Abstract
Purpose
This study was performed to determine the spinal cord tolerance of swine to single-fraction, partial-volume irradiation one year after uniform irradiation to 30Gy in 10 fractions.
Materials/ Methods
A 10cm length of spinal cord (C3-T1) was uniformly irradiated to 30Gy in ten consecutive fractions and reirradiated one year later with a single radiosurgery dose centered within the previously irradiated segment. Radiosurgery was delivered to a cylindrical volume approximately 5cm in length and 2cm in diameter that was positioned lateral to the cervical spinal cord resulting in a dose distribution with the 90%, 50% and 10% isodose lines traversing the ipsilateral, central and contralateral spinal cord, respectively. Twenty-three pigs were stratified into six dose groups with mean maximum spinal cord doses of 14.9±0.1 (n=2), 17.1±0.3 (n=3), 19.0±0.1 (n=5), 21.2±0.1 (n=5), 23.4±0.2 (n=5), and 25.4±0.4 (n=3)Gy. The mean percentage spinal cord volumes receiving >=10Gy for the same groups were 34%±1, 40%±1, 46%±3, 52%±1, 56±3, and 57%±1. The study endpoint was motor neurologic deficit determined by a change in gait during a one year follow-up period.
Results
A steep dose-response curve was observed with an ED50(95% CI) for the maximum point dose of 19.7Gy (17.4-21.4). With two exceptions, histology was unremarkable in animals with normal neurologic status while all animals with motor deficits showed some degree of demyelination and focal white matter necrosis on the irradiated side with relative sparing of the gray matter. Histologic comparison with a companion study of de novo irradiated animals revealed retreatment responders have more extensive tissue damage, including infarction of the gray matter, only at prescription doses >20Gy.
Conclusion
Pigs receiving spinal radiosurgery one year following 30Gy in 10 fractions are not at significantly higher risk of developing motor deficits than pigs that have received radiosurgery alone.
Keywords: reirradiation, spinal cord tolerance, stereotactic spinal radiosurgery, swine
Introduction
Conventional external beam radiation therapy is the most frequently used treatment for metastatic spinal cord compression but the 12-month local failure rate is 39% after a single fraction and 23% after longer courses(1). Although the spinal cord has been shown to possess a large capacity for repair in primates and rodents(2-4), clinical data regarding the retreatment tolerance of the spinal cord are sparse(5) and the risk of spinal cord myelopathy inspires a reluctance to reirradiate recurrent lesions using conventional techniques. In recent years, image-guided stereotactic radiosurgery has been used safely to manage recurrent spinal lesions(6-8) allowing dose escalation and potentially more effective palliation. Despite the increased clinical application of spinal radiosurgery, little is known regarding the tolerance of the spinal cord to reirradiation with single-fraction, partial-volume radiosurgery.
This report describes, in a swine model, re-irradiation tolerance based on partial volume irradiation of the spinal cord. One arm of this study, described previously(9), was designed to determine the dose-related incidence of motor neurologic deficit in swine that receive single-fraction radiosurgery using a lateral partial-volume dose distribution. The second arm, reported here, included a uniform conditioning dose of 30Gy (3Gy times 10 fractions) to the spinal cord one year prior to receiving the radiosurgery distribution described above.
Methods and Materials
This study was approved by the Institutional Animal Care and Use Committee. The study began with the delivery of a total dose of 30Gy in ten consecutive 3Gy fractions (weekdays only) to the spinal cords of twenty-four female, Yucatan minipigs. Twenty-three animals were reirradiated 52-56 weeks later with single-fraction radiosurgery to a 5cm segment within the previously irradiated volume.
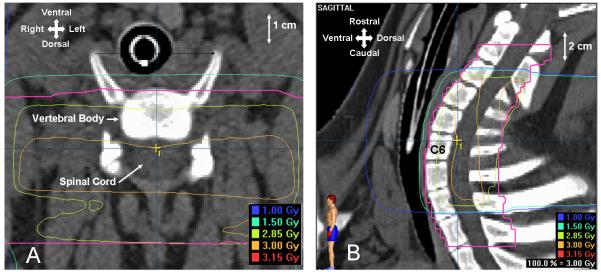
For the fractionated regimen, animals were 42-46 weeks old and weighed approximately 35-45kg on the first day of irradiation. All animals received a treatment planning CT scan with 1.0-1.5mm thick slices and a 340-400cm field of view. Treatment planning was performed using Brainscan 5.31 software (BrainLAB, AG). The spinal cord volume was defined on CT images by contracting the thecal sac contour by 1.5mm in the axial plane. Contouring was then viewed in the sagittal plane for verification/correction. This method was based on CT/MRI fusion of two animals early in the study. Individual treatment plans were created using a three-field static conformal beam arrangement (right/left lateral plus a posterior field) with the goals of sparing the esophagus while minimizing dose inhomogeneity across the irradiated spinal cord volume to ±5%. Radiation was delivered to a 9.8cm-long segment of spinal cord centered at the rostral end of the sixth cervical vertebral body and extending from the third cervical through first thoracic vertebrae. Dose distributions in the axial and sagittal planes are shown in Figure 1. Animals were anesthetized with a mixture of Telazol® and xylazine and maintained on isoflurane. Animals were positioned supine in a body-length, vacuum-molded immobilization cushion that was individually molded for each pig and unchanged throughout the course of radiotherapy.
Figure 1.
Dose distributions for 3Gy times 10 in the axial (A) and sagittal (B) planes.
Image-guided localization was performed using stereoscopic kilovoltage x-rays (Novalis Body X-ray 6D, BrainLAB AG). A pair of digital radiographs was exposed and automatically fused with digitally reconstructed radiographs (DRR’s) generated from the pre-treatment CT scan to determine the shifts necessary to position the target at the isocenter of the linear accelerator. After visual evaluation of the fusion results, the treatment table was shifted until the actual position and required treatment position differed by less than 1mm in the three primary axes. Rotations along the axis of the treatment table were corrected to less than one degree and rotations along the other two axes were corrected to less than two degrees. The image-guidance process was repeated following table adjustments to ensure that shifts were made correctly. Biplanar megavoltage portal images were acquired and evaluated to verify positioning before radiosurgery. Irradiation was performed using a 6MV image-guided linear accelerator (Novalis, BrainLAB AG). Dose was delivered at a rate of 800 monitor units per minute that equated to an instantaneous dose rate of 5.5-6.3Gy/min at the spinal cord, varying with the depth of overlying tissue.
Twenty-three animals were reirradiated with single-fraction radiosurgery 52-56 weeks following their 30Gy fractionated radiation regimen. The twenty-fourth animal was euthanized three months after the fractionated regimen due to a generalized infection. A necropsy with histopathologic analysis of several tissues was performed to rule out study-related causes.
A detailed description of the methods used to deliver the radiosurgery was described previously(9), an abbreviated description is presented here. CT simulation conditions, treatment planning and delivery platforms and positioning methods for radiosurgery were consistent with those presented above for fractionated radiotherapy. Radiosurgery was delivered in a single dose to a cylindrical target volume approximately 5cm in length and 2cm in diameter that was positioned lateral to the cervical spinal cord. In the rostral/caudal direction, the target volume was centered at the level of the rostral end of the sixth cervical vertebral body and extended from mid-C4 through mid-C7. A radiosurgery dose distribution in the axial plane is shown in Figure 2. Arc delivery with dynamically-shaped fields was used to create a dose distribution with the 90%, 50% and 10% isodose lines traversing the ipsilateral, central and contralateral spinal cord, respectively, equating to a lateral dose gradient of approximately 8% per millimeter. The spinal cord was contoured 4.5-7.5mm beyond the irradiated volume in the rostral and caudal directions and the dose calculation grid resolution through the spinal cord ranged from 1.5-1.6mm. Dose distributions were normalized to the global plan maximum and dose was prescribed to the 90% isodose line. Animals were stratified into 6 prescription dose groups as follows: 24Gy (3), 22Gy (5), 20Gy (5), 18Gy (5), 16Gy (3), and 14Gy (2). Treatment planning dose-volume statistics for the spinal cord are summarized in Table 1 including: a) maximum point dose, b) absolute and percentage volume to receive ≥10Gy, c) absolute and percentage volume to receive ≥14Gy, d) maximum dose to 1cc volume.
Figure 2.
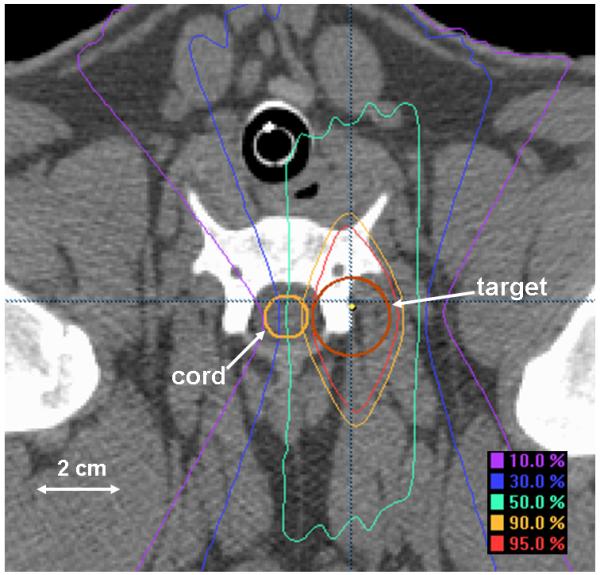
Dose distribution for single-fraction radiosurgery in the axial plane.
Table 1.
Radiosurgery dose-volume histogram statistics for the spinal cord.
| Dose Group (Gy) |
Mean Maximum Cord Dose (Gy) |
Mean Volume >= 10 Gy (cc) |
Mean Percentage Volume >= 10 Gy |
Mean Volume >= 14 Gy (cc) |
Mean Percentage Volume >= 14 Gy |
Mean Maximum Dose to 1.0 cc (Gy) |
|---|---|---|---|---|---|---|
| 14 (n=2) | 14.9±0.1 | 1.33±0.09 | 34±1 | 0.27±0.11 | 7±3 | 11.8±0.4 |
| 16 (n=3) | 17.1±0.3 | 1.40±0.05 | 40±1 | 0.81±0.02 | 23±1 | 12.5±0.5 |
| 18 (n=5) | 19.0±0.1 | 1.72±0.20 | 46±3 | 1.10±0.19 | 29±4 | 14.6±1.0 |
| 20 (n=5) | 21.2±0.1 | 1.98±0.07 | 52±1 | 1.45±0.05 | 38±1 | 17.3±0.3 |
| 22 (n=5) | 23.4±0.2 | 2.09±0.12 | 56±3 | 1.53±0.08 | 41±3 | 18.5±0.4 |
| 24 (n=3) | 25.4±0.4 | 2.18±0.11 | 57±1 | 1.66±0.12 | 43±1 | 20.0±0.9 |
After radiosurgery, animals were followed for 39-53 weeks or until a neurologic response was observed. The general health of animals was observed daily with attention toward unusual restlessness, vocalizing, loss of mobility, licking, biting, or guarding of a painful area, failure to groom, unkempt appearance, open sores, skin lesions, loss of appetite, and weight loss. Gait was observed approximately weekly with the animal walking freely in a large space. Response was defined as any study-related change in gait. Animals recognized to have a change in gait were evaluated by a veterinarian for symptoms indicative of pain. After euthanasia, the cervical spinal cords of all 24 study animals were removed and fixed in formalin before being sectioned and processed for embedding in paraffin. Five uniformly distributed axial sections through the irradiated volume of each animal were cut and stained with a Luxol fast blue/periodic acid Schiff combination and were examined for histology. The dose-related incidence of motor deficit was used as an endpoint to obtain quantal data that was analyzed by probit analysis(10) to establish a dose-response curve and to calculate the probability of a deficit at increasing dose levels with the associated 95% confidence bounds.
Results
Parameters such as prescription dose, maximum spinal cord dose, age at radiosurgery, length of follow-up and latency until response are shown for individual animals in Table 2. Fourteen of twenty-three pigs developed front limb motor changes on the irradiated side (left) while 9 pigs maintained normal neurologic status. No deficits were noted on the unirradiated side (right) in any animal. Six of the fourteen animals that developed front motor deficits were noted to develop hind limb changes within three weeks of front leg deficits. Motor deficits presented initially as a mild limp and progressed to changes ranging from mild paresis (carpal knuckling) to general limb weakness. One animal (#9) is reported as a responder even though its deficits were extremely subtle and inconsistent between observers over a period of three months. The latent period for initial onset of motor deficit ranged from 9-24 weeks. No transient deficits were observed and no animal exhibited behavior indicative of pain throughout this study.
Table 2.
Radiosurgery dose and time parameters for individual animals.
| ID# | Rx Dose (Gy) |
Maximum Cord Dose (Gy) |
Age at SRS (weeks) |
Follow-up (weeks) |
Latency (weeks) |
|---|---|---|---|---|---|
| 1 | 14 | 14.9 | 95 | 41 | NA* |
| 2 | 14 | 14.8 | 94 | 39 | NA* |
| 3 | 16 | 17.3 | 99 | 52 | NA* |
| 4 | 16 | 17.3 | 99 | 52 | NA* |
| 5 | 16 | 16.7 | 99 | 53 | NA* |
| 6 | 18 | 19.0 | 96 | 48 | NA* |
| 7 | 18 | 19.0 | 94 | 48 | NA* |
| 8 | 18 | 19.0 | 100 | 29 | 14 |
| 9 | 18 | 18.8 | 100 | 36 | 24 |
| 10 | 18 | 19.2 | 99 | 53 | NA* |
| 11 | 20 | 21.1 | 96 | 18 | 16 |
| 12 | 20 | 21.3 | 97 | 53 | NA* |
| 13 | 20 | 21.3 | 101 | 14 | 13 |
| 14 | 20 | 21.1 | 101 | 22 | 20 |
| 15 | 20 | 21.1 | 98 | 18 | 16 |
| 16 | 22 | 23.2 | 96 | 20 | 18 |
| 17 | 22 | 23.2 | 97 | 16 | 16 |
| 18 | 22 | 23.7 | 97 | 14 | 13 |
| 19 | 22 | 23.2 | 100 | 17 | 17 |
| 20 | 22 | 23.5 | 97 | 14 | 13 |
| 21 | 24 | 25.3 | 97 | 17 | 9 |
| 22 | 24 | 25.1 | 97 | 19 | 19 |
| 23 | 24 | 25.9 | 99 | 16 | 15 |
No motor neurologic deficits were observed in stated follow-up period.
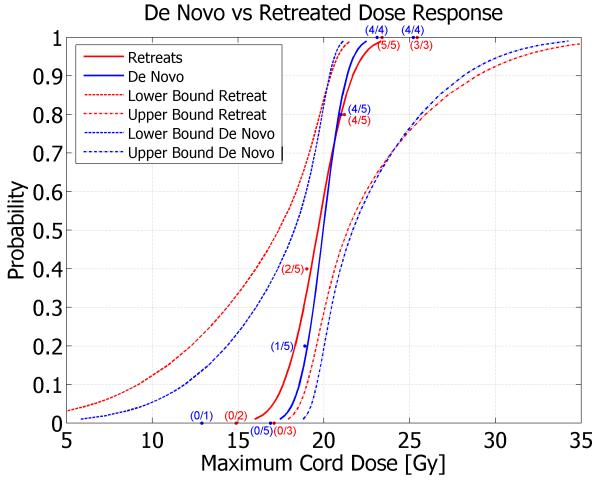
A clear dose-response relationship was observed, with no neurologic response when the maximum spinal cord dose was <18.8Gy and 100% response when the maximum cord dose was >21.3Gy. Calculated estimates of the maximum spinal cord doses resulting in 1, 5, 10, 50 and 90% probability of paralysis along with the associated 95% confidence bounds are shown in Table 3. The dose-response curve for reirradiated pigs presenting ‘maximum spinal cord dose’ versus ‘probability of motor neurologic deficit’ is plotted with the corresponding curve for pigs that received radiosurgery alone(9) in Figure 3. Observed response data points are superimposed on the dose-response curves and annotated to show the number of responders per number of animals at each dose point. The associated upper and lower 95% confidence bounds are also plotted (dashed lines) for each curve. Assuming both dose-response curves (de novo and reirradiation) are parallel (test p-value=0.312) yielded a recovery estimate(2, 11) of 96.2% of the initial 30Gy fractionated dose. The dose associated with a 50% incidence of paralysis (ED50) and 95% confidence limits are 19.7Gy (17.4 - 21.4). A Pearson goodness-of-fit chi-square indicates (p=0.917) that the probit model is an excellent fit. The ED50(95% confidence limits) for histologic and/or neurologic response decreased to 18.8Gy (16.0 – 21.0) in the reirradiation group because two animals (pigs #10 and #12) had histologic response that was not accompanied by a neurologic response.
Table 3.
Probit analysis estimates for the probability of motor neurologic deficit at a given maximum point dose after reirradiation.
| 95% Confidence Limits (Gy) | |||
|---|---|---|---|
| Probability | Dose (Gy) | Lower bound | Upper Bound |
| 0.01 | 16.0 | 1.7 | 17.9 |
| 0.05 | 17.1 | 6.6 | 18.6 |
| 0.10 | 17.6 | 9.2 | 19.0 |
| 0.50 | 19.7 | 17.4 | 21.4 |
| 0.90 | 21.7 | 20.4 | 28.9 |
Figure 3.
Dose-response curves with 95% confidence bounds for radiosurgery de novo versus retreatment.
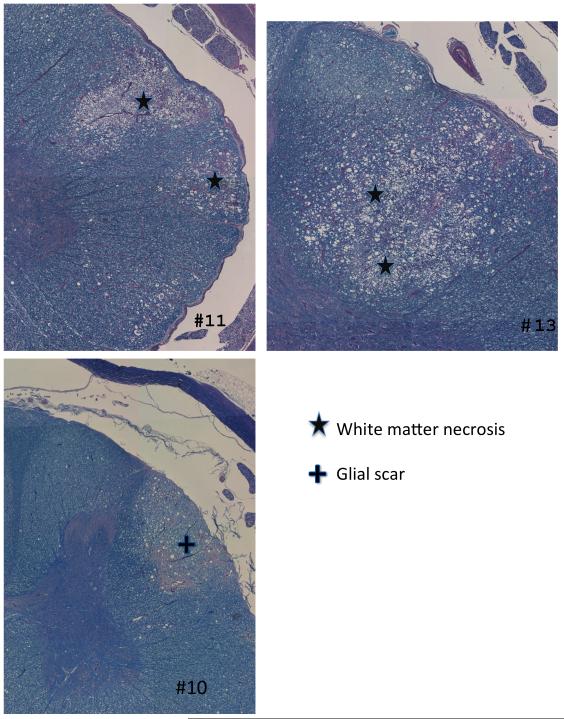
All animals with motor deficits, except pig #8 (18Gy dose group), showed some degree of demyelination and focal white matter necrosis on the irradiated side of the spinal cord with relative sparing of the gray matter. With the exception of pigs #10 and #12 (18 and 20Gy dose groups, respectively), histology was unremarkable in animals with normal neurologic status. For pig #10, unilateral scarring and demyelination were noted in the dorso-lateral region of the spinal cord and the lateral artery next to the demyelinated area was completely obliterated with hyaline deposits. Some minor injury, composed mainly of small glial scars was present in pig #12. Histologic slides of transverse sections through the spinal cord are shown in Figure 4 for three histologic responders (pigs #10, #11, and #13) in the 18Gy and 20Gy prescription groups. Each section of Figure 4 is labeled with the animal identification number as provided in Table 2. The top two images show focal necrosis in the dorso-lateral white matter of pigs #11 and #13. The lower image shows a focal area of glial scarring in an animal with no neurologic change (#10) at one year after retreatment. In a few cases near the threshold dose for myelopathy, only very small foci of demyelination were visible in histology. In addition to diffuse demyelination of the white matter, foci of white matter necrosis were observed increasingly with dose. In the 20Gy prescription group, all animals except one (pig #12) showed extensive lateral demyelination but necrosis was limited to small foci. After reirradiation doses of 22 or 24Gy, extensive white matter necrosis was present in some animals accompanied by gray matter damage, consisting of hemorrhagic infarction and edema. This type of damage was not observed in de novo irradiated animals at similar doses.
Figure 4.
Examples of individual retreated pigs. The top two images represent responders after 20Gy (#11 & 13), each showing focal white matter necrosis in the dorso-lateral white matter on the reirradiated side. The lower picture shows a focal area of glial scarring in an 18Gy non-responder (#10) one year after retreatment.
Discussion
This study was designed to determine the radiation tolerance of the spinal cord in swine that receive single-fraction radiosurgery one year following a dose of 30Gy delivered in ten fractions. A companion study(9) that included identical radiosurgery delivery conditions, determined the spinal cord tolerance to single-fraction radiosurgery alone. Dose-response data for motor neurologic change is nearly identical in both studies with resulting maximum point dose ED50s of 19.7Gy and 20.0Gy for reirradiation and de novo irradiation, respectively.
Radiation repair kinetics have been studied using uniform dose distributions in the rat(3), guinea pig(4) and monkey(2). Wong, et. al. delivered fractionated irradiation to the cervical spines of 10-12 week-old rats followed by reirradiation with graded single doses 20 weeks later. After a minimum 52-week followup, it was concluded that significant long-term spinal cord recovery occurred and that the initial radiation dose influenced both retreatment tolerance and the latency to response(3). Knowles performed split-dose studies to investigate reirradiation tolerance of the spinal cord using guinea pigs. One day old guinea pigs received a single 10Gy dose followed one year later by another single dose. The ED50 for paralysis for retreated animals (19.5 Gy) was only slightly lower than animals treated de novo (20.5 Gy) at one year of age(4). Ang, et. al.(2) investigated the extent and kinetics of recovery from irradiation injury in rhesus monkeys that varied in age from 7-22 years. The cervical/upper thoracic spinal cord was given 44Gy in daily 2.2Gy fractions and then re-irradiated to doses of 57.2Gy (2.2Gy fractions) after one or two year intervals, or 66Gy (2.2Gy fractions) after two or three year intervals. Fitting the observed myeloparesis data with a model assuming all dose-response curves (single course and reirradiation) were parallel resulted in recovery estimates of 33.6Gy (76%), 37.6Gy (85%), and 44.6Gy (101%) of the initial dose after 1, 2, and 3 years, respectively(2). Although significant differences in study design and dose distribution prevent direct comparison of the present study with previous studies, all studies have demonstrated that the spinal cord possesses a large capacity for long-term recovery following single or multi-fraction irradiation.
Five cases of re-irradiation myelopathy following spine stereotactic body radiotherapy have been reported with specific dosimetric analysis comparing re-treatment myelopathy cases to controls(8). Although the SBRT re-irradiation component involved high dose per fraction SBRT ranging from 1 to 3 fractions and thecal sac maximum point dose per fractions ranging from 10.9-14.7Gy, only one case was reported based on conventional radiation followed by single-fraction spinal radiosurgery. This patient received an initial thecal sac dose of 43.2Gy in fifteen fractions based on conventional radiotherapy followed twelve months later by single-fraction radiosurgery with a maximum thecal sac point dose of 14.7Gy. Myelopathy occurred three months following radiosurgery. Although there have been reported single-institution experiences of re-treatment SBRT with a low risk of re-treatment myelopathy,(6-8) the experience is still limited. The correlation between human and animal spinal cord tolerance to single-fraction reirradiation has never been rigorously tested but the results of the present study appear to be consistent with clinical practice and more will be learned as human dosimetric data increases(12).
The accuracy of spinal cord volumes reported in this study is limited by CT-based contouring. Efforts were made to minimize the errors introduced by CT-contouring but CT-myelogram or CT/MRI fusion would have been preferable. The shape of the dose distribution used in the current study makes the dose-volume histogram relatively insensitive to 1-2 mm shifts in the ventral/dorsal and rostral/caudal directions. A lateral 1mm shift of the spinal cord contour towards the treated isocenter would result in an increase in the maximum spinal cord dose of approximately 1-2% while a 1mm shift in the opposite direction would result in a decrease of the maximum spinal cord dose of 3-4%. The follow-up period of the current study (39-53 weeks) is considered long enough to observe the initial phase of radiation myelopathy as commonly reported for guinea pigs(4), rats(13, 14), mice(15) and pigs(9, 10) (2-6 months). The latent period observed for the onset of motor deficits in the present study (9-24 weeks) is in good agreement with the companion study(9), a previous spinal cord tolerance study using pigs(10) and the case of human myelopathy reported by Sahgal, et. al.(8) Potentially more cases of radiation myelopathy would have been observed in a longer follow-up period; a previous spinal cord tolerance study(10) reported two late responding pigs at 65 and 75 weeks following irradiation to doses very close to ED50. The spinal cords of two pigs (pigs #10 and #12) of the present study were found to have histologic changes including vascular hyalinization accompanied by demyelination and glial scarring 53 weeks following radiosurgery but both pigs maintained normal neurologic status. These pigs received maximum spinal cord doses of 19.2 and 21.3Gy that were close to the study ED50 and may have developed neurologic changes with longer followup.
The histological changes observed in the lateral white matter are very similar to lesions observed in other studies in pigs(10), monkeys(16) and rats(17). As reported for most species, including humans, focal white matter necrosis is the most common histology underlying radiation myelopathy of the spinal cord(18). Lesions consist of diffuse demyelination, with focal areas of coalescing necrosis in the higher dose groups. In the current study, the animal showing subtle neurologic signs (#9), had a very small histologic lesion with demyelination, the extent of which is in agreement with the relatively minor character of the motor impairment. No histologic changes were observed in one neurologic responder (pig #8). This animal likely had a focal lesion that occurred between section samples. When comparing histology sections for retreated and de novo irradiated animals, the only difference noted was after prescription doses >20Gy. Although the threshold for induction of neurological damage was the same in both groups, morbidity at higher doses was more severe with gray matter infarction and associated edema. Although a vascular pathology has been suggested to be the basis of most late damage in the spinal cord, it usually does not become manifest as obvious damage to the vasculature such as hemorrhagic infarction. No later signs of vascular gray matter damage have been observed during the one year followup of lower-dosed animals, but a warning is implied that additional vascular damage may occur at longer followup times.
In conclusion, results of the present study are consistent with previous preclinical studies in the rat(3), guinea pig(4) and monkey(2), supporting the observation that the spinal cord possesses a large capacity for recovery following irradiation. Specifically, pigs receiving spinal radiosurgery one year following 30Gy in 10 fractions are not at significantly higher risk of developing motor deficits than pigs that receive radiosurgery alone.
Highlights.
This article describes a preclinical study that was designed to investigate spinal cord tolerance to reirradiation with single-fraction radiosurgery. The study was performed in swine and relates directly to a companion study in which spinal cord tolerance to de novo single-fraction radiosurgery was investigated.
Acknowledgments
This project was funded by the National Institute of Neurological Disorders and Stroke, R01 NS049517.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest Notification
Paul Medin and Timothy Solberg teach in radiosurgery courses sponsored by BrainLAB AG. Remaining authors declare no conflicts of interest.
Contributor Information
Paul M Medin, Department of Radiation Oncology, UT Southwestern Medical Center, Dallas, TX, USA.
Ryan D Foster, Department of Radiation Oncology, UT Southwestern Medical Center, Dallas, TX, USA.
Albert J van Kogel, Department of Radiation Oncology, Radboud University Medical Center Nijmegen, The Netherlands.
James W Sayre, Departments of Biostatistics and Radiology, University of California Los Angeles, CA, USA.
William H McBride, Department of Radiation Oncology, University of California Los Angeles, CA, USA.
Timothy D Solberg, Department of Radiation Oncology, UT Southwestern Medical Center, Dallas, TX, USA.
References
- 1.Rades D, Lange M, Veninga T, et al. Preliminary results of spinal cord compression recurrence evaluation (score-1) study comparing short-course versus long-course radiotherapy for local control of malignant epidural spinal cord compression. Int J Radiat Oncol Biol Phys. 2009;73:228–234. doi: 10.1016/j.ijrobp.2008.04.044. [DOI] [PubMed] [Google Scholar]
- 2.Ang KK, Jiang GL, Feng Y, et al. Extent and kinetics of recovery of occult spinal cord injury. International Journal of Radiation Oncology, Biology, Physics. 2001;50:1013–1020. doi: 10.1016/s0360-3016(01)01599-1. [DOI] [PubMed] [Google Scholar]
- 3.Wong CS, Poon JK, Hill RP. Re-irradiation tolerance in the rat spinal cord: influence of level of initial damage. Radiother Oncol. 1993;26:132–138. doi: 10.1016/0167-8140(93)90094-o. [DOI] [PubMed] [Google Scholar]
- 4.Knowles JF. The radiosensitivity of the guinea-pig spinal cord to X-rays: the effect of retreatment at one year and the effect of age at the time of irradiation. Int J Radiat Biol Relat Stud Phys Chem Med. 1983;44:433–442. doi: 10.1080/09553008314551411. [DOI] [PubMed] [Google Scholar]
- 5.Nieder C, Grosu AL, Andratschke NH, et al. Proposal of human spinal cord reirradiation dose based on collection of data from 40 patients. Int J Radiat Oncol Biol Phys. 2005;61:851–855. doi: 10.1016/j.ijrobp.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 6.Choi CY, Adler JR, Gibbs IC, et al. Stereotactic Radiosurgery for Treatment of Spinal Metastases Recurring in Close Proximity to Previously Irradiated Spinal Cord. Int J Radiat Oncol Biol Phys. 2010;78:499–506. doi: 10.1016/j.ijrobp.2009.07.1727. [DOI] [PubMed] [Google Scholar]
- 7.Gerszten PC, Burton SA, Ozhasoglu C, et al. Radiosurgery for spinal metastases: clinical experience in 500 cases from a single institution. Spine. 2007;32:193–199. doi: 10.1097/01.brs.0000251863.76595.a2. [DOI] [PubMed] [Google Scholar]
- 8.Sahgal A, Ma L, Weinberg V, et al. reirradiation HUMAN Spinal Cord Tolerance for Stereotactic Body Radiotherapy. Int J Radiat Oncol Biol Phys. 2010 doi: 10.1016/j.ijrobp.2010.08.021. In Press. [DOI] [PubMed] [Google Scholar]
- 9.Medin PM, Foster RD, van der Kogel AJ, et al. Spinal cord tolerance to single-fraction partial-volume irradiation: a Swine model. Int J Radiat Oncol Biol Phys. 2011;79:226–232. doi: 10.1016/j.ijrobp.2010.07.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van den Aardweg GJ, Hopewell JW, Whitehouse EM. The radiation response of the cervical spinal cord of the pig: effects of changing the irradiated volume. Int J Radiat Oncol Biol Phys. 1995;31:51–55. doi: 10.1016/0360-3016(94)E0306-5. [DOI] [PubMed] [Google Scholar]
- 11.Bentzen SM, Tucker SL. Quantifying the position and steepness of radiation dose-response curves. Int J Radiat Biol. 1997;71:531–542. doi: 10.1080/095530097143860. [DOI] [PubMed] [Google Scholar]
- 12.Daly ME, Luxton G, Choi CY, et al. Normal Tissue Complication Probability Estimation by the Lyman-Kutcher-Burman Method Does Not Accurately Predict Spinal Cord Tolerance to Stereotactic Radiosurgery. Int J Radiat Oncol Biol Phys. 2011 doi: 10.1016/j.ijrobp.2011.03.004. IN PRESS. [DOI] [PubMed] [Google Scholar]
- 13.Bijl HP, van Luijk P, Coppes RP, et al. Dose-volume effects in the rat cervical spinal cord after proton irradiation. Int J Radiat Oncol Biol Phys. 2002;52:205–211. doi: 10.1016/s0360-3016(01)02687-6. [DOI] [PubMed] [Google Scholar]
- 14.Hopewell JW, Morris AD, Dixon-Brown A. The influence of field size on the late tolerance of the rat spinal cord to single doses of X rays. Br J Radiol. 1987;60:1099–1108. doi: 10.1259/0007-1285-60-719-1099. [DOI] [PubMed] [Google Scholar]
- 15.Lo YC, McBride WH, Withers HR. The effect of single doses of radiation on mouse spinal cord. Int J Radiat Oncol Biol Phys. 1992;22:57–63. doi: 10.1016/0360-3016(92)90982-n. [DOI] [PubMed] [Google Scholar]
- 16.Schultheiss TE, Stephens LC, Jiang GL, et al. Radiation myelopathy in primates treated with conventional fractionation. International Journal of Radiation Oncology, Biology, Physics. 1990;19:935–940. doi: 10.1016/0360-3016(90)90015-c. [DOI] [PubMed] [Google Scholar]
- 17.van der Kogel AJ. Radiation tolerance of the rat spinal cord: time-dose relationships. Radiology. 1977;122:505–509. doi: 10.1148/122.2.505. [DOI] [PubMed] [Google Scholar]
- 18.Wong CS, Van der Kogel AJ. Mechanisms of radiation injury to the central nervous system: implications for neuroprotection. Mol Interv. 2004;4:273–284. doi: 10.1124/mi.4.5.7. [DOI] [PubMed] [Google Scholar]