely due to the fact that the underlying receptors mediating tonic INMDA act persistently, whereas those mediating EPSCs operate within a very narrow time window.
By contrast to synaptic glutamate transmission, the strength of tonic INMDA is expected to depend on factors influencing the concentration and time course of glutamate in the extrasynaptic space. These include the topography of the neuronal-glial microenvironment (59), as well as the activity of glial glutamate transporters, which efficiently remove glutamate from the extracellular space (80, 81). Thus, given that magnocellular neurons’ somata are tightly enwrapped by astroglial processes that express high levels of the glutamate transporter, GLT1 (28, 82), we hypothesized that the strength of tonic INMDA is regulated by astroglial glutamate transporters. This is supported by our results showing that dihydrokainate (DHK), a selective GLT1 blocker, enhanced the magnitude of tonic INMDA to induce robust action potential discharge in SON neurons. The effects of DHK were blocked by the NMDAR blocker AP5 and by memantine, a compound that preferentially blocks extrasynaptic over synaptic NMDARs (83). Taken together, these results indicate that under basal conditions, glial glutamate transporters restrain extrasynaptic ambient glutamate levels, determining the degree of extrasynaptic NMDAR activation, and hence its positive effect on SON neuronal excitability.
A functionally relevant question regarding this novel extrasynaptic signalling modality in SON neurons relates to the source of glutamate contributing to the activation of extrasynaptic NMDARs. Potential sources to be considered include synaptically released glutamate, astroglial release of glutamate, as well as glutamate from metabolic pools. Our studies suggest that the source of glutamate activating extrasynaptic NMDARs, at least under basal conditions, is of a non-synaptic origin. Firstly, under conditions in which vesicular synaptic exocytotic release was blocked (i.e., slices incubated in BafA1, an inhibitor of the vacuolar H+-ATPase pump), the basal tonic INMDA magnitude, and that observed in the presence of the GLT1 blocker, was largely unaffected. Moreover, increasing the on-going frequency of glutamate EPSCs also failed to alter tonic INMDA magnitude. However, we cannot rule out the possibility that spillover of synaptic glutamate during conditions of strong synchronous afferent activity could reach and activate extrasynaptically located glutamate receptors, particularly when astrocytic processes have retracted. Future studies will address whether active release of glutamate by astrocytes can activate extrasynaptic receptors in SON neurons, as shown in hippocampal neurons (27).
Finally, given our results supporting a critical role for astrocytes in influencing the magnitude of the extrasynaptic tonic NMDA excitation, we investigated whether activity-dependent remodelling in the local neuronal-glial microenvironment affected the strength of tonic INMDA. To this end, we measured tonic INMDA and its modulation of glial GLT1 transporters during dehydration by water deprivation for 48 h, a condition that similarly to lactation, results in a rapid and reversible retraction of astroglial processes, diminishing the degree of astrocytic coverage of synaptic and neuronal surfaces in the SON (82, 84–86). Our results show that the basal magnitude of tonic INMDA was larger in SON neurons recorded from dehydrated rats, compared to controls. Furthermore, we found a blunted effect of the GLT1 transporter blocker, DHK, in neurons from dehydrated rats (79). Taken together, these results suggest that there is a larger basal tonic INMDA in dehydrated rats, likely as a result of a diminished efficacy of glial GLT1 transporters under these conditions.
The functional impact of the increased strength of tonic INMDA in dehydrated rats was assessed by obtaining extracellular single unit recordings in vivo from SON neurons in anesthetized rats. These studies showed that microdialysis administration of DHK directly into the SON of control rats induced a progressive and sustained increase in SON neuron action potential discharge, an effect that was almost completely absent in dehydrated rats (79). Taken together, our studies indicate that glial uptake of ambient glutamate restrains the activity of SON neurons under basal conditions, and that a reduction of glial glutamate uptake, likely due to an activity-dependent reorganization of the local neuro-glial microenvironment, contributes to the increased activity of SON neurons during dehydration.
In summary, our studies support the presence of a novel excitatory signalling modality in magnocellular neurons via activation of extrasynaptic NMDARs by ambient glutamate under tight astrocytic control, resulting in a tonic excitatory drive that increases SON neuronal activity. Furthermore, our studies indicate that the strength of tonic INMDA increases during dehydration. Thus, the state-dependent modulation of extrasynaptic glutamate signalling could be a novel mechanism contributing to magnocellular neurons’ homeostatic responses during physiological challenges.
In addition to transporters for glutamate (61), glia express GABA transporters (87) that also modulate ambient neurotransmitter levels. In opposition to the tonic effects of glutamate, ambient GABA generates a persistent hyperpolarization via the activation of extrasynaptic receptors (87, 88). Hence, the tonic drive imposed on magnocellular neurons by ambient neurotransmitter levels will reflect the balance of glutamatergic excitation and GABAergic inhibition. Counter-intuitively, dehydration increases intra-SON GABA release (as well as glutamate release), evident as an increase in the frequency of spontaneous IPSCs (and EPSCs) in magnocellular neurons from dehydrated rats (89). In addition to effects on synaptic activity, ambient glutamate and GABA levels also appear to increase during dehydration and impact magnocellular neuron excitability via activation of extrasynaptic receptors. Just as the excitation of magnocellular neurons evident during blockade of glial glutamate transporters is lost during dehydration (79), the inhibition of magnocellular neurons evident during blockade of glial GABA transporters is also lost during dehydration (C.H. Brown, unpublished observations), suggesting that the tonic effects of endogenous glutamate and GABA are both increased during dehydration. While the role of increased ambient glutamate levels (to excite magnocellular neurons during dehydration) appears straightforward, the role of increased extracellular GABA levels during dehydration is more difficult to reconcile with the required increase in action potential discharge. However, GABA might play a similar role during dehydration to that of increased GABA levels during acute osmotic stimulation; i.e. to reduce the gain of the glutamate-driven hyperosmotic excitation of the magnocellular neuroendocrine system (90), thereby allowing a graded response to graded changes in osmolality over the ‘physiological’ range.
Concluding remarks
Here, we have focussed on recent experiments from our laboratories that were presented at the 9th World Congress on Neurohypophysial Hormones. While our experiments have expanded our understanding of glial regulation of the activity of magnocellular neurons via gliotransmitter modulation of glutamate and GABA receptor activation, and by transporter-mediated regulation of extracellular glutamate levels, these are not the only mechanisms by which glia regulate magnocellular neuron activity.
Another level of complexity in glial regulation of the magnocellular neuroendocrine system is imposed by the release of additional gliotransmitters, such as taurine, which inhibits magnocellular neurons via extrasynaptic glycine receptors, as has been previously reviewed elsewhere (91). Glial taurine release is increased in hypo-osmotic conditions to inhibit magnocellular neurons, and is reduced in hyperosmotic conditions to disinhibit the neurons. Hence, it appears likely that glial taurine release might contribute to the physiological regulation of the magnocellular neuroendocrine system. However, mice that lack the Na+-coupled taurine transporter (taut−/− mice) concentrate urine normally during dehydration, but they continue to generate hyperosmotic urine upon rehydration, when the urine of wild-type littermates rapidly returns to normal osmolality (92). Hence, decreased glial taurine release (e.g. during chronic dehydration) might not substantively impact the activity of magnocellular neurons whereas hypo-osmolality-induced increases in taurine release appear to inhibit the magnocellular neuroendocrine system.
In addition to secretion from their terminals into the bloodstream, magnocellular neurons also release neuromodulators, including oxytocin and vasopressin, from their somata and dendrites (93), and these have been shown to be important for regulation of the activity of individual neurons. In addition to oxytocin and vasopressin, magnocellular neurons also release other neuromodulators from their somata and dendrites; the best characterised of these is somato-dendritic dynorphin release, which modulates the activity of vasopressin neurons (94) via direct inhibition of the intrinsic excitability of vasopressin neurons (95, 96) as well as by inhibition of their excitatory synaptic inputs (97). Clearly, autocrine and retrograde actions of neuromodulators require transit through the extracellular space and we have demonstrated that the inhibitory feedback effects of dynorphin on vasopressin neurons are increased during dehydration (98), consistent with decreased tortuosity in the extracellular environment evident after glial retraction. However, it remains to be determined whether glia actively regulate access of dynorphin, or other somato-dendritic neuromodulators, to their cognate receptors on presynaptic terminals and postsynaptic magnocellular neurons.
For the magnocellular neuroendocrine system, it is the activity of the population as a whole that determines hormone secretion (99). Oxytocin release for fetal expulsion at birth and for milk ejection during suckling is pulsatile, and this pulsatility is underpinned by co-ordinated high frequency bursts of activity across the entire population of oxytocin neurons (100, 101). It is three decades since Theodosis and Poulain questioned whether glial retraction might provide the morphological substrate for inter-neuronal communication required for the co-ordination of bursting activity across the population of magnocellular neurons (4). Recently, these same investigators showed that milk ejection bursts of oxytocin neurones were not affected by disruption of neural cell adhesion molecule (10), which is required for glial retraction in the SON and PVN (102). Hence, glial retraction might not be obligatory for parturition and lactation to proceed. This leaves us with the apparent conundrum that glial retraction takes place, impacts on synaptic inputs to, and excitability of, magnocellular neurons but to no apparent functional effect. However, it should be borne in mind that parturition and lactation are critical to the survival of mammalian species and a failure of either will have catastrophic consequences for the individuals concerned. Hence, it is likely that multiple redundancy is built into the system to prevent a catastrophic systems failure should any one component fail. Glial retraction is likely an integral part of the system under ‘normal’ circumstances but when this component is disrupted, other mechanisms appear able to compensate to permit delivery of the young and delivery of milk to the new-born.
While reproductive functions of oxytocin neurons require the same response to be co-ordinated response across the entire population, the challenge to the magnocellular neuroendocrine system is even greater for its role in body fluid balance that require a mixed response across the population. As plasma osmolality increases (e.g. during dehydration), vasopressin is secreted to promote water reabsorption by the kidney (103) and oxytocin is secreted to stimulate atrial natriuretic peptide release from the heart to promote sodium excretion in the urine (104). The secretory response is graded to the degree of osmotic stimulation (e.g. dehydration) by an average increase in activity across the population of magnocellular neurons (105). Nevertheless, some magnocellular neurons still exhibit very low action potential discharge rates during dehydration (98). Indeed, it is important that they do so because, even during profound dehydration, circulating vasopressin (and oxytocin) levels are far from maximal (103); if all magnocellular neurons increased activity dramatically during dehydration, the outcome would be inappropriate over-secretion of vasopressin and oxytocin. Hence, it is important that co-ordination across the population occurs to generate the appropriate secretory response for the prevailing osmotic conditions, with some magnocellular neurons increasing activity dramatically, some increasing activity modestly and some remaining relatively quiet. As explained above, a single astrocyte interacts with thousands of synapses (21, 22) and can exhibit specificity in the processes that are activated (37). Hence, astrocytes are exquisitely placed to co-ordinate activity across a population of neurons, such as the magnocellular neuroendocrine system; the investigation of whether astrocytes provide this pan-population co-ordination of activity during osmotic stimulation is one of the main challenges facing us today.
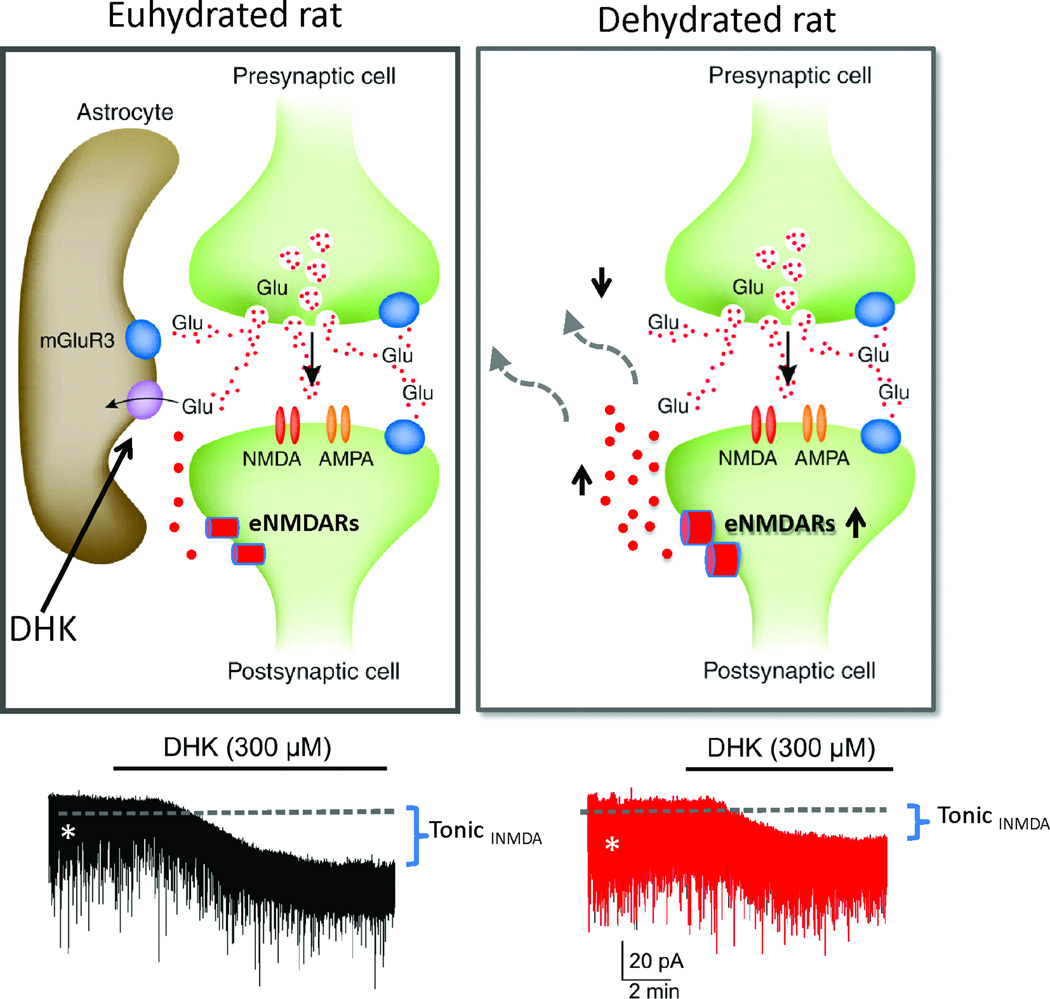
Figure 4.
In control euhydrated rats, astrocyte GLT1 transporters maintain extracellular glutamate (Glu) at relatively low levels, limiting activation of extrasynaptic NMDA receptors (eNMDARs). Blockade of GLT1 activity with dihydrokainate (DHK) results in build-up of extracellular Glu levels, activation of eNMDARs, and progressive development of a tonic eNMDA current (tonic INMDA). In dehydrated rats, astrocytic process withdrawal and/or diminished GLT1 expression results in higher levels of extracellular Glu and greater activation of eNMDARs. This is evident as a larger basal tonic INMDA (shown in part as an increased basal RMS noise, asterisks), as well as a blunted effect of DHK on tonic INMDA in dehydrated rats. Modified from Fleming et al. 2011 (79).
Acknowledgements
This work was supported by Inserm and the Conseil Régional d’Aquitaine (SHO), the Canadian Institutes of Health Research (JSB), the New Zealand Lottery Health Board (grant 223744 to CHB) and the National Institutes of Health (grant R01 HL090948 to JES). JSB is an Alberta Innovates-Health Solutions Senior Scholar.
References
- 1.Wakerly JB, Lincoln DW. Milk ejection in the rat: recordings of intramammary pressure during suckling. J Endocrinol. 1971;51(2):13–14. [PubMed] [Google Scholar]
- 2.Poulain DA, Wakerley JB. Electrophysiology of hypothalamic magnocellular neurones secreting oxytocin and vasopressin. Neuroscience. 1982;7(4):773. doi: 10.1016/0306-4522(82)90044-6. [DOI] [PubMed] [Google Scholar]
- 3.Tweedle CD, Hatton GI. Ultrastructural changes in rat hypothalamic neurosecretory cells and their associated glia during minimal dehydration and rehydration. Cell Tissue Res. 1977;181(1):59–72. doi: 10.1007/BF00222774. [DOI] [PubMed] [Google Scholar]
- 4.Theodosis DT, Poulain DA, Vincent JD. Possible morphological bases for synchronisation of neuronal firing in the rat supraoptic nucleus during lactation. Neuroscience. 1981;6(5):919–929. doi: 10.1016/0306-4522(81)90173-1. [DOI] [PubMed] [Google Scholar]
- 5.Hatton GI, Tweedle CD. Magnocellular neuropeptidergic neurons in hypothalamus: increases in membrane apposition and number of specialized synapses from pregnancy to lactation. Brain Res Bull. 1982;8(2):197. doi: 10.1016/0361-9230(82)90046-6. [DOI] [PubMed] [Google Scholar]
- 6.Tweedle CD, Hatton GI. Magnocellular neuropeptidergic terminals in neurohypophysis: rapid glial release of enclosed axons during parturition. Brain Res Bull. 1982;8(2):205–209. doi: 10.1016/0361-9230(82)90047-8. [DOI] [PubMed] [Google Scholar]
- 7.Theodosis DT, Poulain DA. Evidence that oxytocin-secreting neurones are involved in the ultrastructural reorganisation of the rat supraoptic nucleus apparent at lactation. Cell Tissue Res. 1984;235(1):217–219. doi: 10.1007/BF00213745. [DOI] [PubMed] [Google Scholar]
- 8.Montagnese C, Poulain DA, Theodosis DT. Influence of ovarian steroids on the ultrastructural plasticity of the adult rat supraoptic nucleus induced by central administration of oxytocin. J Neuroendocrinol. 1990;2(2):225–231. doi: 10.1111/j.1365-2826.1990.tb00855.x. [DOI] [PubMed] [Google Scholar]
- 9.Morris JF, Pow DV. New anatomical insights into the inputs and outputs from hypothalamic magnocellular neurons. Ann N Y Acad Sci. 1993;689:16–33. doi: 10.1111/j.1749-6632.1993.tb55534.x. [DOI] [PubMed] [Google Scholar]
- 10.Catheline G, Touquet B, Lombard MC, Poulain DA, Theodosis DT. A study of the role of neuro-glial remodeling in the oxytocin system at lactation. Neuroscience. 2006;137(1):309–316. doi: 10.1016/j.neuroscience.2005.08.042. [DOI] [PubMed] [Google Scholar]
- 11.Todd KJ, Darabid H, Robitaille R. Perisynaptic glia discriminate patterns of motor nerve activity and influence plasticity at the neuromuscular junction. J Neurosci. 2010;30(35):11870–11882. doi: 10.1523/JNEUROSCI.3165-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Todd KJ, Serrano A, Lacaille JC, Robitaille R. Glial cells in synaptic plasticity. J Physiol Paris. 2006;99(2–3):75–83. doi: 10.1016/j.jphysparis.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Jahromi BS, Robitaille R, Charlton MP. Transmitter release increases intracellular calcium in perisynaptic Schwann cells in situ. Neuron. 1992;8(6):1069–1077. doi: 10.1016/0896-6273(92)90128-z. [DOI] [PubMed] [Google Scholar]
- 14.Auld DS, Robitaille R. Perisynaptic Schwann cells at the neuromuscular junction: nerve- and activity-dependent contributions to synaptic efficacy, plasticity, and reinnervation. Neuroscientist. 2003;9(2):144–157. doi: 10.1177/1073858403252229. [DOI] [PubMed] [Google Scholar]
- 15.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22(5):208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 16.Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310(5745):113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- 17.Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6(8):626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- 18.Halassa MM, Fellin T, Haydon PG. Tripartite synapses: roles for astrocytic purines in the control of synaptic physiology and behavior. Neuropharmacology. 2009;57(4):343–346. doi: 10.1016/j.neuropharm.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perea G, Navarrete M, Araque A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci. 2009;32(8):421–431. doi: 10.1016/j.tins.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Halassa MM, Dal Maschio M, Beltramo R, Haydon PG, Benfenati F, Fellin T. Integrated brain circuits: neuron-astrocyte interaction in sleep-related rhythmogenesis. Scientific World Journal. 2010;10:1634–1645. doi: 10.1100/tsw.2010.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci. 2002;22(1):183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haber M, Zhou L, Murai KK. Cooperative astrocyte and dendritic spine dynamics at hippocampal excitatory synapses. J Neurosci. 2006;26(35):8881–8891. doi: 10.1523/JNEUROSCI.1302-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. Eur J Neurosci. 1998;10(6):2129–2142. doi: 10.1046/j.1460-9568.1998.00221.x. [DOI] [PubMed] [Google Scholar]
- 24.Araque A, Sanzgiri RP, Parpura V, Haydon PG. Calcium elevation in astrocytes causes an NMDA receptor-dependent increase in the frequency of miniature synaptic currents in cultured hippocampal neurons. J Neurosci. 1998;18(17):6822–6829. doi: 10.1523/JNEUROSCI.18-17-06822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parpura V, Haydon PG. Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons. Proc Natl Acad Sci U S A. 2000;97(15):8629–8634. doi: 10.1073/pnas.97.15.8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M, Matute C, Tonello F, Gundersen V, Volterra A. Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci. 2007;10(3):331–339. doi: 10.1038/nn1849. [DOI] [PubMed] [Google Scholar]
- 27.Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369(6483):744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- 28.Oliet SH, Piet R, Poulain DA. Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science. 2001;292(5518):923–926. doi: 10.1126/science.1059162. [DOI] [PubMed] [Google Scholar]
- 29.Piet R, Bonhomme R, Theodosis DT, Poulain DA, Oliet SH. Modulation of GABAergic transmission by endogenous glutamate in the rat supraoptic nucleus. Eur J Neurosci. 2003;17(9):1777–1785. doi: 10.1046/j.1460-9568.2003.02611.x. [DOI] [PubMed] [Google Scholar]
- 30.Serrano A, Haddjeri N, Lacaille JC, Robitaille R. GABAergic network activation of glial cells underlies hippocampal heterosynaptic depression. J Neurosci. 2006;26(20):5370–5382. doi: 10.1523/JNEUROSCI.5255-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322(5907):1551–1555. doi: 10.1126/science.1164022. [DOI] [PubMed] [Google Scholar]
- 32.Kang J, Jiang L, Goldman SA, Nedergaard M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci. 1998;1(8):683–692. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- 33.Gordon GR, Baimoukhametova DV, Hewitt SA, Rajapaksha WR, Fisher TE, Bains JS. Norepinephrine triggers release of glial ATP to increase postsynaptic efficacy. Nat Neurosci. 2005;8(8):1078–1086. doi: 10.1038/nn1498. [DOI] [PubMed] [Google Scholar]
- 34.Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA, Oliet SH. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell. 2006;125(4):775–784. doi: 10.1016/j.cell.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 35.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440(7087):1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 36.Tritsch NX, Yi E, Gale JE, Glowatzki E, Bergles DE. The origin of spontaneous activity in the developing auditory system. Nature. 2007;450(7166):50–55. doi: 10.1038/nature06233. [DOI] [PubMed] [Google Scholar]
- 37.Gordon GR, Iremonger KJ, Kantevari S, Ellis-Davies GC, MacVicar BA, Bains JS. Astrocyte-mediated distributed plasticity at hypothalamic glutamate synapses. Neuron. 2009;64(3):391–403. doi: 10.1016/j.neuron.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, Haydon PG, Frank MG. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61(2):213–219. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henneberger C, Papouin T, Oliet SH, Rusakov DA. Long-term potentiation depends on release of D-serine from astrocytes. Nature. 2010;463(7278):232–236. doi: 10.1038/nature08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gregory WA, Tweedle CD, Hatton GI. Ultrastructure of neurons in the paraventricular nucleus of normal, dehydrated and rehydrated rats. Brain Res Bull. 1980;5(3):301. doi: 10.1016/0361-9230(80)90173-2. [DOI] [PubMed] [Google Scholar]
- 41.Hatton GI, Perlmutter LS, Salm AK, Tweedle CD. Dynamic neuronal-glial interactions in hypothalamus and pituitary: implications for control of hormone synthesis and release. Peptides. 1984;5 Suppl 1:121–138. doi: 10.1016/0196-9781(84)90271-7. [DOI] [PubMed] [Google Scholar]
- 42.Chapman DB, Theodosis DT, Montagnese C, Poulain DA, Morris JF. Osmotic stimulation causes structural plasticity of neurone-glia relationships of the oxytocin but not vasopressin secreting neurones in the hypothalamic supraoptic nucleus. Neuroscience. 1986;17(3):679. doi: 10.1016/0306-4522(86)90039-4. [DOI] [PubMed] [Google Scholar]
- 43.Theodosis DT, Chapman DB, Montagnese C, Poulain DA, Morris JF. Structural plasticity in the hypothalamic supraoptic nucleus at lactation affects oxytocin-, but not vasopressin-secreting neurones. Neuroscience. 1986;17(3):661. doi: 10.1016/0306-4522(86)90038-2. [DOI] [PubMed] [Google Scholar]
- 44.Theodosis DT, Poulain DA. Neuronal-glial and synaptic plasticity in the adult rat paraventricular nucleus. Brain Res. 1989;484(1–2):361. doi: 10.1016/0006-8993(89)90382-x. [DOI] [PubMed] [Google Scholar]
- 45.Oliet SH, Piet R, Poulain DA, Theodosis DT. Glial modulation of synaptic transmission: Insights from the supraoptic nucleus of the hypothalamus. Glia. 2004;47(3):258–267. doi: 10.1002/glia.20032. [DOI] [PubMed] [Google Scholar]
- 46.Theodosis DT, Koksma JJ, Trailin A, Langle SL, Piet R, Lodder JC, Timmerman J, Mansvelder H, Poulain DA, Oliet SH, Brussaard AB. Oxytocin and estrogen promote rapid formation of functional GABA synapses in the adult supraoptic nucleus. Mol Cell Neurosci. 2006;31(4):785–794. doi: 10.1016/j.mcn.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 47.Humphrey PP, Buell G, Kennedy I, Khakh BS, Michel AD, Surprenant A, Trezise DJ. New insights on P2X purinoceptors. Naunyn Schmiedebergs Arch Pharmacol. 1995;352(6):585–596. doi: 10.1007/BF00171316. [DOI] [PubMed] [Google Scholar]
- 48.Girdler G, Khakh BS. ATP-gated P2X channels. Curr Biol. 2004;14(1):R6. doi: 10.1016/j.cub.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 49.Jarvis MF, Khakh BS. ATP-gated P2X cation-channels. Neuropharmacology. 2009;56(1):208–215. doi: 10.1016/j.neuropharm.2008.06.067. [DOI] [PubMed] [Google Scholar]
- 50.Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006;442(7102):527–532. doi: 10.1038/nature04886. [DOI] [PubMed] [Google Scholar]
- 51.Washburn DL, Anderson JW, Ferguson AV. A subthreshold persistent sodium current mediates bursting in rat subfornical organ neurones. JPhysiol. 2000;529(Pt 2):359. doi: 10.1111/j.1469-7793.2000.00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuzmiski JB, Bains JS. Metabotropic glutamate receptors: gatekeepers of homeostasis. J Neuroendocrinol. 2010;22(7):785–792. doi: 10.1111/j.1365-2826.2010.02020.x. [DOI] [PubMed] [Google Scholar]
- 53.Ferguson AV, Day TA, Renaud LP. Subfornical organ efferents influence the excitability of neurohypophyseal and tuberoinfundibular paraventricular nucleus neurons in the rat. Neuroendocrinology. 1984;39(5):423. doi: 10.1159/000124015. [DOI] [PubMed] [Google Scholar]
- 54.Day TA, Ferguson AV, Renaud LP. Noradrenergic afferents facilitate the activity of tuberoinfundibular neurons of the hypothalamic paraventricular nucleus. Neuroendocrinology. 1985;41(1):17. doi: 10.1159/000124148. [DOI] [PubMed] [Google Scholar]
- 55.Ferguson AV, Kasting NW. Activation of subfornical organ efferents stimulates oxytocin secretion in the rat. RegulPept. 1987;18(2):93. doi: 10.1016/0167-0115(87)90039-5. [DOI] [PubMed] [Google Scholar]
- 56.Ferguson AV, Kasting NW. Electrical stimulation in subfornical organ increases plasma vasopressin concentrations in the conscious rat. AmJPhysiol. 1986;251(2 Pt 2):R425. doi: 10.1152/ajpregu.1986.251.2.R425. [DOI] [PubMed] [Google Scholar]
- 57.Buller KM, Khanna S, Sibbald JR, Day TA. Central noradrenergic neurons signal via ATP to elicit vasopressin responses to haemorrhage. Neuroscience. 1996;73(3):637–642. doi: 10.1016/0306-4522(96)00156-x. [DOI] [PubMed] [Google Scholar]
- 58.Boudaba C, Di S, Tasker JG. Presynaptic noradrenergic regulation of glutamate inputs to hypothalamic magnocellular neurones. J Neuroendocrinol. 2003;15(8):803–810. doi: 10.1046/j.1365-2826.2003.01063.x. [DOI] [PubMed] [Google Scholar]
- 59.Piet R, Vargova L, Sykova E, Poulain DA, Oliet SH. Physiological contribution of the astrocytic environment of neurons to intersynaptic crosstalk. Proc Natl Acad Sci U S A. 2004;101(7):2151–2155. doi: 10.1073/pnas.0308408100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bonfardin VD, Fossat P, Theodosis DT, Oliet SH. Glia-dependent switch of kainate receptor presynaptic action. J Neurosci. 2010;30(3):985–995. doi: 10.1523/JNEUROSCI.3389-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boudaba C, Linn DM, Halmos KC, Tasker JG. Increased tonic activation of presynaptic metabotropic glutamate receptors in the rat supraoptic nucleus following chronic dehydration. J Physiol. 2003;551:815–823. doi: 10.1113/jphysiol.2003.042739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pinheiro P, Mulle C. Kainate receptors. Cell Tissue Res. 2006;326(2):457–482. doi: 10.1007/s00441-006-0265-6. [DOI] [PubMed] [Google Scholar]
- 63.Schmitz D, Mellor J, Nicoll RA. Presynaptic kainate receptor mediation of frequency facilitation at hippocampal mossy fiber synapses. Science. 2001;291(5510):1972–1976. doi: 10.1126/science.1057105. [DOI] [PubMed] [Google Scholar]
- 64.Jiang L, Xu J, Nedergaard M, Kang J. A kainate receptor increases the efficacy of GABAergic synapses. Neuron. 2001;30(2):503–513. doi: 10.1016/s0896-6273(01)00298-7. [DOI] [PubMed] [Google Scholar]
- 65.Delaney AJ, Jahr CE. Kainate receptors differentially regulate release at two parallel fiber synapses. Neuron. 2002;36(3):475–482. doi: 10.1016/s0896-6273(02)01008-5. [DOI] [PubMed] [Google Scholar]
- 66.Braga MF, Aroniadou-Anderjaska V, Xie J, Li H. Bidirectional modulation of GABA release by presynaptic glutamate receptor 5 kainate receptors in the basolateral amygdala. J Neurosci. 2003;23(2):442–452. doi: 10.1523/JNEUROSCI.23-02-00442.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kerchner GA, Wilding TJ, Li P, Zhuo M, Huettner JE. Presynaptic kainate receptors regulate spinal sensory transmission. J Neurosci. 2001;21(1):59–66. doi: 10.1523/JNEUROSCI.21-01-00059.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Youn DH, Randic M. Modulation of excitatory synaptic transmission in the spinal substantia gelatinosa of mice deficient in the kainate receptor GluR5 and/or GluR6 subunit. J Physiol. 2004;555(Pt 3):683–698. doi: 10.1113/jphysiol.2003.057570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dalby NO, Mody I. Activation of NMDA receptors in rat dentate gyrus granule cells by spontaneous and evoked transmitter release. J Neurophysiol. 2003;90(2):786–797. doi: 10.1152/jn.00118.2003. [DOI] [PubMed] [Google Scholar]
- 70.Le Meur K, Galante M, Angulo MC, Audinat E. Tonic activation of NMDA receptors by ambient glutamate of non-synaptic origin in the rat hippocampus. J Physiol. 2007;580(Pt. 2):373–383. doi: 10.1113/jphysiol.2006.123570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sah P, Hestrin S, Nicoll RA. Tonic activation of NMDA receptors by ambient glutamate enhances excitability of neurons. Science. 1989;246(4931):815–818. doi: 10.1126/science.2573153. [DOI] [PubMed] [Google Scholar]
- 72.Clark BA, Cull-Candy SG. Activity-dependent recruitment of extrasynaptic NMDA receptor activation at an AMPA receptor-only synapse. J Neurosci. 2002;22(11):4428–4436. doi: 10.1523/JNEUROSCI.22-11-04428.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harney SC, Jane DE, Anwyl R. Extrasynaptic NR2D-containing NMDARs are recruited to the synapse during LTP of NMDAR-EPSCs. J Neurosci. 2008;28(45):11685–11694. doi: 10.1523/JNEUROSCI.3035-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jonas P, Bischofberger J, Fricker D, Miles R. Interneuron Diversity series: Fast in, fast out--temporal and spatial signal processing in hippocampal interneurons. Trends Neurosci. 2004;27(1):30–40. doi: 10.1016/j.tins.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 75.Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5(5):405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- 76.Bains JS, Ferguson AV. Nitric oxide regulates NMDA-driven GABAergic inputs to type I neurones of the rat paraventricular nucleus. JPhysiol. 1997;499(Pt 3):733. doi: 10.1113/jphysiol.1997.sp021965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hu B, Bourque CW. NMDA receptor-mediated rhythmic bursting activity in rat supraoptic nucleus neurones in vitro. J Physiol. 1992;458:667–687. doi: 10.1113/jphysiol.1992.sp019440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morsette DJ, Sidorowicz H, Sladek CD. Role of non-NMDA receptors in vasopressin and oxytocin release from rat hypothalamo-neurohypophysial explants. Am J Physiol Regul Integr Comp Physiol. 2001;280(2):R313–R322. doi: 10.1152/ajpregu.2001.280.2.R313. [DOI] [PubMed] [Google Scholar]
- 79.Fleming TM, Scott V, Naskar K, Joe N, Brown CH, Stern JE. State-dependent changes in astrocyte regulation of extrasynaptic NMDA receptor signalling in neurosecretory neurons. J Physiol. 2011;589(Pt 16):3929–3941. doi: 10.1113/jphysiol.2011.207340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16(3):675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 81.Rusakov DA, Kullmann DM. Extrasynaptic glutamate diffusion in the hippocampus: ultrastructural constraints, uptake, and receptor activation. J Neurosci. 1998;18(9):3158–3170. doi: 10.1523/JNEUROSCI.18-09-03158.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Theodosis DT, Poulain DA. Contribution of astrocytes to activity-dependent structural plasticity in the adult brain. Adv Exp Med Biol. 1999;468:175–182. doi: 10.1007/978-1-4615-4685-6_14. [DOI] [PubMed] [Google Scholar]
- 83.Xia P, Chen HS, Zhang D, Lipton SA. Memantine preferentially blocks extrasynaptic over synaptic NMDA receptor currents in hippocampal autapses. J Neurosci. 2010;30(33):11246–11250. doi: 10.1523/JNEUROSCI.2488-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tweedle CD, Hatton GI. Synapse formation and disappearance in adult rat supraoptic nucleus during different hydration states. Brain Res. 1984;309(2):373–376. doi: 10.1016/0006-8993(84)90607-3. [DOI] [PubMed] [Google Scholar]
- 85.Miyata S, Nakashima T, Kiyohara T. Structural dynamics of neural plasticity in the supraoptic nucleus of the rat hypothalamus during dehydration and rehydration. Brain Res Bull. 1994;34(3):169–175. doi: 10.1016/0361-9230(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 86.Tasker JG, Di S, Boudaba C. Functional synaptic plasticity in hypothalamic magnocellular neurons. Prog Brain Res. 2002;139:113–119. doi: 10.1016/s0079-6123(02)39011-3. [DOI] [PubMed] [Google Scholar]
- 87.Park JB, Skalska S, Stern JE. Characterization of a novel tonic gamma-aminobutyric acidA receptor-mediated inhibition in magnocellular neurosecretory neurons and its modulation by glia. Endocrinology. 2006;147(8):3746–3760. doi: 10.1210/en.2006-0218. [DOI] [PubMed] [Google Scholar]
- 88.Park JB, Jo JY, Zheng H, Patel KP, Stern JE. Regulation of tonic GABA inhibitory function, presympathetic neuronal activity and sympathetic outflow from the paraventricular nucleus by astroglial GABA transporters. J Physiol. 2009;587(Pt 19):4645–4660. doi: 10.1113/jphysiol.2009.173435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Di S, Tasker JG. Dehydration-induced synaptic plasticity in magnocellular neurons of the hypothalamic supraoptic nucleus. Endocrinology. 2004;145(11):5141–5149. doi: 10.1210/en.2004-0702. [DOI] [PubMed] [Google Scholar]
- 90.Leng G, Brown CH, Bull PM, Brown D, Scullion S, Currie J, Blackburn-Munro RE, Feng J, Onaka T, Verbalis JG, Russell JA, Ludwig M. Responses of magnocellular neurons to osmotic stimulation involves coactivation of excitatory and inhibitory input: an experimental and theoretical analysis. J Neurosci. 2001;21(17):6967–6977. doi: 10.1523/JNEUROSCI.21-17-06967.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hussy N, Deleuze C, Desarmenien MG, Moos FC. Osmotic regulation of neuronal activity: a new role for taurine and glial cells in a hypothalamic neuroendocrine structure. Prog Neurobiol. 2000;62(2):113–134. doi: 10.1016/s0301-0082(99)00071-4. [DOI] [PubMed] [Google Scholar]
- 92.Huang DY, Boini KM, Lang PA, Grahammer F, Duszenko M, Heller-Stilb B, Warskulat U, Haussinger D, Lang F, Vallon V. Impaired ability to increase water excretion in mice lacking the taurine transporter gene TAUT. Pflugers Arch. 2006;451(5):668–677. doi: 10.1007/s00424-005-1499-y. [DOI] [PubMed] [Google Scholar]
- 93.Brown CH, Ruan M, Scott V, Tobin VA, Ludwig M. Multi-factorial somato-dendritic regulation of phasic spike discharge in vasopressin neurons. Prog Brain Res. 2008;170:219–228. doi: 10.1016/S0079-6123(08)00419-6. [DOI] [PubMed] [Google Scholar]
- 94.Brown CH, Leng G, Ludwig M, Bourque CW. Endogenous activation of supraoptic nucleus kappa-opioid receptors terminates spontaneous phasic bursts in rat magnocellular neurosecretory cells. J Neurophysiol. 2006;95(5):3235–3244. doi: 10.1152/jn.00062.2006. [DOI] [PubMed] [Google Scholar]
- 95.Brown CH, Leng G. In vivo modulation of post-spike excitability in vasopressin cells by kappa-opioid receptor activation. J Neuroendocrinol. 2000;12(8):711–714. doi: 10.1046/j.1365-2826.2000.00547.x. [DOI] [PubMed] [Google Scholar]
- 96.Brown CH, Bourque CW. Autocrine feedback inhibition of plateau potentials terminates phasic bursts in magnocellular neurosecretory cells of the rat supraoptic nucleus. J Physiol. 2004;557:949–960. doi: 10.1113/jphysiol.2004.063818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Iremonger KJ, Bains JS. Retrograde opioid signaling regulates glutamatergic transmission in the hypothalamus. J Neurosci. 2009;29(22):7349–7358. doi: 10.1523/JNEUROSCI.0381-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Scott V, Bishop VR, Leng G, Brown CH. Dehydration-induced modulation of kappa-opioid inhibition of vasopressin neurone activity. J Physiol. 2009;587:5679–5689. doi: 10.1113/jphysiol.2009.180232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Leng G, Brown C, Sabatier N, Scott V. Population dynamics in vasopressin cells. Neuroendocrinology. 2008;88(3):160–172. doi: 10.1159/000149827. [DOI] [PubMed] [Google Scholar]
- 100.Belin V, Moos F, Richard P. Synchronization of oxytocin cells in the hypothalamic paraventricular and supraoptic nuclei in suckled rats: direct proof with paired extracellular recordings. Exp Brain Res. 1984;57(1):201–203. doi: 10.1007/BF00231147. [DOI] [PubMed] [Google Scholar]
- 101.Belin V, Moos F. Paired recordings from supraoptic and paraventricular oxytocin cells in suckled rats: recruitment and synchronization. J Physiol. 1986;377:369–390. doi: 10.1113/jphysiol.1986.sp016192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Theodosis DT, Bonhomme R, Vitiello S, Rougon G, Poulain DA. Cell surface expression of polysialic acid on NCAM is a prerequisite for activity-dependent morphological neuronal and glial plasticity. J Neurosci. 1999;19(23):10228–10236. doi: 10.1523/JNEUROSCI.19-23-10228.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Verbalis JG. Disorders of body water homeostasis. Best Pract Res Clin Endocrinol Metab. 2003;17(4):471–503. doi: 10.1016/s1521-690x(03)00049-6. [DOI] [PubMed] [Google Scholar]
- 104.Gutkowska J, Jankowski M, Lambert C, Mukaddam-Daher S, Zingg HH, McCann SM. Oxytocin releases atrial natriuretic peptide by combining with oxytocin receptors in the heart. Proc Natl Acad Sci U S A. 1997;94(21):11704–11709. doi: 10.1073/pnas.94.21.11704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Scott V, Brown CH. State-dependent plasticity in vasopressin neurones: dehydration-induced changes in activity patterning. J Neuroendocrinol. 2010;22(5):343–354. doi: 10.1111/j.1365-2826.2010.01961.x. [DOI] [PubMed] [Google Scholar]