Abstract
The purpose of this study was to develop and characterize a chitosan gel/gelatin microspheres (MSs) dual delivery system for sequential release of bone morphogenetic protein-2 (BMP-2) and insulin-like growth factor-1 (IGF-1) to enhance osteoblast differentiation in vitro. We made and characterized the delivery system based on its degree of cross-linking, degradation, and release kinetics. We also evaluated the cytotoxicity of the delivery system and the effect of growth factors on cell response using pre-osteoblast W-20-17 mouse bone marrow stromal cells. IGF-1 was first loaded into MSs, and then the IGF-1 containing MSs were encapsulated into the chitosan gel which contained BMP-2. Cross-linking of gelatin with glyoxal via Schiff bases significantly increased thermal stability and decreased the solubility of the MSs, leading to a significant decrease in the initial release of IGF-1. Encapsulation of the MSs into the chitosan gel generated polyelectrolyte complexes by intermolecular interactions, which further affected the release kinetics of IGF-1. This combinational delivery system provided an initial release of BMP-2 followed by a slow and sustained release of IGF-1. Significantly greater alkaline phosphatase activity was found in W-20-17 cells treated with the sequential delivery system than other treatments (p<0.05) after a week of culture.
Keywords: Chitosan, Gelatin microspheres, glyoxal, BMP-2, IGF-1, Sequential delivery, Osteoblastic differentiation, W-20-17
1. Introduction
Therapeutic biomacromolecules such as RGD-like peptides and growth factors have been used to enhance the regeneration of damaged tissues by stimulating cellular activities such as cell migration, proliferation, and differentiation [1–6]. However, they have short biological half lives in physiological conditions due to rapid degradation and deactivation by enzymes and other chemical and physical reactions [1–4]. In addition, the course of wound healing and tissue regeneration is complicated by the interactions of multiple factors [4, 7–10]. Local delivery carriers have been developed for the controlled, sustained release of these active proteins [11–14]. Still, there is a great need for drug delivery systems that allow for improved release kinetics of multiple growth factors in order to enhance their therapeutic efficacy [3, 8, 11–17].
Recent studies have shown that the combined delivery of bone morphogenetic protein-2 (BMP-2) and insulin like growth factor-1 (IGF-1) enhances wound healing and tissue regeneration compared with single growth factor delivery [3, 5, 11, 14, 18]. BMP-2 is an FDA approved growth factor, which plays an important role in the expression of osteogenic markers such as alkaline phosphatase (ALP) and osteocalcin. It is used clinically to help induce osteogenesis [3, 5, 15–16, 19]. IGF-1 is a mitogenic factor affecting the growth of adult cells as well as supporting the growth and differentiation of embryonic cells [17–18, 20–24]. It has been used to stimulate osteoblast growth and proliferation, resulting in enhanced osseointegration at the local site [10, 17].
Raiche et al. used two layers of glutaraldehyde cross-linked gelatin coatings with different concentrations of growth factors to control the release kinetics of BMP-2 and IGF-1 [14, 18]. They found that the sequential release of BMP-2 and IGF-1 resulted in the earliest, most robust elevation of ALP activity of both mouse pluripotent C3H and rat bone marrow stromal cells and that the simultaneous release of BMP-2 and IGF-1 did not promote ALP activity compared with BMP-2 alone. They suggested that treatment with BMP-2 upregulated the expression of the IGF-I receptor, enabling IGF-I to further enhance cell responses [14]. Similarly, Chen et al. reported that the combined delivery of BMP-2 and IGF-1 resulted in the greatest ALP activity of periodontal ligament fibroblasts (PDLFs) [11]. In their experiments, the release of growth factors was controlled by BMP-2 containing basic gelatin microspheres and IGF-1 containing acidic gelatin microspheres, which were incorporated into glycidyl methacrylated dextran (Dex-GMA) scaffolds.
We have previously developed a thermo-sensitive injectable chitosan gel to deliver BMP-2. It has been found to significantly enhance the osteoblastic differentiation of mouse osteoblast precursor cells and the mineralization of human embryonic palatal mesenchymal cells [25]. Chitosan gels have been used due to their excellent biocompatibility, enzyme regulated degradation, and high efficacy of drug therapy [2–3, 9, 25–28]. Additionally, therapeutic agents are released via the diffusion or biodegradation of the chitosan polymers [5, 25–28]. The purpose of this study was to create and characterize a sequential delivery system consisting of a chitosan gel and gelatin microspheres (MSs) to achieve a sequential release of BMP-2 and IGF-1. We hypothesized that an initial release of BMP-2 from the chitosan gel followed by the release of IGF-1 from the gelatin MSs would enhance osteoblastic activity of bone cells. In this study, we made glyoxal cross-linked gelatin MSs for delivery of IGF-1, which were then encapsulated into the chitosan gel formulation. Furthermore, we aimed to characterize the degree of cross-linking, degradation, release rate and cytotoxicity of the delivery system. We also evaluated osteoblastic activity by measuring alkaline phosphatase (ALP) specific activity of preosteoblast W-20-17 mouse bone marrow strmal cells.
2. Materials and Methods
2.1. Materials
Chitosan (≥ 310 kDa, 75% or greater degree of deacetylation), disodium β-GP (glycerol 2-phosphate disodium salt hydrate; cell culture grade), and glyoxal (40 wt. %) were purchased from Sigma-Aldrich (St Louis, MO). Gelatin type B, olive oil, acetone, and ethanol were all purchased from Fisher Scientific (Fair Lawn, NJ). All other chemicals were reagent grade and were used as received. Human bone morphogenetic protein-2 (BMP-2) was obtained from PeproTech (Rocky Hill, NJ) and recombinant human insulin-like growth factor I (IGF-1) was purchased from R&D Systems (Minneapolis, MN). Fetal bovine serum (FBS), Trypsin-EDTA, L-Glutamine, Antibiotic-Antimycotic, phosphate buffered saline (PBS), and Dulbecco's Modified Eagle Medium (DMEM) were all purchased from Invitrogen™ (Eugene, OR). W-20-17 cells were cultured as per American Type Culture Collection (ATCC) instructions.
2.2. Preparation of gelatin microspheres (MSs)
Gelatin MSs were prepared using a water-in-oil emulsion technique. Briefly, a gelatin solution was prepared by dissolving 1g gelatin powder into 10 ml distilled water at 50°C. The solution was then added dropwise to 60 ml olive oil, which was preheated to 50°C while stirring at 500 rpm using a straight-blade impeller. The gelatin solution was allowed to emulsify for 10 min. Subsequently, the entire emulsification bath was chilled to 4°C on ice with continuous stirring at 500 rpm for 40 min, and gelatin MSs were formed. The gelatin MSs were collected by filtration and washed with chilled acetone and ethanol. Finally, the obtained gelatin MSs were freeze-dried overnight.
2.3. Cross-linking of gelatin MSs
The prepared gelatin MSs were dispersed into an aqueous ethanol solution containing different concentrations of glyoxal (10, 20, 50, or 100mM) and stirred at room temperature for cross-linking for 10 hours. The cross-linked gelatin MSs were rinsed twice with an aqueous ethanol solution to remove the residual cross-linking agent on their surfaces and then freeze-dried overnight. They were then sieved to obtain particles ranging from 50 to 100 µm.
2.3.1. Fourier transform infrared spectroscopy (FTIR) spectra
In order to investigate chemical structure of both gelatin MSs and cross-linked gelatin MSs, FTIR spectra were obtained using a Nicolet FTIR Infrared Microscope coupled to a PC with analysis software. Samples were placed in the holder directly in the IR laser beam. All spectra were recorded by transmittance mode (100 times scanning, 650–4000 cm−1).
2.3.2. Degree of cross-linking
Degree of cross-linking of the gelatin MSs was determined by ninhydrin assay, which was used to determine the percentage of free amino groups remaining in the gelatin MSs after cross-linking. The cross-linked gelatin MSs were prepared with different concentrations of glyoxal (10, 20, 50, or 100mM). The samples were heated in the ninhydrin solution at 100°C for 10 minutes, and the light absorbance at 550 nm was recorded using a micro plate reader (TECAN Infinite F50). Glycine (Fisher Scientific, Fair Lawn, NJ) was used as an amino acid nitrogen standard at various known concentrations. The degree of cross-linking (Dc) of the samples was calculated following the equation Dc = [(A−B) / A] × 100, where A is mole fraction of free amino group in uncross-linked gelatin MSs and B is mole fraction of free amino group in cross-linked gelatin MSs.
2.3.3. Swelling of gelatin MSs at different temperatures
To evaluate the effect of cross-linking on water stability of the gelatin MSs, swelling characteristic of the gelatin MSs was investigated at different temperatures. The swelling of the gelatin MSs and the cross-linked gelatin MSs (50 mM) were observed using a microscope (Nikon ECLIPSE TE-2000-U). The dried samples were placed into a container with PBS (pH7.4) and incubated at 4°C or 37°C for 3 days. Photomicrographs of the gelatin MSs were processed at 6 hours, 1 day, and 3 days of incubation using a MetaVue software.
2.3.4. Cytotoxicity
W-20-17 cells were grown and maintained in DMEM media with 10% FBS, 1% antibiotic/antimycotic mixture, 5ml of L-glutamine (200mM), and sodium pyruvate. This cell line has been used in an ASTM F2131 to evaluate activity of BMP-2 in vitro. Cell culture was achieved in an incubator supplied with 5% CO2 at 37°C. The culture medium was changed every 3 days. In order to investigate the cytotoxicity of the gelatin MSs, the W-20-17 cells were cultured in the DMEM media containing the gelatin MSs. Cells were seeded in 24-well plates at a density of 30,000 cells/well and incubated with 10 mg of the gelatin MSs for 3 days. After incubation of 1 and 3 days, the number of viable cells was determined quantitatively using a Cell Titer 96AQueous One Solution (MTS) assay according to the manufacturer’s instructions. Before the assay, the cellular morphology was observed qualitatively using a microscope (Nikon, ECLIPSE TE-2000-U). Photomicrographs of cells were processed using a software (Nikon, MetaVue).
2.4. Gelatin MSs encapsulated chitosan gel composites
A 1.5 % (w/v) chitosan solution was prepared by stirring powdered chitosan in 0.75% (v/v) aqueous acetic acid at room temperature overnight. The insoluble particles in the chitosan solution were removed by filtration. A 50% (w/v) β-GP solution was prepared in distilled water and sterilized using PES syringe filters with 0.22 µm pore size (MillexTM, MA) and stored at 4°C. 50 ml of chitosan solution was dialyzed at room temperature against 1 liter of distilled water for 7 days with daily changes of water (1 L) in an 8 kDa cutoff dialysis membrane to reduce the acetic acid content. The final pH value of the chitosan solution was 6.3. The dialyzed chitosan solution was autoclaved at 121°C for 20 minutes, cooled down to room temperature, and stored at 4°C. The cross-linked gelatin MSs were then encapsulated into the chitosan solution on ice and vortexed. Sterilized, ice-cold β-GP solution (2.31M) was added drop by drop to the chitosan solution under stirring conditions in an ice bath. The final concentration of β-GP in the chitosan solution was 88 mM, and the final pH value of the chitosan gel formulation was 7.2. Each gel forming solution was allowed to completely become a gel in an incubator for 3 hours at 37°C.
2.5. Dissolution rate
Gelatin is an amphoteric protein containing both positively charged and negatively charged amino acids. It easily dissolves in water at body temperature, releasing amino acids. In this study, the cross-linked gelatin MSs or the cross-linked gelatin MSs loaded chitosan gel were placed in a container containing 2 ml of PBS (pH 7.4) and incubated at 37°C for 5 days. At predetermined time points, 500 µl aliquots of the medium were sampled and the same amount of fresh PBS (pH 7.4) was added into each container. In the collected fractions, the cumulative amounts of dissolved proteins from the gelatin MSs or the combination were determined as a function of time by bicinchoninic acid (BCA) assay (Pierce, Rockford, IL). The optical density of each sample was determined using a microplate reader at 562 nm (TECAN Infinite F50).
2.6. Scanning electron microscopy (SEM)
The surface morphology of the materials was observed to examine compatibility of gelatin MSs with a chitosan gel after implantation at body temperature. Three different materials including a chitosan gel, an uncross-linked gelatin MSs loaded chitosan gel, and a cross-linked gelatin MSs loaded chitosan gel were prepared. They were incubated at 37°C for 5 hours and lyophilized overnight (Freezone, LABCONCO). The samples were sputter-coated with gold and examined under a scanning electron microscope (FEI, USA) operated at 15 kV voltages.
2.7. In vitro release studies
2.7.1. IGF-1 release
In vitro IGF-1 release profiles from cross-linked gelatin MSs or a cross-linked gelatin MSs loaded chitosan gel were examined for a week. IGF-1 loading was achieved by a method of adsorption. The cross-linked gelatin MSs were loaded with IGF-1 by swelling in aqueous IGF-1 solutions (IGF-1(MSs)). IGF-1 (IEP=8.6) is positively charged, and therefore, negatively charged type B gelatin forms a polyionic complexation with IGF-1 [23, 29–31]. IGF-1 solution was dripped onto the micro-particles at a volume of 25 µl per mg of the cross-linked gelatin MSs. The resulting mixture was vortexed and incubated at 4°C for 10 hours before freeze-drying. Eventually, 50 ng/ml of IGF-1 was present within each sample (IGF-1(MSs)). The IGF-1 loaded gelatin MSs were then encapsulated into a chitosan gel formulation (IGF-1(gel+MSs)). The IGF-1 loaded micro-particles were added into the chitosan gel formulation on ice and vortexed. 88 mM of cold β-GP solution was added into the mixture to complete the gel forming solution. Each gel forming solution was allowed to completely become a gel in an incubator at 37°C.
IGF-1 (MSs) or IGF-1 (gel+MSs) was placed in a container containing 2 ml of PBS (pH 7.4) and incubated at 37°C for a week. At designated time points, 300 µl aliquots of the release medium were sampled and the same amount of fresh PBS (pH 7.4) was added into each container. In the collected fractions, the cumulative release amounts of IGF-1 from the materials were determined as a function of time by an IGF-1 ELISA kit (RayBio, GA). Briefly, 100 µl of the obtained samples were pipetted into a 96-well IGF-1 microplate coated with anti-human IGF-1 and incubated at 4°C overnight. After washing each well with wash buffer provided by the ELISA kit for a total of 4 washes, 100 µl of biotinylated anti-human IGF-1 was added to each well and incubated at room temperature for 1 hour. After repeating the washing step, each well was filled with 100 µl of HRP-Streptavidin solution and incubated at room temperature for 45 minutes. After the washing step, 100 µl of TMB (3,3’,5,5’ Tetramethylbenzidine) was added to each well and incubated for 30 minutes at room temperature in the dark. Finally, 50 µl of stop solution was added into each well. The optical density of each well was determined using a microplate reader at 450 nm (TECAN Infinite F50).
2.7.2. BMP-2 release
The in vitro BMP-2 release profile from the chitosan gel was investigated for a week. BMP-2 solution was added directly into the chitosan solution on ice and vortexed. 88 mM of cold β-GP solution was added into the mixture to complete the gel forming solution. Each gel forming solution containing BMP-2 was allowed to completely become a gel in an incubator at 37°C. Eventually, 50ng/ml of BMP-2 was present within each sample (BMP-2(Gel)).
BMP-2 (Gel) was placed in a container containing 2 ml of PBS (pH 7.4) and incubated at 37°C for a week. At designated time points, 300 µl aliquots of the release medium were sampled and the same amount of fresh PBS (pH 7.4) was added into each container. In the collected fractions, the cumulative release amounts of BMP-2 from the chitosan gels were determined as a function of time by a BMP-2 ELISA kit (R&D systems, MN). Briefly, 50 µl of the obtained supernatant was pipetted into a 96-well BMP-2 microplate coated with a mouse monoclonal antibody and incubated for 2 hours at room temperature. After washing each well with wash buffer provided by the ELISA kit for a total of 4 washes, 200 µl of BMP-2 conjugate was added to each well and incubated at room temperature for 2 hours. After repeating the washing step, each well was filled with 200 µl of BMP-2 substrate and incubated at room temperature for 30 minutes in the dark. Finally, 50 µl of stop solution was added into each well. The optical density of each well was determined using a microplate reader at 450 nm with a correction setting of 540 nm (TECAN Infinite F50).
2.8. In vitro analysis
2.8.1. Effect of growth factors on ALP specific activity of W-20-17
To better evaluate the sequential delivery of growth factors on the cell responses, we first established a growth factor-cell response calibration model. We studied ALP activity as an indicator of early osteoblastic differentiation to designated singular or combination of BMP-2 and IGF-1 listed in Table 1. In this experiment, W-20-17 cells were treated with growth factors (BMP-2, IGF-1, or combinations), and ALP activity and double stranded DNA (dsDNA) of W-20-17 cells were determined. The cells were seeded in 24-well plates at a density of 30,000 cells/well and cultured for 7 days. On day one and three, 50 ng/ml of each growth factor or their combination was added into the culture medium as shown in Table 1. The culture medium was changed every 3 days.
Table 1.
The group designs for the effect of soluble growth factors on alkaline phosphatase (ALP) specific activity of W-20-17 cells
| A | B | C | D | E | F | G | H | |
|---|---|---|---|---|---|---|---|---|
| Day | N/A | BMP-2 | IGF-1 | BMP-2 | IGF-1 | BMP-2/IGF-1 | BMP-2 | IGF-1 |
| Day | N/A | BMP-2 | IGF-1 | BMP-2/IGF-1 | BMP-2/IGF-1 | BMP-2/IGF-1 | IGF-1 | BMP-2 |
At designated time points (five and seven days) the medium was removed from cell culture. The cell layers were washed twice with PBS (pH 7.4) and then lysed with 1 ml of 0.2% Triton X-100 and three freeze-thaw cycles, which consisted of freezing at −80 °C for 30 minutes immediately followed by thawing at 37 °C for 15 minutes. 50 µl aliquots of the cell lysates were sampled and added to 50 µl of working reagent in a 96-well assay plate. The working reagent contains equal parts (1:1:1) of 1.5M 2-amino-2-methyl-1-propanol (Sigma), 20 mM p-nitrophenyl phosphate (Sigma), and 1 mM magnesium chloride. The samples then were incubated for 1 hour at 37°C. After incubation, the reaction was stopped with 100 µl of 1N sodium hydroxide on ice. ALP activity was determined from the absorbance using a standard curve prepared from p-nitrophenol stock standard (Sigma). The absorbance was measured at 405 nm using a microplate reader (TECAN Infinite F50).
The cell lysates were then examined for dsDNA to estimate cell number. 50 µL aliquots of the cell lysates were added in a 96-well assay plate. Each 50 µl of a 1:200 dilution of PicoGreen (Quant-iT PicoGreen assay kit, Invitrogen) was added to each well and incubated for 5 min in the dark. The assay plate was read at 485 nm excitation and 530 nm emission using a BioTek FLx800 plate reader. The dsDNA content was calculated using a standard curve made by a provided dsDNA standard sample. The ALP activity of cells was then calculated by normalizing to dsDNA content. ALP specific activity was expressed as nmol p-nitrophenol/ng dsDNA.
2.8.2. Effect of growth factors by sequential delivery systems on ALP specific activity of W-20-17
The effect of sequential delivery of growth factors on ALP specific activity of W-20-17 was investigated using the chitosan gel or the gelatin MSs loaded chitosan gel as shown in Table 2. Indirect culture via BD BioCoat™ Control Cell Culture inserts was used as a model system to evaluate the cell responses from the materials. The materials loaded with growth factors were placed in the upper chamber of inserts. Cells were seeded in the bottom of 12-well plates at a density of 30,000 cells/well and cultured for 7 days. The culture medium was changed every 3 days. At designated time points (5, and 7 days), the medium was removed from cell culture. ALP activity and dsDNA content were determined as described above.
Table 2.
The group designs for the effect of sequential delivery systems on alkaline phosphatase (ALP) specific activity of W-20-17 cells
| A | B | C | D | |
|---|---|---|---|---|
| Gelatin MSs | N/A | N/A | N/A | IGF-1 |
| Chitosan gel | N/A | BMP-2 | BMP-2/IGF-1 | BMP-2 |
2.8. Statistical analysis
All data are presented as mean ± standard deviation. For comparing two groups of data, student t-test was performed. For comparing multiple groups of data, one-way ANOVA was performed followed by Dunnett’s test. The differences in groups and experimental time points were considered significant if p<0.05.
3. Results
3.1. Cross-linking of gelatin MSs
3.1.1. FTIR spectra of gelatin MSs
The FTIR spectra obtained from the gelatin MSs and the cross-linked gelatin MSs are shown in Figure 1. The gelatin shows the typical specific amide bands of proteins. The amide I band peaking at 1620 cm−1 is due to C=O stretching vibration while the amide II band at 1535 cm−1 is assigned to C-N stretching and N-H bending vibration. In the cross-linked gelatin MSs, the stronger band centered at 1635 cm−1 is due to C=N stretching vibration from Schiff-bases, and C=O stretching from the mixture of amide I and unreacted aldehyde groups. The Schiff-bases were formed between -amino groups of the gelatin and carbonyl groups of glyoxal [27, 32–33] as a result of cross-linking. The peak at 1540 cm−1 indicates a shift in the amide II band, which becomes stronger after the cross-linking. The absorbance at 1735 cm−1 is due to C=N stretching vibration from Schiff-base reactions.
Figure 1.
FTIR spectra of gelatin microspheres and cross-linked gelatin microspheres. The cross-linking occurred through the formation of Schiff base linkage between amino groups of the gelatin and carbonyl groups of glyoxal.
3.1.2. Degree of cross-linking of gelatin MSs
The gelatin MSs had a dark-yellow color after cross-linking with glyoxal. As shown in Figure 2, the degree of cross-linking significantly increased with increasing concentration of glyoxal (p<0.05). The degree of cross-linking was 8% at 10 mM, 19% at 20 mM, 66% at 50 mM, and 69% at 100 mM of glyoxal. However, there was no significant difference between 50 mM and 100 mM of glyoxal (p=0.5).
Figure 2.
The degree of cross-linking of the gelatin microspheres. The cross-linked gelatin microspheres were prepared according to different concentrations of glyoxal (10, 20, 50, or 100mM). The percentage of free amino groups remaining in the gelatin microspheres was determined by a ninhydrin assay at 550 nm after cross-linking. Each value represents the mean ± SD (n = 4). * denotes significant difference between groups (p<0.05).
3.1.3. Swelling of gelatin MSs at different temperatures
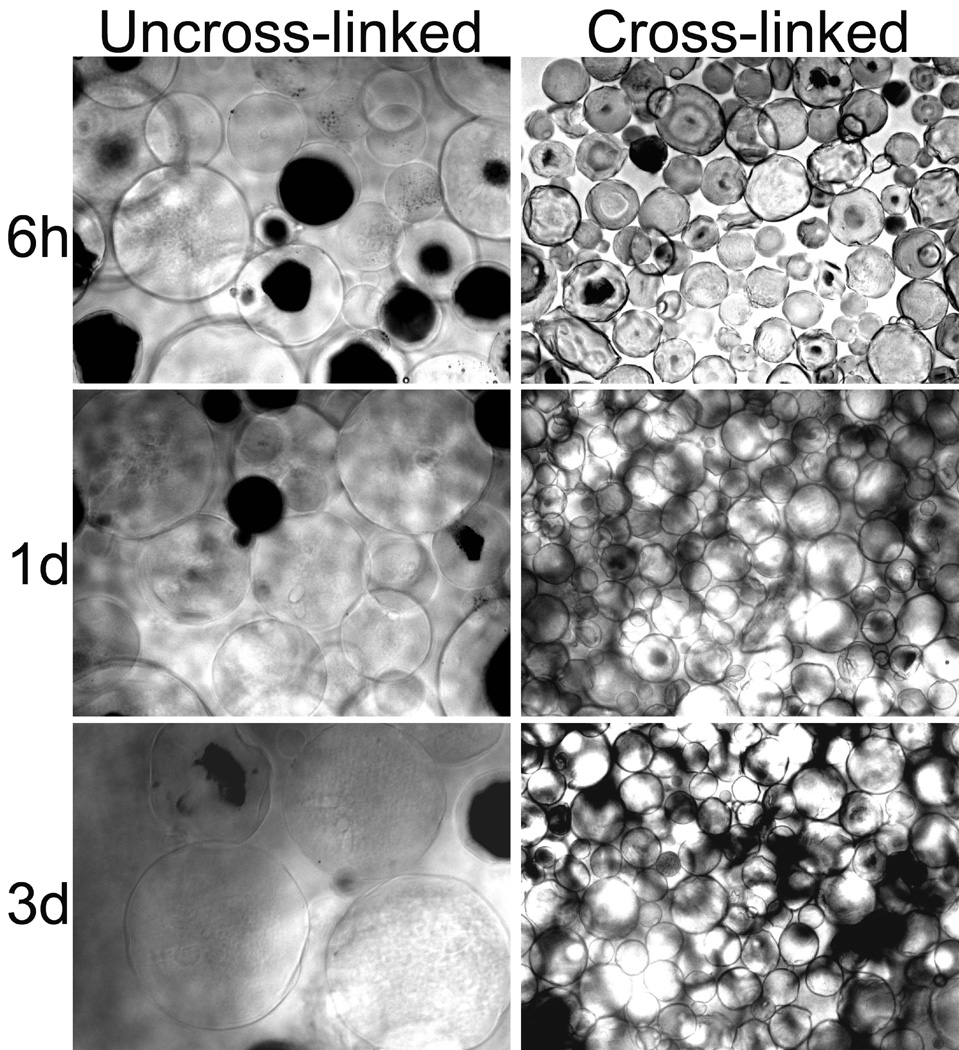
Figure 3 shows the swelling behavior of uncross-linked gelatin MSs or cross-linked gelatin MSs (50 mM) in PBS solution at 4°C or 37°C for 3 days. The swelling behavior of the gelatin MSs changed notably with cross-linking by glyoxal. At 4°C, a halo of diffuse material was visible around the uncross-linked gelatin MSs indicating they became swollen and enlarged while the cross-linked gelatin MSs was much less swollen. At 37°C, the uncross-linked gelatin MSs completely dissolved, but the cross-linked gelatin MSs retained their shape.
Figure 3.
Swelling behaviors of gelatin microspheres and cross-linked gelatin microspheres at (a) 4°C and (b) 37°C. The dried samples were placed into PBS solution, and photomicrographs of the samples were processed at 6 hours, 1 day, and 3 days of incubation. (MAG = ×100).
3.1.4. Cytotoxicity of gelatin MSs
The cytotoxicity of the gelatin MSs on W-20-17 was examined by a MTS assay, and the cell morphology was observed by a microscope for 3 days of incubation. Figure 4a shows the effect of different concentrations of glyoxal in the gelatin MSs on cell viability via an MTS assay. There were significant increases in activity in W-20-17 cells after 3 days of culture (p<0.05). Cells in all the groups showed significantly higher metabolic activity at day 3 than at day 1 (p<0.05), indicating that the cells were viable in the presence of the cross-linked gelatin MSs. However, there were significant differences between lower concentrations (10 and 20 mM) and higher concentration (100 mM) of glyoxal (p<0.05). Consistent with a MTS assay, cellular imaging shows cells in all the groups significantly proliferated for 3 days of culture (Fig 4b), suggesting the non-cytotoxicity of gelatin MSs.
Figure 4.
Cytotoxicity of the cross-linked gelatin microspheres using the preosteoblast mouse stromal cells (W-20-17). (a) The effect of different concentration of glyoxal of gelatin MSs on cell viability via an MTS assay; (b) Photomicrographs of cell morphology using a microscope (Nikon ECLIPSE TE-2000-U). Cells were seeded in 24-well plates at a density of 30,000 cells/well and incubated with 10 mg of the gelatin microspheres for 3 days. (MAG = ×100). Each value represents the mean ± SD (n = 3). * denotes significant difference compared with 1 day of culture (p<0.05). ** denotes significant difference between groups at same time point (p<0.05).
3.2. Dissolution rate of gelatin MSs
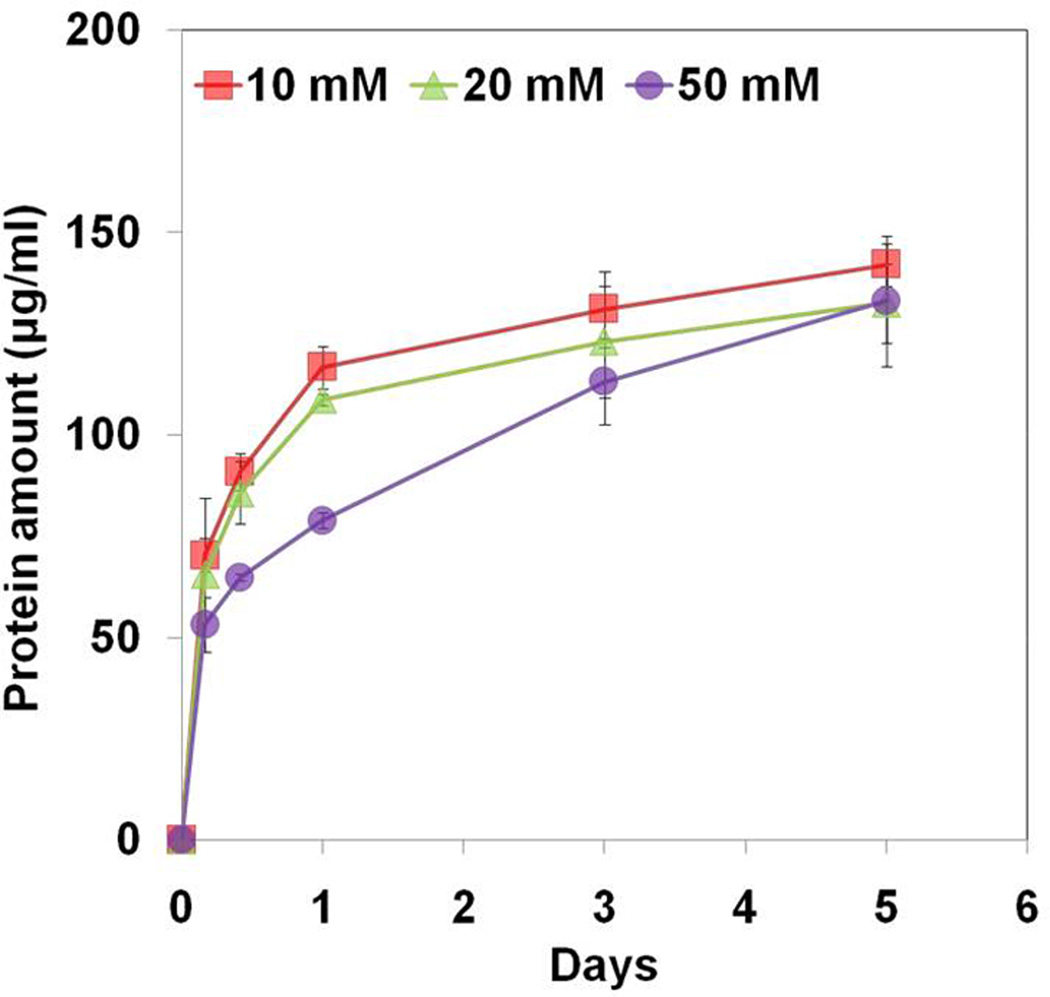
As shown in Figure 5a, there was initial burst release of proteins from the gelatin MSs followed by a sustained slow release. However, there was significant difference between groups for 5 days of incubation (p<0.05). The cumulative amount of proteins released from the gelatin MSs decreased with increasing concentration of glyoxal. In addition, dissolution rate of gelatin MSs was compared to that of the gelatin MSs loaded chitosan gel (Fig 5b). Similarly, there was significant difference in dissolution rate of gelatin MSs with different degree of cross-linking (p<0.05). The gelatin MSs cross-linked by 50 mM glyoxal showed significantly lower dissolution rate compared with the other groups after 10 hours and 1 day of incubation (p<0.05). However, there was no significant difference between groups after 3 days of incubation.
Figure 5.
Dissolution rate of (a) cross-linked gelatin microspheres; (b) cross-linked gelatin microspheres loaded chitosan gel. The samples were placed into PBS solution, and incubated at 37°C for 5 days. The cumulative amounts of dissolved proteins from gelatin MSs or the combination were determined as a function of time by bicinchoninic acid (BCA) assay at 562 nm. Each value represents the mean ± SD (n = 3).
3.3. SEM images
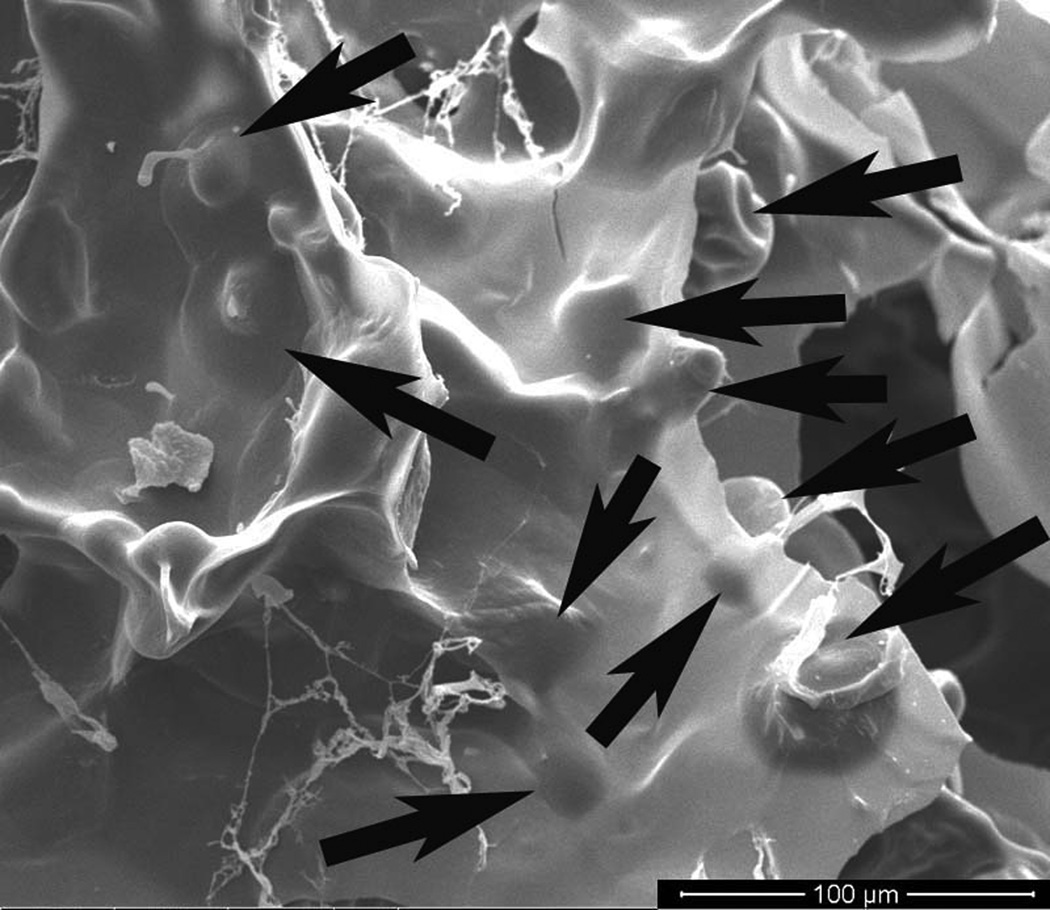
As shown in Figure 6, SEM images show surface structures of different materials. The chitosan gel group shows a porous structure formed by some salts and water during freeze-drying process (Fig. 6a). However, encapsulation of gelatin MSs into the chitosan gel induced a completely different structure. Fig.6b shows that uncross-linked gelatin MSs completely dissolved at 37°C for 5 hours. The newly formed structure became less porous compared to the chitosan gel (Fig. 6b), indicating that the chitosan and dissolved gelatin blended well. On the other hand, the cross-linked gelatin MSs did not completely dissolve, but retained their microspherical structure on the surface of the chitosan gel network (Fig. 6c), indicating the enhanced stability of cross-linked gelatin MSs.
Figure 6.
SEM micrographs on the surface of (a) a chitosan gel; (b) combination of uncross-linked gelatin microspheres and a chitosan gel; (c) combination of crosslinked gelatin microspheres and a chitosan gel. Samples were incubated at 37°C for 5 hours and lypholized overnight before the examination under a scanning electron microscope (FEI, USA) operated at 15 kV voltages. Arrows indicate the remaining microspherical structure on the surface of the chitosan gel network. (Bar = 100µm).
3.4. In vitro release studies
The in vitro release behavior of IGF-1 and BMP-2 from the delivery system was interpreted as the cumulative amount of the growth factors over the time. Figure 7 shows the in vitro release behaviors of IGF-1 from cross-linked gelatin MSs (IGF-1(MSs)) or a chitosan gel encapsulated with the cross-linked gelatin MSs (IGF-1(gel+MSs)) for a week. IGF-1 release behavior from IGF-1(MSs) shows a high initial burst release within 1 day followed by a slow release for 1 week of incubation. The encapsulation of gelatin MSs into the chitosan gel (IGF-1 (gel+MSs)) significantly reduced initial release amount of IGF-1 by 48%, resulting in minimal burst release. There was also a moderate and sustained release from IGF-1 (gel+MSs) between day 1 and 7. The cumulative BMP-2 release profile from the chitosan gels was compared with IGF-1 release from IGF-1 (gel+MSs). The cumulative release amount of BMP-2 was 16.5 ng/ml at day 1 and 28.1 ng/ml at day 3 while the cumulative release amount of IGF-1 was 8.8 ng/ml at day 1 and 10.8 ng/ml at day 3. During this period, the release amount of BMP-2 from the chitosan gel was much higher than that of IGF-1. However, during the release periods between day 3 and 7, release rate of BMP-2 was slower than that of IGF-1, and more amount of IGF-1 was released from the delivery system.
Figure 7.
In vitro cumulative release profiles of growth factors from the materials over a week period. IGF-1 release behavior from cross-linked gelatin MSs (IGF-1(MSs)) was compared with that from cross-linked gelatin MSs loaded chitosan gel (IGF-1(gel+MSs)). BMP-2 release kinetics from a chitosan gel was also compared with IGF-1 release from IGF-1(gel+MSs). The cumulative release amounts of BMP-2 and IGF-1 from the materials were determined as a function of time by either BMP-2 or IGF-1 ELISA Kits at 450 nm. Each value represents the mean ± SD (n = 3).
3.5. In vitro analysis
3.5.1. Effect of growth factors on ALP specific activity of W-20-17
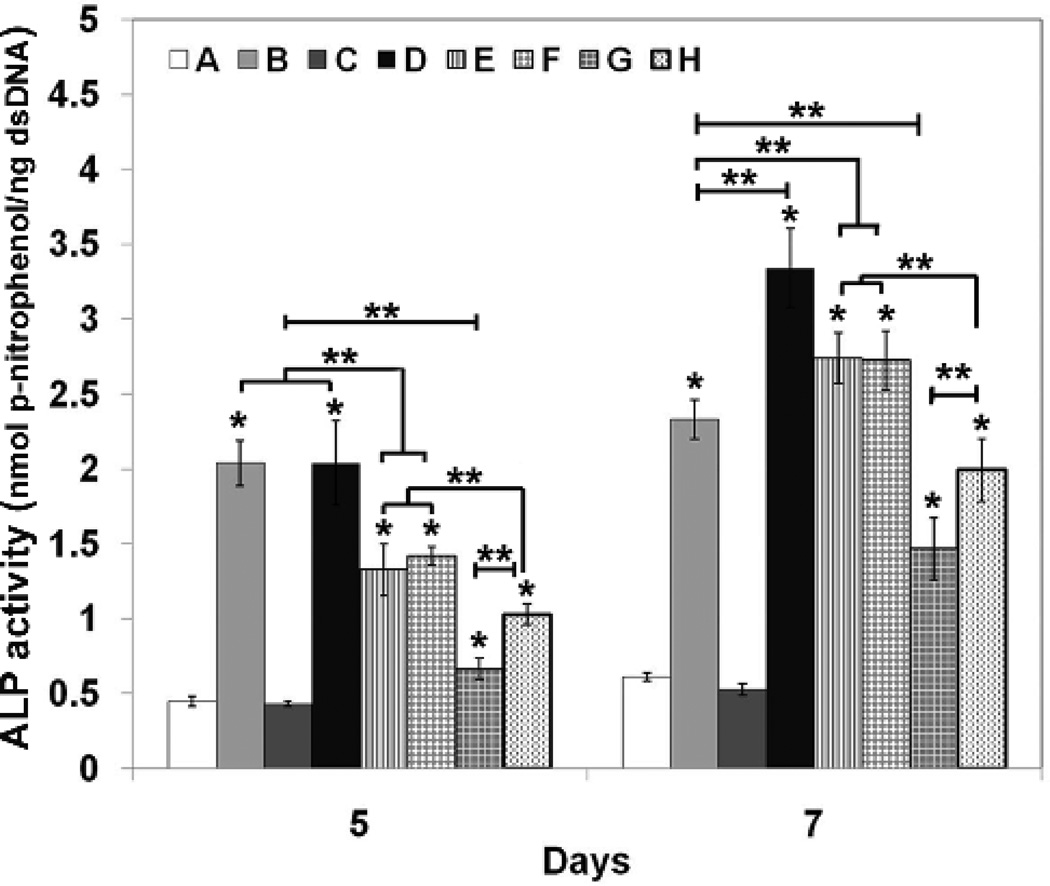
W-20-17 cells were treated with growth factors (BMP-2, IGF-1, or combinations) as shown in Table 1. The ALP specific activity was determined at days 5 and 7 of incubation by normalizing the ALP amount to the dsDNA content per sample. Figure 8 shows ALP specific activity of W-20-17 at different conditions. There were significant differences in ALP specific activity among the various growth factor treatments. At 5 days of cell culture, ALP specific activity was considerably increased with BMP-2 treatment regardless of IGF-I incorporation. Treatments that began with BMP-2 on day 1 followed by BMP-2 alone or in combination with IGF-I on day 3 showed significantly higher ALP activity than any other treatments (p<0.05). However, there was no significant difference between control (no growth factors) and IGF-1 treatment alone, indicating IGF-1 alone did not significantly affect the early osteoblast-associated genes. At 7 days of cell culture, sequential treatment with BMP-2 on day 1 followed by combined BMP-2 and IGF-I on day 3 induced significantly greater ALP activity than any other treatments (p<0.05).
Figure 8.
Effect of growth factors on alkaline phosphatase (ALP) specific activity of W-20-17 cells. The cells were seeded in 24-well plates at a density of 30,000 cells/well and cultured for 7 days. On day 1 and 3, 50 ng/ml of each growth factor or their combination was added as shown in Table 1. The culture medium was changed every 3 days. The ALP activity was determined at 5 and 7 days of cultures and normalized for the dsDNA content. ALP activity is expressed as nmol/ng. Each value represents the mean ± SD (n = 3). * denotes significant increase compared with control group A (no growth factor) at each time point (p<0.05). ** denotes significant difference between groups at same time point (p<0.05). Each group (A to H) is listed at table 1.
Figure 9 shows the effect of sequential delivery of growth factors by the chitosan gel / gelatin MSs on ALP specific activity of W-20-17. Different combinations of BMP-2 and IGF-I loading were shown in Table 2. W-20-17 cells showed increased ALP specific activity at 5 days of culture for treatments with all the delivery systems compared with control group. However, there was significant difference between the treatment with BMP-2 from the chitosan gel and with BMP-2 sequentially followed by IGF-1 from the gelatin MSs loaded chitosan gel (p<0.05). At 7 days of cell culture, ALP specific activity was significantly greater for sequential release of BMP-2 followed by IGF-1 from the gelatin MSs loaded chitosan gel than all other treatments (p<0.05). This result indicates the sequential delivery of BMP-2 followed by IGF-1 by the chitosan gel/gelatin MSs induces significantly greater ALP activity of W-20-17 than single BMP-2 delivery or simultaneous delivery of BMP-2 and IGF-1.
Figure 9.
Effect of sequential delivery systems on alkaline phosphatase (ALP) specific activity of W-20-17 cells. Indirect culture via BD BioCoat™ Control Cell Culture inserts was used as a model system to evaluate the cell responses from the materials. The growth factor loaded materials were placed in the upper chamber of inserts as shown in Table 2. Cells were seeded in the bottom of 12- well plates at a density of 30,000 cells/well and cultured for 7 days. The culture medium was changed every 3 days. The ALP activity was determined at 5 and 7 days of cultures and normalized for the dsDNA content. ALP activity is expressed as nmol/ng. Each value represents the mean ± SD (n = 3). * denotes significant increase compared with control group A (no growth factor) at each time point (p<0.05). ** denotes significant difference between groups at same time point (p<0.05). Each group (A to D) is listed at table 2.
Discussion
Recent evidence has highlighted the importance of the sequential release of growth factors in order to optimize their efficacy [3, 11–18, 34–35]. Similarly, this study presents a sequential release of BMP-2 and IGF-1 significantly enhances osteoblastic differentiation compared to the simultaneous release of BMP-2 and IGF-1. We developed a chitosan gel/gelatin MSs-based delivery system for the sequential administration of two model proteins, BMP-2 and IGF-1. This work was first to develop the cross-linked gelatin MSs for delivery of IGF-1, which were then encapsulated into the chitosan gel for sequential release.
Among natural polymers, gelatin, which is a hydrolysed form of collagen Type I, has been widely studied in the forms of films, hydrogels, and microspheres [29–33, 36–40]. However, gelatin rapidly dissolves in aqueous environment at body temperature, and exhibits uncontrolled, fast release kinetics of growth factors. For controlled release applications, gelatin MSs have been cross-linked to improve their thermal stability [27, 29–30]. In this study, we cross-linked gelatin MSs with glyoxal, which was reported to be less toxic than commonly used cross-linkers, aldehydes such as formaldehyde, glutaraldehyde, and glyceraldehyde [27, 32, 37, 41–43]. The FTIR spectra revealed the formation of Schiff bases between amide groups of the gelatin and carbonyl groups of glyoxal, which was the result of the cross-linking reaction [29, 31]. In this study, the degree of cross-linking significantly increased with increasing concentration of glyoxal (p<0.05) and reached the maximum at 50 mM of glyoxal. The cross-linking considerably reduced swellability of the gelatin MSs and enhanced their water stability in aqueous environment. Cells in all the groups showed significantly increased metabolic activity during 3 days of culture (p<0.05). However, cell growth and proliferation was slower in the gelatin MSs cross-linked with a higher concentration of glyoxal, indicating that optimizing the glyoxal concentration can provide a more suitable for carrier biomaterial. The cross-linking between gelatin molecules and glyoxal increased thermal stability and decreased dissolution, significantly slowing down the release rate of IGF-1. Patel et al. used glutaraldehyde cross-linked gelatin MSs for the controlled release of BMP-2. In their study, gelatin cross-linking and enzymatic degradation significantly reduced the initial burst release and provided linear release kinetics of BMP-2 in vitro thereafter [31]. In another study, Vandelli et al. reported that glyceraldehyde cross-linked gelatin MSs showed a decrease in swelling and the in vitro drug release under physiological conditions [37].
We also investigated the dissolution rates of the gelatin MSs loaded chitosan gel. The findings from this study suggest that encapsulation of gelatin MSs into a chitosan gel significantly reduced the initial dissolution rate of gelatin MSs. This is probably because gelatin molecules form polyelectrolyte complexes with chitosan molecules. Chitosan is a cationic biopolymer with a pKa of 6.5 due to the presence of amino groups [25–28, 44]. Gelatin MSs used in this study are negatively charged type B gelatin (IEP=5) [23, 31, 44]. Oppositely charged chitosan and gelatin molecules can readily form polyelectrolyte complexes. These intermolecular interactions and cross-linking effects are further suggested by the morphological changes seen by SEM. It was found that the gelatin MSs blended well with the chitosan gel. This is probably due to many functional groups of both gelatin and chitosan [28, 44–45]. In addition, Huang et al. reported that a combination of these two biopolymers significantly affect the biological characteristics, and material’s degradation kinetics and mechanical strength [28]. These results suggest that the cross-linking of gelatin MSs and the formation of polyionic complexes by the chitosan and gelatin provide a good medium for controlled release of proteins.
The in vitro release behavior of IGF-1 and BMP-2 from the delivery system showed that the encapsulation of gelatin MSs into a chitosan gel significantly reduced the initial burst release of IGF-1 (p<0.05) and provided a moderate, sustained release. The cumulative amount of BMP-2 released from the chitosan gel was significantly higher than that of the IGF-1 from the gelatin MSs during 3 days of incubation (p<0.05). However, the amount of IGF-1 released was higher than that of BMP-2 during the period between 3 and 7 days of incubation. This result demonstrates that the combination of gelatin MSs and a chitosan gel induces sequential release of BMP-2 followed by IGF-1 over a week.
In addition to the carrier materials’ characteristics such as cross-linking and polyionic complexation, interaction between gelatin and growth factors also affects the product’s loading efficacy and release kinetics [23, 29–30, 37–38]. It is important to minimize the loss of bioactivity resulting from denaturation and deactivation of growth factors during loading process [29]. Chen et al. investigated the controlled delivery of IGF-1 from dextran-co-gelatin microspheres for periodontal regeneration [23]. Their results showed that a polyion complexation between gelatin and IGF-1 is stable and can be used for the sustained release of numerous charged molecules. In this regard, physical absorption using polyion complexation is a relatively simple and non-destructive method [1, 2, 30]. In this study, when positively charged IGF-1, with IEP of 8.6, was loaded into the negatively charged gelatin MSs (IEP=5) [23–24], they formed a polyion complexation. This complex allowed the IGF-1 to be well absorbed into the gelatin MSs while maintaining its bioactivity [23, 29–31].
To better evaluate the sequential delivery of growth factors on the cell responses, we first established a growth factor-cell response calibration model. We used ALP activity as an indicator of cell response to BMP-2 and IGF-1. We found that the combination of BMP-2 followed by BMP-2/IGF-1 resulted in the greatest ALP activity of osteoblastic precursor cells. Sustained exposure of cells to BMP-2 significantly increased ALP activity. However, IGF-I alone did not affect the cell’s proliferation or early osteogenic differentiation. Our results are consistent with previous studies [14, 18] though they used two layers of gelatin gels cross-linked with glutaraldehyde and two other cell lines. Consequently, we selected four experimental groups and used the newly developed delivery system to release growth factors in a controlled fashion, including sequential and simultaneous delivery groups, to achieve the similar trends of cell response. The cell responses induced by the delivery system were consistent with those by addition of growth factors into medium. A sustained delivery of BMP-2 in addition to a controlled, sequentially release of IGF-1 significantly increased the ALP activity of W-20-17 cells.
The recent studies suggested that ideal release strategy for rhBMP-2 includes both a burst and a sustained release for new bone formation [46, 47]. For large critical-sized bone defects, a burst release helps attract osteoprogenitor cells into the delivery system while a sustained release promotes osteoblastic differentiation [46, 47]. Brown et al. reported that a burst followed by a sustained release of rhBMP-2 from biodegradable polyurethane (PUR) scaffolds enhanced bone regeneration relative to a sustained release without the burst [46]. The burst release of rhBMP-2 functions as a chemoattractive protein for the recruitment and condensation of osteoprogenitor cells into the scaffold [47]. On the other hand, the sustained delivery of rhBMP-2 enhances bone regeneration by affecting a larger population of osteoprogenitor cells at the fracture and promoting vasculogenesis [46, 48]. Our study also showed an initial burst followed by a sustained release of BMP-2 over a week. Consequently, the initial burst release of BMP-2 followed by the sustained releases of both BMP-2 and IGF-1 may synergistically enhance bone regeneration.
One of the weaknesses of this study is that it only evaluated one cell type and one osteoblastic differentiation biomarker. ALP activity is a potent marker of early osteoblastic differentiation, but the ultimate marker or goal of in vitro osteoblastic differentiation is whether or not cells stimulate matrix mineralization. Our previous study showed that the late marker of osteoblastic differentiation such as mineralization and osteocalcin was not dependent on the presence of BMP-2 in W-20-17 cultures [25]. Therefore, we had selected ALP activity as a single early marker of osteoblastic differentiation in this study. Additionally, cell responses are highly variable depending on cell types, species, and different phases of phenotype maturation [25, 49, 50]. As the goal of this study was to develop and characterize a sequential delivery system of growth factors, the aforementioned variables were not adjusted and future research should be undertaken to determine the long term effects of the delivery system with regard to release profile, degradation behavior, and calcium mineral deposition.
Conclusions
In this study we have synthesized and characterized a chitosan gel/gelatin MSs based delivery system. We also demonstrated a sequential administration of two model proteins, BMP-2 and IGF-1 by this delivery system. The controlled releases of these two proteins are regulated by the degree of cross-linking of gelatin MSs, the encapsulation of gelatin MSs into the chitosan gel, and the interactions between proteins and carriers. The enhanced effect of sequential administration of BMP-2 and IGF-1 on early osteoblastic differentiation marker activity was clearly validated by the addition of growth factors to the medium and the experimental delivery system. The advantage of this protocol is that the delivery effect can be validated and can be extended to other delivery systems and proteins.
Acknowledgments
We acknowledge the grant supports from DOD W81XWH-10-1-0966, Airlift Research Foundation, Wallace H. Coulter Foundation, March of Dimes Birth Defect Foundation, NIH R01AR057837 from NIAMS and NIH R01DE021468 from NIDCR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tayalia P, Mooney DJ. Controlled growth factor delivery for tissue engineering. Adv Mater. 2009;21:3269–3285. doi: 10.1002/adma.200900241. [DOI] [PubMed] [Google Scholar]
- 2.Tabata Y. Tissue regeneration based on growth factor release. Tissue Eng. 2003;9 Suppl 1:S5–S15. doi: 10.1089/10763270360696941. [DOI] [PubMed] [Google Scholar]
- 3.Lee K, Silva EA, Mooney DJ. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface. 2011;6(55):153–170. doi: 10.1098/rsif.2010.0223. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hing KA. Bone repair in the twenty-first century: biology, chemistry or engineering? Philos Transact A Math Phys Eng Sci. 2004;362(1825):2821–2850. doi: 10.1098/rsta.2004.1466. [DOI] [PubMed] [Google Scholar]
- 5.Salgado AJ, Coutinho OP, Reis RL. Bone tissue engineering: state of the art and future trends. Macromol Biosci. 2004;4(8):743–765. doi: 10.1002/mabi.200400026. [DOI] [PubMed] [Google Scholar]
- 6.Pallu S, Fricain JC, Bareille R, Bourget C, Dard M, Sewing A, et al. Cyclo-DfKRG peptide modulates in vitro and in vivo behavior of human osteoprogenitor cells on titanium alloys. Acta Biomater. 2009;5(9):3581–3592. doi: 10.1016/j.actbio.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 7.Li B, Davidson JM, Guelcher SA. The effect of the local delivery of platelet-derived growth factor from reactive two-component polyurethane scaffolds on the healing in rat skin excisional wounds. Biomaterials. 2009;30(20):3486–3494. doi: 10.1016/j.biomaterials.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 9.Ito Y. Covalently immobilized biosignal molecule materials for tissue engineering. Soft Matter. 2008;4:46–56. doi: 10.1039/b708359a. [DOI] [PubMed] [Google Scholar]
- 10.Di Silvio L, Kayser MV, Downes S. Validation and optimization of a polymer system for potential use as a controlled drug-delivery system. Clin Mater. 1994;16(2):91–98. doi: 10.1016/0267-6605(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 11.Chen FM, Chen R, Wang XJ, Sun HH, Wu ZF. In vitro cellular responses to scaffolds containing two microencapulated growth factors. Biomaterials. 2009;30(28):5215–5224. doi: 10.1016/j.biomaterials.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Basmanav FB, Kose GT, Hasirci V. Sequential growth factor delivery from complexed microspheres for bone tissue engineering. Biomaterials. 2008;29(31):4195–4204. doi: 10.1016/j.biomaterials.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 13.Arosarena OA, Puleo DA. In vitro effects of combined and sequential bone morphogenetic protein administration. Arch Facial Plast Surg. 2007;9(4):242–247. doi: 10.1001/archfaci.9.4.242. [DOI] [PubMed] [Google Scholar]
- 14.Raiche AT, Puleo DA. In vitro effects of combined and sequential delivery of two bone growth factors. Biomaterials. 2004;25(4):677–685. doi: 10.1016/s0142-9612(03)00564-7. [DOI] [PubMed] [Google Scholar]
- 15.Yilgor P, Hasirci N, Hasirci V. Sequential BMP-2/BMP-7 delivery from polyester nanocapsules. J Biomed Mater Res A. 2010;93(2):528–536. doi: 10.1002/jbm.a.32520. [DOI] [PubMed] [Google Scholar]
- 16.Yilgor P, Tuzlakoglu K, Reis RL, Hasirci N, Hasirci V, et al. Incorporation of a sequential BMP-2/BMP-7 delivery system into chitosan-based scaffolds for bone tissue engineering. Biomaterials. 2009;30(21):3551–3559. doi: 10.1016/j.biomaterials.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 17.Jaklenec A, Hinckfuss A, Bilgen B, Ciombor DM, Aaron R, Mathiowitz E. Sequential release of bioactive IGF-I and TGF-beta 1 from PLGA microsphere-based scaffolds. Biomaterials. 2008;29(10):1518–1525. doi: 10.1016/j.biomaterials.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Raiche AT, Puleo DA. Cell responses to BMP-2 and IGF-1 released with different time-dependent profiles. J Biomed Mater Res Part A. 2004;69A(2):342–350. doi: 10.1002/jbm.a.30006. [DOI] [PubMed] [Google Scholar]
- 19.De Groot J. Carriers that concentrate native bone morphogenetic protein in vivo. Tissue Eng. 1998;4(4):337–341. doi: 10.1089/ten.1998.4.337. [DOI] [PubMed] [Google Scholar]
- 20.Meinel L, Zoidis E, Zapf J, Hassa P, Hottiger MO, von Rechenberg B, et al. Localized insulin-like growth factor I delivery to enhance new bone formation. Bone. 2003;33(4):660–672. doi: 10.1016/s8756-3282(03)00207-2. [DOI] [PubMed] [Google Scholar]
- 21.Meinel L, Illi OE, Zapf J, Malfanti M, Peter Merkle H, Gander B. Stabilizing insulin-like growth factor-I in poly(D,L-lactide-co-glycolide) microspheres. J Control Release. 2001;70(1–2):193–202. doi: 10.1016/s0168-3659(00)00352-7. [DOI] [PubMed] [Google Scholar]
- 22.Worster AA, Brower-Toland BD, Fortier LA, Bent SJ, Williams J, Nixon AJ. Chondrocytic differentiation of mesenchymal stem cells sequentially exposed to transforming growth factor-β1 in monolayer and insulin-like growth factor-I in a three-dimensional matrix. J Orthopaed Res. 2001;19(4):738–749. doi: 10.1016/S0736-0266(00)00054-1. [DOI] [PubMed] [Google Scholar]
- 23.Chen FM, Zhao YM, Wu H, Deng ZH, Wang QT, Jin Y. Enhancement of periodontal tissue regeneration by locally controlled delivery of insulin-like growth factor-I from dextran-co-gelatin microspheres. J Control Release. 2006;114(2):209–222. doi: 10.1016/j.jconrel.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Holland TA, Tabata Y, Mikos AG. Dual growth factor delivery from degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds for cartilage tissue engineering. J Control Release. 2005;101(1–3):111–125. doi: 10.1016/j.jconrel.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Kim S, Tsao H, Kang Y, Sen M, Wenke J, Yang Y, et al. In vitro evaluation of an injectable chitosan gel for sustained local delivery of BMP-2 for osteoblastic differentiation. J Biomed Mater Res B Appl Biomater. 2011 doi: 10.1002/jbm.b.31909. [DOI] [PubMed] [Google Scholar]
- 26.Hoemann CD, Chenite A, Sun J, Hurtig M, Serreqi A, Buschmann MD, et al. Cytocompatible gel formation of chitosan-glycerol phosphate solutions supplemented with hydroxyl ethyl cellulose is due to the presence of glyoxal. J Biomed Mater Res A. 2007;83(2):521–529. doi: 10.1002/jbm.a.31365. [DOI] [PubMed] [Google Scholar]
- 27.Gupta KC, Jabrail FH. Glutaraldehyde and glyoxal cross-linked chitosan microspheres for controlled delivery of centchroman. Carbohyd Res. 2006;341(6):744–756. doi: 10.1016/j.carres.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Huang Y, Onyeri S, Siewe M, Moshfeghian A, Madihally SV. In vitro characterization of chitosan–gelatin scaffolds for tissue engineering. Biomaterials. 2005;26(36):7616–7627. doi: 10.1016/j.biomaterials.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 29.Young S, Wong M, Tabata Y, Mikos AG. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J Control Release. 2005;109(1–3):256–274. doi: 10.1016/j.jconrel.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 30.Ikada Y, Tabata Y. Protein release from gelatin matrices. Adv Drug Deliv Rev. 1998;31(3):287–301. doi: 10.1016/s0169-409x(97)00125-7. [DOI] [PubMed] [Google Scholar]
- 31.Patel ZS, Yamamoto M, Ueda H, Tabata Y, Mikos AG. Biodegradable gelatin microparticles as delivery systems for the controlled release of bone morphogenetic protein-2. Acta Biomater. 2008;4(5):1126–1138. doi: 10.1016/j.actbio.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farris S, Song J, Huang Q. Alternative reaction mechanism for the cross-linking of gelatin with glutaraldehyde. J Agric Food Chem. 2010;58(2):998–1003. doi: 10.1021/jf9031603. [DOI] [PubMed] [Google Scholar]
- 33.Stancu IC. Gelatin hydrogels with PAMAM nanostructured surface and high density surface-localized amino groups. React Funct Polym. 2010;70(5):314–324. [Google Scholar]
- 34.Pei M, Seidel J, Vunjak-Novakovic G, Freed LE. Growth factors for sequential cellular de- and re-differentiation in tissue engineering. Biochem Biophys Res Commun. 2002;294(1):149–154. doi: 10.1016/S0006-291X(02)00439-4. [DOI] [PubMed] [Google Scholar]
- 35.Martin I, Suetterlin R, Baschong W, Heberer M, Vunjak-Novakovic G, Freed LE. Enhanced cartilage tissue engineering by sequential exposure of chondrocytes to FGF-2 during 2D expansion and BMP-2 during 3D cultivation. J Cell Biochem. 2001;83(1):121–128. doi: 10.1002/jcb.1203. [DOI] [PubMed] [Google Scholar]
- 36.Solorio L, Zwolinski C, Lund AW, Farrell MJ, Stegemann JP. Gelatin microspheres crosslinked with genipin for local delivery of growth factors. J Tissue Eng Regen Med. 2010;4(7):514–523. doi: 10.1002/term.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vandelli MA, Rivasi F, Guerra P, Forni F, Arletti R. Gelatin microspheres crosslinked with D, L -glyceraldehyde as a potential drug delivery system: preparation, characterisation, in vitro and in vivo studies. Int J Pharm. 2001;215(1–2):175–184. doi: 10.1016/s0378-5173(00)00681-5. [DOI] [PubMed] [Google Scholar]
- 38.Wei HJ, Yang HH, Chen CH, Lin WW, Chen SC, Sung HW. Gelatin microspheres encapsulated with a nonpeptide angiogenic agent, ginsenoside Rg1, for intramyocardial injection in a rat model with infarcted myocardium. J Control Release. 2007;120(1–2):27–34. doi: 10.1016/j.jconrel.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 39.Liang HC, Chang WH, Lin KJ, Sung HW. Genipin-crosslinked gelatin microspheres as a drug carrier for intramuscular administration: in vitro and in vivo studies. J Biomed Mater Res A. 2003;65(2):271–282. doi: 10.1002/jbm.a.10476. [DOI] [PubMed] [Google Scholar]
- 40.de Carvalho RA, Grosso CRF. Properties of chemically modified gelatin films. Braz J Chem Eng. 2006;23(1):45–53. [Google Scholar]
- 41.Yang Q, Dou F, Liang B, Shen Q. Studies of cross-linking reaction on chitosan fiber with glyoxal. Carbohydr Polym. 2005;59(2):205–210. [Google Scholar]
- 42.Vaz CM, van Doeveren PF, Yilmaz G, de Graaf LA, Reis RL, Cunha AM. Processing and characterization of biodegradable soy plastics: Effects of crosslinking with glyoxal and thermal treatment. J Appl Polym Sci. 2005;97(2):604–610. [Google Scholar]
- 43.de Carvalho RA, Grosso CRF. Characterization of gelatin based films modified with transglutaminase, glyoxal and formaldehyde. Food Hydrocolloids. 2004;18(5):717–726. [Google Scholar]
- 44.Mao J, Kondu S, Ji HF, McShane MJ. Study of the near-neutral pH-sensitivity of chitosan/gelatin hydrogels by turbidimetry and microcantilever deflection. Biotechnol Bioeng. 2006;95(3):333–341. doi: 10.1002/bit.20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mao J, Kondu S, Ji HF, McShane MJ. Study on physical properties and nerve cell affinity of composite films from chitosan and gelatin solutions. Biomaterials. 2003;24(17):2871–2880. doi: 10.1016/s0142-9612(03)00117-0. [DOI] [PubMed] [Google Scholar]
- 46.Brown KV, Li B, Guda T, Perrien DS, Guelcher SA, Wenke JC. Improving bone formation in a rat femur segmental defect by controlling bone morphogenetic protein-2 release. Tissue Eng Part A. 2011;17(13–14):1735–1746. doi: 10.1089/ten.TEA.2010.0446. [DOI] [PubMed] [Google Scholar]
- 47.Fiedler J, Roderer G, Gunther KP, Brenner RE. BMP-2, BMP-4, and PDGF-bb stimulate chemotactic migration of primary human mesenchymal progenitor cells. J Cell Biochem. 2002;87:305–312. doi: 10.1002/jcb.10309. [DOI] [PubMed] [Google Scholar]
- 48.Kolambkar YM, Dupont KM, Boerckel JD, Huebsch N, Mooney DJ, Guldberg RE, et al. An alginate-based hybrid system for growth factor delivery in the functional repair of large bone defects. Biomaterials. 2011;32:65–74. doi: 10.1016/j.biomaterials.2010.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diefenderfer DL, Osyczka AM, Garino JP, Leboy PS. Regulation of BMP-induced transcription in cultured human bone marrow stromal cells. J Bone Joint Surg Am. 2003a;85A suppl 2:19–28. doi: 10.2106/00004623-200300003-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Osyczka AM, Diefenderfer DL, Bhargave G, Leboy PS. Different Effects of BMP-2 on Marrow Stromal Cells from Human and Rat Bone. Cells Tissues Organs. 2004;176(1–3):109–119. doi: 10.1159/000075032. [DOI] [PMC free article] [PubMed] [Google Scholar]