Abstract
Current chemotherapeutics are characterized by efficient tumor cell-killing and severe side effects mostly derived from off target toxicity. Hence targeted delivery of these drugs to tumor cells is actively sought. In an in vitro system, we previously demonstrated that targeted drug delivery to cancer cells overexpressing epidermal growth factor receptor (EGFR+) can be achieved by poly(ethylene glycol)-functionalized carbon nanovectors simply mixed with a drug, paclitaxel, and an antibody that binds to the epidermal growth factor receptor, Cetuximab. This construct is unusual in that all three components are assembled through non-covalent interactions. Here we show that this same construct is effective in vivo, enhancing radiotherapy of EGFR+ tumors. This targeted nanovector system has the potential to be a new therapy for head and neck squamous cell carcinomas, deserving of further preclinical development.
Keywords: targeted drug delivery, cancer, nanovectors, hydrophilic carbon clusters, Cetuximab, EGFR+
Head and neck squamous cell carcinoma (HNSCC) represents approximately 3.2% of cancers in the United States and accounted for approximately 49,260 new cancer diagnoses and 7,600 deaths in 2010.1 Despite advances in diagnosis and treatment, the 5-year survival of patients with HNSCC has not improved appreciably over the past few decades.2 One reason for the high treatment failure rate is that the therapeutic ratios of chemotherapy and radiation therapy alone or in combination are not high enough and patients often relapse after these treatments and/or have significant treatment related toxicities. Multimodal therapy that combines targeted drug delivery with radiation therapy and possibly surgical resection has shown promise to overcome this limitation and improve patient outcomes.3
There is a growing awareness that due to the heterogeneity of cancer, such an approach will demand personalized targeting components to realize its potential.4–5 Personalized medicine will involve the characterization of a patient’s tumor(s), the identification of which drugs and targeting ligands will be effective and finally the preparation of a final formulation that will use these ligands to deliver the drugs selectively to the cancer cells. One class of materials identified as potentially suitable platforms for the synergistic assembly of drugs and targeting ligands are nanovectors, which are materials with dimensions in the size range 1–100 nm that are capable of transporting and delivering one or more bioactive molecules.6–7 Nanovectors can carry multiple drugs, imaging agents and targeting ligands, and drug-loaded nanovectors have demonstrated enhanced efficacy with reduced toxic side effects as compared to conventional systemic chemotherapies.8–11 However, for nanovectors to fully enable personalize medicine, the final assembly of drugs and targeting ligands must be simple and modular such that the final therapeutic can be prepared in a timely manner for the patient.
We have been working towards this goal by developing a nanovector that can be loaded with hydrophobic drugs and functionalized with targeting antibodies all by simple mixing. For a proof-of-principle demonstration, paclitaxel (PTX) was selected as the drug to be studied as it is a classic example of a water-insoluble drug with high therapeutic efficacy and severe off-target toxicity. Cetuximab (Erbitux, ImClone Systems) (Cet) is an IgG monoclonal antibody that exclusively binds to epidermal growth factor receptor (EGFR) with high affinity and blocks the normal function of the receptor.12–14 It was chosen as the targeting antibody because it is the most widely studied EGFR targeting agent and is approved by the Food and Drug Administration for the treatment of patients with HNSCC.15–16 Approximately 90% of HNSCCs overexpress the epidermal growth factor receptor (EGFR) and this is correlated with worse clinical outcomes.17
We previously reported on the preparation of extremely small (<40-nm-long and 1-nm-wide) hydrophilic carbon clusters (HCCs) that are poly(ethylene glycol) (PEG) functionalized (PEG-HCCs) (Figure 1).18–21 Preliminary studies suggested that the PEG-HCCs were not toxic.18 When the PEG-HCCs were administered to mice at a dose (1 g/L) of up to 10× that used for drug delivery, no acute toxicity was observed over 5 d. After 5 d the mice were euthanized, a terminal blood sample was collected, and the major organs including the heart, lungs, spleen, kidneys, liver, and brain were removed and examined for gross toxicity. At all tested concentrations, no abnormalities were seen in any of the organs, warranting a longer-term toxicity experiment. Thus, nude mice received a tail vein injection of PEG-HCCs (200 mg/L, 2× the drug delivery concentration) once per week, for up to 10 weeks. All animals were observed daily; the mice did not show any visual signs of fatigue or discomfort and slowly increased in body weight over the 10 week period. Mice were euthanized after 1, 2, 4, 6, 8, and 10 weeks of weekly treatments for histological analysis and no gross toxicity was apparent in any of the organs analyzed. Total blood counts were performed on the mice and all analytes were within normal ranges. Finally, biodistribution studies indicated that the PEG-HCCs had a blood half-life of 2 to 3 h; the large majority of the PEG-HCCs were excreted through the kidneys, the primary accumulation of the trace agglomerated carbon was in the liver and spleen, but no lesions were seen.18
Figure 1.
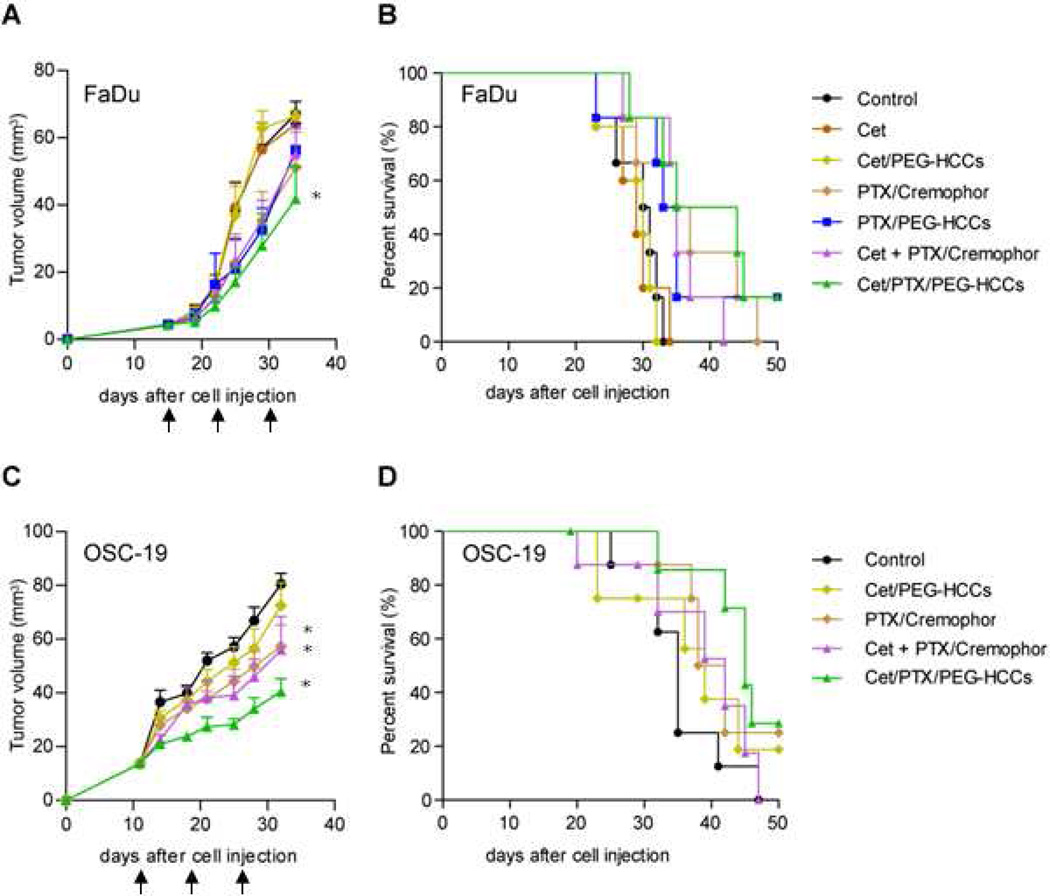
Development of Cet/PTX/PEG-HCCs. A) PEG-HCCs have carbon cores approximately 40 nm × 1 nm functionalized with various oxygen containing functional groups (note that only one each of several representative groups are shown). PEG is conjugated to the core via an amide bond. By simple mixing protocols, the PEG-HCCs can be loaded with PTX and wrapped with Cet. B) Administering PEG-HCCs weekly to mice for 10 weeks results in no detectable toxicity, represented here by an image (100×) of the liver from a mouse treated for 10 weeks. The edge length of the image is 200 µm. The arrows mark the darker spots that likely indicate the presence of trace PEG-HCC aggregates.. C) PTX/PEG-HCCs have efficacy equivalent to Taxol® (PTX/Cremophor) for treating a murine orthotopic model of head and neck cancer. The arrows indicate the time-points of delivered treatment. D) When PTX/PEG-HCCs are functionalized with Cet, they target the delivery of PTX to EGFR+ cells in vitro. * p < 0.05.
We showed that PEG-HCCs are able to sequester PTX (collectively PTX/PEG-HCCs; in this nomenclature, the slash “/” signifies a non-covalent linkage and the dash “-“ signifies a covalent bond) by physisorption and to deliver the drug for killing of cancer cells in vitro and in vivo.18 It was shown that the PTX/PEG-HCCs were stable in solution for at least 5 months. Both clinical formulations of PTX, Taxol® and Abraxane®, make use of a similar strategy of solubilizing unmodified PTX, likely due to both the ease of preparing this class of formulations and the fact that covalently modifying the PTX can alter its efficacy. Both in vitro and in vivo, the efficacy of PTX/PEG-HCCs was equivalent to that of Taxol® (Bristol-Myers-Squibb, Princeton, NJ, USA), which is PTX solubilized in ethanol and a polyethoxylated castor oil, Cremophor EL® (PTX/Cremophor). We further showed that mixing the PTX/PEG-HCCs with Cet results in a targeted drug delivery vehicle (Cet/PTX/PEG-HCCs) in which both the drug and the antibody are physisorbed on the amphiphilic carbon core of the nanovectors. We demonstrated that Cet/PTX/PEG-HCCs is stable for >6 h in the presence of a physiological concentration of albumin, and that it targets in vitro the delivery of PTX to EGFR+ cells via binding to the EGFR.22 Since the blood half life of the PEG-HCCs is 3 h, we hypothesized that the Cet/PTX/PEG-HCCs might be stable during their period of circulation in vivo. Here we demonstrate that this readily prepared targeted drug delivery vehicle is effective in vivo and can be used to radiosensitize tumor cells in in vitro and in vivo models of human HNSCC.
Results and Discussion
We began by comparing the efficacy of Cet/PTX/PEG-HCCs to Cet/PTX/Cremophor and the appropriate controls for the treatment of an orthotopic nude mouse model of tongue cancer derived from FaDu cells (Figure 2A,B). A very low amount of Cet (36 µg/mL) was used to prepare the Cet/PTX/PEG-HCCs. This amount of Cet alone or in combination with the PEG-HCCs did not show any antitumor effect. Consistent with our previous results, PTX/Cremophor and PTX/PEG-HCCs showed an equivalent antitumor effect. Combining Cet with PTX/Cremophor did not increase the efficacy of the treatment as this formulation was as effective as the two non-targeted formulations. However, in this tumor model, while these three treatments appear to be modestly effective, they are not statistically significantly different than the control group. The only treatment in this model that had a significantly enhanced antitumor effect relative to control was the Cet/PTX/PEG-HCCs, but the efficacy of this treatment was not significantly different than that of any treatment containing PTX. The results from this particular cell line were suggestive but not conclusive.
Figure 2.
Treatment effect on tumor growth and survival for mice bearing tumors derived from FaDu cells (A,B) or OSC-19 cells (C,D). Plots A and C represent the tumor size while B and D are the mouse survival plots. Mice with orthotopically established oral tongue tumors were injected via the tail vein once weekly (indicated with black arrows) with the treatment. Survival was analyzed by the Kaplan-Meier method and compared with log-rank tests. * p < 0.05 compared to control at day 34.
Thus, we compared a smaller subset of the treatments in this same tumor model, but with tumors derived from OSC-19 cells (Figure 2C,D). Again, PTX/Cremophor and Cet/PTX/Cremophor showed equivalent efficacy, but, in this case, there was a significant delay in tumor growth and extension of survival. Cet/PTX/PEG-HCCs treatment also resulted in a significant delay in tumor growth and extension of survival, but while this treatment appeared to be more effective than either of the Cremophor-based treatments, that difference was not significant.
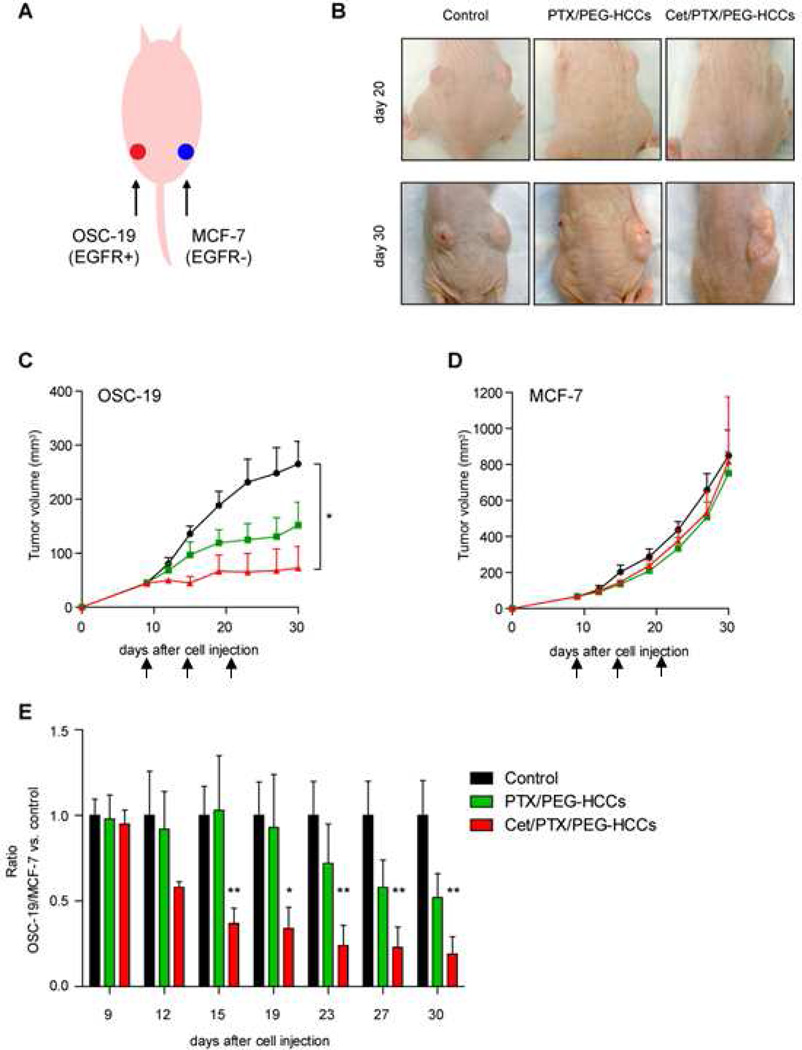
In order to better understand if the Cet/PTX/PEG-HCCs were targeting the delivery of PTX to EGFR-positive tumor cells, we used a dual subcutaneous tumor model with OSC-19 (EGFR positive) and MCF-7 (EGFR negative) tumor cells injected on the opposing flanks of nude mice. These mice were then systemically treated with either saline, PTX/PEG-HCCs or Cet/PTX/PEG-HCCs (Figure 3). The EGFR-positive tumor showed a markedly enhanced response to treatment with the targeted formulation (Figure 3C); however, the EGFR-negative tumor showed no difference in response when treated with either the PTX/PEG-HCCs or the Cet/PTX/PEG-HCCs (Figure 3D). While it is possible that the difference in conditions necessary to establish the two tumors, including the use of Matrigel for the MCF-7 cells, and/or physiological differences between the two tumors might have contributed to the different responses to treatment, the dominant effect could indeed be due to the targeted delivery of PTX to the EGFR-positive tumor by the Cet/PTX/PEG-HCCs. Even though Cet/PTX/PEG-HCCs appear to target the delivery of PTX to EGFR-positive tumor cells, the improvement over PTX/PEG-HCCs is modest. In tumor models where the efficacy of PTX/PEG-HCCs itself is limited, Cet/PTX/PEG-HCCs and PTX/PEG-HCCs might show no significant difference. Thus, radiation treatment was combined with nanovector treatment both because the boost in efficacy from radiation treatment would allow us to confirm a difference in activity for the two treatments and because it models a clinically relevant treatment mode.
Figure 3.
Effect of PTX/PEG-HCCs or Cet/PTX/PEG-HCCs on dual subcutaneous tumors in a nude mouse. (A) OSC-19 tumors (EGFR-positive; 1.5×106 cells per flank) were grown on the left flank and MCF-7 (EGFR-negative; 3×106 cells with Matrigel per flank) tumors on the right flanks of nude mice. (B) The effects of treatment on both OSC-19 and MCF-7 tumors. (C) The effect of treatment on OSC-19 tumors. Mice with established tumors were injected via the tail vein once weekly (indicated with black arrows) with the treatment. (D) The effect of treatment on MCF-7 tumors. Mice with established tumors were injected via the tail vein once weekly (indicated with black arrows) with the treatment. (E) Ratio was determined as the ratio for OSC-19 tumors/MCF-7 tumors divided by one in the control group. Points indicate means; bars, standard errors. *, P < 0.05; **, P < 0.01.
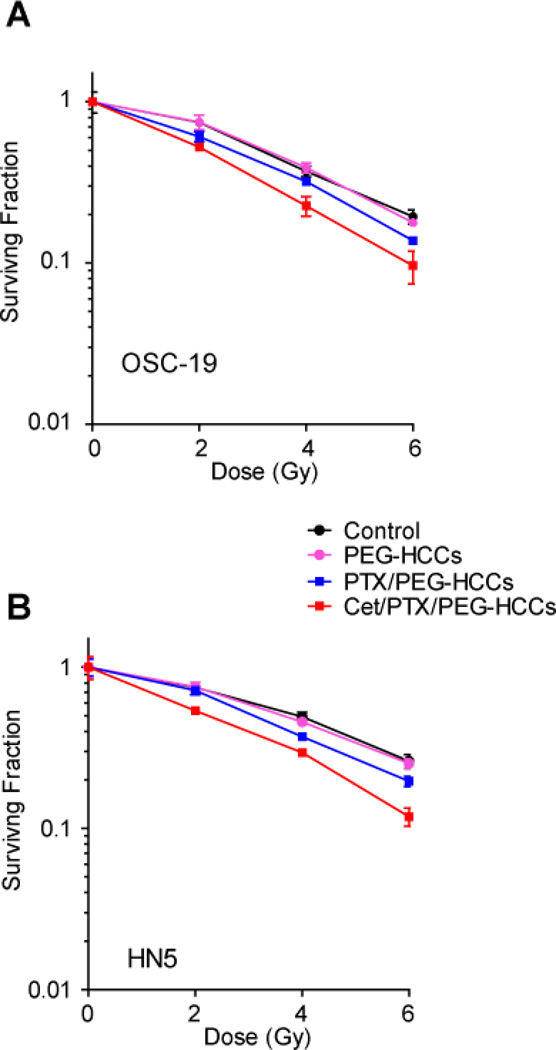
It is known that Cet and PTX each enhance the radiation response of HNSCC.23 In fact, inhibiting the EGFR pathway has been reported to enhance radio responsiveness in both preclinical and clinical study.16,24 Since the use of the Cet/PTX/PEG-HCCs appears to result in the co-localization of Cet and PTX, the radiosensitizing property of this therapy was evaluated. To assess whether Cet/PTX/PEG-HCCs could sensitize HNSCC cells to radiation therapy in vitro, OSC-19 and HN5 cells were treated with saline, Cet, PEG-HCCs, PTX/Cremophor, PTX/PEG-HCCs or Cet/PTX/PEG-HCCs and then exposed to radiation (Figure 4). The effects on the cells were assessed with clonogenic survival assays. In these assays, radiation alone resulted in a dose-dependent decrease in OSC-19 and HN5cell survival. These results are very similar to our previous report.25 Both PEG-HCCs and Cet did not show any additional cell growth inhibition compared to control (radiation alone treatment). Treatment with either PTX/Cremophor (data not shown) or PTX/PEG-HCCs resulted in an identical and significant enhancement in cell growth inhibition, and cells treated with Cet/PTX/PEG-HCCs exhibited the greatest enhancement in growth inhibition.
Figure 4.
Effects of PTX/PEG-HCCs or Cet/PTX/PEG-HCCs on head and neck squamous cell carcinoma cells’ radiosensitivity. (A) The effect of treatment on OSC19 cells. (B) The effect of treatment on HN5 cells. OSC-19 and HN5 cells in culture were exposed to PEG-HCCs (0.96 µg/mL), PTX/PEG-HCCs (PTX 4 nM, PEG-HCCs 0.96 µg/mL) or Cet/PTX/PEG-HCCs (Cet 0.8 pM, PTX 4 nM, PEG-HCCs 0.96 µg/mL) for 1 h, and then irradiated at 2 Gy, 4 Gy, or 6 Gy. After treatments, clonogenic survival assays were performed. Points indicate the means of triplicate experiments; bars, standard errors.
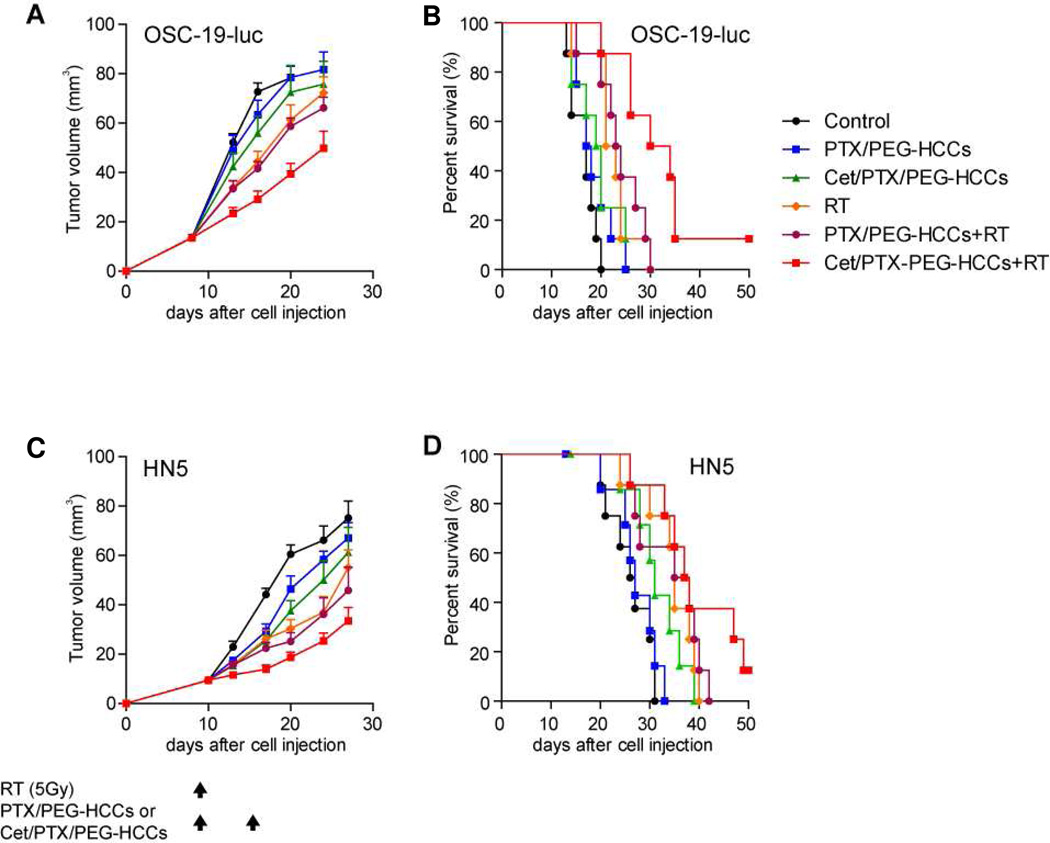
Encouraged by the in vitro results, we evaluated treating nude mice bearing an orthotopic tumor with radiotherapy (RT) alone or in combination with PEG-HCCs combined with PTX or Cet/PTX. As in Figure 4, we evaluated tumors derived from two different HNSCC cell lines, OSC-19 and HN5. In the OSC-19-luc model, there was a significant antitumor effect on day 20 after cell inoculation in the mice treated with Cet/PTX/PEG-HCCs + RT compared with the mice in the control group (P < 0.0001; Figure 5A). Moreover, while several of the other treatments resulted in significant tumor growth delay relative to the control group, the tumor growth delay produced by treatment with Cet/PTX/PEG-HCCs + RT was statistically significant when compared to each of the other treatment groups (vs. PTX/PEG-HCCs P < 0.0001, Cet/PTX/PEG-HCCs P = 0.0136, RT P = 0.0114, and PTX/PEG-HCCs + RT P = 0.0041, respectively). Treatment with Cet/PTX/PEG-HCCs + RT also resulted in the greatest increase in survival time (Figure 5B).
Figure 5.
In vivo effects of treatment with radiation, PTX/PEG-HCCs, Cet/PTX/PEG-HCCs, and their combinations on tumor growth and survival time in mice. (A) The in vivo effects of treatments on tumor growth in OSC-19-luc mice. Points indicate means; bars, standard errors (SE). (B) The in vivo effects of treatments on survival time of OSC-19-luc mice. (C) The in vivo effects of treatments on tumor growth in HN5 mice. Black arrows indicate treatment days. Points, mean; bars, SE. (D) The in vivo effects of treatments on survival time in HN5 mice. Animals were euthanized when they had lost more than 20% of their initial body weight or at 50 d after cell inoculation. Survival was analyzed by the Kaplan-Meier method and compared with log-rank tests.
The antitumor effects of combining the PTX treatments with RT were also evaluated in an orthotopic model derived from a different cell line, HN5 (Figure 5C). Similar results were obtained, as the mice treated with Cet/PTX/PEG-HCCs + RT had a significantly lower mean tumor volume than the mice in the control group at day 27 (P = 0.0002). In addition, the mice treated with Cet/PTX/PEG-HCCs + RT radiation had a significantly lower mean tumor volume than the mice in the PTX/PEG-HCCs alone group, Cet/PTX/PEG-HCCs alone group and the RT alone group (P = 0.0013, P = 0.0351, and P = 0.0335, respectively). Treatment with Cet/PTX/PEG-HCCs + RT also resulted in the greatest increase in survival time (Figure 5D). Finally, for both OSC-19-luc and HN5 cells, all treatments appeared to be well-tolerated, with no evidence of treatment-related weight loss (data not shown) and the experiments were repeated one additional time with similar results (data not shown).
In order to quantify the radiosensitization imparted by Cet/PTX/PEG-HCCs, the degree of growth delay observed for the OSC-19-luc model was expressed as the absolute tumor growth delay (AGD), defined as the average time in d required for the average tumor size in mice given a treatment to grow to 40 mm3 minus the time in d for the average tumor size in the untreated control group to reach the same size; or the normalized growth delay (NGD), defined as the time in d for the average tumor size to reach 40 mm3 in the mice treated with the combination of PTX/PEG-HCCs or Cet/PTX/PEG-HCCs plus radiation, minus the time in d to reach 40 mm3 in mice treated with PTX/PEG-HCCs or Cet/PTX/PEG-HCCs alone. Treatment enhancement factors (EFs) were obtained by dividing the NGD in mice treated with drugs plus radiation by the AGD in mice treated with radiation alone (Table 1).26
Table 1.
Effect of treatment on OSC-19-luc human head and neck squamous cell carcinoma cells’ radioresponse.
| Treatment | Time required to grow to 40 mm3, d |
Absolute growth delay, d |
Normalized growth delay, d |
Enhancement factor |
|---|---|---|---|---|
| Control | 13.5 ± 0.3 | |||
| PTX/PEG-HCCs | 14.7 ± 0.8 | 1.2 ± 0.8 | ||
| Cet/PTX/PEG-HCCs | 16.0 ± 1.7 | 2.5 ± 1.7 | ||
| RT | 17.6 ± 1.1 | 4.1 ± 1.1 | ||
| PTX/PEG-HCCs+RT | 17.4 ± 0.7 | 3.9 ± 0.7 | 2.8 ± 0.7 | 0.68 |
| Cet/PTX/PEG-HCCs+RT * | 21.9 ± 1.0 | 8.4 ± 1.0 | 5.9 ± 1.0 | 1.44 |
NOTE: All data are means ± standard error.
1 of 8 mice whose tumor volume never reached 40 mm3 was not used in the analysis.
Cet/PTX/PEG-HCCs + RT achieved a more than additive effect, resulting in an AGD of 8.4 ± 1.0 d, which was considerably higher than the sum of tumor growth delays caused by individual treatments (2.5 ± 1.7 d with Cet/PTX/PEG-HCCs alone and 4.1 ± 1.1 d with RT alone; EF = 1.44) even though 1 of 8 mice whose tumor never reached 40 mm3 was not used in the analysis. Similarly, while PTX/PEG-HCCs did not enhance the effect of RT in HN5 tumors (EF = 1.00), Cet/PTX/PEG-HCCs enhanced the radioresponsiveness of HN5 tumors, increasing tumor growth delay more than additively even though 1 of 8 mice whose tumor never reached 40 mm3 was not used in the analysis (EF = 1.09/data not shown). Thus, Cet/PTX/PEG-HCCs enhanced HNSCC tumors’ radioresponse.
Conclusion
Cet/PTX/PEG-HCCs, produced by simply mixing PTX/PEG-HCCs with Cet, appear to target tumors overexpressing EGFR in vivo. While the increase in efficacy relative to current clinical therapies is limited, when the therapies are combined with radiation, the Cet/PTX/PEG-HCCs are significantly more effective than the mixture of Cet and PTX/Cremophor. The increased efficacy of the targeted formulation might be due to a synergy between inhibition of the EGFR pathway and the taxane effect.27 Given the fact that most HNSCC patients receive multimodal therapy including radiation, the increased efficacy observed for Cet/PTX/PEG-HCCs is particularly important. Future studies will be required to optimize the formulation and treatment protocol for maximal radiosensitization prior to translation towards the clinic.
Materials and Methods
Animals
We purchased 8-to-12-week-old male athymic nude mice from the National Cancer Institute (Bethesda, MD). The mice were kept in a specific pathogen–free facility approved by the American Association for the Accreditation of Laboratory Animal Care that met all current regulations and standards of the U.S. Department of Agriculture, U.S. Department of Health and Human Services, and the National Institutes of Health. Mice were fed irradiated standard mouse chow and autoclaved, reverse osmosis–treated water. Animal procedures were carried out according to a protocol approved by The University of Texas MD Anderson Cancer Center’s Institutional Animal Care and Use Committee.
Cell lines
FaDu cells were purchased from American Type Culture Collection (Manassas, VA). HN5 cells were provided by Dr. Zhen Fan (Department of Experimental Therapeutics, MD Anderson Cancer Center). OSC-19 was purchased from the Health Science Research Resources Bank (Osaka, Japan). MCF-7 cells were provided by Dr. Francois-Xavier Claret (Department of Systems Biology, MD Anderson Cancer Center). OSC-19 cells were retrovirally infected with the green fluorescent protein and the luciferase gene (OSC-19-luc) as described previously.28 All cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS, L-glutamine, sodium pyruvate, nonessential amino acids, and a 2-fold vitamin solution (Life Technologies, Inc., Grand Island, NY). Adherent monolayer cultures were maintained on plastic plates and incubated at 37°C in 5% carbon dioxide and 95% oxygen. The integrity of all maintained cell lines was clearly established using short tandem repeat genomic profiling. The cultures were Mycoplasma-free and maintained for no longer than 12 weeks after they were recovered from frozen stocks.
Reagents
Hydrophilic carbon clusters (HCCs) that are covalently modified with polyethylene glycol (PEG-HCCs) were synthesized and PEG-HCCs were loaded with paclitaxel (PTX/PEG-HCCs) as described previously.18 As we have done in all past publications,18,22 all concentrations given in this manuscript for the PEG-HCCs are for the carbon cores of the PEG-HCCs, as this can be directly measured by ultraviolet-visible (UV-Vis) spectroscopy. Cet (C225/Erbitux; Imclone, New York, NY), an anti-EGFR monoclonal antibody, is used as the targeting agent to establish Cet/PTX/PEG-HCCs and is attached by simply mixing it with PTX/PEG-HCCs. Paclitaxel (Taxol/Bristol-Myers Squibb, Princeton, NJ) was diluted in PBS to a 982 µg/mL final concentration and Cet was also diluted in PBS to a 35.9 µg/mL final concentration for in vivo experiments.
Orthotopic nude mouse model of HNSCC
To evaluate the effect of Cet/PTX/PEG-HCCs in vivo, we used an orthotopic nude mouse model of HNSCC because its host microenvironment is more similar to that of patients with HNSCC than that of subcutaneous xenograft models of HNSCC.29 An orthotopic nude mouse model of oral tongue cancer was established by injecting FaDu (2.5 × 105) or OSC-19 (5 × 104) cells suspended in 30 µL of serum-free DMEM into the tongues of mice as described previously.30
Twelve to 15 d after the cells were injected, the mice were randomly assigned to 1 of 6 or 8 treatment groups (6 to 8 mice per group): (1) control; (2) PEG-HCCs (100 µg/mL); (3) Cet (35.9 µg/mL); (4) Cet/PEG-HCCs (Cet 35.9 µg/mL, PEG-HCCs 100 µg/mL); (5) PTX/Cremophor (982 µg/mL); (6) PTX/PEG-HCCs (PTX 982 µg/mL, PEG-HCCs 100 µg/mL); (7) Cet/PTX/Cremophor (Cet 35.9 µg/mL, PTX (982 µg/mL); (8) Cet/PTX/PEG-HCCs (Cet 35.9 µg/mL, PTX (982 µg/mL), PEG-HCCs 100 µg/mL). A dose of 200 µL of each treatment was administered intravenously once a week for 3 weeks. Control mice were given 200 µL PBS intravenously once weekly for 3 weeks.
Mice were examined twice a week for tumor size and weight loss. Tongue tumor size was measured with microcalipers. Tumor volume was calculated as (A)(B2)π/6, where A is the longest dimension of the tumor and B is the dimension of the tumor perpendicular to A. We euthanized mice by CO2 asphyxiation when they lost more than 20% of their preinjection body weight or at 50 d after cell injection.
Dual subcutaneous tumors model in a nude mouse
To confirm that Cet/PTX/PEG-HCCs target EGFR-positive cells in vivo, a dual subcutaneous tumors model was established in a nude mouse. As shown in Figure 1A, OSC-19 cells (EGFR-positive; 1.5 × 105) resuspended in PBS and MCF-7 cells (EGFR-negative; 3 × 105) resuspended in 50% Matrigel (BD Bioscience, Bedford, MA) were injected subcutaneously in the left and right flank, respectively, as described previously.31 9 d after implantation of cells, when the average OSC-19 tumor volume reached 44 mm3 and the average MCF-7 tumor volume reached 65 mm3, the mice were randomly assigned to 1 of 3 treatment groups (8 mice per group): control, PTX/PEG-HCCs, or Cet/PTX/PEG-HCCs. PTX/PEG-HCCs and Cet/PTX/PEG-HCCs were administered intravenously injection of 200 µL once a week for 3 weeks. Control mice were given 200 µL PBS intravenously once weekly for 3 weeks. Tumor sizes for both tumors were measured twice a week. The ratio of OSC-19/MCF-7 was also calculated for each time point as
Clonogenic survival assay
OSC-19 and HN5 cells were used, since they are radiation-resistant.25 OSC-19 and HN5 cells in culture were exposed to PEG-HCCs (96 µg/mL), Cet (0.8 pM), PTX/Cremophor (4 nM), PTX/PEG-HCCs (PTX 4 nM, PEG-HCCs 96 µg/mL) or Cet/PTX/PEG-HCCs (Cet 0.8 pM, PTX 4 nM, PEG-HCCs 96 µg/mL) for 1 h, and then exposed to 2, 4, or 6 Gy radiation (γ-rays using a cesium-137 source, 3.055 Gy/min). The cells were then assayed for colony-forming ability by trypsinizing and replating in 100-mm dishes in drug-free medium. After 10–11 d of incubation, the cells were stained with 0.5% crystal violet in absolute ethanol, and colonies with more than 50 cells were counted under a dissection microscope. Plating efficiency was defined as the percentage of cells seeded that grew into colonies under a specific culture condition of a given cell line. The survival fraction, expressed as a function of irradiation, was calculated as the number of colonies counted/(the number of cells seeded × plating efficiency/100) as described previously.25
Enhancement of tumor radioresponse by Cet/PTX/PEG-HCCs in an orthotopic nude mouse model of HNSCC
An orthotopic nude mouse model of oral cancer was established by injecting OSC-19-luc (1 × 105) or HN5 (3 × 105) cells suspended in 30 µL of serum-free DMEM into the tongues of mice as described.
Eight to 10 d after the cells were injected, the mice were randomly assigned to 1 of 6 treatment groups (7 or 8 mice per group): (1) control; (2) PTX/PEG-HCCs (PTX 982 µg/mL, PEG-HCCs 100 µg/mL); (3) Cet/PTX/PEG-HCCs (Cet 35.9 µg/mL, PTX 982 µg/mL, PEG-HCCs 100 µg/mL); (4) radiation; (5) radiation plus PTX/PEG-HCCs (PTX 982 µg/mL, PEG-HCCs 100 µg/mL); (6) radiation plus Cet/PTX/PEG-HCCs (Cet 35.9 µg/mL, PTX 982 µg/mL, PEG-HCCs 100 µg/mL). All drugs were administered intravenously at 200 µL once a week for 2 weeks. Control mice were given 200 µL PBS intravenously once weekly for 2 weeks. Mice bearing tumors in the tongue were locally irradiated with a single dose of 5 Gy using a small-animal irradiator (γ-rays using a cesium-137 source, 4.762 Gy/min). Sodium pentobarbital was administered by intraperitoneal injection at a dose of 50 mg/kg prior to radiation treatment. The mice were immobilized on a customized jig during irradiation with the tumor centered in the 3-cm diameter circular irradiation field as described previously.25 When Cet/PTX/PEG-HCCs or PTX/PEG-HCCs and radiation were combined, drugs were given 1 h before single-dose irradiation.
Mice were examined twice a week for tumor size and weight loss. Tongue tumor size was measured with microcalipers as described above. The degree of growth delay was expressed as the absolute tumor growth delay (AGD), defined as the average time in d required for the average tumor size in mice given a treatment to grow to 40 mm3 minus the time in d for the average tumor size in the untreated control group to reach the same size; or the normalized growth delay (NGD), defined as the time in d for the average tumor size to reach 40 mm3 in the mice treated with the combination of PTX/PEG-HCCs or Cet/PTX/PEG-HCCs plus radiation, minus the time in d to reach 40 mm3 in mice treated with PTX/PEG-HCCs or Cet/PTX/PEG-HCCs alone. Treatment enhancement factors (EFs) were obtained by dividing the NGD in mice treated with drugs plus radiation by the AGD in mice treated with radiation alone.23 Mice were euthanized by CO2 asphyxiation when they lost more than 20% of their preinjection body weight or at 50 d after cell injection.
Statistical Analysis
Two-tailed t tests were used to compare tumor volumes from control groups and treatment groups. Survival was determined using the Kaplan-Meier method and compared using log-rank tests. Statistical analyses were performed with Prism 5.01 software (GraphPad Software). P values < 0.05 were considered statistically significant.
Acknowledgement
We thank the Alliance for NanoHealth through a subcontract from the University of Texas Health Science Center, Houston (Department of Defense: W8XWH-07-2-0101); the Mission Connect Mild Traumatic Brain Injury Consortium, funded by the Department of Defense, W81XWH-08-2-0143; the Nanoscale Science and Engineering Initiative of the National Science Foundation under NSF Award EEC-0647452 for funding through the NSF Center for Biological and Environmental Nanotechnology; The University of Texas M. D. Anderson Cancer Center (UTMDACC) PANTHEON Program; NIH Cancer Center Support Grant CA16672; UTMDACC Cancer Center Support Grant (CA016672).
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J, Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Carvalho AL, Nishimoto IN, Califano JA, Kowalski LP. Trends in Incidence and Prognosis for Head and Neck Cancer in the United States: a Site-Specific Analysis of the SEER Database. Int. J. Cancer. 2005;114:806–816. doi: 10.1002/ijc.20740. [DOI] [PubMed] [Google Scholar]
- 3.Lango MN. Multimodal Treatment for Head and Neck Cancer. Surg. Clin. North Am. 2009;89:43–52. viii. doi: 10.1016/j.suc.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Hamburg MA, Collins FS. The Path to Personalized Medicine. New Engl. J. Med. 2010;363:301–304. doi: 10.1056/NEJMp1006304. [DOI] [PubMed] [Google Scholar]
- 5.Schilsky RL. Personalized Medicine in Oncology: the Future is Now. Nat. Rev. Drug Discov. 2010;9:363–366. doi: 10.1038/nrd3181. [DOI] [PubMed] [Google Scholar]
- 6.Riehemann K, Schneider SW, Luger TA, Godin B, Ferrari M, Fuchs H. Nanomedicine-Challenge and Perspectives. Angew. Chem. Int. Edit. 2009;48:872–897. doi: 10.1002/anie.200802585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrari M. Cancer Nanotechnology: Opportunities and Challenges. Nat. Rev. Cancer. 2005;5:161–171. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 8.Service RF. Materials and Biology. Nanotechnology Takes Aim at Cancer. Science. 2005;310:1132–1134. doi: 10.1126/science.310.5751.1132. [DOI] [PubMed] [Google Scholar]
- 9.Sinha R, Kim GJ, Nie S, Shin DM. Nanotechnology in Cancer Therapeutics: Bioconjugated Nanoparticles for Drug Delivery. Mol. Cancer Ther. 2006;5:1909–1917. doi: 10.1158/1535-7163.MCT-06-0141. [DOI] [PubMed] [Google Scholar]
- 10.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an Emerging Platform for Cancer Therapy. Nat. Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 11.Davis ME, Chen ZG, Shin DM. Nanoparticle Therapeutics: an Emerging Treatment Modality for Cancer. Nat. Rev. Drug Discov. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 12.Bonner JA, Maihle NJ, Folven BR, Christianson TJH, Spain K. The Interaction of Epidermal Growth-Factor and Radiation in Human Head and Neck Squamous-Cell Carcinoma Cell-Lines with Vastly Different Radiosensitivities. Int. J. Radiat. Oncol. 1994;29:243–247. doi: 10.1016/0360-3016(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 13.Huang SM, Bock JM, Harari PM. Epidermal Growth Factor Receptor Blockade with C225 Modulates Proliferation, Apoptosis, and Radiosensitivity in Squamous Cell Carcinomas of the Head and Neck. Cancer Res. 1999;59:1935–1940. [PubMed] [Google Scholar]
- 14.Saleh MN, Raisch KP, Stackhouse MA, Grizzle WE, Bonner JA, Mayo MS, Kim HG, Meredith RF, Wheeler RH, Buchsbaum DJ. Combined Modality Therapy of A431 Human Epidermoid Cancer Using Anti-EGFr Antibody C225 and Radiation. Cancer Biother. Radio. 1999;14:451–463. doi: 10.1089/cbr.1999.14.451. [DOI] [PubMed] [Google Scholar]
- 15.Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol D, et al. Platinum-Based Chemotherapy Plus Cetuximab in Head and Neck Cancer. New Engl. J. Med. 2008;359:1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 16.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, et al. Radiotherapy plus Cetuximab for Squamous-Cell Carcinoma of the Head and Neck. New Engl. J. Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 17.Rubin Grandis J, Melhem MF, Gooding WE, Day R, Holst VA, Wagener MM, Drenning SD, Tweardy DJ. Levels of TGF-Alpha and EGFR Protein in Head and Neck Squamous Cell Carcinoma and Patient Survival. J. Natl. Cancer Inst. 1998;90:824–832. doi: 10.1093/jnci/90.11.824. [DOI] [PubMed] [Google Scholar]
- 18.Berlin JM, Leonard AD, Pham TT, Sano D, Marcano DC, Yan S, Fiorentino S, Milas ZL, Kosynkin DV, Price BK, et al. Effective Drug Delivery, In Vitro and, In Vivo, by Carbon-Based Nanovectors Noncovalently Loaded with Unmodified Paclitaxel. ACS Nano. 2010;4:4621–4636. doi: 10.1021/nn100975c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen ZY, Kobashi K, Rauwald U, Booker R, Fan H, Hwang WF, Tour JM. Soluble Ultra-Short Single-Walled Carbon Nanotubes. J. Am., Chem. Soc. 2006;128:10568–10571. doi: 10.1021/ja063283p. [DOI] [PubMed] [Google Scholar]
- 20.Price BK, Lomeda JR, Tour JM. Aggressively Oxidized Ultra-Short Single-Walled Carbon Nanotubes Having Oxidized Sidewalls. Chem. Mater. 2009;21:3917–3923. [Google Scholar]
- 21.Stephenson JJ, Hudson JL, Leonard AD, Price BK, Tour JM. Repetitive Functionalization of Water-Soluble Single-Walled Carbon Nanotubes. Addition of Acid-Sensitive Addends. Chem. Mater. 2007;19:3491–3498. [Google Scholar]
- 22.Berlin JM, Pham TT, Sano D, Mohamedali KA, Marcano DC, Myers JN, Tour JM. Noncovalent Functionalization of Carbon Nanovectors With an Antibody Enables Targeted Drug Delivery. ACS Nano. 2011;5:6643–6650. doi: 10.1021/nn2021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta AK, McKenna WG, Weber CN, Feldman MD, Goldsmith JD, Mick R, Machtay M, Rosenthal DI, Bakanauskas VJ, Cerniglia GJ, et al. Local Recurrence in Head and Neck Cancer: Relationship to Radiation Resistance and Signal Transduction. Clin. Cancer Res. 2002;8:885–892. [PubMed] [Google Scholar]
- 24.Milas L, Mason K, Hunter N, Petersen S, Yamakawa M, Ang K, Mendelsohn J, Fan Z. In Vivo Enhancement of Tumor Radioresponse by C225 Antiepidermal Growth Factor Receptor Antibody. Clin. Cancer Res. 2000;6:701–708. [PubMed] [Google Scholar]
- 25.Sano D, Matsumoto F, Valdecanas D, Zhao M, Molkentine DP, Takahashi Y, Hanna EY, Papadimitrakopoulou VA, Heymach JV, Milas L, et al. Vandetanib Restores Head and Neck Squamous Cell Carcinoma Cells' Sensitivity to Cisplatin and Radiation In Vivo and In Vitro. Clin. Cancer Res. 2011;17(7):1815–1827. doi: 10.1158/1078-0432.CCR-10-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milas L, Fujii T, Hunter N, Elshaikh M, Mason K, Plunkett W, Ang KK, Hittelman W. Enhancement of Tumor Radioresponse In Vivo by Gemcitabine. Cancer Res. 1999;59:107–114. [PubMed] [Google Scholar]
- 27.Sawai A, Chandarlapaty S, Greulich H, Gonen M, Ye Q, Arteaga CL, Sellers W, Rosen N, Solit DB. Inhibition of Hsp90 Down-Regulates Mutant Epidermal Growth Factor Receptor (EGFR) Expression and Sensitizes EGFR Mutant Tumors to Paclitaxel. Cancer Res. 2008;68:589–596. doi: 10.1158/0008-5472.CAN-07-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou G, Xie TX, Zhao M, Jasser SA, Younes MN, Sano D, Lin J, Kupferman ME, Santillan AA, Patel V, et al. Reciprocal Negative Regulation Between S100A7/Psoriasin and Beta-Catenin Signaling Plays an Important Role in Tumor Progression of Squamous Cell Carcinoma of Oral Cavity. Oncogene. 2008;27:3527–3538. doi: 10.1038/sj.onc.1211015. [DOI] [PubMed] [Google Scholar]
- 29.Sano D, Choi S, Milas ZL, Zhou G, Galer CE, Su YW, Gule M, Zhao M, Zhu Z, Myers JN. The Effect of Combination Anti-Endothelial Growth Factor Receptor and Anti-Vascular Endothelial Growth Factor Receptor 2 Targeted Therapy on Lymph Node Metastasis: a Study in an Orthotopic Nude Mouse Model of Squamous Cell Carcinoma of the Oral Tongue. Arch. Otolaryngol. Head Neck Surg. 2009;135:411–420. doi: 10.1001/archoto.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Myers JN, Holsinger FC, Jasser SA, Bekele BN, Fidler IJ. An Orthotopic Nude Mouse Model of Oral Tongue Squamous Cell Carcinoma. Clin. Cancer Res. 2002;8:293–298. [PubMed] [Google Scholar]
- 31.Choi HS, Liu W, Liu F, Nasr K, Misra P, Bawendi MG, Frangioni JV. Design Considerations for Tumour-Targeted Nanoparticles. Nat. Nanotechnol. 2010;5:42–47. doi: 10.1038/nnano.2009.314. [DOI] [PMC free article] [PubMed] [Google Scholar]