Abstract
Many antidepressant drugs, including the tricyclic antidepressant desipramine (DMI), are broadly understood to function by modulating central noradrenergic neurotransmission. α2 adrenergic receptors (α2ARs) are key regulators of the noradrenergic system, and previous work has implicated α2ARs in mediating the antidepressant activity of DMI in the rodent forced swim test (FST). However, little is known about intracellular regulators of antidepressant drug action. α2AR function is tightly regulated by its intracellular interacting partners arrestin and the dendritic protein spinophilin. We have previously established the competitive and reciprocal nature of these interacting proteins at the α2AR in the context of classic agonist effects, and have shown DMI to be a direct arrestin-biased ligand at the receptor. In the present study, we report that mice deficient in the α2AAR subtype lack DMI-induced antidepressant behavioral effects in the FST. As well, mice deficient in arrestin3 lack antidepressant response to DMI, while spinophilin-null mice have enhanced antidepressant response to DMI compared with wild-type controls, indicating that this α2AAR-mediated response is reciprocally regulated by arrestin and spinophilin. The characteristic of α2AAR-dependence and arrestin3 involvement was shared by the antidepressant effect of the classic α2AR agonist clonidine but not the non-tricyclic norepinephrine reuptake inhibitor reboxetine, supporting a model whereby DMI exerts its antidepressant effect through direct engagement of the α2AAR and arrestin3. Our results implicate arrestin- and spinophilin-mediated regulation of the α2AAR in the pharmacology of the noradrenergic antidepressant DMI, and suggest that manipulation of this mode of receptor regulation may represent a novel and viable therapeutic strategy.
Keywords: α2 adrenergic receptor, arrestin, depression, desipramine, forced swim test, spinophilin
1. Introduction
Current therapeutic strategies in depressive disorders rely almost entirely on modulation of monoamine neurotransmitter systems in the brain, with a large proportion of antidepressants functioning as reuptake inhibitors for the monoamines norepinephrine (NE)1. This reuptake inhibition occurs via blockade of the NE transporter (NET) leading to some modulation of noradrenergic neurotransmission. While this much is known, a complete understanding of the noradrenergic antidepressant mechanism of action remains elusive.
In addition to NET, other key players in the noradrenergic system include the α2 adrenergic receptors (ARs), in particular the α2AAR subtype, which is the predominant subtype expressed in the central nervous system (De et al., 1992; Sastre and Garcia-Sevilla, 1994; Wang et al., 1996). The α2AAR is a key regulator of the noradrenergic system, with important functions as a presynaptic autoreceptor controlling synthesis and release of NE, a postsynaptic heteroreceptor regulating responses to NE, and a presynaptic heteroreceptor regulating release of other neurotransmitters (Hein et al., 1999; Knaus et al., 2007; Gilsbach et al., 2009; Shields et al., 2009). α2AAR function is itself tightly regulated by interacting protein partners. The non-visual arrestins (arrestin2 & 3, also called β-arrestin1 & 2) are key regulators of GPCR function, responsible for attenuation of receptor signaling and mediating endocytosis of receptors via clathrin-coated pits (Shenoy and Lefkowitz, 2003). Further, G protein-independent arrestin-mediated signaling responses are becoming increasingly appreciated at GPCRs (Violin and Lefkowitz, 2007; Rajagopal et al., 2010). As well, we have previously established the dendritic scaffolding protein spinophilin (Allen et al., 1997; Satoh et al., 1998) as a functional antagonist of arrestin at the α2AAR (Wang et al., 2004). The interactions of arrestin and spinophilin with the α2AAR are competitive in nature, and loss of either protein has opposing effects on the in vivo α2AR agonist-induced α2AAR-mediated sedation response (Wang et al., 2004).
The classic tricyclic antidepressant desipramine (DMI) is a potent and selective blocker of NET (Baldessarini, 2006) thought to function primarily through modulation of noradrenergic transmission, modulation which results in antidepressant effects on behavior. As well, we have recently reported that DMI, acting as a direct α2AAR ligand, drives recruitment of arrestin to the receptor thereby modulating receptor function (Cottingham et al., 2011). Importantly, α2ARs have been implicated in mediating the antidepressant activity of DMI, given that α2AR antagonist treatment blocks its antidepressant effects in rodent models (Cervo et al., 1990; Reneric et al., 2001; Zhang et al., 2009). These findings indicate that α2AR activation is required for the antidepressant behavioral effects of DMI in rodents. The antidepressant efficacy of α2AR activation is also supported by the finding that the α2AR agonist clonidine elicits an antidepressant behavioral response (Cervo and Samanin, 1991).
Although arrestin3-null mice are known to have altered behavioral responses to pharmacological manipulations (Schmid and Bohn, 2009) and we have shown that spinophilin-null mice have a range of enhanced responses to α2AR agonists in vivo (Lu et al., 2010), neither of these models has been used to investigate potential roles for these proteins in the antidepressant behavioral response. In particular, whether reciprocal interplay between arrestin and spinophilin applies to the α2AR-mediated antidepressant response to DMI is entirely unknown. Our previous findings do in fact strongly suggest roles for these proteins, given that DMI was shown to be an arrestin-biased ligand at the α2AAR (Cottingham et al., 2011) and spinophilin has been established as an endogenous antagonist of arrestin (Wang et al., 2004).
In the present study, we set out to determine the specific role for the α2AAR subtype in mediating the antidepressant response to DMI, and to investigate whether the interacting partners (arrestin and spinophilin) known to regulate α2AAR function have a role in this response. We chose to model the antidepressant response using the Porsolt's forced swim test (FST) (Porsolt et al., 1977b; Porsolt et al., 1977a), a sensitive and widely accepted model for assessing antidepressant drug activity in rodents. We hypothesized that DMI exerts an antidepressant effect on behavior in the FST in an α2AAR-dependent fashion, and that this antidepressant response is reciprocally regulated by arrestin and spinophilin. The following study tests that hypothesis through the use of transgenic mouse models lacking α2AARs, arrestin3, and spinophilin, primarily in the FST, assaying for responses to acute DMI administration. Our data indicate that the mode of reciprocal regulation by arrestin and spinophilin can be expanded beyond α2AR agonist pharmacology and into the neuropharmacology of antidepressants.
2. Material and methods
2.1 Animals
All animals were housed in the AAALAC-accredited Animal Resources Program (ARP) facility at the University of Alabama at Birmingham in accordance with procedures of the Animal Welfare Act and the 1989 amendments to the Act, and all studies followed protocols approved by the UAB Institutional Animal Care and Use Committee. The ARP provides full animal care services and viral-free barrier facilities. Animals had free access to food (standard chow diet) and water, and were maintained on a 12-hour light/dark cycle. Animals were transported from the animal housing facility to the testing site building 24 hours prior to any behavioral testing. To avoid testing order effects, separate cohorts of animals were used for the behavioral tests. All efforts were made to minimize animal suffering and to reduce the number of animals used in this study.
Male mice of age 3-6 months were used for all studies. Wild-type (WT) control animals were C57BL/6 mice, either obtained from in-house breeding or purchased from Charles River Laboratories. The generation of α2AAR-null (α2AAR-/-) mice (Altman et al., 1999), arrestin3-null (Arr3-/-) mice (Bohn et al., 1999), and spinophilin-null (Sp-/-) mice (Feng et al., 2000) has been previously described. All transgenic animals (α2AAR-/-, Arr3-/-, and Sp-/-) were backcrossed more than 10 generations to and maintained on C57BL/6 genetic background. Consistency in genetic background is vital, as it is well-established that different mouse strains have widely variable baseline phenotypes and responses to pharmacological manipulations in the FST (Petit-Demouliere et al., 2005; Jacobson and Cryan, 2007). Experimental animals were generated by interbreeding of homozygous animals. Pups were weaned at 3-4 weeks of age, and housed in groups of 4-5 males per cage until use for testing. Sp-/- animals were housed in smaller groups (2-3) due to aggression and infighting between males in this mouse line. 2.2 Open field and elevated plus maze (EPM) tests
Data was acquired using the automated EthoVision camera-driven tracker system (Noldus, The Netherlands), the procedures for which have been previously described (Polter et al., 2009). The manufacturer's software was used to extract the relevant parameters from the raw data. Open field trials were conducted in an open field arena of area 42 cm2 and were 10 minutes in length, with total distance traveled and relative time spent in the center of the open field used as endpoints. EPM test was carried out 24 hours following open field, with trials 4 minutes in length; data consisted of entries into and time spent in open versus closed arms of the EPM. Entries into each arm type were expressed as a percentage of total arm entries, and time spent in each arm type was calculated as a percentage of the total time after first closed arm entry. This calculation normalizes for variation which exists in animals’ latency to first closed arm entry.
2.3 Drugs
Desipramine hydrochloride (DMI) and clonidine hydrochloride were obtained from Sigma. Reboxetine mesylate was obtained from Tocris Bioscience. Fluoxetine hydrochloride was generously provided by the NIMH Chemical Synthesis and Drug Supply Program. For animal treatments, drugs were prepared in vehicle (saline), with distilled water (less than 10% of final total volume) used to solubilize DMI, diluted to the appropriate concentration such that the injection volume was 10 ml/kg (e.g. DMI was prepared at a stock concentration of 2 mg/ml for a dose of 20 mg/kg). Drugs (or vehicle alone) were administered acutely via intraperitoneal (i.p.) injection.
2.4 Forced swim test (FST)
In our study, the FST was conducted using the automated Hamilton-Kinder device (Hamilton-Kinder, Poway, CA), controlled by the manufacturer's MotorMonitor software, as has been previously described (Kurtuncu et al., 2005; Polter et al., 2009). Tanks were equipped with two photobeam arrays allowing the animals’ movements to be monitored and filled with 1 L of room temperature water (water changed between each trial). Trials were 6 minutes in length, with the last 4 minutes only counted for data acquisition. The MotorMonitor software was used to extract two primary parameters measuring the antidepressant response from the raw data: rest time (threshold set at 2 seconds), corresponding to time spent immobile; and basic movements (measured by photobeam breaks), corresponding to active swimming behaviors. Animals were tested 30 minutes following i.p. injection
2.5 Tail suspension test (TST)
TST was conducted as previously described (Crowley et al., 2004; Polter et al., 2009), using an automated system (Med Associates Inc., St. Albans, VT). Output for the TST was time spent immobile. As in the FST, trials were 6 minutes in length, with the last 4 minutes only counted for data acquisition, and animals were tested 30 minutes following i.p. injection.
2.6 Statistics
Data were analyzed using GraphPad Prism software (GraphPad, San Diego, CA). Unpaired, two-tailed Student's t-tests were used to compare individual experimental groups with each other. Two-way ANOVA was used to analyze multiple experimental groups and test for drug x genotype interactions.
3. Results
3.1 Basic behavioral assessments
We began by performing basic behavioral assessments in our knockout mouse lines (in comparison with WT controls) to determine baseline activity (open field test) and anxiety (EPM test) levels. These assays provide valuable information about the inherent behavioral phenotypes of these animals, information which is crucial for proper interpretation of the results of any pharmacological manipulations. In the open field, α2AAR-/- mice exhibited slightly but significantly higher locomotor activity compared with WT; otherwise, the transgenic lines were indistinguishable from WT (Figure 1A and 1B). No differences in center exploration (expressed as a ratio of time spent in the center of the open field arena to total trial time), a parameter which can be used as a measure of anxiety (Sweatt, 2010), were observed for any of the knockout lines versus WT (Figure 1C).
Figure 1.
Open field analysis of wild-type (WT, n=7), α2AAR-null (α2AAR-/-, n=6), arrestin3-null (Arr3-/-, n=7), and spinophilin-null (Sp-/-, n=8) mice. A. Total distance traveled during the open field trial. B. Travel velocity during open field trial. C. Time spent in the center of the open field arena expressed as a ratio of center to total trial time. Data are mean ± SEM. *p<0.05 vs. WT.
The EPM test was used as a more sensitive measure for baseline anxiety-like behavior, with less time spent in the EPM open arms (and correspondingly more time in closed arms) taken as an indicator of increased anxiety (Sweatt, 2010). This assay revealed no significant differences in anxiety levels for any of the transgenic lines versus WT, as indicated by percent time spent in and relative proportion of entries into open versus closed arms (Figure 2). Overall, our basic behavioral phenotyping yielded no dramatic abnormalities in activity or anxiety levels in our transgenic models, and therefore no findings which would significantly interfere with our interpretation of the FST data for these animals. As well, these results allow us to move forward with comparisons in the FST responses between the different models.
Figure 2.
Elevated plus maze analysis of WT (n=7), α2AAR-/- (n=6), Arr3-/- (n=7), and Sp-/- (n=8) mice. A. Percent time spent in open versus closed arms of EPM during trial. B. Entries into open versus closed arms of EPM during trial expressed as percent of total arm entries.
2.2 α2AAR-mediated antidepressant responses to DMI
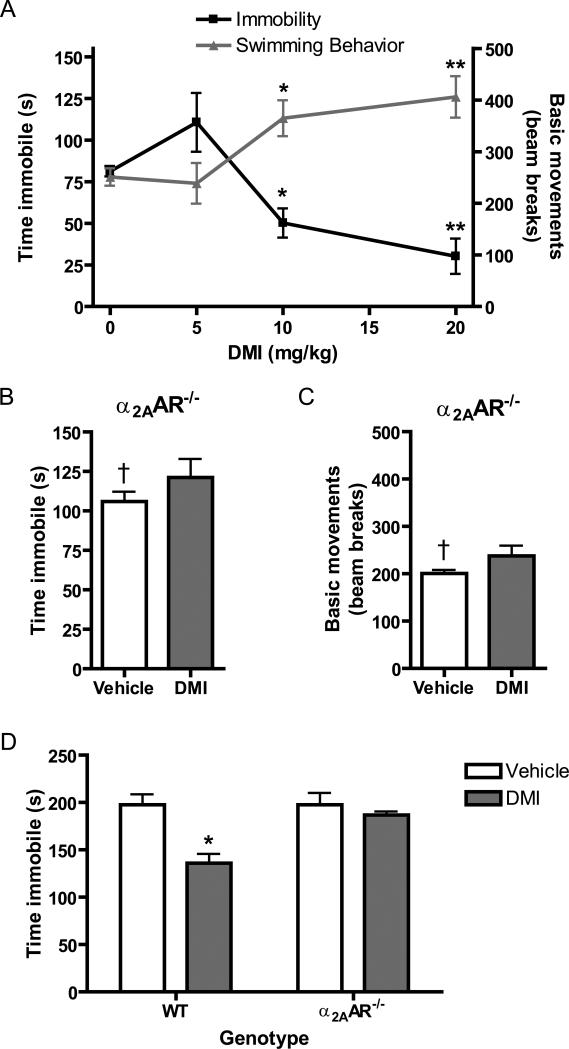
We began by assaying a range of acute doses of DMI for antidepressant activity in the FST. As indicated by both decrease in immobility time and increase in active swimming compared with vehicle, DMI exerted significant antidepressant effects at 10 and 20, but not 5, mg/kg (Figure 3A). To confirm involvement of the α2AAR in mediating the acute antidepressant response to DMI, we performed the FST following a single dose of DMI at the maximum effective level of 20 mg/kg in α2AAR-/- mice. The α2AAR-/- mice exhibited no response in either immobility time or active swimming (Figure 3B and 3C). Two-way ANOVA revealed a significant drug × genotype interaction for both immobility (p=0.0002) and active swimming (p=0.0133) among these experimental groups, further emphasizing the difference in the response to DMI between WT and α2AAR-/- animals. As well, we found that the acute antidepressant response to DMI in the TST (a closely related assay to the FST) observed in WT animals was completely lost in α2AAR-/- animals (Figure 3D). Collectively, these data demonstrate that DMI exerts an acute α2AAR-mediated antidepressant response in rodent behavioral models.
Figure 3.
Acute α2AAR-mediated antidepressant responses to DMI. WT and α2AAR-/- animals were given i.p. injection of DMI or vehicle 30 minutes prior to testing. A. DMI dose-dependently induces an antidepressant effect in WT animals, as indicated by immobility (time, given in seconds) and active swimming behavior (measured in beam breaks). n=12, WT + Vehicle (DMI 0 mg/kg); n=4, WT + DMI 5 mg/kg; n=7, WT + DMI 10 mg/kg; n=6, WT + DMI 20 mg/kg. B & C. α2AAR-/- mice lack antidepressant response to DMI (20 mg/kg) by measures of both immobility (B) and active swimming behavior (C). n=9, α2AAR-/- + Vehicle; n=8, α2AAR-/- + DMI. D. Tail suspension test confirms acute antidepressant effect of DMI (20 mg/kg) in WT animals that is lost in α2AAR-/- animals, as indicated by immobility time. n=3-4 per group. Data are mean ± SEM. *p<0.01, **p<0.001 vs. vehicle, †p<0.05, WT vs. α2AAR-/-.
We next sought to determine whether a non-tricyclic NE reuptake inhibitor (reboxetine), structurally distinct from DMI, also exerts an α2AAR-mediated antidepressant response. Importantly, reboxetine has transporter affinities similar to DMI but lacks the significant non-transporter receptor interactions associated with the tricyclic (Millan et al., 2001). WT and α2AAR-/- mice were administered reboxetine at a dose of 20 mg/kg (selected as the most effective dose based on preliminary studies) and subjected to the FST. Reboxetine was found to exert significant antidepressant effects in both the WT and α2AAR-/- mice (Figure 4), indicating that the α2AAR is not required in the acute response to this drug and suggesting that DMI and reboxetine function via distinct mechanisms. Two-way ANOVA revealed significant drug × genotype interactions for both immobility (p=0.0357) and active swimming (p=0.0008), indicating that reboxetine may in fact be slightly more effective in the α2AAR-/- mice.
Figure 4.
Acute antidepressant responses to reboxetine. WT and α2AAR-/- animals were given i.p. injection of reboxetine (20 mg/kg) or vehicle 30 minutes prior to testing. A. Immobility (time, given in seconds). B. Active swimming behavior (measured in beam breaks). n=12, WT + Vehicle; n=6, WT + Reboxetine; n=9, α2AAR-/- + Vehicle; n=6, α2AAR-/- + Reboxetine. *p<0.05, **p<0.01 vs. vehicle.
2.4 Arrestin3 is required for α2AAR-mediated response to DMI
Given that arrestin3 is a key regulator of α2AAR function (Wang and Limbird, 2007) and DMI drives recruitment of arrestin3 to the α2AAR (Cottingham et al., 2011), we next investigated mice lacking arrestin3 (β-arrestin2) for their responsiveness in the FST. Arr3-/- mice exhibited no antidepressant response to DMI (20 mg/kg), again comparing with the robust response comprising effects on immobility and active swimming, in WT mice (Figure 5A and 5B). Two-way ANOVA revealed a significant drug × genotype interaction for immobility (p=0.0308) and a trend toward significant interaction for active swimming (p=0.0521) among these experimental groups.
Figure 5.
The α2AAR-mediated antidepressant response to DMI is abolished in arrestin3-null mice. WT and Arr3-/- animals were given i.p. injection of DMI (20 mg/kg) or vehicle 30 minutes prior to testing. A. Immobility (time, given in seconds). B. Active swimming behavior (measured in beam breaks). n=12, WT + Vehicle; n=6, WT + DMI; n=7, Arr3-/- + Vehicle; n=7, Arr3-/- + DMI. Data are mean ± SEM. *p<0.001 vs. vehicle.
2.5 α2AAR-/- and Arr3-/- mice remain responsive to fluoxetine
In order to determine if the involvement of the α2AAR and Arr3 is specific for the noradrenergic mechanism of DMI, we tested both α2AAR-/- and Arr3-/- mice were for their ability to respond to the selective serotonin reuptake inhibitor fluoxetine (FLX) in the FST. FLX is an antidepressant with distinct molecular and clinical pharmacological properties from DMI (Baldessarini, 2006), and which primarily functions as a modulator of serotonergic neurotransmission. All genotypes (WT, α2AAR-/-, and Arr3-/-) exhibited a strong antidepressant response to a single dose of FLX (20 mg/kg) in the form of both decreased immobility (Figure 6A) and increased active swimming (Figure 6B). In fact, Arr3-/- mice exhibited a stronger reduction in immobility and increase in active swimming compared with WT. FLX did not induce significant climbing behavior in any of the genotypes. These results indicate that the contributions of α2AARs and Arr3 depend on the system (e.g. noradrenergic versus serotonergic) being manipulated. As well, these data serve as a positive control, demonstrating that α2AAR-/- and Arr3-/- mice are not simply inert in this assay for antidepressant drug activity. Two-way ANOVA revealed no significant drug × genotype interaction for either immobility (p=0.1885) or active swimming (p=0.2647) among these experimental groups.
Figure 6.
Both α2AAR- and arrestin3-null mice retain antidepressant response to the selective serotonin reuptake inhibitor fluoxetine. WT, α2AAR-/-, and Arr3-/- mice were given i.p. injection of fluoxetine (FLX, 20 mg/kg) or vehicle 30 minutes prior to testing. A. Immobility (time, given in seconds). B. Active swimming behavior (measured in beam breaks). n=12, WT + Vehicle; n=6, WT + FLX; n=9, α2AAR-/- + Vehicle; n=4, α2AAR-/- + FLX; n=7, Arr3-/- + Vehicle; n=7, Arr3-/- + FLX. Data are mean ± SEM. *p<0.01, **p<0.001 vs. vehicle, †p<0.05, WT vs. Arr3-/-.
2.6 Spinophilin-null mice exhibit an enhanced response to DMI
We have previously shown that the dendritic scaffolding protein spinophilin serves as a functional antagonist to arrestin at the α2AAR, both in vitro and in vivo (Wang et al., 2004), and that Sp-null mice exhibit enhanced α2AAR-mediated responses to agonists in vivo (Lu et al., 2010) and in vitro2. Therefore, we tested Sp-/- mice for their responsiveness to DMI in the FST. In contrast to the Arr3-/- animals, the Sp-/- mice exhibited a robust response to 5 mg/kg DMI (a dose which is inert in WT animals) and had a dramatically enhanced response to 10 mg/kg DMI versus WT, in particular a greater decrease in immobility (Figure 7A) which is even more apparent when expressed as percent reduction from baseline (vehicle-treated) (Figure 7B). Sp-/- mice also showed enhanced DMI responsiveness in the form of greater increases in active swimming at both 5 and 10 mg/kg DMI (Figure 7C and 7D). Indeed, the dose of 10 mg/kg reaches the same level of effectiveness in Sp-/- mice as the maximal effect induced by 20 mg/kg in WT mice (Figure 1A). Two-way ANOVA on the raw values revealed a significant drug × genotype interaction for both immobility (p=0.0003) and active swimming (p=0.0085) among these experimental groups. These findings indicate that Sp-/- mice have increased sensitivity to DMI in the FST.
Figure 7.
The α2AAR-mediated antidepressant response to DMI is enhanced in spinophilin-null mice. WT and Sp-/- mice were given i.p. injection of DMI (5 or 10 mg/kg) or vehicle 30 minutes prior to testing. A. Immobility (time, given in seconds). B. Percent reduction in immobility by DMI (vehicle set as 100%). C. Active swimming behavior (measured in beam breaks). D. Fold increase in swimming behavior by DMI (vehicle set as 1.0 fold). n=12, WT + Vehicle; n=4, WT + DMI 5; n=7, WT + DMI 10; n=5, Sp-/- + Vehicle; n=4, Sp-/- + DMI 5; n=6, Sp-/- + DMI 10. Data are mean ± SEM. *p<0.05, **p<0.01 vs. vehicle, †p<0.05, WT vs. Sp-/-.
2.7 Antidepressant effects of α2AR agonist treatment
In order to further clarify the mechanism of acute α2AAR-mediated antidepressant responses, we elected to test the α2AR agonist clonidine for antidepressant activity in our mouse models; clonidine has previously been shown to exert antidepressant effects on behavior in the rat FST (Cervo and Samanin, 1991), effects which appear to be independent of the imidazoline activity of the drug (O'Neill et al., 2001). Based on preliminary experiments as well as our own experience with the potent sedative effects of clonidine, we selected doses of 0.1 and 0.3 mg/kg to maximize antidepressant efficacy while limiting the potential confound of sedation. Our experiments revealed that clonidine, at these doses, is an effective antidepressant in WT animals (Figure 8A), and that this antidepressant effect is completely lacking in α2AAR-/- mice (Figure 8B). Arr3-/- animals exhibited reduced sensitivity to clonidine, with the 0.1 mg/kg dose completely ineffective, although significant responses were observed at the higher dose of 0.3 mg/kg (Figure 8C). Two-way ANOVA revealed significant drug × genotype interactions for both immobility (p=0.0006) and active swimming (p<0.0001). Unfortunately, we were unable to satisfactorily assay the antidepressant effect of clonidine in Sp-/- mice due to a predominance of confounding sedative effects; indeed, we have previously shown that Sp-/- mice have enhanced sensitivity to the sedative effects of α2AR agonist treatment (Wang et al., 2004; Lu et al., 2010).
Figure 8.
Acute antidepressant responses to the α2AR agonist clonidine. Animals were given i.p. injection of clonidine (CLON) at 0.1 or 0.3 mg/kg or vehicle 30 minutes prior to testing. A. Immobility (time, given in seconds) and active swimming behavior (measured in beam breaks) in WT mice. n=12, Vehicle; n=5, CLON 0.1; n=6, CLON 0.3. B. Immobility and active swimming behavior in α2AAR-/- mice. n=9, Vehicle; n=4, CLON 0.3. C. Immobility and active swimming behavior in Arr3-/- mice. n=7, Vehicle; n=6, CLON 0.1; n=5, CLON 0.3. Data are mean ± SEM. *p<0.05, **p<0.01 vs. vehicle.
4. Discussion
In the present study, we demonstrate that the acute in vivo antidepressant response to the tricyclic antidepressant DMI is dependent upon the α2A subtype of α2ARs. Our data further indicate that the α2AAR-mediated response to DMI is critically dependent on the presence of arrestin3, as arrestin3-null animals have no response. Conversely, spinophilin-null animals have an exaggerated response to DMI, demonstrating reciprocal interplay between the two receptor interacting partners. Contrastingly, the structurally distinct NE reuptake inhibitor reboxetine, which lacks physiologically significant α2AAR interaction, exerts its antidepressant effect in an α2AAR-independent fashion. Taken together with our previous report on DMI as an arrestin-biased ligand at the α2AAR, our findings lead us to contend that the antidepressant effect modeled in this study results from a mechanism whereby DMI modulates α2AAR function through its direct arrestin-biased interaction with the receptor.
Our open field assessment indicates that α2AAR-/- animals have slightly elevated locomotor activity levels compared with WT controls while those of Sp-/- animals are unchanged (Figure 1). Therefore, these mice do not exhibit general hypoactivity, a potential confound which could account for the elevated immobility and reduced swimming activity observed in the FST assay for vehicle-treated α2AAR-/- and Sp-/- mice compared with WT (Figures 3 & 7). Those data can thus be interpreted as indicating that α2AAR-/- and Sp-/- are more sensitive to the stressors associated with the FST procedure, an observation which has been made previously for the α2AAR-/- model (Schramm et al., 2001). Whether this represents a depressive phenotype per se in these mice is highly debatable, and the soundness of such an interpretation has been well-discussed by others (Nestler et al., 2002; Petit-Demouliere et al., 2005; Nestler and Hyman, 2010). The EPM test results indicate that none of the transgenic lines have any alterations in baseline anxiety levels compared with WT controls (Figure 2), eliminating potential confounds in our interpretation of the FST results.
Previous studies have implicated the α2-type ARs in mediating the antidepressant effect of DMI in the FST (Cervo et al., 1990; Reneric et al., 2001; Zhang et al., 2009). We demonstrate more specifically that the α2A subtype is required for the acute antidepressant response to DMI (Figure 3). Our findings are therefore consistent with those of Zhang et al. (2009), wherein an α2AR antagonist blocked the antidepressant effect of acutely administered DMI, and the involvement of β and α1ARs in the response to DMI was excluded. This study, as well as previous reports by Cervo et al. (1990) and Reneric et al. (2001), is consistent with a mechanism whereby DMI causes a decrease in the activity of noradrenergic locus coeruleus neurons via postsynaptic α2AARs. Contrastingly, a recent report showed that chronic treatment with an α2AR antagonist hastened the effect of a tricyclic antidepressant in promoting hippocampal neurogenesis (Yanpallewar et al., 2010), suggesting a pro-depressive role for α2AARs in the cerebrum. These seemingly conflicting results highlight the complex nature of the α2AAR as a player in depressive disorders and antidepressant drug actions, with roles that likely vary in a brain region-dependent fashion, and emphasize the need for continued investigation of the α2AAR system in this context. As well, although the acute model we have utilized is a valid, commonly used, and informative approach in studying antidepressant pharmacology, we recognize that this paradigm may not recapitulate the clinical situation wherein chronic antidepressant exposure is required for symptom relief. Therefore, further investigation will be needed to validate the α2AAR pharmacology outlined in this study under conditions more closely resembling the clinical setting.
Our contention that the antidepressant effect of DMI modeled in this and other studies depends on the direct interaction of DMI with the α2AAR rather than a general modulation of noradrenergic tone is supported by our findings regarding the structurally distinct NE reuptake inhibitor reboxetine and the classic α2AR agonist clonidine. Reboxetine is not a member of the tricyclic drug class and lacks significant non-transporter interactions with α2AARs or other neurotransmitter receptors at therapeutic levels of the drug (Millan et al., 2001), contrasting with the significant α2AAR interaction of DMI (Cottingham et al., 2011). The antidepressant effect of reboxetine is not α2AAR-mediated (Figure 4), indicating that simple NE reuptake inhibition engages other ARs to exert antidepressant effects on behavior, a mechanism which contrasts with DMI. Clonidine, meanwhile, was found to exert an α2AAR-mediated antidepressant effect (Figure 8), demonstrating that direct α2AAR stimulation leads to an acute antidepressant response in the FST. Other members of the tricyclic drug class may have similar receptor interaction components to their mechanisms as we have observed with DMI. Indeed, the α2AAR-/- model was previously shown to lack antidepressant responses to the tricyclic drug imipramine (Schramm et al., 2001).
The finding that DMI requires arrestin3 for its antidepressant effect (Figure 5) strongly suggests that it is DMI-induced recruitment of arrestin3 to the α2AAR, as we have previously reported (Cottingham et al., 2011), underlying the antidepressant response. Our data on clonidine provide additional support to the contention that arrestin3 is involved in the acute α2AAR-mediated antidepressant response as a direct regulator of the receptor, given that Arr3-/- mice have diminished sensitivity to the antidepressant effects of clonidine (Figure 8C). The fact that the antidepressant effect of clonidine is not completely abolished in Arr3-/- mice as is apparently the case for DMI (Figure 5) is not entirely surprising. DMI, as an α2AAR ligand, functions exclusively in an arrestin-biased fashion without activating heterotrimeric G proteins (Cottingham et al., 2011), while clonidine, as a classic agonist, both recruits arrestin and activates G proteins.
Our data is suggestive of some α2AAR-mediated arrestin-dependent signaling underlying the antidepressant response to DMI. While established for other GPCRs (Violin and Lefkowitz, 2007; Rajagopal et al., 2010), arrestin-dependent signaling has not been reported for the α2AAR. NE itself must, however, be involved in the underlying signaling, given that mice lacking dopamine-β-hydroxylase (rate-limiting enzyme in NE synthesis) do not respond to DMI in the FST (Cryan et al., 2001; Cryan et al., 2004). It therefore seems likely that DMI-induced enrichment of arrestin at the cell surface unmasks some heretofore obscured arrestin-mediated signaling by NE or engages entirely new signaling pathways. The details of such proposed signaling remain to be determined, and accomplishing that will be a goal of future studies. We undertook a preliminary investigation of some candidate signaling molecules previously implicated in antidepressant effects, including MAP kinases (Duman et al., 2007; Galeotti and Ghelardini, 2011) and the Akt/GSK3 pathway (Li and Jope, 2010), but were unable to find any obvious DMI-dependent differences in whole hippocampal or cortical tissue from our experimental animals. It should be noted that, although we propose a critical role for an α2AAR/Arr3 signaling complex, the loss of antidepressant responses in α2AAR-/- and Arr3-/- mice could result from other compensatory changes affecting noradrenergic neurotransmission in these models.
Importantly, both the α2AAR-/- and Arr3-/- mice retained an antidepressant response in the FST to the primarily serotonergic antidepressant fluoxetine (Figure 6). This result suggests that the observed roles for both the α2AAR and arrestin3 are specific for the primarily noradrenergic mechanism associated with DMI. Interestingly, the Arr3-/- mice actually exhibited an enhanced immobility reduction response to fluoxetine (Figure 6A). Arrestin3 has been reported to couple to 5HT2A (Schmid and Bohn, 2010) and 5HT1B (Chen et al., 2011) receptors, and deleting arrestin selectively affects behaviors mediated by these receptors. Although the 5HT receptor subtype mediating the arrestin-dependent effect of fluoxetine remains to be elucidated, our finding nevertheless emphasizes the selective nature of arrestin in regulating GPCR function, and the selective nature of such mediators as α2AARs and arrestin in noradrenergic versus serotonergic antidepressant pharmacology.
Our data provide a novel example of the reciprocal regulation of α2AAR function by arrestin and spinophilin. Knockout of arrestin3 and spinophilin were previously found to have opposing effects on the α2AAR-mediated sedation response to α2AR agonist treatment, with Arr3-/- mice and Sp-/- mice having decreased and enhanced sensitivity, respectively (Wang et al., 2004; Lu et al., 2010). We can now apply this mode of regulation to the α2AAR-mediated acute antidepressant response to DMI, as the Arr3-/- mice are lacking in sensitivity (Figure 5) while the Sp-/- mice have enhanced responsiveness (Figure 7). Presumably, this enhanced responsiveness is due to a loss of the endogenous check on arrestin provided by its functional antagonist spinophilin, leading to enhancement of the clearly critical arrestin-mediated effects of DMI in the Sp-/- mice. Alternatively, it is possible that the DMI response phenotype in Sp-/- mice results from a distinct arrestin-independent mechanism. Additionally, given our use of global knockout models for arrestin3 and spinophilin, we cannot completely rule out the possibility that loss of these proteins affects the α2AAR-mediated antidepressant response indirectly through alterations in the function of other ARs, although any such alternative mechanisms would have to ultimately converge on the α2AAR.
In closing, we contend that the demonstrated interplay between arrestin and spinophilin has implications in the therapeutic targeting of α2AARs in depressive disorders. It may be possible to modulate receptor function by targeting these interacting partners directly or by designing biased receptor ligands that select for interaction with one or the other. Based upon this and our previous study, it seems likely that arrestin-biased α2AAR ligands can have tremendous benefit as efficacious antidepressants. As well, our evidence on DMI suggests that the tricyclic chemical structure shared by many psychiatric medications may be capable of such biased receptor interactions. Therefore, such an approach could be investigated in the context of other neurotransmitter GPCRs, and would represent a new therapeutic strategy in the treatment of depressive disorders.
Highlights.
>In vivo responses to the noradrenergic antidepressant desipramine are investigated.
>Responses to desipramine are entirely dependent on α2A adrenergic receptors.
>Responses are ablated with loss of arrestin and enhanced with loss of spinophilin. >In vivo effect of desipramine is reciprocally regulated by arrestin & spinophilin. >Novel example of reciprocal regulation of α2A adrenergic receptors is revealed.
Acknowledgments
Our thanks to Drs. Brian Kobilka, Paul Greengard and Robert J. Lefkowitz for generously providing the α2AAR-/-, Sp-/- and Arr3-/- lines, respectively, to the UAB Department of Psychiatry and Behavioral Neurobiology for the use of the FST equipment, and to Dr. Thomas van Groen and the UAB Behavioral Core for assistance with the open field and EPM assays.
Funding sources
This work was supported by the National Institutes of Health National Institute of Mental Health [Grant MH 081917, QW], the UAB Training Program in Neurobiology of Cognition and Cognitive Disorders, funded by a National Institutes of Health T32 training grant [NS061788-03, CC], and a National Institutes of Health P30 grant to the UAB Behavioral Core [NIH P30 NS47466].
Role of the funding sources
The funding sources had no involvement in the study design, collection, analysis, and interpretation of data, writing of the report, or decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The abbreviations used are: 5HT, serotonin; AR, adrenergic receptor; Arr, arrestin; DMI, desipramine; EPM, elevated plus maze; FLX, fluoxetine; FST, forced swim test; GPCR, G protein-coupled receptor; NET, norepinephrine transporter; Sp, spinophilin; TST, tail suspension test
Cottingham and Wang, unpublished observation
Reference List
- Allen PB, Ouimet CC, Greengard P. Spinophilin, a novel protein phosphatase 1 binding protein localized to dendritic spines. Proc. Natl. Acad. Sci. U. S. A. 1997;94:9956–9961. doi: 10.1073/pnas.94.18.9956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman JD, Trendelenburg AU, MacMillan L, Bernstein D, Limbird L, Starke K, Kobilka BK, Hein L. Abnormal regulation of the sympathetic nervous system in alpha2A-adrenergic receptor knockout mice. Mol. Pharmacol. 1999;56:154–161. doi: 10.1124/mol.56.1.154. [DOI] [PubMed] [Google Scholar]
- Baldessarini RJ. Drug therapy of depression and anxiety disorders. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman & Gilman's The Pharmacological Basis of Therapeutics. 11th ed. McGraw-Hill, Inc.; New York: 2006. pp. 429–460. [Google Scholar]
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286:2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- Cervo L, Grignaschi G, Samanin R. Alpha 2-adrenoceptor blockade prevents the effect of desipramine in the forced swimming test. Eur. J. Pharmacol. 1990;175:301–307. doi: 10.1016/0014-2999(90)90568-q. [DOI] [PubMed] [Google Scholar]
- Cervo L, Samanin R. Clonidine causes antidepressant-like effects in rats by activating alpha 2-adrenoceptors outside the locus coeruleus. Eur. J. Pharmacol. 1991;193:309–313. doi: 10.1016/0014-2999(91)90144-f. [DOI] [PubMed] [Google Scholar]
- Chen L, Zhou W, Chen PC, Gaisina I, Yang S, Li X. Glycogen synthase kinase-3beta is a functional modulator of serotonin-1B receptors. Mol. Pharmacol. 2011;79:974–986. doi: 10.1124/mol.111.071092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottingham C, Chen Y, Jiao K, Wang Q. The antidepressant desipramine is an arrestin-biased ligand at the alpha2A adrenergic receptor driving receptor downregulation in vitro and in vivo. J. Biol. Chem. 2011;286:36063–36075. doi: 10.1074/jbc.M111.261578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley JJ, Jones MD, O'Leary OF, Lucki I. Automated tests for measuring the effects of antidepressants in mice. Pharmacol. Biochem. Behav. 2004;78:269–274. doi: 10.1016/j.pbb.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Dalvi A, Jin SH, Hirsch BR, Lucki I, Thomas SA. Use of dopamine-beta-hydroxylase-deficient mice to determine the role of norepinephrine in the mechanism of action of antidepressant drugs. J. Pharmacol. Exp. Ther. 2001;298:651–657. [PubMed] [Google Scholar]
- Cryan JF, O'Leary OF, Jin SH, Friedland JC, Ouyang M, Hirsch BR, Page ME, Dalvi A, Thomas SA, Lucki I. Norepinephrine-deficient mice lack responses to antidepressant drugs, including selective serotonin reuptake inhibitors. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8186–8191. doi: 10.1073/pnas.0401080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De VH, Vauquelin G, De KJ, De Backer JP, Van L,I. Regional distribution of alpha 2A- and alpha 2B-adrenoceptor subtypes in postmortem human brain. J. Neurochem. 1992;58:1555–1560. doi: 10.1111/j.1471-4159.1992.tb11378.x. [DOI] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Kodama M, Russell DS, Duman RS. A role for MAP kinase signaling in behavioral models of depression and antidepressant treatment. Biol. Psychiatry. 2007;61:661–670. doi: 10.1016/j.biopsych.2006.05.047. [DOI] [PubMed] [Google Scholar]
- Feng J, Yan Z, Ferreira A, Tomizawa K, Liauw JA, Zhuo M, Allen PB, Ouimet CC, Greengard P. Spinophilin regulates the formation and function of dendritic spines. Proc. Natl. Acad. Sci. U. S. A. 2000;97:9287–9292. doi: 10.1073/pnas.97.16.9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeotti N, Ghelardini C. Selective modulation of the PKCvarepsilon/p38MAP kinase signalling pathway for the antidepressant-like activity of amitriptyline. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.07.020. [DOI] [PubMed] [Google Scholar]
- Gilsbach R, Roser C, Beetz N, Brede M, Hadamek K, Haubold M, Leemhuis J, Philipp M, Schneider J, Urbanski M, Szabo B, Weinshenker D, Hein L. Genetic dissection of alpha2-adrenoceptor functions in adrenergic versus nonadrenergic cells. Mol. Pharmacol. 2009;75:1160–1170. doi: 10.1124/mol.109.054544. [DOI] [PubMed] [Google Scholar]
- Hein L, Altman JD, Kobilka BK. Two functionally distinct alpha2-adrenergic receptors regulate sympathetic neurotransmission. Nature. 1999;402:181–184. doi: 10.1038/46040. [DOI] [PubMed] [Google Scholar]
- Jacobson LH, Cryan JF. Feeling strained? Influence of genetic background on depression-related behavior in mice: a review. Behav. Genet. 2007;37:171–213. doi: 10.1007/s10519-006-9106-3. [DOI] [PubMed] [Google Scholar]
- Knaus AE, Muthig V, Schickinger S, Moura E, Beetz N, Gilsbach R, Hein L. Alpha2-adrenoceptor subtypes--unexpected functions for receptors and ligands derived from gene-targeted mouse models. Neurochem. Int. 2007;51:277–281. doi: 10.1016/j.neuint.2007.06.036. [DOI] [PubMed] [Google Scholar]
- Kurtuncu M, Luka LJ, Dimitrijevic N, Uz T, Manev H. Reliability assessment of an automated forced swim test device using two mouse strains. J. Neurosci. Methods. 2005;149:26–30. doi: 10.1016/j.jneumeth.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Li X, Jope RS. Is glycogen synthase kinase-3 a central modulator in mood regulation? Neuropsychopharmacology. 2010;35:2143–2154. doi: 10.1038/npp.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R, Chen Y, Cottingham C, Peng N, Jiao K, Limbird LE, Wyss JM, Wang Q. Enhanced hypotensive, bradycardic, and hypnotic responses to alpha2-adrenergic agonists in spinophilin-null mice are accompanied by increased G protein coupling to the alpha2A-adrenergic receptor. Mol. Pharmacol. 2010;78:279–286. doi: 10.1124/mol.110.065300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ, Gobert A, Lejeune F, Newman-Tancredi A, Rivet JM, Auclair A, Peglion JL. S33005, a novel ligand at both serotonin and norepinephrine transporters: I. Receptor binding, electrophysiological, and neurochemical profile in comparison with venlafaxine, reboxetine, citalopram, and clomipramine. J. Pharmacol. Exp. Ther. 2001;298:565–580. [PubMed] [Google Scholar]
- Nestler EJ, Gould E, Manji H, Buncan M, Duman RS, Greshenfeld HK, Hen R, Koester S, Lederhendler I, Meaney M, Robbins T, Winsky L, Zalcman S. Preclinical models: status of basic research in depression. Biol. Psychiatry. 2002;52:503–528. doi: 10.1016/s0006-3223(02)01405-1. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat. Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill MF, Osborne DJ, Woodhouse SM, Conway MW. Selective imidazoline I2 ligands do not show antidepressant-like activity in the forced swim test in mice. J. Psychopharmacol. 2001;15:18–22. doi: 10.1177/026988110101500104. [DOI] [PubMed] [Google Scholar]
- Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology (Berl) 2005;177:245–255. doi: 10.1007/s00213-004-2048-7. [DOI] [PubMed] [Google Scholar]
- Polter A, Yang S, Zmijewska AA, van GT, Paik JH, Depinho RA, Peng SL, Jope RS, Li X. Forkhead box, class O transcription factors in brain: regulation and behavioral manifestation. Biol. Psychiatry. 2009;65:150–159. doi: 10.1016/j.biopsych.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 1977a;229:327–336. [PubMed] [Google Scholar]
- Porsolt RD, Le PM, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977b;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat. Rev. Drug Discov. 2010;9:373–386. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reneric JP, Bouvard M, Stinus L. Idazoxan and 8-OH-DPAT modify the behavioral effects induced by either NA, or 5-HT, or dual NA/5-HT reuptake inhibition in the rat forced swimming test. Neuropsychopharmacology. 2001;24:379–390. doi: 10.1016/S0893-133X(00)00214-1. [DOI] [PubMed] [Google Scholar]
- Sastre M, Garcia-Sevilla JA. Alpha 2-adrenoceptor subtypes identified by [3H]RX821002 binding in the human brain: the agonist guanoxabenz does not discriminate different forms of the predominant alpha 2A subtype. J. Neurochem. 1994;63:1077–1085. doi: 10.1046/j.1471-4159.1994.63031077.x. [DOI] [PubMed] [Google Scholar]
- Satoh A, Nakanishi H, Obaishi H, Wada M, Takahashi K, Satoh K, Hirao K, Nishioka H, Hata Y, Mizoguchi A, Takai Y. Neurabin-II/spinophilin. An actin filament-binding protein with one pdz domain localized at cadherin-based cell-cell adhesion sites. J. Biol. Chem. 1998;273:3470–3475. doi: 10.1074/jbc.273.6.3470. [DOI] [PubMed] [Google Scholar]
- Schmid CL, Bohn LM. Physiological and pharmacological implications of beta-arrestin regulation. Pharmacol. Ther. 2009;121:285–293. doi: 10.1016/j.pharmthera.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, Bohn LM. Serotonin, but not N-methyltryptamines, activates the serotonin 2A receptor via a ss-arrestin2/Src/Akt signaling complex in vivo. J. Neurosci. 2010;30:13513–13524. doi: 10.1523/JNEUROSCI.1665-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm NL, McDonald MP, Limbird LE. The alpha(2a)-adrenergic receptor plays a protective role in mouse behavioral models of depression and anxiety. J. Neurosci. 2001;21:4875–4882. doi: 10.1523/JNEUROSCI.21-13-04875.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy SK, Lefkowitz RJ. Multifaceted roles of beta-arrestins in the regulation of seven-membrane-spanning receptor trafficking and signalling. Biochem. J. 2003;375:503–515. doi: 10.1042/BJ20031076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields AD, Wang Q, Winder DG. alpha2A-adrenergic receptors heterosynaptically regulate glutamatergic transmission in the bed nucleus of the stria terminalis. Neuroscience. 2009;163:339–351. doi: 10.1016/j.neuroscience.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD. Mechanisms of Memory. 2nd ed. Academic Press; London: 2010. [Google Scholar]
- Violin JD, Lefkowitz RJ. Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol. Sci. 2007;28:416–422. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Wang Q, Limbird LE. Regulation of alpha2AR trafficking and signaling by interacting proteins. Biochem. Pharmacol. 2007;73:1135–1145. doi: 10.1016/j.bcp.2006.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zhao J, Brady AE, Feng J, Allen PB, Lefkowitz RJ, Greengard P, Limbird LE. Spinophilin blocks arrestin actions in vitro and in vivo at G protein-coupled receptors. Science. 2004;304:1940–1944. doi: 10.1126/science.1098274. [DOI] [PubMed] [Google Scholar]
- Wang R, Macmillan LB, Fremeau RT, Jr., Magnuson MA, Lindner J, Limbird LE. Expression of alpha 2-adrenergic receptor subtypes in the mouse brain: evaluation of spatial and temporal information imparted by 3 kb of 5' regulatory sequence for the alpha 2A AR-receptor gene in transgenic animals. Neuroscience. 1996;74:199–218. doi: 10.1016/0306-4522(96)00116-9. [DOI] [PubMed] [Google Scholar]
- Yanpallewar SU, Fernandes K, Marathe SV, Vadodaria KC, Jhaveri D, Rommelfanger K, Ladiwala U, Jha S, Muthig V, Hein L, Bartlett P, Weinshenker D, Vaidya VA. Alpha2-adrenoceptor blockade accelerates the neurogenic, neurotrophic, and behavioral effects of chronic antidepressant treatment. J. Neurosci. 2010;30:1096–1109. doi: 10.1523/JNEUROSCI.2309-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HT, Whisler LR, Huang Y, Xiang Y, O'Donnell JM. Postsynaptic alpha-2 adrenergic receptors are critical for the antidepressant-like effects of desipramine on behavior. Neuropsychopharmacology. 2009;34:1067–1077. doi: 10.1038/npp.2008.184. [DOI] [PMC free article] [PubMed] [Google Scholar]