Abstract
Cardiotoxicity due to administration of cancer therapeutic agents such as anthracyclines and herceptin are well described. Established guidelines to screen for chemotherapy-related cardiotoxicity (CRC) are primarily based on serial assessment of left ventricular (LV) ejection fraction (EF). However, other parameters such as LV volume, diastolic function, and strain may also be useful in screening for cardiotoxicity. More recent advances in molecular imaging of apoptosis and tissue characterization by cardiac MRI are techniques which might allow early detection of patients at high risk for developing cardiotoxicity prior to a drop in EF. This comprehensive multi-modality review will discuss both the current established imaging techniques as well as the emerging technologies which may revolutionize the future of screening and evaluation for CRC.
Keywords: Chemotherapy, cardiotoxicity, non-invasive, imaging
INTRODUCTION
Cardiotoxicity is an unfortunate but well-established adverse effect of various chemotherapeutic agents, particularly anthracyclines and herceptin. Anthracyclines are used regularly in chemotherapeutic regimens to treat a variety of malignancies, including leukemia, lymphoma, neuroblastoma, sarcoma, ovarian cancer, breast cancer, and gastric cancer. While the exact mechanism of anthracycline-related cardiomyopathy (ARC) is poorly understood, a commonly accepted theory involves myocardial damage caused by mitochondrial injury and free radical formation.1,2 The risk of cardiomyopathy increases with a higher cumulative anthracycline dose: 3% with dose of 400 mg/m2, 7% for a dose of 550 mg/m2, and 18% for a dose of 700 mg/m2.3 However, there are patients who received doses exceeding 1 g/m2 who did not develop cardiomyopathy, which implies that there are clearly other factors involved. It is possible that patients metabolize the drugs differently, with some being more sensitive to the generation of anthracycline-induced free-radical formation than others.4 Many patients receive additional agents, such as taxanes, which are known to increase production of toxic anthracycline metabolites.5 Radiation therapy may also increase the incidence of ARC.6,7
Many patients with invasive breast cancer that overexpresses the human epidermal growth factor (HER-2) receptor receive regimens that include herceptin, a monoclonal antibody targeting the HER-2/neu receptor. The role of herceptin in the development of heart failure has not been elucidated, however, the loss of protective HER-2-mediated signaling pathways in response to stress8 may sensitize the myocardium to cellular injury from anthracyclines,9 as a higher incidence of cardiomyopathy was observed in regimens that involved concurrent herceptin and anthracycline administration compared to those receiving herceptin after completing anthracycline treatment (27% vs 7%, respectively).10,11
The prevalence of clinical heart failure was initially reported to be 2.2% in a large retrospective analysis of over 4,000 patients who received anthracycline-based chemotherapy with a mortality of 71% attributed to heart failure.3 However, that study was performed in 1979, prior to the widespread use of modern heart failure medical therapies and the implementation of routine screening for left ventricular (LV) dysfunction prior to and after dosing anthracyclines. A more recent long-term follow-up study of patients who received anthracyclines suggests that prior studies may have underestimated the number of patients developing heart failure, as they observed a 63% prevalence of LV dysfunction after more than 10 years of follow-up for those who received more than 500 mg/m2 cumulative dose, in contrast to an 18% prevalence in those who had received less than 500 mg/m2.7 Perhaps more concerning is that up to half of patients who develop LV dysfunction after receiving an anthracycline and/or herceptin may not be on medical therapy or have even had a cardiology consultation.12 Although treatment of anthracycline-induced cardiomyopathy with carvedilol and enalapril has shown some promise in small studies,13–15 45% of patients have no improvement in LV function with medical therapy.16 Conversely, cardiotoxicity due to herceptin is considered to be reversible if a prompt diagnosis is made, with discontinuation of herceptin and initiation of medical treatment for heart failure.17 A scheme for classification of cardiotoxicity based on differences in underlying mechanism and reversibility has been developed (Table 1).18 Thus an early diagnosis of cardiotoxicity and the ability to predict which patients are more likely to suffer cardiotoxicity are important for preventing chronic heart failure in patients receiving these agents.
Table 1.
Clinical features distinguishing type I and type II chemotherapy-related cardiac dysfunction
| Type 1 CRCD | Type II CRCD | |
|---|---|---|
| Agent | Doxorubicin | Herceptin |
| Cellular effects | Death | Dysfunction |
| Biopsy findings | Typical anthracycline changes (resolve with time) | No typical anthracycline-like changes |
| Dose response | Cumulative | Not cumulative |
| Reversibility of damage | Permanent | Generally reversible |
Reproduced with permission.18
IMAGING TO DETECT CARDIOTOXICITY
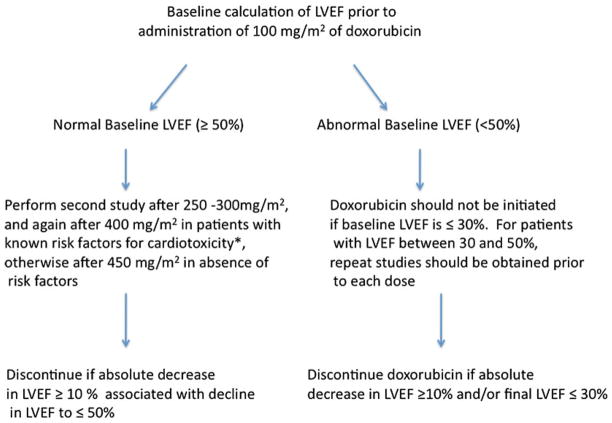
The current guidelines for monitoring patients receiving anthracyclines proposed by Schwartz et al in 198719 recommends obtaining a baseline ejection fraction (EF) by equilibrium radionuclide imaging, with subsequent imaging studies before consideration of any additional doses and specific criteria for drug discontinuation based on interval change in LV function (Figure 1). In patients with a normal resting EF > 50%, a drop in LVEF of >10% or to<50% is considered an indication for discontinuation of these agents. In patients with a baseline resting EF < 50%, the agent should be discontinued for a 10% drop in EF or a drop to <30%. While currently the clinical standard of care, assessment of LVEF or a drop in LVEF has limited ability to predict patients at risk for CRC. Resting EF by equilibrium radionuclide imaging has a sensitivity of only 53% for predicting patients considered moderate-high risk by myocardial biopsy/right heart catheterization criteria for developing heart failure with additional doxorubicin administration.20 Furthermore, higher biopsy grades were observed in patients with normal EF by ERNA or echocardiography who received even moderate cumulative anthracycline doses.21 Because serial myocardial biopsy is not feasible to perform routine screening for CRC, improved non-invasive imaging techniques to identify patients with early signs of cardiotoxicity and to identify patients at high risk for developing CRC are needed. Beyond measuring resting EF, most non-invasive imaging techniques can assess change in diastolic function or LV volumes which typically precede overt drops in EF. The addition of biomarkers to LVEF measurement in screening for cardiotoxicity is still not established. An increase in troponin after anthracycline chemotherapy has been shown to predict those with a larger and sustained drop in LVEF compared to those without troponin rise.22 However, subsequent studies have failed to demonstrate a correlation between troponin rise and anthracycline or herceptin administration.23,24 Interestingly, an increase in pro-BNP occurs early after anthracycline administration, but is not predictive of future LV dysfunction.24
Figure 1.
Current SPECT guidelines for cardiac monitoring during anthracycline treatment, modified from Schwartz et al.19 *Risk factors for cardiotoxicity: known heart disease, abnormal ECG, radiation exposure, cyclophosphamide therapy, or cumulative dose already >450 mg/m2.
In addition to assessing LV function, newer non-invasive methods such as cardiovascular magnetic resonance (CMR) enable imaging of myocardial edema, inflammation, and fibrosis which may provide more specific information about myocardial injury in CRC. Targeted molecular imaging with SPECT, PET, or MRI may be able to detect cell death due to doxorubicin25 and to detect subclinical cardiotoxicity.
With more than 190,000 new diagnoses of invasive breast cancer estimated in 2009,26 as well as the FDA’s expansion of prescribing guidelines for herceptin to include patients with early stage breast cancer, there will likely be an increase in the number of patients presenting with CRC. The updated guidelines provided by the manufacturer of herceptin recommend baseline evaluation of LV function, followed by repeat imaging every 3 months while on treatment, and every 6 months for the immediate 2-year period after completing the regimen.27
Improved methods to identify patients at high risk for CRC as well as a more collaborative approach between oncology and cardiology are needed. This multi-modality review will discuss both the current established imaging techniques as well as the emerging technologies which may revolutionize the future of screening and evaluation for CRC.
RADIONUCLIDE IMAGING
For serial measurements of LVEF, quantification by equilibrium radionuclide angiocardiography (ERNA) is more reproducible than echocardiographic visual assessment,28 and has long been considered the gold-standard for CRC screening. Perhaps the single largest study involving monitoring for CRC involved serial ERNA or single-photon emission computed tomography (SPECT) in 1,487 patients receiving doxorubicin.19 Using this method of screening, 19% of patients will be at high risk for developing cardiotoxicity (defined as a baseline LVEF < 50%, a drop in LVEF by ≥10% to a value <50%, cumulative doxorubicin dose ≥450 mg/m2). Among those at high risk for developing cardiotoxicity, those who developed clinical heart failure had a greater absolute drop in LVEF compared to those who did not (mean drop in LVEF 23% ± 14% vs 12% ± 10%).19 A more recent study demonstrated that 16% of patients receiving doxorubicin will be deemed at risk at some point during their therapy.29 Furthermore, implementation of ERNA screening led to early discontinuation of treatment in 13% of patients due to criteria for cardiotoxicity, the majority of whom were asymptomatic. This highlights the importance of routine screening, as many patients will demonstrate signs of cardiotoxicity by a drop in LVEF the absence of heart failure symptoms. The problem with relying on resting LVEF alone is that many patients will develop histological evidence of anthracycline-related changes without resting LV dysfunction.21 The addition of exercise LVEF to rest LVEF for diagnosing patients at high risk for CRC according to endomyocardial biopsy grade increased sensitivity from 58% to 100%, however, this was accompanied by a drop in specificity.20 While the addition of exercise EF may identify at risk patients who might be missed, there have been no large-scale studies in this population demonstrating whether the routine addition of exercise ERNA to screening would be cost-effective, alter dose recommendations of chemotherapeutic regimens, and not lead to additional unnecessary testing in patients with false-positive findings. Furthermore, the observation that resting LVEF had such a low sensitivity in identifying high-risk histology patients raises the question whether resting LVEF alone is truly the optimal measurement to base therapeutic decisions.
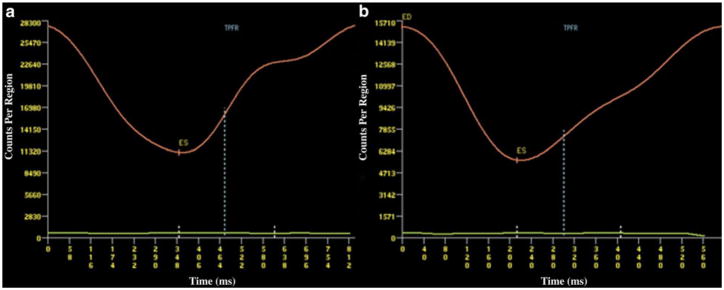
In addition to changes in systolic function, ERNA can provide information regarding diastolic function by generating count-time curves (Figure 2).31,32 Theoretically, diastolic abnormalities should occur prior to a drop in LV systolic function, due to alterations of intracellular calcium handling33 or increased coronary resistance resulting in acute elevations in LV end-diastolic pressure after exposure to anthracyclines.34 The diastolic parameters that can be obtained by RNA include peak filling rate (PFR) expressed as end diastolic volume/s and the time to PFR (TPFR) expressed in milliseconds, which are age dependent.32,35 A significant decrease in PFR occurs 1 month after starting treatment with anthracyclines, but is still within the normal range PFR > 2.5. However, this correlates with a simultaneous decrease in LVEF (also still within normal range), suggesting that the same underlying process impairs both systolic and diastolic functions.35
Figure 2.
Count time curves from a patient prior to (A) and after (B) anthracycline treatment, with marked reduction in the slope of the curve (TPFR) representing abnormal diastolic filling. Reproduced with permission30.
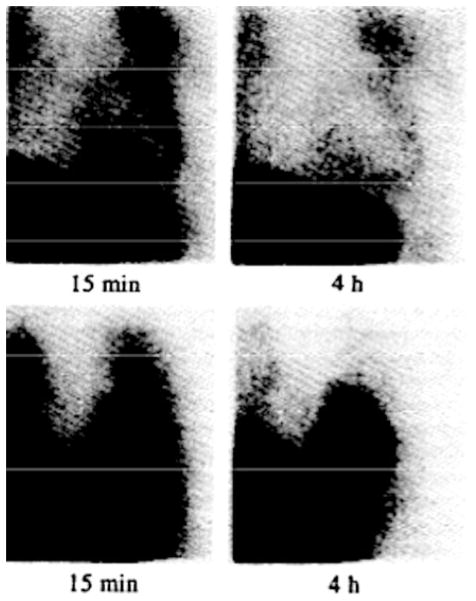
There have been recent advances in nuclear imaging using molecular targets to visualize effects of chemotherapeutic agents on the myocardium. The integrity of the cardiac sympathetic nervous system can be assessed with Iodine-123-metaiodobenbenzylguanidine (123I-MIBG), an analogue of norepinephrine.36 The ratio of heart to mediastinal (H/P) 123I-MIBG uptake is decreased and washout delayed in heart failure patients when compared to normal controls, and decreases with worsening NYHA class.37 Abnormal 123I-MIBG uptake occurs in patients receiving anthracyclines,38 with significantly lower H/P ratios observed with higher cumulative anthracycline dose (Figure 3).39,40 Although a drop in H/P ratio among those receiving higher cumulative doses precedes a drop in LVEF, abnormal uptake in patients receiving low-to-medium anthracyclines also occurs.40 This may be a promising method to detect early anthracycline injury; however, further studies are needed to quantify what interval decrease in uptake portends excessive risk of future LV dysfunction with or without continued treatment, and whether this method is applicable at lower cumulative doses.
Figure 3.
Planar anterior [123I]MIBG images demonstrating reduced uptake and retention in a patient who developed severe adriamycin cardiotoxicity (upper panels), compared to normal uptake and retention in a patient who received a lower cumulative anthracycline dose (lower panels). Reproduced with permission40.
Herceptin labeled with 111Indium has been used to image metastatic breast cancer expressing the HER-2/neu receptor.41,42 A small case series of 20 patients with metastatic breast cancer expressing the HER-2/neu receptor demonstrated that 35% of patients had evidence of myocardial 111Intraztuzumab (111In-TZ) uptake (Figure 4), prior to administration of any chemotherapy; 86% of those patients with tracer uptake developed clinical heart failure, whereas none of the patients without uptake had adverse cardiac events.43 Similarly, all the patients who had tumor tracer uptake had response to treatments including herceptin. These findings demonstrate the potential for targeted radionuclide imaging in weighing risks of treatment with herceptin against likelihood of benefit. In subsequent studies involving patients with HER-2 positive breast cancer who were imaged after receiving herceptin ± paclitaxel or an anthracycline, there fewer patients with myocardial 111In-TZ uptake, and the predictive value of myocardial uptake on the development of heart failure was poor.44,45 This may be due to inherent variations in myocardial expression of HER-2 prior to and during the course of chemotherapy. Larger studies are needed to define what baseline levels of HER2 expression and effects of herceptin and other agents have on development of early and late cardiac events.
Figure 4.
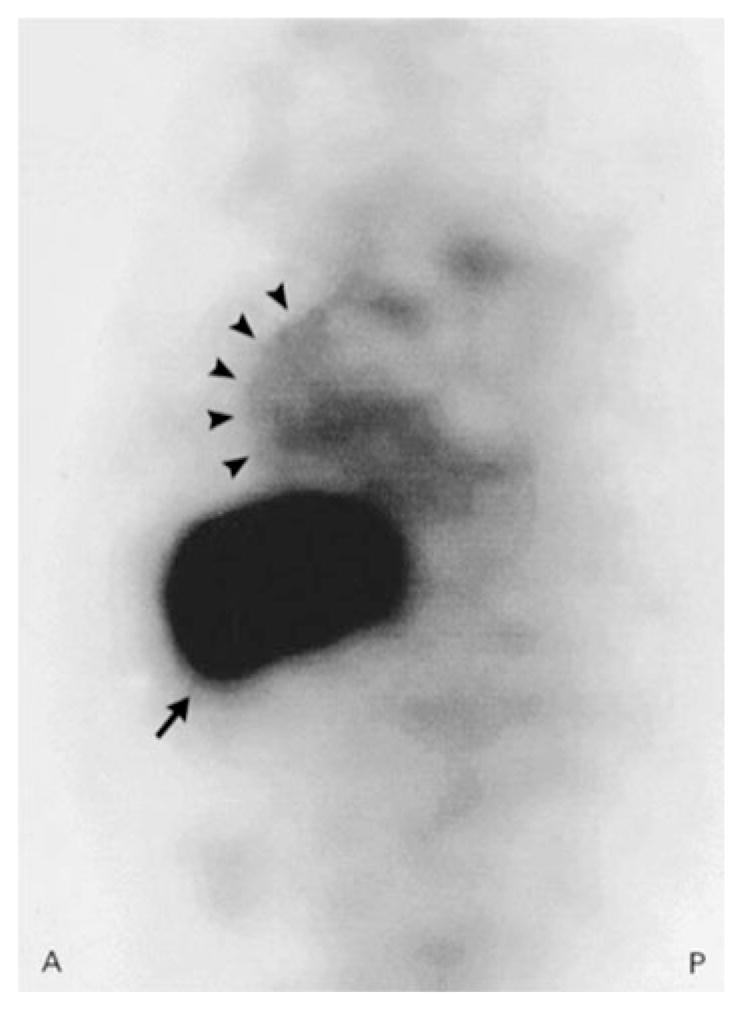
Sagittal slice of a SPECT image in a woman with metastatic breast cancer. Strong uptake of 111In-DTPA-traztuzumab in the liver metastasis (arrow), as well as in the anterior wall of the myocardium (arrowheads). Reproduced with permission43.
POSITRON EMISSION TOMOGRAPHY (PET)
The utility of PET imaging in cancer patients has focused on diagnosis of metastatic lesions and response to chemotherapy. However, fluorine-18-fluorodeoxyglucose (FDG) PET imaging can be useful in diagnosing and monitoring response to treatment of primary cardiac lymphoma, which will be apparent as hypermetabolic areas within the myocardium,46,47 FDG-PET has also been able to evaluate for metastatic pericardial involvement.48 There has been limited investigation applying cardiac PET to monitor for ARC in humans. In patients receiving adriamycin, there has been no early or late change in uptake of carbon-11 acetate, a tracer which is a marker for both myocardial blood flow and an indicator of oxidative metabolism through the TCA cycle.49 However, a preliminary study in rats demonstrated decreased myocardial uptake of β-adrenergic antagonist [3H] CGP12177 in the septum and free wall 3 weeks after treatment with adriamycin.50 It is unclear whether β-receptor density is a good surrogate marker to predict anthracycline cardiotoxicity in humans.
ECHOCARDIOGRAPHY
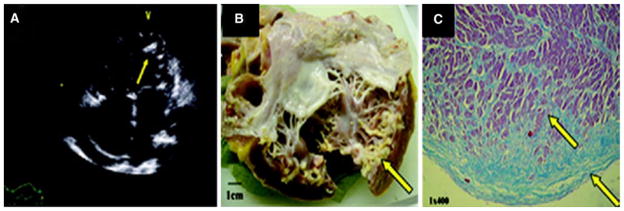
Perhaps the most readily available modality for assessment to screen for cardiotoxicity with serial measurements of LVEF, echocardiography can provide supplemental information that cannot be obtained from SPECT, such as evaluation for valvular disease or pericardial constriction, which are known adverse effects of mediastinal radiation. Recently, a restrictive cardiomyopathy with endocardial calcification has been described by echocardiography in a patient who received an anthracycline-based regimen in childhood who was subsequently referred for orthotopic heart transplant (Figure 5).51 In addition, new-onset mitral regurgitation and papillary muscle dyssynchrony has also been described during treatment with herceptin.52 Thus, echocardiographic screening identifies other cardiac effects of cancer treatment, including valvular disease and pericardial constriction, cannot be evaluated by SPECT.
Figure 5.
A Four-chamber echocardiographic image demonstrating subendocardial LV calcification (arrows) with corresponding to calcification seen on gross specimen after orthotopic heart transplant (B). Masson’s trichrome staining (C) demonstrates fibrosis of the endocardium and myocardium (blue stain). Images reproduced with permission51.
While the accuracy of LVEF measurement by echo has been validated against SPECT and CMR, unenhanced echocardiography tends to underestimate LVEF when compared to contrast echocardiography or against MRI and SPECT.12,53 In patients with inadequate acoustic windows, using contrast echocardiography might increase accuracy of LVEF measurements, as it has been shown to improve the percentage of correct classification of LVEF (normal, mild-moderately reduced, or severely reduced) when compared to CMR measurements of LV volume.54 Using 3D echo volumes to calculate LVEF may also improve accuracy and reproducibility,55 but has not yet been specifically studied for cardiotoxic screening and is not readily available in all laboratories.
As the assessment of LVEF is used as a major guide for determining further therapy, it is important to ensure that physicians skilled in echo interpretation are assessing the images. For example, LVEF measurements obtained from four serial 2D exams performed on healthy volunteers within a 24-hour period varied more when a read by a single unblinded sonographer site reader as compared to a single-blinded physician reader in a core lab (intraobserver variability 19.9% vs 5.5 %).56 This underscores the need for rigorous review of how serial LVEF measurements are performed in individual centers and raises the question whether blinding of echo readers would help decrease variability in LVEF measurements, especially in the case of cardiotoxicity screening where clinical decisions regarding some chemotherapy regimens hinge on criteria for LV function.19 Furthermore, in heart failure patients, there is significant variation among measurements of LVEF by various methods (M-mode, 2D Simpson’s biplane, and Teicholtz), while 2D Simpson’s biplane has the best correlation with CMR in this population.57
For some patients receiving anthracyclines, a decline in systolic function may occur, but the LVEF may still be within the “normal” range.24,35,58,59 Accordingly, there has been interest in finding other echocardiographic parameters which may be of use in detecting subclinical myocardial dysfunction preceding a drop in LVEF to the abnormal range. Diastolic abnormalities including decreased E/A ratio and increased Tei index have been described in adults after receiving anthracycline.29 In pediatric malignancy survivors who received anthracyclines, an increased E/e′ and reduced myocardial performance index has been observed as compared to normal controls.60 However, these differences in diastolic parameters noted in patients were still within the normal range. In addition, the correlation between resting diastolic abnormalities after anthracycline treatment and future reduction in LV systolic function is weak.61
Evaluation for contractile reserve using stress echocardiography can provide important prognostic information in conditions such as severe aortic stenosis,62 but its role in routine screening for LV dysfunction in chemotherapy patients is unclear. In asymptomatic pediatric survivors of malignancy who were treated with relatively low cumulative doses of anthracyclines, increase in LVEF and end-systolic stress-volume index with exercise stress was less pronounced in these patients as compared to gender and age-matched healthy controls,.63 In adults, a change in contractile reserve after anthracycline administration has not been consistently observed, however, a decrease in stress peak E velocity and E/A ratio does occur, and may be a marker of subclinical cardiotoxicity in those with normal LV function after receiving chemotherapy.58,64
More novel techniques such as strain imaging with 2D echocardiography has been used to detect subclinical LV dysfunction and has demonstrated predictive value in disease states such as cardiac amyloidosis,65 hypertrophic cardiomyopathy,66 as well as determining viability prior to revascularization in patients with CAD.67 In a cohort of asymptomatic survivors of pediatric malignancy, strain and strain rate were decreased when compared with normal controls.68 The use of this technology to monitor for subclinical dysfunction during chemotherapy treatment shows promise. In a prospective study of patients receiving herceptin, a decrease in global longitudinal and radial strain but not LVEF was observed as early as 3 months in patients who later developed cardiotoxicity.23 Strain rate also decreased in half of patients receiving herceptin; however, only 16% of those with a decrease in strain rate had a drop in EF ≥ 10%.69 Similarly, in a pilot study of elderly female patients receiving anthracyclines for breast cancer without significant cardiac comorbidities, there was no change in LVEF, however, a significant drop in peak longitudinal systolic LV strain occurred after six cycles of chemotherapy, with mean values below that of normal for age and gender (Figure 6).70 Although these findings suggest strain and strain rate as useful parameters that could be performed with echocardiographic screening for cardiotoxicity, it should be used with caution in patient with comorbidities such as obesity,71 other cardiac conditions such as valvular disease,72 infiltrative disease,73 LV hypertrophy,74 and myocardial infarction,75 all of which can impact LV strain. Age and gender also should be taken into consideration when interpreting strain values.76–78 Another limitation of echocardiographic strain imaging is a dependence on adequate 2D acoustic windows to track endocardial borders for high fidelity measurements. Furthermore, strain analysis is typically performed off-line and requires experienced echocardiographers to interpret.
Figure 6.
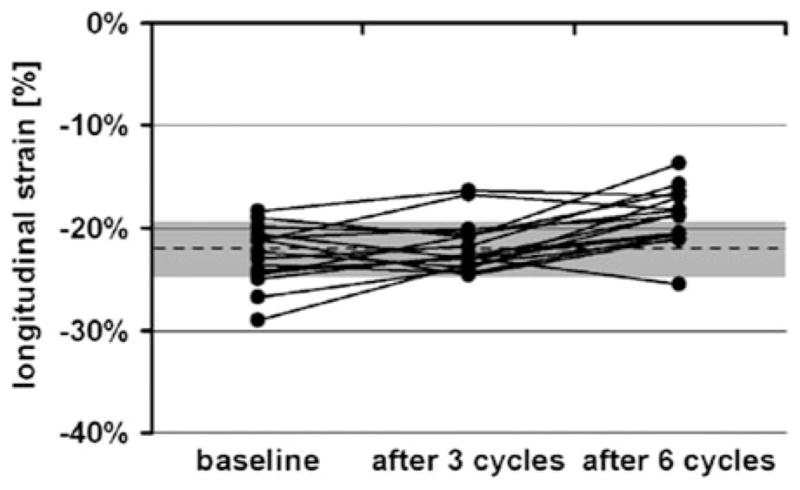
Peak longitudinal LV strain at baseline, after three cycles, and after six cycles of doxorubicin. Dashed line represents published normal value of average peak systolic strain ±1 SD (gray area) for healthy women aged 50–70 years. Reproduced with permission70.
CMR IMAGING
CMR is recognized by the ACC/AHA as method to screen for CRC.79 However, it is less widely used for routine screening for CRC, in part likely due to more widespread availability of echocardiography and SPECT. However, there are some potential advantages of CMR. Particularly in obese patients for whom echocardiography yields suboptimal images, CMR is an excellent modality for obtaining accurate serial measurements of LV function, and is considered the gold standard for measuring LV function.80
CMR has the potential to demonstrate subclinical myocardial changes prior to the onset of LV dysfunction. CMR has the unique ability to detect myocardial edema which may be seen in acute myocardial injury. Increased signal in the myocardium on T2-weighted (T2-W) images has been described in the presence of acute myocardial inflammation and injury, as in myocarditis and myocardial infarction or stress-induced cardiomyopathy,81–83 but T2-W imaging in CRC has not been evaluated clinically. While standardization of T2-W imaging among centers is still needed to ensure fidelity and reproducibility of abnormal T2-W findings, further research is needed to determine whether myocardial edema is of value in identifying high-risk patients prior to receiving chemotherapy as well as monitoring for cardiotoxicity.
As coronary artery disease and prior MI increases the risk for developing CRC,84 CMR might detect occult subendocardial infarcts that would otherwise be missed by SPECT imaging85 and be too small to manifest as a regional wall motion abnormality on 2D echocardiography. In addition to imaging infarct, characteristic patterns of focal or linear mid-myocardial delayed enhancement (DE) CMR in the presence of myocarditis have been described.86 A similar pattern of mid-myocardial delayed enhancement was observed in 10 breast cancer patients treated with an anthracycline and herceptin who were diagnosed with CRC (Figure 7).87 All these patients with focal myocardial delayed enhancement had already developed LV dysfunction.
Figure 7.
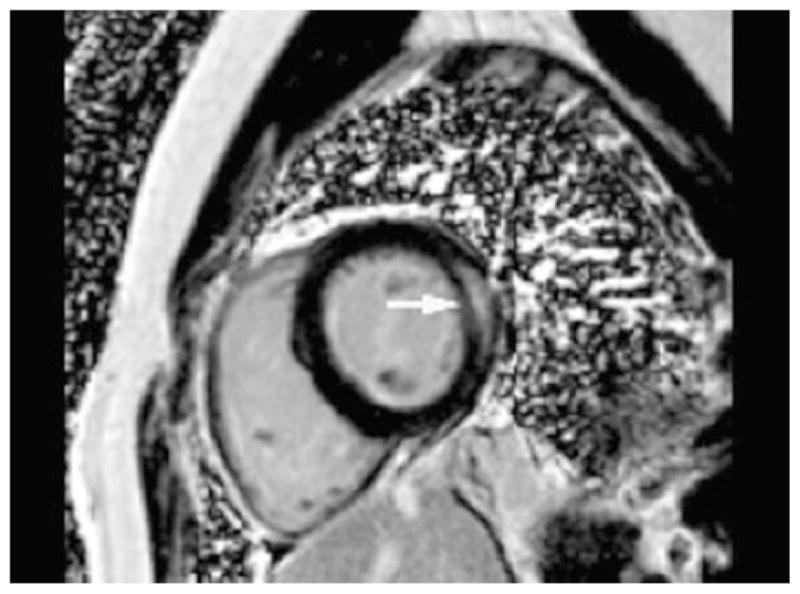
Short-axis mid-ventricular phase-sensitive inversion recovery delayed image in a patient with herceptin-induced cardiomyopathy demonstrating mid-myocardial lateral wall delayed enhancement. Reproduced with permission87.
Myocardial T1 mapping is a new technique which uses T1 relaxation times to calculate the volume of distribution (Vd) of gadolinium contrast in myocardium, a measure which is increased in the presence of diffuse myocardial fibrosis or infiltrative disease.88,89 T1 mapping may prove to be a useful method to identify patients at risk for cardiomyopathy, as discrete areas of fibrosis as seen on DE CMR images may be a late finding, and therefore not useful in detecting early signs of cardiotoxicity. In a cohort of 13 young adults who received anthracyclines as part of their cancer treatment and have achieved remission and have normal LV function, increased fibrosis was associated with increased LV volume, however, there was no correlation between anthracycline dose and degree of fibrosis.90 In adults, a pilot study has demonstrated that patients with significantly increased myocardial signal intensity on post-contrast T1-weighted images obtained within 3 days after the first anthracycline infusion have a significant decrease in EF on day 28 after starting chemotherapy.59 Larger-scale studies in patients receiving cardiotoxic chemotherapeutic agents are still needed to determine whether there is a threshold for myocardial T1 or Vd beyond which there is increased risk for developing LV dysfunction. Correlating these MRI-derived measurements with cumulative doses of chemotherapy administered might demonstrate that some patients are at excess risk after receiving relatively lower doses. Similarly, others may be at low risk even with higher doses and might be able to safely receive more aggressive regimens.
The presence of myocardial fibrosis identified by DE CMR has demonstrated prognostic value in CAD,91 as well as other myocardial diseases such as HCM, amyloidosis, sarcoidosis, and aortic stenosis.92–96 CMR studies in larger populations of patients receiving potentially cardiotoxic agents are needed to see whether the presence of LGE can identify patients who are at higher risk and may benefit from more frequent and long-term follow-up.
MOLECULAR IMAGING OF APOPTOSIS
Molecular imaging agents targeting annexin A5 to detect apoptosis have been developed for multiple imaging modalities including SPECT, echo, and CMR. Non-invasive imaging using 11 In or 99mTc-labeled annexin A5 has been used to monitor response of tumors to treatment, as annexin A5 binds to phosphatidylserine, a cell membrane phospholipid which is exposed during apoptosis.97,98 There has been interest in using annexin as a target to image apoptosis in the myocardium as an indicator of anthracycline-induced cardiotoxicity. Increased binding of annexin-labeled microbubbles to the myocardium in rats treated with doxorubicin has been demonstrated using echocardiography.99 Similar results have been shown using 99m Tc-annexin, with a significant correlation between increasing myocardial uptake of tracer with higher cumulative doses of anthracycline.100 More recently, a molecular MRI probe has been developed to be used with T2* imaging, a technique which has been used clinically to quantify myocardial iron stores in disease states such as hemochromatosis and thalessemia, where regions containing increased iron will have decreased signal when compared to normal myocardium.101,102 This new probe contains superparamagnetic iron oxide conjugated to recombinant human annexin, and has demonstrated diffuse myocardial signal loss when used to image rats treated with adriamycin, indicating areas of adriamycin-induced apoptosis by MRI.103 The development of a molecular target like annexin which can be used screen for CRC with all three modalities is attractive, as individual patients may be better suited to different imaging techniques.
CONCLUSION
Given recent advances in non-invasive cardiac imaging to screen for CRC over the last decade, there is a critical need to re-evaluate the current reliance on LVEF in determining which patients are at high risk for developing cardiomyopathy. The yearly cost of caring for a patient with heart failure due to CRC is offset several times over by the cost of screening an at-risk population by ERNA.29 Screening in a population without established risk factors for CRC is not cost-effective104; however, more sensitive methods have since been discovered for identifying at-risk patients.
As there are strengths and weaknesses of ERNA, echocardiography, and CMR, the future of screening will likely involve an algorithm which includes one or more testing modality to risk stratify prior to and shortly after starting chemotherapy, and subsequently tailored to the patient based on initial risk assessment, chemotherapeutic regimen, and cumulative dose administered. The cost-effectiveness of a multi-modality approach has yet to be performed; however, there is still room for further investigation, particularly in the field of CMR and development of molecular imaging agents to target doxorubicin and herceptin-mediated cell damage.
Footnotes
Disclosures
Ronny S. Jiji, None; Christopher M. Kramer, Research support (significant), Siemens Healthcare; Michael Salerno, AHA research support.
References
- 1.Horenstein MS, Vander Heide RS, L’Ecuyer TJ. Molecular basis of anthracycline-induced cardiomyopathy and its prevention. Mol Genet Metab. 2000;71:436–44. doi: 10.1006/mgme.2000.3043. [DOI] [PubMed] [Google Scholar]
- 2.Tokarska-Schlattner M, Zaugg M, Zuppinger C, Wallimann T, Schlattner U. New insights into doxorubicin-induced cardiotoxicity: The critical role of energetics. J Mol Cell Cardiol. 2006;41:389–405. doi: 10.1016/j.yjmcc.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Von Hoff DD, Layard MW, Basa P, Davis HL, Von Hoff AL, Rozencweig M, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91:710–7. doi: 10.7326/0003-4819-91-5-710. [DOI] [PubMed] [Google Scholar]
- 4.Wojnowski L, Kulle B, Schirmer M, Schlüter G, Schmidt A, Rosenberger A, et al. NAD(P)H oxidase and multidrug resistance protein polymorphisms are associated with doxorubicin-induced cardiomyopathy. Circulation. 2005;112:3754–62. doi: 10.1161/CIRCULATIONAHA.105.576850. [DOI] [PubMed] [Google Scholar]
- 5.Minotti G, Saponiero L, Licata S, Menna P, Calafiore AM, Teodori G, et al. Paclitaxel and docetaxel enhance the metabolism of doxorubicin to toxic species in human myocardium. Clin Cancer Res. 2001;7:1511–5. [PubMed] [Google Scholar]
- 6.Myrehaug S, Pintilie M, Tsang R, Mackenzie R, Crump M, Chen Z, et al. Cardiac morbidity following modern treatment for Hodgkin’s lymphoma: Supra-additive cardiotoxicity of doxorubicin and radiation therapy. Leuk Lymphoma. 2008;49:1486–93. doi: 10.1080/10428190802140873. [DOI] [PubMed] [Google Scholar]
- 7.Steinherz LJ, Steinherz PG, Tan CT, Heller G, Murphy ML. Cardiac toxicity 4 to 20 years after completing anthracycline therapy. JAMA. 1991;266:1672–7. [PubMed] [Google Scholar]
- 8.Hudis C. Traztuzumab—mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 9.Crone SA, Zhao Y, Fan L, Gu Y, Minamisawa S, Liu Y, et al. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med. 2002;8:459–65. doi: 10.1038/nm0502-459. [DOI] [PubMed] [Google Scholar]
- 10.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:784–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 11.Piccart-Gebhart MJ, Proctor M, Leyland-Jones B, Goldhirsch A, Untuch M, Smith I, et al. Traztuzumab after adjunctive chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–71. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 12.Yoon GJ, Telli ML, Kao DP, Matsuda KY, Carlson RW, Witteles RM. Left ventricular dysfunction in patients receiving cardiotoxic cancer therapies. Are clinicians responding optimally? J Am Coll Cardiol. 2010;56:1644–50. doi: 10.1016/j.jacc.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalay N, Basar E, Ozdrogu I, ERO, Cetinkaya Y, Dogan A, et al. Protective effects of carvedilol against anthracycline-induced cardiomyopathy. JACC. 2006;48:2258–62. doi: 10.1016/j.jacc.2006.07.052. [DOI] [PubMed] [Google Scholar]
- 14.Santos DL, Moreno AJM, Leino RL, Froberg MK, Wallace KB. Carvedilol protects against doxorubicin-induced mitochondrial cardiomyopathy. Toxicol Appl Pharmacol. 2002;185:218–27. doi: 10.1006/taap.2002.9532. [DOI] [PubMed] [Google Scholar]
- 15.Cardinale D, Sandri MT, Martinoni A, Tricca A, Lamantia G, Cinieri S, et al. Left ventricular dysfunction predicted by early troponin I release after high-dose chemotherapy. JACC. 2000;36:517–22. doi: 10.1016/s0735-1097(00)00748-8. [DOI] [PubMed] [Google Scholar]
- 16.Cardinale D, Colombo A, Lamantia G, Colombo N, Civelli M, De Giacomi G, et al. Anthracycline-induced cardiomyopathy: Clinical relevance and response to pharmacologic therapy. JACC. 2010;55:213–20. doi: 10.1016/j.jacc.2009.03.095. [DOI] [PubMed] [Google Scholar]
- 17.Ewer MS, Vooletich MT, Durand J, Woods ML, Davis JR, Valero V, et al. Reversibility of traztuzumab-related cardiotoxicity: New insights based on clinical course and response to medical treatment. J Clin Oncol. 2005;23:7820–6. doi: 10.1200/JCO.2005.13.300. [DOI] [PubMed] [Google Scholar]
- 18.Ewer SM, Ewer MS. Cardiotoxicity profile of traztuzumab. Drug Saf. 2008;31:459–67. doi: 10.2165/00002018-200831060-00002. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz RG, McKenzie WB, Alexander J, Sager P, Manatunga A, Schwartz PE, et al. Congestive heart failure and left ventricular dysfunction complicating doxorubicin therapy. Am J Med. 1987;82:1109–18. doi: 10.1016/0002-9343(87)90212-9. [DOI] [PubMed] [Google Scholar]
- 20.McKillop JH, Bristow MR, Goris ML, Billingham ME, Bockemuehl K. Sensitivity and specificity of radionuclide ejection fractions in doxorubicin cardiotoxicity. Am Heart J. 1983;106:1048–56. doi: 10.1016/0002-8703(83)90651-8. [DOI] [PubMed] [Google Scholar]
- 21.Ewer MS, Ali MK, Mackay B, Wallace S, Valdivieso M, Legha SS, et al. A comparison of cardiac biopsy grades and ejection fraction estimations in patients receiving adriamycin. J Clin Oncol. 1984;2:112–7. doi: 10.1200/JCO.1984.2.2.112. [DOI] [PubMed] [Google Scholar]
- 22.Cardinale D, Sandri MT, Martinoni A, Tricca A, Civelli M, Lamantia G, et al. Left ventricular dysfunction predicted by early troponin I release after high-dose chemotherapy. J Am Coll Cardiol. 2000;36:517–22. doi: 10.1016/s0735-1097(00)00748-8. [DOI] [PubMed] [Google Scholar]
- 23.Fallah-Rad N, Walker JR, Wassef A, Lytwyn M, Bohonis S, Fang T, et al. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II-positive breast cancer treated with adjuvant traztuzumab therapy. J Am Coll Cardiol. 2011;57:2263–70. doi: 10.1016/j.jacc.2010.11.063. [DOI] [PubMed] [Google Scholar]
- 24.Dodos F, Halbsguth T, Erdmann E, Hoppe UC. Usefulness of myocardial performance index and biochemical markers for early detection of anthracycline-induced cardiotoxicity in adults. Clin Res Cardiol. 2007;97:318–26. doi: 10.1007/s00392-007-0633-6. [DOI] [PubMed] [Google Scholar]
- 25.Gabrielson KL, Mok GS, Nimmagadda S, Bedja D, Tsao A, Wang Y, et al. Detection of dose response in chronic doxorubicin-mediated cell death with cardiac technetium 99m annexin V single-photon emission computer tomography. Mol Imaging. 2008;3:132–8. [PMC free article] [PubMed] [Google Scholar]
- 26. [Accessed 25 Jan 2012]; http://www.cancer.org/acs/groups/content/@nho/documents/document/f861009final90809pdf.pdf.
- 27. [Accessed 25 Jan 2012]; http://www.gene.com/gene/products/information/pdf/herceptin-prescribing.pdf.
- 28.van Royen N, Jaffe CC, Krumholz HM, Johnson KM, Lynch PJ, Natale D, et al. Comparison and reproducibility of visual echocardiographic and quantitative radionuclide left ventricular ejection fractions. Am J Cardiol. 1996;77:843–50. doi: 10.1016/s0002-9149(97)89179-5. [DOI] [PubMed] [Google Scholar]
- 29.Mitani I, Jain D, Joska TM, Burtness B, Zaret B. Doxorubicin cardiotoxicity: Prevention of congestive heart failure with serial cardiac function monitoring with equilibrium radionuclide angiocardiography in the current era. J Nucl Cardiol. 2003;10:132–9. doi: 10.1067/mnc.2003.7. [DOI] [PubMed] [Google Scholar]
- 30.Salerno M. Multi-modality imaging of diastolic function. J Nucl Cardiol. 2010;17:316–27. doi: 10.1007/s12350-010-9196-4. [DOI] [PubMed] [Google Scholar]
- 31.Muntinga HJ, van den Berg F, Knol HR, Niemeyer MG, Blanksma PK, Louwes H, et al. Normal values and reproducibility of left ventricular filling parameters by radionuclide angiography. Int J Card Imaging. 1997;13:165–71. doi: 10.1023/a:1005704415207. [DOI] [PubMed] [Google Scholar]
- 32.Lee KJ, Southee AE, Bautovich GJ, Freedman B, McLaughlin AF, Rossleigh MA, et al. Normalised radionuclide measures of left ventricular diastolic function. Eur J Nucl Med. 1989;15:123–7. doi: 10.1007/BF00254623. [DOI] [PubMed] [Google Scholar]
- 33.Kusuoka H, Futaki S, Koretsune Y, Kitabatake A, Suga H, Kamada T, et al. Alterations of intracellular calcium homeostasis and myocardial energetics in acute adriamycin-induced heart failure. J Cardiovasc Pharmacol. 1991;18:437–44. doi: 10.1097/00005344-199109000-00017. [DOI] [PubMed] [Google Scholar]
- 34.Pelikan PCD, Weisfeldt ML, Jacobus WE, Miceli MV, Bulkley BH, Gerstenblith G. Acute doxorubicin cardiotoxicity: Functional, metabolic, and morphologic alterations in the isolated, perfused rat heart. J Cardiovasc Pharmacol. 1986;8:1058–66. [PubMed] [Google Scholar]
- 35.Cottin Y, Touzery C, Coudert B, Gilles A, Walker P, Massing JL, et al. Impairment of diastolic function during short-term anthracycline therapy. Br Heart J. 1995;73:61–4. doi: 10.1136/hrt.73.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sisson JC, Shapiro B, Meyers L, Mallette S, Mangner TJ, Wieland DM, et al. Metaiodobenzylguanidine to map scintigraphically the adrenergic system in man. J Nucl Med. 1987;28:1620–4. [PubMed] [Google Scholar]
- 37.Arimoto T, Takeishi Y, Niizeki T, Koyama Y, Okuyama H, Nozaki N, et al. Ongoing myocardial damage relates to cardiac sympathetic nervous disintegrity in patients with heart failure. Ann Nucl Med. 2005;19:535–40. doi: 10.1007/BF02985045. [DOI] [PubMed] [Google Scholar]
- 38.Carrió I, Estorch M, Berná L, López-Pousa J, Tabernero J, Torres G. Indium-111-Antimyosin and Iodine-123-MIBG studies in early assessment of doxorubicin cardiotoxicity. J Nucl Med. 1995;36:2044–9. [PubMed] [Google Scholar]
- 39.Lekakis J, Prassopoulos V, Athanassiadis P, Kostamis P, Moulopoulos S. Doxorubicin-induced cardiac neurotoxicity: Study with 123-labeled metaiodobenzylguanidine scintigraphy. J Nucl Cardiol. 1996;3:37–41. doi: 10.1016/s1071-3581(96)90022-7. [DOI] [PubMed] [Google Scholar]
- 40.Valdés Olmos RA, ten Bokkel Huinink WW, ten Hoeve RFA, van Tinteren H, Bruning PF, van Vlies B, et al. Assessment of anthracycline-related myocardial adrenergic derangement by [123I]metaiodobenzylguanidine scintigraphy. Eur J Cancer. 1995;31A:26–31. doi: 10.1016/0959-8049(94)00357-b. [DOI] [PubMed] [Google Scholar]
- 41.Angerstein C, Behr TM, Behe M, Becker W. Kit formulation for In-111-labeled traztuzumab (Herceptin) for immunoscintigraphy of metastatic breast cancer expressing the HER2/neu receptor [abstract] Eur J Nucl Med. 2000;27:916. [Google Scholar]
- 42.Lub-De Hooge MN, Kosterink JG, Perik PG, Nijnuis H, Tran L, Bart J, et al. Preclinical characterization of 111-In DTPA-traztuzumab. Br J Pharmacol. 2004;143:99–106. doi: 10.1038/sj.bjp.0705915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Behr TM, Behe M. Traztuzumab and breast cancer [letter] N Engl J Med. 2001;345:995–6. doi: 10.1056/NEJM200109273451312. [DOI] [PubMed] [Google Scholar]
- 44.de Korte MA, de Vries EGE, Lub-de Hooge MN, Jager PL, Gietema JA, van der Graaf WTA, et al. 111Indium-trastuzumab visualises myocardial human epidermal growth factor receptor 2 expression shortly after anthracycline treatment but not during heart failure: A clue to uncover the mechanisms of trastuzumab-related cardiotoxicity. Eur J Cancer. 2007;43:2046–51. doi: 10.1016/j.ejca.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 45.Perik PJ, Lub-De Hooge MN, Gietema JA, van der Graaf WTA, de Korte MA, Jonkman S, et al. Indium-111-labeled traztuzumab scintigraphy in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2006;24:2276–82. doi: 10.1200/JCO.2005.03.8448. [DOI] [PubMed] [Google Scholar]
- 46.Kaderli AA, Baran I, Aydin O, Bicer M, Akpinar T, Ozkalemkas F, et al. Diffuse involvement of the heart and great vessels in primary cardiac lymphoma. Eur J Echocardiogr. 2010;11:74–6. doi: 10.1093/ejechocard/jep111. [DOI] [PubMed] [Google Scholar]
- 47.Lee JC, Platts DG, Huang YT, Slaughter RE. Positron emission tomography combined with computed tomography as an integral component in evaluation of primary cardiac lymphoma. Clin Cardiol. 2010;33:106–8. doi: 10.1002/clc.20725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weijs LE, Arsos G, Baarslag HJ, Wittebol S, de Klerk JM. Pericardial involvement in a non-Hodgkin lymphoma patient: Coregistered FDG-PET and CT imaging. Eur Heart J. 2007;28:2698. doi: 10.1093/eurheartj/ehm218. [DOI] [PubMed] [Google Scholar]
- 49.Nony P, Guastalla J, Rebattu P, Landais P, Lievre M, Bontemps L, et al. In vivo measurement of myocardial oxidative metabolism and blood flow does not show changes in cancer patients undergoing doxorubicin therapy. Chemother Pharmacol. 2000;45:375–80. doi: 10.1007/s002800051005. [DOI] [PubMed] [Google Scholar]
- 50.Kenk M, Thackeray JT, Thorn SL, Dhami K, Chow BJ, Ascah KJ, et al. Alterations of pre- and post-synaptic noradrenergic signaling in a rat model of adriamycin-induced cardiotoxicity. J Nucl Cardiol. 2010;17:254–63. doi: 10.1007/s12350-009-9190-x. [DOI] [PubMed] [Google Scholar]
- 51.Guendouz S, Buicuic O, Kirsch M, Benaiem N, Poulard JE, Deux JF, et al. Restrictive cardiomyopathy associated with left ventricle and left atria endocardial calcifications following chemotherapy. J Am Coll Cardiol. 2011;57:1633. doi: 10.1016/j.jacc.2010.07.067. [DOI] [PubMed] [Google Scholar]
- 52.Karabay CY, Kocabay G, Kalayci A, Zehir R, Tanboga H. Mitral regurgitation due to papillary muscle dyssynchrony during traztuzumab treatment. Cardiology. 2010;117:296–300. doi: 10.1159/000323834. [DOI] [PubMed] [Google Scholar]
- 53.Hoffman R, von Bardelben S, ten Cate F, Borges AC, Kasprzak J, Firschke C, et al. Assessment of systolic left ventricular function: A multi-centre comparison of cineventriculography, cardiac magnetic resonance imaging, unenhanced and contrast-enhanced echocardiography. Eur Heart J. 2005;26:607–16. doi: 10.1093/eurheartj/ehi083. [DOI] [PubMed] [Google Scholar]
- 54.Hundley WG, Kizilbash AM, Afridi I, Franco F, Peshock RM, Grayburn PA. Administration of intravenous perfluorocarbon contrast agent improves echocardiographic determination of left ventricular volumes and ejection fraction: Comparison with cine magnetic resonance imaging. J Am Coll Cardiol. 1998;32:1426–32. doi: 10.1016/s0735-1097(98)00409-4. [DOI] [PubMed] [Google Scholar]
- 55.Jenkins C, Bricknell K, Hanekom L, Marwick TH. Reproducibility and accuracy of echocardiographic measurements of left ventricular parameters using real-time three-dimensional echocardiography. J Am Coll Cardiol. 2004;44:878–86. doi: 10.1016/j.jacc.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 56.Bull SC, Main ML, Stevens GR, Goldman JH, Constable SA, Becher H. Cardiac toxicity screening by echocardiography in normal volunteers: A study of the effects of diurnal variation and use of a core laboratory on the reproducibility of left ventricular measurement. Echocardiography. 2011;28:502–7. doi: 10.1111/j.1540-8175.2010.01380.x. [DOI] [PubMed] [Google Scholar]
- 57.Bellenger NG, Burgess MI, Ray SG, Lahiri A, Coats AJS, Cleland JGF, et al. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance. Are they interchangeable? Eur Heart J. 2000;21:1387–96. doi: 10.1053/euhj.2000.2011. [DOI] [PubMed] [Google Scholar]
- 58.Cottin Y, L’huiller I, Casasnovas O, Geoffroy C, Caillot D, Zeller M, et al. Dobutamine stress echocardiography identifies anthracycline cardiotoxicity. Eur J Echocardiogr. 2000;1:180–3. doi: 10.1053/euje.2000.0037. [DOI] [PubMed] [Google Scholar]
- 59.Wassmuth R, Lentszch S, Erdbruegger U, Schulz-Menger J, Doerken B, Dietz R, et al. Subclinical cardiotoxic effects of anthracyclines as assessed by magnetic resonance imaging- a pilot study. Am Heart J. 2001;141:1007–13. doi: 10.1067/mhj.2001.115436. [DOI] [PubMed] [Google Scholar]
- 60.Karakurt C, Kocak G, Ozgen U. Evaluation of the left ventricular function with tissue tracking and tissue doppler echocardiography in pediatric malignancy survivors after anthracycline therapy. Echocardiography. 2008;25:880–7. doi: 10.1111/j.1540-8175.2008.00695.x. [DOI] [PubMed] [Google Scholar]
- 61.Dorup I, Levitt G, Sullivan I, Sorensen K. Prospective longitudinal assessment of late anthracycline cardiotoxicity after childhood cancer: The role of diastolic function. Heart. 2004;90:1214–6. doi: 10.1136/hrt.2003.027516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Monin JL, Monchi M, Gest V, Duval-Moulin AM, Dubois-Rande JL, Gueret P. Aortic stenosis with severe left ventricular dysfunction and low transvalvular pressure gradients: Risk stratification by low-dose dobutamine echocardiography. J Am Coll Cardiol. 2001;37:2101–7. doi: 10.1016/s0735-1097(01)01339-0. [DOI] [PubMed] [Google Scholar]
- 63.Guimaraes-Filho F, Tan D, Braga J, Rodrigues A, Waib P, Matsubara B. Ventricular systolic reserve in asymptomatic children previously treated with low doses of anthracyclines. Am J Cardiol. 2007;100:1303–6. doi: 10.1016/j.amjcard.2007.05.054. [DOI] [PubMed] [Google Scholar]
- 64.Bountioukos M, Doorduijn JK, Roelandt JR, Vourvouri EC, Bax JJ, Schinkel AF, et al. Repetitive dobutamine stress echocardiography for the prediction of anthracycline cardiotoxicity. Eur J Echocardiogr. 2003;4:300–5. doi: 10.1016/s1525-2167(03)00017-9. [DOI] [PubMed] [Google Scholar]
- 65.Koyama J, Falk RH. Prognostic significance of strain doppler imaging in light-chain amyloidosis. JACC Cardiovasc Imaging. 2010;3:333–42. doi: 10.1016/j.jcmg.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 66.Correia E, Rodrigues B, Santos LF, Moreira D, Gama P, Cabral C, et al. Longitudinal left ventricular strain in hypertrophic cardiomyopathy: Correlation with nonsustained ventricular tachycardia. Echocardiography. 2011;7:709–14. doi: 10.1111/j.1540-8175.2011.01427.x. [DOI] [PubMed] [Google Scholar]
- 67.Bansal M, Jeffriess L, Leano R, Mundy J, Marwick TH. Assessment of myocardial viability at dobutamine echocardiography by deformation analysis using tissue velocity and speckle-tracking. JACC Cardiovasc Imaging. 2010;3:121–31. doi: 10.1016/j.jcmg.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 68.Mavinkurve-Groothius AMC, Groot-Loonen J, Marcus KA, Bellersen L, Feuth T, Bökkerink JPM, et al. Myocardial strain and strain rate in monitoring subclinical heart failure in asymptomatic long-term survivors of childhood cancer. Ultrasound Med Biol. 2010;36:1783–91. doi: 10.1016/j.ultrasmedbio.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 69.Hare JL, Brown JK, Leano R, Jenkins C, Woodward N, Marwick TH. Use of myocardial deformation imaging to detect preclinical myocardial dysfunction before conventional measures in patients undergoing breast cancer treatment with traztuzumab. Am Heart J. 2009;158:294–301. doi: 10.1016/j.ahj.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 70.Jurcut R, Wildiers H, Ganame J, D’hooge J, De Backer J, Denys H, et al. Strain rate imaging detects early cardiac effects of pegylated liposomal doxorubicin as adjuvant therapy in elderly patients with breast cancer. J Am Soc Echocardiogr. 2008;21:1283–9. doi: 10.1016/j.echo.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 71.Wong CY, O’Moore-Sullivan T, Leano R, Byrne N, Beller E, Marwick TH. Alterations of left ventricular myocardial characteristics associated with obesity. Circulation. 2004;110:3081–7. doi: 10.1161/01.CIR.0000147184.13872.0F. [DOI] [PubMed] [Google Scholar]
- 72.Mizayaki S, Daimon M, Mizayaki T, Onishi Y, Koiso Y, Nishizaki Y, et al. Global longitudinal strain in relation to the severity of aortic stenosis: A two-dimensional speckle tracking study. Echocardiography. 2011;28:703–8. doi: 10.1111/j.1540-8175.2011.01419.x. [DOI] [PubMed] [Google Scholar]
- 73.Sun JP, Stewart WJ, Yang XS, Donnell RO, Leon AR, Feiner JM, et al. Differentiation of hypertrophic cardiomyopathy and cardiac amyloidosis by two-dimensional strain imaging echocardiography. Am J Cardiol. 2009;103:411–5. doi: 10.1016/j.amjcard.2008.09.102. [DOI] [PubMed] [Google Scholar]
- 74.Takamura T, Dohi K, Onishi K, Tanabe M, Sugiura E, Nakajima H, et al. Left ventricular contraction-relaxation coupling in normal, hypertrophic, and failing myocardium quantified by speckle-tracking global strain and strain rate imaging. J Am Soc Echocardiogr. 2010;23:747–54. doi: 10.1016/j.echo.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 75.Voigt JU, Arnold MF, Karlsson M, Hübbert L, Kukulski T, Hatle L, et al. Assessment of regional longitudinal myocardial strain rate derived from doppler myocardial imaging indexes in normal and infracted myocardium. J Am Soc Echoardiogr. 2000;13:588–98. doi: 10.1067/mje.2000.105631. [DOI] [PubMed] [Google Scholar]
- 76.Marwick TH, Leano RL, Brown J, Sun JP, Hoffman R, Lysansky P, et al. Myocardial strain measurement with 2-dimensional speckle tracking echocardiography. Definition of normal range. J Am Coll Cardiol Imaging. 2009;2:80–4. doi: 10.1016/j.jcmg.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 77.Lindqvist P, Morner S, Henein MY. Cardiac mechanisms of underlying normal exercise tolerance: Gender Impact. Eur J Appl Physiol. 2011;112:451–9. doi: 10.1007/s00421-011-1992-2. [DOI] [PubMed] [Google Scholar]
- 78.Lawton JS, Cupps BP, Knutsen AK, Ma N, Brady BD, Reynolds LM, et al. Magnetic resonance imaging detexts significant sex differences in human myocardial strain. Biomed Eng Online. 2011;10:76. doi: 10.1186/1475-925X-10-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR. Appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging. J Am Coll Cardiol. 2006;48:1475–97. doi: 10.1016/j.jacc.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 80.Cranney GB, Lotan CS, Dean L, Baxley W, Bouchard A, Pohost GM. Left ventricular volume measurement using cardiac axis nuclear magnetic resonance imaging. Validation by calibrated ventricular angiography. Circulation. 1990;82:154–63. doi: 10.1161/01.cir.82.1.154. [DOI] [PubMed] [Google Scholar]
- 81.Zagrosek A, Abdel-Aty H, Boyé P, Wassmuth R, Messroghli D, Utz W, et al. Cardiac magnetic resonance monitors reversible and irreversible myocardial injury in myocarditis. J Am Coll Cardiol Imaging. 2009;2:131–8. doi: 10.1016/j.jcmg.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 82.Abdel-Aty H, Zagrosek A, Schulz-Menger J, Taylor AJ, Messroghli D, Kumar A, et al. Delayed enhancement and T2-weighted cardiovascular magnetic resonance imaging differentiate acute from chronic myocardial infarction. Circulation. 2004;109:2411–6. doi: 10.1161/01.CIR.0000127428.10985.C6. [DOI] [PubMed] [Google Scholar]
- 83.Assomull RG, Lyne JC, Keenan N, Gulati A, Bunce NH, Davies SW, et al. The role of cardiovascular magnetic resonance in patients presenting with chest pain, raised troponin, and unobstructed coronary arteries. Eur Hear J. 2007;28:1242–9. doi: 10.1093/eurheartj/ehm113. [DOI] [PubMed] [Google Scholar]
- 84.Praga C, Beretta G, Vigo PL, Lenaz GR, Pollini C, Bonadonna G, et al. Adriamycin cardiotoxicity: A survey of 1273 patients. Cancer Treat Rep. 1979;63:827–34. [PubMed] [Google Scholar]
- 85.Wagner A, Marholdt H, Holly TA, Elliott MD, Regenfus M, Parker M, et al. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: An imaging study. Lancet. 2003;361:374–9. doi: 10.1016/S0140-6736(03)12389-6. [DOI] [PubMed] [Google Scholar]
- 86.Marholdt H, Goedecke C, Wagner A, Meinhardt G, Athanasiadis A, Vogelsberg H, et al. Cardiovascular magnetic resonance assessment of human myocarditis: A comparison to histology and molecular pathology. Circulation. 2004;109:1250–8. doi: 10.1161/01.CIR.0000118493.13323.81. [DOI] [PubMed] [Google Scholar]
- 87.Fallah-Rad N, Lytwyn M, Fang T, Kirkpatrick I, Jassal DS. Delayed contrast enhancement cardiac magnetic resonance imaging in traztuzumab induced cardiomyopathy. J Cardiovasc Mag Res. 2008;10:5. doi: 10.1186/1532-429X-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Iles L, Pfluger H, Phrommintikul A, Cherayath J, Aksit P, Gupta SN, et al. Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced T 1 mapping. J Am Coll Cardiol. 2008;52:1574–80. doi: 10.1016/j.jacc.2008.06.049. [DOI] [PubMed] [Google Scholar]
- 89.Maciera AM, Joshi J, Prasad SK, Moon JC, Perugini E, Harding I, et al. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2005;111:186–93. doi: 10.1161/01.CIR.0000152819.97857.9D. [DOI] [PubMed] [Google Scholar]
- 90.Tham EB, Chow K, Spavor M, Pagano JJ, Haykowsky M, Thompson R. Degree of diffuse fibrosis measured by cardiac MRI correlates with LV remodeling in childhood cancer survivors after anthracycline chemotherapy [abstract] J Cardiovasc Magn Reson. 2011;13:P276. [Google Scholar]
- 91.Steel K, Broderick R, Gandla V, Larose E, Resnic F, Jerosch-Herold M, et al. Complementary prognostic values of stress myocardial perfusion and late gadolinium enhancement imaging by cardiac magnetic resonance in patients with known or suspected coronary artery disease. Circulation. 2009;120:1390–400. doi: 10.1161/CIRCULATIONAHA.108.812503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bruder O, Wagner A, Jensen CJ, Schneider S, Ong P, Kispert EM, et al. Myocardial scar visualized by cardiovascular magnetic resonance imaging predicts major adverse events in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56:875–87. doi: 10.1016/j.jacc.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 93.Adabag AS, Maron BJ, Appelbaum E, Harrigan CJ, Buros JL, Gibson CM, et al. Occurrence and frequency of arrhythmias in hypertrophic cardiomyopathy in relation to delayed enhancement on cardiovascular magnetic resonance. J Am Coll Cardiol. 2008;51:1369–74. doi: 10.1016/j.jacc.2007.11.071. [DOI] [PubMed] [Google Scholar]
- 94.Mekinian A, Lions C, Leleu X, Duhamel A, Lamblin N, Coiteux V, et al. Prognosis assessment of cardiac involvement of systemic AL amyloidosis by magnetic resonance imaging. Am J Med. 2010;123:864–8. doi: 10.1016/j.amjmed.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 95.Patel MR, Cawley PJ, Heitner JF, Klem I, Parker MA, Jaroudi WA, et al. Detection of myocardial damage in patients with sarcoidosis. Circulation. 2009;120:1969–77. doi: 10.1161/CIRCULATIONAHA.109.851352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Azevedo CF, Nigri M, Higuchi ML, Pomerantzeff PM, Spina GS, Sampaio RO, et al. Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. J Am Coll Cardiol. 2010;56:278–87. doi: 10.1016/j.jacc.2009.12.074. [DOI] [PubMed] [Google Scholar]
- 97.Zhang R, Lu W, Wen X, Huang M, Zhou M, Liang D, et al. Annexin A5-conjugated polymeric micelles for dual SPECT and optical detection of apoptosis. J Nucl Med. 2011;52:958–64. doi: 10.2967/jnumed.110.083220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Blankenberg F. To scan or not to scan, it is a question of timing: Technetium-99m-annexin v radionuclide imaging assessment of treatment efficacy after one course of chemotherapy. Clin Cancer Res. 2002;8:2757–8. [PubMed] [Google Scholar]
- 99.Min P, Lim S, Kang S, Hong S, Hwang K, Chung K, et al. Targeted ultrasound imaging of apoptosis with annexin A5 microbubbles in acute doxorubicin-induced cardiotoxicity. J Cardiovasc Ultrasound. 2010;18:91–7. doi: 10.4250/jcu.2010.18.3.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bennink RJ, van den Hoff MJ, van Hemert FJ, de Bruin KM, Spijkerboer AL, Vanderheyden J, et al. Annexin V imaging of acute doxorubicin cardiotoxicity (apoptosis) in rats. J Nucl Med. 2004;45:842–8. [PubMed] [Google Scholar]
- 101.Anderson LJ, Holden S, Davis B, et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22:2171–9. doi: 10.1053/euhj.2001.2822. [DOI] [PubMed] [Google Scholar]
- 102.Westwood M, Anderson LJ, Firmin DN, et al. A single breath-hold multiecho T2* cardiovascular magnetic resonance technique for diagnosis of myocardial iron overload. J Magn Reson Imaging. 2003;18:33–9. doi: 10.1002/jmri.10332. [DOI] [PubMed] [Google Scholar]
- 103.Dash R, Chung J, Chan T, Yamada M, Barral J, Nishimura D, et al. A molecular MRI probe to detect treatment of cardiac apoptosis in vivo. Magn Reson Med. 2011;66:1152–62. doi: 10.1002/mrm.22876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bristow MR, Lopez MB, Mason JW, Billingham ME, Winchester MA. Efficacy and cost of cardiac monitoring in patients receiving doxorubicin. Cancer. 1982;50:32–41. doi: 10.1002/1097-0142(19820701)50:1<32::aid-cncr2820500108>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]