Abstract
Sigma-1 receptors are associated with Alzheimer's disease, major depressive disorders, and schizophrenia. These receptors show progrowth/antiapoptotic properties via their chaperoning functions to counteract ER (endoplasmic reticulum) stress, to block neurodegeneration, and to regulate neuritogenesis. The sigma-1 receptor knock out mouse offered an opportunity to assess possible mechanisms by which the Sigma-1 receptor modulates cellular oxidative stress. Nuclear magnetic resonance (NMR) metabolomic screening of the WT (wild type) and sigma-1 KO (knockout) livers was performed to investigate major changes in metabolites that are linked to oxidative stress. Significant changes in protein levels were also identified by two-dimensional (2D) gel electrophoresis and mass spectrometry. Increased levels of the antioxidant protein peroxiredoxin 6 (Prdx6), and the ER chaperone BiP (GRP78) compared to WT littermates were detected. Oxidative stress was measured in WT and sigma-1 KO mouse liver homogenates, in primary hepatocytes and in lung homogenates. Furthermore, sigma-1 receptor mediated activation of the antioxidant response element (ARE) to upregulate NAD(P)H quinone oxidoreductase 1 (NQO1) and superoxide dismutase 1 (SOD1) mRNA expression in COS cells was shown by RT PCR. These novel functions of the sigma-1 receptor were sensitive to well-known sigma ligands via their antagonist/agonist properties.
Keywords: Sigma-1 receptor, metabolomics, ER stress, antioxidant response elements, oxidative stress, quinone oxidoreductase 1, superoxide dismutase 1
1. Introduction
Sigma receptors are unique nonopioid binding sites that are distinct from other known neurotransmitters or hormone receptors and ubiquitously expressed in different tissues (Hayashi and Su, 2008; Su et al., 2010). They were initially proposed as opioid receptors based on the binding of N-allyl-normetazocine (SKF-10047) (Martin et al., 1976). This mischaracterization, however, was later corrected when the sigma receptors were found to be insensitive to a common opioid receptor antagonist, naloxone (Iwamoto, 1981; Su, 1982). The sigma-1 receptor subtype has been cloned (Hanner et al., 1996) and shares about 90% identity and 95% similarity across mammalian species. The guinea pig sigma-1 receptor has been expressed in E. coli and purified to homogeneity (Ramachandran et al., 2007). The sigma-2 receptor has yet to be cloned. Pharmacological studies have indicated that the sigma-1 receptor is able to bind a wide range of compounds (Hayashi and Su, 2004; Su et al., 2010) and is able to mediate various cellular events in the nervous system (Monnet, 2005; Hayashi and Su, 2008). Steroids such as progesterone (Hayashi and Su, 2004) and DHEA, amines such as dimethyltryptamine (DMT)(Fontanilla et al., 2009), and lipids such as sphingosine (Ramachandran et al., 2009) have been identified as endogenous ligands for the sigma-1 receptor. A partial classification of some of the primary molecules that have been assessed as sigma-1 receptor agonists and antagonists have been reviewed (Su et al., 2010) (for structures of the agonist and antagonists used in this study, see Supplemental Figure 1).
Sigma-1 receptors have been reported to modulate intracellular calcium (Novakova et al., 1998; Wu and Bowen, 2008) via a direct interaction with L-type voltage gated calcium channels (Tchedre et al., 2008), increased synthesis of inositol triphosphate (IP3) (Novakova et al., 1998), and by interaction through the C-terminal region (amino acids 102–223) with ankyrin-B 220 to disrupt ankyrin-B 220 and IP3 receptor-3 interactions (Wu and Bowen, 2008). Both production of IP3 (Robison et al., 1995) and release of calcium from ER (endoplasmic reticulum) stores (Roveri et al., 1992) have been suggested to occur on exposure of cells to oxidative stress. Recently, sigma-1 receptors have been reported to function as novel ligand-operated chaperones that form a regulated chaperone machinery complex with another ER chaperone, BiP, to counteract ER stress (Hayashi and Su, 2007). Accumulation of misfolded proteins due to ER stress can release calcium from the ER lumen and activate the ER chaperone machinery (Zhang and Kaufman, 2008). This process, simultaneously, leads to generation and accumulation of intracellular reactive oxygen species since correct protein folding is an energy-consuming process that requires oxidizing conditions to form intramolecular and intermolecular disulphide bonds. Reducing equivalents are transferred from thiol groups from protein-folding substrates to molecular oxygen resulting in generation of reactive oxygen species (Cuozzo and Kaiser, 1999). Additional oxidative stress can also result from the depletion of reduced glutathione, which is consumed in reactions that reduce unstable and improperly formed disulphide bonds (Cuozzo and Kaiser, 1999). In addition, the calcium released from the ER is concentrated in the matrix of the mitochondria and causes depolarization of the inner mitochondrial membrane, disrupts electron transport and further increases reactive oxygen species production (Gorlach et al., 2006). Mitochondrial reactive oxygen species can additionally increase calcium release from the ER by sensitizing ER calcium-release channels and increase protein misfolding (Zhang and Kaufman, 2008).
Beneficial effects of reactive oxygen species occur at low/moderate concentrations and involve physiological roles in cellular responses in defense against environmental pathogens (Keisari et al., 1983), in the functioning of cellular signaling pathways (Droge, 2002), and the induction of a mitogenic response (Valko et al., 2007). However, overproduction of reactive oxygen species leads to oxidative stress, a deleterious process that can be an important mediator of damage to cell structure and has been implicated in various pathological conditions involving cardiovascular diseases, cancer, neurological disorders, diabetes, ischemia/reperfusion and aging (Butterfield et al., 2002; Hildeman et al., 2003; Valko et al., 2007; Paravicini and Touyz, 2008).
In this paper, we report that sigma-1 receptors function against cellular oxidative stress as evidenced by metabolic and proteomic examination of the Sigma-1 KO (knockout) and WT (wild type) littermates. Furthermore through activation of Antioxidant Response Element (ARE) genes sigma-1 receptors may provide additional layers of protection during ER stress in addition to its chaperoning activities as reported earlier (Hayashi and Su, 2007).
2. Material and Methods
2.1. Reagents
Primers for quantitative real time RT-PCR were synthesized by Integrated DNA Technologies, Coralville, IA. TransIT- LT1 transfection reagent was purchased from Mirus Bio, Madison, Wisconsin. Radioactive Na[125I] for the preparation of [125I] IAF was from Perkin-Elmer Life Sciences, Wellesley, MA. Luciferase assay kit (cat no E1550) and RNAsin (cat no N2511) were purchased from Promega, Madison, WI. All other chemicals were purchased from Sigma–Aldrich, St. Louis, MO unless otherwise mentioned.
2.2. Preparation of tissue homogenates from Wild type and the sigma-1 receptor KO mice
All animals were handled in accordance with “Animal Care and Use Guidelines” of the University of Wisconsin-Madison. The sigma-1 receptor KO mice were obtained from the “Mutant Mouse Regional Resource Centers” (MMRRC) at University of California, Davis. Liver and lung from WT and the sigma-1 receptor KO littermates (3 months old) (n = 6–10) were minced and homogenized by four bursts of 10 sec each using a Brinkman polytron on setting 6 (10 ml buffer gm−1 of wet tissue). Homogenization was performed in an ice-cold sodium phosphate buffer (10 mM, pH 7.4) containing 0.32 M sucrose and a cocktail of protease inhibitors [20 mg/ml leupeptin, 5 mg/ml soybean trypsin inhibitor, 100 mM phenylmethylsulfonylfluoride (PMSF), 100 mM benzamidine, and 1 mM EDTA]. The homogenates were snap frozen in liquid N2, and were stored at −80°C with a final protein concentration of approximately 10 mg/ml.
2.3. Isolation of primary hepatocytes and cell culture
Primary hepatocytes were isolated from adult WT and sigma-1 KO mice (C57BL/6J) as described by Bumgardner et al (Bumgardner et al., 1998) with slight modifications. Briefly, the livers were sequentially perfused in situ with 2.5 mM EGTA in calcium-free Dulbecco's phosphate buffer (3–4 ml min−1 for 5 min at 37°C) and with 0.05% collagenase type IV in a 1% albumin and balanced salt solution under the same conditions for 15 min for digestion. The livers were transferred to a Petri dish, where the liver tissue was gently minced and filtered (40 µM) to remove large aggregates. Liver cells were washed three times in DMEM with 10% fetal bovine serum (FBS) and centrifuged at 35×g for 5 min between washes. Hepatocytes were purified on a discontinuous 60% Percoll gradient (Pharmacia, Uppsala, Sweden) in a 50-ml conical tube and centrifuged at 140×g for 15 min. The hepatocyte pellet was resuspended in DMEM containing 10% FBS. Both COS-7 cells and primary hepatocytes culture were maintained with DMEM supplemented with 10% FBS containing penicillin and streptomycin.
2.4. Metabolomic Screening
The WT and KO mouse livers were frozen in liquid N2, ground, and extracted with water to assess metabolites. Two 2-D Heteronuclear Single Quantum Coherence (HSQC) spectra were collected on a Bruker DMX 500 MHz along with the metabolite standards at 2 mM, 5 mM, and 10 mM. Raw data were processed by the NMRPIPE program, and the processed data were analyzed by the SPARKY program. Three hundred mg of dried liver yielded 20 mg of dried extract which was dissolved in 0.3 ml of 5 mM HEPES, 0.5 mM DSS, 0.5 mM sodium azide. (NMR experiments were performed at the NMRFAM, Dept. of Biochemistry, UW-Madison).
2.5. Two dimensional gel electrophoresis and Mass spectrometry
The liver homogenates of both WT and the sigma-1 receptor KO mice were centrifuged at 100,000×g to separate membrane and cytosolic fractions. Two dimensional gel electrophoresis (performed by Kendrick Labs, Madison, WI) on 200 µg of the cytosolic and membrane fractions were separated initially using a 17 cm pH 3.5 – 10 linear IPG strip in duplicate followed by conventional 12% SDS-PAGE. The gels were stained with coomassie blue, dried and unique spots were used for identification of proteins using MALDI-TOF-TOF-MS at the Biotechnology Center, University of Wisconsin-Madison.
2.6. Measurement of oxidative stress
Oxidative stress levels were measured using the methods reported by Bejma et. al. (Bejma and Ji, 1999) with slight modifications. Known concentrations of tissue homogenates, primary hepatocytes or COS-7 cell lysates were incubated with 50 mM of 2’,7’-dichlorofluorescin diacetate (DCFH-DA) in DMEM for 30 minutes at 37°C in dark and the fluorescence of 2’,7’- dichlorofluorescin (DCF) was measured at 485/ 530 nm (exi/emi). The fluorescence of the DCFH-DA solution without any samples was taken as the blank.
2.7. Measurement of ARE activation
The ARE-luciferase and GC-ARE (mutant) constructs of the human NQO1 gene were a kind gift from Dr Jeff Johnson, University of Wisconsin-Madison and reported earlier (Lee et al., 2001). Both the sigma-1 receptor and the luciferase reporter construct were co-transfected into COS-7 cells (approximately 1×106 cells) using TransIT- LT1 transfection reagent. After 48 hours of transfection, cells were treated with different sigma ligands for 24 hours with a final concentration of 10 µM and ARE activation was measured using a luciferase assay kit (Promega, Madison) following the manufacturer’s protocol. COS-7 cells, transfected with ARE-Luciferase or mutant GC –ARE, were treated with well-known ARE activator tertiary butyl hydroquinone (t-BHQ) at a concentration of 100 µM for 4 hours after 2 days of transfection, for the positive control and negative control, respectively. Expression of the sigma-1 receptor was confirmed using western blot analysis and further utilized for transfection efficiency.
2.8. Quantitative Real-Time RT-PCR
Total RNA was purified from COS-7 cells (approximately 2×106 cells) with RNeasy Mini Kit (Qiagen) using the manufacturer’s protocol. Complemenray-DNA sequences were prepared by annealing RNA (1 µg) with 250 ng of a 5:1 mixture of random and oligo(dT) primers heated at 68°C for 10 min. This was followed by incubation with Moloney murine leukemia virus (MMLV) reverse transcriptase (50 units) (GIBCO/BRL) combined with 10 mM DTT, RNAsin, and 0.5 mM dNTPs at 42°C for 1 h. Reactions were diluted to a final volume of 150 µl and heat inactivated at 98°C for 5 min. Reactions (25 µl) contained 2.5 µl of cDNA, 12.5 µl of SYBR Green Master Mix (Applied Biosystems, Foster City, CA) and 200 nM of appropriate primers. Product accumulation was monitored by SYBR Green fluorescence. Control reactions lacking reverse transcriptase (RT) yielded very low signals. Relative expression levels were determined from a standard curve of serial dilutions of nontransfected COS-7 cells cDNA samples and were normalized to the expression of RpII 215.
Real-Time RT-PCR Primers
RpII 215
Forward, 5’-CGAATCCGCATCATGAACAG-3'
Reverse, 5'-TGCATCGCAGGAAGACATCA-3'
HMOX-1
Forward, 5'-CCACCAAGTTCAAGCAGCTCTA-3'
Reverse, 5'-GCTCCTGCAACTCCTCAAAGAG-3'
NQO1
Forward, 5'-GAACTTCAATCCCATCATTTCCAG-3’
Reverse, 5'-CAGCTTCTTTTGTTCAGCCACAAT-3’
SOD1
Forward, 5'-AGGTGTCTTTCGAAGATTCTGTGATC-3'
Reverse, 5'-TTTCTTCATTTCCACCTTTGCC-3'
CAT
Forward, 5'-GAGCAGCCCTGACAAAATGC-3'
Reverse, 5'-GGTAGGGACAGTTCACAGGTATCTG-3'
2.9. Statistical Analysis
P values were calculated using the software “Graphpad Prism” version 4.0c (GraphPad Software Inc, San Diego, CA).
3. Results
3.1. Sigma-1 receptor KO mouse showed higher oxidative stress
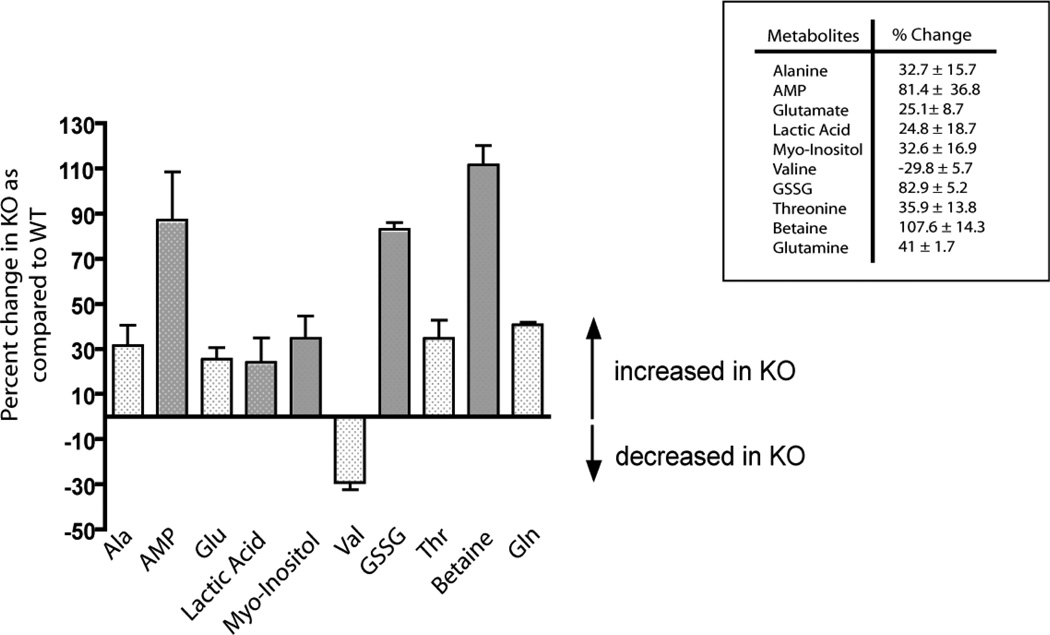
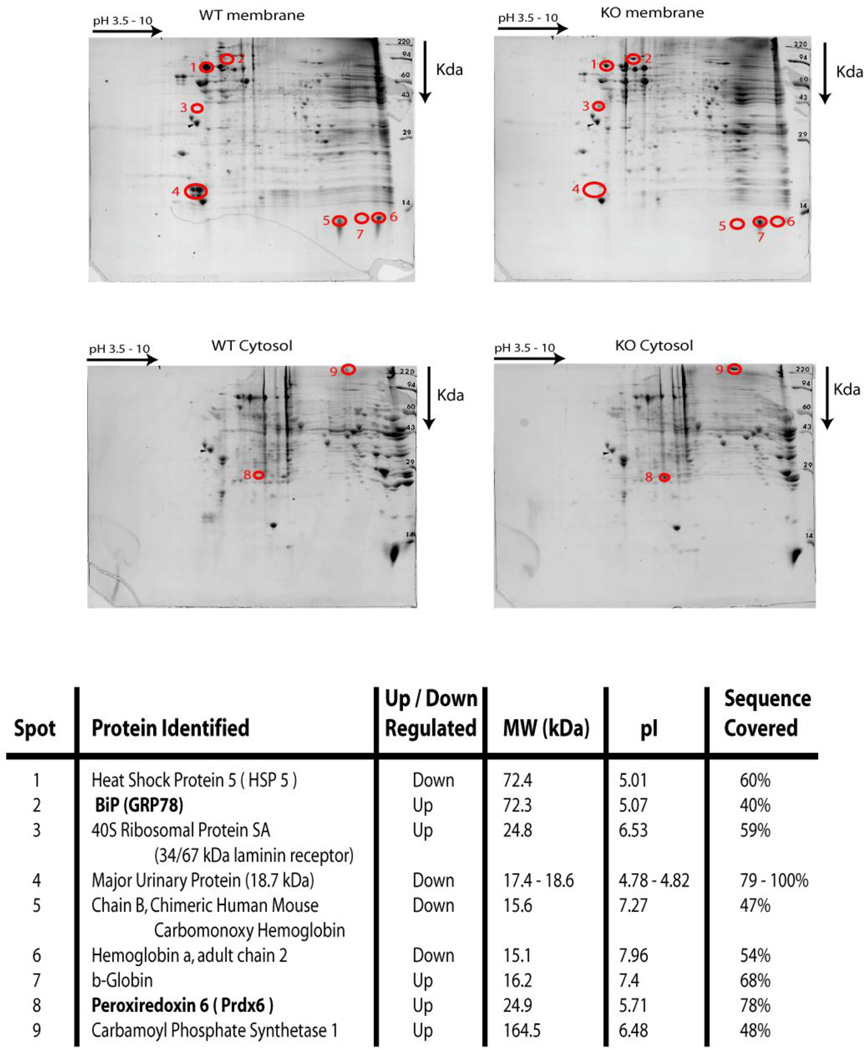
To explore the possible role of the sigma-1 receptor during oxidative stress, we studied primarily the sigma-1 KO mouse liver. We have performed both metabolomic screening and 2D gel electrophoresis of liver homogenates of both the WT and the sigma-1 receptor KO mouse to identify major changes in the metabolites and proteins (Fig 1 and Fig 2). Interestingly, metabolomic screening of the liver samples (n = 6) indicated that the sigma-1 receptor KO mouse livers had significant increases in the levels of metabolites that are well-known signatures of cellular oxidative stress such as oxidized glutathione (GSSG) and glutamate, and those that have protective roles against oxidative stress such as alanine, glutamine, lactic acid, AMP, myo-inositol, and betaine (Fig 1). An increase in threonine levels and a decrease in valine levels were also observed in the sigma-1 receptor KO livers compared to the WT liver (Fig 1). Two dimensional electrophoresis followed by mass spectrometric analysis reveled that the antioxidant protein Prdx6 (Fatma et al., 2005; Power et al., 2008), ER chaperone BiP (Schroder and Kaufman, 2005), 40S ribosomal protein SA, and carbamoyl phosphate synthetase 1 were up regulated in the sigma-1 receptor KO livers compared to the WT livers (Fig 2). In addition, major urinary proteins and HSPA5 were down regulated in KO livers (Fig 2). These results support the general conclusion that the sigma-1 receptor KO mouse has higher levels of oxidative stress than the WT mouse.
Figure 1.
Changes in metabolites in the WT and sigma-1 receptor KO mouse livers. Higher levels of oxidized glutathione (GSSG) and glutamate in the KO livers indicate that the KO mice were under oxidative stress. Error bars represent mean ± S.E.M. from different experiments (n = 6).
Figure 2.
Two dimensional gel electrophoresis of 200 µg of membrane (100,000×g pellet) and cytosolic fractions of the liver homogenates of the WT and sigma-1 receptor KO mice as described in the methods section. The gels were stained with Coomassie blue and marked spots showed the presence and absence of the unique proteins (shown by circled numbered spots), which were selected for sequence identification by mass spectrometry (shown in the lower panel). Up regulation of the antioxidant protein Prdx6 and the ER chaperone BiP was observed in the KO livers.
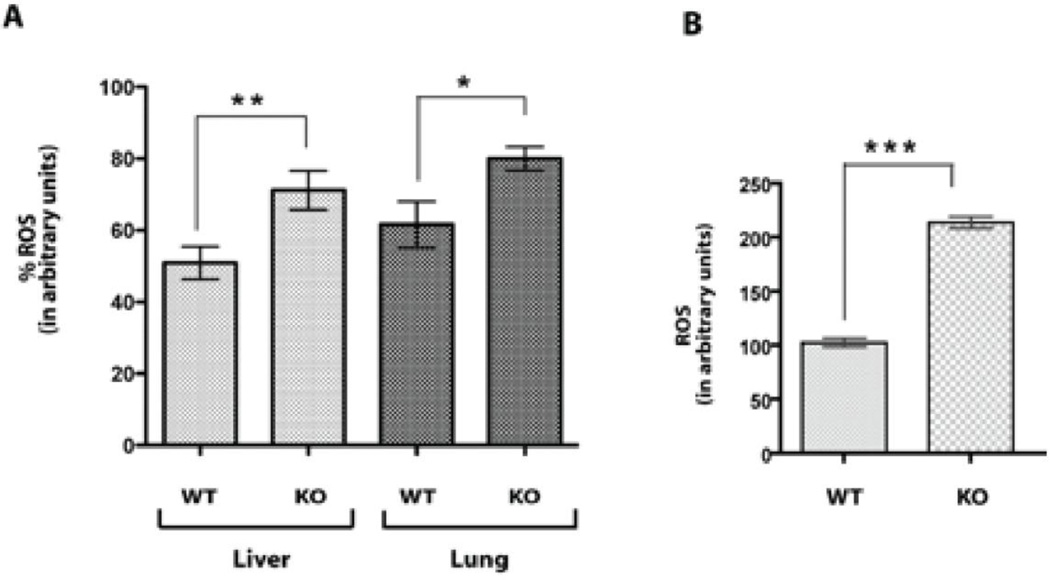
To confirm this view, we also measured the oxidative stress levels in the liver and lung tissues from both the WT (n = 8) and sigma-1 receptor KO mice (n = 9) using the oxidative conversion of DCFH-DA to the highly fluorescent DCF (LeBel et al., 1992) by endogenous reactive oxygen species. The oxidative stress levels in both tissue homogenates of the sigma-1 KO mice were significantly increased compared to that of the WT (Fig 3A). For further confirmation, we measured the oxidative stress levels in the isolated primary hepatocytes from both animals and found a similar pattern; that is, the sigma-1 KO hepatocytes had nearly a two fold higher oxidative stress level compared to the WT hepatocytes (Fig 3B).
Figure 3. Sigma-1 receptor KO mice have higher oxidative stress.
A. Reactive oxygen species (ROS) levels in liver and lung tissue homogenates (50 µg) from WT and sigma-1 receptor KO mice. Both liver and lung tissues from KO mouse showed higher levels of reactive oxygen species compared to that of WT tissues. **P < 0.001, *P < 0.01 by unpaired student t test, mean ± S.E.M of triplicate measurements from different mice (for WT n = 8 and for KO n = 9).
B. Reactive oxygen species levels in primary hepatocytes isolated from the WT and sigma-1 receptor KO mice. Approximately 2.5×106 cells were used in each condition. Cells were lysed using 500 µl of lysis buffer (25 mM Tris-HCl pGH 7.8 supplemented with 2mM DTT, 10% glycerol, 1% TritonX-100) and the protein concentrations in cell lysates were measured. 150 µg of total lysate were used for measurement of reactive oxygen species. The sigma-1 receptor KO hepatocytes showed higher levels of reactive oxygen species compared to that of WT hepatocytes and indicated that sigma-1 receptors are protective against oxidative stress. ***P < 0.0001 by unpaired student t test, mean ± S.E.M of three separate experiments (n = 3).
3.2. Decrease in oxidative stress levels by over-expression of the guinea pig sigma-1 receptor in COS-7 cells
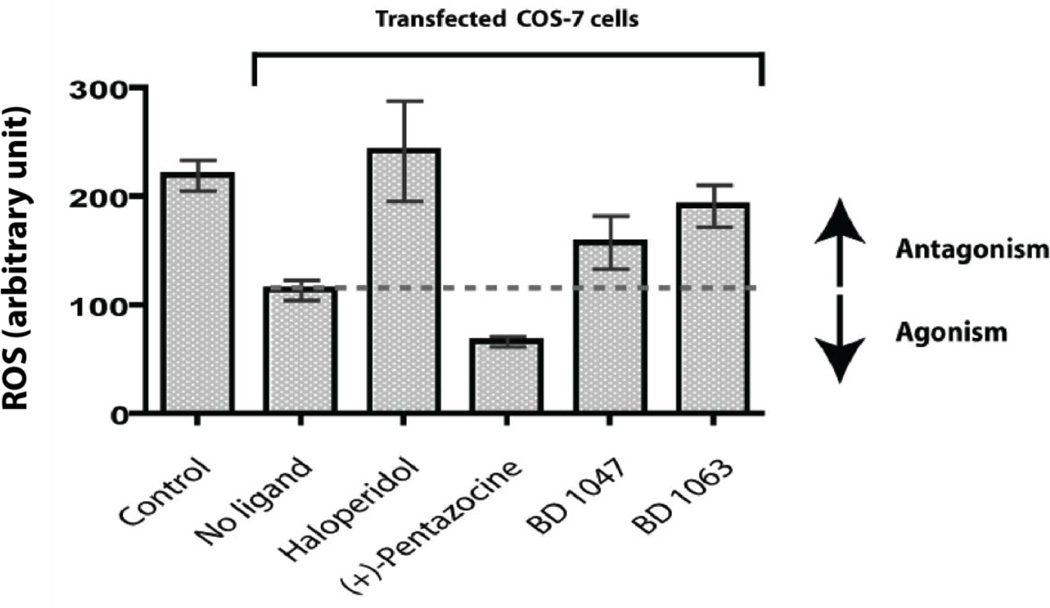
To further validate the functions of the sigma-1 receptor in protection against oxidative stress, we performed a gain-of-function analysis using COS-7 cells as a model cell line [in which the Sigma-1 receptor cannot be detected by photoaffinity labeling, Supplemental Figure 2A, or by western blot (data not shown)] and the guinea pig sigma-1 receptor as the model receptor (the sigma-1 receptor shares approximately 90% identity and 95% similarity across various species). Also no significant effects of Sigma-1 receptor ligands on reactive oxygen species levels in COS cells alone could be detected (Supplemental Figure 2B). Transient expression of the guinea pig sigma-1 receptor in COS-7 cells showed nearly 50% decrease in the measured levels of conversion of DCFH-DA to DCF (n = 4) compared to vehicle transfected COS-7 cells (Fig 4). These results indicated that the over-expressed guinea pig sigma-1 receptor in COS-7 cells is functionally protective against oxidative stress either alone or via endogenous agonist(s). Administration of the sigma-1 receptor antagonists (Hayashi and Su, 2008) haloperidol, BD 1047, and BD 1063 further increased the oxidative stress levels in sigma-1 receptor transfected COS-7 cells compared to control (no drug treatment) whereas (+)-pentazocine, a sigma-1 receptor agonist (Hayashi and Su, 2008), showed a decrease in oxidative stress levels (Fig 4). To check whether this function is cell specific or not, we also measured reactive oxygen species levels in RAW 264.7 cells after over-expressing sigma-1 receptor and found that over-expression of sigma-1 receptor in RAW 264.7 cells reduced the reactive oxygen species levels (nearly 30%) consistently (data not shown).
Figure 4. Sigma-1 receptor protects COS-7 cells from oxidative stress.
Reactive oxygen species (ROS) levels in COS-7 cells after transfection with the guinea pig sigma-1 receptors. Approximately 3×106 cells were used in each condition and cell lysates containing 150 µg of total proteins were used to measure reactive oxygen species in each condition. The sigma-1 receptor transfected COS-7 cells showed nearly a 50% reduction in reactive oxygen species levels compared to vehicle transfected (Mock) COS-7 cells. For determination of agonist/antagonist effects of the various sigma-1 receptor ligands, guinea pig sigma-1 receptor transfected COS-7 cells were treated with sigma-1 ligands for 24 hours with a final concentration of 10 µM before reactive oxygen species level measurements. The sigma-1 receptor antagonists haloperidol, BD 1047, and BD 1063 increased reactive oxygen species levels whereas the agonist (+)-pentazocine lowered the reactive oxygen species levels in the sigma-1 receptor transfected COS-7 cells. Error bars represent mean ± S.E.M. from four separate experiments (n = 4).
3.3. Sigma-1 receptors activate the Antioxidant Response Elements (ARE)
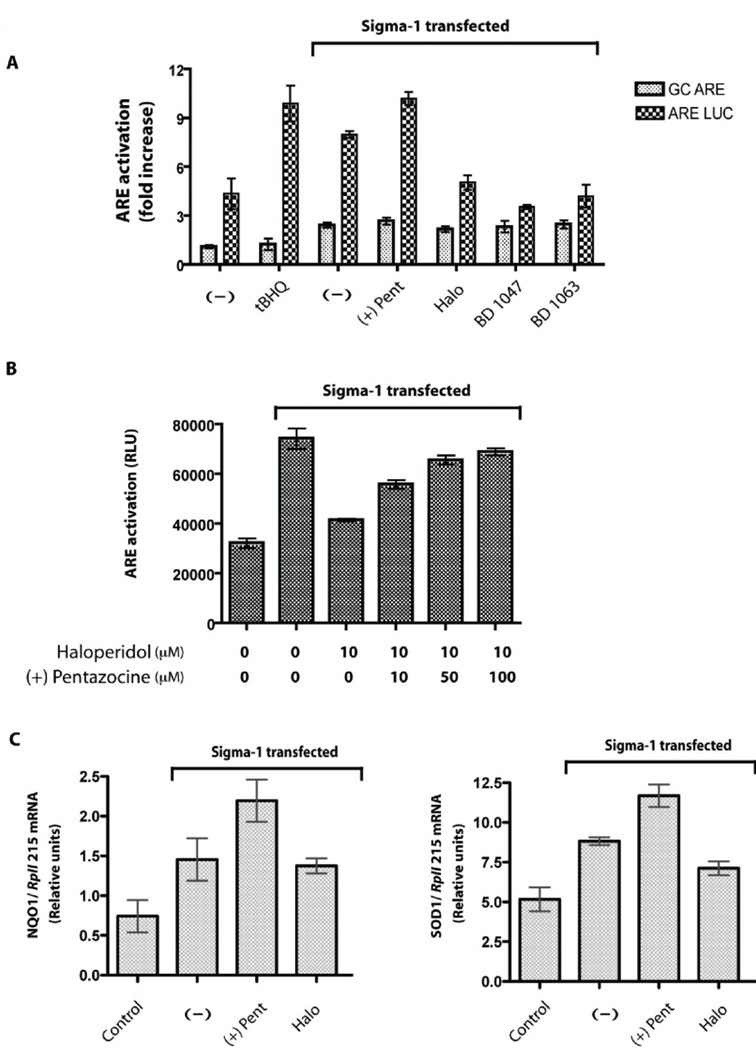
We further investigated whether that the sigma-1 receptor might be involved in signaling pathways which activate the ‘Antioxidant Response Element” (ARE), a cis-acting regulatory enhancer found in the 5’ flanking region of many phase II detoxifixation enzymes and antioxidant proteins such as NAD(P)H:quinone oxidoreductase (NQO), γ-glutamyl cysteine-synthase (γ-GCS), glutathione-S-transferase (GST), superoxide dismutase (SOD), catalase (CAT) and heme oxygenase-1 (HMOX-1) (Nguyen et al., 2003). Therefore, we investigated ARE activation in COS-7 cells transfected with sigma-1 receptors using an ARE- luciferase reporter construct with a minimal promoter region of the NQO1 gene which was reported previously (Lee et al., 2001). Interestingly, we found approximately an 8–10 fold increase in ARE activation in the presence of transfected sigma-1 receptors compared to controls. We also tested the effects of sigma-1 receptor agonists and antagonists and found that the sigma-1 receptor antagonists haloperidol, BD1047, and BD 1063 reduced ARE activation compared to control (no drug treatment) whereas the sigma-1 receptor agonists such as (+)-pentazocine showed increased ARE activation over the control (Fig 5A). The reduction by haloperidol on ARE activation was completely reversed by (+)-pentazocine cotreatment in a dose dependent manner (Fig 5B). In addition, we performed quantitative PCR of the NQO1, HMOX-1, SOD1 and CAT, the gene products under the control of the ARE enhancer (Nguyen et al., 2003). Transfection of the sigma-1 receptor itself increased the NQO1 and SOD1 mRNA levels almost twice over the mock transfected COS-7 cells (Fig 5C) while the mRNA levels of HMOX-1 and CAT remain unchanged (data not shwon). Addition of the sigma-1 agonist (+)-pentazocine further increased the NQO1 and SOD1 mRNA levels significantly over the sigma-1 receptor transfection alone condition (with no ligands) whereas the antagonist haloperidol lowered the NQO1 and SOD1 mRNA levels nearly to that of sigma-1 receptor transfection alone (Fig 5C). No change in the HMOX-1 and CAT mRNAs was observed (data not shown), indicating that the sigma-1 receptor selectively activated the ARE of SOD1 and NQO1 genes.
Figure 5. Sigma-1 receptor activates ‘Antioxidant Response Elements’ (ARE).
A. ARE activation by the sigma-1 receptors and its ligands in COS-7 cells (approximately 1×106 cells) using a luciferase reporter gene assay. Specificity of ARE activation was determined by using mutant GC-ARE luciferase construct, which is not activated by the known ARE activator, tertiary butyl hydroquinone (t-BHQ). Transfections of sigma-1 receptor in COS-7 cells showed almost a two fold specific ARE activation compared to that of the vehicle transfected COS-7 cells. The sigma-1 receptor agonist (+)-pentazocine (10 µM) showed a further increase in the specific ARE activation compared to that no drug treatment [(−) condition], whereas the antagonists haloperidol, BD 1047, and BD 1063 lowered the specific ARE activation. Error bars represent mean ± S.E.M. from three separate experiments (n = 3).
B. Reversal of haloperidol-mediated antagonism of ARE activation (n = 3). COS-7 cells transfected with guinea pig sigma-1 receptor (approximately 1×106 cells) were treated with 10 µM of haloperidol for 24 hours 2 days after transfection and lowered ARE activation was observed. Co-treatment of 50–100 µM (+)-pentazocine, a sigma-1 receptor agonist, reversed the antagonism shown by 10 µM of haloperidol. Error bars represent mean ± S.E.M. from different experiments (n = 3).
C. Real-time RT-PCR analysis of NQO1 and SOD1 mRNAs - the gene products which are under control of the ARE enhancer, in COS-7 cells. Transfection of sigma-1 receptor alone increased the SOD1 and NQO1 mRNA levels almost two fold compared to that of vehicle transfected condition (control) (n = 4). Treatment with the sigma-1 receptor agonist (+)-pentazocine (10 µM) showed a further increase in the NQO1 and SOD1 mRNA levels compared to the no drug treatment condition whereas the treatment of the antagonist haloperidol (10 µM) showed reverse effects. Error bars represent mean ± S.E.M. from four separate experiments (n = 4).
4. Discussion
Reactive oxygen species induce oxidative damage to macromolecular structures such as membranes and DNA and contribute to cellular damage as well as cell death through apoptosis and necrosis (Cave et al., 2005). Increased ligand binding activity to sigma-1 receptors in retinal Muller cells has been observed in vitro when the cells were treated with NO and reactive oxygen species donors (Jiang et al., 2006) and protection of human umbilical vein endothelial cells (HUVEC) from oxidative stress by the antioxidant methyl gallate, has been linked to up-regulation of the sigma-1 receptor gene (Whang et al., 2005). It has been also reported that sigma-1 receptor ligands can protect retinal cells against oxidative stress (Smith et al., 2008) and provide protection by increased transcription of antiapoptotic bcl-2 mRNA to preserve a favorable bcl-2/bax ratio in injured neurons (Yang et al., 2007) as well as in CHO cells via NFkappaB pathway (Meunier and Hayashi, 2010). An inverse correlation between the levels of Bcl2 family proteins and reactive oxygen species has already been established (Hildeman et al., 2003).
The sigma-1 receptor knockout mouse has been reported previously to be viable and fertile showing no overt constitutive phenotype (Langa et al., 2003). Our study provides an explanation for the functional redundancy of the sigma-1 receptor KO mouse. From our study as well as other published reports (Whang et al., 2005; Hayashi and Su, 2007; Yang et al., 2007; Smith et al., 2008), it is clear that the sigma-1 receptor has both antioxidative as well as chaperoning activities, which are sensitive to sigma-1 receptor ligands. Thus, it is expected that the sigma-1KO mouse would have higher levels of proteins and/or metabolites, which have either antioxidative or chaperoning activities in the cells. In fact, metabolomic studies revealed that the KO livers had higher oxidized glutathione (GSSG) and glutamate levels (Fig 1). Glutathione has previously been well characterized as the major cellular “redox buffer” in conjugation with thioredoxins for maintaining intracellular “redox homeostasis” and higher amounts of oxidized glutathione is an indicator of oxidative stress (Valko et al., 2007). Glutamate has also been well documented as an inducer of oxidative stress-related endothelial death by apoptosis (Parfenova et al., 2006). Thus, the increase in GSSG and glutamate indicated the presence of oxidative stress in the KO livers (Fig 1). On the other hand, alanine has been shown to possess cytoprotective effects against free radical-induced injury in various tissues (Grosser et al., 2004) and glutamine, as a precursor of glutathione, is required to maintain high levels of glutathione and to avoid oxidative stress damage (Amores-Sanchez and Medina, 1999). Thus, increases in glutamine and alanine provide protection against oxidative stress (Fig 1). Moreover, organic osmolytes, such as betaine and myo-inositol, which are also upregulated in the KO livers, are involved in cell volume homeostasis as well as in cell protection against oxidative stress (Warskulat et al., 2004) and may act as "chemical chaperones" to stabilize native protein structure and protein function (Welch and Brown, 1996). Increases in lactate and AMP (Fig 1) indicated a metabolic stress which favors anaerobic consumption of glucose to generate ATP in a less effective manner than through the TCA cycle and oxidative phosphorylation, a reactive oxygen species generating process. Identification of changes in protein levels also supports the hypothesis for functional redundancy in KO mice (Fig 2). Two dimensional gel electrophoresis followed by mass spectrometric identification showed dramatic upregulation of the ER chaperon BiP and antioxidant protein Peroxiredoxin 6 (Prdx6) in the sigma-1 receptor KO mouse livers (Fig 2) which most likely occurs to counteract the deficiencies in chaperoning as well as to provide antioxidative functions (Manevich and Fisher, 2005) due to lack of the sigma-1 receptors. A similar increase in Prdx6 has been reported in sigma-1 receptor knockdown experiments, using siRNA in primary cultures of mouse hippocampal neurons (Tsai et al., 2012).
In order to evaluate functionally the sigma-1 receptors further, we investigated the possible role of the sigma-1 receptor in oxidative stress and found that this receptor is protective against cellular oxidative stress (Fig 3A, Fig 3B, and Fig 4) without possessing direct reactive oxygen species scavenging activity (data not shown). The sigma-1 KO mice showed higher levels of oxidative stress (Fig 3A and 3B) and transfection of the sigma-1 receptor into COS-7 cells showed reduced oxidative stress (Fig 4). Transfection of the sigma-1 receptor in COS-7 cells resulted in activation of the ARE in a manner that was enhanced by the Sigma-1 receptor agonist, (+)-Pentazocine and reduced by the Sigma-1 receptor antagonists, Haloperidol, BD1047 and BD1063 (Fig 5A). The inhibition of ARE activity by Haloperidol was reversed by increasing concentrations of (+)-Pentazocine (Fig 5B). Moreover the sigma-1 receptor agonist (+)-pentazocine was able to increase the mRNA levels of the housekeeping antioxidant proteins NQO1 and SOD1 whereas the sigma-1 receptor antagonist, haloperidol, was without effect (Fig 5C). SODs convert superoxide to hydrogen peroxide (Storz, 2007), which is further neutralized by catalase. NQO1 genes encode cytosolic flavoenzymes that catalyze the beneficial two-electron reduction of quinones to hydroquinones and reduce the formation of reactive oxygen species by preventing the unwanted one-electron reduction of quinones in the presence of molecular oxygen (Vasiliou et al., 2006). ARE promoters are under transcriptional control of the transcription factor NF-E2-related factor 2 (Nrf2) (Johnson et al., 2008) which indicates that the sigma-1 receptor is capable of signaling through this transcriptional pathway in an as yet unknown mechanism. The Sigma-1 receptor has been previously linked to the regulation of transcription factor events that are associated with oxidative stress (Meunier and Hayashi, 2010).
In cells, ER calcium levels are regulated by intake through the ER-calcium ATPase and release through the ryanodine and IP3 receptors. Upon depletion of ER calcium due to ER stress or via agonist stimulation, the sigma-1 receptors dissociate from BiP leading to prolonged Ca2+ signaling from ER into mitochondria by chaperoning the IP3 type3 receptors at the ER mitocondrial interface (mitochondrion-associated ER membrane or MAM) (Hayashi and Su, 2007). Increase in the mitochondrial calcium generates more ATP by the TCA cycle and oxidative phosphorylation (ox-phos) via allosteric activation of several enzymes such as pyruvate dehydrogenase, isocitrate dehydrogenase, and alpha-ketoglutarate dehydrogenase, as well as stimulation of ATP synthase (complex V), alpha-glycerophosphate dehydrogenase, and adenine nucleotide translocase (Brookes et al., 2004). As a result, the effect of elevated mitochondrial Ca2+ concentration is the coordinated upregulation of the entire ox-phos machinery, resulting in faster respiratory chain activity and higher ATP output to meet the cellular ATP demand (Brookes et al., 2004). However, stimulation of the TCA cycle and ox-phos by Ca2+ will also enhance oxidative stress (reactive oxygen species) by forcing the mitochondria to function faster and consume more O2 (Brookes et al., 2004). Indeed, mitochondrial reactive oxygen species generation correlates well with metabolic rate where faster metabolism simply results in more respiratory chain electron leakage (Brookes et al., 2004). Thus, the reactive oxygen species generated in the mitochondrial inner membrane (mainly superoxide) is released into the mitochondrial matrix and the cytosol (Storz, 2007). In our study, we found that the NQO1 and SOD1 mRNAs were upregulated in the sigma-1 receptor transfected COS-7 cells via the activation of ARE and the sigma receptor agonists and antagonists further modulated this function (Fig 5). This novel sigma-1 receptor function, in addition to its chaperoning activity and channeling ER calcium to mitochondria via IP3 type 3 receptors, mediates protective effects during ER stress. Although transcription of the ARE gene battery has also been reported previously during ER stress (Cullinan and Diehl, 2004), our results indicate a novel route of ARE activation to increase NQO1 and SOD1 mRNA levels via the sigma-1 receptor to protect the cells against ER stress generated reactive oxygen species as shown schematically in Fig 6.
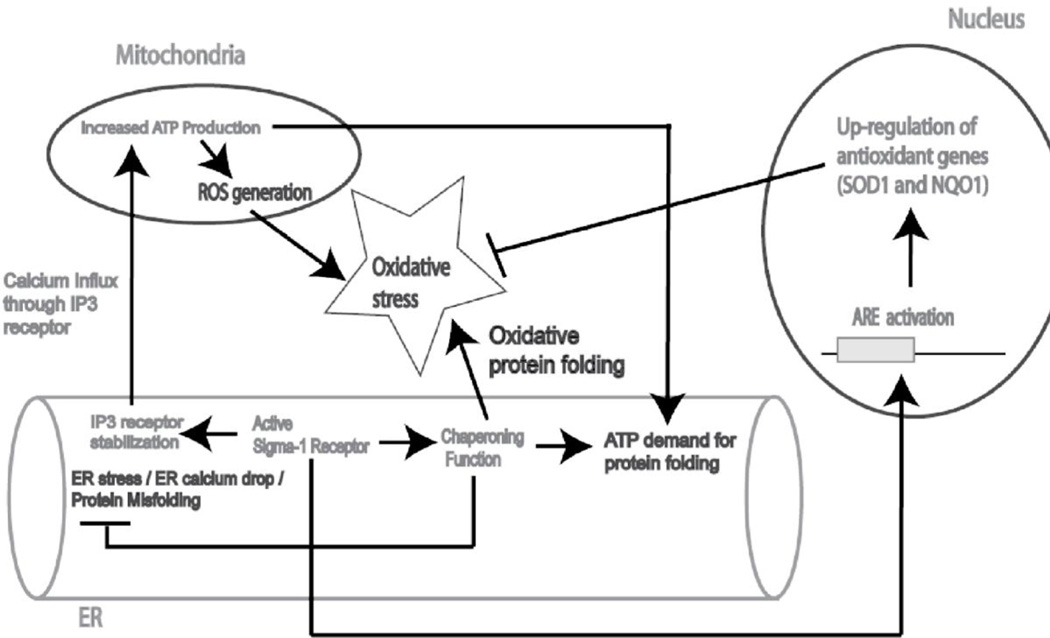
Figure 6.
Putative model for the mechanism of sigma-1 receptor mediated protection against cellular oxidative stress. ER stress or ER calcium drop leads to accumulation of unfolded proteins. Sigma-1 receptor functions as an ER chaperone to protect against ER stress and increase calcium mobilization from ER to mitochondria by stabilizing IP3 receptor(Hayashi and Su, 2007). Calcium influx into the mitochondria leads to increase ATP generation(Brookes et al., 2004) to counteract ATP demand for chaperoning function. Simultaneously, mitochondria also produce more reactive oxygen species (ROS) due to more respiratory chain electron leakage (Brookes et al., 2004). In addition, correct protein folding is an oxidative process, which also increases cellular oxidative stress(Cuozzo and Kaiser, 1999). Sigma-1 receptors counteract cellular oxidative stress by up regulation of antioxidant genes such as SOD1 and NQO1, through the activation of Antioxidant Response Elements (ARE).
5. Conclusion
In summary, the sigma-1 receptors protect the cell from oxidative stress as observed from the modulation of reactive oxygen species levels in the primary hepatocyes from the sigma-1 receptor knockout animal as well as in COS-7 cells when transfected. By reducing levels of reactive oxygen related oxidative stress, sigma-1 receptors are likely to provide additional layers of protection during ER stress in addition to its chaperoning activities (Hayashi and Su, 2007; Tsai et al., 2009). Application of sigma-1 receptor agonists are likely to be useful therapeutic strategies in the treatment of several diseases that involve oxidative stress (Maurice and Su, 2009) such as cardiovascular diseases, cancer, neurodegenerative disorders, diabetes, ischemia/reperfusion, Alzheimer’s (Villard et al., 2009; Su et al., 2010; Villard et al., 2010) and CNS inflammatory conditions associated with cocaine and HIV (Su et al., 2010; Yao et al., 2010).
Supplementary Material
Acknowledgements
This work was supported by National Institutes of Health Grant MH065503 and a Retina Research Foundation Edwin and Dorothy Gamewell Professorship (to A.E.R). We thank Dr. Jeff Johnson, School of Pharmacy, University of Wisconsin-Madison for providing us with both ARE-luciferase and mutant ARE-luciferase (GC-ARE) constructs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amores-Sanchez MI, Medina MA. Glutamine, as a precursor of glutathione, and oxidative stress. Mol. Genet. Metab. 1999;67:100–105. doi: 10.1006/mgme.1999.2857. [DOI] [PubMed] [Google Scholar]
- Bejma J, Ji LL. Aging and acute exercise enhance free radical generation in rat skeletal muscle. J. Appl. Physiol. 1999;87:465–470. doi: 10.1152/jappl.1999.87.1.465. [DOI] [PubMed] [Google Scholar]
- Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium ATP ROS: a mitochondrial love-hate triangle. Am. J. Physiol. Cell. Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- Bumgardner GL, Heininger M, Li J, Xia D, Parker-Thornburg J, Ferguson RM, Orosz CG. A functional model of hepatocyte transplantation for in vivo immunologic studies. Transplantation. 1998;65:53–61. doi: 10.1097/00007890-199801150-00011. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Castegna A, Lauderback CM, Drake J. Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer's disease brain contribute to neuronal death. Neurobiol. Aging. 2002;23:655–664. doi: 10.1016/s0197-4580(01)00340-2. [DOI] [PubMed] [Google Scholar]
- Cave A, Grieve D, Johar S, Zhang M, Shah AM. NADPH oxidase-derived reactive oxygen species in cardiac pathophysiology. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2005;360:2327–2334. doi: 10.1098/rstb.2005.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan SB, Diehl JA. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J. Biol. Chem. 2004;279:20108–20117. doi: 10.1074/jbc.M314219200. [DOI] [PubMed] [Google Scholar]
- Cuozzo JW, Kaiser CA. Competition between glutathione and protein thiols for disulphide-bond formation. Nat. Cell Biol. 1999;1:130–135. doi: 10.1038/11047. [DOI] [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Fatma N, Kubo E, Sharma P, Beier DR, Singh DP. Impaired homeostasis and phenotypic abnormalities in Prdx6−/−mice lens epithelial cells by reactive oxygen species: increased expression and activation of TGFbeta. Cell. Death Differ. 2005;12:734–750. doi: 10.1038/sj.cdd.4401597. [DOI] [PubMed] [Google Scholar]
- Fontanilla D, Johannessen M, Hajipour AR, Cozzi NV, Jackson MB, Ruoho AE. The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science. 2009;323:934–937. doi: 10.1126/science.1166127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlach A, Klappa P, Kietzmann T. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid. Redox. Signal. 2006;8:1391–1418. doi: 10.1089/ars.2006.8.1391. [DOI] [PubMed] [Google Scholar]
- Groser N, Oberle S, Berndt G, Erdmann K, Hemmerle A, Schroder H. Antioxidant action of L-alanine: heme oxygenase-1 and ferritin as possible mediators. Biochem. Biophys. Res. Commun. 2004;314:351–355. doi: 10.1016/j.bbrc.2003.12.089. [DOI] [PubMed] [Google Scholar]
- Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig J, Kempner E, Glossmann H. Purification, molecular cloning, and expression of the mammalian sigma1- binding site. Proc. Natl. Acad. Sci. U. S. A. 1996;93:8072–8077. doi: 10.1073/pnas.93.15.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Sigma-1 receptor ligands: potential in the treatment of neuropsychiatric disorders. CNS Drugs. 2004;18:269–284. doi: 10.2165/00023210-200418050-00001. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP. An update on the development of drugs for neuropsychiatric disorders: focusing on the sigma 1 receptor ligand. Expert. Opin. Ther. Targets. 2008;12:45–58. doi: 10.1517/14728222.12.1.45. [DOI] [PubMed] [Google Scholar]
- Hildeman DA, Mitchell T, Aronow B, Wojciechowski S, Kappler J, Marrack P. Control of Bcl-2 expression by reactive oxygen species. Proc. Natl. Acad. Sci. U. S. A. 2003;100:15035–15040. doi: 10.1073/pnas.1936213100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto ET. Locomotor activity and antinociception after putative mu, kappa and sigma opioid receptor agonists in the rat: influence of dopaminergic agonists and antagonists. J. Pharmacol. Exp. Ther. 1981;217:451–460. [PubMed] [Google Scholar]
- Jiang G, Mysona B, Dun Y, Gnana-Prakasam JP, Pabla N, Li W, Dong Z, Ganapathy V, Smith SB. Expression, subcellular localization, and regulation of sigma receptor in retinal muller cells. Invest. Ophthalmol. Vis. Sci. 2006;47:5576–5582. doi: 10.1167/iovs.06-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JA, Johnson DA, Kraft AD, Calkins MJ, Jakel RJ, Vargas MR, Chen PC. The Nrf2-ARE pathway: an indicator and modulator of oxidative stress in neurodegeneration. Ann. N. Y. Acad. Sci. 2008;1147:61–69. doi: 10.1196/annals.1427.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keisari Y, Braun L, Flescher E. The oxidative burst and related phenomena in mouse macrophages elicited by different sterile inflammatory stimuli. Immunobiology. 1983;165:78–89. doi: 10.1016/S0171-2985(83)80048-5. [DOI] [PubMed] [Google Scholar]
- Langa F, Codony X, Tovar V, Lavado A, Gimenez E, Cozar P, Cantero M, Dordal A, Hernandez E, Perez R, Monroy X, Zamanillo D, Guitart X, Montoliu L. Generation and phenotypic analysis of sigma receptor type I (sigma 1) knockout mice. Eur J. Neurosci. 2003;18:2188–2196. doi: 10.1046/j.1460-9568.2003.02950.x. [DOI] [PubMed] [Google Scholar]
- LeBel CP, Ischiropoulos H, Bondy SC. Evaluation of the probe 2',7'-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 1992;5:227–231. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
- Lee JM, Moehlenkamp JD, Hanson JM, Johnson JA. Nrf2-dependent activation of the antioxidant responsive element by tert-butylhydroquinone is independent of oxidative stress in IMR-32 human neuroblastoma cells. Biochem. Biophys. Res. Commun. 2001;280:286–292. doi: 10.1006/bbrc.2000.4106. [DOI] [PubMed] [Google Scholar]
- Manevich Y, Fisher AB. Peroxiredoxin 6, a 1-Cys peroxiredoxin, functions in antioxidant defense and lung phospholipid metabolism. Free Radic. Biol. Med. 2005;38:1422–1432. doi: 10.1016/j.freeradbiomed.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J. Pharmacol. Exp. Ther. 1976;197:517–532. [PubMed] [Google Scholar]
- Maurice T, Su TP. The pharmacology of sigma-1 receptors. Pharmacol. Ther. 2009;124:195–206. doi: 10.1016/j.pharmthera.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier J, Hayashi T. Sigma-1 receptors regulate Bcl-2 expression by reactive oxygen species-dependent transcriptional regulation of nuclear factor kappaB. J. Pharmacol. Exp. Ther. 2010;332:388–397. doi: 10.1124/jpet.109.160960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnet FP. Sigma-1 receptor as regulator of neuronal intracellular Ca2+: clinical and therapeutic relevance. Biol. Cell. 2005;97:873–883. doi: 10.1042/BC20040149. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Sherratt PJ, Pickett CB. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu. Rev. Pharmacol. Toxicol. 2003;43:233–260. doi: 10.1146/annurev.pharmtox.43.100901.140229. [DOI] [PubMed] [Google Scholar]
- Novakova M, Ela C, Bowen WD, Hasin Y, Eilam Y. Highly selective sigma receptor ligands elevate inositol 1,4,5-trisphosphate production in rat cardiac myocytes. Eur J. Pharmacol. 1998;353:315–327. doi: 10.1016/s0014-2999(98)00398-7. [DOI] [PubMed] [Google Scholar]
- Paravicini TM, Touyz RM. NADPH oxidases, reactive oxygen species, and hypertension: clinical implications and therapeutic possibilities. Diabetes Care. 2008;31 Suppl. 2:S170–180. doi: 10.2337/dc08-s247. [DOI] [PubMed] [Google Scholar]
- Parfenova H, Basuroy S, Bhattacharya S, Tcheranova D, Qu Y, Regan RF, Leffler CW. Glutamate induces oxidative stress and apoptosis in cerebral vascular endothelial cells: contributions of HO-1 and HO-2 to cytoprotection. Am. J. Physiol. Cell Physiol. 2006;290:C1399–C1410. doi: 10.1152/ajpcell.00386.2005. [DOI] [PubMed] [Google Scholar]
- Power JH, Asad S, Chataway TK, Chegini F, Manavis J, Temlett JA, Jensen PH, Blumbergs PC, Gai WP. Peroxiredoxin 6 in human brain: molecular forms, cellular distribution and association with Alzheimer's disease pathology. Acta Neuropathol. 2008;115:611–622. doi: 10.1007/s00401-008-0373-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran S, Chu UB, Mavlyutov TA, Pal A, Pyne S, Ruoho AE. The sigma1 receptor interacts with N-alkyl amines and endogenous sphingolipids. Eur. J. Pharmacol. 2009;609:19–26. doi: 10.1016/j.ejphar.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran S, Lu H, Prabhu U, Ruoho AE. Purification and characterization of the guinea pig sigma-1 receptor functionally expressed in Escherichia coli. Protein Expr. Purif. 2007;51:283–292. doi: 10.1016/j.pep.2006.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison TW, Zhou H, Forman HJ. Modulation of ADP-stimulated inositol phosphate metabolism in rat alveolar macrophages by oxidative stress. Arch. Biochem. Biophys. 1995;318:215–220. doi: 10.1006/abbi.1995.1223. [DOI] [PubMed] [Google Scholar]
- Roveri A, Coassin M, Maiorino M, Zamburlini A, van Amsterdam FT, Ratti E, Ursini F. Effect of hydrogen peroxide on calcium homeostasis in smooth muscle cells. Arch. Biochem. Biophys. 1992;297:265–270. doi: 10.1016/0003-9861(92)90671-i. [DOI] [PubMed] [Google Scholar]
- Schroder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat. Res. 2005;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- Smith SB, Duplantier J, Dun Y, Mysona B, Roon P, Martin PM, Ganapathy V. In vivo protection against retinal neurodegeneration by sigma receptor 1 ligand (+)-pentazocine. Invest. Ophthalmol. Vis. Sci. 2008;49:4154–4161. doi: 10.1167/iovs.08-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz P. Mitochondrial reactive oxygen species --radical detoxification, mediated by protein kinase D. Trends Cell Biol. 2007;17:13–18. doi: 10.1016/j.tcb.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Su TP. Evidence for sigma opioid receptor: binding of [3H]SKF-10047 to etorphine-inaccessible sites in guinea-pig brain. J. Pharmacol. Exp. Ther. 1982;223:284–290. [PubMed] [Google Scholar]
- Su TP, Hayashi T, Maurice T, Buch S, Ruoho AE. The sigma-1 receptor chaperone as an inter-organelle signaling modulator. Trends Pharmacol. Sci. 2010;31:557–566. doi: 10.1016/j.tips.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchedre KT, Huang RQ, Dibas A, Krishnamoorthy RR, Dillon GH, Yorio T. Sigma-1 receptor regulation of voltage-gated calcium channels involves a direct interaction. Invest. Ophthalmol. Vis. Sci. 2008;49:4993–5002. doi: 10.1167/iovs.08-1867. [DOI] [PubMed] [Google Scholar]
- Tsai SY, Hayashi T, Harvey BK, Wang Y, Wu WW, Shen RF, Zhang Y, Becker KG, Hoffer BJ, Su TP. Sigma-1 receptors regulate hippocampal dendritic spine formation via a free radical-sensitive mechanism involving Rac1xGTP pathway. Proc. Natl. Acad. Sci. U. S. A. 2009;106:22468–22473. doi: 10.1073/pnas.0909089106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SY, Rothman RK, Su TP. Insights into the Sigma-1 receptor Chaperone's cellular functions: A microarray report. Synapse. 2012;66:42–51. doi: 10.1002/syn.20984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Vasiliou V, Ross D, Nebert DW. Update of the NAD(P)H:quinone oxidoreductase (NQO) gene family. Hum. Genomics. 2006;2:329–335. doi: 10.1186/1479-7364-2-5-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villard V, Espallergues J, Keller E, Alkam T, Nitta A, Yamada K, Nabeshima T, Vamvakides A, Maurice T. Antiamnesic and neuroprotective effects of the aminotetrahydrofuran derivative ANAVEX1-41 against amyloid beta(25–35)-induced toxicity in mice. Neuropsychopharmacology. 2009;34:1552–1566. doi: 10.1038/npp.2008.212. [DOI] [PubMed] [Google Scholar]
- Villard V, Espallergues J, Keller E, Vamvakides A, Maurice T. Anti-amnesic and neuroprotective potentials of the mixed muscarinic receptor/sigma 1 ({sigma}1) ligand ANAVEX2-73, a novel aminotetrahydrofuran derivative. J. Psychopharmacol. 2010 doi: 10.1177/0269881110379286. [DOI] [PubMed] [Google Scholar]
- Warskulat U, Reinen A, Grether-Beck S, Krutmann J, Haussinger D. The osmolyte strategy of normal human keratinocytes in maintaining cell homeostasis. J. Invest. Dermatol. 2004;123:516–521. doi: 10.1111/j.0022-202X.2004.23313.x. [DOI] [PubMed] [Google Scholar]
- Welch WJ, Brown CR. Influence of molecular and chemical chaperones on protein folding. Cell Stress Chaperones. 1996;1:109–115. doi: 10.1379/1466-1268(1996)001<0109:iomacc>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whang WK, Park HS, Ham IH, Oh M, Namkoong H, Kim HK, Hwang DW, Hur SY, Kim TE, Park YG, Kim JR, Kim JW. Methyl gallate and chemicals structurally related to methyl gallate protect human umbilical vein endothelial cells from oxidative stress. Exp. Mol. Med. 2005;37:343–352. doi: 10.1038/emm.2005.44. [DOI] [PubMed] [Google Scholar]
- Wu Z, Bowen WD. Role of sigma-1 receptor C-terminal segment in inositol 1,4,5-trisphosphate receptor activation: constitutive enhancement of calcium signaling in MCF-7 tumor cells. J. Biol. Chem. 2008;283:28198–28215. doi: 10.1074/jbc.M802099200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Bhardwaj A, Cheng J, Alkayed NJ, Hurn PD, Kirsch JR. Sigma receptor agonists provide neuroprotection in vitro by preserving bcl-2. Anesth. Analg. 2007;104:1179–1184. doi: 10.1213/01.ane.0000260267.71185.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Yang Y, Kim KJ, Bethel-Brown C, Gong N, Funa K, Gendelman HE, Su TP, Wang JQ, Buch S. Molecular mechanisms involving sigma receptor-mediated induction of MCP-1: implication for increased monocyte transmigration. Blood. 2010;115:4951–4962. doi: 10.1182/blood-2010-01-266221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.